Abstract
Simple Summary
Research is still required to establish the relationship between family history (FH) and gastric cancer (GC) in relation to different histological types and anatomical sites. The present work aimed to examine the influence of first-degree FH on the risk of GC, also according to the GC location and histological type, including 5946 cases and 12,776 controls from 17 studies of 11 countries in three continents participating in the Stomach Cancer Pooling (StoP) Project consortium. This analysis confirms the effect of FH on the risk of GC, reporting an approximately doubled risk, and provides further quantification of the risk of GC according to the subsite and histotype.
Abstract
Although there is a clear relationship between family history (FH) and the risk of gastric cancer (GC), quantification is still needed in relation to different histological types and anatomical sites, and in strata of covariates. The objective was to analyze the risk of GC according to first-degree FH in a uniquely large epidemiological consortium of GC. This investigation includes 5946 cases and 12,776 controls from 17 studies of the Stomach Cancer Pooling (StoP) Project consortium. Summary odds ratios (OR) and the corresponding 95% confidence intervals (CIs) were calculated by pooling study-specific ORs using fixed-effect model meta-analysis techniques. Stratified analyses were carried out by sex, age, tumor location and histological type, smoking habit, socioeconomic status, alcohol intake and fruit consumption. The pooled OR for GC was 1.84 (95% CI: 1.64–2.04; I2 = 6.1%, P heterogeneity = 0.383) in subjects with vs. those without first-degree relatives with GC. No significant differences were observed among subgroups of sex, age, geographic area or study period. Associations tended to be stronger for non-cardia (OR = 1.82; 95% CI: 1.59–2.05 for subjects with FH) than for cardia GC (OR = 1.38; 95% CI: 0.98–1.77), and for the intestinal (OR = 1.92; 95% CI: 1.62–2.23) than for the diffuse histotype (OR = 1.62; 95% CI: 1.28–1.96). This analysis confirms the effect of FH on the risk of GC, reporting an approximately doubled risk, and provides further quantification of the risk of GC according to the subsite and histotype. Considering these findings, accounting for the presence of FH to carry out correct prevention and diagnosis measures is of the utmost importance.
Keywords: gastric cancer, family history, international consortium, meta-analyses
1. Introduction
Gastric cancer (GC) is the fifth leading cause of cancer by incidence and the third leading cause of cancer death in both sexes worldwide. In 2018, there were an estimated one million new GC cases and nearly 800 thousand deaths [1].
The most accepted model of human gastric carcinogenesis is a multistage model in which both environmental and genetic factors are involved [2]. This includes family history (FH), genetic susceptibility, shared environmental or lifestyle factors and/or a combination of interactions. Between 80 and 90% of GCs are sporadic, 10 and 20% have a positive FH and only between 1 and 3% show a clear Mendelian inheritance pattern [3]. Various studies have investigated the role of FH in relation to GC, often reporting relative risks around or over two for subjects with a positive FH of GC [4]. Such a strong association may be explained, besides the genetic component, by environmental exposures—including smoking habits, diet and particularly Helicobacter pylori infection—shared by family members. Still, an unexplained large variability between risk estimates has been reported according to geographic area, ethnic group and sex, as well as the histological type and location of GC [5]. Given the relatively small proportion of GC cases with a positive FH, only a few large studies to date have been able to examine the role of FH on different locations and histological types of GC, as well as in strata of covariates.
Five-year GC survival in most countries is less than 30% [6]. Therefore, it is important to consider FH in prevention or early detection. The present work aims to examine the influence of first-degree FH on the risk of GC, also according to the GC location and histological type, in 17 studies from 11 countries in three continents participating in the Stomach Cancer Pooling (StoP) Project consortium.
2. Materials and Methods
The StoP Project is a consortium of epidemiological studies on gastric cancer. A detailed description of its aims and methods has been provided elsewhere [7]. Inclusion criteria for study participation were: case–control study design, including nested case–control within cohort studies, and inclusion of at least 80 cases of incident, histologically confirmed GC (including both cardia and non-cardia locations). In addition, the original questionnaires and useful information were collected to help with data handling from studies, in order to optimize data harmonization.
For this analysis, the studies were selected from the StoP consortium studies that had both the family history information and the covariates that we used in the models. This work is based on the second data release of the StoP Project, where 17 studies [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] conducted in 11 countries had data on the FH of GC and were examined, including a total of 5946 cases and 12,776 controls. The following data were extracted: (i) main study variables, including study design, geographic area, study period, single center/multicentric study and study center if multicentric; (ii) relevant covariates, including sex, age, education/social class, body mass index, total alcohol consumption, smoking habit, H. pylori infection and consumption of fruit and vegetables; (iii) specific cancer-related variables (for cases only), including cancer subsite and histotype; (iv) FH of GC among first-degree relatives (parents, siblings and offspring). All of the above variables were harmonized centrally according to a pre-specified format. Furthermore, any additional information related to the FH of GC available in each study (e.g., age at GC occurrence in relatives, type of relative affected) was considered.
