Abstract
Introduction
The anti-inflammatory effect of macrolides prompted the study of oral clarithromycin in moderate COVID-19.
Methods
An open-label non-randomized trial in 90 patients with COVID-19 of moderate severity was conducted between May and October 2020. The primary endpoint was defined at the end of treatment (EOT) as no need for hospital re-admission and no progression into lower respiratory tract infection (LRTI) for patients with upper respiratory tract infection and as at least 50% decrease of the respiratory symptoms score without progression into severe respiratory failure (SRF) for patients with LRTI. Viral load, biomarkers, the function of mononuclear cells and safety were assessed.
Results
The primary endpoint was attained in 86.7% of patients treated with clarithromycin (95% CIs 78.1–92.2%); this was 91.7% and 81.4% among patients starting clarithromycin the first 5 days from symptoms onset or later (odds ratio after multivariate analysis 6.62; p 0.030). The responses were better for patients infected by non-B1.1 variants. Clarithromycin use was associated with decreases in circulating C-reactive protein, tumour necrosis factor-alpha and interleukin (IL)-6; by increase of production of interferon-gamma and decrease of production of interleukin-6 by mononuclear cells; and by suppression of SARS-CoV-2 viral load. No safety concerns were reported.
Conclusions
Early clarithromycin treatment provides most of the clinical improvement in moderate COVID-19.
Trial Registration
ClinicalTrials.gov, NCT04398004
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00505-8.
Keywords: Clarithromycin, COVID-19, Th1, Th2, Viral load
Key Summary Points
| Why carry out this study? |
| Published data are controversial on the efficacy of macrolides for COVID-19. This may be due to the varying time delay from infection onset until start of treatment |
| In the ACHIEVE study the clinical efficacy of clarithromycin was associated with the time delay since symptoms onset and with the mechanism of action |
| What was learned from the study? |
| Early clarithromycin treatment in patients with moderate COVID-19 is associated with most of clinical improvement |
| Treatment should start in the first 5 days from symptoms onset |
| The effect of clarithromycin is associated with increase of interferon-gamma production, decrease of interleukin-6 production and decrease of viral load |
Introduction
Early at the beginning of the COVID-19 pandemic, azithromycin, a macrolide drug, was introduced in the treatment algorithm because of its anti-inflammatory properties [1, 2]. The anti-inflammatory properties of macrolides for the empirical management of community-acquired pneumonia (CAP) are reflected in the guidelines of the American Thoracic Society published in 2019 [3]. These guidelines are influenced by observational studies showing that the addition of a macrolide decreases the risk of death from severe CAP [4–8]. Two double-blind, randomized, placebo-controlled clinical trials of our group have also shown substantial reduction of the risk of death among critically ill patients with gram-negative sepsis and ventilator-associated pneumonia with clarithromycin adjunctive treatment [9–11]. We have also recently shown that 28-day mortality of severe CAP was 20.8% among patients treated with a combination of β-lactam and clarithromycin and 33.8% when treated with a combination of β-lactam and azithromycin [12].
Based on the above evidence, it seems likely that treatment of COVID-19 with oral clarithromycin may attenuate the hyper-inflammatory responses of the host and decrease the risk of progression into severe respiratory failure (SRF). We conducted the ACHIEVE trial (Anti-inflammatory Clarithromycin to Improve SARS-CoV 2 Infection Early) as a proof of concept to investigate whether early administration of oral clarithromycin in patients with upper or lower respiratory tract infection (RTI) due to SARS-CoV-2 may attenuate the inflammatory burden, modulate the immune response of the host and result in early clinical improvement. The clinical efficacy of clarithromycin was also compared with a group of propensity-score matched concurrent comparators receiving standard-of-care treatment (SOC) with azithromycin and hydroxychloroquine.
Methods
Patient Population
ACHIEVE was an open-label non-randomized trial conducted in four study sites in tertiary hospitals in Greece (3rd Department of Internal Medicine, Sotiria General Hospital; 1st Department of Internal Medicine, Ioannina University General Hospital; 2nd Department of Internal Medicine, Alexandroupolis University General Hospital; 2nd Department of Internal Medicine, Tzaneion Piraeus General Hospital; EudraCT no. 2020-001882-36; ClinicalTrials.gov NCT04398004). Registration at the public EU repository EudraCT was done on 15 April 2020 before submission for regulatory approval according to the law applying for clinical trials in the EU. Since ACHIEVE is an interventional study, only central ethical approval by the National Ethics Committee of Greece is warranted. The study was approved for all sites by the National Ethics Committee of Greece (approval no. 45/20) and subsequently by the National Organization for Medicines of Greece (approval no. ISO 36/20). Patients were screened for eligibility and enrolment after providing written informed consent. Concurrent comparators receiving azithromycin plus hydroxychloroquine/chloroquine and hospitalized at the same time period in five other medical departments of tertiary hospitals in Greece were prospectively registered after written informed consent. Comparators were hospitalized in departments participating in the registry of the Hellenic Sepsis Study Group (www.sepsis.gr) and approval was provided by their Institution Review Boards. The first patient was enrolled on 17 May 2020 and the last visit of the last patient was on 22 October 2020.
Enrolled patients were adults (age ≥ 18 years) with confirmed infection by SARS-CoV-2 virus by real-time PCR of nasopharyngeal secretions and infection of the upper respiratory tract (URTI) or of the lower respiratory tract (LRTI). URTI was defined as the acute presentation of at least two of the following signs in a patient without radiological evidence of lung infection: (1) core temperature ≥ 37.5 °C; (2) new onset of cough; (3) chills or rigour; (4) total absolute lymphocyte count < 1500/mm3. LRTI was defined as the presence of infiltrates compatible with lower respiratory tract infections in chest x-ray or in chest computed tomography accompanied by at least one of the following: (1) new onset of cough or worsening cough; (2) dyspnea; (3) respiratory rales compatible with lung infection; (4) total absolute lymphocyte count < 1500/mm3. Exclusion criteria were: age < 18 years; intake of any other macrolide and of hydroxychloroquine/chloroquine for the infection under study; ratio of partial oxygen pressure to the fraction of inspired oxygen (pO2/FiO2) < 150; need for mechanical ventilation (MV) or non-invasive ventilation under positive pressure (NIV); neutropenia (< 1000/mm3); any intake of corticosteroids at a daily dose ≥ 0.4 mg/kg prednisone or equivalent the last 15 days; QTc interval at rest electrocardiogram ≥ 500 ms; pregnancy or lactation.
To derive the most appropriate comparators, the inclusion and exclusion criteria of the ACHIEVE trial were applied to all available concurrent comparators.
