Abstract
Simple Summary
In the context of a growing variety in treatment strategies for patients with cancer, especially approaches based on antiangiogenetic pathways, we aimed to identify a useful biomarker for patients with head and neck squamous cell carcinoma (HNSCC). Our experimental results detected vascular endothelial growth factor (VEGF) in patients’ pre-therapeutic plasma, and not serum, which serves as a suitable biomarker for outcome prognostication. Results were validated in an independent cohort, confirming VEGF as an independent predictor (Pi) of outcomes in HNSCC patients. Therefore, pre-therapeutic VEGF in plasma may be an attractive biomarker in future HNSCC studies.
Abstract
Vascular endothelial growth factor (VEGF) is centrally involved in cancer angiogenesis. We hypothesized that pre-therapeutic VEGF levels in serum and plasma differ in their potential as biomarkers for outcomes in head and neck squamous cell carcinoma (HNSCC) patients. As prospectively defined in the study protocols of TRANSCAN-DietINT and NICEI-CIH, we measured VEGF in pretreatment serum and plasma of 75 HNSCC test cohort (TC) patients. We analyzed the prognostic value of VEGF concentrations in serum (VEGFSerum) and plasma (VEGFPlasma) for event-free survival (EFS) utilizing receiver-operating characteristics (ROC). Mean VEGF concentrations in plasma (34.6, 95% CI 26.0–43.3 ng/L) were significantly lower (p = 3.35 × 10−18) than in serum (214.8, 95% CI 179.6–250.0 ng/L) but, based on ROC (area under the curve, AUCPlasma = 0.707, 95% CI 0.573–0.840; p = 0.006 versus AUCSerum = 0.665, 95% CI 0.528–0.801; p = 0.030), superiorly correlated with event-free survival (EFS) of TC patients. Youden indices revealed optimum binary classification with VEGFPlasma 26 ng/L and VEGFSerum 264 ng/L. Kaplan–Meier plots demonstrated superiority of VEGFPlasma in discriminating patients regarding outcome. Patients with VEGFPlasma < 26 ng/L had superior nodal (NC), local (LC) and loco-regional control (LRC) leading to significant prolonged progression-free survival (PFS) and EFS. We successfully validated VEGFPlasma according the cut-off <26 ng/L as predictive for superior outcome in an independent validation cohort (iVC) of 104 HNSCC patients from the studies DeLOS-II and LIFE and found better outcomes including prolonged tumor-specific (TSS) and overall survival (OS). Outcomes in TC and iVC combined again was related to VEGFPlasma, and multivariate Cox regression revealed that VEGFPlasma was an independent outcome predictor. In HNSCC, pre-therapeutic VEGFPlasma is prognostic for outcomes.
Keywords: head and neck squamous cell carcinoma (HNSCC), head and neck cancer (HNC), prognostic biomarker, vascular endothelial growth factor (VEGF), outcome research, survival, angiogenesis, anti-angiogenesis, biomarker validation, multivariate Cox proportional hazard regression
1. Introduction
Vascular endothelial growth factor (VEGF), and its main representative isoform VEGFA, is a main driver of angiogenesis, a hallmark of cancer [1,2]. Produced by a multitude of cell types, e.g., megakaryocytes and platelets [3,4,5], neutrophil granulocytes [6,7], T-lymphocytes [8], and also tumor cells [9,10], VEGF interacts with surrounding stroma and directly or indirectly affects cell proliferation and processes for vessel growth [11,12]. In comparison to healthy controls, VEGF concentrations are upregulated in the tissue, serum, and plasma of patients suffering from various cancers [13,14]. Because VEGF is a central player in physiological and pathophysiological vascularization and angiogenesis and is, not only causatively involved, but also reflects oncological processes, it has already been under investigation as a potential biomarker in several cancer entities [15,16,17,18,19].
In line with this, anti-angiogenetic treatment targeting VEGF with monoclonal antibodies, e.g., Bevacizumab, has been successfully established in 1st and 2nd line treatments for numerous cancer entities [20,21]; however, this has not yet been done for head and neck squamous cell carcinoma (HNSCC). There may be various reasons for this and may include prior difficulties to demonstrate a substantial survival benefit justifying acceptance of adverse events [22]. Such difficulties may result from the limited availability of specimen collected alongside clinical trials [23] and, even more relevant, difficulties in comparing the previous literature because of inconsistent standards in VEGF measurements [24]. A clear link between circulating VEGF in HNSCC patient blood and the relevance of pre-therapeutic levels of VEGF for outcomes appears to be underreported. Moreover, biomarkers identifying patients with a higher risk of relapse or being eligible for targeted anti-angiogenetic therapy would be useful and could lead to more individualized and effective treatments for HNSCC patients.
In this study, we established regular sampling of serum and EDTA-anticoagulated plasma from venous blood draws of HNSCC patients participating in either randomized clinical trials or cohort studies with prospective blood sampling. We demonstrate the superiority of circulating VEGF from plasma over serum of therapy-naïve HNSCC patients as a potential biomarker and further validate its potential as an independent predictor (Pi) of outcome in an independent cohort.
2. Materials and Methods
2.1. Patient Description and Samples
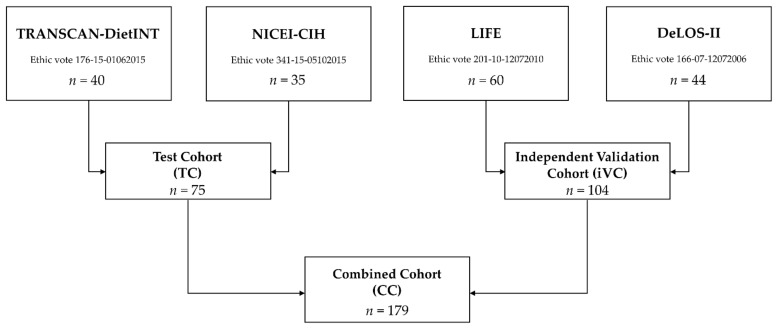
Included in this study were two independent cohorts of therapy-naïve patients with histopathologically confirmed HNSCC (ICD-O-M-8070/3, 8071/3), serving as a test (TC) and an independent validation cohort (iVC), from studies approved by the ethics committee of the Medical Faculty of the University Leipzig (Figure 1). All patients provided written informed consent according to the declaration of Helsinki II prior to participation in the trials and cohort studies described below.
Figure 1.
CONSORT diagram illustrating the selection process of n = 179 HNSCC patients under study.
TC patients participated in one of two trials, either TRANSCAN-DietINT, a randomized phase II study for tertiary prevention of HNSCC with a dietary intervention (ethic vote 176-15-01062015), or NICEI-CIH, a prospective cohort study to analyze the neoantigen spectrum, immunogenicity, and clinical efficacy of immune-checkpoint inhibitors in HNSCC (ethic vote 341-15-05102015). The iVC patients are a subsample from the LIFE cohort (vote 201-10-12072010) [25,26,27] or DeLOS II trial (vote 166-07-12072006) [28,29].
Serum and plasma samples from venous blood of patients were collected prospectively before treatment at the time of diagnosis, aliquoted and stored at −80 °C until enzyme-linked immunosorbent assay (ELISA) measurements (see below). Biopsies of HNSCC were taken under general anesthesia.
Clinical data including TNM categories [30] and staging according to criteria of Union for International Cancer Control (UICC), patient characteristics, such as their Eastern Cooperative Oncology Group performance scores (ECOG) or Charlson comorbidity scores (CS) [31], as well as their clinical course were taken from the tumor database of the Otorhinolaryngology Department of our university hospital. At date of first contact, we collected data comprising epidemiologic information, including self-reported tobacco smoking, and alcohol consumption. Patient characteristics are shown in Table 1 and Table 2.
Table 1.