The StoP Project received ethical approval from the University of Milan Review Board (reference 19/15 on 1 April 2015).
To estimate the association between FH and GC, a two-stage modeling approach was used [26]. First, the association in each study was assessed by calculating the odds ratio (OR) and the corresponding 95% confidence interval (CI) using multivariate logistic regression models including terms for sex, age, socioeconomic level, smoking status and alcohol consumption. In the second stage, summary effect estimates were computed by pooling study-specific ORs using fixed-effect model meta-analysis techniques. Heterogeneity between studies was evaluated using the Q test statistics and quantified using I2—that is, the proportion of total variation contributed by between-study variance [27].
To investigate whether the role of FH was heterogeneous across strata of selected covariates, analyses stratified by sex, age, socioeconomic status, GC location (cardias/non cardias), histological subtype (intestinal, diffuse), tobacco smoking, socioeconomic status, alcohol intake, fruit consumption and H. pylori infection were carried out. Additional analyses were carried out according to type of study, control selection system (matched or by frequency), source of controls (population or hospital) and study period (XX or XXI century).
3. Results
The main characteristics of the StoP Project studies included in the present analysis are shown in Table 1. Most studies were conducted in European countries (82.3% of the controls, and 77.9% of the cases). Overall, 15.8% of cases (n = 942) and 7.7% of the controls (n = 979) had an FH of stomach cancer in first-degree relatives, ranging from 4.4 to 23.0% among cases, and from 1.0 to 16.8% among controls.
Table 1.
Main characteristics of the StoP Project studies included in the analyses.
| StoP Study ID | Study Area(s) | Period | Study Type | Cases | Controls | References | ||
|---|---|---|---|---|---|---|---|---|
| With FH | Total | With FH | Total | |||||
| 1 | Milan, Italy | 1985–1997 | CC, hospital-based | 97 | 768 | 110 | 2081 | La Vecchia et al. [8] |
| 3 | Milan, Italy | 1997–2007 | CC, hospital-based | 30 | 230 | 31 | 547 | Foschi et al. [9] |
| 4 | Rome, Italy | 2006–2010 | CC, hospital-based | 12 | 152 | 16 | 411 | De Feo et al. [10] |
| 5 | Four areas, Italy | 1985–1987 | CC, population-based | 213 | 1016 | 138 | 1159 | Palli et al. [11] |
| 6 | Athens, Greece | 1981–1984 | CC, hospital-based | 8 | 86 | 1 | 97 | Lagiou et al. [12] |
| 8 | Taixing, Jiangsu, China | 2000 | CC, population-based | 31 | 206 | 14 | 415 | Mu et al. [13] |
| 9 | Moscow, Russia | 1996–1997 | CC, hospital-based | 74 | 433 | 76 | 593 | Zaridze et al. [14] |
| 10 | Ardabil, Iran | 2004–2005 | CC, population-based | 31 | 217 | 24 | 394 | Pourfarzi et al. [15] |
| 11 | Ardabil, Iran | 2005–2007 | CC, population-based | 27 | 286 | 19 | 304 | Pakseresht et al. [16] |
| 13 | Yangzhong, China | 1995 | CC, population-based | 9 | 133 | 16 | 433 | Setiawan et al. [17] |
| 17 | North of Portugal | 2001–2006 | CC, population-based | 134 | 584 | 68 | 612 | Lunet et al. [25] |
| 21 | Ten provinces, Spain | 2008–2013 | CC, population-based | 70 | 435 | 208 | 3418 | Castaño-Vinyals et al. [18] |
| 22 | Five counties, Sweden | 1989–1995 | CC, population-based | 111 | 561 | 155 | 1164 | Ye et al. [19] |
| 23 | Two provinces, Spain | 1995–1999 | CC, hospital-based | 30 | 367 | 23 | 433 | Santibañez et al. [20] |
| 28 | Brazil-Brazilian origin | 1991–1994 | CC, hospital-based | 10 | 226 | 4 | 226 | Nishimoto et al. [22] |
| 29 | Brazil-Japanese origin | 1991–1994 | CC, hospital-based | 17 | 93 | 25 | 186 | Hamada et al. [23] |
| 30 | Japan | 1998–2002 | CC, hospital-based | 38 | 153 | 51 | 303 | Machida-Montani et al. [24] |
Table 2 shows the frequency distribution of cases and controls according to the selected covariates. Cases were more frequently male (63.7% vs. 58.3%), aged 65 or older (50.5% vs. 43.0%) and of a low socioeconomic status (63.9% vs. 52.1%) compared to controls. Furthermore, cases were more frequently current smokers (28.9% vs. 25.8%) and reported a high alcohol intake (14.5% vs. 10.6%) and a low consumption of fruit (36.3% vs. 29.0%) compared to controls.
Table 2.