Trial Interventions
Enrolled patients received one tablet of 500 mg of clarithromycin every 12 h for 7 days. All other drugs except macrolides and hydroxychloroquine/chloroquine phosphate were allowed. Study visits were done daily starting from day 1 before start of treatment until day 8 at end of treatment (EOT visit). The test-of-cure (TOC) visit was done on day 14. For patients discharged earlier from hospital, EOT and TOC visits were done by phone calls. Recorded baseline information was severity assessed by APACHE II score, SOFA score and pneumonia severity index (PSI), comorbidities and laboratory information. On each visit follow-up, the state of the patient was assessed and the respiratory symptoms score (RSS) was calculated (see Supplementary methods). Fifteen millilitres of whole blood was collected on day 1 before start of treatment and on EOT into EDTA-coated tubes and sterile and pyrogen-free tubes for the isolation of peripheral blood mononuclear cells (PBMCs), serum and plasma. PBMCs were isolated after gradient centrifugation over Ficoll (Biochrom, Berlin, Germany) for 20 min at 1400 g. After three washings in ice-cold PBS pH 7.2, PBMCs were counted in a Neubauer plate with trypan blue exclusion of dead cells. They were then diluted in RPMI 1640 enriched with 2 mM of l-glutamine, 500 μg/ml of gentamicin, 100 U/ml of penicillin G, 10 mM of pyruvate and 10% foetal bovine serum (Biochrom) and suspended in wells of a 96-well plate. The final volume per well was 200 μl with a density of 2 × 106 cells/ml. PBMCs were exposed in duplicate for 24 h or 5 days at 37 °C in 5% CO2 to different stimuli: 10 ng/ml of Escherichia coli O55:B5 lipopolysaccharide (LPS, Sigma, St. Louis, USA) or 5 × 105 colony-forming units of heat-killed Candida albicans. Following incubation, cells were removed and analysed for flow cytometry. Concentrations of tumour necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-10 and interferon-gamma (IFNγ) were measured in cell supernatants or serum in duplicate by an enzyme immunoassay; the lower limit of detection was 20 pg/ml (Invitrogen, Carlsbad, CA, USA). C-reactive protein was measured by nephelometry; the lower limit of detection was 0.5 mg/l.
Nasopharyngeal swabs were collected using the 350C UTM© mini 1 ml collection and transport system (COPAN, Italia, S.p.A.) on day 1 before start of treatment, on day 4 and on EOT. The viral load was expressed as the Ct of the reaction positivity and as the relative log2 copies of the E gene and of the RdRp gene. Viral RNA was extracted from the transport medium using the QIAamp® Viral RNA Mini kit (Qiagen, Hilden, Germany) and all quantified with the Qubit™ RNA HS Assay Kit on a Qubit Fluorometer (Thermo Fisher Scientific Waltham, MA. USA). Sequencing libraries were generated using the CleanPlex® SARS-CoV-2 Research and Surveillance Panel powered by SOPHiA (Paragon Genomics, Hayward, CA, USA) using CleanPlex Indexed PCR Primers for Illumina (Paragon Genomics). The generated DNA libraries were loaded in a 300-cycle sequencing cartridge and sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA, USA).
The generated analysis output, in the FASTQ file format, was uploaded to the SOPHiA DDM tool (SOPHiA GENETICS Inc., Boston, MA, USA). A total of 5,598,884 read pairs were generated of which 5,351,919 pairs (95.59%) were successfully mapped. The reference sequence used was MN908947 (NC_045512.2). Four quality indicators generated for all samples (coverage uniformity < 90%, effective reads < 90%, pool 1 and 2 balance < − 1 and > 1 log 2 ratio, target covered < 95%) allow easy assessment of sequencing results quality. The genome sequences of SARS-CoV-2 were assigned to viral lineages according to the dynamic nomenclature system proposed by Rambaut et al. [13] using the SOPHiA DDM tool (https://pangolin.cog-uk.io).
Blood was sampled from concurrent SOC comparators on day 1 before start of treatment and on day 8 for the measurement of biomarkers.
Outcomes
The primary study endpoint was assessed at EOT and it was a composite endpoint defined differently for patients with URTI and for patients with LRTI. For patients with URTI the primary endpoint was defined as either no need for hospital re-admission in case of earlier discharge or as lack of progression into lower RTI. For patients with LRTI the primary endpoint was defined as any at least 50% decrease of the RSS from the baseline provided that the patient did not progress into SRF or die. SRF was defined as any decrease of the pO2/FiO2 < 150 mmHg necessitating MV or NIV. This endpoint was compared between patients who started early clarithromycin and those who started late clarithromycin. The same endpoints were captured for the SOC concurrent comparators. Secondary outcomes were the development of SRF by TOC visit; hospital readmission until TOC; the change of viral load from baseline in respiratory secretions on day 4 and EOT; the change of circulating biomarkers; the changes of cytokine responses. Study exploratory endpoints were the impact of time delay from the onset of COVID-19 symptoms on the change of the 11-point WHO clinical progression scale [14] and the association with the lineage of SARS-CoV-2.
Similar definitions of the study endpoints were applied for concurrent SOC comparators. Adverse Events (AE) (Common Terminology Criteria for Adverse Events, version 4.03) and Severe Adverse Events (SAE) (see Supplementary Appendix) were captured.
Statistical analysis
Qualitative data were presented as percentages with confidence intervals (CI) and quantitative data as means and SD; biomarkers and cytokines were expressed as means and SE. Using the median of the time between symptoms onset and start of clarithromycin, patients were divided into those with an early start and into those with a late start; comparisons were done by the binomial test. The impact of early start of clarithromycin on the achievement of the primary endpoint was assessed after multivariate analysis. Variables being different between patients who met and patients who did not meet the primary endpoint at p < 0.100 entered the multivariate equation; the odds ratio (OR) and 95% CIs were calculated. Propensity score 1:1 matching was applied to select the best matched comparators to the 90 patients treated with clarithromycin. Matching variables were: age, Charlson’s Comorbidity Index (CCI) and admission PSI; the frequency of URTI and LRTI; and admission C-reactive protein (CRP). Comparisons were performed by Fisher’s exact test using confirmatory forward stepwise logistic regression analysis (IBM SPSS Statistics v. 25.0). Paired comparisons were done by the Wilcoxon’s signed rank test. Any two-sided p value < 0.05 was statistically significant.
