Abstract
Fish serves as the principal source of animal protein for the indigenous people of the Amazon, ensuring their food and nutritional security. However, gold mining causes mercury (Hg) contamination in fish, and consequently increases health risks associated with fish consumption. The aim of this study was to assess the health risk attributed to the consumption of mercury-contaminated fish by Munduruku indigenous communities in the Middle-Tapajós Region. Different fish species were collected in the Sawré Muybu Indigenous Land to determine mercury levels. The health risk assessment was carried out according to the World Health Organization (WHO 2008) methodology and different scenarios were built for counterfactual analysis. Eighty-eight fish specimens from 17 species and four trophic levels were analyzed. Estimates of Hg ingestion indicated that the methylmercury daily intake exceeds the U.S. EPA (United States Environmental Protection Agency) (2000) reference dose from 3 to 25-fold, and up to 11 times the FAO (Food and Agriculture Organization)/WHO (2003) dose recommendation. In all situations analyzed, the risk ratio estimates were above 1.0, meaning that the investigated Munduruku communities are at serious risk of harm as a result of ingestion of mercury-contaminated fish. These results indicate that, at present, fish consumption is not safe for this Munduruku population. This hazardous situation threatens the survival of this indigenous population, their food security, and their culture.
Keywords: mercury, indigenous, health risk assessment, Munduruku, fish, Brazilian Amazon
1. Introduction
Fish has an important role in food security, since 17% of all animal protein consumed in the world is provided by fish [1]. In addition, fish consumption contributes to nutritional security, given its high content of essential nutrients (i.e., vitamins and minerals) and polyunsaturated fatty acids (e.g., omega-3 and omega-6) [2,3]. The food and nutritional security attributed to fish consumption is especially important for low-income populations in developing countries where over 90% of inland water caught fish are directed for local human consumption [4,5]. Indeed, fish is often the only quality protein accessible to poor people [6,7]. These reasons make fish a vital element for Amazonian populations, especially for indigenous and riverine populations.
The Amazon is the largest freshwater ecosystem and displays the greatest diversity of freshwater fish in the world [8,9]. Therefore, it is not surprising that Amazonian people have one of the highest fish consumption rates in the world [10,11,12]. An archaeological study found that fish was the species of vertebrates most consumed (>75% of total) in an ancient settlement of indigenous populations in the Central Brazilian Amazon. Fish consumption is historically related to culture and to food security of the original peoples from the Amazon [13,14]. However, several anthropogenic activities threaten the survival of Amazonian peoples, such as deforestation promoted by agribusiness (e.g., soybean cultivate and cattle), construction of hydroelectric dams, and artisanal gold mining [15,16,17,18,19,20,21,22]. The indigenous populations are particularly affected by the impact of these activities because they live in socially and environmentally vulnerable conditions caused by historical government neglect.
In this sense, the artisanal gold mining (also called garimpos) can be considered one of the most harmful economic activities in the Amazon, because it causes not only deforestation, river siltation, and soil erosion, but also releases large amounts of mercury into the environment. Mercury is a toxic heavy metal that contaminates the atmosphere, waters, sediments, and organisms [23,24].
Nevertheless, the current policies of the Brazilian federal government aim to allow oil and natural gas extraction, agribusiness, mining, and other economic activities in protected areas and indigenous lands. These policies are responsible for the highest rate of deforestation in the past 10 years, weakening the environmental protection legislation and human rights of traditional and indigenous populations [20,25,26].
In addition, recent studies carried out in the Amazon region indicate that illegal gold mining has had a sharp increase in the past years due to incentives from the federal government, mainly due to Bill 191/2020 presented by President Jair Bolsonaro to Parliament. The situation becomes even more serious because this increase is largely concentrated in indigenous areas, mainly affecting the Yanomami and Munduruku traditional territories [20,25].
The mercury used in garimpos is converted into methylmercury (MeHg), the most dangerous mercurial form to human health. Methylmercury undergoes bioaccumulation and biomagnification through aquatic trophic chains and, consequently, the consumption of contaminated fish and other organisms (e.g., crabs, shrimp, turtle, etc.) provides the main route of human exposure to this persistent environmental contaminant [27]. Ingested methylmercury is rapidly absorbed by the human gastrointestinal tract, principally affecting the central nervous and cardiovascular systems [28,29,30]. This mercurial form is especially harmful to pregnant women because the fetal brain is more sensitive to the action of methylmercury, causing many neurodevelopment problems to occur, including mental retardation, learning delays, visual and auditory alterations, and other harmful effects [31,32,33].
Considering this critical situation of mercury contamination within the Amazon and the consequences of human exposure to this contaminant, the present study aimed to assess the health risks from consumption of fish by mercury-exposed Munduruku indigenous communities in the Middle-Tapajós Region, in the state of Pará, one of the areas most threatened by illegal mining in the Brazilian Amazon. Risk assessment studies are of fundamental importance to identify population groups with a higher risk of exposure to a certain contaminant and can be the basis for the development of public policies to mitigate contamination.
2. Materials and Methods
2.1. Study Area
The present study was developed in the Sawré Muybu Indigenous Land (also known as Pimental), where a proportion of the Munduruku indigenous people live. This indigenous land is in the municipalities of Itaituba and Trairão, in the state of Pará, Brazil. The data collection and the fish capture were carried out between 29 October and 9 November 2019, in the villages Poxo Muybu, Sawré Aboy, and Sawré Muybu (see Basta et al. [34] to access the map and more details).
2.2. Fish Capture
The fish samplings were conducted in the mornings (8:00 to 12:00 a.m.) for seven consecutive days. All fish catches were conducted by the indigenous Munduruku themselves using their own fishing gears (i.e., gillnets and handlines) accompanied by field researchers. Indigenous fishermen employed gillnets with different sized mesh, one of them with 25 mm between opposite knots to catch bait to handline and other gillnets with 35 and 45 mm between opposite knots aiming to catch their target fish species. All fish caught were measured to standard length (cm), weighed (g), and the popular names recorded by researchers. Each fish specimen caught was identified to a species level in the field with further analyses in the laboratory, and the trophic level was recorded based on Santos et al. [35,36]. After the measurement and identification of the fish species, samples of 2 to 5 g of dorsal muscle tissue without skin or scales were collected from each specimen and stored in liquid nitrogen.
2.3. Mercury Analysis
The total mercury (THg) determined in the fish muscle tissue was obtained using the methodology proposed by Akagi et al. [37]. For each sample, 0.3 to 0.5 g of muscle tissue was weighed (wet weight) in a 50 mL Pyrex® volumetric flask (Corelle Brands, Charleroi, Belgium). Then, 1 mL of deionized water, 2 mL of HNO3 and HClO4 (1:1), and 5 mL of H2SO4 were added for digestion. The vials were exposed to a hot plate (200 to 230 °C) for 30 min. After cooling to room temperature, the flasks were measured with deionized water and the digested samples were homogenized. The THg determination was made by cold vapor atomic absorption system (CVAAS), using semi-automatic mercury analyzer equipment Analyzer Model Hg-201 (Sanso Seisakusho Co. Ltd., Tokyo, Japan) [38]. To guarantee the Quality Assurance (QA)/ Quality Control (QC), we used for the mercury analysis in fish samples the following parameters: (i) reference materials dogfish liver certified reference material for trace metals (DOLT-4) (% of recovery: 92.24 ± 7.73; 70.92 to 100) and fish protein certified reference material for trace metals (DORM-3) (% of recovery: 96.22 ± 4.69; 87.16 to 100) from the National Research Council of Canada; (ii) a method blank; (iii) a 6-point calibration curve; and (iv) the relative standard deviation (RSD) of 8.32%. The detection and quantification limits (LOD/LOQ) obtained were 0.0083 ng/mg and 0.027 ng/mg, respectively.