Distribution of cases and controls according to selected covariates.
| Variables | Cases | Controls | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 3790 | 63.7 | 7454 | 58.3 |
| Female | 2156 | 36.3 | 5322 | 41.7 |
| Age | ||||
| 18–49 | 759 | 12.8 | 2553 | 20.0 |
| 50–65 | 2184 | 36.7 | 4724 | 37.0 |
| 65–74 | 2171 | 36.5 | 3940 | 30.8 |
| 75–106 | 832 | 14.0 | 1557 | 12.2 |
| Missing | 0 | 0.0 | 2 | 0.0 |
| Socioeconomic status | ||||
| Low | 3799 | 63.9 | 6488 | 50.8 |
| Medium | 1543 | 26.0 | 3760 | 29.4 |
| High | 501 | 8.4 | 2198 | 17.2 |
| Missing | 103 | 1.7 | 330 | 2.6 |
| Smoking habit (1) | ||||
| Current | 1720 | 28.9 | 3255 | 25.5 |
| No | 4132 | 69.5 | 9386 | 73.4 |
| Missing | 94 | 1.6 | 135 | 1.1 |
| Alcohol intake g of ethanol/day | ||||
| Never | 1526 | 25.7 | 3375 | 26.4 |
| Low (≤12) | 1190 | 20.0 | 3468 | 27.1 |
| Intermediate (>12–47) | 1745 | 29.4 | 3047 | 23.9 |
| High (>47) | 860 | 14.5 | 1353 | 10.6 |
| Missing | 625 | 10.5 | 1533 | 12.0 |
| Fruit intake (2) | ||||
| Low | 2157 | 36.3 | 3701 | 29.0 |
| Medium | 1872 | 31.5 | 4109 | 32.2 |
| High | 1685 | 28.3 | 4293 | 33.6 |
| Missing | 232 | 3.9 | 673 | 5.3 |
| H. pylori infection | ||||
| Positive | 688 | 11.6 | 3662 | 28.7 |
| Negative | 1661 | 27.9 | 1252 | 9.8 |
| Missing | 3597 | 60.5 | 7862 | 61.5 |
(1) No: never and former; (2) defined according to study-specific tertiles.
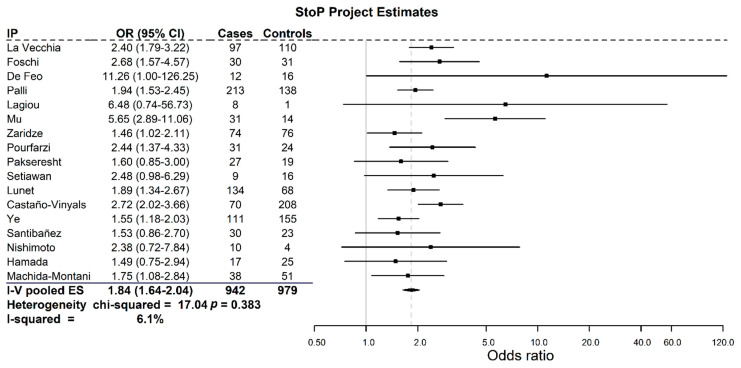
In all studies, first-degree FH was positively related to GC, with ORs ranging between 1.46 and 11.26 (Figure 1). The differences observed were statistically significant in 10 out of the 17 studies included. The pooled OR of all studies was 1.84 (95% CI = 1.64–2.04; I2 = 6.1%, Pheterogeneity = 0.383).
Figure 1.
Pooled adjusted odds ratios with 95% confidence intervals for GC related to family history in the StoP Project.
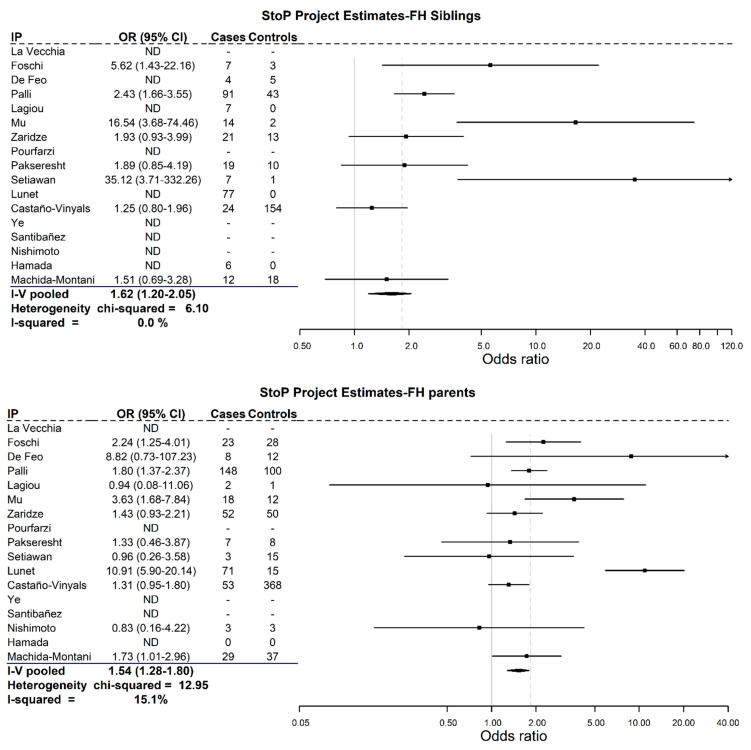
Regarding the analysis of the type of family member affected, Figure 2 shows that the pooled OR for siblings was higher than for parents (1.62; 95% CI = 1.20–2.05, and 1.54; 95% CI = 1.28–1.80, respectively). No significant heterogeneity was observed.
