Abstract
Background:
Urgency and frequency are common lower urinary tract symptoms (UF-LUTS) in women. There is limited evidence to guide physical therapist-led treatment.
Objectives:
To compare hip and pelvic floor muscle strength between women with and without UF-LUTS. We hypothesized women with UF-LUTS would demonstrate 1) diminished hip external rotator and abductor strength and 2) equivalent pelvic floor strength and diminished endurance compared to controls.
Study Design:
A matched case-control study
Methods:
Women with UF-LUTS (cases) and controls were matched on age, body mass index (BMI), vaginal parity. Examiner measured participants’ 1) hip external rotator and abductor strength via dynamometry (maximum voluntary effort against fixed resistance) and 2) pelvic floor muscle strength (peak squeeze pressure) and endurance (squeeze pressure over a 10 second hold) via vaginal manometry. Values compared between cases and controls with paired-sample t-tests (hip) or Wilcoxon signed rank tests (pelvic floor).
Results:
21 pairs (42 women): Hip external rotation (67.0 ± 19.0 N vs 83.6 ± 21.5 N; P=0.005) and hip abduction strength (163.1 ± 48.1 N vs 190.1 ± 53.1 N; P=0.04) were significantly lower in cases than controls. There was no significant difference in pelvic floor strength (36.8 ± 19.9 cmH20 vs 41.8 ± 21.0 cmH20; P=0.40) or endurance (234.0 ± 149.6 cmH20*seconds vs 273.4 ± 149.1 cmH20*seconds; P=0.24).
Conclusion:
Women with UF-LUTS had weaker hip external rotator and abductor muscles, but similar pelvic floor strength and endurance compared to controls. Hip strength may be important to assess in patients with UF-LUTS, further research is needed.
Keywords: urinary urgency, urinary frequency, overactive bladder, musculoskeletal system, case-control studies
INTRODUCTION
Urgency and frequency are common lower urinary tract symptoms (LUTS) in women1 and substantially interfere with daily activities.2,3 Medical management begins with ruling out organ pathologies, typically followed by fluid intake and timed voiding often combined with pharmacologic management, and may progress to nerve stimulation, botulinum injections, or surgery where indicated.4,5 Despite abundant management options for urgency and frequency predominant LUTS (UF-LUTS) and high financial burden,6 patients often experience only partial relief from medical treatments for LUTS.7–9 To develop better treatment options for patients with UF-LUTS, we must first understand factors that may be associated with UF-LUTS.
Pelvic floor muscle (PFM) training is a common nonpharmacological management strategy with strong evidence to support its use in those with stress incontinence.10 One theory behind using PFM training in women with UF-LUTS is that PFM contraction inhibits detrusor contraction through the “voluntary urinary inhibition reflex.”11 However, researchers who have studied PFM training typically use reduction of urinary incontinence as the primary outcome and rarely report outcomes associated with urgency and frequency.12 Therefore, women with UF-LUTS but without incontinence may not benefit from PFM training in the same way. Another assumption behind using PFM training as a treatment is that PFM strength and/or endurance are impaired in patients with UF-LUTS, however this assumption has not been investigated. Further, a small treatment trial demonstrated that 43% of patient with UF-LUTS did not have resolution of urgency after 12 weeks of standard PFM training.8 Many have suggested that PFM training may not be effective for those with UF-LUTS because their PFMs are overactive rather than weak.13–16 We theorized that increased tonic activity of the PFMs, as their system attempts to inhibit the detrusor muscle or prevent urine loss throughout the day, may lead poorer endurance induced by muscle fatigue. To test the assumption that PFM training is beneficial for women with UF-LUTS, we must first understand whether UF-LUTS is associated with PFM weakness or poor endurance.
One factor often not considered in treatment of UF-LUTS is hip muscle strength. A small but growing number of studies suggests that hip muscle performance may be pertinent to PFMs and pelvic floor disorders, including LUTS.17–20 The obturator internus, a deep external rotator of the hip, attaches to the PFMs via the arcus tendineus fasciae pelvis (arcus tendineus). The connection between the obturator internus and the arcus tendinous is important because the PFMs have very small physiological cross-sectional area and have been theorized to require sufficient tension in the arcus tendinous and obturator internus muscle to produce forces associated with normal use.21 Because the hip abductor muscles work in concert with the hip external rotator muscles to stabilize the hip in the transverse plane,22 the hip abductor muscles were also of interest. Habitual hip adduction during activities of daily living has been observed among women with urgency,17 and may be associated with hip abductor muscle (particularly gluteus medius) weakness.23 Significant weakness in hip external rotators19 and abductors19,20 has been found among patients with stress urinary incontinence compared to asymptomatic participants; however, the relationship between hip muscle performance and UF-LUTS has not been studied.
The objective of this study was to compare hip and pelvic floor muscle strength between women with and without UF-LUTS via hip muscle force dynamometry and PFM manometry. We hypothesized that 1) women with UF-LUTS would demonstrate decreased hip external rotator and abductor strength as compared to women without UF-LUTS, and 2) women with UF-LUTS would demonstrate comparable PFM strength and diminished endurance as compared to women without UF-LUTS.
MATERIALS AND METHODS
Study Design
The study was a 1:1 matched case-control study of women with and without UF-LUTS. The study was approved by the Human Research Protection Office of Washington University in St. Louis (approval #201810086) and conducted in accordance with the Declaration of Helsinki. Participants gave written informed consent prior to participation.
Participants
From April to December 2019, female participants 18–60 years of age were recruited from the community via paper and social media advertisements, emails, and research recruitment fairs. Participants with UF-LUTS (“cases”) were included if they experienced bothersome urinary urgency (sudden need to rush to urinate) and/or frequency (more frequent than every 2 hours)24 on a typical day in the past 4 weeks, as reported during a phone screen. Because the current International Continence Society (ICS) definition of frequency is not specific, “complaint that voiding occurs more frequently than deemed normal by the individual,”25 we used “more frequent than every 2 hours” as an additional criteria to ensure discrimination between cases and controls. Women without UF-LUTS (“controls”) were matched 1:1 to cases based on age ± 5 years, body mass index (BMI) ± 5 kg/m2 and vaginal parity (0, 1, >1). Women with stress or mixed incontinence were excluded. Women with urgency urinary incontinence26 were included. Full inclusion and exclusion criteria are listed in Table 1.
TABLE 1.
Inclusion and exclusion criteria for all participants with and without urgency and frequency predominant lower urinary tract symptoms (UFLUTS)
| Inclusion Criteria | Exclusion Criteria (n=42) |
|---|---|
| All | • Stress urinary incontinence or mixed incontinence more than once per month |
| • Women, ages 18–60 | • Current or recurrent urinary tract infectiona or gynecologic infection or cancer |
| • Able to speak and understand English | • Symptomatic pelvic organ prolapsea |
| • Previous surgery for prolapse or incontinence | |
| Cases w/ UFLUTS (n=21) | • Hip, pelvic, or trunk trauma or cancer |
| • Bothersome urgency or frequency in the past 4 weeks | • Abdominal or pelvic surgery in the past year |
| • Current injury that would limit their ability to participate in testing | |
| • Onabotulinumtoxin injections to the bladder, pelvic floor or hip muscles | |
| Controls (n=21) | • Vulvovaginal dermatological conditions associated with UFLUTS |
| • No UFLUTS in the past 6 months | • Diabetes |
| • Current pregnancya or birth/termination/miscarriage in the past 12 weeks | |
| • Age, BMI, & parity matched to cases | • Neurological involvement that would influence their coordination or balance |
| • Implanted devices that impair the ability to visualize / make ultrasound measuresb |
Items ruled out during final eligibility exam
A separate study with the same participants called for ultrasound imaging
UF-LUTS and Activity Assessment
Study data were collected and managed using REDCap electronic data capture tools.27 Prior to examination, participants completed questionnaires including the LUTS Tool28 for a comprehensive assessment of participants’ LUTS, the Pelvic Floor Impact Questionnaire short form (PFIQ-7)29 for a general overview of impact of symptoms on daily activities and quality of life, and the UCLA Activity Score30 for a general overview of activity and exercise level.
