Abstract
Background:
Studies using structural magnetic resonance imaging (MRI) volumetrics showed smaller hippocampal volume in patients with post-traumatic stress disorder (PTSD). These studies were cross-sectional and did not address whether smaller volume is secondary to stress-induced damage, or whether pre-existing factors account for the findings. The purpose of this study was to use a co-twin case control design to assess the relative contribution of genetic and environmental factors to hippocampal volume in PTSD.
Methods:
Monozygotic (N = 13 pairs) and dizygotic (N = 21 pairs) twins with a history of Vietnam Era military service, where one brother went to Vietnam and developed PTSD, while his brother did not go to Vietnam or develop PTSD, underwent MR imaging of the brain. Structural MRI scans were used to manually outline the left and right hippocampus on multiple coronal slices, add the areas and adjust for slice thickness to determine hippocampal volume.
Results:
Twins with Vietnam combat-related PTSD had a mean 11% smaller right hippocampal volume in comparison to their twin brothers without combat exposure or PTSD (p < .05). There was no significant interaction by zygosity, suggesting that this was not a predisposing risk factor or genetic effect.
Conclusions:
These findings are consistent with smaller hippocampal volume in PTSD, and suggest that the effects are primarily due to environmental effects such as the stress of combat.
1. Introduction
Posttraumatic stress disorder (PTSD) affects a large number of persons in the United States and is associated with considerable morbidity and loss of productivity (Hoge et al., 2006; Kessler et al., 1995). PTSD, which can follow exposure to combat or civilian traumatic experiences, is associated with intrusive memories, avoidance, hyperarousal, and negative cognitions. Cognitive deficits, including problems with concentration, new learning, and remembering directions, are also commonly seen clinically (Elzinga and Bremner, 2002; Roca and Freeman, 2001). Studies have linked PTSD to alterations in the hippocampus, a brain area involved in learning and memory that is sensitive to stress (Arbel et al., 1994; Bremner and Vermetten, 2012; Campanella and Bremner, 2016; Diamond et al., 1996; Kunimatsu et al., 2019; Luine et al., 1994; McEwen et al., 1992; Nibuya et al., 1995; Sapolsky, 1996; Sapolsky et al., 1990; Smith et al., 1995; Zoladz and Diamond, 2016). These findings have led to the alternative hypotheses that prolonged stress results in smaller hippocampal volume in PTSD due to cortisol or other factors that is potentially plastic and reversible (Bremner, 2001, 2002; Bremner and Campanella, 2016; Davis et al., 2016), or that smaller hippocampal volume is a vulnerability factor for chronic PTSD (Gilbertson et al., 2002; Pitman, 2001).
Patients with PTSD secondary to combat stress or childhood abuse compared to individuals without PTSD showed deficits in hippocampal-based verbal declarative memory and smaller volume of the hippocampus as measured with magnetic resonance imaging (MRI) volumetrics (Apfel et al., 2011; Averill et al., 2017; Bossini et al., 2008; Bremner et al., 1993, 1995a, 1995b, 1997, 2003, 2004b; Chao et al., 2013, 2014; Emdad et al., 2006; Felmingham et al., 2009; Gilbertson et al., 2002; Golier et al., 1997; Gurvits et al., 1996; Hedges et al., 2003; Lindauer et al., 2004, 2006; Logue et al., 2018; Schuff et al., 1997; Shin et al., 2004; Stein et al., 1997; Tischler et al., 2006; Uddo et al., 1993; Vasterling and Bremner, 2006; Vasterling et al., 2002; Villarreal et al., 2002a; Vythilingam et al., 2005; Wang et al., 2010; Wignall et al., 2004; Yehuda et al., 1995, 2006; Zhang et al., 2011). Other studies showed evidence of loss or alterations in neuronal integrity in PTSD as measured with Magnetic Resonance Spectroscopy studies of hippocampal N-acetyl aspartate (NAA) (Freeman et al., 1998; Guo et al., 2012; Li et al., 2006; Lim et al., 2003; Mahmutyazicioglu et al., 2005; Rosso et al., 2017; Schuff et al., 1997, 2008; Villarreal et al., 2002b; Wang et al., 2010). Multiple independent meta-analyses found smaller hippocampal volume for both the left and the right sides, equally in adult men and women with chronic PTSD, related to either combat or childhood trauma (Bromis et al., 2018; Kitayama et al., 2005; Kühn and Gallinat, 2013; Meng et al., 2016; O’Doherty et al., 2015; Smith, 2005; Woon et al., 2010).
Not all MRI studies, however, showed smaller hippocampal volume in PTSD. Some studies in adults with chronic PTSD did not show smaller hippocampal volume or lower NAA when compared to controls (Golier et al., 2005; Hedges et al., 2007; Jatzko et al., 2006; Landre et al., 2010; Mueller et al., 2015; Nardo et al., 2009; Pederson et al., 2004; Shin et al., 2017; Starcevic et al., 2014; Veer et al., 2015; Yamasue et al., 2003; Yehuda et al., 2007). Some studies found smaller hippocampal volume in PTSD patients compared to trauma exposed individuals without PTSD (Bremner et al., 2003; van Rooij et al., 2015) while others found smaller volume in both trauma-exposed PTSD and non-PTSD relative to non-trauma exposed non-PTSD individuals (Luo et al., 2016; Winter and Irle, 2004). Studies of new onset PTSD found smaller hippocampal volume in some studies (Li et al., 2006; Wignall et al., 2004; Xie et al., 2018; Zhang et al., 2011) but not others (Bonne et al., 2001; Fennema-Notestine et al., 2002). Smaller hippocampal volume in PTSD was correlated with duration (Chao et al., 2014; Felmingham et al., 2009) and severity of PTSD symptoms (Neylan et al., 2010; Xie et al., 2018), resistance to treatment (Rubin et al., 2016; van Rooij et al., 2015) and elevated cortisol (Neylan et al., 2003). Although several studies of adults with PTSD related to childhood abuse found smaller hippocampal volume, studies in children with PTSD related to abuse and neglect have not shown smaller hippocampal volume (Carrion et al., 2001; De Bellis et al., 1999, 2001; Keding and Herringa, 2015; Mehta et al., 2009; Morey et al., 2016; Tupler and De Bellis, 2006). Longitudinal studies in children with PTSD showed elevated cortisol levels (Carrion et al., 2002) that were associated with increased PTSD symptoms and a reduction in hippocampal volume over time (Carrion et al., 2002). Other studies found a delayed effect of early life stress on hippocampal volume that manifests in adulthood (Andersen and Teicher, 2004; Andersen et al., 2008; Teicher, 2002; Teicher et al., 2012). Studies of medications with known effects on the hippocampus (Aribi and Stringer, 2002; Duman, 2004) have shown increased hippocampal volume with successful treatment of PTSD (Bossini et al., 2007; Bremner et al., 2004a; Vermetten et al., 2003). Some studies showed increased hippocampal volume with psychotherapy (Bossini et al., 2011) while others did not (Lindauer et al., 2005; Rubin et al., 2016; van Rooij et al., 2015). Studies in veterans that found a relationship between premorbid low Intelligence Quotient (IQ) and the development of PTSD were interpreted as representing a vulnerability factor for PTSD (Macklin et al., 1998; McNally and Shin, 1995). Individuals with smaller hippocampal volume and deficits in memory have been posited to be at increased risk for the development of PTSD after psychological trauma (Pitman, 2001). It is known that not all individuals exposed to the same level of trauma are at equal risk for the development of PTSD, and more than 10% of the variance in PTSD is estimated to be genetic, based on prior twin studies of PTSD (Goldberg et al., 1990). A study in monozygotic twins where both twins were veterans in the Vietnam Era found a correlation between smaller hippocampal volume in a non combat-exposed twin and increased PTSD symptoms in his identical brother with combat-related PTSD (Gilbertson et al., 2002). This study, however, did not examine both monozygotic and dizygotic twin pairs discordant for combat exposure and PTSD, which is helpful to examine the relative importance of environmental and genetic contributions.
