Abstract
Simple Summary
Ovarian cancer (OC) has the highest mortality rate of all gynecological cancers. It is usually diagnosed in late stages (FIGO III-IV), and therefore, overall survival is very poor. If diagnosed at the early stages, ovarian cancer has a 90% five-year survival rate. Liquid biopsy has a good potential to improve early ovarian cancer detection and is discussed in this review.
Abstract
Current diagnostic tools used in clinical practice such as transvaginal ultrasound, CA 125, and HE4 are not sensitive and specific enough to diagnose OC in the early stages. A lack of early symptoms and an effective asymptomatic population screening strategy leads to a poor prognosis in OC. New diagnostic and screening methods are urgently needed for early OC diagnosis. Liquid biopsies have been considered as a new noninvasive and promising method, using plasma/serum, uterine lavage, and urine samples for early cancer detection. We analyzed recent studies on molecular biomarkers with specific emphasis on liquid biopsy methods and diagnostic efficacy for OC through the detection of circulating tumor cells, circulating cell-free DNA, small noncoding RNAs, and tumor-educated platelets.
Keywords: ovarian cancer, liquid biopsies, uterine lavage, high-throughput methods, NGS-based multigene panels
1. Introduction
Ovarian cancer (OC) has the highest mortality rate of all gynecologic malignancies [1]. The overall five-year survival is 46% and varies depending on the stage and histological type of the tumor [2]. High-grade serous carcinoma (HGSOC) accounts for 75% of all epithelial ovarian malignancies and is diagnosed mainly at FIGO stage III (51%) or IV (29%), reflecting the aggressive nature [3]. In contrast, nonepithelial and more rare epithelial tumors such as endometrioid, mucinous. and clear-cell carcinomas are more frequently diagnosed at FIGO stages I–II [3]. Consequently, the five-year survival for HGSOC is 43%, compared with 82%, 71%, and 66% for endometrioid, mucinous, and clear-cell carcinoma, respectively. The five-year OS rate is only 9% for FIGO stage IV HGSOC patients [1].
Risk factors for OC can be categorized into genetic and nongenetic risk factors associated with reproductive history, exogenous hormone use, medical history, lifestyle, and environmental influence [4]. It is well established that a family history of OC, especially if a relative was diagnosed under the age of 50, germline BRCA1/BRCA2 mutations, are the strongest risk factor for this pathology. Lynch syndrome-associated mutations in MSH2, MSH6, MLH1, PMS2, and EPCAM, as well as mutations in BRIP1, PALB2, RAD51C, and RAD51D increase the risk for OC. It is considered that approximately 18% of epithelial cancers, in particular high-grade serous carcinomas, are caused by inherited mutations [5,6].
Currently, conventional tools for diagnosing OC are serum cancer antigen 125 (CA 125), Human Epididymis Protein 4 (HE4), and transvaginal ultrasonography (TU). According to the majority of studies, the routine use of CA 125 alone is not adequate for differential diagnosis, as it might increase in other conditions [7,8]. A number of large prospective studies reported that CA 125 ± TU is not sensitive and specific enough for early OC diagnostics, detecting only 30 to 45% of OC in the early stages [7,9,10,11,12]. The use of this approach for OC screening did not demonstrate a survival benefit, and the high rate of false-positive results leads to unnecessary surgery in cancer-free women and is not recommended in the general population due to the potential harms outweighing the potential benefits. An exception could be made for patients with a heredity predisposition to ovarian cancer [7,13,14]. The recent UKCTOS study suggested that in order to improve OC survival rates, we need to reduce the incidence of stage III by more than 10% [15]. Therefore, the development of more sensitive and specific methods for diagnosing OC at the earliest possible stage of the disease would be likely to impact the outcome.
Until recently, OC classification was based on morphology and immunohistochemistry (IHC), but more modern diagnostic approaches take into account molecular genetics, protein post-translational transformations, and immune cell infiltrates [16,17]. Over the last few decades, two distinct pathogenesis models were defined dividing ovarian malignancies into ovarian-origin OC and extra ovarian-origin OC. Ovarian-origin malignancies are very rare, mostly occurring at a young age or in childhood, and are presented by two main groups: (1) sex-cord stromal tumors tend to manifest as low-grade disease with a nonaggressive clinical course and are usually diagnosed at the early stages; (2) predominantly malignant germ cell tumors stand out due to their very fast tumor growth and the progression of clinical symptoms. Therefore, detailed screening tests do not seem mandatory for this category of tumors. The majority of epithelial ovarian cancers (EOCs) and epithelial–stromal ovarian tumors are suspected to be of extra ovarian origin, as the derivative cell is not ovarian (serous, mucinous, endometrioid, clear cell, and others). For clinical decision making, surface epithelial malignancies were further divided into two categories as a function of their pathogenetic pathways: type I and type II [2,18,19,20].
Most malignant tumors of the ovary are surface epithelial (90%). In 2014, the World Health Organization (WHO) recognized five principal epithelial OC histotypes: high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous carcinoma. Other malignancies such as carcinosarcoma, adenosarcoma, and endometrioid stromal sarcoma are very rare; therefore, there is very little data concerning their pathogenesis and molecular features. Moreover, not otherwise specified ovarian tumors such as neuroendocrine, rete ovarii adenocarcinoma, Wilm‘s tumor, and others are exceptionally rare with an incidence of less than 0.1%. The most frequent mutation characteristics according to tumor morphology are presented in Table 1 [18,19,20,21,22,23,24,25,26,27,28,29,30,31].
Table 1.
Discriminatig features of major histotypes of epithelial ovarian cancer.
| Histology | Cells of Origin | Precursors | More Frequent Somatic Mutations |
|---|---|---|---|
| Low-Grade Serous Carcinoma | Fallopian tube progenitor cell or secretory cell | Serous cystadenoma, adenofibroma, atypical proliferative serous tumor, noninvasive micropapillary serous borderline tumor | KRAS (30%), BRAF (30%), NRAS, EIF1AX, USP9X, ERBB2, FRAR1, NF1, HRAS |
| Mucinous Carcinoma | Unknown | Mucinous adenoma, mucinous borderline tumor | CDKN2A (76%), KRAS and TP53 (both 64%), ERBB2 (26%), RNF43, BRAF, PIK3CA, ARID1A (8–12%) |
| Endometrioid Carcinoma | Endometrial epithelial cells | Endometriosis and endometrial cell-like hyperplasia, endometrioid borderline tumor | ARID1A (30%), PIK3CA (30%), TERT, CTNNB1, TP53 |
| Clear-Cell Carcinoma | Endometrial epithelial cells | Endometriosis, endometrioid borderline tumors | PIK3CA (50%), ARID1A (50%), KRAS, MET, PTEN, CTNNB1, RPL22, TP53 |
| High-Grade Serous Carcinoma | Fallopian tube progenitor cell or secretory cell | SCOUT, P53 signature, STIC |
TP53 (96–98%) BRCA1/BRCA2 (10%, 25% somatic + germline); CNAs of CCNE1 amplification, PTEN deletion, RB1 and NF1 loss |
| Carcinosarcomas | Unknown | Carcinomatous component | TP53, CTNNB1 |
STIC—serous tubal intraepithelial carcinomas, SCOUT—secretory cell OUT growth.
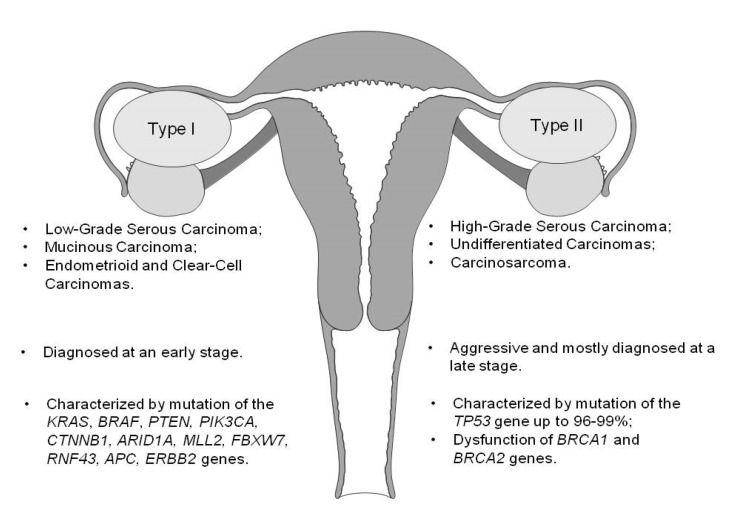
Predominantly type I tumors clinically present as large cystic masses, without ascites, tend to be less aggressive, grow more slowly, and usually are diagnosed at an early stage. They are relatively genetically stable, as well as characterized by mutations in different genes (Figure 1) and rarely harbor TP53 mutations [18,19,20].