Final Eligibility Exam
A single assessor, a physical therapist trained in intravaginal pelvic floor examination, completed all measurements. To determine final eligibility, urine and pelvic organ prolapse screens were completed. Using 10SG and human chorionic gonadotropin (HCG) test strips (McKesson, Irving, TX, USA), participants were excluded if their urine tested positive for glucose, nitrite, blood, leukocyte esterase, or HCG. After urine screening, a transabdominal ultrasound scan confirmed whether the bladder was empty. If postvoid residual urine was evident, participants were asked to void again prior to intravaginal exam. A screening exam for symptomatic prolapse was performed with the participant supine with knees bent and feet flat on the exam table. A lubricated sterile wide tongue depressor was used to retract the vaginal wall as the participant bore down. Participants were excluded if any intravaginal landmark descended beyond the vaginal introitus (hymenal ring) because prolapse at or beyond this point has been associated with symptomatic prolapse.31,32
Hip Muscle Strength
A microFET3 hand-held dynamometer (Hogan Health Industries, Salt Lake City, UT, USA) was used to assess hip muscle strength. Isometric make tests33 were used, incorporating a strap for fixed resistance to minimize the effect of assessor strength on measurements. Isometric make tests with a hand dynamometer and strap have been found to be valid for measuring hip strength compared to isokinetic dynamometry.34 Hip external rotation force was measured in sitting with the hip at 90 degrees of flexion and neutral hip rotation (Figure 1). Hip abduction was measured in side-lying with pillows supporting the test hip at neutral abduction, rotation and flexion/extension (Figure 2). Participants completed one submaximal practice trial and three maximal 3 second measurement trials for each muscle group on each hip. The three trials for each hip were averaged for analysis. To estimate the size of the difference between readings, 10 participants completed a second testing session within two weeks of the first testing session. The average between-reading difference for external rotation was 1.7 Newtons (N) (95% CI: 0.4 to 3.8) and for abduction was 7.7 N (95% CI: 8.5 to 23.9).
Figure 1. Hip External Rotator Isometric Make Test.
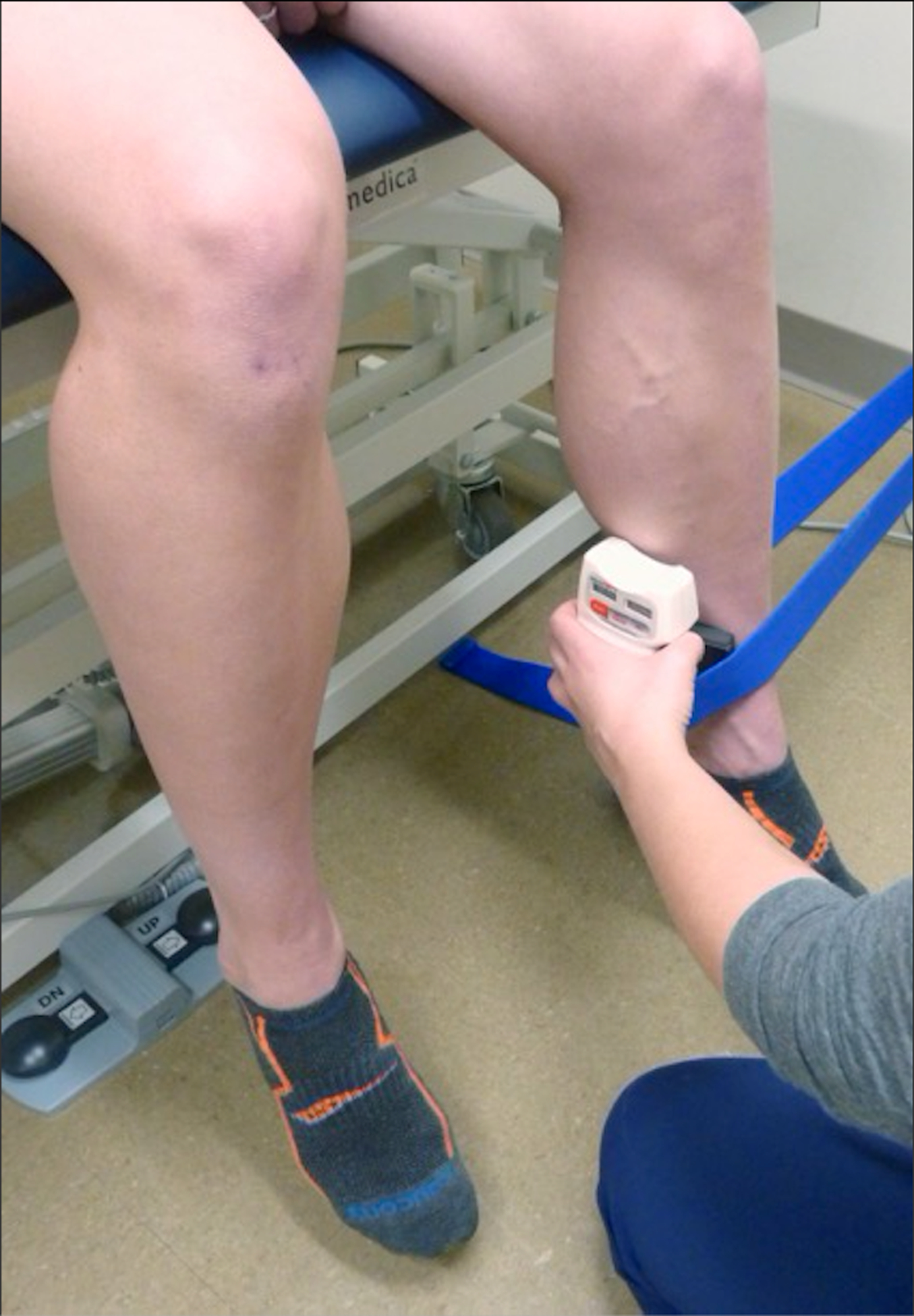
With hip flexed to 90 degrees, participants externally rotated their hip as strongly as possible against fixed resistance of the strap. Peak force in Newtons was measured by a handheld dynamometer inside the strap at 4cm proximal to the medial malleolus.
Figure 2. Hip Abductor Isometric Make Test.
