Abstract
The objective of this study was to describe the association of enrollment in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC), the Supplemental Nutrition Assistance Program (SNAP), and infant growth and neurodevelopmental outcomes. Z scores and Bayley Scales of Infant and Toddler Development–Third Edition (Bayley-III) and Vineland Adaptive/Behavior Scale–II (VABS-II) scores represented primary outcomes. We conducted bivariate analyses and linear regression. Children who were enrolled in WIC or WIC/SNAP had weight z scores U (95% confidence interval [CI]) that were 1.32 (0.42–2.21) or 1.19 (0.16–2.23) units higher. Enrollment in WIC or WIC/SNAP was associated with a higher score (95% CI) of 11.7 U (1.2–22.2 U) or 11.5 (0.1–22.9) for Bayley-III cognitive score and 10.1 U (1.9–19.1 U) or 10.3 (0.9–19.7) for the VABS-II composite score. These findings support increased advocacy for participation in WIC or WIC/SNAP for families with high-risk infants.
Keywords: Growth, development, WIC, outcomes
Introduction
A recent policy statement by the American Academy of Pediatrics, “Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health,” supports that adequate maternal and child nutrition during this period is foundational for children’s growth and development.1 Unfortunately, many preterm infants who are hospitalized in neonatal intensive care units (NICUs) face considerable nutritional insufficiency.1 Provision of important macro- and micronutrients at certain points in development after discharge are crucial for growth and brain development.2,3 Therefore, possible malnutrition that concurs with a period of brain growth may explain why preterm infants have a higher risk of cognitive delay.4 Fortification of breast milk and nutrient-enriched formula has been recommended to address issues of postnatal growth and development.5 Caring for children with special medical and nutritional needs may burden families with financial consequences, which often disproportionately affect those who are low income and minorities, making it harder for them to receive the nutritional support they may need.6,7
Food assistance programs such as the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) provide “nutritional supplementation, health care referrals, and nutrition education to low-income children younger than 5 years and pregnant, breastfeeding, and postpartum mothers meeting financial and nutritional eligibility criteria.”8,9 WIC serves “8 million low-income women and young children” each month, which makes it the most important public food assistance program for young children.9,10 WIC often also sponsors reimbursements for therapeutic formulas such as nutrient-rich (calorie-dense) formulas for premature infants.8,9 Additionally, WIC centers and educators serve as a primary medical resource for under-served communities by recognizing early developmental delays and issues with child health.11 Many families enrolled in WIC are also enrolled in the Supplemental Nutrition Assistance Program (SNAP), which provides financial support to buy food at retail outlets to eat at home.10
Prenatal assessments have shown that maternal involvement in WIC is associated with decreased risk for neonatal prematurity and mortality.12–14 Infants and toddlers receiving WIC demonstrate faster weight gains during infancy15 and diets with more exposure to iron and zinc.13,16 However, there has been limited evaluation linking WIC participation for preterm infants in terms of growth or developmental outcomes. Our research question was, “Is enrollment in publically funded food assistance programs associated with improved growth and developmental outcomes of preterm infants after discharge from the NICU independent of other risk factors?” The specific aims of this work were (1) to describe the prevalence of enrollment in food assistance programs and (2) to evaluate whether enrollment in food assistance programs is associated with improved growth and developmental outcomes of preterm infants after discharge from the NICU in this analytic sample.
Methods
Study Design and Participants
This cross-sectional study consisted of a questionnaire/survey administered to participants. As in previous work conducted, the survey contained validated components in English and Spanish and queried families about the use of community services, medication use, feeding patterns, quality of life, and financial burden.17 We enrolled 1 caregiver of a preterm (<37-week gestation) infant attending a high-risk infant follow-up clinic at a quaternary urban hospital between 2013 and 2015. We included English- or Spanish-speaking parents of infants who were up to 24 months corrected age with completed developmental assessments. Consent was obtained when the parent agreed to complete the questionnaire, which was self-administered via laptop, or via an in-person or telephone interview. Participants were provided a small incentive to complete the survey. The Human Subjects Protection Program approved the study protocol. Of the 199 eligible participants, 169 enrolled (85% response rate), and 71 completed the developmental assessments and had growth data available (Figure 1).
Figure 1.