The use of the co-twin study design as in the current study with both monozygotic (identical) and dizygotic (fraternal) twins in which one twin went to Vietnam and developed PTSD and his twin brother was in military service but did not go to Vietnam and did not develop PTSD, permits the assessment of the relative environmental (e.g., combat stress) and genetic contributions to outcomes of interest such as hippocampal volume in PTSD (Eisen et al., 1989; Goldberg et al., 1987; Henderson et al., 1990; True et al., 1993). The co-twin design also has the advantage of controlling a number of other unmeasured potentially confounding factors such as differences in genetic predisposition, family environment and socio-economic status. The purpose of this study was to compare hippocampal volume in monozygotic and dizygotic twins with a history of Vietnam combat-related PTSD in comparison to their brothers with a history of military service but without a history of Vietnam combat exposure or PTSD. We hypothesized that twins with PTSD would have smaller hippocampal volume than their non-PTSD twin brothers, and these effects would not be due to familial or genetic confounding factors.
2. Methods
2.1. Participants
Participants in the study included 34 twin pairs (13 monozygotic (MZ) and 21 dizygotic (DZ) twins) recruited from the Vietnam Era Twin Registry where both brothers served in the United States military during the Vietnam era (Forsberg et al., 2010; Tsai et al., 2012) (Goldberg et al., 1987, 1990; Henderson et al., 1990; True et al., 1993) who agreed to participate and completed the protocol. This study was approved by the Emory University Investigational Review Board and all participants provided written, informed consent.
Participants were identified by initial screening as discordant for combat exposure based on the Combat Exposure Scale, a validated measure of the severity and quantity of combat-related trauma experienced in Vietnam (Keane et al., 1989). Twins were also screened as being discordant for PTSD based on the Mississippi Scale for Combat-Related PTSD, a validated measure of combat-related PTSD symptom severity (Keane et al., 1988), where one twin had a Mississippi Scale score of greater than 95 and his twin brother had a score of less than 70. Twin pairs were excluded where one of the twins had a history of current alcohol or substance abuse or dependence, neurological disorder, major medical illness, or indwelling metal that would preclude scanning. Ninety eight twin pairs, including monozygotic (MZ) and dizygotic (DZ) twins, were identified from the Registry database who met these initial criteria (Fig. 1). 42 twin pairs agreed to participate, traveled to Atlanta for participation, and signed a written informed consent. Twins were re-screened in Atlanta with the Structured Clinical Interview for DSMIV (First et al., 1995). Twin pairs where one of the brothers had a history of schizophrenia, traumatic brain injury or loss of consciousness of greater than 1 min were excluded. Nine pairs did not complete scanning because they were excluded due to a history of schizophrenia in one of the twins, a current major psychiatric disorder in the non-exposed twin, the exposed twin did not have a history of PTSD, claustrophobia that precluded scanning, or technical problems with scan acquisition. Thirty-four twin pairs (13 MZ and 21 DZ) met final diagnostic criteria and completed the imaging protocol.
Fig. 1.
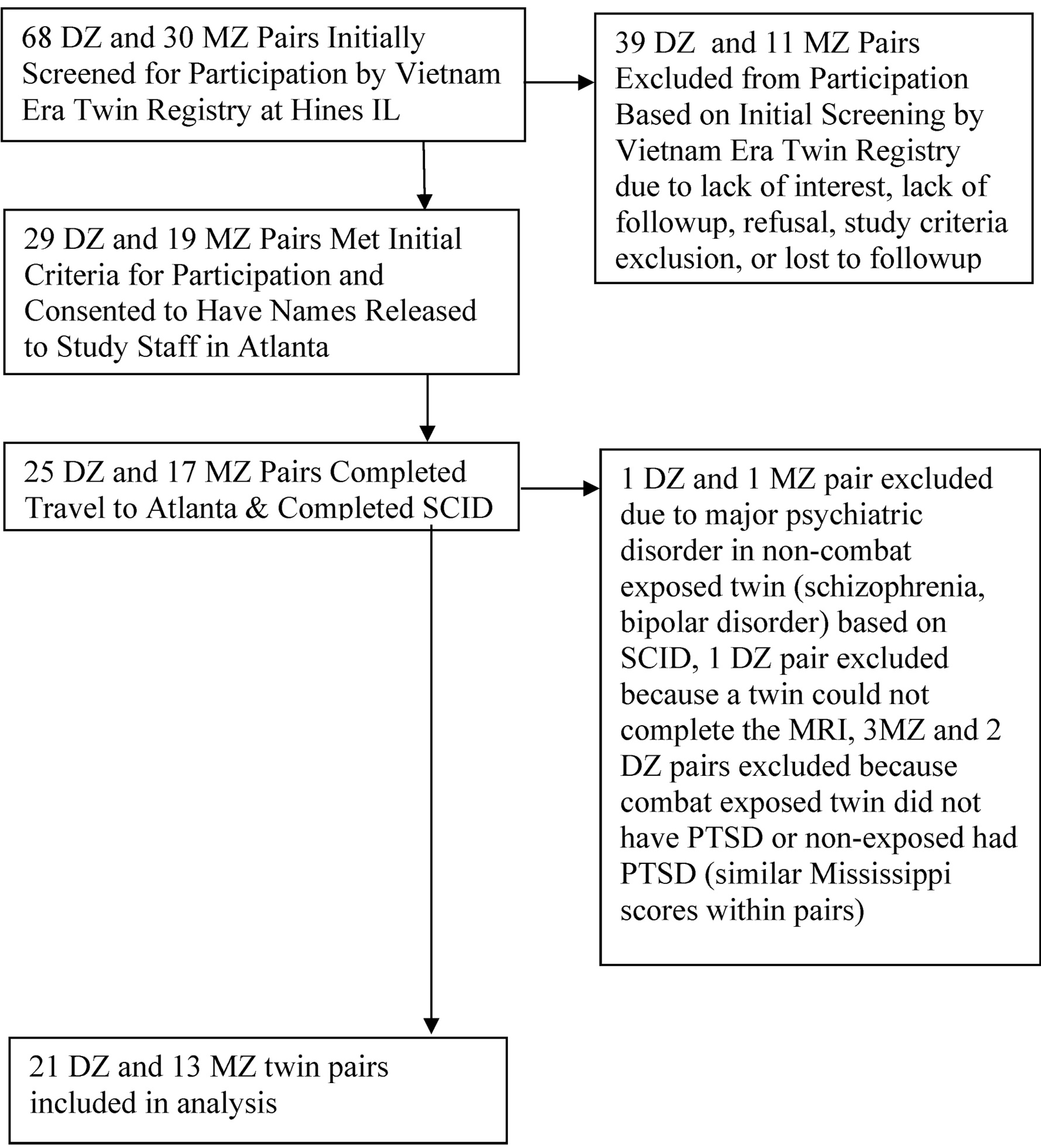
Subject flow diagram. 68 DZ and 30 MZ pairs initially screened for participation by vietnam era twin registry at hines IL
2.2. Psychometric assessments
The Structured Clinical Interview for the Diagnostic and Statistical Manual (DSM) IV (First et al., 1995) (with PTSD module) was used to establish psychiatric diagnosis. The PTSD module of the SCID has a kappa of .93 for test-retest reliability, and excellent sensitivity (0.81) and specificity (0.98) against a composite PTSD diagnosis. Alcohol and substance abuse was evaluated with the Addiction Severity Index (ASI). The ASI has an inter-rater reliability of 0.89 and a high degree of test-retest reliability and concurrent and discriminant validity (McClellan et al., 1985). Level of combat exposure in Vietnam was assessed with the Combat Exposure Scale (Keane et al., 1989). This reliable and valid measure was used to document history of combat exposure in the PTSD patients. Current PTSD symptom severity was assessed with the Mississippi Scale for PTSD (Keane et al., 1988; Vreven et al., 1995). The Clinician Administered PTSD Scale (CAPS) is a clinician administered measure of PTSD symptomatology that provides continuous measures of symptom severity and frequency (Blake et al., 1995; Weathers et al., 1999). The CAPS has a coefficient alpha of .94, test-retest reliability of 0.9–0.98, a sensitivity of 0.91 and specificity of .86 for diagnosis of PTSD, and good convergent and divergent validity. The CAPS was used as a measure of PTSD symptom severity based on DSM criteria and complements the Mississippi also is a measure of PTSD severity but which was developed prior to the DSM. The Clinician Administered Dissociative States Scale (CADSS) is a 28-item measure of current “state” level of dissociative symptomatology; the CADSS shows excellent inter-rater reliability (0.92), has a coefficient alpha of .94, and shows excellent test-retest and convergent validity (Bremner et al., 1998). The Early Trauma Inventory (ETI) is an instrument for measurement of childhood traumatic experiences; inter-rater reliability (0.99) and test-retest reliability (0.91) are excellent, coefficient alpha is .95, and the ETI has excellent convergent validity. The self-report version of the ETI was used (Bremner et al., 2000).