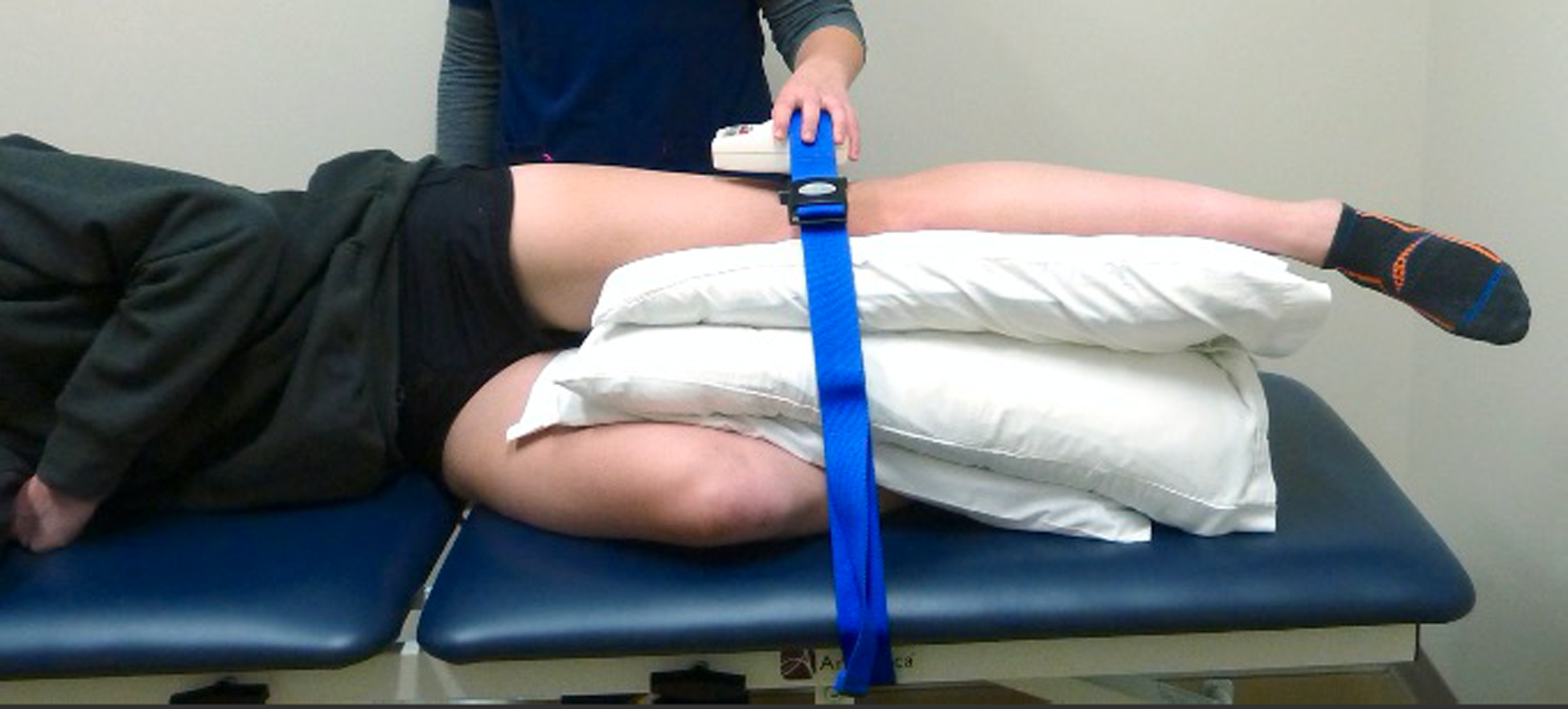
With hip in neutral flexion, abduction and rotation, participants abducted their hip as strongly as possible against fixed resistance of the strap. Peak force in Newtons was measured by a handheld dynamometer inside the strap at 4cm proximal to the lateral knee joint line.
Pelvic Floor Muscle Strength and Endurance
Prior to vaginal squeeze pressure measurement, the examiner palpated vaginally while participants were cued to “squeeze and lift the PFMs as if you’re stopping your urine stream or trying to hold back gas.” If participants were unable to perform the movement appropriately, brief verbal instruction with confirmatory palpation was given in an effort to obtain valid squeeze pressure measurements. Vaginal manometry is commonly used as a “gold standard” and considered to be a valid measure of PFM strength when accompanied by an inward lift of the perineum.35
Vaginal squeeze pressure was measured using a Peritron Perineometer and single-participant-use vaginal pressure sensors (Laborie, Williston, VT, USA). Participants were examined in supine with knees bent, feet flat on the exam table, and hips in neutral internal/external rotation. The sensor was lubricated and inserted vaginally until 1 cm of the blue sheath remained external. If, due to pain, the participant was unable to tolerate sensor insertion, vaginal manometry was not completed. After insertion and a 60 second acclimation period, the manometer was zeroed. For each strength trial, participants were instructed to squeeze and lift the PFMs as strongly as possible, hold for 10 seconds while the assessor counted audibly with a clock, then relax completely. Participants performed a brief submaximal practice trial with no hold followed by 3 trials in which peak pressure, duration of contraction, and average pressure over the trial were recorded by the manometer. The participants were given 30 seconds rest between trials. For each of the 3 trials, area under the curve (AUC) metric was computed by multiplying duration by average pressure of the trial. Peak pressure and AUC were averaged across the 3 trials for each participant. PFM strength was assessed via peak vaginal squeeze pressure in cmH20, and PFM endurance was assessed using the AUC metric over the 10 second hold in cmH20*sec. To estimate the size of the difference between readings, 9 participants completed a second testing session within two weeks of the first testing session. The average between-reading difference for peak was 2.2 cmH20 (95% CI: 0.0 to 10.3) and for AUC was 57.0 cmH20*seconds (95% CI: 10.6 to 103).
Sample size
Because no data had been published on the primary study measures in patients with UF-LUTS by the start of the study, we computed interim effect sizes for hip external rotation and abduction force using means and standard deviations of the differences between the first 10 complete case-control pairs and G*Power (Heinrich-Heine-Universität Düsseldorf, DE). We powered the study on hip strength variables because we were most interested in hip strength as a novel variable in patients with UF-LUTS. Cohen’s d effect sizes were computed for hip abduction force as 0.84 and for hip external rotation as 0.56. With Cohen’s d having a minimum of 0 (no effect) and a theoretical maximum value of 1.00, these effect sizes can be interpreted as large and medium, respectively.36 These effect sizes were similar to another study measuring hip external rotation in similarly aged healthy women (d = 0.78).18 Based on these estimates, we determined we needed a total sample size of 44 participants (22 matched case-control pairs) to detect differences in hip external rotation strength with 80% power, an alpha level of 0.05, and a paired sample two-tailed t-test.
Statistical Analysis
Statistical analyses were computed using SAS (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were computed for participant characteristics and questionnaire scores. Because case-control pairs were 1:1 matched, paired-samples tests were used37 to compare participant characteristics, hip external rotator and abductor force, vaginal squeeze pressure peak and endurance, UCLA activity, LUTS Tool scores, and PFIQ-7 scores between cases and matched controls. One-tailed tests were used for hip strength and pelvic floor endurance outcomes due to a priori directional hypotheses. Assumptions were checked graphically with histograms and Q-Q plots and statistically with the Shapiro Wilk test. When the between-pair difference was not normally distributed, Wilcoxon’s signed rank test was used as a nonparametric alternative. An alpha level of 0.05 was chosen a priori.
RESULTS
A total of 21 case-control pairs (42 women) were enrolled with adherence to 1:1 matching rules. We were unable to enroll 22 total pairs during our predetermined study timeline. No participants were excluded based on the prolapse screening exam. Mean age of our sample was 28.5 years and all but one pair were nulliparous. There were minimal, nonsignificant differences between cases and controls by age, BMI and vaginal parity (Table 2). Women with UF-LUTS had significantly worse LUTS Tool Storage and Voiding Symptom and Bother scores and significantly worse PFIQ-7 Urogenital Impact scores (Table 2). Median LUTS Tool Scores for those with UF-LUTS represented mild to moderate symptoms and bother. None of our sample had ever used medications for LUTS. Complete data for 21 pairs were available for hip external rotator and abductor strength. For PFM strength and endurance, data for 17 pairs were available because 4 participants (3 cases, 1 control) were unable to tolerate insertion of the vaginal manometer due to pain.
TABLE 2.