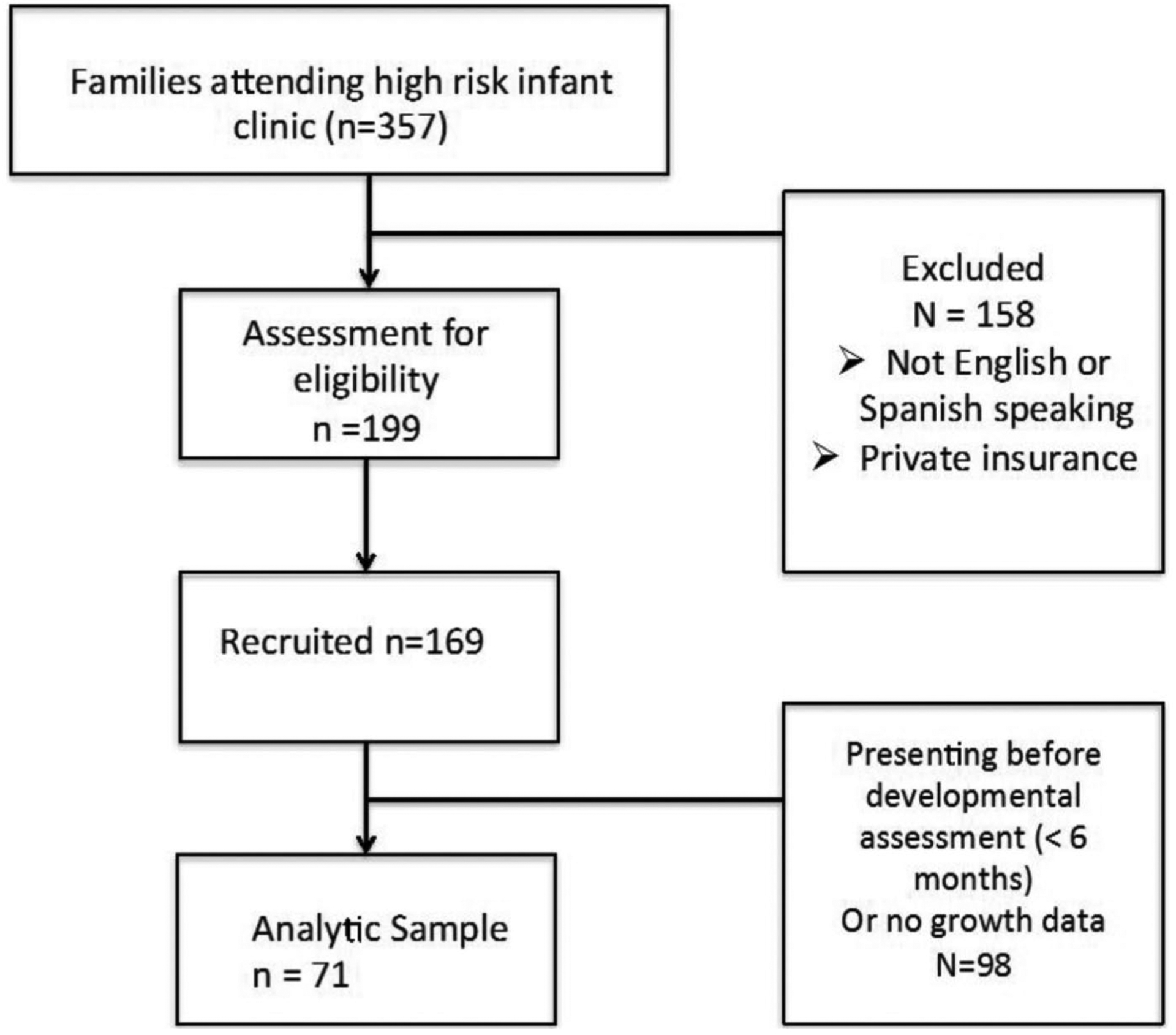
Patient recruitment.
Measurements
Measurements of primary outcomes and enrollment in food assistance programs are summarized below.
Anthropometrics.
Birth weight was obtained from the infants’ medical records. Current weight, length, and head circumference were obtained at the clinic visit. Subjects were weighed on a standard electronic scale to the nearest gram, and length was measured to the nearest centimeter on an infant length growth board. Head circumference (centimeters) was obtained with a standard clinical tape measure. Birth-weight z scores were calculated using the 2013 Fenton growth charts.18 All subsequent weight, length, and head circumference z scores were calculated from the World Health Organization reference data.19
Neurodevelopmental Assessment.
An occupational therapist, a physical therapist, or a member of the research team administered the cognitive and motor subscales of the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) and the Vineland Adaptive Behavioral Scales, second edition (VABS-II) in English and Spanish. The Bayley-III is the most widely used tool for assessing early development.20 Currently in its third edition, the Bayley Scales’ primary objective is “to identify children with developmental delay and to provide information for intervention planning” via individually administered assessment of children aged 1 to 42 months.20,21 The Bayley-III has an age-corrected mean score of 100 with a standard deviation of 15; higher scores indicate better development.
We assessed social skills and communication using the VABS-II by interviewing caregivers who were Spanish speaking.22 Specifically, the
VABS-II assesses adaptive behavior in four domains: Communication, Daily Living Skills, Socialization and Motor Skills. It also provides a composite score that summarizes an individual’s performance across all four domains. The VABS-II has an age-corrected mean score of 100 and a standard deviation of 15; higher scores indicate better function.22–24
Clinical Data.
We obtained information from medical records regarding delivery and complications during neonatal hospitalization. We asked the parents questions about their infants’ health status since discharge, and we queried about the use of durable medical equipment and the administration of prescription medications.25
Enrollment in Public Assistance Programs.
Participants were asked yes/no questions about their eligibility and use of community-based developmental resources such as early intervention programs, use of social services such as food assistance programs (enrollment in WIC or WIC/SNAP). Self-report for enrollment in public assistance programs has been validated in other studies.6,26 These questions were adapted from HelpSteps. Help-Steps.com is survey designed to identify health-related social problems. Details about the HelpSteps survey have been detailed in previous studies.25,27–30
Statistical Analysis
We described the characteristics of the study population using means and proportions. We compared the frequency of covariates (race/ethnicity, maternal education, language, income level, infant birth weight, neonatal comorbidities, postdischarge diagnoses, and medical technology) across developmental scores (Bayley-III, VABS-II). P values were obtained from t-tests for 2-category comparison and analysis of variance if there were more than 2 categories.
As per previous literature,6,26 we modeled food assistance as enrollment in either WIC or WIC and SNAP (WIC/SNAP).
We used least squares adjusted difference of means to quantify the effects of enrollment in food assistance programs on growth, adjusting for covariates. These covariates included race/ethnicity, maternal education, language, birth weight, neonatal comorbidity, and post-discharge diagnosis. This method has been used previously.31
Linear regression was used to evaluate the association between food assistance and neurodevelopmental scores. Covariates that changed the β coefficients by 10% were retained in the multivariable models. To evaluate goodness of fit of the linear multivariable model, we evaluated the F test and R2 for the linear models and found no differences from fit. To identify whether income assistance or infant chronologic age was an effect modifier for food assistance (stratified analysis), we fit linear regression models containing a multiplicative interaction term.
A sample size of at least 71 with unequal groups (52:19) achieves 99% power to reject the null hypothesis of equal means when the population difference is 10 with a standard deviation (SD) of 10 with a significance level (α) of .05 using a 2-sided, 2-sample equal variance t test (summary statement generated in PASS).
All of the statistical analyses were carried out using SAS, v. 9.4 (SAS Institute, Cary, NC).