Based on chemical analysis results, the mercury potential for biomagnification (between different trophic levels) and bioaccumulation (between different fish sizes of the same trophic level) was evaluated. The mercury biomagnification between four trophic levels sampled (i.e., piscivorous, omnivorous, herbivorous, and detritivorous) was checked by Kruskal–Wallis tests (residuals were not normal and variance non-homogeneous), with a post-hoc Dunn test. Bioaccumulation was analyzed using linear regression between THg levels and different fish sizes (standard length in cm) inside each trophic level. The linear regression was run due to expected relation of cause–effect between fish size and THg level. The residuals of regressions were checked, and one outlier, an individual of Piranha-preta (Serrasalmus rhombeus), was excluded from the analysis to ensure normality of the data and homogeneity of variances.
2.4. Data Collection: Participants’ Weight, Family Composition and Fish Consumption
To perform the health risk assessment related to consumption of mercury-contaminated fish, it was necessary to collect data about participants’ average weight (i.e., women, men, and children), fish consumption by Munduruku indigenous families (i.e., most consumed species and frequency), and family composition (i.e., number of individuals, age, and gender). The access to the amount of fish consumed (in grams) by the family members is described in the next section.
Data about diet were obtained through interviews with the head of households (husband/father), followed by weight measurements of all individuals living in the three investigated villages. The interview answers were recorded on electronic forms with the aid of portable devices (i.e., tablets). To measure weight, a portable digital scale from Seca® (model 877) (Seca GmbH, Hamburg, Germany) was used, with a maximum capacity of 150 kg and precision of 0.1 kg.
2.5. Potential Fish Consumption Estimative from Catch Effort
The average amount of fish captured in one fishing day by the Munduruku indigenous was used to estimate the fish consumption by a family.
For the calculation of the family members’ fish consumption, we assumed as a premise that the effort of the Munduruku fishermen to catch fish during this fieldwork was similar to the fishing effort usually devoted by the heads of households. Due to this assumption, the average quantity of fish obtained could be a proxy for the quantity of fish available at home to feed a family for a week.
Taking into consideration that the amount of fish consumed varies according to the gender and age of the family member, we assumed that adult men consume 45% of the fish captured, adult women consume 35%, children aged 5 to 12 years consume 15%, while children from 2 to 5 years old consume only 5%.
2.6. Health Risk Assessment
The health risk assessment was carried out according to the methodology proposed by World Health Organization (WHO) [39]. We made two assumptions to calculate the daily mercury intake: (i) 100% of the mercury detected in the fish sample is in the form of methylmercury; (ii) 100% of the methylmercury available in the fish muscle tissue is absorbed in the human’s gastrointestinal tract. The amount of mercury ingested was estimated from the equation:
| (1) |
where MI is methylmercury intake per kilogram body weight per day (µg methylmercury per kg body weight per day); FI is amount of fish ingested per day (g/day); MC is mercury concentration in the fish ingested (µg/g); KBW is kilogram body weight (kg bw).
We created different scenarios of methylmercury exposure from the data collected: (1) Rainy Season, (2) Dry Season, (3) Current, and (4) Critical. Scenarios 1 and 2 were constructed from the average mercury levels detected in fish species most consumed by the indigenous in the different seasons of the year, based on interview data. Scenario 3 was constructed from the weighted average of medium mercury concentrations detected in piscivorous and non-piscivorous species. The percentages of piscivorous and non-piscivorous species caught by the fishermen (34% and 66%, respectively) were multiplied by the average mercury levels detected. Scenario 4 was constructed from the 95th percentile of mercury concentrations in piscivorous and non-piscivorous species (1.42 and 0.29 µg/g, respectively). The 95th percentile was multiplied by the occurrence of the fish species, similarly to the previous scenario.
The risk ratio was calculated from the ratio between “the methylmercury estimated intake” and the reference doses proposed by U.S. EPA (United States Environmental Protection Agency) [40] and by FAO (Food and Agriculture Organization)/WHO [41]. According to U.S. EPA, the safe daily intake, also known as Reference Dose (RfD), is equal to 0.1 µg Hg/Kg bw/day. With the same purpose, FAO/WHO limits of 0.23 µg Hg/Kg bw/day for childbearing age women and for children and 0.45 µg Hg/Kg bw/day for adults in general.
When the risk ratio is less than 1, the risk of exposure is below the reference levels and, consequently, the risk of becoming ill is low. On the other hand, when the risk ratio is equal to or greater than 1, the risk of becoming ill due to mercury exposure must be considered. Therefore, the higher the risk ratio, the greater the risk of becoming ill due to mercury exposure.
3. Results
3.1. Fish Catch and Mercury Contamination
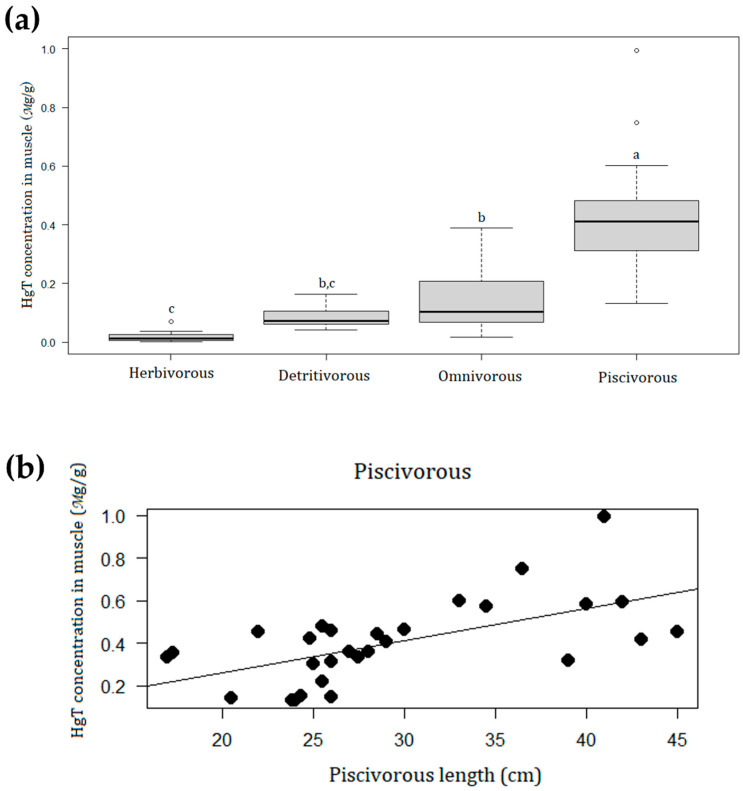
In total, 88 fish specimens were captured, distributed across 17 species and four trophic levels, as described in Table 1 showing the general characterization of the fish caught by the Munduruku fishermen during the fieldwork. Three piscivorous species showed average mercury levels above 0.5 µg/g. The biomagnification in the trophic chain of fish was confirmed, as there was a significant difference in the concentration of total mercury in muscle tissues among trophic levels (H = 60.2; df = 3; p < 0.0001) (Figure 1a). The average mercury levels in samples of non-piscivorous fish (n = 57) was 0.10 µg/g (SD = 0.09) and the average for piscivorous fish (n = 31) was 0.44 µg/g (SD = 0.34). In addition, bioaccumulation was found only in piscivorous species, where there was a positive and significant relationship between the size of the fish and the THg concentration in the muscle tissues (y = −0.041 + 0.0151X; R2 = 0.36; F = 15.4; df = 27; p = 0.0005) (Figure 1b). On the other hand, among other analyzed trophic levels (i.e., omnivorous, detritivorous, and herbivorous), there was no significant relationship between size and concentration of mercury (p > 0.05).