Characteristics of women with and without urgency and frequency predominant lower urinary tract symptoms (UFLUTS)
| Characteristic | UFLUTS (n=21) | Control (n=21) | Summary statistics (UFLUTS minus Control) | P |
|---|---|---|---|---|
| Age (years, mean ± SD [range]) | 28 ± 10 [19–56] | 29 ± 9 [21–57] | −0.9 ± 3 [−5 – 4] | 0.12 |
| BMI (kg/m2, mean ± SD [range]) | 24 ± 4 [18–35] | 25 ± 4 [19–33] | −0.4 ± 2 [−4 – 4] | 0.40a |
| Vaginal parity (%) | ||||
| 0 | 95 | 95 | ||
| 2–3 | 5 | 5 | ||
| Race (%) | ||||
| Asian | 19 | 5 | ||
| Black or African American | 5 | 0 | ||
| White | 76 | 81 | ||
| Other or Mixed-race | 0 | 14 | ||
| Ethnicity (%) | ||||
| Hispanic or Latino | 14 | 10 | ||
| Not Hispanic or Latino | 86 | 90 | ||
| UCLA Activity Score (Median [IQR]) | 9 [5] | 6 [5] | 0.3 [3.6] | 0.64 |
| LUTS Tool (Median [IQR]) | ||||
| LUTS Storage Symptom | 9 [3] | 1 [2] | 7 [3] | <0.0001 |
| LUTS Storage Bother | 8 [4] | 0 [0] | 8 [5] | <0.0001 |
| LUTS Voiding Symptom | 6 [4] | 0 [1] | 5 [4] | <0.0001 |
| LUTS Voiding Bother | 2 [3] | 0 [0] | 2 [3] | <0.0001 |
| Pelvic Floor Impact Questionnaire - 7 (median [IQR]) | ||||
| PFIQ-7 Urogenital | 24 [24] | 0 [0] | 24 [24] | <0.0001 |
| PFIQ-7 Colorectal | 0 [5] | 0 [0] | 0 [5] | 0.004 |
| PFIQ-7 Vagina/Pelvis | 0 [5] | 0 [0] | 0 [5] | 0.02 |
LUTS=Lower Urinary Tract Symptoms; SD=standard deviation; IQR=interquartile range defined as the 75th minus the 25th percentile.
P value from two-tailed paired samples t-test (all others from two-tailed Wilcoxon signed rank)
The UCLA Activity Score is an ordinal self-report scale from 1 (No activity) to 10 (Regularly participates in high impact activity)
LUTS Tool scores were obtained by summing the frequency of LUTS (0–5) within each respective domain. The number of component items summed for each scale is: Storage Symptom 5, Storage Bother 4, Voiding Symptom 8, Voiding bother 7.
Hip Muscle Strength
A bilateral mean was used for analysis. A sensitivity analysis was performed where strength from a single hip was selected at random. The conclusions were unchanged. Participants with UF-LUTS had significantly less hip external rotation strength (67.0 ± 19.0 N vs 83.6 ± 21.5 N; P=0.005) and hip abductor strength (163.1 ± 48.1 N vs 190.1 ± 53.1 N; P=0.04) compared to control participants (Figures 3 and 4).
Figure 3. Hip External Rotator Strength in women with and without urgency and frequency predominant lower urinary tract symptoms (UF-LUTS).
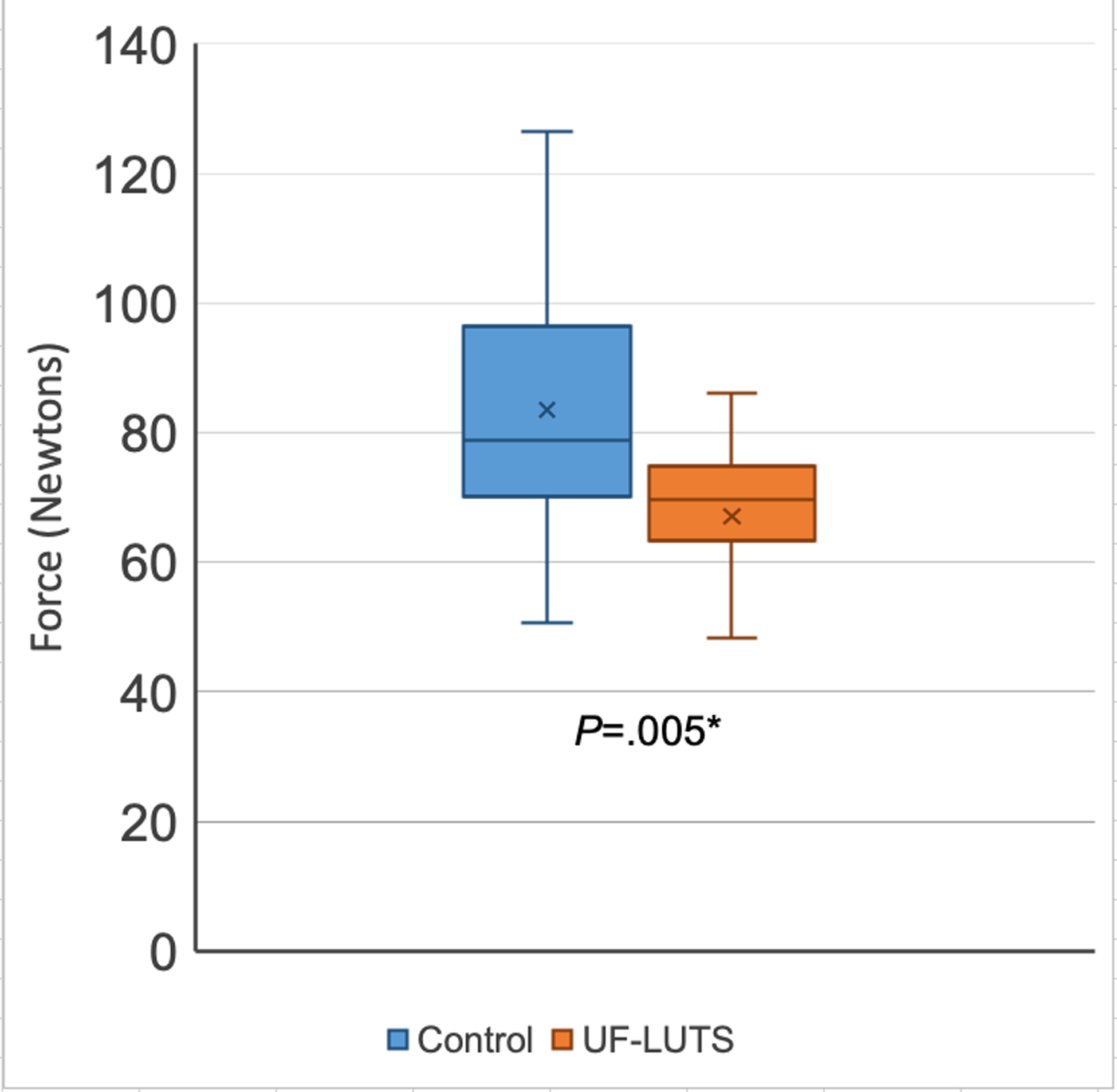
*Significantly greater in Control compared to UF-LUTS, per one-tailed paired t-test
X represents mean, line represents 50th percentile, error bars represent minimum and maximum
Figure 4. Hip Abductor Strength in women with and without urgency and frequency predominant lower urinary tract symptoms (UF-LUTS).
