INTRODUCTION:
Histopathological alterations in the ileum and colon in irritable bowel syndrome (IBS) are controversial, and normal values are poorly established. We hypothesized that changes in mucosal immune cells characterize IBS and key changes in immune composition are associated with the mucosa-associated microbiota (MaM).
METHODS:
A nested case-control study (48 IBS and 106 controls included) from 745 colonoscopy participants in a random population sample. Intraepithelial lymphocytes (IELs)/100 enterocytes and eosinophils/5 nonoverlapping high-power fields counted; mast cells identified by immunocytochemistry (CD117)/5 high-power fields. Paneth cells quantified per 5 crypts. 16S rRNA gene amplicon sequencing performed on available sigmoid MaM, n = 55 and fecal microbiota, n = 20. Microbiota profiles compared between samples with high and low IEL counts.
RESULTS:
IBS had increased IELs in the terminal ileum (relative risk ratio = 1.70, 95% confidence interval 1.08–2.76, P = 0.022 adjusted for age, sex, and smoking). Cecal IELs were increased in IBS—diarrhea (relative risk ratio = 2.03, 95% confidence interval 1.13–3.63, P = 0.017). No difference was observed in alpha diversity of MaM or fecal microbiota based on IEL count. There was no difference in beta diversity of the MaM according to IEL count in the terminal ileal (TI) (P = 0.079). High TI IEL counts associated with a significant expansion of the genus Blautia (P = 0.024) and unclassified Clostridiales (P = 0.036) in colon MaM.
DISCUSSION:
A modest but significant increase in IELs was observed in IBS vs. controls in a population-based setting. Subtle TI and cecal inflammation may play a pathogenic role in IBS but needs confirmation. Modest but discernible differences in the colonic MaM were seen according to TI IEL count but not IBS status.
INTRODUCTION
The irritable bowel syndrome (IBS) is a common, chronic disorder characterized by unexplained abdominal pain associated with disturbed defecation (1,2). In those with typical symptoms, an underlying organic explanation has been reported to be unlikely, although mimics of IBS include celiac disease and microscopic colitis (1,2). The etiopathogenesis of IBS is unclear, but it is likely a heterogeneous collection of gut-brain diseases (3).
Emerging data suggest immune activation is present in a major subset with IBS, especially in those with diarrhea, indirectly implicating subtle intestinal inflammation (4). Similar findings of immune activation have been observed in functional dyspepsia (FD), a disorder that overlaps with IBS, and increased eosinophils (and in some cases mast cells) have been observed in the upper small intestinal (duodenum) in a major subgroup with FD (4,5). If immune homeostasis is dysregulated in IBS and FD, with a Th2 or Th17 response, then subtle increases in intestinal mast cells and eosinophils may occur (6). Although studies of intestinal eosinophilia are few in IBS (7), there is major interest in the potential role of mast cells, but this is controversial; studies from Italy and Spain suggest colonic mast cells are increased in IBS, whereas studies from Northern Europe and the United States have not confirmed the findings (8–12). Increased intraepithelial lymphocytes (IELs) in colonic biopsies were observed in IBS in a New Zealand study (13), but the small intestine was not examined, and little other data are available. However, none of the past studies were population-based, and selection bias is difficult to exclude in the reports to date.
As there may be intestinal dysbiosis and increased intestinal permeability in IBS (14), we speculated Paneth cells may be altered in IBS which has not been previously explored, although they are reduced in Crohn disease (15). Paneth cells are located throughout the small intestine and importantly play a role in host-microbe interactions through secretion of antimicrobial peptides, as well as helping to maintain intestinal integrity (16). Increased metaplastic Paneth cells are seen in the colon in damage and infection (17).
We aimed to determine what is normal in terms of immune cell counts in the ileocolonic region in subjects identified from a population-based colonoscopy study and determine whether there is histological evidence of increased IELs, mast cells, eosinophils, or Paneth cell changes in the terminal ileum and colon in subjects with IBS. On finding alterations in ileocolonic IELs in IBS, we then investigated whether the mucosa-associated microbiota (MaM) and fecal microbiota (FM) differed between subjects with high and low IEL counts where samples were available.