Table 1.
Characterization of the species of fish caught, Sawré Muybu Indigenous Land, Pará, Amazon, Brazil, 2019.
| Fish Species | Popular Name | N | Size (cm) | Weight (g) | Trophic Level | Hg (µg/g) (SD) | Min–Max (Hg) |
|---|---|---|---|---|---|---|---|
| Serrasalmus rhombeus | Piranha Preta | 6 | 17–34.5 | 140–1305 | Piscivorous | 0.71 (±0.61) | 0.33–1.95 |
| Pseudoplatystoma fasciatum | Surubim | 6 | 23.8–45 | 141–907 | Piscivorous | 0.24 (±0.15) | 0.13–0.45 |
| Pinirampus pirinampu | Barbado | 8 | 17.3–42 | 109–961 | Piscivorous | 0.49 (±0.14) | 0.31–0.75 |
| Cichla ocellaris | Tucunaré | 6 | 25–29 | 347–571 | Piscivorous | 0.33 (±0.06) | 0.22–0.41 |
| Rhaphiodon vulpinus | Peixe Cachorro | 2 | 39–41 | 328–469 | Piscivorous | 0.66 (±0.48) | 0.32–1.00 |
| Ageneiosus inermis | Mandubé | 1 | 33 | 550 | Piscivorous | 0.6 | - |
| Pachyurus junki | Corvina | 1 | 20.5 | 148 | Piscivorous | 0.14 | - |
| Geophagus proximus | Caratinga | 10 | 10.5–18.5 | 36–171 | Omnivorous | 0.07 (±0.03) | 0.03–0.10 |
| Pimelodus blochii | Mandii | 7 | 14.5–17.3 | 60–84 | Omnivorous | 0.20 (±0.05) | 0.13–0.28 |
| Leporinus fasciatus | Aracu Flamengo | 5 | 17.7–23.3 | 102–244 | Omnivorous | 0.09 (±0.02) | 0.05–0.11 |
| Caenotropus labyrinthicus | João Duro | 6 | 13.7–14.8 | 63–73 | Omnivorous | 0.28 (±0.07) | 0.17–0.39 |
| Hemiodus unimaculatus | Charuto | 1 | 17.5 | 95 | Omnivorous | 0.02 | - |
| Schizodon vittatus | Aracu | 4 | 21.3–27.3 | 163–351 | Herbivorous | 0.03 (±0.01) | 0.02–0.04 |
| Myloplus rubripinnis | Pacu Branco | 7 | 12.5–20.5 | 89–390 | Herbivorous | 0.02 (±0.03) | 0.01–0.07 |
| Semaprochilodus insignis | Jaraqui Escama Grossa | 6 | 21–23.5 | 223–329 | Detritivorous | 0.11 (±0.05) | 0.05–0.16 |
| Prochilodus nigricans | Curimatá | 6 | 20.5–24 | 253–369 | Detritivorous | 0.07 (±0.02) | 0.04–0.10 |
| Curimata sp. | Branquinha | 6 | 12.7–14 | 64–85 | Detritivorous | 0.09 (±0.03) | 0.06–0.13 |
Figure 1.
(a) Concentrations of mercury total (µg/g) in the muscle tissue of fish sampled (n = 88) compared among different trophic levels. Median (darker line in the box plot), minimum and maximum values (vertical lines), and outer lines of boxplot (25% and 75%). Dunn test: a > b > c, p < 0.05. Circles are outliers. (b) Linear regression between standard length (cm) of piscivorous fish (n = 29) and the concentration of mercury total (µg/g) in the muscle tissue of fish (y = −0.041 + 0.0151X; R2 = 0.36; F = 15.4; df = 27; p = 0.0005). One individual of Piranha-preta (Serrasalmus rhombeus) was excluded from the analysis to maintain normality and homogeneity of variances.
3.2. Weight Measurement, Family Composition and Fish Consumption
During the fieldwork, our team visited 35 domiciles: 20 in the Sawré Muybu village, 8 in the Poxo Muybu village, and 7 in the Sawré Aboy village. Among the participants, 53 were women of childbearing age (12 to 49 years old), 58 were adult men (≥12 years old), 24 were children aged 5 to 12 years old, and 42 were children aged 2 to 5 years. The interviews revealed that families are composed on average of 4 members (i.e., two adults and two children). According to the data collected in the fieldwork, the women of childbearing age had an average weight of 49.89 kg, adult men were 56.45 kg, children over 5 years old were 24.45 kg, and children under 5 years old were 14.07 kg (Table 2).
Table 2.
Data collected and estimates, Sawré Muybu Indigenous Land, Pará, Amazon, Brazil, 2019.
| Fish Catch | ||
| Total fish caught (n°) | 88 | |
| Catch period (days) | 7 | |
| Fish caught per day (n°) | 12.6 | |
| Average weight of fish (grams) | 268.2 | |
| Amount of fish per family (grams) | 3371.7 | |
| Family composition and weight measurements (Kg) | ||
| Average number of individuals per family | 4 | |
| Average number of adults | 2 | |
| Average number of children | 2 | |
| Average weight of childbearing age women (n = 53) | 49.89 | |
| Average weight of adult men (≥12 years) (n = 58) | 56.45 | |
| Average weight of children (from 5|−12 years old) (n = 24) | 24.45 | |
| Average weight of children (2|−5 years) (n = 42) | 14.07 | |
| Fish consumption estimative (grams) | ||
| Weekly Intake | Daily Intake | |
| Adult men (45%) | 1517.2 | 216.75 |
| Childbearing age women (35%) | 1180.1 | 168.58 |
| Children 5|−12 years old (15%) | 505.7 | 72.25 |
| Children aged 2|−5 years (5%) | 168.6 | 24.08 |
The questions about fish consumption revealed that 96% of the families consume fish regularly (≥3 times a week) and the most consumed species varied according to the season. During the rainy season, the most consumed species, in order of frequency related, are surubim (Pseudoplatystoma spp.), barbado (Pinirampus pirinampu), aracu (family Anostomidae), tucunaré (Cichla spp.), and caratinga (Geophagus spp.). The species most consumed in the dry season are caratinga, curimatá (Prochilodus nigricans), surubim, pacu (family Serrasalmidae), and barbado.