2.3. Structural magnetic resonance imaging and volumetric assessments
Magnetic resonance imaging (MRI) and volumetric assessment of the whole hippocampus and whole brain was performed utilizing measures previously described in detail (Bremner et al., 1995a, 1997, 2003). Magnetic resonance (MR) images were obtained with a 1.5 T device. Coronal images were acquired with a T1 weighted gradient echo 3D sequence with, TR = 35 msec, TE = 12 msec, flip angle 35, NEX = 2, 1.2 mm3 voxel with no gap and 256 × 256 matrix and FOV 240 mm or 1.5 mm thick contiguous slice with matrix 512 × 512 and FOV 220. Images were then transferred via internet to a SunSparc Workstation for image processing, with archiving on optic disk media. MRI scans were resliced to correct for head rotation and to create slices perpendicular to the long axis of the hippocampus using the ANALYZE program. First, two mid-hippocampal points separated by 15 mm were selected to construct a line that defines the long axis of the hippocampus. A third mid-hippocampal point in the opposite hippocampus was then selected to define a plane parallel to the long axes of both hippocampi. A series of oblique images were then constructed 90° perpendicular to this plane and parallel to the first two hippocampal points to create images orthogonal to the long axis of the hippocampus. The outline of the hippocampus was then traced using a mouse-driven cursor using the method of Watson et al. (1992) and methods described by us previously in detail (Vermetten et al., 2003; Vythilingam et al., 2002). The anterior border of the hippocampus was defined by the amygdala. The uncal recess of the temporal horn of the lateral ventricle was determined to be the most reliable way to separate the hippocampal head from the amygdala. However in cases where the uncal recess was not clearly visible, traces were made along the alveus that connects the inferior horn of the lateral ventricle to the sulcus at the inferior margin of the semilunar gyrus (i.e. the semianular or amygdaloid sulcus). If neither the uncal recess nor the alveus were clearly visible, a horizontal line was drawn connecting the plane of the inferior horn of the lateral ventricle with the surface of the uncus. The posterior most slice of the hippocampus was defined as the slice of the hippocampus where the pulvinar interrupts the fornix superiorly. The superior border of the hippocampus includes gray matter visible within the hippocampus as well as the alveus and fimbriae. The inferior border of the hippocampus includes the subiculum. A straight line from the inferior subcortical white matter extending medially was used to disconnect the parahippocampal gyrus from the subiculum. Cross sectional areas were measured in each of these slices, summed, and multiplied by the slice thickness to obtain the volume of these structures. Initially measurements were performed in a sub-sample by two raters for determination of reliability of the measurements. Intraclass correlation coefficients (R) for inter-rater reliability between two raters were as follows: left hippocampus (R = 0.93; df = 1,31; p < .05) and right hippocampus (R = 0.94; df = 1,31; p < .05). Then measurements were repeated in the entire sample by a single rater as this provides greater uniformity of the final measurements. Final volumetric measurements of the whole hippocampus were performed independently by a single rater blinded to subject diagnosis. Measurements of the left and right whole brain were performed on non resliced images and included gray and white matter as well as ventricles and were performed by a single blinded rater.
2.4. Statistical analysis
All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). Significance level was set at 0.05, two-sided.
All P values were corrected for the correlation between co-twins using generalized estimating equations or mixed-effects models with a random intercept for pair, depending on variable distribution. Initially, hippocampal volume data was displayed in bins and chi-square statistics were used to determine if the data were normally distributed. Hippocampal volumes were then compared within brothers in PTSD-discordant twin pairs. This was a matched co-twin analysis which controlled by design for all factors shared by the twins. Twins share maternal factors, genes (50% if dizygotic and 100% if monozygotic), and childhood and adolescent environment if they are raised together. The within-pair comparison minimizes confounding by these factors.
The within-pair analyses were further stratified by zygosity so we could examine the potential influence of genetic factors on the association between PTSD and hippocampal volume. Since monozygotic twins share 100% of their segregating genes, the analysis of within-pair differences in monozygotic twins is fully controlled for genetic factors shared between PTSD and hippocampal volume. Dizygotic twins only share on average 50% of genes and differences among these twins are only partially controlled for shared genetic factors. In general, shared genetic factors would be indicated if the within-pair difference in PTSD discordant pairs is smaller in monozygotic than in dizygotic pairs.
3. Results
Mean score on the Combat Exposure Scale in the combat-exposed twin was 17 (6 SD). PTSD patients had higher levels of PTSD symptoms as measured with the CAPS and the Mississippi Scale and dissociative symptoms as measured with the CADSS than twins without PTSD (Table 1). They had similar years of education and years of past alcohol and substance abuse (Table 1). Co-morbid psychiatric disorders based on the SCID are presented in Table 1.
Table 1.