Results
Trial Conduct
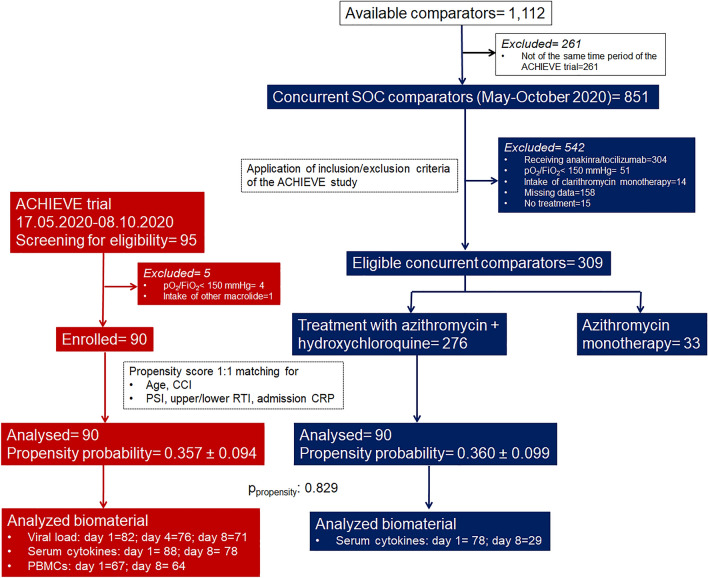
Patients participating in the ACHIEVE open-label trial were enrolled between 17 May 2020 and 8 October 8 2020. After propensity matching, 90 concurrent SOC comparators were selected within the same time frame. The propensity score of patients enrolled in the ACHIEVE trial was 0.357 ± 0.094; the respective score of the concurrent SOC comparators was 0.360 ± 0.099 (p = 0.829). The study flow chart is shown in Fig. 1. None of the 180 participants received biological anti-cytokine drugs. The administered dose of azithromycin was 500 mg daily for 7 days and of hydroxychloquine 500 mg every 12 h for 7 days. Baseline demographics of patients receiving clarithromycin and of concurrent comparators were not different (supplementary Table 1).
Fig. 1.
ACHIEVE Trial profile and selection of concurrent standard-of-care (SOC) comparators. Azithromycin plus hydroxychloroquine/chloroquine comparators were hospitalized at the same time period in five departments of internal medicine in tertiary hospitals and they were selected in two steps: at the first step after application of the inclusion and exclusion criteria of patients receiving clarithromycin; at the second step after propensity score matching. CCI Charlson’s Comorbidity Index, CRP C-reactive protein, FiO2 fraction of inspired oxygen, PSI pneumonia severity index, pO2 partial oxygen pressure, RTI respiratory tract infection
Study Endpoints
The primary endpoint was met in 78 patients treated with clarithromycin (86.7% 95% CIs 78.1–92.2%). Using the median of distribution, patients treated with clarithromycin were divided into those who started treatment within the first 5 days from start of symptoms (n = 48, range 1–5 day) and into those who started treatment 6 days or later from start of symptoms (n = 42, range 6–12 days). No differences were found in baseline characteristics of the enrolled patients divided into those with early and late start of clarithromycin (Table 1). The primary endpoint was achieved in 91.7% of early starters (44 out of 48 patients; 95% CIs 80.4–96.7%) and in 81.4% (34 out of 42 patients; 95% CIs 66.7–90.0%) of late starters. Multivariate logistic regression analysis showed that early start of clarithromycin was the only independent variable to be positively associated with the favourable primary endpoint (OR = 6.62; 95% CIs 1.19–32.63; p = 0.030; Table 2).
Table 1.
Baseline laboratory values and treatment modalities for participants in the ACHIEVE trial
| Variable | Late start (N = 42) | Early start (N = 48) | p value |
|---|---|---|---|
| Age, years, mean (SD) | 57.0 (17.4) | 58.5 (16.9) | 0.959 |
| Male gender, n (%) | 24 (57.1) | 29 (60.4) | 0.831 |
| Upper/lower respiratory tract infection, n (%) | 5 (11.9)/37 (88.1) | 7 (14.6)/41 (85.4) | 0.765 |
| Severity indexes, mean (SD) | |||
| Charlson’s Comorbidity Index | 2.50 (2.68) | 2.29 (2.20) | 0.687 |
| Admission APACHE II score | 5.28 (3.97) | 5.70 (3.33) | 0.604 |
| Admission Pneumonia Severity Index | 60.3 (27.9) | 60.1 (27.1) | 0.966 |
| Admission SOFA score | 1.14 (1.09) | 1.33 (1.26) | 0.449 |
| Comorbidities, no. (%) | |||
| Type 2 diabetes mellitus | 6 (14.3) | 11 (22.9) | 0.419 |
| Chronic heart failure | 2 (4.8) | 2 (4.2) | 1.00 |
| Coronary heart disease | 4 (9.5) | 4 (8.3) | 1.00 |
| Chronic renal disease | 1 (2.4) | 5 (10.4) | 0.209 |
| Atrial fibrillation | 3 (7.1) | 0 (0) | 0.098 |
| Hypertension | 17 (40.5) | 19 (39.6) | 1.00 |
| Chronic obstructive pulmonary disease | 1 (2.4) | 3 (6.3) | 0.620 |
| Hypothyroidism | 6 (14.3) | 6 (12.5) | 1.00 |
| Solid tumor malignancy | 5 (11.9) | 2 (4.2) | 0.245 |
| Dyslipidemia | 11 (26.2) | 11 (22.9) | 0.808 |
| Laboratory values, mean (SD) | |||
| Total white blood cells (/mm3) | 5930.7 (1700.1) | 6159.1 (2716.3) | 0.642 |
| Neutrophils (/mm3) | 3835.7 (1674.5) | 4370.6 (2528.5) | 0.251 |
| Lymphocytes (/mm3) | 1331.7 (574.7) | 1336.2 (491.5) | 0.968 |
| Platelets (× 103/mm3) | 221.4 (83.7) | 208.8 (61.6) | 0.424 |
| International normalized ratio | 1.10 (0.25) | 1.10 (0.21) | 0.887 |
| AST (U/l) | 33.9 (16.2) | 34.1 (24.7) | 0.984 |
| ALT (U/l) | 30.6 (14.9) | 34.6 (26.2) | 0.395 |
| pO2/FiO2 (mmHg) | 373.7 (103.3) | 370.6 (72.8) | 0.872 |
| C-reactive protein (mg/l) | 77.2 (108.4) | 62.7 (58.3) | 0.439 |
| Concomitant treatment, n (%) | |||
| 3rd-generation cephalosporin | 27 (64.3) | 36 (76.6) | 0.246 |
| Piperacillin/tazobactam | 7 (16.7) | 4 (8.5) | 0.337 |
| Moxifloxacin/levofloxacin | 1 (2.4) | 1 (2.1) | 1.00 |
Patients are divided onto those who started early treatment with clarithromycin (≤ 5 days from symptom onset) and those who started late treatment with clarithromycin (6 days or more from symptoms onset)
ALT alanine aminotransferase, AST aspartate aminotransferase, APACHE Acute Physiology and Chronic Health Evaluation, n number, pO2/FiO2 ratio of partial oxygen pressure to the fraction of inspired oxygen, APTT activate partial thromboplastin time, SOFA sequential organ failure assessment, SD standard deviation
Table 2.