Characteristics of HNSCC patients of NICEI-CIH and the Leipzig subsample of TRANSCAN-DietINT constituting the test cohort binary classified based on VEGF in plasma (<26 vs. >26 ng/L) or serum (<264 vs. >264 ng/L). Significant differences between groups (p < 0.05) in Pearson’s Chi-square tests are highlighted bold.
| Characteristics | TC Cohort | VEGF Plasma | VEGF Serum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <26 ng/L | >26 ng/L | <264 ng/L | >264 ng/L | ||||||||||
| n | (%) | n | (%) | n | (%) | p Value ‡ | n | (%) | n | (%) | p Value ‡ | ||
| Study | DietINT | 40 | (53.3) | 24 | (61.5) | 16 | (44.4) | 0.1382 | 29 | (56.9) | 11 | (45.8) | 0.3718 |
| NICEI | 35 | (46.7) | 15 | (38.5) | 20 | (55.6) | 22 | (43.1) | 13 | (54.2) | |||
| Sex | female | 11 | (14.7) | 7 | (17.9) | 4 | (11.1) | 0.4030 | 9 | (17.6) | 2 | (8.3) | 0.2875 |
| male | 64 | (85.3) | 32 | (82.1) | 32 | (88.9) | 42 | (82.4) | 22 | (91.7) | |||
| BMI ¶ | 15–24.9 | 36 | (48.0) | 18 | (46.2) | 18 | (50.0) | 0.5578 | 23 | (45.1) | 13 | (54.2) | 0.5227 |
| 25–29.9 | 32 | (42.7) | 16 | (41.0) | 16 | (44.4) | 22 | (43.1) | 10 | (41.7) | |||
| >30 | 7 | (9.3) | 5 | (12.8) | 2 | (5.6) | 6 | (11.8) | 1 | (4.2) | |||
| p16 IHC | negative | 15 | (20.0) | 10 | (25.6) | 5 | (13.9) | 0.4453 | 9 | (18.4) | 6 | (25) | 0.5100 |
| (CINtec+) | positive | 58 | (77.3) | 28 | (71.8) | 30 | (83.3) | 40 | (81.6) | 18 | (75) | ||
| unknown | 2 | (2.7) | 1 | (2.6) | 1 | (2.8) | 2 | (3.9) | -- | (--) | |||
| Number of | 0 | 12 | (16.0) | 7 | (17.9) | 5 | (13.9) | 0.7700 | 8 | (15.7) | 4 | (16.7) | 0.6833 |
| positive | 1–2 | 26 | (34.7) | 14 | (35.9) | 12 | (33.3) | 18 | (35.3) | 8 | (33.3) | ||
| nodes | 3–4 | 23 | (30.7) | 11 | (28.2) | 12 | (33.3) | 15 | (29.4) | 8 | (33.3) | ||
| 5–8 | 10 | (13.3) | 6 | (15.4) | 4 | (11.1) | 6 | (11.8) | 4 | (16.7) | |||
| >8 | 4 | (5.3) | 1 | (2.6) | 3 | (8.3) | 4 | (7.8) | -- | (--) | |||
| Extranodal | negative | 22 | (29.3) | 14 | (35.9) | 8 | (22.2) | 0.4714 | 18 | (35.3) | 4 | (16.7) | 0.4399 |
| extension | positive | 37 | (49.3) | 20 | (51.3) | 17 | (47.2) | 27 | (52.9) | 10 | (41.7) | ||
| unknown | 16 | (21.3) | 5 | (12.8) | 11 | (30.6) | 6 | (11.8) | 10 | (41.7) | |||
| Grading | 1 | 1 | (1.3) | 1 | (2.6) | -- | (--) | 0.242 | 1 | (2.0) | -- | (--) | 0.6376 |
| 2 | 33 | (44.0) | 14 | (35.9) | 19 | (52.8) | 21 | (41.2) | 12 | (50.0) | |||
| 3 | 41 | (54.7) | 24 | (61.5) | 17 | (47.2) | 29 | (56.9) | 12 | (50.0) | |||
| Lymphatic | no | 7 | (9.3) | 3 | (7.7) | 4 | (11.1) | 0.4659 | 4 | (7.8) | 3 | (12.5) | 0.3472 |
| invasion | yes | 54 | (72.0) | 31 | (79.5) | 23 | (63.9) | 40 | (78.4) | 14 | (58.3) | ||
| unknown | 14 | (18.7) | 5 | (12.8) | 9 | (25.0) | 7 | (13.7) | 7 | (29.2) | |||
| Vascular | no | 57 | (76.0) | 31 | (79.5) | 26 | (72.2) | 0.6769 | 41 | (80.4) | 16 | (66.7) | 0.2839 |
| invasion | yes | 3 | (4.0) | 2 | (5.1) | 1 | (2.8) | 3 | (5.9) | -- | (--) | ||
| unknown | 15 | (20.0) | 6 | (15.4) | 9 | (25.0) | 7 | (13.7) | 8 | (33.3) | |||
| Perineural | no | 51 | (68.0) | 30 | (76.9) | 21 | (58.3) | 0.1564 | 38 | (74.5) | 13 | (54.2) | 0.6237 |
| invasion | yes | 9 | (12.0) | 3 | (7.7) | 6 | (16.7) | 6 | (11.8) | 3 | (12.5) | ||
| unknown | 15 | (20.0) | 6 | (15.4) | 9 | (25.0 | 7 | (13.7) | 8 | (33.3) | |||
| Any soft risk | no | 5 | (6.7) | 3 | (7.7) | 2 | (5.6) | 0.8144 | 3 | (5.9) | 2 | (8.3) | 0.4813 |
| factor | yes | 55 | (73.3) | 30 | (76.9) | 25 | (69.4) | 41 | (80.4) | 14 | (58.3) | ||
| unknown | 15 | (20.0) | 6 | (15.4) | 9 | (25.0) | 7 | (13.7) | 8 | (33.3) | |||
| T category | T1 | 9 | (12.0) | 6 | (15.4) | 3 | (8.3) | 0.4759 | 7 | (13.7) | 2 | (8.3) | 0.1460 |
| TNM 2017 | T2 | 28 | (37.3) | 15 | (38.5) | 13 | (36.1) | 19 | (37.3) | 9 | (37.5) | ||
| T3 | 24 | (32.0) | 13 | (33.3) | 11 | (30.6) | 19 | (37.3) | 5 | (20.8) | |||
| T4 | 12 | (16.0) | 4 | (10.3) | 8 | (22.2) | 5 | (9.8) | 7 | (29.2) | |||
| T4a | 1 | (1.3) | -- | (--) | 1 | (2.8) | -- | (--) | 1 | (4.2) | |||
| Tx | 1 | (1.3) | 1 | (2.6) | -- | (--) | 1 | (2.0) | -- | (--) | |||
| N category | 0 | 15 | (20.0) | 9 | (23.1) | 6 | (16.7) | 0.7821 | 10 | (19.6) | 5 | (20.8) | 0.7048 |
| TNM 2017 | 1 | 23 | (30.7) | 13 | (33.3) | 10 | (27.8) | 19 | (37.3) | 4 | (16.7) | ||
| 2 | 21 | (28.0) | 8 | (20.5) | 13 | (36.1) | 12 | (23.5) | 9 | (37.5) | |||
| 2a | 3 | (4.0) | 2 | (5.1) | 1 | (2.8) | 2 | (3.9) | 1 | (4.2) | |||
| 2c | 3 | (4.0) | 2 | (5.1) | 1 | (2.8) | 2 | (3.9) | 1 | (4.2) | |||
| 3a | 3 | (4.0) | 1 | (2.6) | 2 | (5.6) | 2 | (3.9) | 1 | (4.2) | |||
| 3b | 7 | (9.3) | 4 | (10.3) | 3 | (8.3) | 4 | (7.8) | 3 | (12.5) | |||
| M category | 0 | 72 | (96.0) | 38 | (97.4) | 34 | (94.4) | 0.5089 | 50 | (98.0) | 22 | (91.7) | 0.1889 |
| TNM 2017 | 1 | 3 | (4.0) | 1 | (2.6) | 2 | (5.6) | 1 | (2.0) | 2 | (8.3) | ||
| UICC 2017 | I | 20 | (26.7) | 10 | (25.6) | 10 | (27.8) | 0.6652 | 15 | (29.4) | 5 | (20.8) | 0.6624 |
| II | 17 | (22.7) | 11 | (28.2) | 6 | (16.7) | 13 | (25.5) | 4 | (16.7) | |||
| III | 22 | (29.3) | 9 | (23.1) | 13 | (36.1) | 14 | (27.5) | 8 | (33.3) | |||
| IV | 3 | (4.0) | 1 | (2.6) | 2 | (5.6) | 1 | (2.0) | 2 | (8.3) | |||
| IVA | 6 | (8.0) | 4 | (10.3) | 2 | (5.6) | 4 | (7.8) | 2 | (8.3) | |||
| IVB | 7 | (9.3) | 4 | (10.3) | 3 | (8.3) | 4 | (7.8) | 3 | (12.5) | |||
| Received | no OP | 16 | (21.3) | 6 | (15.4) | 10 | (27.8) | 0.1906 | 7 | (13.7) | 9 | (37.5) | 0.0191 |
| surgery | OP | 59 | (78.7) | 33 | (84.6) | 26 | (72.2) | 44 | (86.3) | 15 | (62.5) | ||
| Received | no RT | 7 | (9.3) | 2 | (5.1) | 5 | (13.9) | 0.1926 | 4 | (7.8) | 3 | (12.5) | 0.5178 |
| radiotherapy | RT | 68 | (90.7) | 37 | (94.9) | 31 | (86.1) | 47 | (92.2) | 21 | (87.5) | ||
| Received | no RChT | 29 | (38.7) | 15 | (38.5) | 14 | (38.9) | 0.9697 | 22 | (43.1) | 7 | (29.2) | 0.2465 |