Figure 1.
A schematic representation of type I and type II EOC.
Type II tumors are characterized as highly aggressive neoplasms accounting for 75% of all EOCs, which are usually diagnosed at a late stage. They include high-grade serous carcinoma (HGSOC)—the most common type—and rare types such as high-grade endometrioid, undifferentiated carcinomas, and malignant epithelial mesenchymal tumors (carcinosarcomas). Type II ovarian tumors have a high level of genetic instability; the majority harbors TP53 mutations [18,19,20]. Recent data suggest that HGSOC tumors originate from the epithelium of the fallopian tube. Mutation of TP53 is the first known molecular event in the transformation of fallopian tube secretory cells to serous tubal intraepithelial carcinomas (STICs), which leads to HGSOC initiation. Mutated TP53 can be identified as an early tumor precursor of HGSOC. It has been estimated that it takes approximately seven years from STIC to clinically evolve into HGSOC [18,29,32]. Almost 80% of women present with advanced (stages III-IV) disease and poor prognosis (the five-year survival rate is around 25%). Since up to 98% of all HGSOC cases are characterized by TP53 somatic mutations, this biomarker is widely investigated as a potential diagnostic tool for OC diagnostics [18,20,29,31].
2. Materials and Methods
We performed a literature search in NCBI PubMed from January 2014 to September 2020 with a specific emphasis on liquid biopsy biomarkers for early OC detection. We used the keywords “ovarian cancer” together with “circulating free DNA”, ”circulating tumor DNA”, ”circulating tumor cells”, “small non coding RNA”, “microRNA”, “PIWI- interactingRNA”, “Transfer-RNA-derivated small RNA”, “liquid biopsy”, “TEPS”, and “uterine lavage”. We identified 2193 abstracts in NCBI PubMed and selected 30 reports considered inclusion criteria—evaluating the efficacy of liquid biopsies as a diagnostic tool for OC detection. We summarize the results of these studies in Table 2. This work provides deeper understanding of the aspects of OC pathogenesis and existing challenges for liquid biopsy applications in clinical practice.
Table 2.
Studies on ctDNA, DNA, CTC and microRNA in ovarian cancer.
| Author (Year), References | Number of OC Patients | Specimen | Method | Genetic Marker/Antigen | Detection Rate (%) | Detection Rate (%) (I-II Stage) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| K.K Lin et al. (2019) [33] | 112 germline or somatic BRCA-mutant HGOC | Plasma (ctDNA) | Targeted-NGS | BRCA1, BRCA2, TP53 | 96 for TP53 | NR | NR | NR |
| Y. Wang et al. (2018) [34] | 83 OC | Plasma (ctDNA) | Pap SEEK-PCR-based error-reduction technology Safe-SeqS | 18 genes + assay for aneuploidy | 43 | 35 | NR | 100 |
| Y. Wang et al. (2018) [34] | 83 OC | Plasma (ctDNA) + Pap Brush samples | Pap SEEK-PCR-based error-reduction technology Safe-SeqS | 18 genes + assay for aneuploidy | 63 | 54 | NR | 100 |
| P.A. Cohen et al. (2018) [35] | 54 OC | Plasma (ctDNA) + proteins | CancerSEEK Targeted NGS |
16 gene panel + 41 protein biomarkers | 98 | 38 | NR | >99 AUC = 0.91 |
| J. Phallen et al. (2017) [36] | 42 OC | Plasma (ctDNA) | Targeted NGS (TEC-Seq) and ddPCR | 55 gene panel | 71 | 68 | NR | 100 |
| E. Pereira et al. (2015) [37] | 22 HGSOC | Serum (ctDNA) | ddPCR, NGS, WES | TP53, PTEN, PIK3CA, MET, KRAS, FBXW7, BRAF | 93.8 | NR | 81-91 | 60-99 |
| A. Piskorz et al. (2016) [37] | 18 OC | Plasma (ctDNA) | Targeted NGS | TP53 | 100 | NR | NR | NR |
| R.C. Arend et al. (2018) [38] | 14 OC | Plasma (cfDNA) | Targeted NGS | 50 gene | 100 | NR | NR | NR |
| J.D. Cohen et al. (2016) [39] | 32 HGSOC | Plasma cfDNA (instability) |
WEG (WISECONDOR) | CNV | 38 | 40.6 | NR | 93.8 |
| A. Vanderst-ichele et al. [40] | 57 OC and bordline tumors | Plasma cfDNA | WGS | CNV | 67 | NR | NR | 99.6 AUC = 0.89 |
| Y. Wang et al. (2018) [34] | 245 OC | Cervix Pap brush samples (DNA) | Pap SEEK-PCR-based error-reduction technology Safe-SeqS, | 18 genes + assay for aneuploidy | NR | 33 | 34 | 99 |
| Tao Brush (DNA) | Pap SEEK-PCR-based error-reduction technology Safe-SeqS | 18 genes + assay for aneuploidy | NR | 45 | 47 | 100 | ||
| Salk et al. (2019) [41] | 10 OC | Uterine lavage (DNA) | Duplex Sequencing | TP53 | 80 | NR | 70 | 100 |
| E.Maritschnegg (2018) [42] | 33 OC | Uterine lavage (DNA) | Deep-sequencing | AKT1, APC, BRAF, CDKN2A, CTNNB1, EGFR, FBXW7, FGFR2, KRAS, NRAS, PIK3CA, PIK3R1, POLE, PPP2R1A, PTEN, TP53 | 80 for TP53 | NR | NR | NR |
| E.Maritschnegg (2015) [43] | 30 OC | Uterine lavage (DNA) | Massively parallel sequencing | AKT1, APC, BRAF, CDKN2A, CTNNB1, EGFR, FBXW7, FGFR2, | 60 for TP53 | 100 for TP53 | NR | NR |
| With ddPCR and SafeSeqS | KRAS, NRAS, PIK3CA, PIK3R1, POLE, PPP2R1A, PTEN, TP53 | 80 for TP53 | ||||||
| B.K Erickson et al. (2014) [44] | 5 OC | Vaginal tampon (DNA) | Massively parallel sequencing | NR | 60 | NR | 60 | NR |
| Kinde et al. (2013) [45] | 22 OC | Liquid Pap smear tests (DNA) | Massively parallel sequencing | NR | 41 | NR | NR | NR |
| N. Li et al (2019) [46] | 30 EOC | Plasma (CTC) | Magnetic nanospheres (MNs) + IHC | EpCAM, FRα | 92 | NR | 75 | 90 AUC = 0.8 |
| Zhang et al. (2018) [47] | 109 EOC | Plasma (CTC) | Imunomagnetic beads (EpCAM, HER2 and MUC1) + multiplex RT-PCR | EpCAM, HER2, MUC1, WT1, P16, PAX8 | 90 | 93 | NR | NR |
| Q Rao et al. (2017) [48] | 23 EOC | Plasma (CTC) | Microfluidic system with immunomagnetic beads (EpCAM) + IHC | EpCAM, CK3-6H5, panCK | 87 | NR | NR | NR |
| M. Lee et al. (2017) [49] | 54 EOC | Plasma (CTC) | Incorporating a nanoroughened microfluidic platform + IHC | EpCAM, TROP-2, EGFR, Vimentin, N-cadherin | 98.1 | NR | NR | NR |
| Dong Hoon Suh et al. (2017) [50] | 87 EOC, bordline, benigh | Plasma (CTC) | Tapered-slit membrane filters + IHC | EpCAM, CK9 | 56.3 | NR | 77.4 | 55.8 AUC = 0.61–0.75 |
| I. Chebouti et al. (2017) [51] | 95 EOC | Plasma (CTC) | Adna Test Ovarian Cancer and EMT-1 Select/Detect + Multiplex RT-PCR | EpCAM, ERCC1, MUC1, MUC16, PI3Ka, Akt-2, Twist | 82 | NR | >90 | >90 |
| K. Kolostova et al. (2016) [52] | 40 OC | Plasma (CTC) | MetaCell + IHC/qPCR | ICC: NucBlueTM, CelltrackerTM. EpCAM, MUC1, MUC16, KRT18, KRT19, ERCC1, WT1 |
58 | NR | NR | NR |
| K. Kolostova et al (2015) [53] | 118 OC | Plasma (CTC) | MetaCell + IHC/qPCR | ICC: NucBlueTM, CelltrackerTM. EpCAM, MUC1, MUC16, KRT18, KRT19, |
65.2 | NR | NR | NR |
| M. Pearl et al. (2015) [54] | 31 EOC | Plasma (CTC) | CAM uptake-cell enrichment + IHC/RT-qPCR | EpCAM, Ca 125, CD44, seprase EpCAM, CD44, MUC16, FAP |
100 | NR | 83 | 97 |
| Pearl et al. (2014) [55] | 129 EOC | Plasma (CTCs) | CAM uptake – cell enrichment + IHC | EpCAM, Ca 125, CD44, seprase | 88. 6 | 41.2 | 83 | 95.1 |