Univariate and multivariate logistic regression analysis of baseline variables associated with the achievement of the study primary endpoint in the ACHIEVE trial
| Variable | Incidence of primary endpoint | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| No (total = 12) | Yes (total = 78) | OR (95% CIs) | p | OR (95% CIs) | p | |
| Start of clarithromycin within the first 5 days for symptoms onset, n (%) | 4 (33.3) | 44 (56.4) | 3.49 (0.85–14.34) | 0.082 | 6.62 (1.19–32.63) | 0.030 |
| CCI, mean (SD) | 4.00 (2.95) | 2.14 (2.25) | 0.76 (0.60–0.96) | 0.019 | * | |
| Admission APACHE II score, mean (SD) | 8.00 (3.13) | 5.12 (3.79) | 0.84 (0.72–0.97) | 0.021 | * | |
| Admission SOFA score, mean (SD) | 2.16 (1.11) | 1.10 (1.13) | 0.51 (0.31–0.84) | 0.008 | 0.39 (0.22–0.68) | 0.001 |
| Admission PSI, mean (SD) | 80.7 (20.7) | 57.0 (26.9) | 0.97 (0.95–0.99) | 0.008 | * | |
| Lymphocytes/mm3, mean (SD) | 1126.9 (432.3) | 1366.5 (536.9) | 1.00 | 0.148 | ||
| CRP, mg/l, mean (SD) | 110.1 (180.2) | 63.7 (61.4) | 0.99 (0.99–1.00) | 0.094 | * | |
APACHE Acute Physiology and Chronic Health Evaluation, CCI Charlson’s Comorbidity Index, CI confidence interval, CRP C-reactive protein, n number, OR odds ratio, PSI pneumonia severity index, SOFA sequential organ failure assessment
*Variable did not enter the equation after three steps of the multivariate model
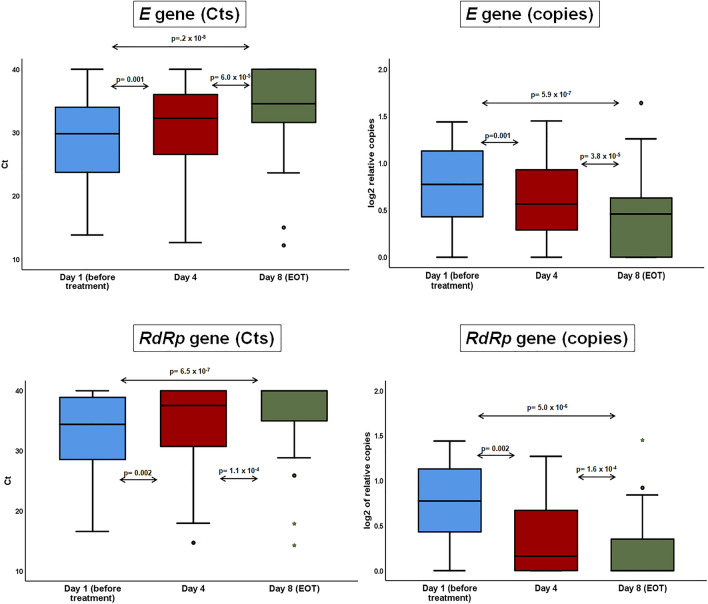
RSS was significantly decreased within the first 24 h from start of clarithromycin (supplementary Fig. 1). The viral loads of patients at baseline (before start of the study drug), on day 4 of treatment with clarithromycin and at the EOT visit with clarithromycin are shown in Fig. 2. The time of Ct to positivity was increased over study visits to be considered an indirect index of the decrease of the copies of E gene and RdRp gene. The relative log2 copies of both genes were decreased over time.
Fig. 2.
Viral loads of E gene and RdRp gene in the nasopharynx. Viral load of SARS-CoV-2 was assessed in the nasopharynx of clarithromycin-treated patients over visit using the relative expression of the constitutive E gene and of the specific RdRP gene. The expression of both genes was expressed as both the absolute time cycle (Ct) of the real-time PCR reaction and the log2 relative copies. The arrows indicate the comparisons; the respective p value by the Wilcoxon rank-sum test is provided. EOT end of treatment
NGS analysis of viral isolates did not detect any single nucleotide variant (SNV) in either the E gene or RdRp gene. SARS-CoV-2 viral isolates could be determined following quality control amputation in isolates coming from 49 patients. In total, analysis included 32 viral isolates of the B1.1 lineage and 17 isolates of the non-B.1.1 lineage. Patients starting early clarithromycin met the primary endpoint of treatment response similarly for both types of viral lineage. However, among patients starting late clarithromycin the rate of the positive treatment response was significantly greater among those infected by the non-B1.1 SARS-CoV-2 viral isolates (Table 3).
Table 3.