| RChT | RChT | 46 | (61.3) | 24 | (61.5) | 22 | (61.1) | 29 | (56.9) | 17 | (70.8) | ||
| Event-free | no event | 53 | (70.7) | 33 | (84.6) | 20 | (55.6) | 0.0048 | 38 | (74.5) | 15 | (62.5) | 0.2866 |
| survival | event | 22 | (29.3) | 6 | (15.4) | 16 | (44.4) | 13 | (25.5) | 9 | (37.5) | ||
| Progression- | no event | 55 | (73.3) | 33 | (84.6) | 22 | (61.1) | 0.0215 | 38 | (74.5) | 17 | (70.8) | 0.7370 |
| free survival | event | 20 | (26.7) | 6 | (15.4) | 14 | (38.9) | 13 | (25.5) | 7 | (29.2) | ||
| Local control | no event | 62 | (82.7) | 35 | (89.7) | 27 | (75.0) | 0.0920 | 43 | (84.3) | 19 | (79.2) | 0.5828 |
| event | 13 | (17.3) | 4 | (10.3) | 9 | (25.0) | 8 | (15.7) | 5 | (20.8) | |||
| Nodal | no event | 62 | (82.7) | 36 | (92.3) | 26 | (72.2) | 0.0217 | 43 | (84.3) | 19 | (79.2) | 0.5828 |
| control | event | 13 | (17.3) | 3 | (7.7) | 10 | (27.8) | 8 | (15.7) | 5 | (20.8) | ||
| Distant | no event | 67 | (89.3) | 37 | (94.9) | 30 | (83.3) | 0.1058 | 45 | (88.2) | 22 | (91.7) | 0.6534 |
| control | event | 8 | (10.7) | 2 | (5.1) | 6 | (16.7) | 6 | (11.8) | 2 | (8.3) | ||
| Loco-regional | no event | 58 | (77.3) | 34 | (87.2) | 24 | (66.7) | 0.0340 | 41 | (80.4) | 17 | (67.8) | 0.3564 |
| control | event | 17 | (22.7) | 5 | (12.8) | 12 | (33.1) | 10 | (19.6) | 7 | (29.2) | ||
| Overall | alive | 67 | (89.3) | 35 | (89.7) | 32 | (88.9) | 0.9046 | 47 | (92.2) | 20 | (83.3) | 0.2482 |
| survival | dead | 8 | (10.7) | 4 | (10.3) | 4 | (11.1) | 4 | (7.8) | 4 | (16.7) | ||
‡ p value from Pearson’s Chi square (χ2) tests. ¶ Classification of anthropometric height/weight characteristics according to WHO (2000) “Obesity: Preventing and Managing the Global Epidemic” into underweight (BMI 15–19.9 kg/m2) and normal weight (BMI 20–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obesity summarizing adiposity I (BMI 30–34.9 kg/m2), adiposity II (BMI 35–39.9 kg/m2), and adiposity III (BMI ≥ 40 kg/m2). p16 IHC (CINtec+), combined immunohistochemical tests for p16INK4A and Ki-67 expression; T, Tumor size; N, Nodal involvement; M, distant metastasis; UICC, Union for International Cancer Control; OP, surgical operation of the primary and/or neck dissection; RT, radiotherapy; RChT, radio-chemo-therapy.
Table 2.
Characteristics of HNSCC patients in the test cohort (TC; n = 75) and independent validation cohort (iVC; n = 104). Significant differences between groups (p < 0.05) in Pearson’s Chi-square tests are highlighted bold.
| Characteristics | All Patients | TC | iVC | |||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | p Value ‡ | ||
| Sex | female | 33 | (18.4) | 11 | (14.7) | 22 | (21.2) | 0.2695 |
| male | 146 | (81.6) | 64 | (85.3) | 82 | (78.8) | ||
| Age Score | <50 years | 24 | (13.4) | 8 | (10.7) | 16 | (15.4) | 0.4982 |
| <60 years | 77 | (43.0) | 31 | (41.3) | 46 | (44.2) | ||
| <70 years | 50 | (27.9) | 21 | (28.0) | 29 | (27.9) | ||
| ≥70 years | 28 | (15.6) | 15 | (20.0) | 13 | (12.5) | ||
| ECOG | 0 | 118 | (65.9) | 53 | (70.7) | 65 | (62.5) | 0.2554 |
| >0 | 61 | (34.1) | 22 | (29.3) | 39 | (37.5) | ||
| Charlson score | 0 | 98 | (54.7) | 43 | (57.3) | 55 | (52.9) | 0.0722 |
| >0 | 81 | (45.3) | 32 | (42.7) | 49 | (47.1) | ||
| Pack years | ≤30 PY | 103 | (55.3) | 44 | (58.7) | 59 | (56.7) | 0.0752 |
| >30 PY | 76 | (42.5) | 31 | (41.3) | 45 | (43.3) | ||
| Alcohol | none | 20 | (11.2) | 7 | (9.3) | 13 | (12.5) | 7.16 × 10−5 |
| consumption | <30 g/d | 74 | (41.3) | 40 | (53.3) | 34 | (32.7) | |
| <60 g/d | 27 | (15.1) | 6 | (8.0) | 21 | (20.2) | ||
| >60 g/d | 50 | (27.9) | 14 | (18.7) | 36 | (34.6) | ||
| unknown | 8 | (4.5) | 8 | (10.7) | -- | (--) | ||
| Localization | LHSCC | 66 | (36.9) | 9 | (12.0) | 57 | (54.8) | 4.03 × 10−9 |
| OPSCC | 88 | (49.2) | 56 | (74.7) | 32 | (30.8) | ||
| OSCC | 23 | (12.8) | 8 | (10.7) | 15 | (14.4) | ||
| other | 2 | (1.1) | 2 | (2.7) | -- | (--) | ||
| OPSCC vs. Other | p16+ OPSCC | 70 | (39.1) | 49 | (65.3) | 21 | (20.2) | 1.02 × 10−9 |
| other | 109 | (60.9) | 26 | (34.7) | 83 | (79.8) | ||
| T category TNM | T1 | 26 | (14.5) | 9 | (12.0) | 17 | (16.3) | 1.06 × 10−4 |
| 2017 | T2 | 55 | (30.7) | 28 | (37.3) | 27 | (26.0) | |
| T3 | 49 | (27.4) | 24 | (32.0) | 25 | (24.0) | ||
| T4 | 18 | (10.1) | 12 | (16.0) | 6 | (5.8) | ||
| T4a | 29 | (16.2) | 1 | (1.3) | 28 | (26.9) | ||
| Tx | 2 | (0.6) | 1 | (1.3) | 1 | (1.0) | ||
| N category TNM | N0 | 44 | (24.6) | 15 | (20.0) | 29 | (27.9) | 5.10 × 10−6 |
| 2017 | N1 | 42 | (23.5) | 23 | (30.7) | 19 | (18.3) | |
| N2 | 30 | (16.8) | 21 | (28.0) | 9 | (8.7) | ||
| N2a | 3 | (1.7) | 3 | (4.0) | -- | (--) | ||
| N2b | 14 | (7.8) | -- | (--) | 14 | (13.5) | ||
| N2c | 17 | (9.5) | 3 | (4.0) | 14 | (13.5) | ||
| N3 | 3 | (1.7) | 3 | (4.0) | -- | (--) | ||
| N3a | 1 | (0.6) | -- | (--) | 1 | (1.0) | ||
| N3b | 25 | (14.0) | 7 | (9.3) | 18 | (17.3) | ||
| M category TNM | M0 | 175 | (97.8) | 72 | (96.0) | 103 | (99.0) | 0.1748 |
| 2017 | M1 | 4 | (2.2) | 3 | (4.0) | 1 | (1.0) | |
| UICC 2017 | I | 38 | (21.2) | 20 | (26.7) | 18 | (17.3) | 4.73 × 10−4 |
| II | 29 | (16.2) | 17 | (22.7) | 12 | (11.5) | ||
| III | 44 | (24.6) | 22 | (29.3) | 22 | (21.2) | ||
| IV | 3 | (1.7) | 3 | (4.0) | -- | (--) | ||
| IVA | 38 | (21.2) | 6 | (8.0) | 32 | (30.8) | ||
| IVB | 26 | (14.5) | 7 | (9.3) | 19 | (18.3) | ||
| IVC | 1 | (0.6) | -- | (--) | 1 | (1.0) | ||
‡ p value from Pearson’s Chi square (χ2) tests; ECOG, Eastern Cooperative Oncology Group performance score; Charlson comorbidity score; LHSCC, laryngeal- and hypopharyngeal squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinoma; OSCC, oral squamous cell carcinoma; T, Tumor size; N, Nodal involvement; M, distant metastasis; UICC, Union for International Cancer Control.
