Abstract
DNA replication and repair proteins play an important role in cancer initiation and progression by affecting genomic instability. The DNA endonuclease Mus81 is a DNA structure-specific endonuclease, which has been implicated in DNA replication and repair. In this study, we found that Mus81 promotes gastric metastasis by controlling the transcription of ZEB1, a master regulator of the epithelial-mesenchymal transition (EMT). Our results revealed that Mus81 is highly expressed in gastric cancer samples from patients and cell lines compared with their normal counterparts. Particularly, Mus81 expression positively correlated with ZEB1 expression and Mus81 overexpression was significantly associated with higher incidence of lymph node metastasis in patients. Furthermore, Mus81 promoted migration of gastric cancer cells both in vitro and in vivo. We conducted a drug screen using a collection of pre-clinical and FDA proved drugs and found that the BRD4 inhibitor AZD5153 inhibited the expression of Mus81 and ZEB1 by regulating the epigenetic factor Sirt5. As expected, AZD5153 treatment significantly reduced the migration of gastric cancer cells overexpressing Mus81 in vitro and in vivo. Collectively, we show that Mus81 is a regulator of ZEB1 and promotes metastasis in gastric cancer. Importantly, we demonstrate that the BRD4 inhibitor AZD5153 can potentially be used as an effective anti-metastasis drug because of its effect on Mus81.
Keywords: Mus81, BRD4, EMT, gastric cancer, ZEB1
Introduction
Gastric cancer is one of the most common malignant diseases worldwide, especially in East Asian countries such as China (1, 2). Complete surgery combined or not with chemoradiotherapy is the standard strategy for advanced gastric cancer treatment (3), but the overall outcome is still unsatisfying, with tumor metastasis being the main cause of the negative prognosis of gastric cancer (4). Herein, it is essential to study the underlying mechanisms of gastric cancer metastasis and identify novel targets that might allow the development of effective therapies and better outcomes of this disease (5, 6).
The maintenance of the genomic integrity is a key process in cell survival and is enabled by numerous DNA damage repair (DDR) networks (7, 8). Deficient DDR pathways cause genomic instability and lead to the accumulation of chromosomal aberrations and mutations (9), which not only promote the initiation of cancer but are also involved in the progress of this disease (10, 11). Methyl methanesulfonate and ultraviolet-sensitive gene 81 (Mus81), a DDR protein, was firstly identified in yeast as a member of the endonuclear Xeroderma pigmentosum type F/Cockayne syndrome (XPF) family, whose highly conserved members EME1 or EME2 promote nucleolytic cleavage and restart stalled replication forks induced by DNA damage via homologous recombination (12-14). Interestingly, there are some discrepancies about the correlation between Mus81 and carcinogenesis. A study from McPherson and collogues demonstrated that Mus81 mutated mice had higher susceptibility to various malignant diseases, such as lymphoma (15); however, a follow-up work showed there was no difference in Mus81 mutant and wild type mice (16). Additionally, the expression of Mus81 differs in various cancer types: it is higher in ovary cancer and lower in hepatocellular carcinoma compared with normal tissues (17, 18).
The discordance on the biological function of Mus81 and its importance in DDR prompted us to study the function of this molecule in different cancer types (19, 20). However, Mus81 function in gastric cancer is still not clear. Therefore, in this study, we investigated the role of Mus81 on gastric cancer metastasis, and the molecular mechanisms involved.
Materials and Methods
Cell culture
The human gastric cancer cell lines AGS, SGC7901, MGC803, BGC823, HGC27 and MKN45 and the immortalized human gastric mucosal cell line GES-1 were purchased from China Center for Type Culture Collection (Wuhan, Hubei, China). Cells authentication were determined by STR profiling. All cell lines were cultured in RPMI-1640 medium supplemented with 10% FBS (ScienCell, Carlsbad, CA, USA) and 1% penicillin-streptomycin, and incubated in a humidified incubator with 5% CO2 at 37 °C.
Cells transfection or infection
The GV248 lentiviral vector expressing two different short hairpin RNAs (shRNA) targeting human Mus81 (shRNA#1: TACCAACAAACAGCAAGTGGG, shRNA#2: CACGCGCTTCGTATTTCAGAA) or a control shRNA (TTCTCCGAACGTGTCACGT) and Mus81 overexpressing lentivirus (NM_025128) were purchased from GenePharma (Shanghai, China). Mus81 depleted or overexpressing cells were generated according to the manufacturer’s recommendations.
siRNAs (RiboBio, Guangzhou, China) were used to silence the expression of BRD4 (BRD4 siRNA#1: GACACTATGGAAACACCAG and BRD4 siRNA#2: GCTTAGTGGGAAATTGTAA), and Sirt5 (Sirt5 siRNA#1: CCAATTTGTCCAGCTTTAT and Sirt5 siRNA#2: GGAGATCCATGGTAGCTTA). The control siRNA was TTCTCCGAACGTGTCACGT. Transient transfection was performed using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) with siRNAs.
RNA extraction and quantitative PCR (qPCR)
Total RNA was extracted using TRIzol (cat#9109, TaKaRa, Dalian, China) and reverse transcribed into cDNA using the PrimeScript RT Master Mix (cat#RR036A, TaKaRa) according to the manufacturer’s recommendations. Gene expression was determined using SYBR® Premix Ex TaqTM (cat#RR820A, TaKaRa) on a StepOnePlus™ Real-Time PCR System and calculated using the 2−ΔΔCt method. GAPDH was used as an internal control. Experiments were carried out in triplicate. The primer sequences are in Supplementary Table S1.
Western blotting
Tissues and cells were lysed in RIPA buffer (cat#V900854, Sigma, MO, USA) and protein concentration was measured with the BCA method (cat#P0012, Beyotime, Shanghai, China). Proteins were isolated by SDS-PAGE, transferred to PVDF membranes (Millipore, Billerica, USA), incubated with primary and secondary antibodies and imaged with the ECL detection reagent (cat#12630, CST, California, USA) using a ChemiDoc™ XRS+ System. The primary antibodies included anti-Mus81 (1:1,000; cat#ab14387, Abcam, Cambridge, UK), anti-BRD4 (1:1,000; cat#ab128874, Abcam), anti-ZEB1 (1:1,000; cat#ab180905, Abcam), anti-E-cadherin (1:1,000; cat#3195, CST, California, USA), anti-N-cadherin (1:1,000; cat#13116, CST), anti-Snail1 (1:1,000; cat#9782, CST), anti-ZEB2 (1:1,000; cat#14026-1-AP, Proteintech, Wuhan, China), anti-Sirt5 (1:500; cat#15122-1-AP, Proteintech), anti-GAPDH (1:5,000; cat#G9545, Sigma). The secondary antibodies included HRP conjugated goat anti-Rabbit (1:3,000; SA00001-15, Proteintech) and anti-Mouse (1:3000; cat# SA00001-1, Proteintech).
Cell migration assays
For wound healing assays, cells were plated in 6-well plates and incubated till 85% confluency. The monolayer was then scratched with a 10 μL sterile tip, washed with PBS and cultured in RPMI-1640 with 2% FBS. Photographs were recorded using a camera connected to a light microscope at 0 h and 48 h.
For Transwell migration assays, the cells were starved with free FBS culture medium overnight and then plated (5-8 × 104 cells) in medium with 2% FBS into the upper chamber of a Transwell insert; the bottom chamber of the insert was filled with medium plus 10% FBS. After incubation for 16 h, the cells that had migrated through the filter were fixed with formaldehyde and stained with crystal violet (cat#G1014, Servicebio, Wuhan, China). Images were acquired and the number of migrated cells was then calculated.