Demographic factors in twins with (N = 34) and without (N = 34) PTSD.
| Non-PTSD (N = 34) | PTSD (N = 34) | |
|---|---|---|
| Age | 51.9 (1.6) | 51.9 (1.6) |
| Years of Education | 14.4 (2.6) | 13.8 (2.3) |
| CAPS (PTSD) | 7.6 (10.4) | 41.6 (32.2)*** |
| CADSS (Dissociation) | 1.8 (3.9) | 10.5 (13.2) *** |
| Mississippi (PTSD) | 72.5 (17.6) | 100.2 (29.1) *** |
| Early Trauma Inventory | 22.3 (15.2) | 23.4 (18.1) |
| Major Depression^ | 10 (29.4%) C, 17 (50.0%) L | 0 (0%) C, 1 (2.9%) L |
| Dysthymia^ | 2 (5.8%) C/L | 0 (0%) C/L |
| Panic Disorder without Agoraphobia^ | 3 (8.8%) C, 6 (17.6%) L | 0 (0%) C/L |
| Panic Disorder with Agoraphobia^ | 3 (8.8%) C/L | 0 (0%) C/L |
| Agoraphobia without Panic Disorder^ | 1 (2.9%) C/L | 0 (0%) C/L |
| Social Phobia^ | 3 (8.8%) C, 4 (11.8%) L | 0 (0%) C, 1 (2.9%) L |
| Simple Phobia^ | 4 (11.8%) C/L | 0 (0%) C, 3 (8.8%) L |
| Obsessive Compulsive Disorder^ | 1 (2.9%) C, 2 (5.8%) L | 0 (0%) C/L |
| Generalized Anxiety Disorder^ | 3 (8.8%) C/L | 0 (0%) C/L |
| Alcohol Abuse^ | 0 (0%) C, 7 (20.6%) L | 0 (0%) C, 6 (17.6%) L |
| Alcohol Dependence^ | 0 (0%) C, 13 (38.2%) L | 0 (0%) C, 3 (8.8%) L |
| Years of Alcohol Abuse | 5.1 (7.5) | 4.6 (4.6) |
| Substance Abuse^ | 0 (0%) C, 9 (26.5%) L | 0 (0%) C, 5 (14.7%) L |
| Substance Dependence^ | 0 (0%) C, 3 (8.8%) L | 0 (0%) C/L |
| Years of Substance Abuse | 1.5 (3.3) | 3.8 (7.4) |
Data for hippocampal volume were normally distributed. Twins with Vietnam combat-related PTSD had a 11% smaller right hippocampal volume in comparison to their twin brothers without combat exposure or PTSD (measured as the mean of the percent differences between pairs) (Table 2, Fig. 2) (p = .002). Right hippocampal volume was 12% smaller in DZ PTSD twins (N = 21 pairs) compared to their unaffected DZ twin brothers and 8% smaller in MZ PTSD twins (N = 13 pairs) compared to their unaffected MZ brothers with no significant interaction by zygosity (no difference between MZ and DZ twin pairs). Left hippocampal volume was 2% smaller in the DZ PTSD twins compared to their non-PTSD brothers and 4% smaller in the MZ PTSD twins compared to their non-PTSD brothers (Table 2). These differences were not statistically significant. There was no difference between twins with and without PTSD in left (521 cm3 (62 SD) v. 538 (50 SD); p = .39) or right (535 cm3 (54 SD) v 542 cm3 (49 SD); p = .68) whole brain volumes.
Table 2.
Hippocampal volumes in monozygotic and dizygotic twins discordant for PTSD.
| All Twins (N = 34 pairs) | Monozygotic (N = 13 pairs) | Dizygotic (N = 21 pairs) | ||||||||||
| PTSD | Non-PTSD | % diff | P value | PTSD | Non-PTSD | % diff | P value | PTSD | Non-PTSD | % diff | P value | |
| Left Hippocampus | 2114 (364) | 2180 (394 | −3% | .188 | 2259 (334) | 2357 (356) | −4% | .288 | 2024 (359) | 2071 (383) | −2% | .444 |
| Interaction by zygosity p=.61 | ||||||||||||
| PTSD | Non-PTSD | % diff | P value | PTSD | Non-PTSD | % diff | P value | PTSD | Non-PTSD | % diff | P value | |
| Right Hippocampus | 2049 (335) | 2229 (380) | −11% | .002 | 2115 (179) | 2287 (367) | −8% | .069 | 2009 (402) | 2193 (393) | −12% | .013 |
| Interaction by zygosity p=.91 | ||||||||||||
Data expressed as Mean volume in mm3 and (Standard Deviation) or (percentage).
Fig. 2.
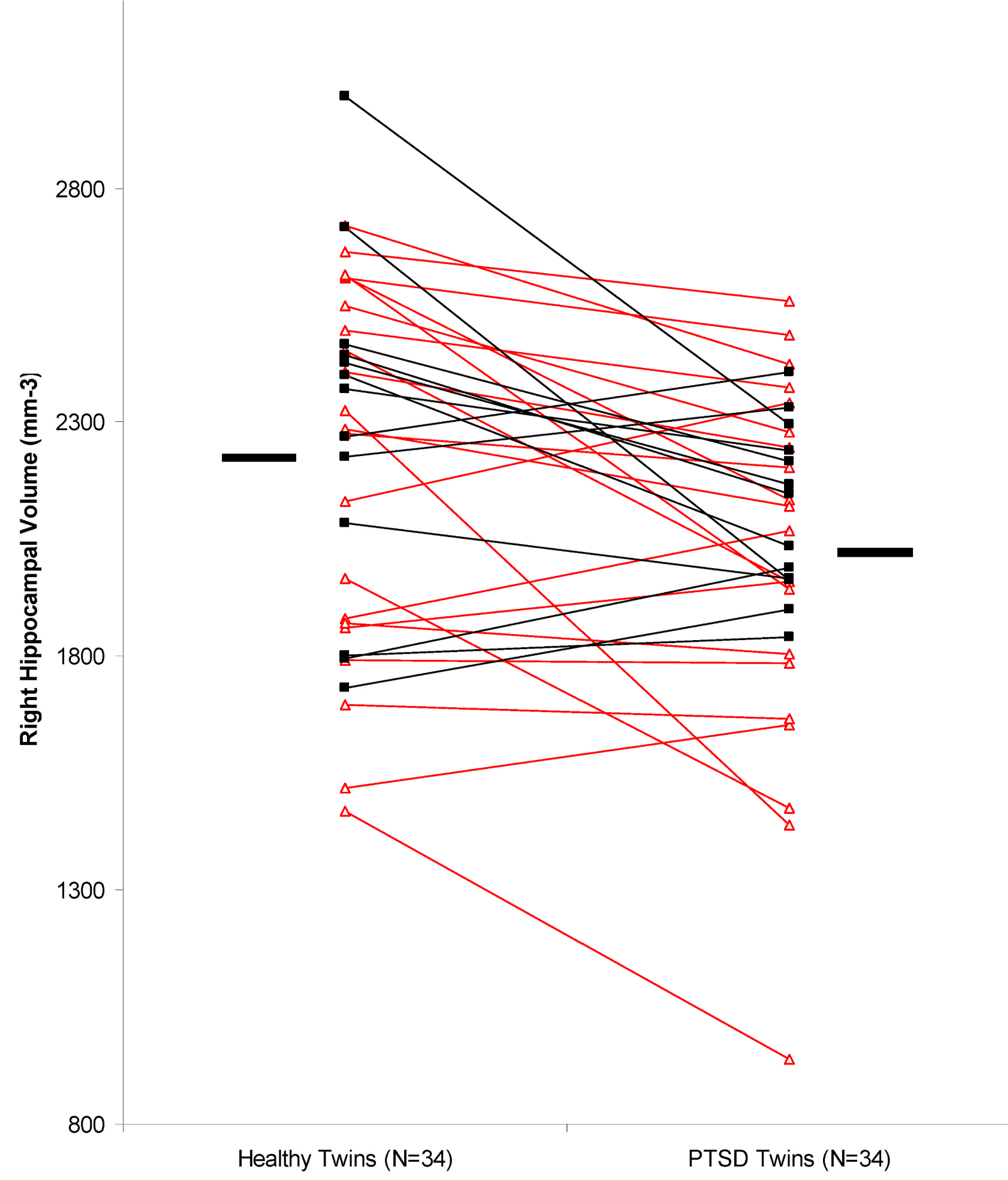
Right hippocampal volume in monozygotic (N = 13 pairs; closed squares) and dizygotic (N = 21 pairs; open triangles) discordant for combat exposure and PTSD. There was a mean 11% smaller volume in the PTSD affected twin compared to each non-PTSD twin brother (2049 mm3 (335 SD) v 2229 mm3 (380 SD) (p = .002).