2.2. Material and Chemicals
Serum-gel and EDTA-plasma S-Monovettesdfd (Sarstedt, Nümbrecht, Germany) were used to collect venous blood samples from patients. Blood samples were centrifuged (2343× g, 10 min). Aliquoted serum and plasma were stored at –80 °C until the measuring of VEGF using indirect sandwich-ELISA based on the Human VEGFA ELISA Development Kit (EDK 0709010; 900-K10) from PeproTech GmbH (Hamburg, Germany). Dulbecco’s phosphate buffered saline (PBS) from Biochrom AG (Berlin, Germany) was used for coating the microtiter plates (Greiner Bio-One, Nürtingen, Germany) with 0.5 µg/mL anti-VEGFA antibodies, overnight at 4 °C. PBS containing 0.025% v/v Tween®20 from Sigma-Aldrich (Darmstadt, Germany) was used for washing. After a 60-min blocking step with PBS containing 5% v/v heat-inactivated fetal calf serum (FCS; Thermo-Fisher Scientific, Waltham, MA, USA), 50 µL of sample (plasma or serum) and a serial dilution of VEGF for calibration were incubated for 120 min, followed by washing and adding biotinylated anti-VEGFA antibodies. After three further washing steps, streptavidin-horseradish conjugate was incubated for 60 min followed by six washing steps before adding tetra-methylbenzidine (TMB) 1-Stepᵀᴹ Ultra (Pierce via Thermo-Fisher Scientific, Waltham, MA, USA).
2.3. VEGF Quantification
TMB 1-Stepᵀᴹ Ultra conversion by horseradish peroxidase (HRP) was stopped by adding the same volume of 1 M sulfuric acid. Optical density (OD) was measured at λ1 = 450 nm and λ2 = 620 nm using a Synergy2 microplate reader equipped with Gen5 software (BioTek Instruments Inc., Winooski, VT, USA). OD (λ1–λ2) and was converted to concentration (ng/L) according to 4-parameter calibration curves. Lower limit of detection (LLD) and lower limit of quantification (LLQ) were determined in 30 replicates and triplicate log2 dilutions and were <2 ng/L and <4 ng/L, respectively. There were no detectable matrix-related differences in serum versus EDTA-plasma.
2.4. Statistical Analysis
SPSS Version 25 (IBM, Corporation, Armonk, New York, NY, USA) was used for statistical analyses. Differences between quantitative parameters were analyzed using t-tests and associations between categorical variables examined by Pearson’s chi-square (Χ2) test. Receiver operating characteristic (ROC) curves served to calculate Youden score and Youden index in order to define the optimum cut-off values for quantitative parameters for binary classification of patients in TC. We measured time-dependent survival parameters from the date of diagnosis to the date of an event. Analyses included overall survival (OS), tumor-specific survival (TSS), event-free survival (EFS), as well as progression-free survival (PFS). OS (the time from diagnosis to death of any cause, censoring patients who remained alive at the end of follow-up), TSS (the time from diagnosis to cancer-related death, censoring patients who remained alive at the end of follow-up or died from other causes), as well as EFS (the time from diagnosis to relapse or death from any cause, censoring patients at the time of the last follow-up who remained alive without signs of any cancer) and PFS (the time from diagnosis to relapse or cancer-related death censoring patients who remained alive at the end of follow-up or who died from other causes) are present censored at 60 months follow-up. Additionally, we assessed the kind of treatment failure to detect a potential link between VEGF and local control (LC), nodal control (NC), loco-regional control (LRC, the combined LC and NC), and distant control (DC).
By censoring all other PFS events, we measured LC as the time of diagnosis to local recurrence or second primary squamous cell carcinoma in the head and neck region. NC was measured from time of diagnosis to relapse in the neck by focusing on squamous cell carcinoma (SCC)-positive lymph node status only. DC was measured from the time of diagnosis to detection of distant metastasis (M1).
Survival parameters in TC, iVC, and the combined cohort (CC) were analyzed using Kaplan–Meier plots for cumulative survival applying log-rank tests. Hazard ratios (HR) were analyzed using multivariate Cox proportional hazard regression models (mCox) applying the conditional logistic regression and bootstrapping utilizing 1000 iterations. We considered 2-sided p ≤ 0.05 as significant.
3. Results
3.1. Patients Characteristics
Table 1 and Table 2 show patients characteristics in detail. In TC, 75 blood samples of 11 female and 64 male patients with HNSCC, more specifically, 9 laryngeal and hypopharyngeal squamous cell carcinoma (LHSCC), 56 oropharyngeal squamous cell carcinoma (OPSCC), 8 oral squamous cell carcinoma (OSCC) and 2 with cancer of another head and neck side, served for measurements of VEGF concentrations in pretherapeutic plasma and serum. Median age was 59.0 (95% CI 58.0–62.6) years. The iVC consisted of 22 female and 82 male HNSCC patients of which 57 patients had LHSCC, 32 OPSCC, and 15 OSCC. Median age was 57.0 (95% CI 56.3–59.9) years and compared well to the TC.
At database lock, median follow-up time in TC and iVC were 26.4 (95% CI 22.8–30.0) and 45.0 (95% CI 39.6–50.4) months (p = 9.48 × 10−6).
3.2. TNM Staging and Outcome in TC
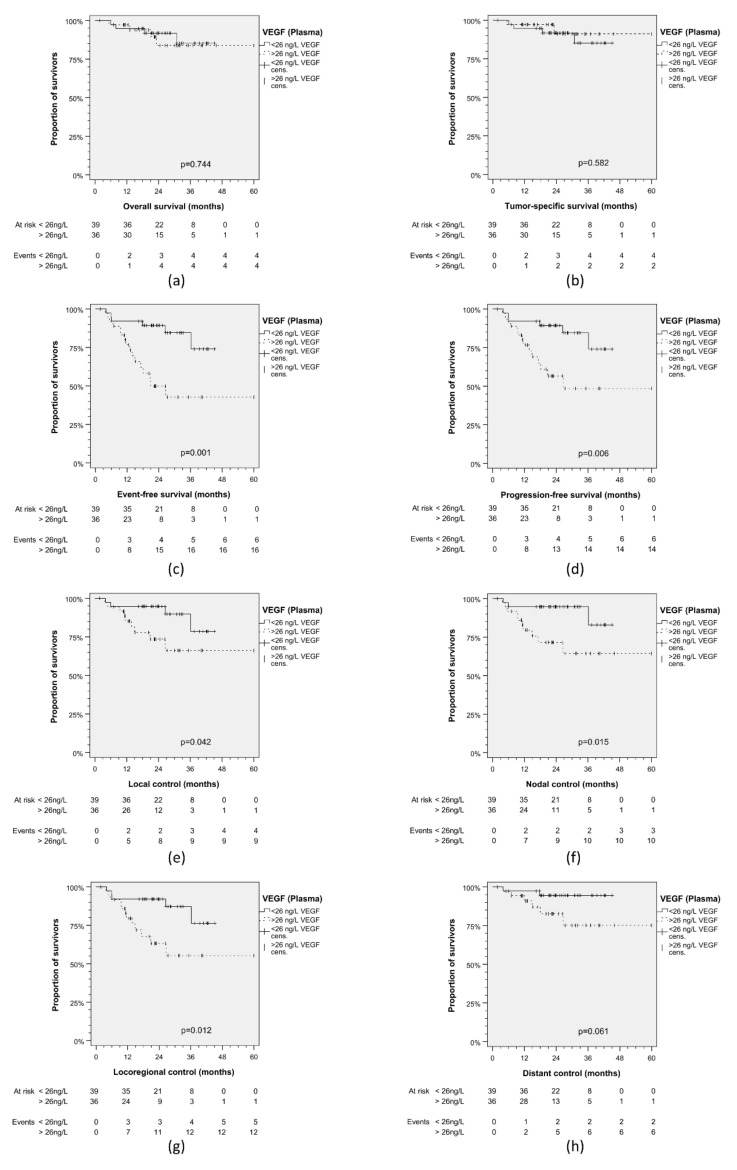
In TC, pre-therapeutic EDTA-plasma and serum samples stored at −80 °C were available from all patients and VEGF measurements were performed using ELISA. VEGF concentrations in plasma (mean VEGF 34.6, 95% CI 26.0–43.3 ng/L) were significantly lower (p = 3.35 × 10−18) than in serum (214.8, 95% CI 179.6–250.0 ng/L). Based on ROC (area under the curve, AUCPlasma = 0.707, 95% CI 0.573–0.840; p = 0.006 versus AUCSerum = 0.665, 95% CI 0.528–0.801; p = 0.030) VEGFPlasma was superiorly correlated with EFS of patients and according to the Youden index (the maximum product of sensitivity and specificity observed) achieved 75% sensitivity and 61.8% specificity when 26 ng/L were used as cut-off (Figure S1). Kaplan–Meier plots of cumulative EFS revealed superiority of plasma versus serum VEGF levels (VEGFPlasma 26 ng/L; VEGFSerum 264 ng/L) as a predictive biomarker for outcomes (Table 1). In line with the Youden index for the optimum split of TC patients, Kaplan–Meier analysis (Figure 2) showed that patients with VEGFPlasma > 26 ng/L had significantly worse EFS (p = 0.001), PFS (p = 0.006), LC (p = 0.042), NC (p = 0.015), and LRC (p = 0.012), and a trend towards impaired DC (p = 0.061); however, this was not the case for OS (p = 0.744) and TSS (p = 0.582). This was not seen after stratification according to VEGFSerum <264 versus >264 ng/L (Figure S2).