Figure 2.
Pooled adjusted odds ratios with 95% confidence intervals considering the type of relative affected.
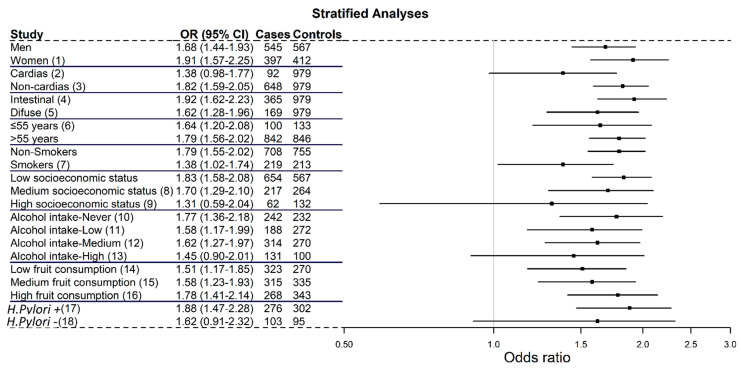
Figure 3 shows the results from the stratified analyses. No significant differences were found by sex, +68% in men and +91% in women. Thirteen and fourteen studies provided information on the location of cardia and non-cardia GC, respectively. Of the 695 cardia GC cases, 92 (13.2%) had a first-degree FH of GC, while 648 (17.6%) of the 3676 non-cardia GC cases had a first-degree FH of GC. The pooled ORs were 1.38 (95% CI = 0.98–1.77) and 1.82 (95% CI = 1.59–2.05), respectively. Twelve studies reported information on the histological classification of GC. Of the 2030 intestinal GC cases, 365 (18.0%) had a first-degree FH of GC, yielding a pooled OR of 1.92 (95% CI = 1.62–2.23). The diffuse histological GC type was reported in 1170 cases, with 169 (14.4%) having a first-degree FH of GC. The pooled OR for all studies was 1.62 (95% CI = 1.28–1.96). There were no significant differences according to age, smoking habit, socioeconomic status, alcohol intake, fruit consumption or H. pylori infection.
Figure 3.
Pooled adjusted odds ratios with 95% confidence intervals for gastric cancer associated with a family history of gastric cancer stratified by sex, age, socioeconomic status, gastric cancer location, histological subtype, tobacco smoking, alcohol intake, fruit consumption and Helicobacter pylori infection in the StoP Project. No information for studies: (1) De Feo, Lagiou; (2) Foschi, Lagiou, Mu, Setiawan; (3) Mu, Setiawan; (4) La Vecchia, Lagiou, Mu, Setiawan, Machida-Montani; (5) La Vecchia, Lagiou, Mu, Setiawan, Machida-Montani; (6) De Feo, Lagiou, Pourfarzi, Pakseresht, Castaño-Vinyals; (7) De Feo, Lagiou, Pourfarzi, Pakseresht, Castaño-Vinyals, Nishimoto; (8) De Feo, Lagiou, Pourfarzi, Pakseresht, Castaño-Vinyals, Nishimoto; (9) De Feo, Palli, Lagiou, Pourfarzi, Setiawan, Santibañez; (10) De Feo, Lagiou, Pakseresht, Setiawan; (11) De Feo, Lagiou, Pourfarzi, Pakseresht, Setiawan, Nishimoto, Hamada; (12) Mu, Pourfarzi, Pakseresht, Setiawan, Nishimoto, Hamada; (13) De Feo, Lagiou, Mu, Pourfarzi, Pakseresht, Setiawan, Ye, Nishimoto, Hamada; (14) Lagiou, Hamada; (15) De Feo, Satiawan, Nishimoto; (16) De Feo, Lagiou, Setiawan; (17) La Vecchia, Foschi, De Feo, Palli, Lagiou, Setiawan, Santibañez; (18) La Vecchia, Foschi, De Feo, Palli, Lagiou, Pakseresht, Setiawan.
Furthermore, no significant differences were observed according to whether the studies were performed in European (OR = 1.84, 95% CI = 1.62–2.06) or non-European populations (OR = 1.86, 95% CI = 1.33–2.38); were matched (OR = 1.83, 95% CI = 1.30–2.36) or not-matched (OR = 1.85, 95% CI = 1.63–2.07); and were multicentric (OR = 1.83; 95% CI = 1.57–2.09) or not (OR = 1.87, 95% CI = 1.54–2.21). Modest differences were observed according to the type of controls (hospital-based controls: OR = 1.76, 95% CI = 1.43–2.09; population-based controls: OR = 1.90, 95% CI = 1.64–2.15), and to the study period before the year 2000 (OR = 1.75, 95% CI = 1.51–1.98) or after the year 2000 (OR = 2.11, 95% CI = 1.72–2.50).