The average daily fish consumption estimates for the studied groups were the following: adult men consume 216.75 g (corresponding to 45% of the fish available for consumption), childbearing age women consume 168.58 g (35%), children over 5 years consume 72.25 g (15%), and children under 5 years consume 24.08 g (5%) (Table 2).
3.3. Health Risk Assessment
The analysis of the estimates reveals that the daily intake of methylmercury exceeds the reference limits recommended by the U.S. EPA [40] and FAO/WHO [41] in all scenarios built and in all studied population strata (Table 3). It means that in all hypothetical situations created in this study, the risk ratio estimates have values greater than 1.0. In summary, the Munduruku indigenous people living in the Middle-Tapajos River are at high risk of illness by the ingestion of mercury-contaminated fish. We can say that the less alarming risk ratio estimates (between 1.0 to 2.0) were observed in the population stratum represented by children aged 2 to 5 years, and by adults in general. Considering the safe dose proposed by FAO/WHO, risk ratio estimates under 2.0 were observed in children under 5 years old and adults in all hypothetical situations, except for the critical scenario. The risk ratio estimate for the critical scenario was 5.0 and 5.75 in children and adults, respectively (Table 3).
Table 3.
Estimated of methylmercury intake dose and risk ratio in different scenarios, Sawré Muybu Indigenous Land, Pará, Amazon, Brazil, 2019.
| Scenarios Constructed | Hg-intake Dose µg/kg bw/day |
Risk Ratio | ||
|---|---|---|---|---|
| U.S. EPA | FAO/WHO (Women and Children) |
FAO/WHO (Adults in General) |
||
| Scenario 1—Rainy Season | ||||
| Women of childbearing age | 0.78 | 7.84 | 3.41 | N.A. |
| Adult Men | 0.89 | 8.91 | N.A. | 1.98 |
| Children 5|−12 years old | 0.69 | 6.86 | 2.98 | N.A. |
| Children 2|−5 years | 0.40 | 3.97 | 1.73 | N.A. |
| Scenario 2—Dry Season | ||||
| Women of childbearing age | 0.59 | 5.95 | 2.59 | N.A. |
| Adult Men | 0.68 | 6.76 | N.A. | 1.50 |
| Children 5|−12 years old | 0.52 | 5.20 | 2.26 | N.A. |
| Children 2|−5 years | 0.30 | 3.01 | 1.31 | N.A. |
| Scenario 3—Current | ||||
| Women of childbearing age | 0.73 | 7.29 | 3.17 | N.A. |
| Adult Men | 0.83 | 8.28 | N.A. | 1.84 |
| Children 5|−12 years old | 0.64 | 6.37 | 2.77 | N.A. |
| Children 2|−5 years | 0.37 | 3.69 | 1.60 | N.A. |
| Scenario 4—Critical | ||||
| Women of childbearing age | 2.28 | 22.76 | 9.90 | N.A. |
| Adult Men | 2.59 | 25.86 | N.A. | 5.75 |
| Children 5|−12 years old | 1.99 | 19.90 | 8.65 | N.A. |
| Children 2|−5 years | 1.15 | 11.53 | 5.01 | N.A. |
U.S. EPA = United States Environmental Protection Agency; FAO = Food and Agriculture Organization; WHO = World Health Organization.
In our opinion, the current scenario is the closest to the reality of fish consumption by the Munduruku indigenous villages, and it indicates that all population segments ingest mercury in quantities above what is considered acceptable or safe. According to the U.S. EPA, women of childbearing age, who represent the most vulnerable demographic group to the effects of methylmercury, ingest 7 times more mercury than the reference dose proposed by this agency. According to FAO/WHO, the ingestion is 3 times higher than the safe intake. The critical scenario represents the levels of exposure observed in approximately 5% of the Munduruku population. In this case, women of childbearing age ingest 10 times more mercury than the limit proposed by the FAO/WHO, and 23 times more mercury than the safe limit proposed by the U.S. EPA. The analysis of the mercury levels in hair of the Munduruku population revealed that, in fact, there are individuals which presented mercury levels above 20 µg/g (more details in Basta et al. [34]).
4. Discussion
Fish are not only an essential source of protein, but many species are also rich in polyunsaturated fatty acids that reduce cholesterol levels in the blood, reduce the risk of myocardial infarction, and promote cognitive development [42,43]. Some authors point out that the annual average consumption of fish is 23 kg per capita in the Brazilian Amazon [44]. Frequently, the fish intake of riverside communities exceeds 300 g per day, resulting in annual average consumption that could surpass 100 kg per capita [10,45,46].
Despite the undeniable nutritional potential of fish, contaminants such as methylmercury has provoked significant debate about the balance between risks and benefits associated with fish consumption. Hu et al. [47] in a meta-analysis suggest that hair mercury concentration of 2–3 µg/g might be considered as a threshold for risk of developing hypertension. Fillion et al. [30] investigated riverine communities in the Amazon and showed an odds ratio equal to 2.91 (CI 95% 1.26–7.28) for elevated systolic blood pressure among individuals with hair Hg levels above 10 µg/g. In addition to these studies, Salonen et al. [29], in a longitudinal study with Finnish men, concluded that hair mercury levels above 2.0 µg/g represent a risk 69% higher for an acute myocardial infarction. Besides that, the neurotoxic effects of methylmercury have been known for a long time, since the Minamata tragedy in the 1950s and 1960s. The cohort studies conducted in the Faroe Islands and New Zealand indicate that even in low doses, the consumption of mercury-contaminated fish during pregnancy can cause important cognitive alterations in children [48,49]. The mercury neurotoxic potential effects in children and adults of the Amazon have been reported in recent publications [50,51,52,53,54]. The most common effects in children are cognitive problems, neurodevelopmental impairment, and psychomotor disorders. In adults, decreased visual field, neurobehavioral, and motor coordination disorders are most frequently reported [27].
Given the current federal government’s effort to create strategies to facilitate the invasion of protected areas in the Amazon by garimpeiros and mining industries, it is essential to clarify that the contamination of fish by mercury and all related health damages are caused (or intensified) by exploitation of gold. Many studies have already shown mercury contamination in the fauna of the Tapajós River Basin at least two decades ago [55,56,57,58,59,60,61,62,63,64,65]. Dórea et al. [59] detected mean mercury levels in piscivorous fish of 0.578 µg/g and 0.052 µg/g Hg in non-piscivorous in the upper Tapajós basin, whilst Brabo et al. [56] investigated fish contamination in the Sai Cinza region, also inhabited by indigenous Munduruku. They observed that the piscivorous species had mean mercury levels of 0.293 µg/g, while the non-piscivorous species had average mercury levels equal to 0.112 µg/g. These studies corroborate the present results that the mean mercury levels detected in piscivorous species are about 4 times greater than non-piscivorous species, highlighting the methylmercury biomagnification. Indeed, piscivorous fish were the only trophic level where we found a positive relationship between fish size and total Hg concentration, indicating bioaccumulation. Therefore, the larger the size of piscivorous fish, the higher the mercury concentration in their tissues and thus the higher the health risk for people who eat the larger ones.