Figure 2.
Kaplan–Meier plots for cumulative survival in HNSCC patients from the test cohort stratified according to VEGF in pre-therapeutic plasma. (a) Overall survival; (b) tumor-specific survival; (c) event-free survival; (d) progression-free survival; (e) local control; (f) nodal control; (g) loco-regional control; (h) distant control. p values shown are from 2-sided log-rank test.
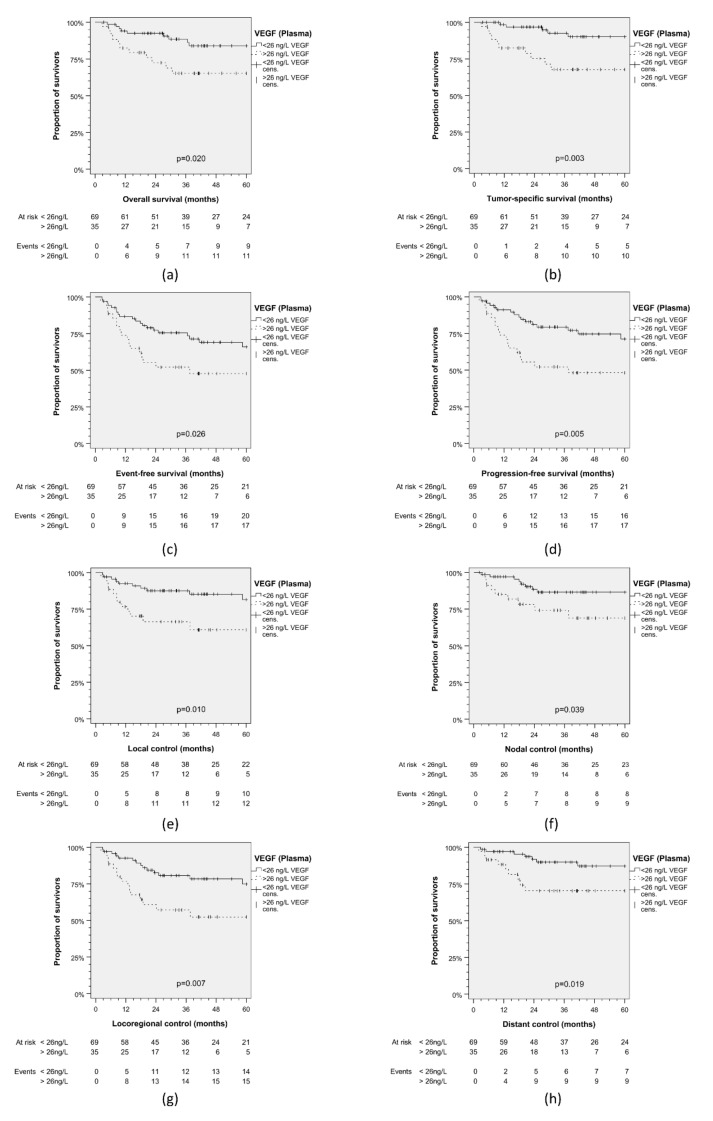
3.3. Validation of VEGFPlasma Cut-Off for Dicriminating Outcome Groups
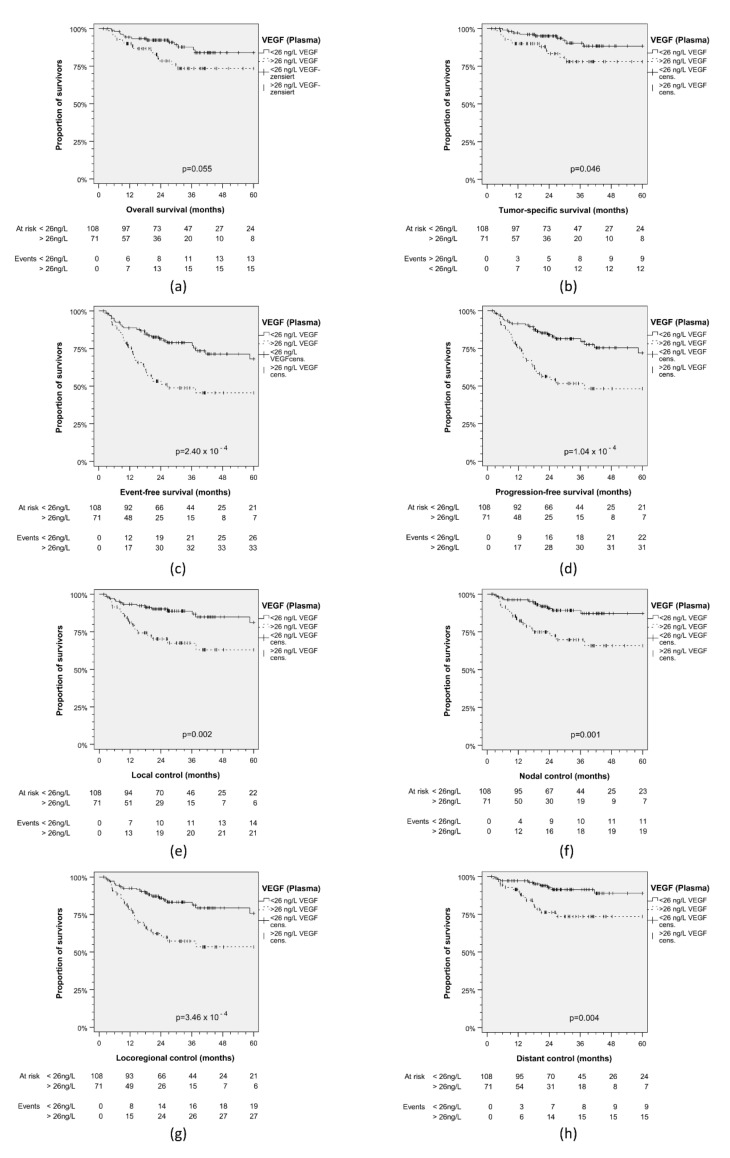
Outcome differences in 104 iVC patients (Table 2) confirmed these results (Figure 3), as patients with VEGFPlasma > 26 ng/L demonstrated significantly impaired EFS (p = 0.026), PFS (p = 0.005), LC (p = 0.010), NC (p = 0.039), LRC (p = 0.007) and even significantly reduced DC (p = 0.019), TSS (p = 0.003) and OS (p = 0.020). Statistical analysis for the CC similarly showed impaired OS (p = 0.055), TSS (p = 0.046), EFS (p = 2.40 × 10−4), PFS (p = 1.04 × 10−4), LC (p = 0.002), NC (p = 0.001), LRC (p = 3.46 × 10−4), and DC (p = 0.004) linked to VEGFPlasma > 26 ng/L (Figure 4). Figure 2, Figure 3 and Figure 4 consistently demonstrate an early split of survival curves for patients grouped according to the cut-off value of 26 ng/L VEGFPlasma.
Figure 3.
Kaplan–Meier plots for cumulative survival in HNSCC patients from the independent validation cohort stratified according to VEGF in pre-therapeutic plasma. (a) Overall survival; (b) tumor-specific survival; (c) event-free survival; (d) progression-free survival; (e) local control; (f) nodal control; (g) loco-regional control; (h) distant control. p values shown are from 2-sided log-rank test.
Figure 4.
Kaplan–Meier plots for cumulative survival in HNSCC patients from combined test cohort and independent validation cohort stratified according to VEGF in pre-therapeutic plasma. (a) Overall survival; (b) tumor-specific survival; (c) event-free survival; (d) progression-free survival; (e) local control; (f) nodal control; (g) loco-regional control; (h) distant control. p values shown are from 2-sided log-rank test.