METHODS
Participants
In this study, ileocolonic biopsies from subjects from the population-based colonoscopic (PopCol) study with a normal colonoscopy, i.e., no inflammatory bowel disorder or spirochetosis or other detected intestinal pathogens that were not taking proton pump inhibitors (PPIs) or nonsteroidal anti-inflammatory drugs (NSAIDs) at the time of investigation, were evaluated histologically.
The PopCol study has previously been described in detail (18). In short, a validated questionnaire was sent out to a sample of 3,556 randomly selected persons of the general population in 2 parishes in Stockholm, Sweden. All 2,293 responders were contacted by phone with an invitation to a hospital visit comprising an interview by a gastroenterologist for medical history and further questionnaires, plus a subsequent research ileocolonoscopy and biopsies. Of the 1,643 responders reached by the invitation, 1,244 accepted a hospital visit, and of these, 745 had the ileocolonoscopy.
The mean age was 44.5 years, and 51% were women in the original study population (the 3,556 randomly selected from the inhabitants in the 2 parishes). It was more difficult to reach younger participants as they may have their registered address with their parents while living elsewhere and often had unregistered prepaid cell phone cards. Also, they were less inclined to take time off work to do the investigation. In addition, IBS is more common in women. Of those who had the colonoscopy, the mean age was 51.7 years and 57.2% were women (18). Otherwise responders were very similar to nonresponders in terms of clinical characteristics, as previously reported in detail (18).
Of the 745 subjects who had the colonoscopy, 13 had inflammatory bowel disease including microscopic colitis, 17 had spirochetosis, 12 had other intestinal pathogens such as Clostridium difficile, Salmonella, Candida, or spring worms, and PPIs and NSAIDs were taken by 32 and 52 subjects, respectively. Only subjects who had a completely normal colonoscopy and no evidence of organic or infectious gastrointestinal (GI) disease and who were not taking PPIs or NSAIDs are included in this study. All the subjects in this study were born in Sweden; the Swedish population is genetically similar to other populations of Northern Europe (19) and the diet in Sweden, and especially Stockholm, is also similar to Europe including the intake of fermentable carbohydrates (20).
IBS cases
IBS was defined by applying criteria congruent to Rome criteria to the validated Abdominal Symptom Questionnaire (ASQ) (2,18), as previously described. IBS cases were defined as having abdominal pain or discomfort at least weekly in the last 3 months paired with at least 2 of 3 of the following: (i) pain (or discomfort) associated with defecation (pain at defecation or pain improved or relieved by defecation), (ii) onset of pain or discomfort associated with a change in usual number of bowel movements, or (iii) onset of pain or discomfort associated with change in stool consistency, plus a normal colonoscopy. Subtypes were based on stool form reports: IBS—diarrhea was defined as having loose but not hard stools, IBS—constipation was defined as having hard but not loose stools. IBS—mixed was defined as having both, and IBS—unspecified as having neither.
Controls
Controls were similarly identified from the PopCol cohort and were selected if they had reported no abdominal pain or discomfort, or reported pain or discomfort not more than monthly in the past 3 months, plus a normal colonoscopy.
Assessment of possible confounders
Age, sex, and smoking are potential risk factors for IBS and were included in the analyses to control for confounding (21). Participants reported their current smoking status (current smoker or not currently smoking) in a questionnaire at the hospital visit.
The 36-Item Short-Form Survey (SF-36) is a validated measure of health-related quality of life including mood. We included in a post hoc secondary analysis the emotional well-being scale as a potential confounder (22).
Histopathology
Biopsies were taken at ileocolonoscopy from the terminal ileum, cecum, transverse colon, sigmoid colon, and rectum. Blinded assessment of histology was undertaken, and slides were dual read and any disagreement resolved by consensus. The counts were all performed in well-orientated areas of the sections, with epithelial nuclei in parallel by an expert GI pathologist (M.M.W.) (23).