4. Discussion
The results of this uniquely large collaborative study confirm and quantify the influence of FH on the development of GC better than previously available studies. History of GC in a first-degree relative has been found to increase the risk of GC by about 85%. In this pooled investigation, results were suggestive of a possible higher risk of non-cardia than of cardia GC in subjects with a positive FH of gastric cancer, whereas no relevant differences were observed in strata of sex, in the histological type of GC or in the characteristics analyzed in epidemiological studies.
The family aggregation of GC is due to a complex interaction between genetic inheritance and environmental and lifestyle factors [28]. It is known that between 10 and 20% of people who develop GC have a FH, but only part of this can be attributed to hereditary syndromes. The three primary familial gastric cancers include hereditary diffuse gastric cancer [3], familial intestinal gastric cancer and gastric adenocarcinoma and proximal polyposis of the stomach, caused by germline mutations in genes such as CDH1, CTNNA1 and APC [29]. The remaining part may be due to low-penetrance genes, which, due to their interaction with the family-shared environment, have an important influence [30]. In this way, the genetic inheritance, the unique environment of each individual and the aforementioned family-shared environment can be divided into three pathways of GC development. A study conducted among twins found that these causes account for 28%, 62% and 10% of the variation in GC susceptibility, respectively [31].
Our results do not show relevant differences according to sex. Though findings of different studies have varied, with some of them reporting a stronger risk related to FH among women [32], our finding is broadly consistent with the conclusions of several reviews [8,11,16,20]. Additionally, our results on the GC subsite are consistent with those from a meta-analysis, indicating that FH has a greater relative risk (RR) on non-cardia GC (RR = 1.97; 95% CI = 1.72–2.25) than on cardia GC (RR = 1.46; 95% CI = 0.89–2.39) [28]. Kharazmi et al. also showed similar results in a nationwide Swedish cohort study, where the standardized incidence ratio (SIR) of cardia GC associated with a positive FH of cancer at the same subsite was lower (SIR = 1.70; 95% CI = 1.10–2.50) compared to non-cardia subclasses—antrum (SIR = 5.5; 95% CI = 2.4–11.00) and body (SIR = 4.6; 95% CI = 2.6–7.4) [33]. A large percentage of non-cardia GC cases are attributed to H. pylori infection and, therefore, are more likely associated with family transmission [33]. On the other hand, cardia GC is more likely related to lifestyle factors, with issues such as obesity (increases the risk of cardia GC by 82%) [34,35,36], gastroesophageal reflux (increases the risk two to four times) [19,37] and tobacco smoking [38].
The results of the present study show a higher risk of GC when the affected relatives were siblings rather than parents, which is in line with previous studies reporting that the association tends to be stronger among siblings than between parents and offspring [5,28,39,40]. However, one of the limitations of our study is that neither offspring nor the age at diagnosis of the affected family member could be included due to the small sample available for these specific analyses.
Regarding the histological type of GC, our results show that a FH of GC is associated with both intestinal and diffuse subtypes, which is consistent with findings from the prospective Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study [28], which suggested similarly increased risks in both histological subtypes of GC (intestinal: HR 1.53, 95% CI 0.92–2.55; diffuse: HR 1.47, 95% CI 0.72–3.03). However, by taking advantage of the study consortium approach, our findings were based on a larger number of cases for both histotypes, and therefore our estimates are more robust.
H. pylori infection is the major environmental factor in gastric carcinogenesis. As in our study, another investigation [41] showed that patients with an FH and H. pylori infection had a slightly higher risk of GC than those without H. pylori infection. However, these differences were not significant.
No significant differences were found when the geographical origin of the samples of the studies included (European vs. non-European populations) was analyzed. Most previous studies on FH and GC were conducted in Asian countries, in North America and in Northern Europe [9], and this analysis has the advantage of including studies from less frequently considered areas (i.e., eight studies were from the Mediterranean region, two from Iran and two from Brazil). Furthermore, the pooled analysis patient-level approach allows for a direct comparison between estimates of different geographic areas, by limiting methodological variation (through centralized data harmonization, using the same adjustment terms in the models, etc.).
Only moderate differences were observed in relation to studies based on hospital- and population-based controls. A higher risk of GC related to FH emerged for studies conducted after the year 2000, as compared to those conducted earlier. This may be due to a decrease in the weight of environmental factors, particularly H. pylori infection and tobacco smoking, with respect to FH [42,43].
As strengths of the study, there are 5946 cases and 12,776 controls available for the analysis, accompanied by a wide variety of predictor variables, providing an adequate statistical power. Furthermore, this project is a collaborative framework, contributing with varied geographical origins. In addition, we assessed the relationship between FH and GC by location and histological types. The most important difference between our work and the rest of the studies and meta-analyses is that, in our case, we worked with a consortium of studies, meaning we directly used the study data instead of the ORs of the published articles. This allows us to adjust all studies for the same variables and to have more homogeneity in the results.