3.4. VEGF Is an Independent Predictor for Outcome
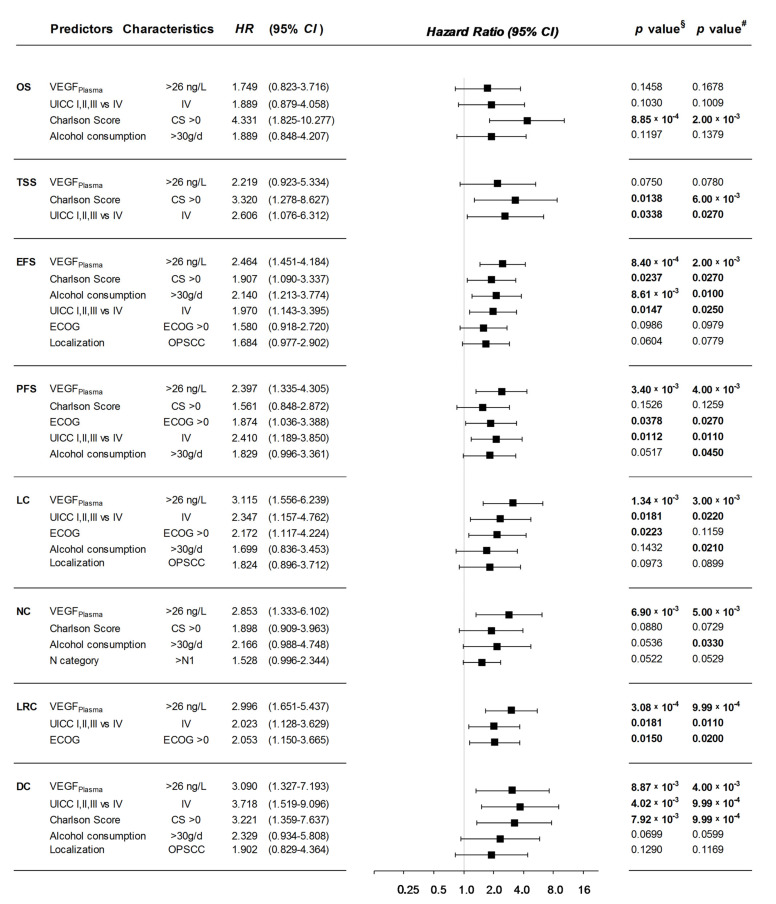
Using CC data, multivariate Cox proportional hazard regression (mCox) models demonstrate VEGF as a pre-dominant Pi for outcome, even after applying bootstrapping. VEGFPlasma > 26 ng/L in this regard is a superior Pi compared to well-known classical risk factors for survival of HNSCC patients like tobacco smoking, alcohol consumption, age, and ECOG (Figure 5). VEGFPlasma > 26 ng/L had the highest impact on LC (HR 3.12, 95% CI 1.56–6.24; p = 1.34 × 10−3), DC (HR 3.09, 95% CI 1.33–7.19; p = 8.87 × 10−3), LRC (HR 2.97, 95% CI 1.65–5.44; p = 3.08 × 10−4), NC (HR 2.85, 95% CI 1.33–6.10; p = 6.90 × 10−3), EFS (HR 2.46, 95% CI 1.45–4.18; p = 8.40 × 10−4), and PFS (HR 2.40, 95% CI 1.34–4.31; p = 3.40 × 10−3). However, VEGFPlasma > 26 ng/L was a relevant Pi in mCox for TSS and OS, but failed to demonstrate a significant impact on TSS (HR 2.22, 95% CI 0.92–5.33; p = 0.075) and OS (HR 1.75, 95% CI 0.82–3.72; p = 0.146) in these models.
Figure 5.
Forrest plots for independent predictors for (OS) overall survival, (TSS) tumor-specific survival, (EFS) event-free survival, (PFS) progression-free survival, (LC) local control, (NC) nodal control, (LRC) loco-regional control, and (DC) distant control from multivariate Cox proportional hazard models build step wise using the likelihood-ratio forward method reaching the highest significance in combined cohorts of HNSCC patients. § p values from multivariate Cox proportional hazard model; # p values from multivariate Cox proportional hazard model applying bootstrapping using 1000 iterations.
4. Discussion
According to our results, based on VEGF quantification in plasma and serum of 75 therapy-naïve TC patients, VEGFPlasma is superior to VEGFSerum as a prognostic biomarker for outcomes in EFS (p = 0.001), PFS (p = 0.006), LC (p = 0.042), NC (p = 0.015), and LRC (p = 0.012). Using the same cut-off for binary classification of 104 iVC patients, we found superior OS (p = 0.020), TSS (p = 0.003), EFS (p = 0.026), PFS (p = 0.005), LC (p = 0.010), NC (p = 0.039), LRC (p = 0.007), and DC (p = 0.019) for patients with VEGFPlasma < 26 ng/L. The results were confirmed with the 179 CC patients and multivariate Cox regression models.
The VEGF concentrations were measured in plasma and serum obtained during the same blood draw. Both differed substantially, but are comparable to concentrations measured in blood from treatment-naïve HNSCC, reported for either VEGFPlasma [15] or VEGFSerum [32,33]. In our study, no proportionality was detected for VEGFSerum and VEGFPlasma as they were sometimes nearly the same, but there were huge differences between both. Hence, our results would support former assumptions, whereas VEGFSerum may depend on the platelet’s ability to release VEGF during coagulation [34,35,36] and may be modified under anti-coagulant treatment, which is often administered to comorbid HNSCC; VEGFPlasma may reflect the level of circulating VEGF more accurately [7], thereby, more reliably, representing the pre-therapeutic angiogenetic pressure caused by the malignancy.
This would lead to the hypothesis, that, according to our binary classification, a proportion of our HNSCC patients with <264 ng/L VEGFSerum could potentially be wrongly categorized as belonging to the “low VEGF—low risk group” and having superior outcomes, whereas VEGFPlasma > 26 ng/L correctly classifies them as belonging to the “high VEGF—high risk group”. Demonstrating the superiority of VEGF in plasma over serum (Table 1), VEGFPlasma may therefore allow for better outcome prognoses. Otherwise, it points to the role of the VEGF-signaling pathway in HNSCC development and defines VEGF and its receptors as promising targets for targeted therapies.
Angiogenesis is necessary for the shift from tumor dormancy to exponential tumor growth [37] and belongs to the hallmarks of cancer [1,2]. VEGF as potent inducer of angiogenesis leads in combination with basic Fibroblast Growth Factor (FGF) to a higher microvessel density in HNSCC [38]. The immunohistochemically identified VEGF overexpression in tumor tissue is associated with a poorer prognosis (DFS) [39,40] and a higher mortality of HNSCC patients [40,41].
Thereby, VEGF in patient plasma and serum has been under investigation as a potential biomarker for HNSCC [15,32,33,42,43], but decision making regarding sampling plasma or serum for VEGF measurements, with the achievement of biomarker identification, has not been standardized [24] and is inconsistently used, as seen in several studies [15,32]. Nevertheless, elevated levels of VEGF in serum and plasma in comparison to non-cancer control groups have been detected throughout studies [13,14,15] and thereby highlight that VEGF should be considered more for investigations, especially in regards to actual studies and the development of anti-angiogenetic drugs targeting for instance VEGF and its receptors in HNSCC. Indeed, anti-angiogenetic therapies might be based on either targeting VEGF using antibodies, such as bevacizumab, or receptor-tyrosine kinase inhibitors (TKI) of VEGF receptors, for example sorafenib, sunitinib, lenvatinib, or others.
The sole use of bevacizumab or TKI in HNSCC are not yet FDA-approved. As reported by Argiris et al. [22,32], or demonstrated by ASCO 2020 through the presentation of the LEAP-010 study, evaluating the efficacy of lenvantinib in combination with pembrolizumab in patients with HNSCC [44], targeting the VEGF pathway, remains of interest. A combined use with cisplatin-based chemotherapy or immunotherapy may be useful for this cancer entity. In line with this, several clinical trials are currently underway, as reviewed by Micaily et al. [45]. Such trials, and also treatment of HNSCC in the curative setting, may benefit from using VEGFPlasma as a biomarker for treatment stratification.
Biomarker identification often is only a secondary or surrogate endpoint in recently ongoing phase II RCTs. So far, especially in combined use with anti-angiogenetic targeted therapy, VEGF levels have been reported as potential marker in several studies, e.g., for breast cancer [46], in regards of better patients stratification or treatment success. Nevertheless, VEGF in HNSCC patients’ blood has not being approved being a solid biomarker for outcome prognostication yet. This could be linked to differences between VEGF in serum and plasma and the role of anti-coagulation or thrombotic events in modifying VEGF levels observed to be mostly ignored.