Association between delay start of clarithromycin and onset of symptoms and lineage of SARS-CoV-2 viral isolate and achievement of the primary treatment outcome
| Achievement of primary endpoint | Non-B1.1 lineage*, n (%) | B1.1 lineage, n (%) | p value | OR (95% CIs) |
|---|---|---|---|---|
| Early start of clarithromycin (≤ 5 days) | ||||
| No | 1 (14.3) | 2 (9.5) | 1.00 | 1.58 |
| Yes | 6 (85.7) | 19 (90.5) | 0.12–20.69 | |
| Late start of clarithromycin (> 5 days) | ||||
| No | 0 (0) | 5 (45.5) | 0.035 | NC** |
| Yes | 10 (100) | 6 (54.5) | ||
CI confidence intervals, OR odds ratio
*Isolates were matched as B1.1 (n = 30, 61.2%); B1.1. with 75% matching to the parent B1.1 (n = 2, 4.1%); B1.1. with 50% matching to the parent B1.1 (n = 8, 16.3%); B1.1.2 (n = 3; 6.1%); B1 (n = 2; 4.1%), B1.1.25 (n = 2; 4.1%), B1.1.10 (n = 1, 2.1%); B1.1.70 (n = 1, 2.1%). Viral isolates B1.1 and B1.1 with 75% matching to the parent B1.1 were analysed together as viral isolates of the B1.1 lineage
**NC: cannot be calculated because one value is zero
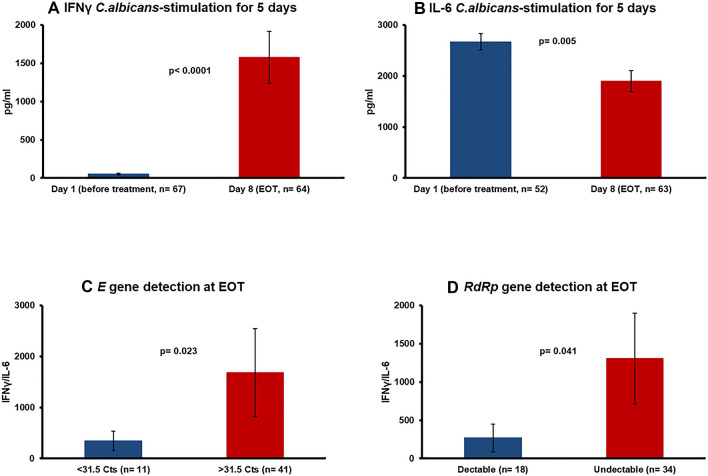
Clarithromycin treatment was associated with increased production of IFNγ in response to stimulation with heat-killed C.albicans for 5 days (Fig. 3A) and decreased production of IL-6 (Fig. 3B). The ratio of IFNγ/IL-6 production from PBMCs at the EOT was greater among patients with lower viral load (Fig. 3C and D). Contrarily, no change of the monocyte function was found (supplementary Fig. 2).
Fig. 3.
Modulation of cytokines responses at the end of treatment as the proposed mechanism of action of clarithromycin. Production of A interferon-gamma (IFNγ) and of B interleukin (IL)-6 from isolated peripheral blood mononuclear cells following stimulation with heat-killed Candida albicans before start of treatment with clarithromycin and at end of treatment (EOT) with clarithromycin. Comparison of the IFNγ/IL-6 ratio of PBMCs at the EOT between patients with high and low viral load of SARS-CoV-2 on the same treatment visit. The viral load is expressed as the Ct of positivity of the real-time PCR separately for C the E gene and for D the RdRp gene. The N values in parentheses refer to the existing number of patients analysed. The p values of comparisons by the Mann-Whitney U test are provided
The selection of SOC concurrent comparators was appropriate and no differences in baseline characteristics were found (supplementary Table 1). The primary endpoint was met in 66 patients among the concurrent SOC comparators (73.3%; 95% CIs 63.4–81.4%) and this was significantly lower than in patients treated with clarithromycin. When concurrent SOC comparators and patients treated with clarithromycin were analyzed as one cohort, clarithromycin treatment and nine other variables showed an association with the endpoint in univariate logistic regression. However, in a multivariate step-wise logistic regression analysis, only three variables retained a robust independent association with the endpoint (Table 4), including clarithromycin treatment (odds ratio 3.30; 95% CIs 1.10–9.87; p = 0.033).
Table 4.
Univariate and multivariate logistic regression analysis of baseline variables associated with the achievement of the study primary endpoint
| Variable | Incidence of primary endpoint | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| No (total = 36) | Yes (total = 144) | OR (95% CIs) | p value | OR (95% CIs) | p value | |
| Clarithromycin treatment, n (%) | 12 (33.3) | 78 (54.2) | 2.36 (1.09–5.09) | 0.039 | 3.30 (1.10–9.87) | 0.033 |
| CCI, mean (SD) | 3.30 (2.50) | 1.84 (2.07) | 0.77 (0.66–0.89) | 0.001 | * | |
| Admission APACHE II score, mean (SD) | 7.52 (3.18) | 4.82 (3.67) | 0.82 (0.75–0.92) | < 0.0001 | * | |
| Admission SOFA score, mean (SD) | 2.60 (1.55) | 0.99 (1.03) | 0.39 (0.27–0.54) | < 0.0001 | 0.38 (0.25–0.59) | < 0.0001 |
| Admission PSI, mean (SD) | 86.8 (27.4) | 58.3 (24.9) | 0.96 (0.95–0.97) | < 0.0001 | 0.96 (0.94–0.98) | 0.001 |
| Lymphocytes/mm3, mean (SD) | 1052.8 (581.8) | 1299.9 (575.3) | 1.01 (1.00–1.02) | 0.023 | * | |
| Hypertension, n (%) | 18 (50.0) | 45 (31.3) | 0.45 (0.21–0.95) | 0.037 | * | |
| Coronary heart disease, n (%) | 7 (19.4) | 7 (4.9) | 0.21 (0.07–0.65) | 0.009 | * | |
| Atrial fibrillation, n (%) | 6 (16.7) | 1 (0.7) | 0.04 (0.00–0.30) | < 0.0001 | * | |
| CRP, mg/l, mean (SD) | 104.9 (115.8) | 58.2 (68.4) | 0.99 (0.99–0.99) | 0.005 | * | |
In the analysis patients enrolled in the ACHIEVE trial and concurrent standard-of-care comparators are analysed together
APACHE Acute Physiology and Chronic Health Evaluation, CCI Charlson’s Comorbidity Index, CI confidence interval, CRP C-reactive protein, n number, OR odds ratio, PSI pneumonia severity index, SOFA sequential organ failure assessment
*Variable did not enter the equation after three steps of the multivariate model
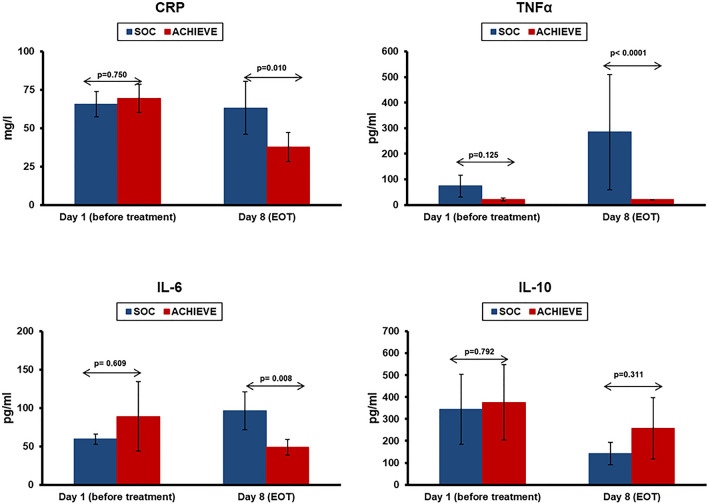
Clarithromycin treatment was associated with attenuation of pro-inflammatory responses. On day 8 the serum concentrations of C-reactive protein (CRP), tumour necrosis factor-alpha (TNFα) and interleukin (IL)-6 were lower among patients treated with clarithromycin than among concurrent SOC comparators. No differences were found in IL-10 (Fig. 4).