Chromatin immunoprecipitation (ChIP)
The EpiQuikTM Chromatin Immunoprecipitation Kit (cat#P-2002, EpiGentek, Farmingdale, USA) was used to perform ChIP assays. Briefly, the process included cell lysis and DNA sharing, protein-antibody immunoprecipitation, purification of the protein-DNA complexes and reversion of the cross-link, isolation of the DNA and qPCR analysis. As a negative control, experiments with IgG control were carried out. For the qPCR, the amplification consisted of an initial denaturation at 95 °C for 30 s, followed by 30 amplification cycles (denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 60 s) and a final extension step at 72 °C for 10 min. The PCR products were analyzed by 3% agarose gel electrophoresis. The qPCR results are presented as Input% = 2^-{Ct(ChIP) – [Ct(Input) – Log2(Input Dilution Factor)]} and enrichment fold = 2^{Ct(ChIP) – [Ct(Input) – Log2(Input Dilution Factor)]-Ct(IgG)}. ChIP Primers are listed in Supplementary Table S2.
Tissue samples
Twenty-nine human specimens including gastric cancer tissues and paired adjacent normal tissues were obtained from the Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, Hubei, China) after the investigators obtained informed written consent from the patients involved in this study. The specimens were fixed in 4% neutral formaldehyde for paraffin embedding and stored at −80°C. The research was approved by the Ethics Committees of Union Hospital of Huazhong University of Science and Technology and performed in accordance with the Declaration of Helsinki.
Immunohistochemistry (IHC) and H&E staining
Four micrometers-thick tissue sections were cut from the paraffin blocks. The sections were then deparaffinized with xylene and rehydrated in a graded series of alcohol. After antigen retrieval and blocking of non-specific antibody-binding sites, the sections were incubated with a primary antibody (anti-Mus81 1:200; anti-ZEB1 1:300) and relative secondary antibody. An SABC reagent kit (cat#SA1020, BOSTER Bio-technology, Wuhan, China) was then used to visualize the immunocomplexes, according to the manufacturer’s recommendations.
For statistical analysis, we assessed the staining intensity and area. The staining intensity score was: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong); the staining area score was: 0 (none), 1 ((1-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%) . The staining intensity score and staining area score were then multiplied to produce a final score. Scores 0 to 5 were regarded as negative staining and score 6 to 12 were considered positive staining.
For H&E staining, the sections were stained with hematoxylin and eosin after being rehydrated.
Drug screening
Cells were treated with the following drugs: AZD5153 (BRD4 inhibitor), MK2206 (AKT inhibitor), Rapamycin (mTOR inhibitor), MK1775 (WEE1 inhibitor), LY3214996 (ERK1/2 inhibitor), AZD7762 (Chk1 inhibitor), Panobinostat (HDAC inhibitor), AZD6244 (MEK inhibitor), VE821 (ATR inhibitor), KU55933 (ATM inhibitor), Olaparib (PARP inhibitor). After incubation with the drugs for 24 h, qPCR assays were performed to assess Mus81 level. All drugs were purchased from SELLECK (Houston, TX, USA).
Bioinformatics analysis
We used bioinformatic analysis to elucidate the relationship between BRD4 target genes and Mus81. Microarray quantitative analysis performed on human gastric cancer tissue samples and gastric cancer cells was kindly provided by Dr. Jae-Ho Cheong (21). The raw datasets were preprocessed individually using the LIMMA software package with log2 transformation and annotated by converting different probe IDs to the respective gene symbols. Duplicate gene expression values were averaged. Genes co-expressed with Mus81 were screened in cell and tissue samples according to the Pearson correlation coefficient (P < 0.05). We selected BRD4 targets using the Biogrid database (https://thebiogrid.org/). The genes that were co-expressed with Mus81 and were BRD4 targets, were visualized in a Venn diagram.
In vivo metastasis assays
Five-week-old Balb/C null male mice were purchased from HFK Bio-technology (Beijing, China). All animal experiments were approved by the Animal Care Committee of the Huazhong University of Science and Technology. For the metastasis assays, 2 × 106 control or Mus81-depleted SGC7901 cells/200 μL PBS were injected into mice through the tail vein. Two weeks later, mice were randomly dived into two subgroups: treated or untreated with AZD5153, for a total of four groups: control group, Mus81-depleted group, AZD5153-treated group and Mus81-depleted plus AZD5153-treated group. AZD5153 was administered by gavage (5 mg/kg in 2% DMSO + 30% PEG300 + ddH2O) every three days for four weeks. After treatment, mice were sacrificed and the lungs were fixed in 4% neutral formaldehyde. The number of lung metastases was counted and after paraffin embedding, the sections were used to perform H&E staining and immunohistochemistry.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 and GraphPad Prism 5.0 software. Results are presented as the mean ± SD. The t test or ANOVA were used to determine the statistical differences between groups. The χ2 text was used to evaluate the correlation between Mus81 expression and the clinical characteristics of the patients. The correlation between Mus81 and ZEB1 in human gastric cancer tissues was assessed by Pearson’s analysis. P < 0.05 was regarded as statistically significant. * indicates P < 0.05 and ** represents P < 0.01.
Results
Mus81 is overexpressed and positively correlates with lymph node metastasis in patients with gastric cancer
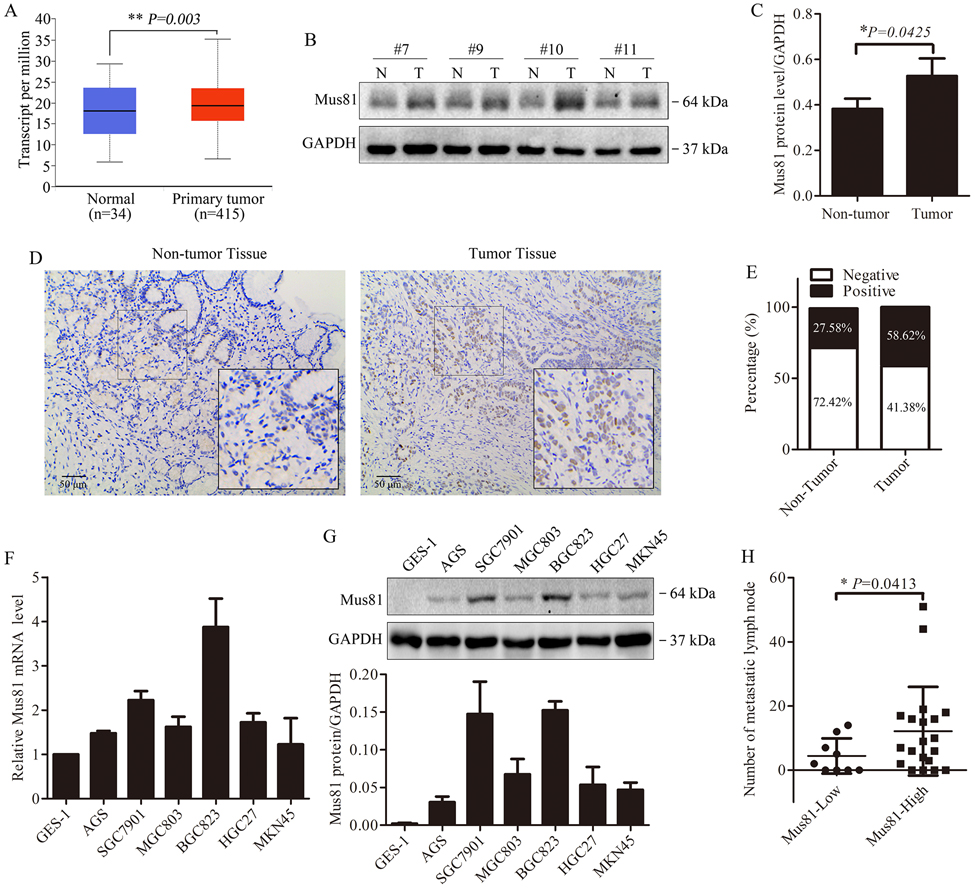
To investigate the expression and potential role of Mus81 in gastric cancer, we analyzed the TCGA database including 415 patients with gastric cancer. We found higher expression of Mus81 in the patients (P = 0.003, Fig. 1A). Then we examined the protein level of Mus81 in the surgical specimens from 29 patients with gastric cancer from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Compared with adjacent tissues, Mus81 was elevated in gastric cancer tissues (P = 0.0425, Fig. 1B, Fig. 1C and Supplementary Fig. 1). Additionally, IHC staining revealed that Mus81 mainly located in the nucleus and was increased in gastric cancer tissues compared with normal tissues (Fig. 1D). According to the IHC scores described in the Methods section, 27.58% of the normal tissues (8/29) were scored positive for Mus81 expression, while 58.62% of the gastric cancer samples (17/29) were positive (Fig. 1E).