Results
As outlined in Table 1, 73% of eligible families were enrolled in WIC and 21% were enrolled in WIC/SNAP. Most of our sample was Hispanic (81%), 73% of participants were non-English speaking and 93% had an annual income less than $40 000. All the subjects were enrolled in Medicaid. The median (interquartile range [IQR]) birth weight and gestational age of the infants was 1168 g (654 g) and 28 weeks (4 weeks), respectively, and their median (IQR) chronologic age was 14 months (8 months). Thirty-eight percent had a postdischarge diagnosis such as global developmental delay or cerebral palsy, and 24% used some sort of medical equipment such as supplemental oxygen, tracheostomy, a wheelchair, an adaptive stroller, or a feeding tube.
Table 1.
Characteristics of Eligible for the WIC Families (N = 71).
| Enrolled in WIC, n (%) | Eligible, But Not Enrolled in WIC, n (%) | P | ||
|---|---|---|---|---|
| 52 (73) | 19 (27) | |||
| Socio-demographic characteristics | ||||
| Race/ethnicity | ||||
| White non-Hispanic | 3 (4) | 2 (4) | 1 (6) | .04 |
| Black non-Hispanic | 6 (9) | 6 (12) | 0 (0) | |
| Hispanic | 56 (81) | 43 (82) | 13 (76) | |
| Other | 4 (6) | 1 (2) | 3 (18) | |
| Maternal education | ||||
| ≤High school | 23 (35) | 19 (40) | 4 (24) | .23 |
| Some college | 42 (65) | 29 (60) | 13 (76) | |
| Primary language | ||||
| English | 20 (29) | 13 (25) | 7 (39) | .26 |
| Non-English | 50 (71) | 39 (75) | 11 (61) | |
| Annual household family income ($) | ||||
| <40 000 | 65 (93) | 50 (96) | 15 (83) | .10 |
| ≥40 000–<80 000 | 4 (6) | 2 (4) | 2 (11) | |
| ≥80 000 | 1 (1) | 0 (0) | 1 (6) | |
| SNAP recipient | ||||
| Yes | 17 (24) | 15 (29) | 2 (11) | .13 |
| No | 54 (76) | 37 (71) | 17 (89) | |
| Enrollment in income assistance programs | ||||
| Supplemental Security Income recipient | ||||
| Yes | 25 (35) | 22 (42) | 3 (16) | .04 |
| No | 46 (65) | 30 (58) | 16 (84) | |
| Temporary assistance to needy families recipient | ||||
| Yes | 2 (3) | 2 (4) | 0 (0) | .99 |
| No | 69 (97) | 50 (96) | 19 (100) | |
| Enrollment in other public assistance programs | ||||
| Early Intervention Program | ||||
| Yes | 35 (49) | 24 (46) | 11 (58) | .38 |
| No | 36 (51) | 28 (54) | 8 (42) | |
| Reliance on energy assistance (Low-Income Home Energy Assistance Program) | ||||
| Yes | 3 (4) | 2 (4) | 1 (5) | .99 |
| No | 68 (96) | 50 (96) | 18 (95) | |
| Infant characteristics | ||||
| Birth weight (g), median (IQR) | 1168 (654) | 1110 (515) | 1195 (835) | .18 |
| Gestational age, median (IQR) | 28 (4) | 28 (4) | 30 (4) | .10 |
| Chronologic age, median (IQR) | 14 (8) | 14 (7) | 14 (10) | .77 |
| Neonatal comorbiditiesa | ||||
| Yes | 55 (77) | 40 (77) | 15 (79) | .99 |
| No | 16 (23) | 12 (23) | 4 (21) | |
| Post discharge diagnosesb | ||||
| Yes | 27 (38) | 19 (37) | 8 (42) | .67 |
| No | 44 (62) | 33 (63) | 11 (58) | |
| Use of medical equipmentc | ||||
| Yes | 17 (24) | 7 (13) | 10 (53) | .001 |
| No | 54 (76) | 45 (87) | 9 (47) | |
Abbreviations: WIC, Women, Infants, and Children; SNAP, Supplemental Nutrition Assistance Program; IQR, interquartile range.
Neonatal comorbidities include at least 1 diagnosis of fetal growth restriction, surfactant deficiency, necrotizing enterocolitis, intraventricular hemorrhage grade 3 or 4, patent ductus arteriosus, and retinopathy of prematurity.
Postdischarge diagnoses include at least 1 diagnosis of attention deficit hyperactivity disorder, autism, global developmental delay, and cerebral palsy.
Use of medical equipment includes oxygen, tracheostomy, wheelchair, adaptive stroller, and feeding tube.
Anthropometric measures at the clinic visit differed between the groups who were enrolled in food assistance programs and those who were not (Table 2). Specifically, children who were enrolled in food assistance programs (WIC or WIC/SNAP) had weight z scores U (95% CI) that was either 1.32 (0.42–2.21) or 1.19 (0.16–2.23) higher than those children who were not; enrollment in WIC/SNAP was associated with length z scores U (95% CI) that was 1.42 (0.19–2.65) higher than those children who were not (Table 2 and Figure 2). Finally, we identified that WIC or WIC/SNAP enrollment was associated with a change weight z score U (95% CI) from birth to current by 1.15 (95% CI = 0.10–2.20) or 1.2 (95% CI = 0.13–2.28), independent of other risk factors (Figure 3).
Table 2.