There were no significant correlations between hippocampal volume and age, education, past alcohol abuse, PTSD symptoms as measured with the CAPS or dissociative symptoms as measured with the CADSS.
4. Discussion
The current study showed 11% smaller right hippocampal volume in Vietnam combat veterans with PTSD in comparison to their twin brothers with a history of military service, but without a history of combat exposure or PTSD. Monozygotic twins share identical genetic material, therefore any differences between them must be due to an environmental effect. Dizygotic twins share 50% of their genetic material, therefore a larger association in dizygotic than monozygotic twins could signify a shared genetic effect between PTSD and hippocampal volume. In the current study there was a smaller hippocampal volume in both monozyptic and dizygotic twins with PTSD in comparison to their brothers, with no significant difference by zygosity. Therefore these findings do not support a genetic effect as an explanation for smaller hippocampal volume in PTSD or the idea that smaller hippocampal volume is a predisposing factor for the development of PTSD.
A significant environmental contribution to smaller hippocampal volume in PTSD is not consistent with previous hypotheses from other authors that smaller hippocampal volume represents a risk factor for PTSD (Gilbertson et al., 2002). This hypothesis was based on studies showing that more than 10% of the variance in PTSD symptomatology was genetic (Banerjee et al., 2017; Binder et al., 2008; Goldberg et al., 1990; Lee et al., 2005; Stein et al., 2002; Xian et al., 2000) and that lower IQ before trauma exposure predicted risk for PTSD (McNally and Shin, 1995). Other studies found smaller hippocampal volume in twins whose brothers developed PTSD secondary to combat exposure (Gilbertson et al., 2002). Genetic contributions to smaller hippocampal volume in PTSD were argued to be mediated either by smaller hippocampal volume at birth (Pitman, 2001), and/or increased vulnerability for stress exposure or increased responsiveness to stressors (Kendler et al., 2000). A prior twin study compared hippocampal volume between veterans with Vietnam combat-related PTSD and their non-exposed non-PTSD monozygotic twin brothers with a history of military service in the Vietnam Era. These twin pairs were compared to a group of monozygotic twin pairs with a history of Vietnam Era military service where neither twin had PTSD but one brother had a history of Vietnam combat exposure. Hippocampal volume was smaller in PTSD patients as a whole compared to non-PTSD. Hippocampal volume in the brothers of PTSD patients was correlated with level of PTSD symptom severity in the PTSD patients, and was smaller than in the brothers of combat-exposed veterans who did not develop PTSD. The authors concluded that smaller hippocampal volume in PTSD was genetically determined (Gilbertson et al., 2002). However, this study did not include both monozygotic and dizygotic twin pairs, which permit a determination of the relative contribution of genetic and environmental factors. Therefore, this study did not exclude the possible contribution of environmental factors to smaller hippocampal volume in PTSD. The results of our current showing no significant interaction by zygosity are in contradiction to these prior conclusions, and in fact argue for a largely environmental contribution to smaller hippocampal volume in PTSD, although given the small sample size a genetic contribution cannot be excluded. Given the fact that twins in this study have similar familial and environmental exposures, including similar levels of alcohol exposure and childhood trauma, apart from exposure to Vietnam combat, the most salient explanation for our findings is that exposure to the stress of combat results in smaller hippocampal volume in individuals who later develop PTSD. The hypothesis that stress results in smaller hippocampal volume in PTSD is in fact supported by a large literature in both animals and humans (Czeh et al., 2001; Duman et al., 2001; Sapolsky, 2001; van der Beek et al., 2004).
The use of the co-twin study design minimizes a number of the potentially confounding factors that have affected prior studies of hippocampal volume in PTSD. Twins share the same intra-uterine and early family environment, as well as socioeconomic status in childhood, reducing differences between twins in factors other than the factor of interest, which in this case is the stress of combat exposure. The twin pairs in this study had identical age and race, and similar years of education. We did not find a correlation between age and hippocampal volume in the current study, which is to be expected as the twins were in a restricted age range due to their military service during the period of the Vietnam War. Although prior studies have found reductions in gray matter with age (Jernigan et al., 1991), the most significant results for hippocampal volume were found in more elderly persons than the current sample (Lupien et al., 1998). As noted above, duration of PTSD symptoms has been correlated with hippocampal volume. In the current study we did not collect detailed information on symptom duration, although all twins had PTSD related to their combat experiences, so duration would have been limited by the range of years during which the Vietnam war took place. Twin pairs in the current study had similar levels of exposure to trauma in childhood as measured by the ETI. In the current study, twin pairs had similar levels of alcohol exposure as measured by the ASI, unlike prior studies in which PTSD patients had higher levels of alcohol exposure. These findings suggest that smaller hippocampal volume is related to PTSD and not the confounding factors that have been associated with PTSD in prior studies.
This study has several limitations. The sample was restricted to veterans from the Vietnam Era and PTSD was related to combat, and therefore the results may not be generalizable to civilians with non combat-related PTSD. The current study employed manual based tracings of hippocampal volume, which have been widely used in the literature and have demonstrated differences between PTSD patients and controls in numerous studies. Although automated methods are available, we are not aware of any evidence for superior reliability or accuracy for hippocampal measurements. Findings of smaller hippocampal volume could possibly be accounted for by factors related to military service other than combat stress. Up to 20% of veterans have a history of traumatic brain injury (TBI) (Chapman and Diaz-Arrastia, 2014), and TBI has been associated with smaller hippocampal volume (Bigler et al., 1997; Jorge et al., 2007; Monti et al., 2013; Tate and Bigler, 2000). Prior studies of TBI, however, mostly included patients with moderate or greater TBI, who were excluded in the current study, as were patients with loss of consciousness of greater than 1 min. As noted above the sample size was limited, confined by the total number of twins discordant for Vietnam combat and PTSD currently alive in the United States, which limits conclusions about the results. Based on this we cannot conclude that there is not a genetic contribution to smaller hippocampal volume in PTSD.
This study is consistent with smaller hippocampal volume in PTSD, and suggests that it is largely due to environmental exposures, such as combat, rather than genetic factors. Based on our data, the effect of genetic factors in explaining the association between PTSD and hippocampal volume is likely to be small, although a partial effect cannot be excluded. Our results are not consistent with the idea that the association between PTSD and hippocampal volume is exclusively due to a genetic effect, as previously postulated. Twin brothers share familial and environmental backgrounds, however in this case the twin brothers differed in terms of Vietnam combat exposure, which is a likely determinant of their PTSD. A finding of a significant environmental contribution to smaller hippocampal volume in PTSD would be consistent with an extended literature in animals showing both genetic and environmental contributions to hippocampal structure. Future studies are indicated to replicate this finding.
Data expressed as Mean and (Standard Deviation) or (percentage); C = current; CADSS=Clinician Administered Dissociative States Scale; ^ = Data expressed as number of individuals with the diagnosis and the percentage within that group in parentheses; CAPS=Clinician Administered PTSD Scale; PTSD=Posttraumatic Stress Disorder; L = lifetime; ***p < .001.
Acknowledgements:
The authors would like to thank Mary Ellen Vitek, Birute Curran, the Hines IL and VA Puget Sound Healthcare System and Vietnam Era Twin Registry, for assistance in performing these studies. This study was supported by a VA Merit Review Grant, a VA Career Development Award to Dr. Bremner, and R01MH56120 to Dr Bremner.