| Gao et al. (2015) [56] | 143 all 74 EOC | Serum microRNA | qRT-PCR | miR-200c | NR | NR | 72 | 70, AUC = 0.79 |
| miR-141 | 69 | 72, AUC = 0.75 | ||||||
| Meng et al. (2016) [57] | 163 EOC | Serum microRNA | TaqMan microRNA assays and ELISA | miR-200a | NR | NR | 83 | 90, AUC = 0.91 |
| miR-200b | 52 | 100, AUC = 0.81 | ||||||
| miR-200C | 31 | 100, AUC = 0.65 | ||||||
| 3miRNAs set | 88 | 90, AUC = 0.92 | ||||||
| Yokoi et al. in (2017) [58] | 269 all 155EOC | Serum microRNA | qRT-PCR + statistical cross-validation methods | 8 miRNA combination | NR | 86 | 92 | 91, AUC = 0.96 |
| Yokoi et al. in (2018) et al. [59] | EOC 333 | Serum microRNA | Microarrays | 10 miRNAs set miRNA-320a, -665, -1275, -3184-5p, -3185, -3195, -4459, 4640-5p, -6076, and -6717-5p. EOS vs. non cancer |
NR | NR | 99 | 100, AUC = 0.72–1.0 |
| Kim S. (2019) [60] | 68 all 39HGOC | Serum microRNA | qRT-PCR | miRNA-145 | NR | NR | 91.7 | 86.8, AUC = 86.8 |
| miRNA-200C | 72.9 | 90.0, AUC = 77.9 |
NR: not reported; OC- ovarian cancer; EOC: epithelial ovarian cancer; ddPCR: Droplet digital PCR; RT-PCR: real time PCR technology; qRT-PCR: quantitative real time PCR; NGS: next generation sequencing; CAM: cell adhesion matrix; WES: whole exome sequencing; TGS: targeted gene sequences; HGSOC: high grade serous ovarian cancer; ddPCR: droplet digital PCR; AUC- areas under the ROC curves; IHC: immunocytochemistry staging; CNV: Copy number variation; WES: Whole exome sequencing; Safe-SeqS: Safe-sequencing system; WGS: Whole genome sequencing.
3. Modern Means for Early Detection of OC
The essential aspect of any screening test is that it should be cost-effective and easily incorporated into standard medical practice. Furthermore, an ideal test should be reproducible, no-invasive, and able to distinguish between a healthy woman and a patient at an early stage of disease. High specificity to avoid false positives and a sensitivity of at least 75% are needed [61]. Due to the prevalence of <1% of OC in the population, a positive predictive value (PPV) of 10% should be obtained for a cost-effective screening tool. This explains the need for a 75% sensitivity and a 99.6% specificity for early-stage disease. New, potentially promising early diagnostic tools can be based on the fact that the ovarian surface, the fallopian tube, and the uterine cavity form a communicating space. The peristaltic waves of the fallopian tube allow the transport of exfoliated cells from HGSCs or STICs into the uterine cavity and peritoneum. Several different ways can be applied for collecting cancer cells from the Müllerian duct: cytological specimens or uterine lavage samples. Sequencing of TP53 exons is widely performed due to the fact that the majority of HGSOCs are characterized by TP53 mutations [44,62].
3.1. Uterine Cavity Lavage Biomarkers
An approach for the lavage of the uterine cavity to detect cancer cells that have been shed was developed by Paul Speiser, Professor at Medical College of Vienna, and colleagues [63].
A study published by Kinde et al. in 2013 analyzed the liquid Pap test from the uterine cervix for detecting ovarian and uterine cancers. Massively parallel sequencing for tumor-specific mutations using a 12-gene panel was performed on DNA extracted from liquid Pap smear tests. This technique was successfully applied to 100% of patients. Detectible DNA mutations were found in 24 (100%) for endometrial cancer patients and in 9 of 22 (41%) OC, mainly in late stages [45]. A pilot study showed that tumor cells and fragments containing tumor DNA can be found and collected in the vagina using a vaginal tampon and studied by using genetic analysis. They succeeded in revealing TP53 mutations in 60% of advanced HGSOCs [44]. Y. Wang et al. 2018 published data of DNA analysis in Pap brush samples from 245 OC patients, and the detection sensitivity was 33%, including 34% for patients with stage I–II disease [34].
In 2015, E. Maritschnegg et al. collected samples closer to the ovaries and fallopian tubes; uterine cavity lavage was used from 65 patients: 30 with OC, 27 with benign gynecological disease, and 8 with other malignancies. The lavage technique was applied successfully, and sufficient amounts of DNA were obtained. Lavage and tumor specimens were analyzed by massive parallel sequencing. Amplicons comprised gene regions of: AKT1, APC, BRAF, CDKN2A, CTNNB1, EGFR, FBXW7, FGFR2, KRAS, NRAS, PIK3CA, PIK3R1, POLE, PPP2R1A, PTEN, and TP53. Mutations, mainly in TP53, were detected in 60% of OC lavage samples using next-generation sequencing (NGS) approaches. Using additional methods with higher sensitivity, such as digital droplet polymerase chain reaction (ddPCR) and the Safe-sequencing system (SafeSeqS), the mutation detection rate was increased up to 80%. Moreover, TP53 mutation in lavage samples was identified in all patients (N = 5) with FIGO stage IA and one patient with occult OC. Mutations in KRAS were identified (eight of twenty-seven cases) in a benign tumor group mainly, and none of them had the TP53 mutation [43].
Later, in 2018, E. Maritschnegg published data on the uterine lavage technique’s feasibility [42]. The technique was successfully performed in 98.9% of gynecological patients by six different gynecologists in four centers. The median absolute amount of DNA was 2.23 μg. As a result, in 80% (24 of 30) of OC patients, specific mutations could be identified in the samples. The molecular analysis of uterine lavage holds great potential and significant promise for the earlier diagnosis of OC.
Deep sequencing was reported as the method of choice for detecting low-level signatures of tumor-derived mutations in liquid biopsies [63]. Duplex sequencing (DS) uses double-stranded molecular barcodes for error correction and decreases the error rate of sequencing from 10-3 to <10-7, so currently, it is the highest-accuracy sequencing NGS method. J. Salk in a 2018–2019 study demonstrated a high sensitivity (80%) of TP53 mutation detection in OC patients’ uterine lavage using DS. However, low-frequency TP53 mutations can be detected in healthy women without cancer. The TP53 mutation rate progressively increased with age and shared the selection traits of clonal TP53 mutations commonly found in human tumors. These results illustrate that in order to avoid false-positive results, the mutant allele frequency threshold should be used for careful differentiation of cancer-specific changes from age-associated mutations. The combined approach of uterine lavage and DS allows the collection of cancer cells very close to the anatomical site of the tumor’s origin, and an ultra-accurate DNA sequencing technology can detect exceptionally low-frequency mutations [41,64].