Figure 1. Mus81 expression is elevated in gastric cancer and associated with lymph node metastasis.
(A) Expression of Mus81 in gastric cancer tissues and normal tissues from the analysis of the TCGA database (t test). (B) Western blots of Mus81 protein expression in gastric cancer patient specimens (N, non-tumor tissue; T, tumor tissue). (C) Quantification of western blots as in (B) and data are presented as the mean ± SD (t test). (D) Representative images of IHC Mus81 staining in gastric cancer and adjacent tissues from patient specimens. (E) Frequency of Mus81 negative and positive IHC staining in normal and gastric cancer tissues from patient specimens (n=29). (F and G) qPCR analysis of mRNA expression levels (F) and Western blot analysis of protein (G) expression levels of Mus81 in a gastric normal epithelial cell line (GES-1) and 6 indicated gastric cancer cell lines. (H) Correlation between Mus81 expression and the number of metastatic lymph node in 29 patients with gastric cancer (t test). *, P < 0.05; **, P < 0.01.
Next, we checked the expression of Mus81 in a normal gastric mucosal cell line (GES-1) and six human gastric cancer cell lines, and found that the expression in gastric cancer lines was elevated both at the mRNA and protein levels (Fig. 1F and Fig. 1G). Furthermore, we evaluated the correlation between the expression of Mus81 and clinicopathological parameters in the 29 gastric cancer patients aforementioned. Interestingly, high expression of Mus81 positively correlated to the number of lymph node metastases (P = 0.0413, Fig. 1H). We also analyzed the correlation between Mus81 expression and cell migration in gastric cancer cells. As shown in Supplementary Fig. 2, we observed that SGC7901 and BGC823 cells, with the highest Mus81 expression, had stronger migration capabilities compared with the other cells.
These data indicate that high levels of Mus81 probably correlate with gastric cancer progression and are involved in lymph node metastasis in patients with gastric cancer.
Mus81 promotes gastric cancer cell migration in vitro
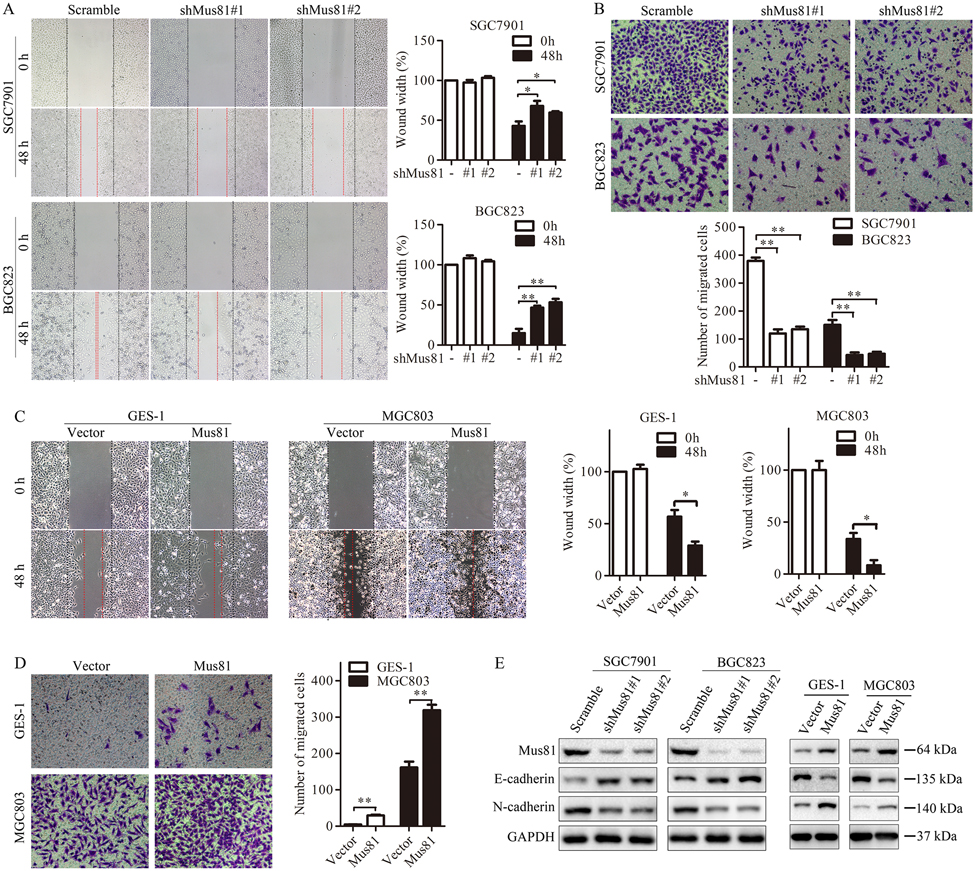
Our preliminary data suggested the potential role of Mus81 in gastric cancer metastasis. Next, we confirmed this correlation. First, we established SGC7901 and BGC823 cells stably knocked down for Mus81, GES-1 and MGC803 cells stably overexpressing Mus81. We confirmed the altered Mus81 expression by qPCR (Supplementary Fig. 3). Then, we performed wound healing and Transwell assays using these cells. Mus81 knockdown decreased the migration ability of gastric cancer cells (Fig. 2A and Fig. 2B), while Mus81 overexpression promoted cell migration (Fig. 2C and Fig. 2D). On the other hand, Mus81 knockdown alone has limited effect on cell proliferation and cell cycle distribution (Supplementary Fig. 4 and Supplementary Fig. 5), suggesting that Mus81 does not affect gastric cancer cell growth.
Figure 2. Mus81 promotes gastric cancer cell migration by regulating EMT in vitro.
(A and B) Cell migration analyses by using wound healing (A) and Transwell (B) assays were performed in Mus81-depleted SGC7901 and BGC823 cells. Data are reported as the mean ± SD of three independent experiments (t test). (C and D) Cell migration analyses were performed in Mus81-overexpressing GES-1 cells and MGC803 cells after transfecting with the Mus81 overexpression vector. Data are presented as the mean ± SD (t test). (E) Western blots showing that the expression of E-cadherin and N-cadherin in Mus81 depleted or overexpressing gastric cancer cells. *, P < 0.05; **, P < 0.01.
E-cadherin and N-cadherin play an important role in EMT, whose dysregulation is linked to cancer metastasis. Loss of E-cadherin indicates EMT and increased ability to metastasis. We found that Mus81 knockdown increased E-cadherin expression and inhibited the expression of N-cadherin in SGC7901 and BGC823 cells. Mus81 overexpression was associated with the opposite effect in GES-1 and MGC803 cells (Fig. 2E).