Growth Parameters and Enrollment in Food Assistance Programs (N = 71).
| Weight-for-Age z Score | Length-for-Age z Score | Head Circumference-for-Age z Score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SE)a | Adjusted Differenceb | Pc | Mean (SE)a | Adjusted Differenceb | Pc | Mean (SE)a | Adjusted Differenceb | Pc | |
| Enrollment in the Supplemental Nutrition Program for Women, Infant, and Children (WIC) | |||||||||
| Yes | −1.13 (0.18) | 1.32 (0.42 to 2.21) | .005 | −2.64 (0.23) | 1.02 (−0.07 to 2.12) | .07 | −1.53 (0.24) | 0.18 (−0.97 to 1.33) | .76 |
| No | −1.91 (0.32) | −3.15 (0.35) | −1.65 (0.36) | ||||||
| Enrollment in the Supplemental Nutrition Assistance Program (SNAP) | |||||||||
| Yes | −1.38 (0.39) | 0.17 (−0.73 to 1.1) | .71 | −2.34 (0.36) | 0.77 (−0.24 to 1.77) | .14 | −1.40 (0.35) | −0.18 (−1.18 to 0.82) | .71 |
| No | −1.32 (0.18) | −2.90 (0.23) | −1.61 (0.24) | ||||||
| Enrollment in WIC and SNAP | |||||||||
| Yes | −1.20 (0.18) | 1.19 (0.16 to 2.23) | .03 | −2.66 (0.22) | 1.42 (0.19 to 2.65) | .03 | −1.56 (0.23) | −0.11 (−1.44 to 1.22) | .87 |
| No | −1.78 (0.35) | −3.16 (0.22) | −1.58 (0.41) | ||||||
Abbreviation: SE = standard error.
Expressed as unadjusted mean (SE).
Adjusted difference of means after adjusting for race/ethnicity, maternal education, language, birth weight, neonatal comorbidity, and postdischarge diagnosis.
P value for the adjusted difference.
Figure 2.
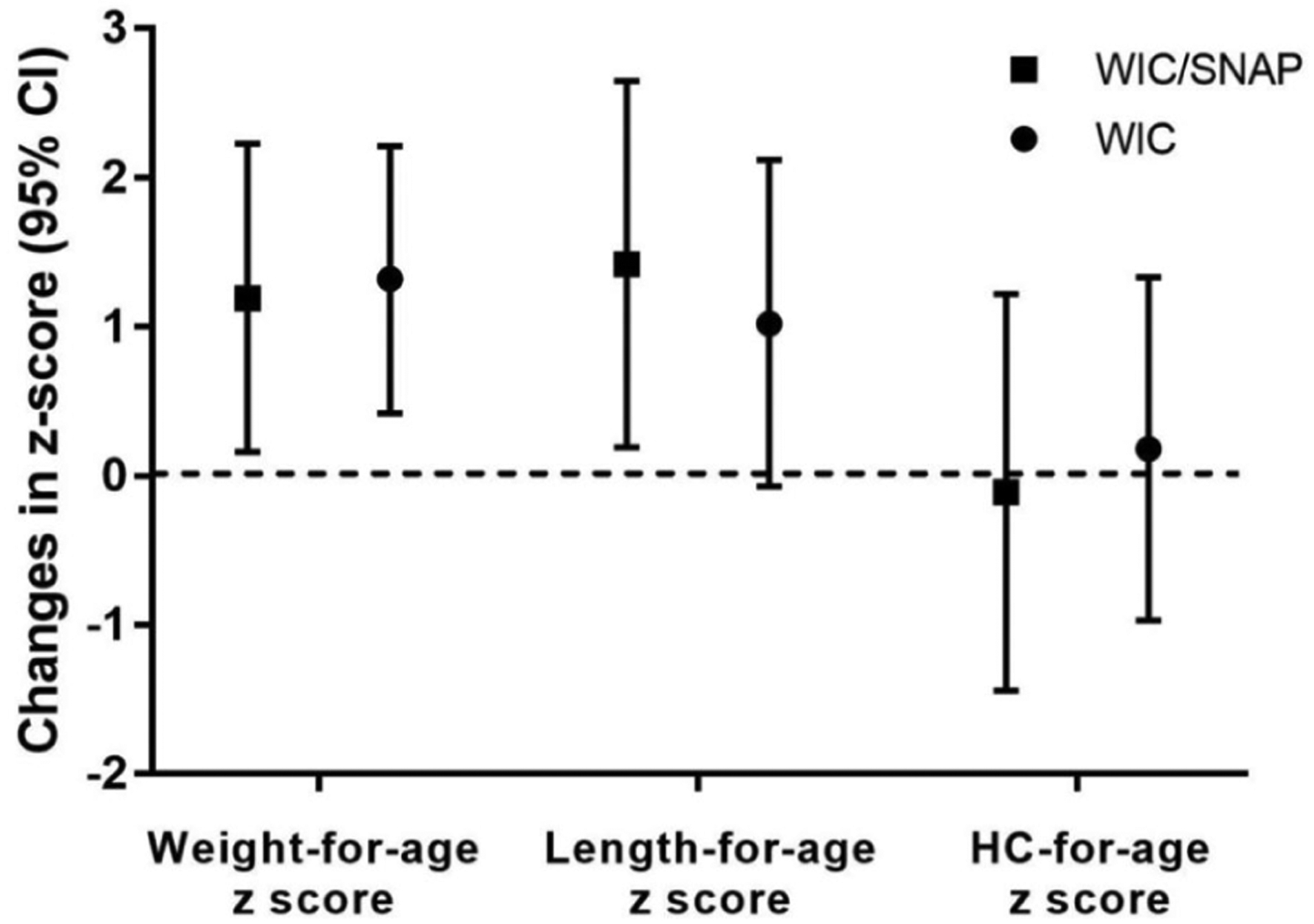
Adjusted difference of means of growth parameters and enrollment in food assistance (N = 71). WIC, Women, Infants, and Children.
*Adjusted difference of means after adjusting for race/ethnicity, maternal education, language, birth weight, neonatal comorbidity, post discharge diagnosis, and enrollment in early intervention (reference, no enrollment in WIC).
Figure 3.