3.2. Circulating Tumor Cells
Circulating tumor cells (CTCs) are living tumor cells that are released into the bloodstream. They can be released either by primary tumor or metastases and very rarely can be found in healthy individuals. Various CTC isolation techniques were reported based either on physical (microfluidic platforms, density gradient centrifugation, and others) or biological characteristics (immunoaffinity based, immunomagnetic beads, and the functional cell adhesion molecule (CAM) uptake cell enrichment method). Immunohistochemistry (IHC), analysis by real-time PCR (RT-PCR), fluorescent in situ hybridization, and the detection of epithelial and mesenchymal markers are usually used for CTCs’ identification. Analysis of the CTC detection methods, the diagnostic and prognostic significance in OC by Du-Bois Asante et al. revealed that using only IHC for CTCs’ quantification had detection rates from 7.7 to 98%, while using RT-PCR had a 14–91% rate A combination of these two methods for the identification of CTCs had the detection rates ranging from 65 to 100% [65]. Pearl M. et al. demonstrated CTCs’ detection and isolation by using a CAM-based functional cell enrichment and identification platform and IHC from 129 OC patients before surgery. The detection rate was 88.6%, and the PPV (positive predictive value) for all stages was 97.3% with 83% sensitivity. The sensitivity for the detection of EOC stages I and II was 41.2%, the specificity 95.1% and the PPV 77.8% [55]). When qRT-PCR was added to the IHC for identification of CTCs in the next study, the detection rate increased up to 100% (N = 31/31) with the same specificity and sensitivity [54,55]. Zhang et al. performed CTC detection by immunomagnetic bead screening, targeting epithelial antigens on OC cells, in combination with a multiplex RT-PCR. In early-stage disease (IA-IB), the CTC detection rate was 93%, which compared favorably with the 64% of patients with elevated CA 125 levels [47]. While diagnostic techniques, such as the CAM uptake cell enrichment method for CTC isolation, RT-PCR, and qRT-PCR for CTC quantification have been a major addition to IHC and serum markers, molecular profiling has provided important information on genetic alterations linked to resistance to chemotherapy, which should in the future help clinical decision making.
3.3. Cell-Free DNA and Circulating Tumor DNA
Cell-free DNA (cfDNA) comprises small DNA fragments that circulate freely in the bloodstream. In healthy individuals, cfDNA derives from apoptotic cells and can increase in the case of exercise, inflammation, or trauma. Recent studies confirmed that cancer patients have higher levels of cfDNA in the blood with an average of 180 ng/L (range from 0 to 1000 ng/mL), while healthy individuals or patients with benign ovarian pathologies have an average of 30 ng/mL (range from 0 to 100 ng/mL) [66,67,68]. Kamat et al. found elevated plasma cfDNA levels before surgery in OC patients compared to benign ovarian disease or healthy individuals, suggesting the use of cfDNA as a diagnostic and prognostic marker [69]. A meta-analysis of nine studies by Q. Zhou et al. evaluated the accuracy of cfDNA for the diagnosis of OC. The results showed unsatisfactory sensitivity at 70%, but acceptable specificity at 90% for the diagnosis of OC. Quantitative analysis of cfDNA can hardly be used as an independent diagnostic biomarker for OC detection, but the combination with OC-specific biomarkers detectable in cfDNA may be a promising tool [70].
Apart from evaluating quantitative changes, various investigators focused on qualitative changes, including somatic mutations, aberrant DNA methylation, and chromosomal abnormalities in circulating cell-free DNA. Plasma circulating tumor DNA (ctDNA) has the potential to serve as a minimally invasive diagnostic tool with the detection sensitivity ranging from 76 to 83% and a specificity of 55–95%. Some studies examined gene fusion and somatic copy number variations. Analysis of ctDNA by using NGS and digital polymerase chain reaction (dPCR) has the advantage of identifying alterations that are specific to the tumor. On the other hand, pre-identification of mutant gene targets is required. Most researchers performing ctDNA analysis in OC are currently focused on HGSOC patients, and targeting of mutant TP53 has demonstrated high sensitivity (>75–100%) and specificity (>80%) [36,65,71,72]. According to our literature review, J. Phallen et al. achieved the highest—68%—detection rate of OC FIGO stages I–II with 100% specificity. Targeted error correction sequencing (TEC-Seq) and digital droplet PCR for ctDNA detection were used in this study. The analytical performance characteristics of TEC-Seq, as well as ultrasensitive direct evaluation of sequence changes in ctDNA using massively parallel sequencing suggested that it may be suitable for early-stage OC. A variety of experimental and bioinformatic aspects may contribute to the high specificity of the TEC-Seq method such as deep sequencing and others [36]. A limitation of this study was the small number of patients included. Cohen et al. used a commercial blood test called CancerSEEK to analyze circulating proteins and mutations in cell-free DNA. On the basis of this study, the ctDNA detection rate was 98% for OC patients, but the early-stage detection rate was only up to 38%. The sensitivity of this test for OC was 99% [35]. Investigators at Johns Hopkins Kimmel Cancer Center applied the PapSEEK test (assay for mutations in 18 genes and assay for aneuploidy) to fluids from Pap brush, Tao brush, and plasma ctDNA. There were 1002 healthy controls, 382 with endometrial cancer, and 245 OC patients analyzed. ctDNA was found in 43% of 83 OC patients with the available plasma sample. When both Pap brush and plasma samples were tested, the sensitivity for OC patients was at 63% for all stages and 54% for early stages with 100% specificity [34].
3.4. Circulating Small Noncoding RNAs
3.4.1. sncRNAs Are a Large Group of RNA Molecules with Size below <200 nt That Have No Protein Coding Potency
Small noncoding RNAs (sncRNAs), such as microRNAs (miRNAs), transfer RNA-derived small RNAs (tsRNAs), and P-element induced wimpy testis (PIWI) interacting small RNAs (piRNAs), are a hot spot in the field of biomedical research due to their active involvement in the initiation and development of various malignancies. Relative stability, short length, association with the Argonaute (Ago) family of proteins, and in most cases, the downregulation or silencing of target gene expression are the main common features of all sncRNAs [73].
3.4.2. PIWI-Interacting RNA
PIWI-interacting RNA (piRNAs) interact with PIWIs—germline-specific Ago family nuclear RNA-binding proteins—and form piRNA-induced silencing complexes (piRISCs). The latest data demonstrate the contribution of piRNAs and PIWI proteins to the main carcinogenesis events: cell proliferation, resisting cell death, genome instability, invasion, and metastasis. PIWIs are essential for germline tissues and gametogenesis. Due to their restricted expression in reproductive tissue and tumors, PIWIs are classified as cancer/testis antigens (CTA). They are considered as excellent objects for diagnostic/prognostic biomarkers and targeted therapies. piRNAs regulate mechanistic RNA-based inhibition of transposable elements in germlines. They can target nontransposable elements as well—such as protein-coding messenger RNAs (mRNAs) —and modulate their expression, not only in germlines, but also in somatic cells, by a mechanism similar to that of miRNAs. piRISCs contribute to cancer development and progression by promoting a stem-like state of cancer cells, or cancer stem cells. The expression of germline genes in cancer reflects the ectopic activation in somatic tissues of a naturally silenced developmental program managing the escape from cell death, immune circumvention, and invasiveness [73,74]. In gynecologic malignancies, the study of piRNA pathophysiological significance, expression levels, and diagnostic performance remains exploratory.
3.4.3. Transfer RNA-Derived Small RNAs
Transfer RNAs (tRNAS) are ncRNAs that deliver amino acids to ribosomes during protein biosynthesis. Transfer RNA-derived small RNAs (tsRNAs) are unique sequences generated in the nucleus and derived from tRNA precursors or mature molecules and can be divided into two main groups: (1) transfer RNA-derived RNA fragments (tRFs) and (2) tRNA halves (tiRNAs). The production of tiRNAs is induced by stress such as starvation, hypoxia, heat shock, viral infection, and others. tsRNAs’ functional mechanism remains largely unknown. They play important roles in carcinogenesis, and their stability and higher expression levels place them as ideal diagnostic and prognostic biomarkers and therapeutic targets as well. We found no data reporting the diagnostic performance in terms of specificity and sensitivity of tRFs and tiRNAs in gynecological malignancies [73].