Unfortunately, the very important findings from phase II and III studies from Argiris et al. [22,32] demonstrating the benefit achievable by VEGF targeting were not appropriately perceived by the community of HNSCC specialists. These studies highlighted both, the value of VEGF as biomarker (despite VEGF was measured in serum) as they were able to demonstrate in 1st line treatment of R/M HNSCC a reduction in median VEGF concentrations from baseline 547.7 ng/L to 59.45 ng/L post treatment along with VEGF-targeting by bevacizumab [32] and the improved outcome achieved through VEGF targeting [22]. Dual targeting of EGFR and VEGF pathways, however, offers a potential opportunity for a subgroup of patients unable to receive a cisplatin-based first-line therapy for R/M HNSCC [22,32,42] and represents a potential strategy to improve efficiency, as demonstrated by the studies by Cohen et al. [42] and Argiris et al. [32]. As both studies reported VEGF and VEGFR2 as biomarkers for superior outcomes, using them for treatment stratification could be possible [22,32,42]. Whereas these phase II studies reported lower grade and rate of toxicity compared to EXTREME, the first-line regimen for R/M HNSCC utilizing cisplatin/carboplatin, 5-fluorouracil and cetuximab [47,48], the phase III study by Argiris et al. reported serious (but manageable) side effects occurring in a substantial proportion of patients [22].
Unfortunately, and to the best of our knowledge, no data are available for randomized controlled trial utilizing VEGF targeting in the curative setting and reporting baseline and concentrations of VEGF in context of patient’s clinical outcome.
Our study has limitations. Despite efforts made to record all data within our prospective studies completely, some patients did not report pack years of tobacco smoking history and daily alcohol consumption, causing missing data regarding these risk factors in TC and iVC. The follow-up in TC was about 3 years and, consequently, few fatal events occurred limiting the chance to make reliable conclusions regarding the impact of circulating VEGF on survival. Hence, all outcome parameters analyzed consistently revealed VEGFPlasma as Pi, and we are confident that improved LC, NC, LRC, PFS, and EFS will translate into improved TSS and OS. Compared to other published studies, a strength of our study is the standardization and uniform procedure in blood sampling and storage since 2007, which is necessary for the discovery of plasma biomarkers [49]. The classification characteristics of the cut-off value of 26 ng/L VEGFPlasma obtained based on EFS in the TC were successfully validated for multiple outcome parameters in the iVC.
Moreover, a further strength of this study were consistent findings of VEGFPlasma being a Pi in the CC in all mCox models for outcome parameters and stably remaining a significant Pi, even after internal validation with bootstrapping by applying 1000 iterations. The only exception in this regard were OS and TSS based on limited follow-up time in the TC in particular.
In summary, VEGF in plasma is an Pi for outcome superior to a variety of well-established outcome predictors, for instance tobacco smoking, p16-positivity and age [50,51,52] based on mCox and applying bootstrapping. Those three covariates were not found to be among the Pi in any mCox model whenever VEGF was included in the analyses. Most mCox models identified UICC stages I, II, and III vs. IV as Pi, but neither T nor N categories (N category >1 was the only exception in mCox for NC). Furthermore, VEGFPlasma outperformed CS as Pi for LRC and LC, as well as alcohol consumption as Pi for LRC and TSS (Figure 5).
5. Conclusions
In the curative setting, circulating VEGF in plasma of therapy-naïve HNSCC was superior to VEGF in serum as a prognostic biomarker. VEGF levels below 26 ng/L are associated with improved outcome. In the light of clinical trials reporting benefit of a subgroup of R/M HNSCC patients from VEGF-targeting by bevacizumab, targeting the VEGF pathway may have the potential to improve their outcome. Quantification of VEGF in plasma may potentially facilitate identification of patients that are also at risk in the curative setting.
Acknowledgments
We thank all patients who participated in clinical studies, providing blood and tumor tissue samples and data. We thank all current and former employees of our hospital and laboratory who supported clinical studies with their daily efforts and all contributors who added data to the tumor database of our department.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13153781/s1, Figure S1: Receiver-operator characteristics (ROC) of vascular endothelial growth factor (VEGF) concentration in plasma [(a), (c)] and serum [(b), (d)] for event-free survival of therapy-naïve head and neck squamous cell carcinoma patients. (a) ROC for VEGFPlasma; (b) ROC for VEGFSerum; (c) tabulated sensitivity, false-discovery rate (FDR), specificity, and Youden indices for VEGFPlasma to define the cut-off for VEGFPlasma of 26 ng/L according the maximum Youden score (underlined); (d) tabulated sensitivity, false-discovery rate (FDR), specificity, and Youden indices for VEGFSerum to define the cut-off for VEGFSerum of 264 ng/L according the maximum Youden score (underlined), Figure S2: Kaplan-Meier plots for cumulative survival in head and neck squamous cell carcinoma patients from the test cohort stratified according to vascular endothelial growth factor (VEGF) in pre-therapeutic serum. (a) overall survival; (b) tumor-specific survival; (c) event-free survival; (d) progression-free survival; (e) local control; (f) nodal control; (g) loco-regional control; (h) distant control. p values shown are from 2-sided log-rank test.
Author Contributions
Conceptualization, A.D. and G.W.; data curation, J.S., T.W., M.K., I.P. and G.W.; formal analysis, J.S. and G.W.; funding acquisition, S.W. and G.W.; investigation, J.S., M.K., I.P., U.B., M.P., A.D. and G.W.; methodology, J.S. and G.W.; project administration, J.S., M.K., S.W., A.D. and G.W.; resources, J.S., M.K., A.D. and G.W.; software, J.S. and G.W.; supervision, S.W., A.D. and G.W.; validation, J.S., T.W. and G.W.; visualization, G.W.; writing—original draft, J.S. and G.W.; writing—review and editing, J.S., T.W., M.K., I.P., U.B., M.P., S.W., A.D. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and biomarker analyses approved by the Ethics Committee of the Medical Faculty of the University Leipzig: TRANSCAN-DietINT (ethic vote 176-15-01062015), NICEI-CIH (ethic vote 341-15-05102015), LIFE (ethic vote 201-10-12072010) DeLOS II trial (ethic vote 166-07-12072006).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because of patient confidentiality and participant privacy terms. Requests to access the datasets should be directed to G.W., Gunnar.Wichmann@medizin.uni-leipzig.de.
Conflicts of Interest
The authors J.S., G.W., T.W., I.P., U.B., S.W. and A.D. declare no conflicts of interest. M.K. is now an employee of Eisai GmbH (Germany) and declares no potential conflicts of interest related to this research.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D., Weinberg R.A. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Verheul H.M., Hoekman K., Luykx-de Bakker S., Eekman C.A., Folman C.C., Broxterman H.J., Pinedo H.M. Platelet: Transporter of vascular endothelial growth factor. Clin. Cancer Res. 1997;3:2187–2190. [PubMed] [Google Scholar]
- 4.Möhle R., Green D., Moore M.A., Nachman R.L., Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salgado R., Benoy I., Bogers J., Weytjens R., Vermeulen P., Dirix L., van Marck E. Platelets and vascular endothelial growth factor (VEGF): A morphological and functional study. Angiogenesis. 2001;4:37–43. doi: 10.1023/A:1016611230747. [DOI] [PubMed] [Google Scholar]
- 6.Kusumanto Y.H., Dam W.A., Hospers G.A.P., Meijer C., Mulder N.H. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 7.Werther K., Christensen I.J., Nielsen H.J. Determination of vascular endothelial growth factor (VEGF) in circulating blood: Significance of VEGF in various leucocytes and platelets. Scand. J. Clin. Lab. Investig. 2002;62:343–350. doi: 10.1080/00365510260296492. [DOI] [PubMed] [Google Scholar]
- 8.Freeman M.R., Schneck F.X., Gagnon M.L., Corless C., Soker S., Niknejad K., Peoples G.E., Klagsbrun M. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: A potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 9.Senger D.R., Galli S.J., Dvorak A.M., Perruzzi C.A., Harvey V.S., Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 10.Senger D.R., Perruzzi C.A., Feder J., Dvorak H.F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 11.Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N., Henzel W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291X(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 13.Kut C., Mac Gabhann F., Popel A.S. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br. J. Cancer. 2007;97:978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z., Malhotra P.S., Thomas G.R., Ondrey F.G., Duffey D.C., Smith C.W., Enamorado I., Yeh N.T., Kroog G.S., Rudy S., et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin. Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 15.Kulapaditharom B., Boonkitticharoen V., Sritara C. Plasma vascular endothelial growth factor dysregulation in defining aggressiveness of head and neck squamous cell carcinoma. J. Oncol. 2012;2012:687934. doi: 10.1155/2012/687934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferroni P., Spila A., Martini F., D’Alessandro R., Mariotti S., Del Monte G., Graziano P., Buonomo O., Guadagni F., Roselli M. Prognostic value of vascular endothelial growth factor tumor tissue content of colorectal cancer. Oncology. 2005;69:145–153. doi: 10.1159/000087838. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama Y., Sako T., Shibao K., Okazaki K., Rempo N., Onitsuka K., Minagawa N., Akahane K., Nagashima N., Nagata N., et al. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res. 2002;22:2437–2442. [PubMed] [Google Scholar]