5. Conclusions
Our results confirm and further quantify the effect of FH on the development of GC. Subjects with an FH of GC among first-degree relatives have an approximately doubled risk of GC occurrence. It is important to take into account the presence of an FH to carry out GC prevention measures, both primary and secondary, i.e., early diagnosis.
Author Contributions
Conceptualization, F.V.-S., M.R.-G., C.P. and Y.B.; methodology, M.R.-G., F.V.-S., V.M. and C.L.V.; software F.V.-S., M.R.-G. and V.M.; validation, C.P., C.L.V., R.B. and E.N.; formal analysis M.R.-G., F.V.-S. and V.M.; investigation, M.R.-G., F.V.-S., M.R., D.P., M.F., N.L., S.M. and W.Y.; data curation, M.R.-G., F.V.-S., V.M., C.P., R.B. and M.F.; writing—review and editing, A.P., R.M., S.T., A.H., N.A., G.C.-V., D.G.Z., D.M., J.V., M.G.-d.-l.-H., Z.-F.Z., G.S.H., M.P., F.P., L.M., S.B., R.P., G.-P.Y., A.L., P.L., E.N., C.L.V. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Project no. 21378 (Investigator Grant). The Unidade de Investigação em Epidemiologia—Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020) was funded by the Foundation for Science and Technology—FCT (Portuguese Ministry of Science, Technology and Higher Education). SM was also funded by the project “NEON-PC—Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline” (POCI-01-0145-FEDER-032358; ref. PTDC/SAU-EPI/32358/2017), which is funded by FEDER through the Operational Program competitiveness and Internationalization, and national funding from FCT. This work was conducted with the contribution of the Carlos III Health Institute CIBERESP CB06/02/0073, co-financed by the European Regional Development Fund ERDF, a way to build Europe. It also acknowledges the support of the Secretariat for Universities and Research of the Department of Business and Knowledge of the Generalitat de Catalunya, grants to support the activities of research groups 2017SGR01085. We thank CERCA Programme/Generalitat de Catalunya for institutional support. M.R.-G is supported by the Ministry of Education of Spain (FPU17/06488).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Milan (Ref 19/5—01/04/2015).
Informed Consent Statement
Informed consent was not obtained from all studies because some of them were carried out during the 1990s.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yusefi A.R., Bagheri Lankarani K., Bastani P., Radinmanesh M., Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. APJCP. 2018;19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corso G., Roncalli F., Marrelli D., Carneiro F., Roviello F. History, Pathogenesis, and Management of Familial Gastric Cancer: Original Study of John XXIII’s Family. BioMed Res. Int. 2013;2013:385132. doi: 10.1155/2013/385132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghoobi M., Bijarchi R., Narod S.A. Family History and the Risk of Gastric Cancer. Br. J. Cancer. 2010;102:237–242. doi: 10.1038/sj.bjc.6605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi Y.J., Kim N. Gastric Cancer and Family History. Korean J. Intern. Med. 2016;31:1042–1053. doi: 10.3904/kjim.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C.J., Esteve J., et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet. 2018;39:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelucchi C., Lunet N., Boccia S., Zhang Z.F., Praud D., Boffetta P., Levi F., Matsuo K., Ito H., Hu J., et al. The Stomach Cancer Pooling (StoP) Project: Study Design and Presentation. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2015;24:16–23. doi: 10.1097/CEJ.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia C., D’Avanzo B., Negri E., Decarli A., Benichou J. Attributable Risks for Stomach Cancer in Northern Italy. Int. J. Cancer. 1995;60:748–752. doi: 10.1002/ijc.2910600603. [DOI] [PubMed] [Google Scholar]
- 9.Foschi R., Lucenteforte E., Bosetti C., Bertuccio P., Tavani A., La Vecchia C., Negri E. Family History of Cancer and Stomach Cancer Risk. Int. J. Cancer. 2008;123:1429–1432. doi: 10.1002/ijc.23688. [DOI] [PubMed] [Google Scholar]
- 10.De Feo E., Simone B., Persiani R., Cananzi F., Biondi A., Arzani D., Amore R., D’Ugo D., Ricciardi G., Boccia S. A Case-Control Study on the Effect of Apolipoprotein E Genotypes on Gastric Cancer Risk and Progression. BMC Cancer. 2012;12:494. doi: 10.1186/1471-2407-12-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palli D., Galli M., Caporaso N.E., Cipriani F., Decarli A., Saieva C., Fraumeni J.F., Buiatti E. Family History and Risk of Stomach Cancer in Italy. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1994;3:15–18. [PubMed] [Google Scholar]