3.4.4. microRNA
The investigation of the microRNA (miRNA) class has received the most attention of all sncRNAs to date. At present, >2800 human mature miRNAs have been identified and are registered at miRbase Release 22 [75]. miRNAs are involved in post-transcriptional regulation of gene expression through their binding to a complementary target. Depending on the region they bind to, they can lead to suppression or degradation of the target. miRNAs, which are overexpressed and targeted to tumor-suppressive protein-coding transcripts, are classified as oncogenic or onco-miRs. Tumor-suppressive miRNAs are responsible for the downregulation of oncogenes and are usually lost in cancer. The expression rate of the same miRNA may be different depending on the biological substance being tested. After biogenesis, miRNAs are secreted from cells to a variety of body fluids, such as plasma/serum, urine and vaginal discharge, breast milk, and others. They are bound to specific proteins or high-density lipoproteins or packed in extracellular vesicles (EV), such as exosomes, to avoid RNase degradation, and as a result, they acquire high stability. Exosomes play an important role in the information exchange between cells, and cancer derived exosomes reflect the tumor-specific miRNA profile [73].
The regulatory roles of miRNAs have been demonstrated in tumorigenesis, cell differentiation, proliferation, and apoptosis. For example, exosomal miRNA-200a, miRNA-26a are reported to be involved in OC cell proliferation, while miR-21–3p, miR-125 b-5p, miR-181 d-5p, and miRNA-205 promote tumor invasion and metastasis. miRNA-125b was shown to inhibit angiogenesis, while miRNA-374a, miRNA-374, miRNA-622, and miRNA-223 were shown to participate in cisplatin resistance mechanisms. miRNAs have been reported to have faster biogenesis and activation rates and longer half-lives relative to mRNA and proteins, which make them suitable for OC diagnostics at early stages [76,77,78,79].
A number of studies dedicated to the diagnostic, prognostic, and therapeutic potential of circulating miRNAs in OC have been published during the last decade. The pioneering study by Taylor et al. in 2008 documented eight exosome miRNAs: miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205, and miR-214 were reported to be elevated in the serum of OC patients compared to normal controls. Quantitatively, the eight different investigated miRNAs were not significantly different in early and late OC stages [80]. Subsequently, several lines of evidence reported that serum miRNAs (miRNA-141, miRNA-200a, miRNA-200b, and miRNA-200c) were upregulated in OC patients compared to normal controls or borderline tumors [56,57]. Moreover, differences in miRNA-200c expression levels between OC stages might contribute to the cancer staging system, since more advanced tumors have lower miRNA-200c levels. On the contrary, the level of miRNA-141 displayed a trend towards increasing from FIGO stage I to stage IV [56]. Kim S. et al. analyzed seven serum exosomal miRNAs and reported that miRNA-141, 200a, and 200b were expressed at extremely low levels and qualified them as inappropriate serological biomarkers. Expression levels of miRNA-93, -145, and -200c were significantly more elevated in cancer tissues as compared to benign and borderline tumors. miRNA-145 was identified as the best-performing single marker with a sensitivity of 91.7% and accuracy of 86.8%. Even higher sensitivity (97.9%) was observed when miRNA-145 was combined with CA 125 assessment [60]. However, altered levels of miRNA-145, as well as some other widely investigated miRNAs (miRNA-21, miRNA-221, miRNA-155) were observed not only in OC, but in other malignancies as well [81]. Numerous studies reported the excellent behavior of the selected miRNAs or their combinations as biomarkers for OC, but did not investigate whether the profile of OC patients is distinct from those of other cancers, so it becomes clear that any single miRNA is unlikely to be a reliable biomarker. Yokoi et al. in 2017 performed miRNA sequencing to identify candidate miRNAs that could be useful in the early detection of OC and cancer subtype classification. They identified eight miRNAs, which were validated by qRT-PCR and statistical cross-validation with a large research cohort and were applied to determine the optimal combination of miRNAs. They succeeded in distinguishing early-stage OC from benign tumors with 86% sensitivity and 83% specificity and OC patients versus healthy controls with 92% and 91% respectively [58].
Later Yokoi et al. constructed three kinds of discrimination models: (1) OC vs. noncancer, (2) OC vs. other cancers + noncancer, and (3) OC vs. borderline/benign ovarian tumors + noncancer. A total of 4046 serum samples, including 333 ovarian cancers, 66 borderline ovarian tumors, 29 benign ovarian tumors, 2759 noncancer controls, and 859 other solid cancers, were analyzed by a miRNA microarray, yielding comprehensive miRNA expression profiles. This is the first large-scale comprehensive analysis of circulating miRNAs in OC, which identified promising miRNA combinations for early–stage detection of OC. Data revealed that selected combined miRNAs could be successfully used to discriminate OC from lung, gastric, breast, hepatic, colorectal, and pancreatic carcinoma, but not from sarcoma and esophageal squamous cell carcinoma. While using circulating miRNA profiles, a sensitivity of 99% and a specificity of 100% were observed discriminating between OC and noncancer patients, but discrimination was more difficult between OC and borderline or benign ovarian tumors [59].
3.5. Other Potential Biomarkers
3.5.1. Long Noncoding RNAs
Long noncoding RNAs (lncRNAs) are class of mRNA-like transcripts that are longer than 200 nucleotides. They lack a protein-coding ability and are involved in various biological roles. Similar to miRNAs, lncRNAs are frequently aberrantly expressed in different types of cancer. Few studies have examined lncRNAs or their combination with other circulating markers for colorectal, hepatocellular, and lung cancer detection and showed their diagnostic potential [82,83,84].
3.5.2. Extracellular Vesicle-Associated Proteins
Extracellular vesicles (EVs) contain cell surface proteins, as well as miRNAs and other molecules. EV-associated proteins and lncRNAs were investigated as potential biomarkers and showed greater sensitivity comparing to conventional biomarkers, but there are no data about the value to OC patients [82,83,84,85].
3.5.3. Tumor-Educated Platelets
Tumor-educated platelets (TEPs) are known for their function as the main player in the systemic/local responses to tumor growth and their ability to change the RNA profile. Tumor-associated biomolecules are transferred to platelets, resulting in their “education”, and also, platelets are activated to induce specific splicing of premessenger RNAs (pre-mRNAs). TEPs may offer certain advantages, including their abundance and easy isolation, their high-quality RNAand their capacity to process RNA in response to external signals. In 2015, Best et al. first suggested the diagnostic potential of TEPs by mRNA sequencing. Their patient cohort included six tumor types: nonsmall-cell lung carcinoma, colorectal cancer, glioblastoma, pancreatic cancer, hepatobiliary cancer, and breast cancer. Their study revealed that TEPs can help to distinguish cancer patients from healthy individuals with a very high accuracy up to 96% and the primary tumor location with 71% accuracy [86]. Piek et al. in 2019 investigated TEPs as a diagnostic tool in differentiating OC FIGO stages I–II from benign ovarian tumors and reached 80% accuracy [87]. In the future, combinatorial analysis of TEPs with ctDNA/CTC can function as a potential blood-based source for cancer diagnostics. An ongoing clinical trial (NCT04022863), evaluating the accuracy of TEPs and ctDNA to determine the nature of an ovarian tumor, will bring more precise information on TEPs’ utility in OC detection [88].
4. Discussion
The early detection of OC, especially as early as FIGO stage II, appears critical to reduce mortality. The development of an efficient and cost-effective early OC screening test could be the path towards improved diagnostics for OC. This might lead to a substantial increase in patients who can undergo complete tumor resection and have less surgical complications due to smaller tumor burdens at the start.
Liquid biopsy has an advantage over traditional biopsies in providing easy access and potentially additional biological information that might be useful for treatment decisions such as by the molecular analysis of a variety of material: CTC, ctDNA, and sncRNAs.
A present limitation to assessing the value of liquid biopsies as a suitable diagnostic tool is the small number of patients enrolled in the studies, especially at early disease stages. Our review of the literature also showed significant variability in the terminology, in the timing and methodology of sampling, in the selection of gene panels assessed, in the methodology of isolation, and in the histological types of OC.