In Brazil, the National Health Surveillance Agency (ANVISA) establishes the maximum concentrations of mercury in fish tissues that are judged appropriate for commercialization. Resolution No. 42 establishes that the maximum limit for inorganic mercury in fish is 0.5 µg/g for non-predatory species and 1.0 µg/g for predatory species [66]. Reflection on the applicability of these concentration limits promotes questions. The first question is, why propose limits for inorganic-mercury species, when almost all mercury present in the fish muscle is methylmercury, an organic mercury form? The second question is, how effective is the use of mercury concentration limits in fish in protecting the health of the population that consumes this fish?
We believe that the National Health Surveillance Agency (ANVISA) resolution does not promote any regulatory or normative effects, since the limits proposed by this agency are basically a wrong adaptation of the limits recommended by FAO/WHO [67] (0.5 µg MeHg/g for non-piscivorous fish and 1.0 µg MeHg/g for piscivorous fish). Furthermore, it is vital to clarify that these limits established by the FAO/WHO were adopted in 1991 and do not consider health effects produced by the ingestion of methylmercury in the fish [67]. The calculations for defining these maximum limits were performed based on data of the average mercury levels in fish samples of different trophic levels. Unfortunately, these values are frequently cited as safe levels for consumption.
The people’s diet is an extremely important cultural characteristic, as well as language and spiritualistic rituals. In indigenous communities, the inclusion of fish and other aquatic organisms as a diet items and the consumption frequency vary considerably among the groups living in the Amazon. The consumption of these items depends not only on the availability in the environment but also on the individual’s preferences as well as cultural patterns. For example, the Yanomami people who live in the Auaris region in the extreme northwest of Roraima state, as well as the Yanomami living in Venezuela, rarely eat fish [68,69]. On the other hand, fish consumption among Munduruku indigenous people can be considered high (at least three meals per day), varying slightly between different groups [56,58,59]. Studies that focus on the characterization of indigenous people’s diet face numerous difficulties, ranging from cultural and linguistic barriers to the choice of an effective method for quantifying the consumption of certain foods. Memory-based methods (recall method) about what was consumed by the family in the past 24 h or in the past few days generally produce over or underestimated data that rarely translate into reality and cause errors in estimates. Taking into consideration all the aspects exposed previously, it is extremely hard to measure during a study’s fieldwork the quantity in grams of fish consumed by each person in a day and the number of daily meals that include fish (and other aquatic organisms such as fish, crabs, mollusks, shrimp, turtles, etc.). From this point of view, the present study proposed a methodology for estimating potential fish consumption based on the catching of fish by indigenous themselves and then recorded by field researchers. The time devoted to fishing and the catching strategies were defined based on the reports of Munduruku fishermen.
With this challenge to estimate the food intake in culturally differentiated communities in mind, the goal of this study was to simulate an ordinary fishing day for the head of a Munduruku household. Thus, the mean amount of fish caught on a typical fishing day represents the amount of fish that a Munduruku family consumes over a week. Since there is no electricity in the homes visited, neither a refrigerator nor any other way of preserving food, the “moquém” technique (which is a type of smoking) is used to conserve fish.
To assess the accuracy of the empirical methodology accomplished in this investigation for estimating fish consumption, the daily mercury intake doses calculated for the current scenario were compared to the mercury levels detected in hair samples of the Munduruku indigenous people in the studied communities (data available in Basta et al. [34]). The current scenario was built to represent the mercury exposure scenario that most closely matches the local reality. The estimated mercury daily intake in this scenario for adult men and women was 0.828 and 0.729 µg/bw kg, respectively. The mean mercury level in male hair was 8.83 µg/g (SD: 4.56) and in women of childbearing age, the mean hair mercury level was 7.71 µg/g (SD: 3.88). If we consider that the intake of 0.1 µg/bw kg/day corresponds to hair mercury levels of 1.0 µg/g [39], the calculation of the amount of mercury intake daily is well-matched with the mercurial concentration detected in hair samples. Children from 5 to 12 years old also had a mercury daily intake dose matching with the levels of mercury in hair. The intake dose was estimated at 0.64 µg/bw kg/day and the average level of mercury in hair was 7.62 µg/g (SD: 5.44). Only the population stratum constituted by children aged 2 to 5 years did not indicate compatibility between intake and concentration in hair. In this case, the daily intake was estimated at 0.37 µg/bw kg/day and the average concentration in hair was 6.68 µg/g (SD: 3.44). Most likely, the difference found may be due to the influence of other routes of exposure, besides to fish consumption, such as breastfeeding and remnants of intrauterine transfer. However, there is a possibility that fish consumption was underestimated by our team for this age group.
The risk ratio estimated in this study indicates that there is no safe consumption of fish by the Munduruku population in any of the scenarios created for counterfactual analysis. Comparing the mercury intake doses in the different scenarios, we observed that mercury ingestion during the rainy season is higher than in the dry season. This result reflects the mercury levels detected in the most consumed fish species in this season of the year, according to reports by the study participants. In the rainy season, according to the interview reports, 60% of fish most consumed by the Munduruku families are piscivorous and therefore have higher levels of mercury.
According to the safety parameters proposed by FAO/WHO [41] and U.S. EPA [40], the entire study population is at risk of becoming ill due to the consumption of methylmercury contaminated fish. It is important to remember that the safe intake doses proposed by these international agencies were calculated from data produced in longitudinal studies. The dose of FAO/WHO [41] (PTWI: 1.6 µg MeHg/Kg bw/week) was derived from data produced in Seychelles, Faroe Islands, and New Zealand cohort studies. The dose recommended by U.S. EPA [40] was based only on the findings of the Faroe Islands cohort. However, the longitudinal studies mentioned above considered populations that differ strongly from the Amazonian populations and, probably, present mercury exposure thresholds for toxic health outcomes quite different from the indigenous communities.
The native people of the Brazilian Amazon are neglected by the State, which often becomes evident from the difficulty in accessing health services, the lack of sewage sanitation, and the high prevalence of many infectious diseases and child stunting. Besides this, the Amazonian ecosystem sees several risk factors for human exposure to mercury alongside each other which combine to create a uniquely dangerous situation. These factors include the presence of natural mercury in the soil as well as the development of activities that significantly change the mercury biogeochemical cycle in the region (e.g., artisanal gold mining, industrial gold mining, construction of dams and hydroelectric plants and agribusiness, which promotes forest burning and deforestation). This becomes evident that the development of a longitudinal study involving different population groups in the Amazon, such as indigenous, riverine, and urban populations, is especially important. Only after a long-term study will it be possible to estimate safe doses of mercury intake for the Amazonian population.
5. Conclusions
The current gold mining activity in the Middle-Tapajós Region is causing environmental devastation, social conflict, and increasing mercury levels in the environment. This activity causes mercury accumulation in fish, especially in piscivorous. Consequently, the population living in this region consumes contaminated fish and compromises their health. The present study revealed that the fish collected in the rivers that cross the Sawré Muybu Indigenous Land have mercury concentrations with the potential to harm the health of the Munduruku population, particularly women of childbearing age and children. In all of the scenarios created for counterfactual analysis, the estimated risk ratios are greater than 1.0, indicating that the intake of mercury by the groups studied is higher than the limits proposed by health agencies. However, we highlight that fish is an important element of Munduruku culture and is an essential animal protein for riverside and indigenous populations across the Amazon. The mercury contamination observed in fish and the indigenous Munduruku is a direct consequence of gold mining and the Brazilian authorities’ longstanding refusal to condemn this activity, threatening the health and rights of the native peoples. Therefore, we refute the current policies of the Brazilian federal government regarding the permission of mining in Indigenous Land. In conclusion, we recommend the immediate closure of illegal gold mining in the Brazilian Amazon, the principal cause of mercury contamination in the region.