Therefore, we confirmed that Mus81 plays an important role in enhancing the migration ability of gastric cancer cells.
Mus81 enhances the expression of ZEB1
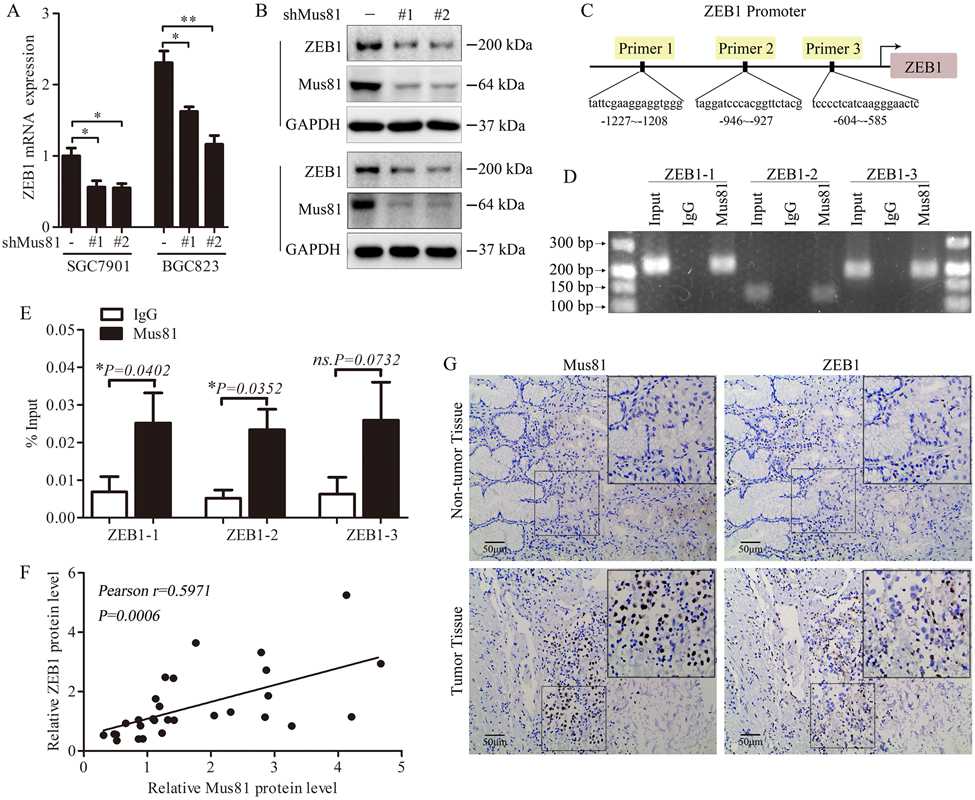
Previous studies have shown that loss of E-cadherin is an important marker of EMT (22), a process that is regulated by several EMT inducers, including ZEB1, ZEB2, Twist1 and Snail1 (23, 24). The data we showed above indicate that Mus81 is involved in the metastasis of gastric cancer. Next, we investigated the mechanism through which Mus81 exerts its function. For this purpose, we checked the correlation between Mus81 and EMT inducers. Knockdown of Mus81 decreased the mRNA and protein expression of ZEB1 (Fig. 3A and Fig. 3B) and did not affect the expression of ZEB2, Twist1 and Snail1 (Supplementary Fig. 6). Therefore, we hypothesized that Mus81 regulates the expression of ZEB1 at the transcriptional level. To confirm our hypothesis, we performed ChIP assays and investigated whether Mus81 binds the ZEB1 promoter (Fig. 3C). We observed significant binding between Mus81 and the promoter region of ZEB1 (−1227 to −1208 and −946 to −927 regions; Fig.3D and Fig.3E). Therefore Mus81 might act as a novel transcriptional regulator of ZEB1 in gastric cancer cells.
Figure 3. Mus81 regulates ZEB1 gene expression in gastric cancer.
(A) qPCR analysis of ZEB1 mRNA level in Mus81-depleted gastric cancer cells. Data are reported as mean ± SD of three independent experiments (t test). (B) Western blots of ZEB1 expression in Mus81-depleted gastric cancer cells. (C) Diagram of ZEB1 primers used for ChIP assays. (D and E) ChIP-qPCR was used to evaluate of Mus81 enrichment at the promoter region of ZEB1. Representative agarose gel image of DNA fragments amplified in the ChIP assays (D); q-PCR analysis of ChIP assays (E). Data presented as the mean ± SD of three independent experiments (t test). (F) Pearson correlation analysis to determine the correlation between Mus81 and ZEB1 expression in patients with gastric cancer (n = 29). (G) Representative images of IHC staining of Mus81 and ZEB1 in gastric cancer and adjacent tissues (n=29). *, P < 0.05; **, P < 0.01; ns., not significant.
To further validate the correlation between Mus81 and ZEB1, we examined ZEB1 expression in gastric cancer tissues. As showed in Fig. 3F, Mus81 expression positively correlated with the expression of ZEB1 in the 29 patients with gastric cancer investigated in this study (P = 0.0006, r = 0.5893). IHC staining also confirmed the result (Fig. 3G). Therefore, Mus81 can bind to the promoter region of ZEB1 and enhance its transcription. These data are consistent with the effect of Mus81 on the migration of gastric cancer cells.
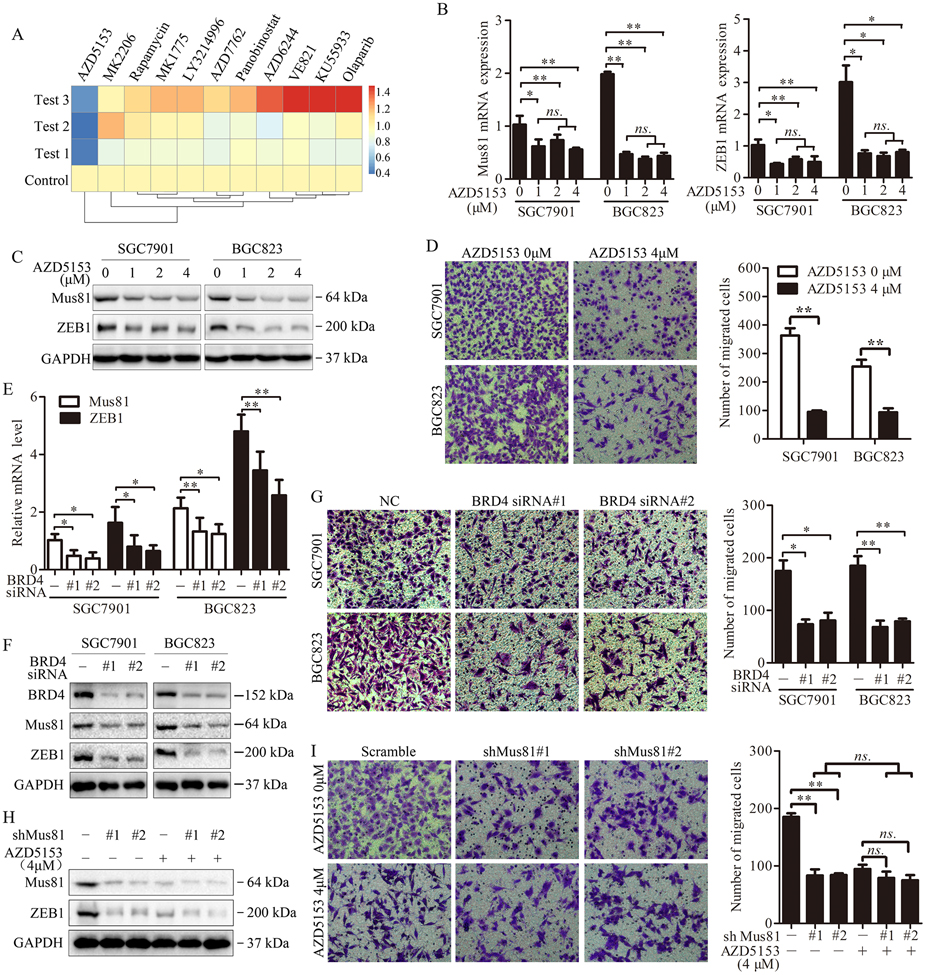
The BRD4 inhibitor AZD5153 suppresses the expression of Mus81 and impairs the migration of gastric cancer cells
Next, we investigated whether we could use drugs or chemical compounds to suppress the expression of Mus81 and impair metastasis. Therefore, we performed a drug screening in SGC7901 cells by using several promising drugs which have been used in clinical trials or have been proved by the FDA, such as PARP inhibitors, BRD4 inhibitors and WEE1 inhibitors (25-28). We treated the cells with these drugs at the indicated concentrations for 24 h, and assessed the expression of Mus81 by qPCR. The BRD4 inhibitor AZD5153 significantly inhibited the expression of Mus81 in SGC7901 cells (Fig. 4A). We further confirmed that AZD5153 suppressed Mus81 and ZEB1 expression at both the mRNA and protein level in SGC7901 and BGC823 cells (Fig. 4B and 4C). Transwell assays also showed that AZD5153 significantly impairs the migration of gastric cancer cells (Fig. 4D). To confirm the specificity of AZD5153 effect, we also inhibited BRD4 using specific siRNAs, and observed similar results of AZD5153 (Fig. 4E-G), confirming that BRD4 regulates the expression of Mus81 and inhibits cell migration in gastric cancer.