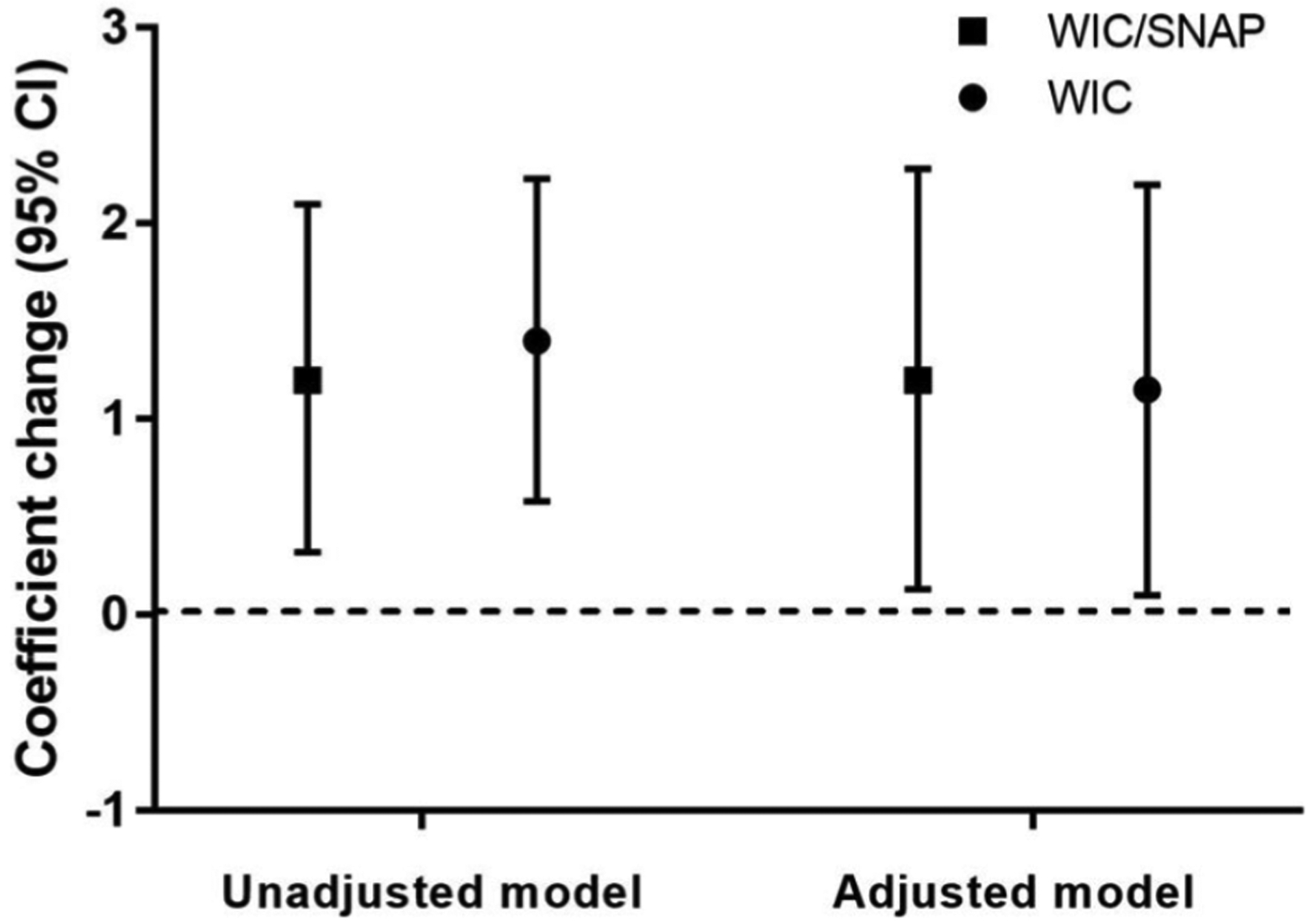
Association of enrollment in food assistance programs (WIC or WIC and SNAP) and z-score change from birth to follow-up (N = 71). WIC, Women, Infants, and Children; SNAP, Supplemental Nutrition Assistance Program.
*Model adjusted for infant age in months, race/ethnicity, maternal education, language, neonatal comorbidity, post discharge diagnosis, use of medical equipment, and enrolment in early intervention.
When we examined differences in development between groups using bivariate analysis as a function of enrollment in food assistance programs (Table 3), we did not find any.
Table 3.
Unadjusted Association of Enrollment Food Assistance Programs and Neurodevelopmental Outcomes (n = 71).
| Food Assistance | Total, n (%) | Bayley Scales of Infant Development III Composite Cognitive Score | Bayley Scales of Infant Development III Composite Motor Score | Vineland Adaptive Behavior II Score | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | P | Mean (SD) | P | Mean (SD) | P | ||
| Enrollment in the Supplemental Nutrition Program for Women, Infant and Children (WIC) | |||||||
| Yes | 52 (73) | 90 (17) | 0.14 | 82 (21) | 0.35 | 82 (15) | .20 |
| No | 19 (27) | 84 (18) | 77 (24) | 77 (17) | |||
| Enrollment in the Supplemental Nutrition Assistance Program (SNAP) | |||||||
| Yes | 17 (24) | 94 (19) | 0.09 | 87 (22) | 0.07 | 82 (15) | .51 |
| No | 54 (76) | 87 (17) | 80 (21) | 80 (16) | |||
| Enrollment in WIC and SNAP | |||||||
| Yes | 15 (29) | 90 (17) | 0.20 | 81 (21) | 0.67 | 81 (15) | .30 |
| No | 37 (71) | 84 (18) | 79 (24) | 77 (17) | |||
After adjusting for race/ethnicity, maternal education, primary language, language, birth weight, neonatal comorbidities, postdischarge diagnoses, the use of medical equipment, and enrollment in early intervention, we identified that enrollment in WIC or WIC/SNAP was associated with a higher score (95% CI) of 11.7 U (1.2–22.2 U) or 11.5 (0.1–22.9) for Bayley-III cognitive score and 10.1 U (1.9–19.1 U) or 10.3 (0.9–19.7) for the VABS-II composite score (Figure 4).
Figure 4.