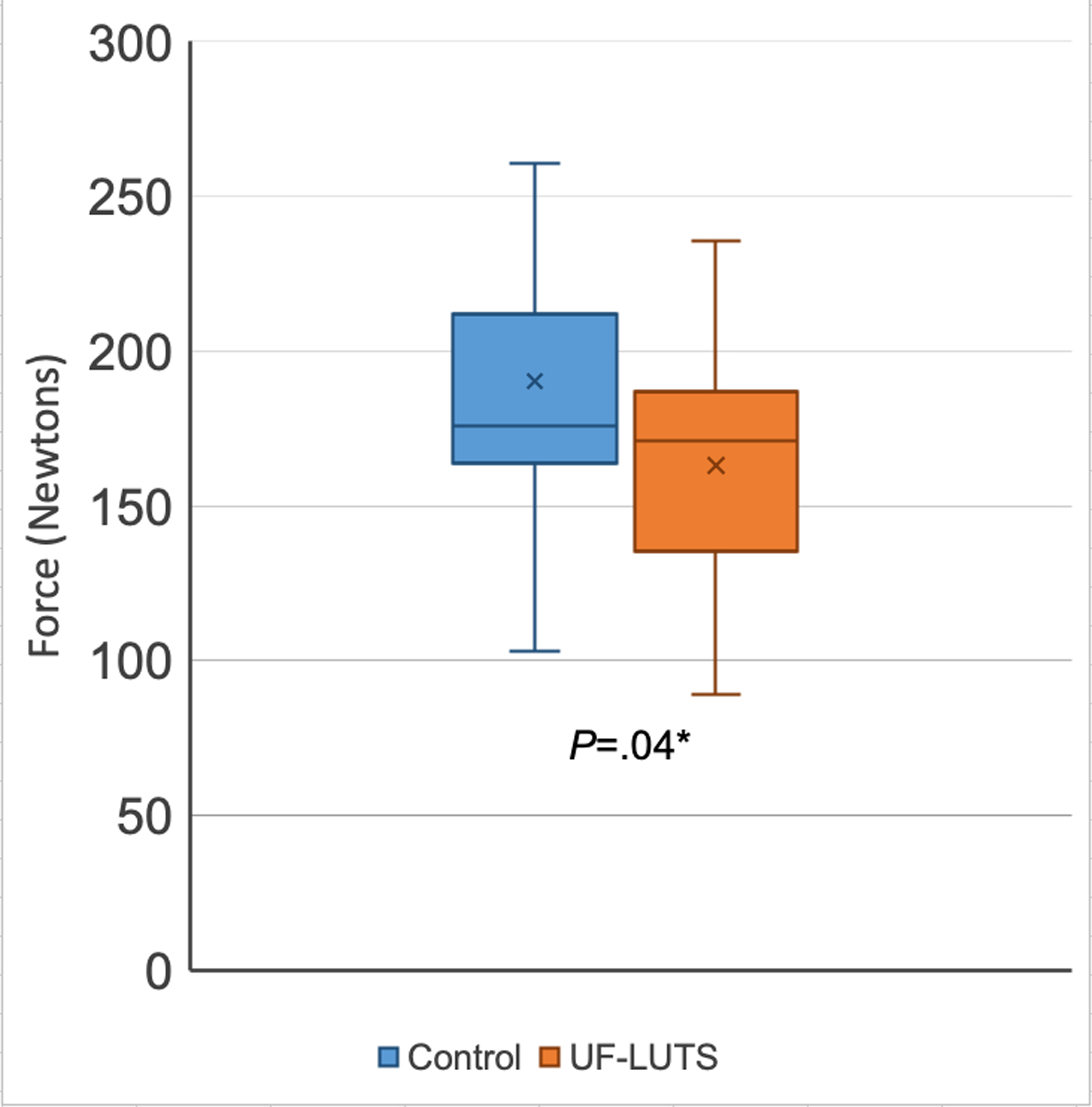
*Significantly greater in Control compared to UF-LUTS, per one-tailed t-test
X represents mean, line represents 50th percentile, error bars represent minimum and maximum
Pelvic Floor Muscle Strength
No significant differences were observed between women with UF-LUTS and matched controls in PFM strength (36.8 ± 19.9 cmH20 vs 41.8 ± 21.0 cmH20; P=0.40) or endurance (234.0 ± 149.6 cmH20*seconds vs 273.4 ± 149.1 cmH20*seconds; P=0.24) (Figures 5 and 6).
Figure 5. Pelvic Floor Muscle Strength - Peak Vaginal Squeeze Pressure (cmH20) in women with and without urgency and frequency predominant lower urinary tract symptoms (UF-LUTS).
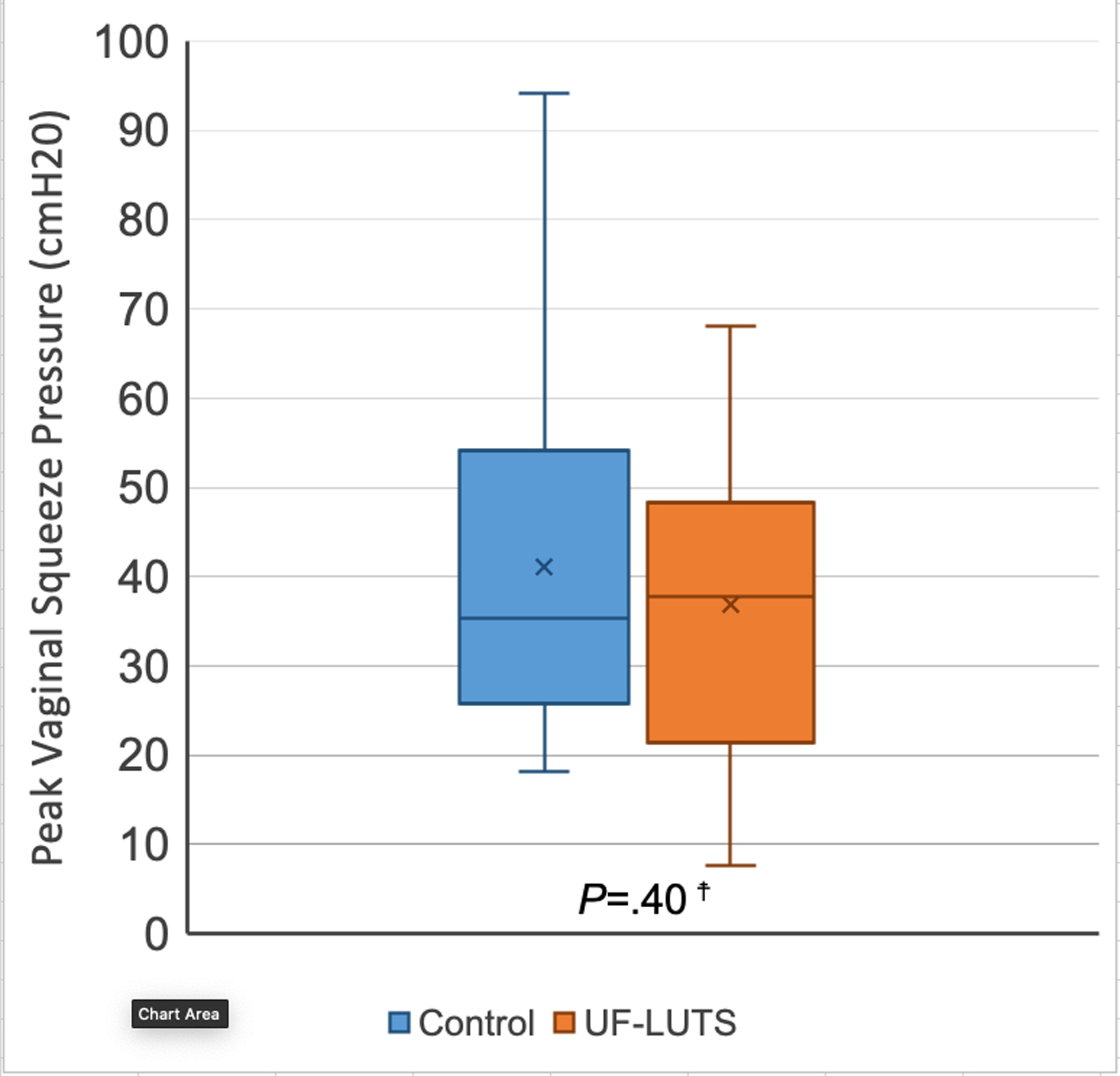
☨No significant paired differences, per two-tailed Wilcoxon signed ranks test
X represents mean, line represents 50th percentile, error bars represent minimum and maximum
Figure 6. Pelvic Floor Muscle Endurance – Vaginal Squeeze Pressure over 10 second hold (cmH20*seconds) in women with and without urgency and frequency predominant lower urinary tract symptoms.