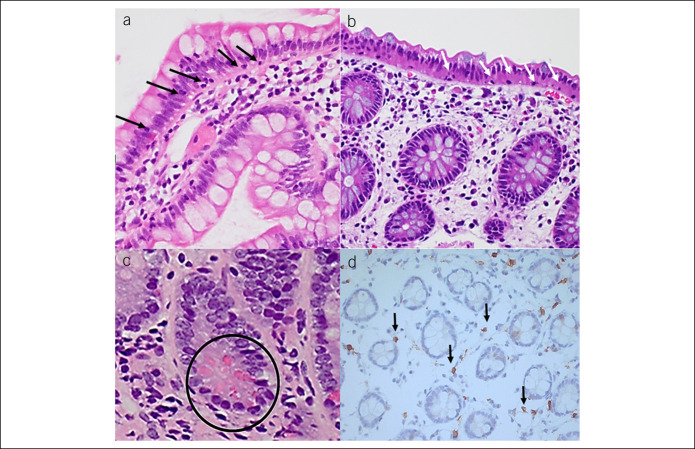
A biopsy from each site was fixed in 10% formalin and routinely processed and stained with hematoxylin and eosin. IELs, eosinophils, and mast cells were counted with an Olympus BX51 light microscope using ×40 objective. IELs were counted per 100 enterocytes/colonocytes, excluding crypts and enterocytes/colonocytes overlying lymphoid aggregates. Immunohistochemistry for mast cells was performed using rabbit anti-human polyclonal antibodies for mast cells, CD117 (c-kit, Dakocytomation) and counterstained with Harris's hematoxylin. Eosinophils and mast cells were counted in 5 nonoverlapping high-power fields and summed to calculate the number of cells per 5 high-power fields. When counting CD117 immunostained mast cells, only those cells with unquestionable cytoplasmic staining or a visible blue nucleus were recorded positive. Paneth cells were identified by their secretory granules on hematoxylin and eosin and location at the base of the crypts and number of Paneth cells/5 crypts counted (Figure 1a–d).
Figure 1.
Ileocolonic histopathological alterations in the irritable bowel syndrome. (a) Terminal ileum biopsy hematoxylin and eosin stain, intraepithelial lymphocytes, arrowed. (b) Cecal biopsy hematoxylin and eosin stain, scant eosinophils in lamina propria, intraepithelial lymphocytes, arrowed. (c) Terminal ileum biopsy hematoxylin and eosin stain, paneth cells, circled. (d) Cecal biopsy immunohistochemistry CD117 stain, mast cells, arrowed.
For the purposes of the microbiome analysis, terminal ileal (TI) IEL counts were dichotomized using >15 per 100 enterocytes to define a high IEL count (based on control data).
Microbiota analyses
16S rRNA gene sequencing data from sigmoid MaM and FM were taken from the PopCol study, with sequencing and bioinformatics processing performed as described previously (24). The data were further subsampled at 10,000 reads, such that 57 samples were removed, and singleton and rare sequences were discarded. Chao and Shannon indices were generated, and Bray-Curtis distance measures were calculated as a measure of Beta diversity. Nonmetric dimensional scaling plots and permutational multivariate analysis of variance tests were performed using the Vegan library within R. Taxonomic profiles were compared at genus level in STAMP (25).
Statistics
Differences in background variables between cases and controls were calculated with the χ2 test for dichotomous variables and the Mann-Whitney rank-sum test for ordinal variables.
Contrasts of cell counts proceeded in 2 stages. First, IBS and controls were compared using binary unconditional logistic regression with cell counts as the independent variables and probability of IBS as the outcome. Second, the probability of being in any IBS subtype (with controls as the reference) was modeled using multinomial logistic regression with cell counts as the outcome. In both sets of models, an odds ratio >1.0 indicates higher cell counts that are associated with increased probability of having IBS (or a specific subtype) as opposed to controls. Conversely, an odds ratio <1.0 indicates higher cell counts that are associated with decreased probability of IBS. Both univariate associations and multivariable associations adjusted for variables selected a priori (age, sex, and smoking) were calculated. A sensitivity analysis was performed also adjusting for mood applying the SF-36 in addition to age, sex, and smoking.