Acknowledgments
Through chiefs Juarez Saw, Jairo Saw, and Valdemar Poxo, and the leader Alessandra Korap, we thank the Munduruku people for the trust placed in our team and the support in carrying out the research. On behalf of Cleidiane Carvalho Ribeiro dos Santos we thank the team from the Rio Tapajós Indigenous Special Sanitary District, who spared no efforts to support us in all stages of the fieldwork. In particular, we thank nurses Alan Marcelo Simon and Lygia Catarina de Oliveira. We thank João Paulo Goes Pereira for mercury analysis in the fish samples. We also thank Marcelo Oliveira-Costa from the WWF-Brasil for financial and technical support.
Author Contributions
Proposal design, study design, and methodology, A.C.S.V., G.H. and P.C.B.; Fieldwork data collection, A.C.S.V., J.G.B., A.N.S.A. and P.C.B.; Laboratory analysis, J.G.B., A.N.S.A., H.N.M.M., G.H., I.M.J. and M.O.L.; Writing—original draft preparation, A.C.S.V., G.H. and P.C.B.; Writing—review and editing, A.C.S.V., G.H., P.C.B. and S.S.H.; Supervision, obtaining resources, and project management, P.C.B. and S.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the National School of Public Health at Oswaldo Cruz Foundation (REC/ENSP) and the Brazilian National Research Ethics Commission of the National Health Council (CONEP/CNS), CAAE: 65671517.1.0000.5240, with Opinion No. 2.262.686 favorable to its performance. In compliance with Convention No. 169 of the International Labor Organization (ILO), the study began with a pre-study consultation, carried out in August 2019, during a visit to the villages, in which two authors (S.S.H. and P.C.B.) and local indigenous leaders participated. At the time, the study objectives were presented and discussed (https://www.youtube.com/watch?v=oFEYEGxNmns&t=704s accessed on 3 March 2021). After answering questions and obtaining approval for the proposal by the communities, we received support from the coordination of the Special Indigenous Sanitary District of the Tapajós River through the multidisciplinary indigenous health team to carry out the study. In addition, the interviews and data collection started only after the participants had their questions answered and given formal consent in the Informed Consent Form (ICF) by the children’s guardians.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO . The State of World Fisheries and Aquaculture. FAO; Rome, Italy: 2020. [Google Scholar]
- 2.Kawarazuka N., Béné C. Linking small-scale fisheries and aquaculture to household nutritional security: A review of the literature. Food Secur. 2010;2:343–357. doi: 10.1007/s12571-010-0079-y. [DOI] [Google Scholar]
- 3.Hicks C.C., Cohen P.J., Graham N.A.J., Nash K.L., Allison E.H., D’Lima C., Mills D.J., Roscher M., Thilsted S.H., Thorne-Lyman A.L., et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature. 2019;566:378–382. doi: 10.1038/s41586-019-1592-6. [DOI] [PubMed] [Google Scholar]
- 4.Funge-Smith S., Bennett A.A. Fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019;20:1176–1195. doi: 10.1111/faf.12403. [DOI] [Google Scholar]
- 5.Fluet-Chouinard E., Funge-Smith S., McIntyre P.B. Global hidden harvest of freshwater fish revealed by household surveys. Proc. Natl. Acad. Sci. USA. 2018;115:7623–7628. doi: 10.1073/pnas.1721097115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Béné C., Steel E., Kambala L.B., Gordon A. Fish as the “bank in the water”: Evidence from chronic-poor communities in Congo. Food Policy. 2009;34:108–118. doi: 10.1016/j.foodpol.2008.07.001. [DOI] [Google Scholar]
- 7.McIntyre P.B., Liermann C.A.R., Revenga C. Linking freshwater fishery management to global good security and biodiversity conservation. Proc. Natl. Acad. Sci. USA. 2016;113:12880–12885. doi: 10.1073/pnas.1521540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welcomme R.L. River Fisheries. FAO; Rome, Italy: 1985. [Google Scholar]
- 9.Jézéquel C., Tedesco P.A., Bigorne R., Maldonado-Ocampo J.A., Ortega H., Hidalgo M., Martens K., Torrente-Vilara G., Zuanon J., Acosta A., et al. A Database of freshwater fish species of the Amazon basin. Sci. Data. 2020;7:96. doi: 10.1038/s41597-020-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaac V.J., Almeida M.C. El Consumo de Pescado en la Amazonía Brasileña. FAO; Rome, Italy: 2011. [Google Scholar]
- 11.Isaac V.J., Almeida M.C., Giarrizzo T., Deus C.P., Vale R., Klein G., Begossi A. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An. Acad. Bras. Ciências. 2015;87:2229–2242. doi: 10.1590/0001-3765201520140250. [DOI] [PubMed] [Google Scholar]
- 12.Begossi A., Salivonchyk S., Hallwass G., Hanazaki N., Lopes P., Silvano R.A.M., Dumaresq D., Pittock J. Fish consumption on the Amazon: A review of biodiversity, hydropower and food security issues. Braz. J. Biol. 2019;79:345–357. doi: 10.1590/1519-6984.186572. [DOI] [PubMed] [Google Scholar]
- 13.Prestes-Carneiro G., Béarez P., Bailon S., Py-Daniel A.R., Neves E.G. Subsistence fishery at Hatahara (750-1230 CE), a pre-Columbian central Amazonian village. J. Archaeol. Sci. Rep. 2015;8:454–462. doi: 10.1016/j.jasrep.2015.10.033. [DOI] [Google Scholar]
- 14.Prestes-Carneiro G., Béarez P., Shock M.P., Prümers H., Betancourt C.J. Pre-Hispanic fishing practices in interfluvial Amazonia: Zooarchaeological evidence from managed landscapes on the Llanos de Mojos savanna. PLoS ONE. 2019;14:e0214638. doi: 10.1371/journal.pone.0214638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearnside P.M. Social impacts of Brazil’s Tucuruí Dam. Environ. Manag. 1999;24:483–495. doi: 10.1007/s002679900248. [DOI] [PubMed] [Google Scholar]
- 16.Nevado J.B., Martín-Doimeadios R.R., Bernardo F.G., Moreno M.J., Herculano A.M., Do Nascimento J.L.M., Crespo-López M.E. Mercury in the Tapajós River basin, Brazilian Amazon: A review. Environ. Int. 2010;36:593–608. doi: 10.1016/j.envint.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Hallwass G., Lopes P.F., Juras A.A., Silvano R.A.M. Fishers’ knowledge identifies environmental changes and fish abundance trends in impounded tropical rivers. Ecol. Appl. 2013;23:392–407. doi: 10.1890/12-0429.1. [DOI] [PubMed] [Google Scholar]
- 18.Arrifano G.P., Martín-Doimeadios R.C.R., Jiménez-Moreno M., Ramírez-Mateos V., Da Silva N.F., Souza-Monteiro J.R., Augusto-Oliveira M., Paraense R.S.O., Macchid B.M., Nascimento J.L.M., et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018;147:299–305. doi: 10.1016/j.ecoenv.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 19.RAISG. [(accessed on 3 March 2021)];2018 Available online: https://www.amazoniasocioambiental.org/pt-br/radar/mapa-inedito-indica-epidemia-de-garimpo-ilegal-na-panamazonia/