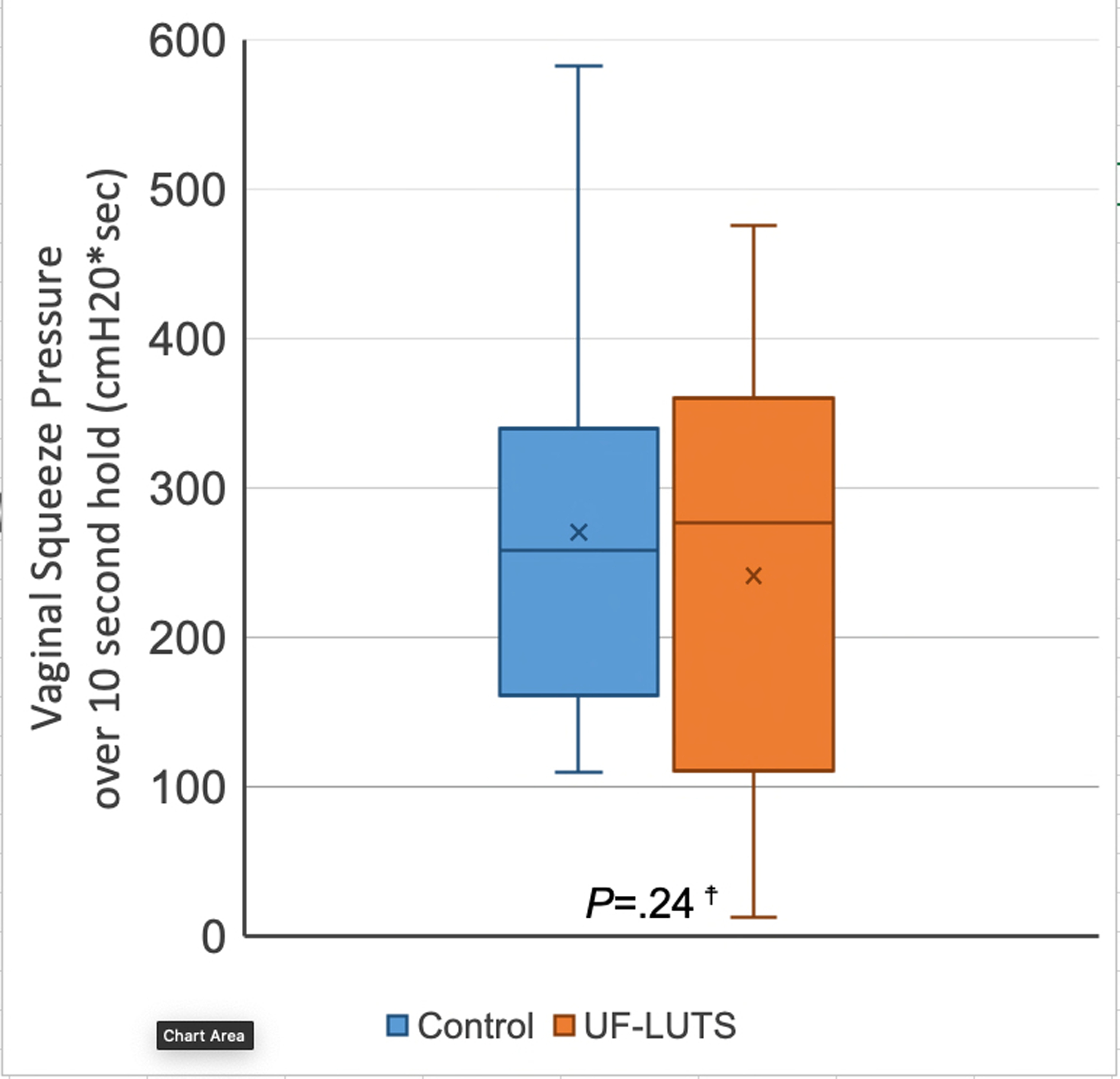
☨No significant paired differences, per one-tailed Wilcoxon signed ranks test
X represents mean, line represents 50th percentile, error bars represent minimum and maximum
DISCUSSION
Principle Findings
Our study observed that women with UF-LUTS had less hip external rotator and abductor strength, but similar PFM strength and endurance compared to women without UF-LUTS. We report novel information about the role of hip muscle and PFM strength in women with UF-LUTS without stress incontinence. Our results suggest that assessing hip muscle performance may be important for those with UF-LUTS. Intervention studies for this treatment strategy have not yet been conducted. Further research is required to better understand the role of PFM performance in UF-LUTS.
Research Implications
We found weakness in hip external rotators and abductors in women with UF-LUTS. Our results are consistent with studies of women with stress urinary incontinence that found women with stress urinary incontinence had less hip abductor strength19,20 than controls; previous studies that compared hip external rotator strength in women with and without stress incontinence have reported mixed results.19,20 Future research to better understand the relationship of hip muscle strength and symptoms should investigate whether strengthening hip external rotators and abductors can improve UF-LUTS. Previous studies have found hip strengthening can improve symptoms of stress incontinence38 and other pelvic floor dysfunction.39 The fact that hip strength differed between women with and without UF-LUTS, but PFM strength and endurance did not, suggests the influence of hip strength on UF-LUTS is not purely via an effect on PFMs. Investigation into potential musculoskeletal underpinnings of UF-LUTS from the hip and pelvis are warranted. Hip muscle weakness may be related to habitual hip adduction motion or posturing that has been associated with UF-LUTS,17 but the causal pathway is not yet understood. Gluteus maximus, which contributes to hip abduction, also has a morphological and functional connection to the PFMs via connective tissue septa in the ischioanal fossa.40 Future studies would benefit from measuring global hip muscle function, including hip extension.
We did not find PFM weakness in women with UF-LUTS as measured by peak volitional vaginal squeeze pressure and a ten second hold. Our results are consistent with studies that found women with stress urinary incontinence did not have worse PFM strength than women without.19,20,41 This may indicate force production capability alone may not be the most important factor among patients with UF-LUTS. Perhaps different parameters of PFM performance are more important to UF-LUTS such as PFM mobility and coordination42 in response to daily activity demands. Mobility of the PFMs (ability to move through full range of motion for contraction and relaxation) may be important as evidenced by known associations between difficulty relaxing PFMs and LUTS such as urgency, frequency, hesitancy, dysuria and pain.13 However, PFM mobility has yet to be studied systematically in patients with UF-LUTS. PFM function in response to demands of activities of daily living and exercise may be more important than a voluntary response to verbal cuing. UF-LUTS respond to qualitative changes in common daily movements,17 and thus, the response of PFMs to movement and activities of daily living may be important to capture in future studies.
Clinical implications
Given that women with UF-LUTS demonstrated hip muscle weakness compared to asymptomatic controls, a more thorough musculoskeletal assessment may be warranted, including assessment of hip muscle strength. Based on our findings and previous literature, patients with LUTS with or without incontinence may benefit from hip strengthening. Previous work has demonstrated improved pelvic floor muscle strength18,39 and decreased pelvic floor dysfunction38,39 after a hip strengthening program. Further research is required to assess the efficacy of hip strengthening in improving UF-LUTS. As this type of intervention poses minimal risk, a hip strengthening protocol under physical therapist supervision may be warranted on a trial basis for patients with UF-LUTS.