Declaration of competing interest
This research was supported by a Veterans Administration (VA) Merit Review Grant, a VA Research Career Development Award, K24 MH076955 and R01MH56120 to Dr. Bremner. None of the authors have any other financial or other conflict to disclose, including direct or indirect financial or personal relationships, interests, and affiliations relevant to the subject matter of the manuscript, or that are expected in the foreseeable future, including, but is not limited to, grants or funding, employment, affiliations, patents (in preparation, filed, or granted), inventions, honoraria, consultancies, royalties, stock options/ownership, or expert testimony.
Role of the funding source
The funding source NIHhad no role in the drafting of the manuscript or interpretation of the results. This study was supported by a VA Merit Review Grant, and NIMH R01MH56120.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2020.10.042.
References
- Andersen SL, Teicher MH, 2004. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29 (11), 1988–1993. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH, 2008. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 20 (3), 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC, 2011. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol. Psychiatr. 69 (6), 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel I, Kadar T, Silberman M, Levy A, 1994. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-aged rats. Brain Res. 657, 227–235. [DOI] [PubMed] [Google Scholar]
- Aribi AM, Stringer JL, 2002. Effects of antiepileptic drugs on extracellular pH regulation in the hippocampal CA1 region in vivo. Epilepsy Res. 49, 143–151. [DOI] [PubMed] [Google Scholar]
- Averill CL, Satodiya RM, Scott JC, Wrocklage KM, Schweinsburg B, Averill LA, Akiki TJ, Amoroso T, Southwick SM, Krystal JH, Abdallah CG, 2017. Posttraumatic stress disorder and depression symptom severities are differentially associated with hippocampal subfield volume loss in combat veterans. Chronic Stress 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SB, Morrison FG, Ressler KJ, 2017. Genetic approaches for the study of PTSD: advances and challenges. Neurosci. Lett. 649, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B, 1997. Hippocampal volume in normal aging and traumatic brain injury. Am. J. Neuroradiol. 18, 11–23. [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 299, 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a clinician-administered PTSD scale. J. Trauma Stress 8 (1), 75–90. [DOI] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY, 2001. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am. J. Psychiatr. 158, 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Calossi S, Lombardelli A, Polizzotto NR, Galli R, Vatti G, Pieraccini F, Castrogiovanni P, 2008. Magnetic resonance imaging volumes of the hippocampus in drug-naive patients with post-traumatic stress disorder without comorbidity conditions. J. Psychiatr. Res. 42 (9), 752–762. [DOI] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Calossi S, Polizzotto NR, Vatti G, Marino D, Castrogiovanni P, 2011. EMDR treatment for posttraumatic stress disorder, with focus on hippocampal volumes: a pilot study. J. Neuropsychiatry Clin. Neurosci. 23 (2), E1–E2. [DOI] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Lombardelli A, Calossi S, Polizzotto NR, Galli R, Vatti G, Pieraccini F, Castrogiovanni P, 2007. Changes in hippocampal volume in patients with post-traumatic stress disorder after sertraline treatment. J. Clin. Psychopharmacol. 27 (2), 233–235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2001. Hypotheses and controversies related to the effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder (PTSD). Hippocampus 11, 75–81. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2002. Does Stress Damage the Brain? Understanding Trauma-Related Disorders from a Mind-Body Perspective. W.W. Norton, New York. [Google Scholar]
- Bremner JD, Campanella C, 2016. Effects of psychotherapy for psychological trauma on PTSD symptoms and the brain. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: from Neurobiology to Treatment. Wiley-Blackwell, Hoboken, N.J., pp. 413–420 [Google Scholar]
- Bremner JD, Krystal JH, Putnam F, Marmar C, Southwick SM, Lubin H, Charney DS, 1998. Measurement of dissociative states with the clinician administered dissociative states scale (CADSS). J. Trauma Stress 11, 125–136. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Mletzko T, Welter S, Siddiq S, Reed L, Williams C, Heim CM, Nemeroff CB, 2004a. Treatment of posttraumatic stress disorder with phenytoin: an open label pilot study. J. Clin. Psychiatr. 65 (11), 1559–1564. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB, 1995a. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatr. 152 (7), 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Capelli S, Scott TM, McCarthy G, Charney DS, 1995b. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatr. Res. 59, 97–107. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS, 1997. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol. Psychiatr. 41, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS, 1993. Deficits in short-term memory in post-traumatic stress disorder. Am. J. Psychiatr. 150, 1015–1019. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, 2012. The hippocampus and post-traumatic stress disorders. In: Bartsch T (Ed.), The Clinical Neurobiology of the Hippocampus: an Integrative View. Oxford University Press, pp. 262–272. [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM, 2000. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety 12, 1–12. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Nafzal N, Vythilingam M, 2004b. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD). J. Nerv. Ment. Dis. 192 (10), 643–649. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg P, Ng CK, Staib LH, Duncan JS, Charney DS, 2003. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD). Am. J. Psychiatr. 160 (5), 924–932. [DOI] [PubMed] [Google Scholar]
- Bromis K, Calem M, Reinders AATS, Williams CCR, Kempton MJ, 2018. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am. J. Psychiatr. 175 (10), 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella C, Bremner JD, 2016. Neuroimaging of PTSD. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: from Neurobiology to Treatment. Wiley-Blackwell, Hoboken, New Jersey, pp. 291–320. [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL, 2001. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol. Psychiatr. 50, 943–951. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL, 2002. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol. Psychiatr. 5 (7), 575–582. [DOI] [PubMed] [Google Scholar]
- Chao L, Weiner M, Neylan T, 2013. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res. Neuroimaging. 213 (3), 193–201. [DOI] [PubMed] [Google Scholar]
- Chao LL, Yaffe K, Samuelson K, Neylan TC, 2014. Hippocampal volume is inversely related to PTSD duration. Psychiatry Res. Neuroimaging. 222 (3), 119–123. [DOI] [PubMed] [Google Scholar]
- Chapman JC, Diaz-Arrastia R, 2014. Military traumatic brain injury: a review. Alzheimers Dement 10 (3 Suppl. l), S97–S104. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E, 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. U.S.A. 98 (22), 12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Hamner M, Bremner JD, 2016. Pharmacotherapy for PTSD: effects on PTSD symptoms and the brain. Posttraumatic Stress Disorder: from Neurobiology to Treatment. Wiley-Blackwell, Hoboken, NJ, pp. 389–412. [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G, 2001. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatr. 50, 305–309. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND, 1999. A.E. Bennett research award: developmental traumatology: Part II. Brain development. Biol. Psychiatr. 45, 1271–1284. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM, 1996. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav. Neurosci. 110 (4), 661–672. [DOI] [PubMed] [Google Scholar]
- Duman RS, 2004. Depression: a case of neuronal life and death? Biol. Psychiatr. 56, 140–145. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg JE, Nakagawa S, 2001. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Therapeut. 299, 401–407. [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W, 1989. Determining zygosity in the Vietnam era twin registry: an approach using questionnaires. Clin. Genet. 35, 423–432. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD, 2002. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 70 (1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad R, Bonekamp D, Sondergaard HP, Bjorklund T, Agartz I, Ingvar M, Theorell T, 2006. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother. Psychosom. 75 (2), 122–132. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA, 2009. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport 20 (16), 1402–1406. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL, 2002. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol. Psychiatr. 52 (11), 1089–1101. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M, 1995. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P). American Psychiatric Press, Washington, D.C. [Google Scholar]