Fig. 4.
Attenuation of pro-inflammatory responses in COVID-19 following treatment with clarithromycin. Serum concentrations of C-reactive protein (CRP), tumour necrosis factor-alpha (TNFα) and interleukin (IL)-6 were measured before start of treatment and at the end-of-treatment visit (EOT) of day 8 among patients treated with clarithromycin (ACHIEVE trial) and among concurrent standard-of-care (SOC) comparators. The p-values of comparisons by the Mann–Whitney U test are provided. EOT: end of treatment
At the TOC visit, the incidence of SRF was 12.2% (n = 11; 95% CIs 6.9–20.6%) among patients treated with clarithromycin. This was 26.7% (n = 24; 95% CIs 18.6–36.6%) among SOC concurrent comparators (p: 0.023). Multivariate logistic regression analysis revealed that the only independent variable associated with protection from SRF at TOC was the intake of clarithromycin (odds ratio 0.22; 95% CIs 0.06–0.79; p 0.022; supplementary Table 2).
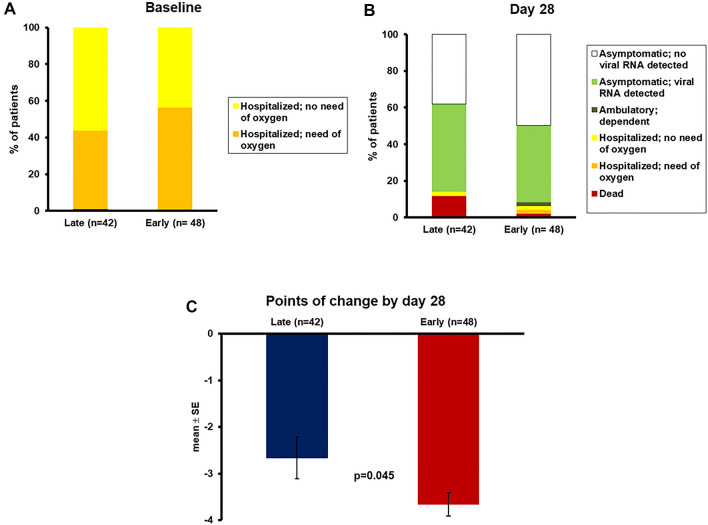
The benefit coming from the early start of clarithromycin was reflected in the exploratory outcome of the 11-point WHO CPS by day 28. The decrease in points on the symptoms scale was significantly greater among early starters of clarithromycin (Fig. 5).
Fig. 5.
Change of the 11-point WHO clinical progression scale (CPS) after 28 days in association with the time to start of clarithromycin. Patients are divided into those who started early treatment (the first 5 days from symptoms onset) and those who started late treatment (≥ 6 days from symptoms onset). Panel A demonstrates the allocation of patients at the WHO-CPS scale at baseline before start of treatment. Panel B demonstrates the allocation of patients at the WHO-CPS scale after 28 days. Panel C demonstrates the comparative change of points of the WHOC-CPS scale on day 28 from baseline. The p value of comparison is provided
Safety
The serious adverse events (SAEs) and the non-serious AEs that were captured during the study period of 14 days are listed in Table 5. Reported events probably or possibly related to the study drug with incidence > 1% were: increase of aminotransferases (grade I 18.8%; grade II 1.1%); diarrhea (mild 12.1%; moderate 1.1%); mild vomiting in 1.1%, grade I increase of bilirubin in 2.22%; mild allergic reaction in 1.1%. No drug-related SAEs were reported.
Table 5.
Serious and non-serious adverse events reported by day 14
| Event | No. of patients (%) | Relationship with study drug, n (%) |
|---|---|---|
| Serious adverse events | ||
| Mechanical ventilation | 4 (4.4) | None reported to be related |
| Hypoxemia prolonging hospitalization | 11 (12.2) | None reported to be related |
| Death | 3 (3.3) | None reported to be related |
| Non-serious adverse events | ||
| Increase of aminotransferases | 56 (62.2) | |
| Grade I | 52 (57.8) | Probably-related 1 (1.1); possibly related 16 (17.7) |
| Grade II | 4 (4.4) | Possibly related 1 (1.1) |
| Electrolyte abnormalities | ||
| Grade I hyperkalemia | 7 (7.7) | None reported to be related |
| Grade I hypokalemia | 12 (13.3) | None reported to be related |
| Grade II hypokalemia | 2 (2.2) | None reported to be related |
| Grade I hyponatremia | 17 (18.9) | None reported to be related |
| Grade II hyponatremia | 2 (2.2) | None reported to be related |
| Grade I hypocalcemia | 3 (3.3) | None reported to be related |
| Gastrointestinal events | ||
| Mild diarrhea | 16 (17.1) | Probably related 3 (3.3); possibly related 8 (8.8) |
| Moderate diarrhea | 2 (2.2) | Possibly related 1 (1.1) |
| Mild vomiting | 2 (2.2) | Possibly related 2 (2.2) |
| Moderate vomiting | 1 (1.1) | None reported to be related |
| Grade I bilirubin increase | 3 (3.3) | Possibly related 2 (2.2) |
| Grade I amylase increase | 7 (7.7) | None reported to be related |
| Metabolic events | ||
| Grade I hyperglycemia | 24 (26.7) | None reported to be related |
| Grade II hyperglycemia | 7 (7.7) | None reported to be related |
| Grade I hypoglycemia | 7 (7.7) | None reported to be related |
| Grade I hypertriglyceridemia | 8 (8.8) | None reported to be related |
| Blood and lymphatic tissue | ||
| Grade I anemia | 16 (17.1) | None reported to be related |
| Grade II anemia | 2 (2.2) | None reported to be related |
| Grade I decrease of neutrophils | 2 (2.2) | None reported to be related |
| Grade II decrease of neutrophils | 1 (1.1) | None reported to be related |
| Grade I decrease of lymphocytes | 11 (12.2) | None reported to be related |
| Grade II decrease of lymphocytes | 11 (12.2) | None reported to be related |
| Grade I decrease of platelets | 6 (6.6) | None reported to be related |
| Grade II decrease of platelets | 1 (1.1) | None reported to be related |
| Grade I increase of INR | 3 (3.3) | None reported to be related |
| Grade II increase of INR | 3 (3.3) | None reported to be related |
| Grade I increase of aPTT | 1 (1.1) | None reported to be related |
| Mild allergy | 4 (4.4) | Possibly related 1 (1.1) |
| Grade I creatinine increase | 8 (8.9) | None reported to be related |
| Cardiovascular events | ||
| Grade I tachycardia | 1 (1.1) | None reported to be related |
| Grade I bradycardia | 6 (6.6) | None reported to be related |
| Grade I increase of blood pressure | 1 (1.1) | None reported to be related |
| Episode of chest pain | 1 (1.1) | None reported to be related |
aPTT activated partial thromboplastin time, INR international normalized ratio
Discussion
Based on previous experience with clarithromycin [9–12], we sought to investigate the potential of this antibiotic in reducing the hyper-inflammatory response in moderate COVID-19 and contributing to better clinical outcomes than currently available treatment approaches. Analysis revealed that clarithromycin treatment was associated with a significantly greater clinical benefit at the EOT visit of 86.7% compared with 73.3% achieved by the concurrent SOC comparators. This early benefit shown at the end of the 7-day course with clarithromycin was further reflected in the incidence of SRF at the TOC visit of day 14 when treatment with clarithromycin provided 78% relative decrease of the risk for SRF compared to the SOC regimen. The benefit was pronounced for patients starting clarithromycin within the first 5 days from the onset of COVID-19-related symptoms. The benefit of clarithromycin was also linked with a decrease of CRP and IL-6 at the EOT.