Our results suggest PFM weakness may not be a primary factor in UF-LUTS and other factors may need to be considered. Stress incontinence literature suggests PFM training results in patient-reported symptom improvement.10 Interestingly, studies comparing PFM strength between those with and without incontinence report no differences in baseline PFM strength, similar to our findings.19,20,41 PFM training may be targeting mechanisms additional to PFM force production. Among PFM training programs is a wide variety in emphasis on improving parameters of PFM performance including strength, endurance, power, muscle tone, coordination, motor control, flexibility, and myofascial pain. This varying emphasis may explain why intervention studies have shown positive effects of PFM training while case-control studies have not shown differences in PFM strength and endurance. There may also be a subset of individuals who benefit from particular PFM-focused interventions. Because PFM performance parameters other than strength and endurance may contribute to UF-LUTS, we recommend assessment of PFM and surrounding muscle coordination during activities meaningful to the patient,17,43,44 and systematic palpation of each PFM for myofascial pain or symptom reproduction45 to determine an individualized approach.
Strengths and Limitations
A major strength of this study is the inclusion of control participants matched one to one to cases on age, BMI and vaginal parity which helped to focus the study on the relationship of muscle performance to UF-LUTS and reduce confounding factors. Systematic procedures for examination were tested for reliability, and a single examiner performed all testing. The similarity of our findings in women with UF-LUTS without stress incontinence and previous studies of women with stress incontinence lends support to our observation that in symptomatic women compared to controls, hip external rotators19 and hip abductors19,20 are weaker, but PFM strength19,20,41 and endurance are no different.19
Our sample size for PFM measures was reduced because 4 women (3 UF-LUTS and 1 control) were unable to tolerate the vaginal manometer probe. These 4 women were not statistical outliers on any other variables tested, and variable means and dispersions looked similar whether PFM measures were analyzed pairwise with 17 pairs, or unpaired with 38 total participants. Our study was not powered to detect differences in PFM strength or endurance, but the within-pair differences we found in PFM strength are likely not clinically important. Effect sizes for PFM strength (peak squeeze pressure in cmH20) and endurance (cmH20*seconds) were small, and a much larger sample size would be needed to achieve 80% power at alpha=0.05 (n=177 for peak cmH20 and n=134 for cmH20*seconds). Peak vaginal squeeze pressures measured for women with UF-LUTS in our study (mean 36.9 cmH20, 95% CI: 27.3, 46.5) were comparable to those in healthy asymptomatic women of similar age in previous studies (summary mean 38.1 cmH20, 95% CI: 34.6, 41.5).18,43,46,47 It was not possible to blind the single examiner to participant symptom status, but systematic examination procedures and equipment were in place to minimize bias. Most of our participants were between age 18 and 35 (only 3 pairs were over age 35) and nulliparous. Our participants tended to report low to moderate UF-LUTS severity, likely due to being recruited from the general population rather than a population of women seeking treatment. Because we were interested in clinical tests that could be completed by physical therapists and under budgetary constraints, we do not have urodynamic data on participants in our sample. General activity levels, as reported in the UCLA Activity Score, were similar between cases and controls. Given our small sample size, we limited our analyses to those we had determined a priori and did not assess the relationship between activity level and our variables of interest. We do not know how our results may have differed in a population aged over 35, parous, less physically active, and more severely symptomatic. Although our sample may not be reflective of most clinical populations, UF-LUTS are present in women under age 453,48,49 and our data points to the presence of bothersome symptoms prior to seeking medical attention and a willingness of these women to participate in research. Our hope is that continued inclusion of women in studies who have not yet sought medical attention for UF-LUTS will improve early education and treatment efforts in the future.
Conclusions
Our study found women with UF-LUTS have weaker hip external rotators and abductors than women without UF-LUTS, but similar PFM strength and endurance. Further research is needed to determine whether strengthening hip external rotators and abductors can improve UF-LUTS.
ACKNOWLEGEMENTS
The authors would like to thank Darrah Snozek for her assistance with screening and data collection.
Funding for the study was provided by The Foundation for Barnes-Jewish Hospital, NIH T32HD007434 and UL1TR002345, Washington University in St. Louis Program in Physical Therapy. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Darrah Snozek
Washington University in St Louis School of Medicine, Program in Physical Therapy
Public trials registry: N/A
REFERENCES
- 1.Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104(3):352–360. doi: 10.1111/j.1464-410X.2009.08427.x [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7(4):455–463. doi: 10.1111/j.1524-4733.2004.74008.x [DOI] [PubMed] [Google Scholar]
- 3.Sutcliffe S, Bavendam T, Cain C, et al. The Spectrum of Bladder Health: The Relationship Between Lower Urinary Tract Symptoms and Interference with Activities. J Women’s Heal. 2019;28(6):827–841. doi: 10.1089/jwh.2018.7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi S, Takei M, Nishizawa O, et al. Clinical Guideline for Female Lower Urinary Tract Symptoms. Low Urin Tract Symptoms. 2016;8(1):5–29. doi: 10.1111/luts.12111 [DOI] [PubMed] [Google Scholar]
- 5.Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J Urol. 2019;202(3):558–563. doi: 10.1097/JU.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 6.Ganz ML, Smalarz AM, Krupski TL, et al. Economic Costs of Overactive Bladder in the United States. Urology. 2010;75(3):526–532.e18. doi: 10.1016/j.urology.2009.06.096 [DOI] [PubMed] [Google Scholar]
- 7.Anger JT, Nissim HA, Le TX, et al. Women’s experience with severe overactive bladder symptoms and treatment: Insight revealed from patient focus groups. Neurourol Urodyn. 2011;30(7):n/a–n/a. doi: 10.1002/nau.21004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arruda RM, Castro RA, Sousa GC, Sartori MGF, Baracat EC, Girão MJBC. Prospective randomized comparison of oxybutynin, functional electrostimulation, and pelvic floor training for treatment of detrusor overactivity in women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(8):1055–1061. doi: 10.1007/s00192-008-0586-y [DOI] [PubMed] [Google Scholar]
- 9.Kalder M, Pantazis K, Dinas K, Albert U-S, Heilmaier C, Kostev K. Discontinuation of Treatment Using Anticholinergic Medications in Patients With Urinary Incontinence. Obstet Gynecol. 2014;124(4):794–800. doi: 10.1097/AOG.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 10.Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. Published online October 4, 2018. doi: 10.1002/14651858.CD005654.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafik A, Shafik IA. Overactive bladder inhibition in response to pelvic floor muscle exercises. World J Urol. 2003;20(6):374–377. doi: 10.1007/s00345-002-0309-9 [DOI] [PubMed] [Google Scholar]
- 12.Fitz F, Sartori M, Girão MJ, Castro R. Pelvic floor muscle training for overactive bladder symptoms – A prospective study. Rev Assoc Med Bras. 2017;63(12):1032–1038. doi: 10.1590/1806-9282.63.12.1032 [DOI] [PubMed] [Google Scholar]