Figure 4. The BRD4 inhibitor AZD5153 suppresses cell migration via targeting Mus81 in gastric cancer cells.
(A) Heat map of Mus81 expression levels in SGC7901 cells treated with the indicated drugs for 24 h. Data are presented for three independent experiments. (B and C) qPCR (B) and western blot (C) analyses of Mus81 and ZEB1 expression levels in SGC7901 and BGC823 cells treated with indicated concentrations of AZD5153 (one-way ANOVA). (D) Cell migration analysis using Transwell assays in SGC7901 and BGC823 treated with AZD5153 at the indicated concentration for 24 hours. Data are reported as the mean ± SD of three independent experiments (one-way ANOVA). (E and F) qPCR and western blot analyses of Mus81 and ZEB1 expression levels in SGC7901 and BGC823 cells after transfected with BRD4 siRNAs (G) Cell migration analysis using Transwell assays in BRD4 knockdown SGC7901 and BGC823 cells. Data are reported as the mean ± SD of three independent experiments (t test). (H) Western blot showed Mus81 expression in Mus81-depleted BGC823 cells treated with AZD5153 at the indicated concentration. (I) Cell migration analysis using Transwell assays in Mus81-depleted BGC823 cells treated with AZD5153 at indicated the concentration. Data are reported as the mean ± SD of three independent experiments. *, P < 0.05; **, P < 0.01; ns., not significant.
To further prove that the effect of BRD4 on cell migration is exerted via Mus81, BGC823 cells and Mus81-depleted BGC823 cells were treated with AZD5153. As shown in Fig. 4H and Fig. 4I, AZD5153 had limited effect on cell migration in Mus81-depleted cells, indicating that BRD4 affects cell migration in a Mus81-dependent manner.
Collectively, our data demonstrated that BRD4 regulates the expression of Mus81 in gastric cancer and BRD4 inhibition suppresses cell migration in a Mus81-dependent manner.
BRD4 regulates the expression of Mus81 via Sirt5
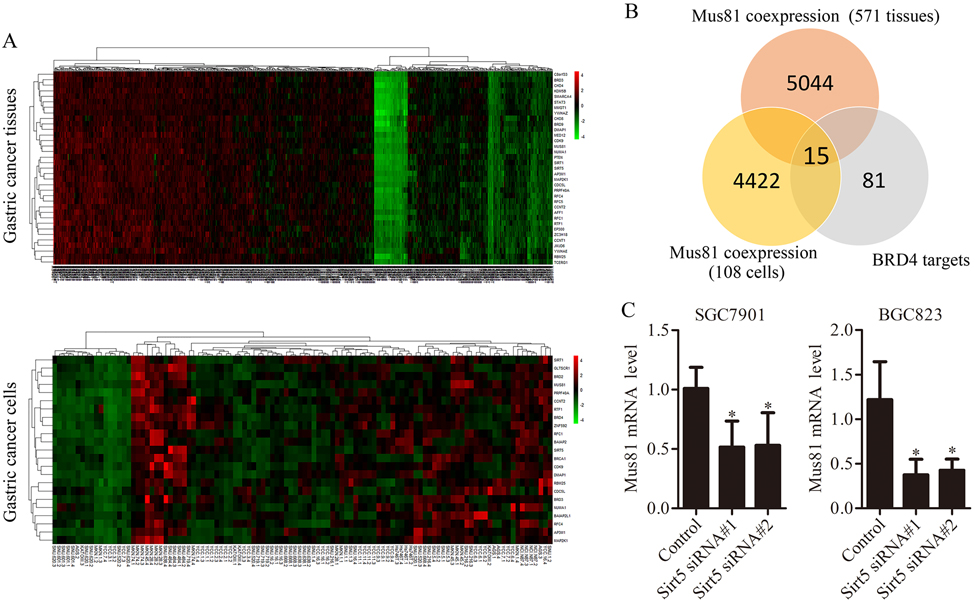
Next, we investigated how BRD4 regulates Mus81 expression. First, we tested whether BRD4 directly regulates Mus81 expression. As showed in Supplementary Fig. 7, Immunoprecipitation (IP) and ChIP assays showed there no direct interaction between BRD4 and Mus81. Therefore, we performed quantitative analysis of a chipset of 517 human gastric cancer specimens and 36 gastric cancer cell lines (triplicate for every cell lines) from Dr. Jae-Ho Cheong. As shown in Fig. 5A and Supplementary Fig. 8, there were 4,422 and 5,044 genes in the specimens and in the gastric cancer cell lines, respectively, co-expressed with Mus81 (P < 0.05). Within them, we selected 15 reported BRD4 target genes, which were co-expressed with Mus81 in gastric cancer tissues and cells (Fig. 5B) (29). Next, we designed specific siRNA to target them found that the Sirt5 was involved in BRD4-mediated Mus81 regulation (Fig. 5C). Taken together, these results suggest that BRD4 might regulate Mus81 in gastric cancer cells through Sirt5.
Figure 5. BRD4 regulates the expression of Mus81 via Sirt5 in gastric cancer cells.
(A) Heat map of genes co-expressed with Mus81 in 517 gastric cancer tissues and 36 gastric cancer cell lines through transcriptomic analysis. (B) Intersection between genes co-expressed with Mus81 (in tissues and cell lines) and BRD4 target genes. (C) Mus81 mRNA levels in Sirt5 knockdown gastric cancer cells. Data was presented as the mean ± SD of three independent experiments (t test). *, P < 0.05; **, P < 0.01.