Emerging data suggest that active virus replication and persistence in the upper respiratory tissue are associated with the severity of the illness [15, 16]. Patients treated with clarithromycin showed remarkable reduction of the viral load in the nasopharynx, which was associated with clinical improvement. The effect of clarithromycin could be exerted either directly on viral replication or indirectly through improvement of the viral clearance capacity of the host. Clarithromycin belongs to the macrolide family of antibiotics. Azithromycin, another macrolide antibiotic, was suggested for treatment early during the first months of the COVID-19 pandemic. Azithromycin facilitates the intracellular entrance of zinc ions, which inhibit the activity of the RNA-dependent RNA-polymerase (RdRp) of SARS-CoV-2 [17]. SARS-CoV-2 replicates intracellularly and exploits host ribosomes for synthesis of the proteins of maturing virions. Viral replication is inhibited by azithromycin which binds directly on the human ribosomes [18]. Clarithromycin may also possess this direct antiviral effect. Furthermore, our data show that at the end of clarithromycin treatment, the IFNγ production by PBMCs was increased and the IL-6 production by PBMCs was decreased. This shift of cytokine production may suggest an enhancement of Th1 responses which are responsible for the containment of viral replication [19].
In the randomized COALITION trial from Brazil, patients with moderate-to-severe COVID-19 were randomized to treatment with placebo (n = 227), hydroxychloroquine (n = 221) and a combination of hydroxychloroquine and azithromycin (n = 217). The achievement of the primary endpoint was similar among the three treatment groups [20]. The same group of investigators performed the COALITION II trial where participants were allocated to treatment with SOC with (n = 214) or without azithromycin (n = 183). Although the two groups did not differ in the achievement of the primary endpoint after 15 days, patients allocated to azithromycin treatment had a better overall health state after 7 days of treatment [21]. Two aspects of data analysis may explain the difference in the outcome clarithromycin treatment in the ACHIEVE trial and the outcome of azithromycin treatment in the COALITION I and COALITION II trials: (1) the time delay from symptoms onset to start of therapy was not analysed in the COALITION trials; (2) the viral variants were not taken into consideration in the COALITION trials.
In another trial from Egypt, patients with mild COVID-19 were randomized to 7 days of oral treatment with azithromycin (n = 106), clarithromycin (n = 99) or standard of care (n = 99). Both macrolide antibiotics exhibited equal efficacy (which was superior to of standard of care) on time to achievement of negative viral load and of radiological improvement [22].
The main limitation of the ACHIEVE study is the lack of inclusion of a randomization arm of patients receiving SOC. Instead, one group of comparators was selected through propensity score among available concurrent comparators of the same time period receiving the same SOC in other tertiary hospitals. The study was designed in mid-March 2020 at the beginning of the pandemic in Greece. It was decided to endorse an open-label and single-arm design in an attempt to include (and benefit) as many patients as possible since no SOC was framed at that time period. The azithromycin plus hydroxychloroquine concurrent comparators were optimally matched without differences in baseline severity and co-administered treatment. When the study was started, most of the patients with COVID-19 hospitalized in the Greek hospitals were co-administered azithromycin and hydroxychloroquine as an off-label treatment option. In the following months, emerging data showed that hydroxychloroquine does not affect the natural course of COVID-19 [23, 24]. As a consequence our data showing superior clinical efficacy of clarithromycin over the combination of azithromycin and hydroxychloroquine may point toward the superiority of clarithromycin over azithromycin taking into consideration the reported lack of efficacy of hydroxychloroquine.
Conclusions
The results of the ACHIEVE trial clearly indicate that clarithromycin treatment in patients with moderate COVID-19 is associated with early clinical improvement and containment of viral load. This is associated with an increase of the ratio of IFNγ/IL-6 production from PBMCs. To the best of our knowledge, this is the first study to show the anti-inflammatory impact of clarithromycin on COVID-19. Further studies are needed to better define its future role in mild and moderate COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Hughes Associates, Oxford, UK, for supporting us with manuscript preparation. The authors thank the patients, families, clinical, laboratory and research staff who contributed to the trial.
Funding
This work was supported by Abbott Operation Products AG. Funding body had no role in the design, conduct, analysis and interpretation of data and decision to publish. Hughes Associates, Oxford, UK, assisted publication administration, including settlement of the journal’s Rapid Service Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authorship Contributions
Konstantinos Tsiakos participated in data analysis, drafted the manuscript, had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis. Antonios Tsakiris, Georgios Tsibris, Pantazis-Michael Voutsinas, Periklis Panagopoulos, Maria Kosmidou, Vasileios Petrakis, Areti Gravvani, Eleftherios Klouras, Iro Rapti, Emmanouil Vrentzos, Christina Damoulari, Vagia Zarkada, Chrysanthi Sidiropoulou, Sofia Artemi, Androniki Papapostolou, Evangelos Michelakis, Maria Georgiopoulou, Dimitra-Melia Myrodia, Panteleimon Tsiamalos, Konstantinos Syrigos, George Chrysos, Thomas Nitsotolis, Haralampos Milionis and Garyphallia Poulakou recruited patients in this study, collected data and revised the manuscript for important intellectual content. Theologia Gkavogianni, Konstantina Katrini, Panagiotis Koufargyris, Athanassios Karageorgos and Anastasios Ioannidis performed laboratory experiments and revised the manuscript for important intellectual content. Evangelos J. Giamarellos-Bourboulis conceptualized the study design, participated in data analysis and drafting the manuscript, had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis. All authors gave final approval for the version to be published.