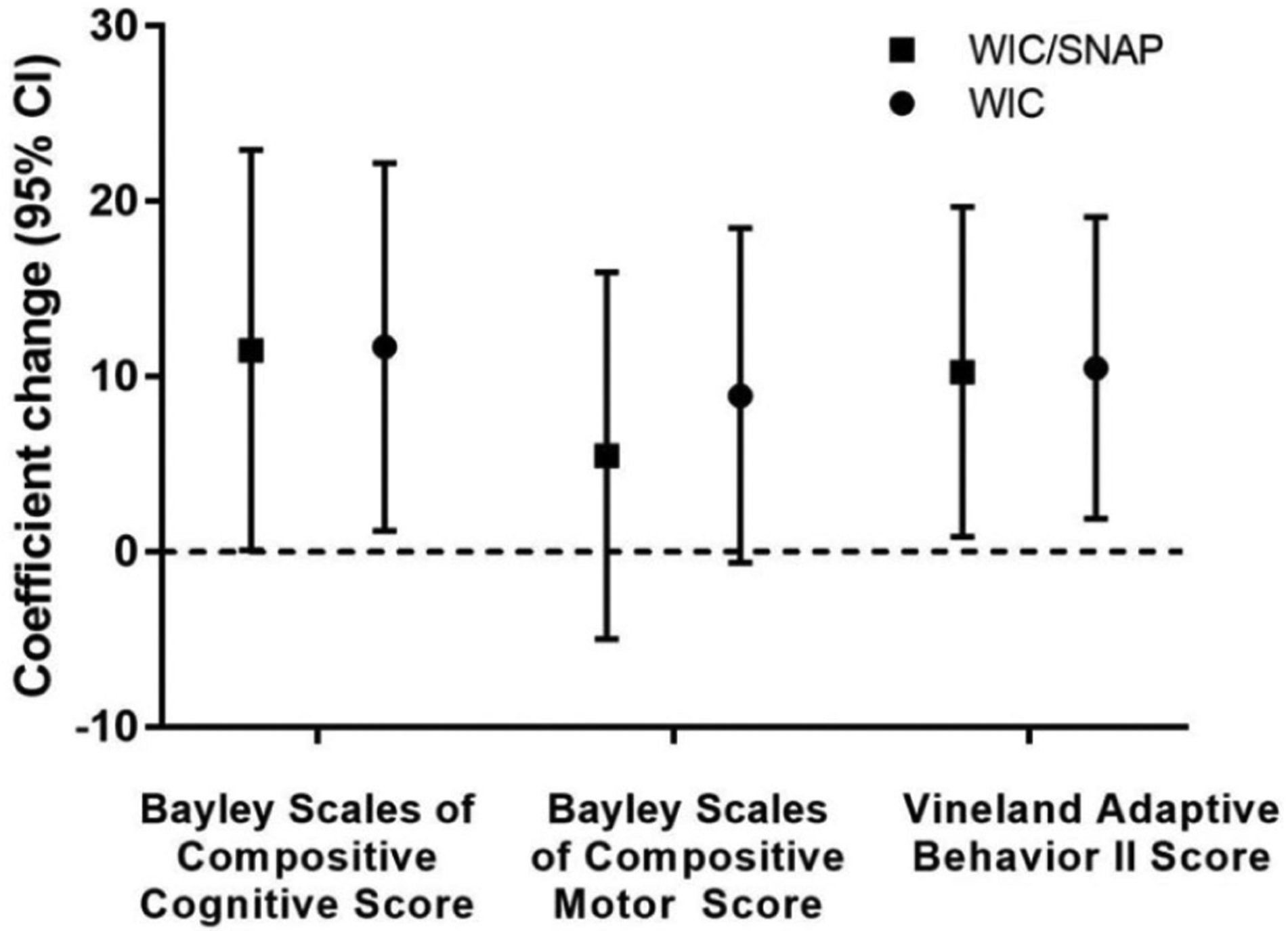
Adjusted association of food assistance (enrollment in WIC or WIC and SNAP) and neurodevelopmental outcomes (N = 71). WIC, Women, Infants, and Children; SNAP, Supplemental Nutrition Assistance Program.
*Model adjusted for infant age in months, race/ethnicity, maternal education, language, birth weight, neonatal comorbidity, postdischarge diagnosis, use of medical equipment, and enrolment in early intervention.
All of the interaction terms were nonsignificant when stratified by income assistance (food assistance × income assistance) or by chronologic age (food assistance × chronologic age) in all models. Therefore, neither income assistance nor infant chronologic age were effect modifiers of food assistance.
Discussion
In a vulnerable population of low-income, minority families with high-risk infants after NICU discharge, we found that enrollment in food assistance programs, independent of risk factors, was associated with higher weight and length z scores for the children of families enrolled in food assistance and found a change in weight z score from birth weight to follow-up when families were enrolled in WIC or WIC/SNAP. We also identified enrollment was associated positive neurodevelopmental outcomes, specifically cognitive and communication/adaptive behavior scores.