All cell counts were standardized in the regression analyses, so that relative risk ratios (RRRs) are comparable between cell types and localities as a measure of strength of association. Missing data for histology due to missing biopsies or unreadable slides differ between cell types and locations and numbers included in the analyses are provided.
Five subjects had missing data on IBS status, and 6 subjects without IBS but with daily or monthly abdominal pain were excluded leaving 154 subjects.
All statistical analyses were performed in Stata 15.1 (StataCorp, College Station, TX). All P values calculated were 2-tailed, and an alpha level of 0.05 was used for statistical significance in the reporting of the results.
The sample size available was constrained by that available in the original study; however, statistical power was evaluated as adequate (power = 0.87 at α = 0.05) for a minimally important odds ratio of 1.5 or greater when contrasting IBS overall with controls. However, statistical power is low (power = 0.35 at α = 0.05) when contrasting the smallest IBS subtypes with controls under the same conditions.
RESULTS
Demographic data in IBS cases and controls
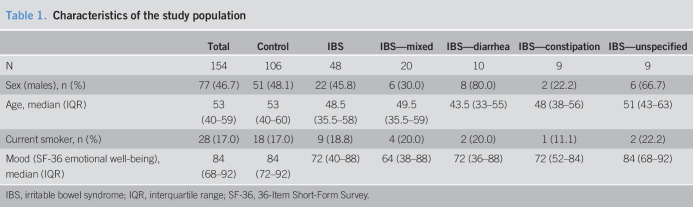
Study group characteristics are given in Table 1. About half of the participants were men (47%), and the mean age was 53 years. IBS subjects (n = 48) and controls (n = 106) were similar in terms of age, sex, and smoking. IBS cases had lower (worse) mood scores than controls on the SF-36 (P = 0.002).
Table 1.
Characteristics of the study population
Histopathology in IBS cases and controls
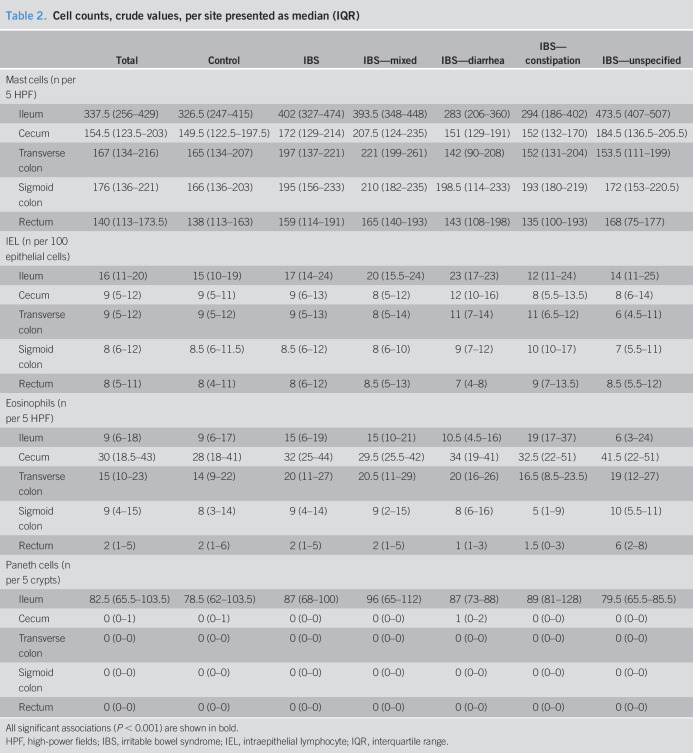
Median and interquartile range of cells counts in total and divided on IBS status are given in Table 2. In total, 39 of the 106 controls reported monthly abdominal pain; there were no significant differences in histological cell counts between controls reporting monthly pain and controls reporting no pain (data not shown).
Table 2.