Sirt5 inhibition impairs the transcriptional function of Mus81 in gastric cancer cells
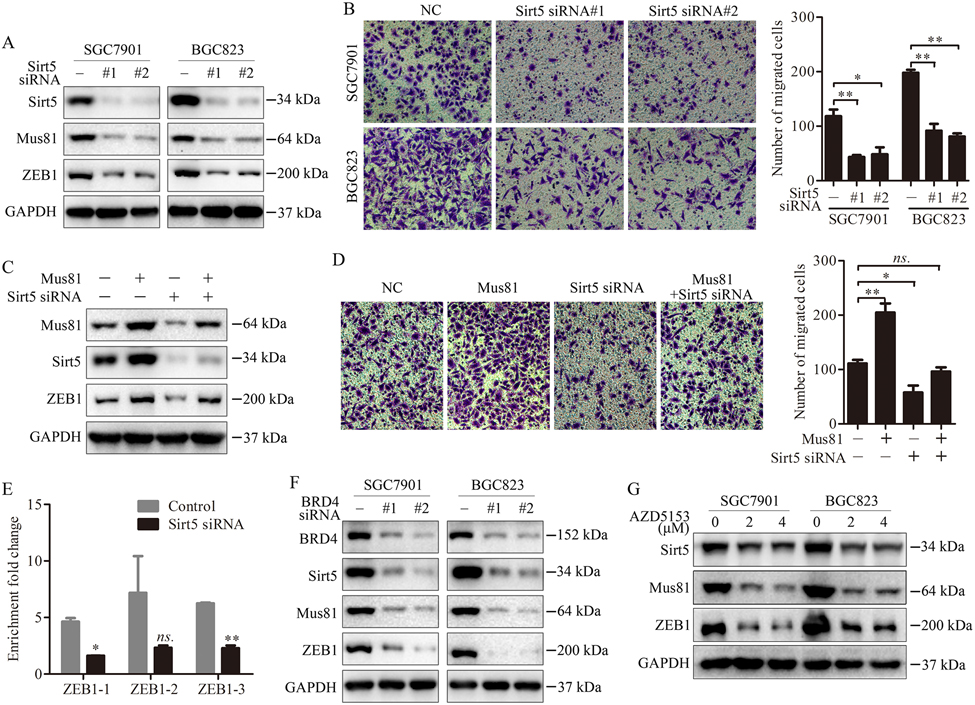
We further investigated how Sirt5 regulates Mus81. We detected the protein levels of Mus81 in Sirt5-silenced gastric cancer cells, and found that Sirt5 inhibition significantly suppressed the expression of Mus81 and ZEB1 in SGC7901 and BGC823 cells (Fig. 6A). In addition, knockdown of Sirt5 inhibited cell migration (Fig. 6B). We also inhibited Sirt5 expression in Mus81-overexpressing SGC7901 cells. As expected, we observed that knockdown of Sirt5 could inhibit the ectopic expression of Mus81 and Mus81-induced cell migration (Fig. 6C and 6D). Additionally, ChIP assays indicated that Sirt5 inhibition significantly suppressed the transcriptional function of Mus81 (Fig. 6E).
Figure 6. Sirt5 inhibition impairs the Mus81-induced transcriptional activity in gastric cancer cells.
(A) Western blot showed the expression of Mus81 and ZEB1 in SGC7901 and BGC823 cells treated with Sirt5 siRNAs. (B) Cell migration analysis using Transwell assays in Sirt5 knockdown SGC7901 and BGC823 cells. Data are reported as the mean ± SD of three independent experiments (t test). (C) Western blot showed Mus81 expression in Mus81-overexpressing SGC7901 cells transfected with Sirt5 siRNAs. (D) Cell migration analysis using Transwell assays in Mus81-overexpressing SGC7901 cells transfected with Sirt5 siRNAs. Data are reported as the mean ± SD of three independent experiments (t test). (E) ChIP assays of Mus81 enrichment on ZEB1 promoter regions in cells transfected with Sirt5 siRNAs. Data are reported as the mean ± SD of three independent experiments (t test). (F) Western blot showed the expression of Sirt5, Mus81 and ZEB1 in SGC7901 and BGC823 cells treated with BRD4 siRNAs. (G) Western blot showed the expression of Sirt5, Mus81 and ZEB1 in SGC7901 and BGC823 cells treated with AZD5153. *, P < 0.05; **, P < 0.01; ns., not significant.
To confirm our findings, we also examined the effect of BRD4 inhibition (ADZ5153 or BRD4 knockdown) on the expression of Sirt5, Mus81 and ZEB1 in gastric cancer cells, and found that BRD4 inhibition suppressed Sirt5, Mus81 and ZEB1 expression cells (Fig. 6F and Fig. 6G). Taken together, our data demonstrate that BRD4 regulates the expression of Mus81 via its target Sirt5 in gastric cancer cells.
AZD5153 decreases gastric cancer cells metastasis via Mus81 in vivo
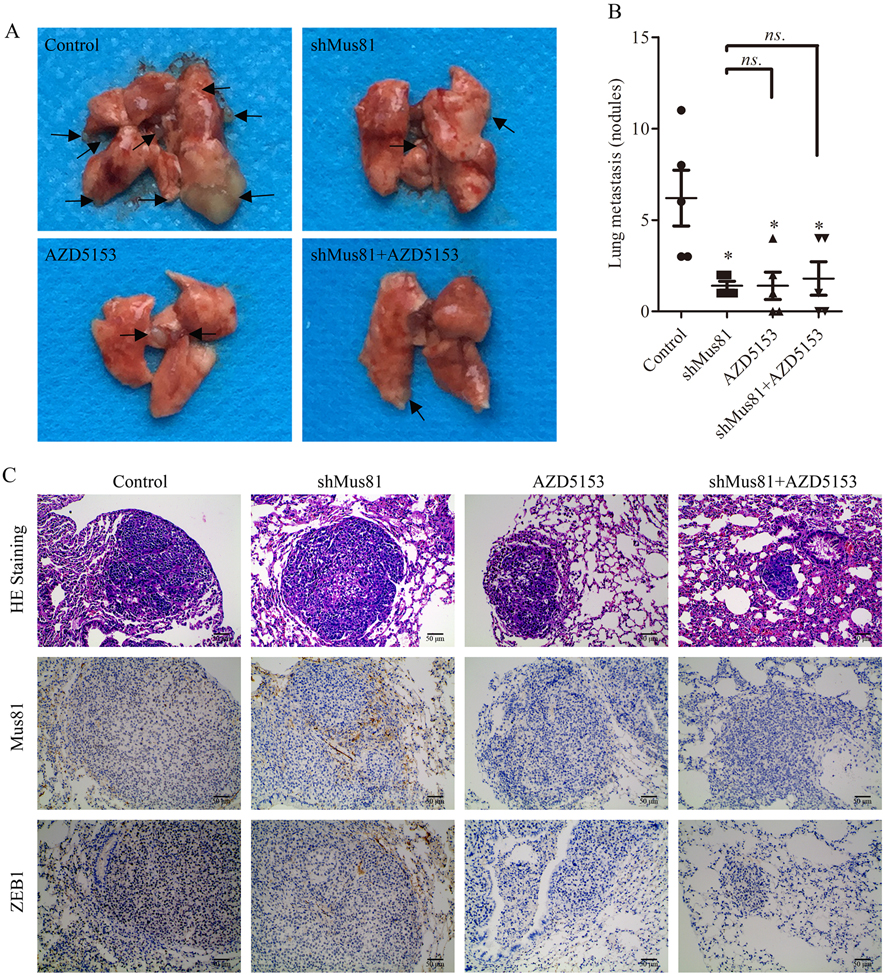
To further confirm our findings, control or Mus81-depleted SGC7901 cells were intravenously injected via tail vein in nude mice. As shown in Fig. 7A and 7B and Supplementary Fig. 9, mice injected with Mus81 knockdown cells had reduced number of lung metastasis nodes than the control group (P < 0.05). Similarly, AZD5153 treatment significantly inhibited lung metastasis compared with the control group (P < 0.05). However, AZD5153 had limited effect in the Mus81-depleted SGC7901 cells group. H&E staining showed the pathology of lung metastasis, which confirmed that the lumps in the lung of the nude mice was formed by gastric cancer cells. IHC staining showed that AZD5153 inhibited the expression of Mus81 and ZEB1 in gastric cancer cells (Fig. 7C). Therefore, AZD5153 negatively regulated the metastasis-promoting effect of Msu81 in vivo.