- 13.Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc. 2012;87(2):187–193. doi: 10.1016/j.mayocp.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitznagle T, Cabelka C, Clinton S, Abraham K, Norton B. Diagnosis Dialog for Womenʼs Health Conditions. J Womenʼs Heal Phys Ther. 2017;41(3):154–162. doi: 10.1097/JWH.0000000000000086 [DOI] [Google Scholar]
- 15.Bendaña EE, Belarmino JM, Dinh JH, et al. Efficacy of transvaginal biofeedback and electrical stimulation in women with urinary urgency and frequency and associated pelvic floor muscle spasm. Urol Nurs. 2009;29(3):171–176. http://www.ncbi.nlm.nih.gov/pubmed/19579410 [PubMed] [Google Scholar]
- 16.Aw HC, Ranasinghe W, Tan PHM, O’Connell HE. Overactive pelvic floor muscles (OPFM): improving diagnostic accuracy with clinical examination and functional studies. Transl Androl Urol. 2017;6(S2):S64–S67. doi: 10.21037/tau.2017.05.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wente KR, Spitznagle TM. Movement-Related Urinary Urgency. J Womenʼs Heal Phys Ther. 2017;41(2):83–90. doi: 10.1097/JWH.0000000000000075 [DOI] [Google Scholar]
- 18.Tuttle LJ, DeLozier ER, Harter KA, Johnson SA, Plotts CN, Swartz JL. The Role of the Obturator Internus Muscle in Pelvic Floor Function. J Womenʼs Heal Phys Ther. 2016;40(1):15–19. doi: 10.1097/JWH.0000000000000043 [DOI] [Google Scholar]
- 19.Hartigan E, McAuley JA, Lawrence M, et al. Pelvic Floor Muscle Performance, Hip Mobility, and Hip Strength in Women With and Without Self-Reported Stress Urinary Incontinence. J Womenʼs Heal Phys Ther. Published online June 2019:1. doi: 10.1097/JWH.0000000000000141 [DOI] [Google Scholar]
- 20.Underwood DB, Calteaux TH, Cranston R, Novotny SA, Hollman JH. Hip and pelvic floor muscle strength in women with and without stress urinary incontinence : a case-control study. J Women’s Heal Phys Ther. 2012;36:55–61. doi: 10.1097/JWH.0b013e31824fbee5 [DOI] [Google Scholar]
- 21.Tuttle LJ, Nguyen OT, Cook MS, et al. Architectural design of the pelvic floor is consistent with muscle functional subspecialization. Int Urogynecol J Pelvic Floor Dysfunct. 2014;25(2):205–212. doi: 10.1007/s00192-013-2189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann DA. Kinesiology of the Hip: A Focus on Muscular Actions. J Orthop Sport Phys Ther. 2010;40(2):82–94. doi: 10.2519/jospt.2010.3025 [DOI] [PubMed] [Google Scholar]
- 23.Dix J, Marsh S, Dingenen B, Malliaras P. The relationship between hip muscle strength and dynamic knee valgus in asymptomatic females: A systematic review. Phys Ther Sport. 2019;37:197–209. doi: 10.1016/j.ptsp.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 24.Lukacz ES, Whitcomb EL, Lawrence JM, Nager CW, Luber KM. Urinary frequency in community-dwelling women: what is normal? Am J Obstet Gynecol. 2009;200(5):552.e1–552.e7. doi: 10.1016/j.ajog.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi: 10.1007/s00192-009-0976-9 [DOI] [PubMed] [Google Scholar]
- 26.D’Ancona C, Haylen B, Oelke M, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433–477. doi: 10.1002/nau.23897 [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coyne KS, Barsdorf AI, Thompson C, et al. Moving towards a comprehensive assessment of lower urinary tract symptoms (LUTS). Neurourol Urodyn. 2012;31(4):448–454. doi: 10.1002/nau.21202 [DOI] [PubMed] [Google Scholar]
- 29.Barber MD, Chen Z, Lukacz E, et al. Further validation of the short form versions of the pelvic floor Distress Inventory (PFDI) and pelvic floor impact questionnaire (PFIQ). Neurourol Urodyn. 2011;30(4):541–546. doi: 10.1002/nau.20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66(2):228–241. http://www.ncbi.nlm.nih.gov/pubmed/6693450 [PubMed] [Google Scholar]
- 31.Ghetti C, Gregory WT, Edwards SR, Otto LN, Clark AL. Pelvic organ descent and symptoms of pelvic floor disorders. Am J Obstet Gynecol. 2005;193(1):53–57. doi: 10.1016/j.ajog.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am J Obstet Gynecol. 2008;199(6):683.e1–683.e7. doi: 10.1016/j.ajog.2008.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stratford PW, Balsor BE. A Comparison of Make and Break Tests Using a Hand-Held Dynamometer and the Kin-Com. J Orthop Sport Phys Ther. 1994;19(1):28–32. doi: 10.2519/jospt.1994.19.1.28 [DOI] [PubMed] [Google Scholar]
- 34.Katoh M, Hiiragi Y, Uchida M. Validity of Isometric Muscle Strength Measurements of the Lower Limbs Using a Hand-held Dynamometer and Belt: a Comparison with an Isokinetic Dynamometer. J Phys Ther Sci. 2011;23(4):553–557. doi: 10.1589/jpts.23.553 [DOI] [Google Scholar]
- 35.Bø K, Kvarstein B, Hagen R. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence. II: Validity of vaginal pressure measurements of pelvic floor muscle strength and the necessity of supplementary methods for control of correct contraction. Neurourol Urodyn. 1990;9:479–487. [Google Scholar]
- 36.Cohen J Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; 1988. [Google Scholar]
- 37.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. Int Agency Res Cancer. 1980;(32):5–338. http://www.ncbi.nlm.nih.gov/pubmed/7216345 [PubMed] [Google Scholar]
- 38.Jordre B, Schweinle W. Comparing Resisted Hip Rotation With Pelvic Floor Muscle Training in Women With Stress Urinary Incontinence. J Womenʼs Heal Phys Ther. 2014;38(2):81–89. doi: 10.1097/JWH.0000000000000008 [DOI] [Google Scholar]
- 39.Tuttle LJ, Autry T, Kemp C, et al. Hip exercises improve intravaginal squeeze pressure in older women. Physiother Theory Pract. Published online February 2019:1–8. doi: 10.1080/09593985.2019.1571142 [DOI] [PubMed] [Google Scholar]
- 40.Soljanik I, Janssen U, May F, et al. Functional interactions between the fossa ischioanalis, levator ani and gluteus maximus muscles of the female pelvic floor: a prospective study in nulliparous women. Arch Gynecol Obstet. 2012;286(4):931–938. doi: 10.1007/s00404-012-2377-4 [DOI] [PubMed] [Google Scholar]
- 41.Bø K, Stien R, Kulseng-Hanssen S, Kristofferson M. Clinical and urodynamic assessment of nulliparous young women with and without stress incontinence symptoms: a case-control study. Obstet Gynecol. 1994;84(6):1028–1032. http://www.ncbi.nlm.nih.gov/pubmed/7970459 [PubMed] [Google Scholar]
- 42.Bø K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221–244. doi: 10.1002/nau.23107 [DOI] [PubMed] [Google Scholar]
- 43.Bø K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85(3):269–282. http://www.ncbi.nlm.nih.gov/pubmed/15733051 [PubMed] [Google Scholar]
- 44.Smith MD, Coppieters MW, Hodges PW. Postural response of the pelvic floor and abdominal muscles in women with and without incontinence. Neurourol Urodyn. 2007;26(3):377–385. doi: 10.1002/nau.20336 [DOI] [PubMed] [Google Scholar]
- 45.Meister MR, Sutcliffe S, Ghetti C, et al. Development of a standardized, reproducible screening examination for assessment of pelvic floor myofascial pain. Am J Obstet Gynecol. 2019;220(3):255.e1–255.e9. doi: 10.1016/j.ajog.2018.11.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbosa PB, Franco MM, Souza F de O, Antônio FI, Montezuma T, Ferreira CHJ. Comparison between measurements obtained with three different perineometers. Clinics (Sao Paulo). 2009;64(6):527–533. doi: 10.1590/s1807-59322009000600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahmani N, Mohseni-Bandpei MA. Application of perineometer in the assessment of pelvic floor muscle strength and endurance: a reliability study. J Bodyw Mov Ther. 2011;15(2):209–214. doi: 10.1016/j.jbmt.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 48.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4 [DOI] [PubMed] [Google Scholar]
- 49.van Breda HMK, Bosch JLHR, de Kort LMO. Hidden prevalence of lower urinary tract symptoms in healthy nulligravid young women. Int Urogynecol J. 2015;26(11):1637–1643. doi: 10.1007/s00192-015-2754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