CTC: The impact of CTC isolation, detection, and molecular profiling and the cell capture technique selection for the purpose of early OC diagnosis is not clear. The detection rate of CTC in OC patients varies from 56–100% depending on the techniques [46,47,49,50,51,52,53,54,55]. Most studies evaluated CTC in advanced OC patients with only two studies reporting detection rates of 93% [47] and 41% [55] in early-stage disease. Presently, there is a high variability between platforms for the techniques of CTC isolation, and different IHC markers and/or gene panels are used for CTC content evaluation. Large blood samples are required. CTC surface markers suffer from a lack of tumor specificity. Only half of the studies reported on the diagnostic efficacy of the assay, and the sensitivity of the method was between 70 and 90% [46,50,51,54,55]. The feasibility of the technique in early-stage disease remains questionable.
ctDNA: Highly divergent detection rates of ctDNA in plasma samples have been reported, ranging from 38–100% and 35–68% for advanced and early-stage OC, respectively. The diagnostic performance of ctDNA can be evaluated via mutation and aberrant DNA methylation detection in selected genes (mainly TP53) or through the analysis of chromosomal abnormalities. The limitation of such a diagnostic approach could be met in occult OC patients with clinically and radiologically undetectable disease, a quite frequent situation in early HGSOC stages. Somatic mutations in selected genes, such as TP53, KRAS, BRAF, PTEN, or PIK3CA, are detectable in various human cancers and not specific for OC.
The application of NGS-based genetic or epigenetic panels offers a wide possibility to detect early OC-specific changes for cancer screening, but these NGS panels need to be developed and validated for clinical usage along with the standardization of sample collection and library preparation protocols. The development of protocols for tumor DNA collection from the uterine cavity and proximal tube lavage samples offers a new source of tumor-specific liquid biopsies collected from the sites very close to the primary tumor, confirming the likely origin as a gynecological cancer. Several ongoing clinical trials [83,84,86,87,89,90,91] evaluating the uterine lavage approach for OC detection are expected to clarify the most specific and sensitive biomarkers and molecular profiling methods.
miRNA: Despite the great clinical potential of miRNAs, it is also known that selected miRNAs are not specific for one tumor type. In most of the studies, combinations of miRNAs were analyzed with the aim to create specific discrimination models for disease detection and monitoring [57,58,59]. Before miRNAs may be considered as reliable biomarkers for clinical use, there are many issues to be solved, concerning the standardization of miRNA processing (from sample collection and storage to RNA isolation and data analyses) and large-scale validation. Most investigators used qRT-PCR for miRNA expression detection; however, this is a time-consuming and high-tech procedure, therefore not suitable for daily clinical testing. A less complicated, more rapid, more specific diagnostic test for OC is needed. To improve the biomarker sensitivity and specificity, further studies continue such as a national project in Japan, entitled the Development and Diagnostic Technology for Detection of miRNAs in Body fluids, which investigates serum miRNA profiles in 13 types of human cancers, including OC, which plans to include 10,000 patients. The aim of this study is to develop an algorithm allowing differentiating cancer from noncancer controls using expression levels of serum miRNA. Future research directions may also be highlighted. Despite the great clinical potential of miRNAs, the selection of OC-specific miRNAs remains a challenge due to the secretion of miRNAs from various cells, including blood cells.
5. Conclusions and Future Prospects
Innovative technologies based on very small samples are likely to drastically change medical practice in the near future. Presently available liquid biopsy assessments are not ready for use in clinical practice. Significant efforts remain to create reliable tests for early OC detection. Uterine lavage techniques are easy to apply and safe, and this approach appears very promising for implementation in daily clinical practice. miRNAs are promising biomarkers for cancer diagnosis and prognosis, and large-scale prospective clinical studies are ongoing. Research efforts directed toward single-cell analysis are likely to shed more light on diagnostic biomarkers and potential therapeutic targets in the future.
Author Contributions
Conceptualization, R.Č., D.Ž.; formal analysis R.Č., D.Ž., S.J.; data curation, R.Č. and D.Ž.; writing—original draft preparation R.Č., D.Ž., S.J. and R.S.; writing—review and editing all authors, visualization R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jessmon P., Boulanger T., Zhou W., Patwardhan P. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev. Anticancer. Ther. 2017;17:5427–5437. doi: 10.1080/14737140.2017.1299575. [DOI] [PubMed] [Google Scholar]
- 2.Testa U., Petrucci E., Pasquini L., Castelli G., Pelosi E. Ovarian cancers: Genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicine. 2018;5:16. doi: 10.3390/medicines5010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics. CA Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb P.M., Jordan S.J. Epidemiology of epithelial ovarian cancer. Best Pr. Res. Clin. Obstet. Cancer Biol. Med. 2017;14:9–32. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Weiderpass E., Tyczynski J.E. Epidemiology of Patients with Ovarian Cancer with and without a BRCA1/2 Mutation. Mol. Diagn. Ther. 2015;19:351–364. doi: 10.1007/s40291-015-0168-x. [DOI] [PubMed] [Google Scholar]
- 6. [(accessed on 20 November 2020)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf.
- 7.Temkin S.M., Miller E.A., Samimi G., Berg C.D., Pinsky P., Minasian L. Outcomes from ovarian cancer screening in the PLCO trial: Histologic heterogeneity impacts detection, overdiagnosis and survival. Eur. J. Cancer. 2017;87:182–188. doi: 10.1016/j.ejca.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Moss E., Hollingworth J., Reynolds T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005;58:308–312. doi: 10.1136/jcp.2004.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia M., Deng J., Cheng X., Cheng Z., Yan L.Q.C., Xing Y.Y., Fan D.M., Tina X.Y. Diagnostic accuracy of urine HE4 in patients with ovarian cancer: A meta-analysis. Oncotarget. 2017;8:9660–9671. doi: 10.18632/oncotarget.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romagnolo C., Leon A.E., Fabricio A.S., Taborelli M., Polesel J., Del Pup L., Steffan A., Cervo S., Ravaggi A., Zanotti L., et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: An Italian multicenter study. Gynecol. Oncol. 2016;141:303–311. doi: 10.1016/j.ygyno.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Wei S., Li H., Zhang B. The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer. Biomed. Rep. 2016;5:41–44. doi: 10.3892/br.2016.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H., Yamada Y., Sado T., Sakata M., Yoshida S., Kawaguchi R., Kanayama S., Shigetomi H., Haruta S., Tsuji Y., et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer. 2008;18:414–420. doi: 10.1111/j.1525-1438.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 13.Campbell S., Gentry-Maharaj A. The role of transvaginal ultrasound in screening for ovarian cancer. Climacteric. 2018;21:221–226. doi: 10.1080/13697137.2018.1433656. [DOI] [PubMed] [Google Scholar]
- 14.U.S.Preventive Services Task Force Screening for Ovarian Cancer: Recommendation Statement. [(accessed on 11 November 2019)];Am. Fam. Physician. 2005 71:759–762. Available online: https://www.aafp.org/afp/2005/0215/p759.html. [PubMed] [Google Scholar]
- 15.Menon U., Gentry-Maharaj A., Burnell M., Singh N., Ryan A., Karpinskyj C., Carlino G., Taylor J., Massingham S.K., Raikou M., et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2021;397:2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng W., Rongting H., Liang Y. Crosstalk of intracellular post-translational modifications in cancer. Arch. Biochem. Biophys. 2019;676:108138. doi: 10.1016/j.abb.2019.108138. ISSN 0003-9861. [DOI] [PubMed] [Google Scholar]
- 17.Herrera F.G., Irving M., Kandalaft L., Coukos G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019;20:e417–e433. doi: 10.1016/S1470-2045(19)30401-2. [DOI] [PubMed] [Google Scholar]
- 18.Kurman R. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann. Oncol. 2013;24:x16–x21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 19.Koshiyama M., Matsumura N., Konishi I. Recent Concepts of Ovarian Carcinogenesis: Type I and Type II. BioMed Res. Int. 2014;2014:934261. doi: 10.1155/2014/934261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Bairi K., Al Jarroudi O., Le Page C., Afqir S. Does the “Devil” originate from the fallopian tubes? Semin. Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.03.018. in press; ISSN 1044-579X. [DOI] [PubMed] [Google Scholar]
- 21.Vang R., Shih I.M., Kurman R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009;16:267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer G., Kurman R.J., Chang H.-W., Cho S.K., Shih I.-M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am. J. Pathol. 2002;160:1223–1228. doi: 10.1016/S0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer G., Oldt R., Cohen Y., Wang B., Sidransky D., Kurman R.J., Shih I.-M. Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. J. Natl. Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 24.Sieben N.L.G., Macropoulos P., Roemen G.M.J.M. In ovarian neoplasms, BRAF, but not KRAS mutations are restricted to low-grade serous tumours. J. Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 25.Seidman J.D., Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with walthard cell nests: A study of 120 tumors. Arch. Pathol. Lab. Med. 2008;132:1753–1760. doi: 10.5858/132.11.1753. [DOI] [PubMed] [Google Scholar]