Figure 7. The BRD4 inhibitor AZD5153 decreases gastric cancer cells metastasis via targeting Mus81 in vivo.
(A) Images of nude mice lung metastases (arrows point to the metastasis nodules). (B) Quantification of the nodules in the control, Mus81-depleted, AZD5153-treated and Mus81-depleted plus AZD5153-treated groups, presented as the mean ± SD (n = 5, t test). (C) H&E staining of lung nodules in the control, Mus81-depleted, AZD5153-treated and Mus81-depleted plus AZD5153-treated mice. IHC staining was used to determine the expression of Mus81 and ZEB1 in the control, Mus81-depleted, AZD5153-treated and Mus81-depleted plus AZD5153-treated mice. *, P < 0.05; ns., not significant.
Discussion
DNA damage-induced genome instability is a hallmark of malignant diseases (30), and may promote tumor metastasis (31). Mus81 has been identified as an important component of the HR-mediated pathway for the repair of double strand breaks in mammalian cells, and is considered a potential therapeutic target in many cancer types (32). However, its role in gastric cancer has not been fully understood. In this study, we demonstrated the role of Mus81 in the metastasis of gastric cancer. Firstly, we found that elevated expression of Mus81 was significantly associated with the number of metastatic lymph node in patients with gastric cancer. Secondly, Mus81 was showed to be involved in the EMT program, through the decrease of the expression of the epithelial marker E-cadherin and the increased expression of the mesenchymal inducer ZEB1. Thirdly, we showed that Mus81 promoted migration in gastric cancer cells in vitro and in vivo. Taken together, our data demonstrate that Mus81 is involved in gastric cancer metastasis and highlight a novel function of Mus81 in cancer. Our findings provide yet another piece of evidence that genome instability is involved in cancer metastasis.
Previous studies on Mus81 have mainly focused on its function on DNA structure, such its role in sustaining replication forks (33). It has also been reported that Mus81 has a comprehensive role in cancer initiation, cell proliferation, apoptosis and sensitivity to chemotherapy agents (34). In this study, we first demonstrated that Mus81 is required for the migration of gastric cancer cells. Importantly, this is the first study to demonstrate that Mus81 can act as a ZEB1 transcriptional regulator. EMT is related to carcinogenesis and cancer metastasis, and ZEB1 is a well-characterized EMT inducer. ZEB1 not only plays a vital role in EMT and cancer metastasis but is also correlated with drug resistance in cancer therapy. Therefore, to search for molecules which could regulate ZEB1 expression is a reasonable strategy to negatively affect cancer metastasis and drug resistance.
A variety of molecules have been reported to regulate ZEB1, such as microRNAs (35). However, without any enzymatic activities, ZEB1 itself does not serve as a good drug target. Thus, as an alternative approach, it might be more promising to identify and target transcription regulators of ZEB1. We identified Mus81 as such regulator. Additionally, we screened several promising drugs and found that the BRD4 inhibitor AZD5153 suppresses the expression of Mus81 at both the mRNA and protein level and inhibits migration via Mus81 in gastric cancer cells. Dysregulation of the epigenome has been recognized as a key step in the activation and maintenance of abnormal transcription in cancer (36, 37). Therefore, targeting chromatin regulators, such as BET proteins, including BRD2 and BRD4, might be therapeutically effective. BRD4 inhibitors are the most promising BET inhibitors and are therapeutically effective in several cancer types, such as melanoma and ovary cancer (38, 39). Additionally, BRD4 inhibition can impair metastasis formation in many cancer types, including gastric cancer (40), though the mechanism of this effect is still unclear. Our data showed that Mus81 is regulated by BRD4 in gastric cancer cells, and BRD4 inhibition suppresses migration in a Mus81-dependent manner.
We also investigated the mechanism through which BRD4 regulates Mus81 expression in gastric cancer. Our data indicated that BRD4 does not directly regulate Mus81. BRD4 is a transcriptional regulator of many DNA regulatory molecules (41). Therefore, we supposed that Mus81 was regulated by a BRD4 target. We performed bioinformatics analyses on an expression dataset to search for potential BRD4 targets involved in Mus81 regulation, and confirmed that the BRD4 target Sirt5 can regulate Mus81 in gastric cancer. Sirt5 is a member of the Sirtuin family, whose members are lysine deacetylases and desuccinylase (42). Sirt5 promotes cell proliferation and drug resistance in many cancer types (43, 44). It is worthwhile to investigate the mechanism trough which Sirt5 regulates Mus81. Because of the important role of Sirt5 in cell metabolism, many pathways may be involved in this process. Future studies might focus on this aspect.
In conclusion, we have revealed a novel functional role of Mus81: the promotion of the migration of gastric cancer in vitro and in vivo. Mus81 transcriptional effect on ZEB1 gives it potential value as a target for cancer therapy to interfere with metastases formation. Finally, we demonstrated that BRD4 inhibitors are good candidates to inhibit gastric cancer metastasis.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant Nos.81572413 (to K.X. Tao), 81874184 (to K.X. Tao), and 81702386 (to P. Zhang) , the Foundation of Independent Innovation Fund of Huazhong University of Science and Technology grant No.2017KFYXJJ230 (to K.X. Tao) and the Natural Science Foundation of Hubei Province grant No.2016CFA100 (to K.X. Tao).