Cell counts, crude values, per site presented as median (IQR)
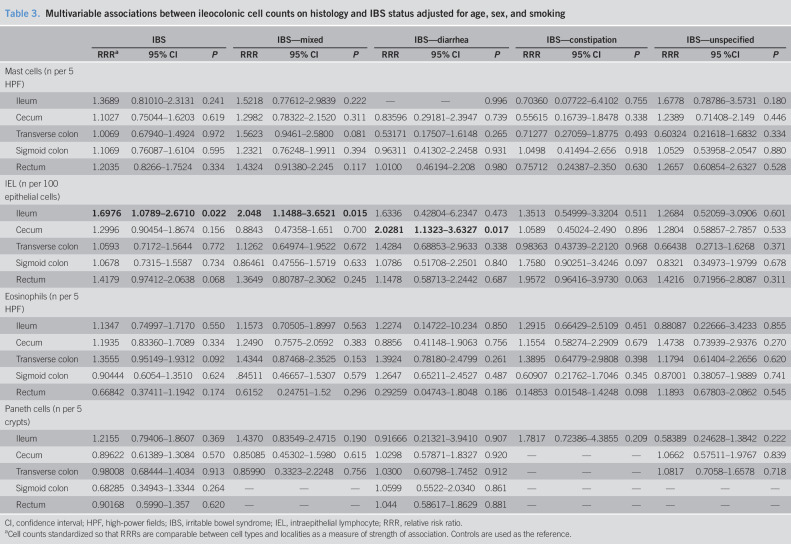
Univariately, there was a statistically significant increase of IELs (Figure 1a) in the terminal ileum in IBS (P = 0.016) and IBS-mixed (P = 0.017). These associations remained after adjustment of age, sex, and smoking (Table 3). There were also increased IELs in IBS—diarrhea in the cecum univariately (P = 0.005) (Figure 1b), and this also held true after adjustment for age, sex, and smoking (Table 3). In a post hoc sensitivity analysis also adjusting for mood, a major component of the mental health scale of the SF-36, in the multivariable models as well as age, sex, and smoking, the association of increased cecal IELs in IBS—diarrhea was retained (RRR = 2.43, 95% confidence interval [CI] 1.26–4.71, P = 0.008), as was the association of TI IELs with IBS overall (RRR = 1.60, 95% CI 1.00–2.56, P = 0.048) and IBS—mixed (RRR = 1.89, 95% CI 1.02–3.49, P = 0.042).
Table 3.
Multivariable associations between ileocolonic cell counts on histology and IBS status adjusted for age, sex, and smoking
Paneth cells (Figure 1c) were not reduced in the terminal ileum in IBS or increased in the colon (Table 3). Eosinophils (Figure 1b) and mast cells (Figure 1d) were not increased in the terminal ileum or colon in IBS (Table 3).
Mucosa-associated and FM in IBS cases and controls
There were 55 participants in whom paired IEL count and microbiota data were available. All 55 participants had MaM data, and 20 of these participants also had FM data; 23 (42%) had IBS, and 31 (56%) had a high IEL count in the terminal ileum. There was no statistical difference in sex (P = 0.83, χ2 test) or age (P = 0.30, Mann-Whitney rank-sum test) between subjects with available microbiota data and subjects without data.
No difference was observed in Chao richness and Shannon diversity of MaM or FM between participants with low and high TI IEL counts (Figure 2a–d). Neither Chao richness nor Shannon diversity differentiated participants based on IBS diagnosis (data not shown).
Figure 2.