- Forsberg CW, Goldberg J, Sporleder J, Smith NL, 2010. Determining zygosity in the Vietnam era twin registry: an update. Twin Res. Hum. Genet. 13 (5), 461–464. [DOI] [PubMed] [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, Komoroski RA, 1998. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn. Reson. Med. 40, 66–71. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK, 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 5 (11), 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, True W, Eisen SA, Henderson W, Robinette CD, 1987. The Vietnam era twin (VET) registry: ascertainment bias. Acta Genetica Med. Gemellol. 36, 67–78. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True WR, Eisen SA, Henderson WG, 1990. A twin study of the effects of the Vietnam war on posttraumatic stress disorder. J. Am. Med. Assoc. 263, 1227–1232. [PubMed] [Google Scholar]
- Golier J, Yehuda R, Cornblatt B, Harvey P, Gerber D, Levengood R, 1997. Sustained attention in combat-related posttraumatic stress disorder. Integr. Physiol. Behav. Sci. 32 (1), 52–61. [DOI] [PubMed] [Google Scholar]
- Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, de Leon MJ, 2005. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatr. Res. 139 (1), 53–64. [DOI] [PubMed] [Google Scholar]
- Guo M, Chen F, Guo J-C, Lu C-Z, Jiang X-L, Liu T, Li M, Song W, 2012. Study of the hippocampus and the anterior cingulate gyrus by proton MR spectroscopy in patients with post-traumatic stress disorder. Asian Pac. J. Trop. Med. 5 (2), 162–164. [DOI] [PubMed] [Google Scholar]
- Gurvits TG, Shenton MR, Hokama H, Ohta H, Lasko NB, Gilbertson MB, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK, 1996. Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol. Psychiatr. 40 (11), 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Allen S, Tate DF, Thatcher GW, Miller MJ, Rice SA, Cleavinger HB, Sood S, Bigler ED, 2003. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cognit. Behav. Neurol. 16 (4), 219–224. [DOI] [PubMed] [Google Scholar]
- Hedges DW, Thatcher GW, Bennett PJ, Sood S, Paulson D, Creem-Regehr S, Brown BL, Allen S, Johnson J, Froelich B, Bigler ED, 2007. Brain integrity and cerebral atrophy in Vietnam combat veterans with and without posttraumatic stress disorder. Neurocase 13 (5), 402–410. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME, 1990. The Vietnam era twin registry: a resource for medical research. Publ. Health Rep. 105, 368–373. [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Auchterlonie JL, Milliken CS, 2006. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. J. Am. Med. Assoc. 295 (9), 1023–1032. [DOI] [PubMed] [Google Scholar]
- Jatzko A, Rothenhofer S, Schmitt A, Gaser C, Demirakca T, Weber-Fahr W, Wessa M, Magnotta V, Braus DF, 2006. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J. Affect. Disord. 94 (1–3), 121–126. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR, 1991. Cerebral structure on MRI, Part I: localization of age-related changes. Biol. Psychiatr. 28, 55–67. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Starkstein SE, Magnotta V, 2007. Hippocampal volume and mood disorders after traumatic brain injury. Biol. Psychiatr. 62 (4), 332–338. [DOI] [PubMed] [Google Scholar]
- Keane TM, Caddell JM, Taylor KL, 1988. Mississippi scale for combat-related post-traumatic stress disorder: three studies in reliability and validity. J. Consult. Clin. Psychol. 56, 85–90. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, 1989. Clinical evaluation of a scale to measure combat exposure. Psychol. Assess. 56, 85–90. [Google Scholar]
- Keding TJ, Herringa RJ, 2015. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40 (3), 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO, 2000. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the "kindling" hypothesis. Am. J. Psychiatr. 157 (8), 1243–1251. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatr. 52 (12), 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD, 2005. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 88 (1), 79–86. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J, 2013. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol. Psychiatr. 73 (1), 70–74. [DOI] [PubMed] [Google Scholar]
- Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, Abe O, 2019. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imag 52, 380–396. [DOI] [PubMed] [Google Scholar]
- Landre L, Destrieux C, Baudry M, Barantin L, Cottier JP, Martineau J, Hommet C, Isingrini M, Belzung C, Gaillard P, Camus V, El Hage W, 2010. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatr. Res. 183 (3), 181–186. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, Kim YH, Kim YK, Kim JB, Yeon BK, Oh KS, Oh BH, Yoon JS, Lee C, Jung HY, Chee IS, Paik IH, 2005. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress. Anxiety 21 (3), 135–139. [DOI] [PubMed] [Google Scholar]
- Li L, Chen S, Liu J, Zhang J, He Z, Lin X, 2006. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Can. J. Psychiatr. 51 (7), 431–437. [DOI] [PubMed] [Google Scholar]
- Lim MK, Suh CH, Kim HJ, Kim ST, Lee JS, Kang MH, Kim JH, Lee JH, 2003. Fire-related post-traumatic stress disorder: brain 1H-MR spectroscopic findings. Korean J. Radiol. 4 (2), 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP, 2006. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol. Psychiatr. 59 (2), 171–177. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP, 2004. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol. Psychiatr. 56 (5), 356–363. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, Den Heeten GJ, Gersons BP, 2005. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychol. Med. 35 (10), 1421–1431. [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawjin L, O-Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage KM, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett MH, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, Morey RA, 2018. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatr. 83 (3), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villages M, Martinex C, McEwen BS, 1994. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 639, 167–170. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shan H, Liu Y, Wu L, Zhang X, Ma T, Zhu W, Yang Y, Wang J, Cao Z, 2016. Decreased left hippocampal volumes in parents with or without posttraumatic stress disorder who lost their only child in China. J. Affect. Disord. 197, 223–230. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ, 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1 (1), 69–73. [DOI] [PubMed] [Google Scholar]
- Macklin ML, Metzger LJ, Litz BT, McNally RJ, Lasko NB, Orr SP, Pitman RK, 1998. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J. Consult. Clin. Psychol. 66, 323–326. [DOI] [PubMed] [Google Scholar]
- Mahmutyazicioglu K, Konuk N, Ozdemir H, Atasoy N, Atik L, Gundogdu S, 2005. Evaluation of the hippocampus and the anterior cingulate gyrus by proton MR spectroscopy in patients with post-traumatic stress disorder. Diagn. Interv. Radiol. 11 (3), 125–129. [PubMed] [Google Scholar]
- McClellan AT, Luborsky A, Cacciola J, Griffith J, Evans F, Bar HL, O’Brien CP, 1985. New data from the addiction severity index: reliability and validity in three centers. J. Nerv. Ment. Dis. 73, 412–423. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley CS, 1992. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol. Psychiatr. 31 (2), 177–199. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Shin LH, 1995. Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. Am. J. Psychiatr. 152, 936–938. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, S.C W, Rutter M, Sonuga-Barke E, 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. JCPP (J. Child Psychol. Psychiatry) 50 (8), 943–951. [DOI] [PubMed] [Google Scholar]
- Meng L, Jiang J, Jin C, Liu J, Zhao Y, Wang W, Li K, Gong Q, 2016. Trauma-specific grey matter alterations in PTSD. Sci. Rep. 6, 33748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ, 2013. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 5, 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, De Bellis MD, 2016. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology 41, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Ng P, Neylan T, Mackin S, Wolkowitz O, Mellon S, Yan X, Flory J, Yehuda R, Marmar CR, Weiner MW, 2015. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. Neuroimaging. 234 (2), 194–201. [DOI] [PubMed] [Google Scholar]
- Nardo D, Högbert G, Looi JCL, Larsson S, Hällström T, Pagani M, 2009. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. J. Psychiatr. Res. 44, 477–485. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N, 2010. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol. Psychiatr. 68 (5), 494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Schuff N, Lenoci M, Yehuda R, Weiner MW, Marmar CR, 2003. Cortisol levels are positively correlated with hippocampal N-acetylaspartate. Biol. Psychiatr. 54 (10), 1118–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS, 1995. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15 (11), 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J, 2015. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. Neuroimaging. 232 (1), 1–33. [DOI] [PubMed] [Google Scholar]
- Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, Osborn RE, 2004. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J. Trauma Stress 17 (1), 37–40. [DOI] [PubMed] [Google Scholar]
- Pitman RK, 2001. Hippocampal dimunition in PTSD: more (or less?) than meets the eye. Hippocampus 11 (2), 73–74. [DOI] [PubMed] [Google Scholar]
- Roca V, Freeman TW, 2001. Complaints of impaired memory in veterans with PTSD. Am. J. Psychiatr. 158, 1738. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Crowley DJ, Silveri MM, Rauch SL, Jensen JE, 2017. Hippocampus glutamate and N-acetyl aspartate markers of excitotoxic neuronal compromise in posttraumatic stress disorder. Neuropsychopharmacology 42 (8), 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, Mann JJ, Neria Y, 2016. Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Res. Neuroimaging. 252, 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 1996. Why stress is bad for your brain. Science 273 (5276), 749–750. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, 2001. Atrophy of the hippocampus in posttraumatic stress disorder: how and when? Hippocampus 11 (2), 90–91. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE, 1990. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 10 (9), 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Marmar CR, Weiss DS, Neylan TC, Schoenfeld F, Fein G, Weiner MW, 1997. Reduced hippocampal volume and n-acetyl aspartate in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 821, 516–520. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, Kornak J, Marmar CR, Weiner MW, 2008. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatr. Res. 162 (2), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Choi C-H, Lee JM, Kwon JS, Lee SH, Kim H-C, Han N-Y, Choi S-H, Yoo SY, 2017. Association between memory impairment and brain metabolite concentrations in North Korean refugees with posttraumatic stress disorder. PLoS One 12 (12), e0188953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL, 2004. Hippocampal function in posttraumatic stress disorder. Hippocampus 14 (3), 292–300. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM, 1995. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J. Neurosci. 15, 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, 2005. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus 15 (6), 798–807. [DOI] [PubMed] [Google Scholar]
- Starcevic A, Postic S, Radojicic Z, Starcevic B, Milovanovic S, Ilankovic A, Dimitrijevic I, Damjanovic A, Aksić M, Radonjic V, 2014. Volumetric analysis of amygdala, hippocampus, and prefrontal cortex in therapy-naive PTSD participants. BioMed Res. Int. 968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley J, 2002. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatr. 159 (10), 1675–1681. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B, 1997. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 27 (4), 951–959. [DOI] [PubMed] [Google Scholar]
- Tate DF, Bigler ED, 2000. Fornix and hippocampal atrophy in traumatic brain injury. Learn. Mem. 7 (6), 442–446. [DOI] [PubMed] [Google Scholar]
- Teicher MH, 2002. Scars that won’t heal: the neurobiology of child abuse. Sci. Am. 286 (3), 68–75. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U.S.A. 109 (9), E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler L, Brand SR, Stavitsky K, Labinsky E, Newmark R, Grossman R, Buchsbaum MS, Yehuda R, 2006. The relationship between hippocampal volume and declarative memory in a population of combat veterans with and without PTSD. Ann. N. Y. Acad. Sci. 1071, 405–409. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J, 1993. A twin study of genetic and environmental contributions to liability for posttraumatic stress disorder symptoms. Arch. Gen. Psychiatr. 50, 257–264. [DOI] [PubMed] [Google Scholar]
- Tsai M, Mori AM, Forsberg CW, Waiss N, Sporleder JL, Smith NL, Goldberg J, 2012. The Vietnam era twin registry: a quarter century of progress. Twin Res. Hum. Genet. 1–8. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD, 2006. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatr. 59 (6), 523–529. [DOI] [PubMed] [Google Scholar]
- Uddo M, Vasterling JJ, Braily K, Sutker PB, 1993. Memory and attention in posttraumatic stress disorder. J. Psychopathol. Behav. Assess. 15, 43–52. [Google Scholar]
- van der Beek EM, Wiegant VM, Schouten WGP, van Eerdenburg FJCM, Loijens LWS, van der Plas C, Benning MA, de Vries H, de Kloet ER, Lucassen PJ, 2004. Neuronal number, volume, and apoptosis of the left dentate gyrus of chronically stressed pigs correlate negatively with basal saliva cortisol levels. Hippocampus 14, 688–700. [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E, 2015. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol. Med. 45 (13), 2737, 2346. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Bremner JD, 2006. The impact of the 1991 Gulf War on the mind and brain: findings from neuropsychological and neuroimaging research. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 361 (1468), 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr., Sutker PB, 2002. Attention, learning, and memory performance and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology 16, 5–14. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB, 2015. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatr. Res. 233 (3), 436–442. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD, 2003. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatr. 54 (7), 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM, 2002a. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol. Psychiatr. 52 (2), 119–125. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Petropoulos H, Hamilton DA, Rowland LM, Horan WP, Griego JA, Moreshead M, Hart BL, Brooks WM, 2002b. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: preliminary results. Can. J. Psychiatr. 47, 666–670. [DOI] [PubMed] [Google Scholar]
- Vreven DL, Gudanowski DM, King LA, King DW, 1995. The civilian version of the Mississippi PTSD Scale: a psychometric evaluation. J. Trauma Stress 8, 91–109. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport CD, Miller AH, Vermetten E, Anderson E, Bronen R, Staib L, Charney DS, Nemeroff CB, Bremner JD, 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatr. 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA 3rd, Lipschitz D, Charney DS, Bremner JD, Southwick SM, 2005. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatr. Res. 139 (2), 89–99. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N, 2010. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatr. 67 (3), 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G, 1992. Anatomic basis of amygdaloid and hippocampal measurement by magnetic resonance imaging. Neurology 42, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM, 1999. Psychometric properties of nine scoring rules for the clinician-administered posttraumatic stress disorder scale. Psychol. Assess. 11 (2), 124–133. [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TF, Wilkinson ID, Hunter MD, Woodruff PW, 2004. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol. Psychiatr. 56 (11), 832–836. [DOI] [PubMed] [Google Scholar]
- Winter H, Irle E, 2004. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am. J. Psychiatr. 161 (12), 2194–2200. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW, 2010. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 34 (7), 1181–1188. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR, 2000. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 61 (1), 95–102. [DOI] [PubMed] [Google Scholar]
- Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, Kaminski B, McLean SA, Liberzon I, Wang X, 2018. Relationship of hippocampal Volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (11), 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N, 2003. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc. Natl. Acad. Sci. U.S.A. 100 (15), 9039–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, Buchsbaum MS, 2007. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J. Psychiatr. Res. 41 (5), 435–445. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ, 1995. Learning and memory in combat veterans with posttraumatic stress disorder. Am. J. Psychiatr. 152, 137–139. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Tischler L, Golier JA, Grossman R, Brand SR, Kaufman S, Harvey PD, 2006. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol. Psychiatr. 60, 714–721. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, Wang H, Xu J, Li L, 2011. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. Neuroimaging. 192 (2), 84–90. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond D, 2016. Psychosocial predator stress model of PTSD based on clinically relevant risk factors for trauma-induced psychopathology. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: from Neurobiology to Treatment. Wiley & Sons, Hoboken, N.J., pp. 125–144 [Google Scholar]