Disclosures
P. Panagopoulos has received honoraria from Gilead Sciences, Janssen and MSD. G. Poulakou has received independent educational grants from Pfizer, MSD, Angelini and Biorad. H. Milionis reports receiving honoraria, consulting fees and non-financial support from healthcare companies, including Amgen, Angelini, Bayer, Mylan, MSD, Pfizer and Servier. E.J. Giamarellos-Bourboulis has received honoraria from Abbott CH, Angelini Italy, InflaRx GmbH, MSD Greece, XBiotech Inc. and B·R·A·H·M·S GmbH (Thermo Fisher Scientific); independent educational grants from AbbVie Inc., Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux Inc., Novartis, InflaRx GmbH, Sobi and XBiotech Inc.; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens) and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). The other authors do not report any conflict of interest.
Compliance with Ethics Guidelines
The ACHIEVE study was approved by the National Ethics Committee of Greece (approval number 45/20) and subsequently by the National Organization for Medicines of Greece (approval number ISO 36/20). Patients were screened for eligibility and enrolment after written informed consent. Concurrent comparators receiving azithromycin plus hydroxychloroquine/chloroquine were hospitalized at the same time period in five other medical departments of tertiary hospitals of Greece. The collection of clinical information and blood samples from comparators was done by Institutional Review Board approvals for the registry of the Hellenic Sepsis Study Group (www.sepsis.gr). Written informed consent was also provided for screening and enrolment of comparators. The first patient was enrolled on 17 May 17 2020 and the last visit of the last patient was on 22 October 2020. The full protocol is provided in the supplement.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Antonios Tsakiris, Georgios Tsibris, Pantazis-Michael Voutsinas have contributed equally to this work.
References
- 1.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and treatment of adults with community-acquired pneumonia. Am J Resp Crit Care Med. 2019;200:45–67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García Vázquez E, Mensa J, Martínez JA, Marcos MA, Puig J, Ortega M, Torres A. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis. 2005;24:190–195. doi: 10.1007/s10096-005-1295-9. [DOI] [PubMed] [Google Scholar]
- 5.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Resp J. 2009;33:153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 8.Nie W, Li B, Xiu Q. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, Zervakis D, Baziaka F, Koronaios A, Antonopoulou A, Markaki V, Koutoukas P, Papadomichelakis E, Tsaganos T, Armaganidis A, Koussoulas V, Kotanidou A, Roussos C, Giamarellou H. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin Infect Dis. 2008;46:1157–1164. doi: 10.1086/529439. [DOI] [PubMed] [Google Scholar]
- 10.Giamarellos-Bourboulis EJ, Mylona V, Antonopoulou A, Tsangaris I, Koutelidakis I, Marioli A, Raftogiannis M, Kopterides P, Lymberopoulou K, Mouktaroudi M, Papageorgiou C, Papaziogas B, Georgopoulou AP, Tsaganos T, Papadomichelakis E, Gogos C, Ladas M, Savva A, Pelekanou A, Baziaka F, Koutoukas P, Kanni T, Spyridaki A, Maniatis N, Pelekanos N, Kotsaki A, Vaki I, Douzinas EE, Koratzanis G, Armaganidis A. Effect of clarithromycin in patients with suspected Gram-negative sepsis: results of a randomized controlled trial. J Antimicrob Chemother. 2014;2014(69):1111–1118. doi: 10.1093/jac/dkt475. [DOI] [PubMed] [Google Scholar]
- 11.Tsaganos T, Raftogiannis M, Pratikaki M, Christodoulou S, Kotanidou A, Papadomichelakis E, Armaganidis A, Routsi C, Giamarellos-Bourboulis EJ. Clarithromycin leads to long-term survival and cost benefit in ventilator-associated pneumonia and sepsis. Antimicrob Agents Chemother. 2016;60:3640–3646. doi: 10.1128/AAC.02974-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriazopoulou E, Sinapidis D, Halvatzis S, Velissaris D, Alexiou N, Kosmas V, Adami ME, Kyprianou M, Kyprianou A, Stefos A, Lada M, Koutoukas P, Pavlaki M, Kyriakoudi A, Makina A, Gogos C, Niederman M, Giamarellos-Bourboulsi EJ. Survival benefit associated with clarithromycin in severe community-acquired pneumonia: a matched comparator study. Int J Antimicrob Agents. 2020;55:105836. doi: 10.1016/j.ijantimicag.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C, du Plessis L, Pybus OG. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO working group on the clinical characterization and management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poupaert JH, Aguida B, Hountondji C. Study of the interaction of zinc cation with azithromycin and its significance for COVID-19 treatment: a molecular approach. Open Biochem J. 2020;14:33–40. doi: 10.2174/1874091X02014010033. [DOI] [Google Scholar]
- 18.Hountondji C, Bansaïnou G, Gaudet E, Popaert JH. Repositioning adequate antibiotics to treat/cure the coronavirus disease 2019 (COVID-19): current treatments and future directions. Open Biochem J. 2021;15:1–19. doi: 10.2174/1874091X02115010001. [DOI] [Google Scholar]
- 19.Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162:30–43. doi: 10.1111/imm.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VV, Avezum A, Veiga VV, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hofmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020;38:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, Zampieri FG, Veiga VC, Azevedo LCP, Rosa RG, Lopes RD, Avezum A, Manoel ALO, Piza EMT, Martins PA, Lisboa TC, Pereira AJ, Olivato GB, Dantas VCS, Milan EP, Gebara OCE, Amazonas RB, Oliveira MB, Sorasr RVP, Moia DDF, Piano LPA, Castilho K, Momesso RGRAP, Schettino GPP, Rizzo LV, Neto AS, Machado FR, Cavalcanti AB. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomized clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rashad A, Nafady A, Hassan MH, Taya U, Bazeed SES, Aref ZF, Sayed MAA, Nafady-Hego H, Abdelmaksoud AA. Therapeutic efficacy of macrolides in management of patients with mild COVID-19. Res Sq. 2021 doi: 10.2103/rs.3.rs-181996/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, Chang SY, Collins SP, Eppensteiner JC, Filbin MR, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashour Z, Riaz M, Garbati MA, AlDosary MA, AlDosary O, Tlayjeh H, Gerberi D, Murad MH, Sohail MR, Kashour T, Tleyjeh IM. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76:30–42. doi: 10.1093/jac/dkaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.