- 12.Lagiou P., Samoli E., Lagiou A., Peterson J., Tzonou A., Dwyer J., Trichopoulos D. Flavonoids, Vitamin C and Adenocarcinoma of the Stomach. Cancer Causes Control CCC. 2004;15:67–72. doi: 10.1023/B:CACO.0000016619.18041.b0. [DOI] [PubMed] [Google Scholar]
- 13.Mu L.N., Lu Q.Y., Yu S.Z., Jiang Q.W., Cao W., You N.C., Setiawan V.W., Zhou X.-F., Ding B.-G., Wang R.-H., et al. Green Tea Drinking and Multigenetic Index on the Risk of Stomach Cancer in a Chinese Population. Int. J. Cancer. 2005;116:972–983. doi: 10.1002/ijc.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaridze D., Borisova E., Maximovitch D., Chkhikvadze V. Aspirin Protects against Gastric Cancer: Results of a Case-Control Study from Moscow, Russia. Int. J. Cancer. 1999;82:473–476. doi: 10.1002/(SICI)1097-0215(19990812)82:4<473::AID-IJC1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Pourfarzi F., Whelan A., Kaldor J., Malekzadeh R. The Role of Diet and Other Environmental Factors in the Causation of Gastric Cancer in Iran--a Population Based Study. Int. J. Cancer. 2009;125:1953–1960. doi: 10.1002/ijc.24499. [DOI] [PubMed] [Google Scholar]
- 16.Pakseresht M., Forman D., Malekzadeh R., Yazdanbod A., West R.M., Greenwood D.C., Crabtree J.E., Cade J.E. Dietary Habits and Gastric Cancer Risk in North-West Iran. Cancer Causes Control CCC. 2011;22:725–736. doi: 10.1007/s10552-011-9744-5. [DOI] [PubMed] [Google Scholar]
- 17.Setiawan V.W., Zhang Z.F., Yu G.P., Li Y.L., Lu M.L., Tsai C.J., Cordova D., Wang M.-R., Guo C.H., Yu S.-Z., et al. GSTT1 and GSTM1 Null Genotypes and the Risk of Gastric Cancer: A Case-Control Study in a Chinese Population. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2000;9:73–80. [PubMed] [Google Scholar]
- 18.Castano-Vinyals G., Aragones N., Perez-Gomez B., Martin V., Llorca J., Moreno V., Altzibar J.M., Ardanaz E., Sanjose S.D., Jimenez-Moleon J.J., et al. Population-Based Multicase-Control Study in Common Tumors in Spain (MCC-Spain): Rationale and Study Design. Gac. Sanit. 2015;29:308–315. doi: 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Ye W., Chow W.H., Lagergren J., Yin L., Nyren O. Risk of Adenocarcinomas of the Esophagus and Gastric Cardia in Patients with Gastroesophageal Reflux Diseases and after Antireflux Surgery. Gastroenterology. 2001;121:1286–1293. doi: 10.1053/gast.2001.29569. [DOI] [PubMed] [Google Scholar]
- 20.Santibanez M., Alguacil J., de La Hera M.G., Navarrete-Munoz E.M., Llorca J., Aragones N., Kauppinen T., Vioque J. Occupational Exposures and Risk of Stomach Cancer by Histological Type. Occup. Environ. Med. 2012;69:268–275. doi: 10.1136/oemed-2011-100071. [DOI] [PubMed] [Google Scholar]
- 21.Fujioka N., Fahey M.T., Hamada G.S., Nishimoto I.N., Kowalski L.P., Iriya K., Rodrigues J.J.G., Tajiri H., Tsugane S. Serological Immunoglobulin G Antibody Titers to Helicobacter Pylori in Japanese Brazilian and Non-Japanese Brazilian Gastric Cancer Patients and Controls in Sao Paulo. Jpn. J. Cancer Res. Gann. 2001;92:829–835. doi: 10.1111/j.1349-7006.2001.tb01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimoto I.N., Hamada G.S., Kowalski L.P., Rodrigues J.G., Iriya K., Sasazuki S., Hanaoka T., Tsugane S. São Paulo--Japan Cancer Project Gastric Cancer Study Group. Risk Factors for Stomach Cancer in Brazil (I): A Case-Control Study among Non-Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002;32:277–283. doi: 10.1093/jjco/hyf060. [DOI] [PubMed] [Google Scholar]
- 23.Hamada G.S., Kowalski L.P., Nishimoto I.N., Rodrigues J.J., Iriya K., Sasazuki S., Hanaoka T., Tsugane S. São Paulo--Japan Cancer Project Gastric Cancer Study Group. Risk Factors for Stomach Cancer in Brazil (II): A Case-Control Study among Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002;32:284–290. doi: 10.1093/jjco/hyf061. [DOI] [PubMed] [Google Scholar]
- 24.Machida-Montani A., Sasazuki S., Inoue M., Natsukawa S., Shaura K., Koizumi Y., Kasuga Y., Hanaoka T., Tsugane S. Association of Helicobacter Pylori Infection and Environmental Factors in Non-Cardia Gastric Cancer in Japan. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2004;7:46–53. doi: 10.1007/s10120-004-0268-5. [DOI] [PubMed] [Google Scholar]