- 20.Silva-Junior C.H.L., Pessôa A.C.M., Carvalho N.S., Reis J.B.C., Anderson L.O., Aragão L.E.O.C. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat. Ecol. Evol. 2021;5:144–145. doi: 10.1038/s41559-020-01368-x. [DOI] [PubMed] [Google Scholar]
- 21.Baird I.G., Silvano R.A.M., Parlee B., Poesch M., Maclean B., Napoleon A., Lepine M., Hallwass G. The downstream impacts of hydropower dams and indigenous and local knowledge: Examples from the Peace–Athabasca, Mekong, and Amazon. Environ. Manag. 2021;67:682–696. doi: 10.1007/s00267-020-01418-x. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante L., Andrade M.B.T., Leite L., Silva-Junior C.A., Lima M., Coelho-Junior M.G., Da Silva Neto E.C., Campolina D., Carolino K., Diele-Viegas L.M., et al. Brazils Highway BR-319: The road to the collapse of the Amazon and the violation of indigenous rights. J. Geogr. Soc. Berl. 2021;152:65–70. doi: 10.12854/erde-2021-552. [DOI] [Google Scholar]
- 23.Malm O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ. Res. 1998;77:73–78. doi: 10.1006/enrs.1998.3828. [DOI] [PubMed] [Google Scholar]
- 24.Kalamandeen M., Gloor E., Johnson I., Agard S., Katow M., Vanbrooke A., Ashley D., Batterman S.A., Ziv G., Holder-Collins K., et al. Limited biomass recovery from gold mining in Amazonian forests. J. Appl. Ecol. 2020;57:1730–1740. doi: 10.1111/1365-2664.13669. [DOI] [Google Scholar]
- 25.Ferrante L., Fearnside P.M. Brazil’s new president and “ruralists” threaten Amazonia’s environment, traditional peoples and the global climate. Environ. Conserv. 2019;46:261–263. doi: 10.1017/S0376892919000213. [DOI] [Google Scholar]
- 26.Siqueira-Gay J., Soares-Filho B., Sanchez L.E., Oviedo A., Sonter L.J. Proposed legislation to mine Brazil’s indigenous lands will threaten Amazon forests and their valuable ecosystem services. One Earth. 2020;3:356–362. doi: 10.1016/j.oneear.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crespo-Lopez M.E., Augusto-Oliveira M., Lopes-Araújo A., Santos-Sacramento L., Yuki Takeda P., Macchi B.M., Do Nascimento J.L.M., Maia C.S.F., Lima R.R., Arrifano G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021;146:106223. doi: 10.1016/j.envint.2020.106223. [DOI] [PubMed] [Google Scholar]
- 28.Lacerda E.M.D.C.B., Souza G.D.S., Cortes M.I.T., Rodrigues A.R., Pinheiro M.C.N., Silveira L.C.D.L., Ventura D.F. Comparison of visual functions of two Amazonian populations: Possible consequences of different mercury exposure. Front. Neurosci. 2020;13:1428. doi: 10.3389/fnins.2019.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salonen J.T., Seppänen K., Nyyssönen K., Korpela H., Kauhanen J., Kantola M., Tuomilehto J., Esterbauer H., Tatzber F., Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.CIR.91.3.645. [DOI] [PubMed] [Google Scholar]
- 30.Fillion M., Mergler D., Passos C.J.S., Larribe F., Lemire M., Guimarães J.R.D. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ. Health. 2006;5:1–9. doi: 10.1186/1476-069X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasconcellos A.C.S., Barrocas P.R.G., Ruiz C.M.V., Mourão D.D.S., Hacon S.D.S. Burden of Mild Mental Retardation attributed to prenatal methylmercury exposure in Amazon: Local and regional estimates. Cienc. Saude Coletiva. 2018;23:3535–3545. doi: 10.1590/1413-812320182311.15812016. [DOI] [PubMed] [Google Scholar]
- 32.Poulin J., Gibb H., Prüss-Üstün A., World Health Organization . Mercury: Assessing the Environmental Burden of Disease at National and Local Levels. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 33.Bose-O’Reilly S., McCarty K.M., Steckling N., Lettmeier B. Mercury exposure and children’s health. Curr. Probl. Pediatric Adolesc. Health Care. 2010;40:186–215. doi: 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basta P.C., Viana P.V.S., Vasconcellos A., Périssé A., Hofer C., Paiva N.S., Kempton J.W., Ciampi de Andrade D., Oliveira R.A.A., Achatz R.W., et al. Mercury exposure in Munduruku indigenous communities from Brazilian Amazon: Methodological background and an overview of the principal results. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18179222. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos G.M., De Mérona B., Juras A.A., Jégu M. Peixes do Baixo Rio Tocantins: 20 Anos Depois da Usina Hidrelétrica Tucuruí. Eletronorte; Brasília, Brasil: 2004. p. 216. [Google Scholar]
- 36.Santos G.M., Ferreira E.J.G., Zuanon J.A.S. Peixes Comerciais de Manaus. Ibama/PróVárzea; Manaus, Brazil: 2006. [Google Scholar]
- 37.Akagi H., Suzuki T., Arimura K., Ando T., Sakamoto M., Satoh H., Matsuyama A. Mercury Analysis Manual. 105th ed. Ministry of the Environmental; Tokyo, Japan: 2004. [(accessed on 21 November 2019)]. Available online: http://nimd.env.go.jp/kenkyu/docs/march_mercury_analysis_manual(e).pdf. [Google Scholar]
- 38.da Silva S.F., de Oliveira Lima M. Mercury in fish marketed in the Amazon triple frontier and health risk assessment. Chemosphere. 2000;248:125989. doi: 10.1016/j.chemosphere.2020.125989. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization . Guidance for Identifying Populations at Risk from Mercury Exposure. Mercury Publications; Geneva, Switzerland: 2008. [Google Scholar]
- 40.U.S.-EPA . Reference Dose for Methylmercury. U.S. Environmental Protection Agency; Washington, DC, USA: 2000. [Google Scholar]
- 41.FAO/WHO Evaluation of certain food additives and contaminants: Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives; Proceedings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Rome, Italy. 10–19 June 2003. [Google Scholar]
- 42.Domingo J.L. Omega-3 fatty acids and the benefits of fish consumption: Is all that glitters gold? Environ. Int. 2007;33:993–998. doi: 10.1016/j.envint.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Sidhu K.S. Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 2003;38:336–344. doi: 10.1016/j.yrtph.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Arruda M.C.F. Avaliação Dos Indicadores da Política de Pesca do Programa Zona Franca Verde: Perspectivas Econômicas e Ambientais. Universidade Federal do Amazonas; Manaus, Brasil: 2017. [Google Scholar]