- 26.Ricci F., Affatato R., Carrassa L., Damia G. Recent insights into mucinous ovarian carcinoma. Int. J. Mol. Sci. 2018;19:1569. doi: 10.3390/ijms19061569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brilhante A.V.M., Augusto K.L., Portela M.C., Sucupira L.C.G., Oliveira L.A.F., Magalhãe A.J., Pouchaim V., Nóbrega L.R.M., Magalhães T.F., Sobreira L.R.P. Endometriosis and ovarian cancer: An integrative review (endometriosis and ovarian cancer) Asian Pac. J. Cancer Prev. 2017;18:11–16. doi: 10.22034/APJCP.2017.18.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell D., Berchuck A. Birrer MIntegrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroeger P., Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr. Opin. Obstet. Gynecol. 2017;29:26–34. doi: 10.1097/GCO.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheasley D., Wakefield M.J., Ryland G.L., Allan P.E., Alsop K., Amarasinghe K.C., Ananda S., Anglesio M.S., Au-Yeung G., Böhm M., et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019;10:3935. doi: 10.1038/s41467-019-11862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisio M.-A., Fu L., Goyeneche A., Gao Z.-H., Telleria C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019;20:952. doi: 10.3390/ijms20040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labidi-Galy S., Papp E., Hallberg D., Niknafs N., Adleff V., Noe M., Bhattacharya R., Novak M., Jones Phallen J. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017;8:1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin K.K., Harrell M.I., Oza A.M., Oaknin A., Coquard I.R., Tinker A.V., Helman E., Radke M.R., Say C., Vo L.T., et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9:210–219. doi: 10.1158/2159-8290.CD-18-0715. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Li L., Douville C., Cohen J.D., Yen T.T., Kinde I., Sundfelt K., Kjær K.S., Hruban R.H., Shih I.M., et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018;10:eaap8793. doi: 10.1126/scitranslmed.aap8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J.D., Lu Li Y., Wang C., Thoburn B., Afsari Danilova L., Douville C., Javed A.A., Wong F., Mattox A. Detection and localization of surgically respectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phallen J., Sausen M., Adleff V., Leal A., Hruban C., White J., Anagnostou V., Fiksel J., Cristiano S., Papp E. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017;9:403. doi: 10.1126/scitranslmed.aan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira E., Camacho-Vanegas O., Anand S., Sebra R., Camacho S.C., Garnar-Wortzel L., Nair N., Moshier E., Wooten M., Uzilov A., et al. Personalized circulating tumor DNA biomarkers dynamically predict treatment response and survival in gynecologic cancers. PLoS ONE. 2015;10:12. doi: 10.1371/journal.pone.0145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arend R.C., Londoño A.I., Montgomery A.M., Smith H.J., Dobbin Z.C., Katre A.A., Martinez A., Yang E.S., Alvarez R.D., Huh W.K., et al. Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Mol. Cancer Res. 2018;16:813–824. doi: 10.1158/1541-7786.MCR-17-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen P.A., Flowers N., Tong S., Hannan N., Pertile M.D., Hui L. Abnormal plasma DNA profiles in early ovarian cancer using a non-invasive prenatal testing platform: Implications for cancer screening. BMC Med. 2016;14:126. doi: 10.1186/s12916-016-0667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderstichele A., Busschaert P., Smeets D., Landolfo C., Nieuwenhuysen E.V., Leunen K., Neven P., Amant F., Mahner S., Braicu E.I., et al. Chromosomal instability in cell-free DNA as a highly specific biomarker for detection of ovarian cancer in women with adnexal masses Clin. Cancer Res. 2016;23:2223–2231. doi: 10.1158/1078-0432.CCR-16-1078. [DOI] [PubMed] [Google Scholar]
- 41.Salk J.K., Loubent-Senear K., Maritschnegg E., Valentine C.C., Williams L.N., Higgins J.E., Horvat R., Vanderstichele A., Nachmanson D., Baker K.T., et al. Ultra-Sensitive TP53 Sequencing for Cancer Detection Reveals Progressive Clonal Selection in Normal Tissue over a Century of Human Lifespan. Cell Rep. 2019;28:132–144. doi: 10.1016/j.celrep.2019.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maritschnegg E., Heitz F., Pecha N., Bouda J., Trillsch F., Grimm C., Vanderstichele A., Agreiter C., Harter P., Obermayr E., et al. Uterine and Tubal Lavage for Earlier Cancer Detection Using an Innovative Catheter: A Feasibility and Safety Study. Int. J. Gynecol. Cancer. 2018;28:1692–1698. doi: 10.1097/IGC.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maritschnegg E., Wang Y., Pecha N., Horvat R., Van Nieuwenhuysen E., Vergote I., Heitz F., Sehouli J., Kinde I., Diaz L.A., et al. Lavage of the Uterine Cavity for Molecular Detection of Müllerian Duct Carcinomas: A Proof-of-Concept Study. J. Clin. Oncol. 2015;33:4293–4300. doi: 10.1200/JCO.2015.61.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson B.K., Kinde I., Dobbin Z.C., Wang Y., Martin J.Y., Alvarez R.D., Conner M.G., Huh W.K., Roden R.B.S., Kinzler K.W., et al. Detection of somatic TP53 mutations in tampons of patients with high-grade serous ovarian cancer. Obstet. Gynecol. 2014;124:881–885. doi: 10.1097/AOG.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinde I., Bettegowda C., Wang Y., Wu J., Agrawal N., Shih I.M., Kurman R., Dao F., Levine D.A., Giuntoli R., et al. Evaluation of DNA from the papanicolaou test to detect ovarianand endometrial cancers. Sci. Transl Med. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N., Cheng Y., Chen L., Zuo H., Weng Y., Zhou J., Yao Y., Xu B., Gong H., Weng Y., et al. 1428P—Circulating tumour cell detection in epithelial ovarian cancer using dual-component antibodies targeting EpCAM and FRα. Ann. Oncol. 2019;30:5. doi: 10.1093/annonc/mdz095.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Li H., Yu X., Li S., Lei Z., Li C., Zhang Q., Han Q., Li Y., Zhang K., et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell Physiol. Biochem. 2018;48:1983–1994. doi: 10.1159/000492521. [DOI] [PubMed] [Google Scholar]
- 48.Rao Q., Zhang Q., Zhen C., Dai W., Zhang B., Ionescu-Zanetti C., Lin Z., Zhang L. Detection of circulating tumour cells in patients with epithelial ovarian cancer by a microfluidic system. Int. J. Clin. Exp. Pathol. 2017;10:9599–9606. [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M., Kim E.J., Cho Y., Kim S., Chung H.H., Park N.H., Song Y.S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017;145:2361–2365. doi: 10.1016/j.ygyno.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 50.Dong Hoon S., Suh D.H., Kim M., Choi J.Y., Bu J., Kang Y.T., Lee B., Kim K., No J.H., Kim Y.B., et al. Circulating tumor cells in the differential diagnosis of adnexal masses. Oncotarget. 2017;8:77195–77206. doi: 10.18632/oncotarget.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Issam CheboutiKasimir-Bauer S., Buderath P., Wimberger P., Hauch S., Kimmig R., Kuhlmann D. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget. 2017;8:48820. doi: 10.18632/oncotarget.16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolostova K., Pinkas M., Jakabova A., Pospisilova E., Svobodova P., Spicka J., Cegan M., Matkowski R., Bobek V. Molecular characterization of circulating tumor cells in ovarian cancer. Am. J. Canc. Res. 2016;6:973. [PMC free article] [PubMed] [Google Scholar]
- 53.Kolostova K., Pinkas M., Jakabova A., Pospisilova E., Svobodova P., Spicka J., Cegan M., Matkowski R., Bobek V. The added value of circulating tumor cells examination in ovarian cancer staging. Am. J. Canc. Res. 2015;5:3363. [PMC free article] [PubMed] [Google Scholar]
- 54.Pearl M.L., Dong H., Tulley S., Zhao Q., Golightly M., Zucker S., Chen W.-T. Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs) Gynecol. Oncol. 2015;137:229–238. doi: 10.1016/j.ygyno.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearl M., LlZhao Q., Yang Y., Dong H., Tulley S., Zhang Q., Golightly M., Zucker S., Chen W.T. Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol. Oncol. 2014;134:581–590. doi: 10.1016/j.ygyno.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Y.-C., Wu J. microRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumor Biol. 2015;36:4843–4850. doi: 10.1007/s13277-015-3138-3. [DOI] [PubMed] [Google Scholar]
- 57.Meng X., Müller V., Milde-Langosch K., Trillsch F., Pantel K., Schwarzenbach H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget. 2016;7:16923–16935. doi: 10.18632/oncotarget.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokoi A., Yoshioka Y., Hirakawa A., Yamamoto Y., Ishikawa M., Ikeda S.-I., Kato T., Niimi K., Kajiyama H., Kikkawa F., et al. A combination of circulating miRNAs for the early detection of ovarian cancer. Oncotarget. 2017;8:89811–89823. doi: 10.18632/oncotarget.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokoi A., Matsuzaki J., Yamamoto Y., Yoneoka Y., Takahashi K., Shimizu H., Uehara T., Ishikawa M., Ikeda S., Sonoda T., et al. Integrated extracellular microRNA profiling for ovarian cancer screening. Nat. Commun. 2018;9:4319. doi: 10.1038/s41467-018-06434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S., Choi M.C., Jeong J.-Y., Hwang S., Jung S.G., Joo W.D., Park H., Song S.H., Lee C., Kim T.H., et al. Serum exosomal miRNA-145 and miRNA-200c as promising biomarkers for preoperative diagnosis of ovarian carcinomas. J. Cancer. 2019;10:1958–1967. doi: 10.7150/jca.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristjánsdóttir B. Ph.D. Thesis. University of Gothenburg; Gothenburg, Sweden: 2013. Early Diagnosis of Epithelial Ovarian Cancer Analysis of Novel Biomarkers. [Google Scholar]
- 62.Otsuka I., Kameda S., Hoshi K. Early detection of ovarian and fallopian tube cancer by examination of cytological samples from the endometrial cavity. Br. J. Cancer. 2013;109:603–609. doi: 10.1038/bjc.2013.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. [(accessed on 30 May 2014)]; Available online: https://patents.justia.com/patent/10004484.