- 18.Chin K.F., GREENMAN J., Gardiner E., Kumar H., Topping K., Monson J. Pre-operative serum vascular endothelial growth factor can select patients for adjuvant treatment after curative resection in colorectal cancer. Br. J. Cancer. 2000;83:1425–1431. doi: 10.1054/bjoc.2000.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alabi A.A., Suppiah A., Madden L.A., Monson J.R., Greenman J. Preoperative serum vascular endothelial growth factor-a is a marker for subsequent recurrence in colorectal cancer patients. Dis. Colon Rectum. 2009;52:993–999. doi: 10.1007/DCR.0b013e31819ed3bc. [DOI] [PubMed] [Google Scholar]
- 20.Garcia J., Hurwitz H.I., Sandler A.B., Miles D., Coleman R.L., Deurloo R., Chinot O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 21.Roche Registration GmbH Fachinformation Avastin®, Grenzach-Wyhlen, Deutschland. [(accessed on 12 December 2020)];2020 Available online: https://www.patienteninfo-service.de/a-z-liste/a/avastinR-25-mgml-konzentrat-zur-herstellung-einer-infusionsloesung/
- 22.Argiris A., Li S., Savvides P., Ohr J.P., Gilbert J., Levine M.A., Chakravarti A., Haigentz M., Saba N.F., Ikpeazu C.V., et al. Phase III Randomized Trial of Chemotherapy With or Without Bevacizumab in Patients With Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019;37:3266–3274. doi: 10.1200/JCO.19.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goossens N., Nakagawa S., Sun X., Hoshida Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hormbrey E., Gillespie P., Turner K., Han C., Roberts A., McGrouther D., Harris A.L. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: Is the current literature meaningful? Clin. Exp. Metastasis. 2002;19:651–663. doi: 10.1023/A:1021379811308. [DOI] [PubMed] [Google Scholar]
- 25.Wichmann G., Rosolowski M., Krohn K., Kreuz M., Boehm A., Reiche A., Scharrer U., Halama D., Bertolini J., Bauer U., et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int. J. Cancer. 2015;137:2846–2857. doi: 10.1002/ijc.29649. [DOI] [PubMed] [Google Scholar]
- 26.Wichmann G., Herchenhahn C., Boehm A., Mozet C., Hofer M., Fischer M., Kolb M., Dietz A. HLA traits linked to development of head and neck squamous cell carcinoma affect the progression-free survival of patients. Oral Oncol. 2017;69:115–127. doi: 10.1016/j.oraloncology.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Wichmann G., Gaede C., Melzer S., Bocsi J., Henger S., Engel C., Wirkner K., Wenning J.R., Wald T., Freitag J., et al. Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets. Cancers. 2019;11:814. doi: 10.3390/cancers11060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichmann G., Krüger A., Boehm A., Kolb M., Hofer M., Fischer M., Müller S., Purz S., Stumpp P., Sabri O., et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and 18F-FDG-PET/CT. Eur. J. Cancer. 2017;72:144–155. doi: 10.1016/j.ejca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Dietz A., Wichmann G., Kuhnt T., Pfreundner L., Hagen R., Scheich M., Kölbl O., Hautmann M.G., Strutz J., Schreiber F., et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann. Oncol. 2018;29:2105–2114. doi: 10.1093/annonc/mdy332. [DOI] [PubMed] [Google Scholar]
- 30.Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K., Meyer L., Gress D.M., Byrd D.R., Winchester D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 31.Charlson M.E., Pompei P., Ales K.L., MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Argiris A., Kotsakis A.P., Hoang T., Worden F.P., Savvides P., Gibson M.K., Gyanchandani R., Blumenschein G.R., Chen H.X., Grandis J.R., et al. Cetuximab and bevacizumab: Preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2013;24:220–225. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HOMER J.J., Anyanwu K., Ell S.R., GREENMAN J., STAFFORD N.D. Serum vascular endothelial growth factor in patients with head and neck squamous cell carcinoma. Clin. Otolaryngol. 1999;24:426–430. doi: 10.1046/j.1365-2273.1999.00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Banks R.E., Forbes M.A., Kinsey S.E., Stanley A., Ingham E., Walters C., Selby P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeulen P.B., Salven P., Benoy I., Gasparini G., Dirix L.Y. Blood platelets and serum VEGF in cancer patients. Br. J. Cancer. 1999;79:370–373. doi: 10.1038/sj.bjc.6690059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunsilius E., Gastl G. Platelets and VEGF blood levels in cancer patients. Br. J. Cancer. 1999;81:185–186. doi: 10.1038/sj.bjc.6690673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folkman J., Hochberg M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedel F., Götte K., Schwalb J., Bergler W., Hörmann K. Ko-Expression von VEGF und bFGF ist assoziiert mit einer erhöhten Gefässdichte in Kopf-Hals-Karzinomen. Laryngorhinootologie. 2000;79:730–735. doi: 10.1055/s-2000-9131. [DOI] [PubMed] [Google Scholar]
- 39.Mineta H., Miura K., Ogino T., Takebayashi S., Misawa K., Ueda Y., Suzuki I., Dictor M., Borg A., Wennerberg J. Prognostic value of vascular endothelial growth factor (VEGF) in head and neck squamous cell carcinomas. Br. J. Cancer. 2000;83:775–781. doi: 10.1054/bjoc.2000.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith B.D., Smith G.L., Carter D., Sasaki C.T., Haffty B.G. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2000;18:2046–2052. doi: 10.1200/JCO.2000.18.10.2046. [DOI] [PubMed] [Google Scholar]
- 41.Kyzas P.A., Cunha I.W., Ioannidis J.P.A. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: A meta-analysis. Clin. Cancer Res. 2005;11:1434–1440. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 42.Cohen E.E.W., Davis D.W., Karrison T.G., Seiwert T.Y., Wong S.J., Nattam S., Kozloff M.F., Clark J.I., Yan D.-H., Liu W., et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: A phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Druzgal C.H., Chen Z., Yeh N.T., Thomas G.R., Ondrey F.G., Duffey D.C., Vilela R.J., Ende K., McCullagh L., Rudy S.F., et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005;27:771–784. doi: 10.1002/hed.20246. [DOI] [PubMed] [Google Scholar]
- 44.Siu L.L., Burtness B., Cohen E.E., Harrington K.J., Licitra L.F., Rischin D., Zhu Y., Lee C.P., Pinheiro C., Swaby R.F., et al. Phase III LEAP-010 study: First-line pembrolizumab with or without lenvatinib in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) J. Clin. Oncol. 2020;38:TPS6589. doi: 10.1200/JCO.2020.38.15_suppl.TPS6589. [DOI] [Google Scholar]
- 45.Micaily I., Johnson J., Argiris A. An update on angiogenesis targeting in head and neck squamous cell carcinoma. Cancers Head Neck. 2020;5:5. doi: 10.1186/s41199-020-00051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos L.V.d., Cruz M.R., Lopes G.d.L., Da Lima J.P.S.N. VEGF-A levels in bevacizumab-treated breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2015;151:481–489. doi: 10.1007/s10549-015-3410-7. [DOI] [PubMed] [Google Scholar]
- 47.Vermorken J.B., Trigo J., Hitt R., Koralewski P., Diaz-Rubio E., Rolland F., Knecht R., Amellal N., Schueler A., Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 48.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.-R., Cupissol D., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 49.Gyllensten U., Bosdotter Enroth S., Stålberg K., Sundfeldt K., Enroth S. Preoperative Fasting and General Anaesthesia Alter the Plasma Proteome. Cancers. 2020;12:2439. doi: 10.3390/cancers12092439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyss A., Hashibe M., Chuang S.-C., Lee Y.-C.A., Zhang Z.-F., Yu G.-P., Winn D.M., Wei Q., Talamini R., Szeszenia-Dabrowska N., et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013;178:679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farsi N.J., Rousseau M.-C., Schlecht N., Castonguay G., Allison P., Nguyen-Tan P.F., Souliéres D., Coutlée F., Hier M., Madathil S., et al. Aetiological heterogeneity of head and neck squamous cell carcinomas: The role of human papillomavirus infections, smoking and alcohol. Carcinogenesis. 2017;38:1188–1195. doi: 10.1093/carcin/bgx106. [DOI] [PubMed] [Google Scholar]
- 52.Chen S., Lin Z., Chen J., Yang A., Zhang Q., Xie C., Zhang X., Yang Z., Chen W., Song M. Older age is a risk factor associated with poor prognosis of patients with squamous cell carcinoma of the oral cavity. Eur. Arch. Otorhinolaryngol. 2020;277:2573–2580. doi: 10.1007/s00405-020-05963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because of patient confidentiality and participant privacy terms. Requests to access the datasets should be directed to G.W., Gunnar.Wichmann@medizin.uni-leipzig.de.