- 45.Cerdeira R.G.P., Ruffino M.I., Isaac V.J. Consumo de pescado e outros alimentos pela população ribeirinha do lago grande de Monte Alegre, PA-Brasil. Acta Amaz. 1997;27:213–227. doi: 10.1590/1809-43921997273228. [DOI] [Google Scholar]
- 46.Batista V.D., Isaac V.J., Viana J.P. Exploração e Manejo Dos Recursos Pesqueiros da Amazônia. A Pesca e os Recursos Pesqueiros na Amazônia Brasileira. Ibama/ProVárzea; Manaus, Brasil: 2004. pp. 63–151. [Google Scholar]
- 47.Hu X.F., Singh K., Chan H.M. Mercury exposure, blood pressure, and hypertension: A systematic review and dose–response meta-analysis. Environ. Health Perspect. 2018;126:076002. doi: 10.1289/EHP2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weihe P., Grandjean P., Jørgensen P.J. Application of hair-mercury analysis to determine the impact of a seafood advisory. Environ. Res. 2005;97:201–208. doi: 10.1016/j.envres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Crump K.S., Kjellström T., Shipp A.M., Silvers A., Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/B:RIAN.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- 50.Santos Serrão de Castro N., de Oliveira Lima M. Hair as a biomarker of long-term mercury exposure in Brazilian Amazon: A systematic review. Int. J. Environ. Res. Public Health. 2018;15:500. doi: 10.3390/ijerph15030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reuben A., Frischtak H., Berky A., Ortiz E.J., Morales A.M., Hsu-Kim H., Pendergast L.L., Pan W.K. Elevated hair mercury levels are associated with neurodevelopmental deficits in children living near artisanal and small-scale gold mining in Peru. Geo. Health. 2020;4:e2019GH000222. doi: 10.1029/2019GH000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costa Junior J.M.F., Lima A.A.D.S., Rodrigues Junior D., Khoury E.D.T., Souza G.D.S., Silveira L.C.D.L., Pinheiro M.D.C.N. Emotional and motor symptoms in riverside dwellers exposed to mercury in the Amazon. Rev. Bras. De Epidemiol. 2017;20:212–224. doi: 10.1590/1980-5497201700020003. [DOI] [PubMed] [Google Scholar]
- 53.Marques R.C., Bernardi J.V., Abreu L., Dórea J.G. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch. Environ. Contam. Toxicol. 2015;68:432–441. doi: 10.1007/s00244-014-0103-x. [DOI] [PubMed] [Google Scholar]
- 54.Marques R.C., Bernardi J.V., Cunha M.P., Dórea J.G. Impact of organic mercury exposure and home delivery on neurodevelopment of Amazonian children. Int. J. Hygen Environ. Health. 2016;219:498–502. doi: 10.1016/j.ijheh.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Malm O., Castro M.B., Bastos W.R., Branches F.J., Guimarães J.R., Zuffo C.E., Pfeiffer W.C. An assessment of Hg pollution in different goldmining areas, Amazon Brazil. Sci. Total Environ. 1995;175:127–140. doi: 10.1016/0048-9697(95)04909-6. [DOI] [Google Scholar]
- 56.Brabo E.D.S., Santos E.D.O., Jesus I.M.D., Mascarenhas A.F., Faial K.F. Mercury levels in fish consumed by the Sai Cinza indigenous community, Munduruku Reservation, Jacareacanga County, State of Para, Brazil. Cad. Saúde Pública. 1999;15:325–332. doi: 10.1590/S0102-311X1999000200017. [DOI] [PubMed] [Google Scholar]
- 57.Roulet M., Lucotte M., Canuel R., Farella N., Courcelles M., Guimaraes J.R., Amorim M. Increase in mercury contamination recorded in lacustrine sediments following deforestation in the central Amazon. Chem. Geol. 2000;165:243–266. doi: 10.1016/S0009-2541(99)00172-2. [DOI] [Google Scholar]
- 58.de Oliveira Santos E.C., de Jesus I.M., da Silva Brabo E., Loureiro E.C.B., da Silva Mascarenhas A.F., Weirich J., Cleary D. Mercury exposures in riverside Amazon communities in Para, Brazil. Environ. Res. 2000;84:100–107. doi: 10.1006/enrs.2000.4088. [DOI] [PubMed] [Google Scholar]
- 59.Dórea J.G., Barbosa A.C., Ferrari Í., De Souza J.R. Fish consumption (Hair Mercury) and nutritional status of Amazonian Amer-Indian Children. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2005;17:507–514. doi: 10.1002/ajhb.20410. [DOI] [PubMed] [Google Scholar]
- 60.Telmer K., Costa M., Angélica R.S., Araujo E.S., Maurice Y. The source and fate of sediment and mercury in the Tapajós River, Pará, Brazilian Amazon: Ground-and space-based evidence. J. Environ. Manag. 2006;81:101–113. doi: 10.1016/j.jenvman.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 61.Hacon S., Barrocas P.R., Vasconcellos A.C.S.D., Barcellos C., Wasserman J.C., Campos R.C., Azevedo-Carloni F.B. An overview of mercury contamination research in the Amazon basin with an emphasis on Brazil. Cad. Saúde Pública. 2008;24:1479–1492. doi: 10.1590/S0102-311X2008000700003. [DOI] [PubMed] [Google Scholar]
- 62.Passos C.J.S., Da Silva D.S., Lemire M., Fillion M., Guimaraes J.R.D., Lucotte M., Mergler D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008;18:76–87. doi: 10.1038/sj.jes.7500599. [DOI] [PubMed] [Google Scholar]
- 63.Faial K., Deus R., Deus S., Neves R., Jesus I., Santos E., Brasil D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015;30:66–76. doi: 10.1016/j.jtemb.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Freitas J.S., Lacerda E., Maria C., Rodrigues D., Jr., Corvelo T.C.O., Silveira L.C.L., Souza G.S. Mercury exposure of children living in Amazonian villages: Influence of geographical location where they lived during prenatal and postnatal development. An. Acad. Bras. Ciências. 2019;91 doi: 10.1590/0001-3765201920180097. [DOI] [PubMed] [Google Scholar]
- 65.Lino A.S., Kasper D., Guida Y.S., Thomaz J.R., Malm O. Total and methyl mercury distribution in water, sediment, plankton, and fish along the Tapajós River basin in the Brazilian Amazon. Chemosphere. 2019;235:690–700. doi: 10.1016/j.chemosphere.2019.06.212. [DOI] [PubMed] [Google Scholar]
- 66.Brasil. Resolução RDC nº42. Dispõe Sobre o Regulamento Técnico MERCOSUL sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos. Diário Oficial da União da República Federativa do Brasil; Brasília, Brasil: 2013. [Google Scholar]
- 67.FAO/WHO Joint FAO/WHO Expert Committee on Food Additives (JECFA), Report of the Tenth Section, Rotterdam, The Netherlands 4 to 8 April 2016. [(accessed on 22 June 2020)]; Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-10%252FReport%252FREP16_CFe.pdf.
- 68.Holmes R. Non-dietary modifiers of nutritional status in tropical forest populations of Venezuela. Interciencia. 1984;9:386–390. [Google Scholar]
- 69.Dufour D.L. Diet and nutritional status of Ameridians: A review of the literature. Cad. Saúde Pública. 1991;7:481–502. doi: 10.1590/S0102-311X1991000400003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.