- 25.Lunet N., Valbuena C., Vieira A.L., Lopes C., Lopes C., David L., Carneiro F., Barros H. Fruit and Vegetable Consumption and Gastric Cancer by Location and Histological Type: Case-Control and Meta-Analysis. Eur. J. Cancer Prev. 2007;16:312–327. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Warner S.A., Spiegelman D., Ritz J., Albanes D., Beeson W.L., Bernstein L., Berrino F., Van Den Brandt P.A., Buring J.E., Cho E., et al. Methods for Pooling Results of Epidemiologic Studies: The Pooling Project of Prospective Studies of Diet and Cancer. Am. J. Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring Inconsistency in Meta-Analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song M., Camargo M.C., Weinstein S.J., Best A.F., Mannisto S., Albanes D., Rabkin C.S. Family History of Cancer in First-Degree Relatives and Risk of Gastric Cancer and Its Precursors in a Western Population. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018;21:729–737. doi: 10.1007/s10120-018-0807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lott P.C., Carvajal-Carmona L.G. Resolving Gastric Cancer Aetiology: An Update in Genetic Predisposition. Lancet Gastroenterol. Hepatol. 2018;3:874–8838. doi: 10.1016/S2468-1253(18)30237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butterworth A. Family History as a Risk Factor for Common, Complex Disease. Public Health Genetics Unit PHG Found; Cambridge, UK: 2007. pp. 1–2. [Google Scholar]
- 31.Lichtenstein P., Holm N.V., Verkasalo P.K., Iliadou A., Kaprio J., Koskenvuo M., Pukkala E., Skytthe A., Hemminki K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 32.Yatsuya H., Toyoshima H., Mizoue T., Kondo T., Tamakoshi K., Hori Y., Tokui N., Hoshiyama Y., Kikuchi S., Sakata K., et al. Family History and the Risk of Stomach Cancer Death in Japan: Differences by Age and Gender. Int. J. Cancer. 2002;97:688–694. doi: 10.1002/ijc.10101. [DOI] [PubMed] [Google Scholar]
- 33.Kharazmi E., Babaei M., Fallah M., Chen T., Sundquist K., Hemminki K. Importance of Tumor Location and Histology in Familial Risk of Upper Gastrointestinal Cancers: A Nationwide Cohort Study. Clin. Epidemiol. 2018;10:1169–1179. doi: 10.2147/CLEP.S168152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Martel C., Forman D., Plummer M. Gastric Cancer: Epidemiology and Risk Factors. Gastroenterol. Clin. N. Am. 2013;42:219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Liu L., Wang X., Wang J., Yan Z., Cheng J., Gong G., Li G. Body Mass Index and Risk of Gastric Cancer: A Meta-Analysis of a Population with More than Ten Million from 24 Prospective Studies. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2013;22:1395–1408. doi: 10.1158/1055-9965.EPI-13-0042. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Zhou Y., Chen B., Wan H.W., Jia G.Q., Bai H.L., Wu X.T. Overweight, Obesity and Gastric Cancer Risk: Results from a Meta-Analysis of Cohort Studies. Eur. J. Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Figueroa J.D., Terry M.B., Gammon M.D., Vaughan T.L., Risch H.A., Zhang F.F., Kleiner D.E., Bennett W.P., Howe C.L., Dubrow R., et al. Cigarette Smoking, Body Mass Index, Gastro-Esophageal Reflux Disease, and Non-Steroidal Anti-Inflammatory Drug Use and Risk of Subtypes of Esophageal and Gastric Cancers by P53 Overexpression. Cancer Causes Control CCC. 2009;20:361–368. doi: 10.1007/s10552-008-9250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez C.A., Pera G., Agudo A., Palli D., Krogh V., Vineis P., Tumino R., Panico S., Berglund G., Siman H., et al. Smoking and the Risk of Gastric Cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Int. J. Cancer. 2003;107:629–634. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 39.Bakir T., Can G., Erkul S., Siviloglu C. Stomach Cancer History in the Siblings of Patients with Gastric Carcinoma. Eur. J. Cancer Prev. 2000;9:401–408. doi: 10.1097/00008469-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Bakir T., Can G., Siviloglu C., Erkul S. Gastric Cancer and Other Organ Cancer History in the Parents of Patients with Gastric Cancer. Eur. J. Cancer Prev. 2003;12:183–189. doi: 10.1097/00008469-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Lee J., Chung S.J., Choi J.M., Han Y.M., Kim J.S. Clinicopathologic Characteristics and Long-Term Outcome of Gastric Cancer Patients with Family History: Seven-Year Follow-Up Study for Korean Health Check-Up Subjects. Gastroenterol. Res. Pract. 2020;2020:4028136. doi: 10.1155/2020/4028136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Esquinas E., Perez-Gomez B., Pollan M., Boldo E., Fernandez-Navarro P., Lope V., Vidal E., Lopez-Abente G., Aragones N. Gastric Cancer Mortality Trends in Spain, 1976-2005, Differences by Autonomous Region and Sex. BMC Cancer. 2009;9:346. doi: 10.1186/1471-2407-9-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadtlander C.T., Waterbor J.W. Molecular Epidemiology, Pathogenesis and Prevention of Gastric Cancer. Carcinogenesis. 1999;20:2195–2208. doi: 10.1093/carcin/20.12.2195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.