We found that participation in WIC was positively associated with growth, which may be due to a variety of reasons. Infants may receive direct benefit from the food supplements and nutritional recommendations that their families received. The WIC food package including items such as specifically therapeutic (calorie- and nutrient-rich formula especially designed for premature babies) formula that may be beyond the food budget of low-income families.32 Moreover, a recent qualitative study found that caregivers valued the WIC infant package and were influenced by the high cost of formula.33 Other studies have demonstrated that WIC participants are more likely to use preventative health services even beyond infant care.34
Furthermore, we found that use of WIC services was associated with better neurodevelopmental outcomes. Previous studies have found that intervention in early nutritional deficiency can be effective.1,35 Nutrients that affect
early brain development are (1) macronutrients such as protein, long-chain polyunsaturated fatty acids (LC-PFAs), and glucose; (2) micronutrients such as zinc, copper, iron, iodine, selenium; and (3) vitamins and cofactors such as B-vitamins, vitamin A, vitamin K, folate, and choline.1
Prenatal and early infancy iron deficiency can be associated with long-term behavioral changes that may not be reversible.36 Long-chain polyunsaturated fatty acids, which include docosahexaenoic acid and archidonic acid, are important for vision and neurocognitive development.3 Food packages from WIC often provide more balanced nutrition that includes these nutrients. In addition, participation in WIC may suggest better follow-up with other programs such as enrollment in neurodevelopmental follow-up (such as early intervention and high-risk infant follow-up programs).37
While not adequately powered in post hoc analysis or presented in this article, we found that 48% of families cited difficulty enrolling in food assistance (WIC or SNAP) due to transportation, lack of information, or burdensome paperwork. Unfortunately, many low-income and minority families do not receive many of the follow-up services prescribed.38–41 This incongruence can be attributed to several factors: Minority and immigrant mothers often utilize safety-net health care for themselves and may not be aware of the services for which their child is eligible; the prevalence of parenting stress, and postpartum depression.42,43 If more eligible families enrolled in food assistance programs, we believe that our results would have only been more positive (ie, we underestimated our presented results). Unfortunately, many children and families do not qualify for WIC services. Children whose families are on the edge for qualifying for WIC may liver on cheaper, less nutritionally sound diets. Many families fail to take advantage of the program after the first year because of the challenge of access or the contents of the WIC food packages.33 Keeping families in the program longer will make supplemental food available to growing toddlers and support neurodevelopment. Addressing these barriers to participation will be crucial to assisting these families improve outcomes.
Our study was one of the first to examine preterm infant outcomes among minority, low-income families who receive WIC. The analysis was appropriately powered and minimized chances of a type II error. Furthermore, the results are reproducible and generalizable to similar populations. However, there are limitations to the study. One is that it is cross-sectional, which makes it difficult to infer causality. Our study may also be prone to selection bias, specifically self-selection or volunteer bias. However, on examination of the source population (eligible patients from the high-risk infant follow-up clinic), we found the patient variables were very comparable with our analytic population, which increased the internal validity of our results. Although our outcomes and exposure measures have been well validated, there is some concern that Bayley-III may overinflate developmental scores compared with the previous edition of this scale.20 In such a case, the effect of enrollment in WIC may be underestimated.
Conclusions/Implications
We found that enrollment in food assistance programs was associated, independent of risk factors, with positive growth and neurodevelopmental outcomes. Specifically, we identified higher weight and length z scores for families enrolled in food assistance and found a change in weight z score from birth weight to follow-up when families were enrolled in WIC or WIC/SNAP. Moreover, cognitive and communication/adaptive behavior scores were higher for infants whose families were enrolled in WIC or WIC/SNAP.
The WIC and SNAP programs help 45 million low-income Americans (half of them children) pay for food each month.9,10 WIC is a federal grant program, not an entitlement program. Questions about the effectiveness of WIC and concerns about cost-effectiveness have endangered the existing program.14 Recently, the Agricultural Improvement Act of 2018 (Farm Bill) reauthorized SNAP and largely preserved benefits and eligibility but did not greatly increase the budget.44 The findings from this study only strengthen the platform that these food assistance programs should be protected and expanded to help infants and especially preterm infants after discharge from the NICU.
Acknowledgments
We acknowledge the support of the Teresa and Byron Pollitt Family Chair in Fetal & Neonatal Medicine, Sylvia Magallon, RN, for her assistance as the manager of the High-Risk Infant Follow-Up Program at the Fetal and Neonatal Institute for her assistance with recruitment. We also thank Ms Griselda Monroy, Ms Rachel Polcyn, and Ms Bernadette Lam for their assistance with data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored and supported by the Packard Foundation for Children’s Health, the Saban Research Career Development Award, and the Confidence Foundation. Dr Lakshmanan is supported by grant KL2TR001854 from the National Center for Advancing Translational Science (NCATS) of the US National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Schwarzenberg SJ, Georgieff MK; Committee on Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. 2018;141:e20173716. [DOI] [PubMed] [Google Scholar]
- 2.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–284. [DOI] [PubMed] [Google Scholar]
- 3.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teller IC, Embleton ND, Griffin IJ, van Elburg RM. Post-discharge formula feeding in preterm infants: a systematic review mapping evidence about the role of macronutrient enrichment. Clin Nutr. 2016;35:791–801. [DOI] [PubMed] [Google Scholar]
- 5.ESPGHAN Committee on Nutrition; Aggett PJ, Agostoni C, et al. Feeding preterm infants after hospital discharge: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42:596–603. [DOI] [PubMed] [Google Scholar]
- 6.Rose-Jacobs R, Fiore JG, de Cuba SE, et al. Children with special health care needs, supplemental security income, and food insecurity. J Dev Behav Pediatr. 2016;37: 140–147. [DOI] [PubMed] [Google Scholar]
- 7.Hodek JM, von der Schulenburg JM, Mittendorf T. Measuring economic consequences of preterm birth—methodological recommendations for the evaluation of personal burden on children and their caregivers. Health Econ Rev. 2011;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.California Department of PublicHealth. Women, Infants & Children (WIC). WIC Program overview. https://www.cdph.ca.gov/Programs/CFH/DWICSN/Pages/AboutWIC.aspx. Accessed June 19, 2017.