Footnotes
Conflict of interest: All authors declare no conflict of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- 3.Kankeu Fonkoua L, Yee NS. Molecular Characterization of Gastric Carcinoma: Therapeutic Implications for Biomarkers and Targets. Biomedicines 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinoshita K, Beppu T, Miyata T, Kuramoto K, Yoshida Y, Umesaki N, et al. A Case of 15-Year Recurrence-free Survival After Microwave Coagulation Therapy for Liver Metastasis from Gastric Cancer. Anticancer Res 2018;38:1595–8. [DOI] [PubMed] [Google Scholar]
- 5.Till JE, Yoon C, Kim BJ, Roby K, Addai P, Jonokuchi E, et al. Oncogenic KRAS and p53 Loss Drive Gastric Tumorigenesis in Mice That Can Be Attenuated by E-Cadherin Expression. Cancer Res 2017;77:5349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian Y, Wong CC, Xu J, Chen H, Zhang Y, Kang W, et al. Sodium Channel Subunit SCNN1B Suppresses Gastric Cancer Growth and Metastasis via GRP78 Degradation. Cancer Res 2017;77:1968–82. [DOI] [PubMed] [Google Scholar]
- 7.Limpose KL, Trego KS, Li Z, Leung SW, Sarker AH, Shah JA, et al. Overexpression of the base excision repair NTHL1 glycosylase causes genomic instability and early cellular hallmarks of cancer. Nucleic Acids Res 2018;46:4515–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prado F. Homologous recombination maintenance of genome integrity during DNA damage tolerance. Mol Cell Oncol 2014;1:e957039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi K, Zhao X, Tock AJ, Lambing C, Underwood CJ, Hardcastle TJ, et al. Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res 2018;28:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickoloff JA. Paths from DNA damage and signaling to genome rearrangements via homologous recombination. Mutat Res 2017;806:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner E, Wang Y, Doetsch PW. A Single Exposure to Low- or High-LET Radiation Induces Persistent Genomic Damage in Mouse Epithelial Cells In Vitro and in Lung Tissue. Radiat Res 2017;188:373–80. [DOI] [PubMed] [Google Scholar]
- 12.Lai X, Broderick R, Bergoglio V, Zimmer J, Badie S, Niedzwiedz W, et al. MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells. Nat Commun 2017;8:15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Marco S, Hasanova Z, Kanagaraj R, Chappidi N, Altmannova V, Menon S, et al. RECQ5 Helicase Cooperates with MUS81 Endonuclease in Processing Stalled Replication Forks at Common Fragile Sites during Mitosis. Mol Cell 2017;66:658–71 e8. [DOI] [PubMed] [Google Scholar]
- 14.Ciccia A, Constantinou A, West SC. Identification and characterization of the human mus81-eme1 endonuclease. J Biol Chem 2003;278:25172–8. [DOI] [PubMed] [Google Scholar]
- 15.McPherson JP, Lemmers B, Chahwan R, Pamidi A, Migon E, Matysiak-Zablocki E, et al. Involvement of mammalian Mus81 in genome integrity and tumor suppression. Science 2004;304:1822–6. [DOI] [PubMed] [Google Scholar]
- 16.Dendouga N, Gao H, Moechars D, Janicot M, Vialard J, McGowan CH. Disruption of murine Mus81 increases genomic instability and DNA damage sensitivity but does not promote tumorigenesis. Mol Cell Biol 2005;25:7569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie S, Zheng H, Wen X, Sun J, Wang Y, Gao X, et al. MUS81 is associated with cell proliferation and cisplatin sensitivity in serous ovarian cancer. Biochem Biophys Res Commun 2016;476:493–500. [DOI] [PubMed] [Google Scholar]
- 18.Wu F, Liu SY, Tao YM, Ou DP, Fang F, Yang LY. Decreased expression of methyl methansulfonate and ultraviolet-sensitive gene clone 81 (Mus81) is correlated with a poor prognosis in patients with hepatocellular carcinoma. Cancer 2008;112:2002–10. [DOI] [PubMed] [Google Scholar]
- 19.Rondinelli B, Gogola E, Yucel H, Duarte AA, van de Ven M, van der Sluijs R, et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat Cell Biol 2017;19:1371–8. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Ning S, Wei Z, Xu R, Xu X, Xing M, et al. 53BP1 and BRCA1 control pathway choice for stalled replication restart. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol 2018;19:629–38. [DOI] [PubMed] [Google Scholar]
- 22.Kim WK, Byun WS, Chung HJ, Oh J, Park HJ, Choi JS, et al. Esculetin suppresses tumor growth and metastasis by targeting Axin2/E-cadherin axis in colorectal cancer. Biochem Pharmacol 2018;152:71–83. [DOI] [PubMed] [Google Scholar]
- 23.Campbell K, Lebreton G, Franch-Marro X, Casanova J. Differential roles of the Drosophila EMT-inducing transcription factors Snail and Serpent in driving primary tumour growth. PLoS Genet 2018;14:e1007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Zhang W, Li Y, Alvarez A, Li Z, Wang Y, et al. SHP-2-upregulated ZEB1 is important for PDGFRalpha-driven glioma epithelial-mesenchymal transition and invasion in mice and humans. Oncogene 2016;35:5641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchtel KM, Vogel Postula KJ, Weiss S, Williams C, Pineda M, Weissman SM. FDA Approval of PARP Inhibitors and the Impact on Genetic Counseling and Genetic Testing Practices. J Genet Couns 2018;27:131–9. [DOI] [PubMed] [Google Scholar]
- 26.Mendez E, Rodriguez CP, Kao MC, Raju S, Diab A, Harbison RA, et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res 2018;24:2740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschos SJ, Sullivan RJ, Hwu WJ, Ramanathan RK, Adjei AA, Fong PC, et al. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun C, Yin J, Fang Y, Chen J, Jeong KJ, Chen X, et al. BRD4 Inhibition Is Synthetic Lethal with PARP Inhibitors through the Induction of Homologous Recombination Deficiency. Cancer Cell 2018;33:401–16 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol Cell Biol 2011;31:2641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018;553:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia J, Shi Y, Chen L, Lai W, Yan B, Jiang Y, et al. Decrease in Lymphoid Specific Helicase and 5-hydroxymethylcytosine Is Associated with Metastasis and Genome Instability. Theranostics 2017;7:3920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemacon D, Jackson J, Quinet A, Brickner JR, Li S, Yazinski S, et al. MRE11 and EXO1 nucleases degrade reversed forks and elicit MUS81-dependent fork rescue in BRCA2-deficient cells. Nat Commun 2017;8:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisakova A, Altmannova V, Sebesta M, Krejci L. Role of PCNA and RFC in promoting Mus81-complex activity. BMC Biol 2017;15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu F, Su SC, Tan GQ, Yan L, Li TY, Zhang HL, et al. Mus81 knockdown sensitizes colon cancer cells to chemotherapeutic drugs by activating CHK1 pathway. Clin Res Hepatol Gastroenterol 2017;41:592–601. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Men X, Zhao R, Han J, Fan Z, Wang Y, et al. miR-200c inhibits TGF-beta-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther 2018;25:68–76. [DOI] [PubMed] [Google Scholar]
- 36.Kuser-Abali G, Gong L, Yan J, Liu Q, Zeng W, Williamson A, et al. An EZH2-mediated epigenetic mechanism behind p53-dependent tissue sensitivity to DNA damage. Proc Natl Acad Sci U S A 2018;115:3452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Takenobu H, Setyawati AN, Akita N, Haruta M, Satoh S, et al. EZH2 regulates neuroblastoma cell differentiation via NTRK1 promoter epigenetic modifications. Oncogene 2018;37:2714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrieu GP, Denis GV. BET Proteins Exhibit Transcriptional and Functional Opposition in the Epithelial-to-Mesenchymal Transition. Mol Cancer Res 2018;16:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riganti C, Lingua MF, Salaroglio IC, Falcomata C, Righi L, Morena D, et al. Bromodomain inhibition exerts its therapeutic potential in malignant pleural mesothelioma by promoting immunogenic cell death and changing the tumor immune-environment. Oncoimmunology 2018;7:e1398874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong X, Hu X, Chen J, Hu D, Chen LF. BRD4 regulates cellular senescence in gastric cancer cells via E2F/miR-106b/p21 axis. Cell Death Dis 2018;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Baek G, Ramanand SG, Sharp A, Gao Y, Yuan W, et al. BRD4 Promotes DNA Repair and Mediates the Formation of TMPRSS2-ERG Gene Rearrangements in Prostate Cancer. Cell Rep 2018;22:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1beta Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep 2017;19:2331–44. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Wang Z, Li X, Liu B, Liu M, Liu L, et al. SHMT2 Desuccinylation by SIRT5 Drives Cancer Cell Proliferation. Cancer Res 2018;78:372–86. [DOI] [PubMed] [Google Scholar]
- 44.Du Z, Liu X, Chen T, Gao W, Wu Z, Hu Z, et al. Targeting a Sirt5-Positive Subpopulation Overcomes Multidrug Resistance in Wild-Type Kras Colorectal Carcinomas. Cell Rep 2018;22:2677–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.