- 64.Salk J., Loubet-Senear K., Maritschnegg E., Valentine C.C., Williams L.N., Jacob E., Horvat E., Vanderstichele A., Nachmanson D., Baker K.T., et al. Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat. Rev. Genet. 2018;19:269–285. doi: 10.1038/nrg.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du-Bois Asante L., Calapre M., Ziman T.M., Meniawy E.G. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020;468:59–71. doi: 10.1016/j.canlet.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Mari R., Mamessier E., Lambaudie E., Provansal M., Birnbaum D., Bertucci F., Sabatier R. Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease. Cancers. 2019;11:774. doi: 10.3390/cancers11060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbosa A., Peixoto A., Pinto P., Pinheiro M., Teixeira M.R. Potential clinical applications of circulating cell-free DNA in ovarian cancer patients. Expert Rev. Mol. Med. 2018;20:e6. doi: 10.1017/erm.2018.5. [DOI] [PubMed] [Google Scholar]
- 68.Li B., Pu K., Ge L., Wu X. Diagnostic significance assessment of the circulating cell-free DNA in ovarian cancer: An updated meta-analysis. Gene. 2019;714:143993. doi: 10.1016/j.gene.2019.143993. [DOI] [PubMed] [Google Scholar]
- 69.Kamat M., Baldwin D., Urbauer D., Dang L.Y., Han A. Godwin Karlan BY, Simpson JL, Gershenson DM, Coleman RL. Plasma cell-free DNA I ovarian cancer. Cancer. 2010;116:1918–1925. doi: 10.1002/cncr.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Q., Li W., Leng B., Zheng W., He Z., Zuo M., Chen A. Circulating cell free DNA as the diagnostic marker for ovarian cancer: A systematic review and meta-analysis. PLoS ONE. 2016;11:e0155495. doi: 10.1371/journal.pone.0155495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci. Transl. Med. 2014;6:224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piskorz A., Lin K., Morris J.A., Mann E., Oza A.M., Coleman R.L. Feasibility of Monitoring Response to the PARP Inhibitor Rucaparib with Targeted Deep Sequencing of Circulating Tumor DNA (ctDNA) in Women with High-Grade Serous Carcinoma on the ARIEL2 Trial. J. Clin. Oncol. 2016;34:15. doi: 10.1200/JCO.2016.34.15_suppl.5549. [DOI] [Google Scholar]
- 73.Dwivedi S., Rao G., Dey A., Mukherjee P., Wren J., Bhattacharya R. Small Non-Coding-RNA in Gynecological Malignancies. Cancers. 2021;13:1085. doi: 10.3390/cancers13051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan Y., Liu L., Liao M., Zhang C., Hu S., Zou M., Gu M., Li X. Emerging roles for PIWI proteins in cancer. Acta Biochim. Biophys. Sin. 2015;47:315–324. doi: 10.1093/abbs/gmv018. [DOI] [PubMed] [Google Scholar]
- 75. [(accessed on 1 October 2018)]; Available online: http://www.mirbase.org/
- 76.Mateescu B., Batista L., Cardon M., Gruosso T., de Feraudy Y., Mariani O., Nicolas A., Meyniel J.P., Cottu P., Sastre-Garau X., et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat. Med. 2011;17:1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- 77.Shen W., Song M., Liu J., Qiu G., Li T., Hu Y., Liu H. MiR-26a Promotes Ovarian Cancer Proliferation and Tumorigenesis. PLoS ONE. 2014;9:e86871. doi: 10.1371/journal.pone.0086871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li N., Yang L., Wang H., Yi T., Jia X., Chen C., Xu P. MiR-130a and MiR-374a Function as Novel Regulators of Cisplatin Resistance in Human Ovarian Cancer A2780 Cells. PLoS ONE. 2015;10:e0128886. doi: 10.1371/journal.pone.0128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L., Zhao F., Xiao Z., Yao L. Exosomal microRNA-205 is involved in proliferation, migration, invasion, and apoptosis of ovarian cancer cells via regulating VEGFA. Cancer Cell Int. 2019;19:281. doi: 10.1186/s12935-019-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor D.D., Gercel-Taylor C. microRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 81.Matsuzaki J., Ochiya T. Circulating microRNAs and extracellular vesicles as potential cancer biomarkers: A systematic review. Int. J. Clin. Oncol. 2017;22:413–420. doi: 10.1007/s10147-017-1104-3. [DOI] [PubMed] [Google Scholar]
- 82.Liu T., Zhang X., Gao S., Jing F., Yang Y., Du L., Zheng G., Li P., Li C., Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–85563. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang J., Zhuo H., Zhang X., Jiang R., Ji J., Deng L., Qian X., Zhang F., Sun B. A novel biomarker Linc00974 interacting with KRT19 promotes proliferation and metastasis in hepatocellular carcinoma. Cell Death Dis. 2014;5:e1549. doi: 10.1038/cddis.2014.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng H., Wang J., Li J., Zhao M., Huang S.H., Gu Y.Y., Li Y.L., Sun X.J., Yang L., Luo Q. A circulating non-coding RNA panel as an early detection predictor of non-small cell lung cancer. Life Sci. 2016;151:235–242. doi: 10.1016/j.lfs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Chang L., Ni J., Zhu Y., Pang B., Graham P., Zhang H., Li Y. Liquid biopsy in ovarian cancer: Recent advances in circulating extracellular vesicle detection for early diagnosis and monitoring progression. Theranostics. 2019;9:4130–4140. doi: 10.7150/thno.34692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Best M.G., Sol N., Kooi I.J., Tannous B.A., Westerman F., Rustenburg P., Schellen H., Verschueren E., Post E., Koster J., et al. Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. [(accessed on 1 November 2019)]; Available online: https://ijgc.bmj.com/content/29/Suppl_4/A291.3.
- 88. [(accessed on 17 July 2017)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04022863.
- 89. [(accessed on 17 January 2014)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02039388.
- 90. [(accessed on 7 August 2015)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02518256.
- 91. [(accessed on 30 July 2018)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03606486.