- 9.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review WIC Food Packages. Review of WIC Food Packages: Improving Balance and Choice: Final Report. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 10.Bleich SN, Rimm EB, Brownell KD. US Nutrition Assistance, 2018—modifying SNAP to promote population health. N Engl J Med. 2017;376:1205–1207. [DOI] [PubMed] [Google Scholar]
- 11.Zuckerman KE, Chavez AE, Reeder JA. Decreasing disparities in child development assessment: identifying and discussing possible delays in the special supplemental nutrition program for women, infants, and children (WIC). J Dev Behav Pediatr. 2017;38:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy ET, Gershoff S, Reed R, Austin JE. Evaluation of the effect of WIC supplemental feeding on birth weight. J Am Diet Assoc. 1982;80:220–227. [PubMed] [Google Scholar]
- 13.Rush D, Sloan NL, Leighton J, et al. The National WIC evaluation: evaluation of the Special Supplemental Food Program for Women, Infants, and Children. V. Longitudinal study of pregnant women. Am J Clin Nutr. 1988;48(2 suppl): 439–483. [DOI] [PubMed] [Google Scholar]
- 14.Black MM, Cutts DB, Frank DA, et al. ; Children’s Sentinel Nutritional Assessment Program Study Group. Special Supplemental Nutrition Program for Women, Infants, and Children participation and infants’ growth and health: a multisite surveillance study. Pediatrics. 2004;114: 169–176. [DOI] [PubMed] [Google Scholar]
- 15.Heimendinger J, Laird N, Austin JE, Timmer P, Gershoff S. The effects of the WIC program on the growth of infants. Am J Clin Nutr. 1984;40:1250–1257. [DOI] [PubMed] [Google Scholar]
- 16.Rose D, Habicht JP, Devaney B. Household participation in the Food Stamp and WIC programs increases the nutrient intakes of preschool children. J Nutr. 1998;128: 548–555. [DOI] [PubMed] [Google Scholar]
- 17.Flores-Fenlon N, Song AY, Yeh A, et al. Smartphones and text messaging are associated with higher parent quality of life scores and enrollment in early intervention after NICU discharge. Clin Pediatr (Phila). 2019;58:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PJ, Burnett A. Assessing developmental delay in early childhood—concerns with the Bayley-III scales. Clin Neuropsychol. 2017;31:371–381. [DOI] [PubMed] [Google Scholar]
- 21.Belfort MB, Santo E, McCormick MC. Using parent questionnaires to assess neurodevelopment in former preterm infants: a validation study. Paediatr Perinat Epidemiol. 2013;27:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. Circle Pines, MN: Pearson Assessment; 2005. [Google Scholar]
- 23.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meert KL, Slomine BS, Christensen JR, et al. ; Therapeutic Hypothermia after Pediatric Cardiac Arrest Trial Investigators. Family burden after out-of-hospital cardiac arrest in children. Pediatr Crit Care Med. 2016;17:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshmanan A, Agni M, Lieu T, et al. The impact of preterm birth <37 weeks on parents and families: a cross-sectional study in the 2 years after discharge from the neonatal intensive care unit. Health Qual Life Outcomes. 2017;15:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black MM, Quigg AM, Cook J, et al. WIC participation and attenuation of stress-related child health risks of household food insecurity and caregiver depressive symptoms. Arch Pediatr Adolesc Med. 2012;166:444–451. [DOI] [PubMed] [Google Scholar]
- 27.Hassan A, Scherer EA, Pikcilingis A, et al. Improving social determinants of health: effectiveness of a web-based intervention. Am J Prev Med. 2015;49:822–831. [DOI] [PubMed] [Google Scholar]
- 28.Hassan A, Blood EA, Pikcilingis A, et al. Youths’ health-related social problems: concerns often overlooked during the medical visit. J Adolesc Health. 2013;53:265–271. [DOI] [PubMed] [Google Scholar]
- 29.Wylie SA, Hassan A, Krull EG, et al. Assessing and referring adolescents’ health-related social problems: qualitative evaluation of a novel web-based approach. J Telemed Telecare. 2012;18:392–398. [DOI] [PubMed] [Google Scholar]
- 30.Fleegler EW, Lieu TA, Wise PH, Muret-Wagstaff S. Families’ health-related social problems and missed referral opportunities. Pediatrics. 2007;119:e1332–e1341. [DOI] [PubMed] [Google Scholar]
- 31.Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whaley SE, Ritchie LD, Spector P, Gomez J. Revised WIC food package improves diets of WIC families. J Nutr Educ Behav. 2012;44:204–209. [DOI] [PubMed] [Google Scholar]
- 33.Weber S, Uesugi K, Greene H, Bess S, Reese L, Odoms-Young A. Preferences and perceived value of WIC foods among WIC caregivers. J Nutr Educ Behav. 2018;50: 695–704. [DOI] [PubMed] [Google Scholar]
- 34.Buescher PA, Horton SJ, Devaney BL, et al. Child participation in WIC: Medicaid costs and use of health care services. Am J Public Health. 2003;93:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollitt E, Gorman KS, Engle PL, Rivera JA, Martorell R. Nutrition in early life and the fulfillment of intellectual potential. J Nutr. 1995;125(4 suppl):1111S–1118S. [DOI] [PubMed] [Google Scholar]
- 36.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnett AC, Cheong JLY, Doyle LW. Biological and social influences on the neurodevelopmental outcomes of preterm infants. Clin Perinatol. 2018;45:485–500. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg SA, Robinson CC, Shaw EF, Ellison MC. Part C early intervention for infants and toddlers: percentage eligible versus served. Pediatrics. 2013;131:38–46. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Ellison MC, Fast B, Robinson CC, Lazar R. Computing theoretical rates of part C eligibility based on developmental delays. Matern Child Health J. 2013;17: 384–390. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg SA, Zhang D, Robinson CC. Prevalence of developmental delays and participation in early intervention services for young children. Pediatrics. 2008;121: e1503–e1509. [DOI] [PubMed] [Google Scholar]
- 41.Enlow E, Herbert SL, Jovel IJ, Lorch SA, Anderson C, Chamberlain LJ. Neonatal intensive care unit to home: the transition from parent and pediatrician perspectives, a prospective cohort study. J Perinatol. 2014;34: 761–766. [DOI] [PubMed] [Google Scholar]
- 42.Ell K, Vourlekis B, Xie B, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115:4606–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. [DOI] [PubMed] [Google Scholar]
- 44.Conaway. Agricultural Improvement Act of 2018. Conference report. docs.house.gov/billsthisweek/20181210/CRPT-20181115hrpt20181072.pdf.Accessed April 11, 2019. [Google Scholar]