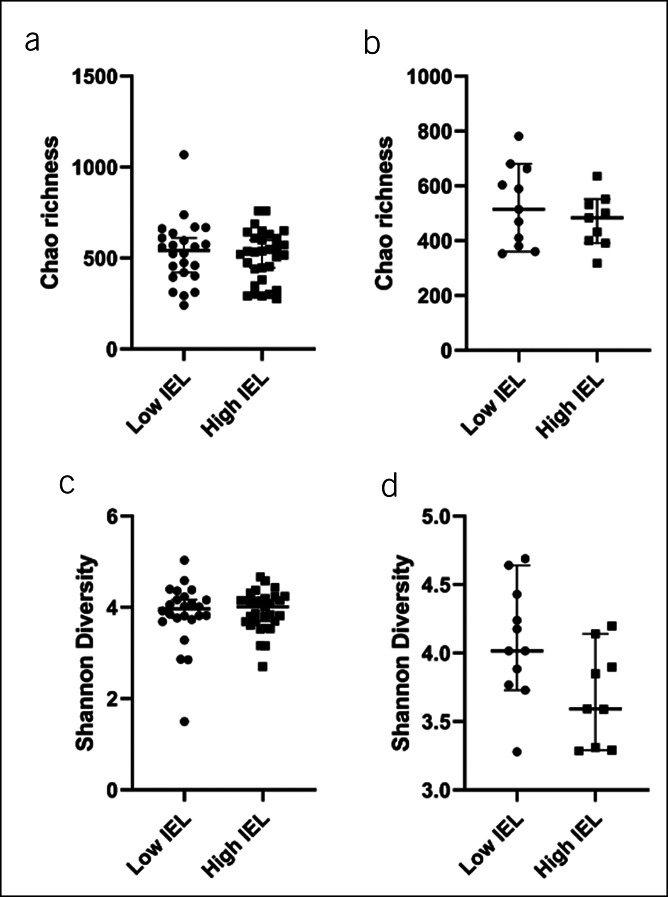
Alpha diversity indices in cases with low intraepithelial lymphocytes (IELs) and high IELs. There was no association between IEL count in the terminal ileum (TI) and Chao richness of (a) the mucosal microbiota (P = 0.717) and (b) the fecal microbiota (FM) (P = 0.503). There was no association between IEL count in TI with (c) Shannon diversity of the mucosal microbiota (P = 0.953). (d) The association between reduced Shannon diversity of the FM and high IEL count in the TI did not reach statistical significance (P = 0.067).
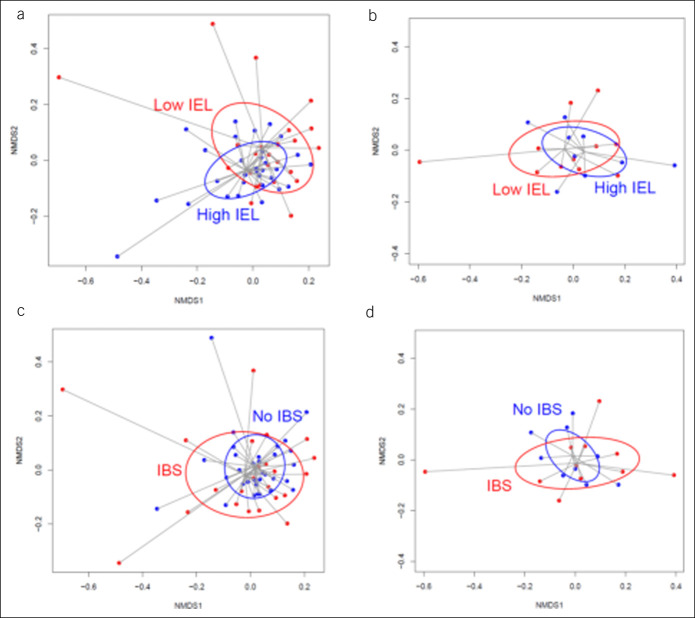
Difference in beta diversity of the MaM between low and high terminal ileum IEL count groups did not reach statistical significance (Figure 3a P = 0.08), and no difference in FM beta diversity was observed (Figure 3b P = 0.85). There was no difference in beta diversity of either the MaM or FM between IBS and non-IBS participants (Figure 3c,d; P = 0.20 and 0.54 respectively).
Figure 3.
Beta diversity (Bray-Curtis index) of mucosal microbiota in samples with low intraepithelial lymphocytes (IELs) and high IELs in the terminal ileum. Nonmetric dimensional scaling (NMDS) plots comparing subjects with high and low IELs (high IEL samples colored blue; low IEL samples colored red). (a) A difference in beta diversity of the mucosa-associated microbiota (MaM) between low and high IEL groups, which does not reach statistical significance (permutational multivariate analysis of variance test; P value = 0.079). There was no difference in fecal microbiome (FM) beta diversity in high and low IEL groups (b; P = 0.854). There was no difference in beta diversity of either the MaM or FM between IBS and non-IBS participants (c and d; P = 0.195 and 0.544, respectively).
When taxonomic profiles were compared in the MaM, the genus Blautia and unclassified Clostridiales were associated with high TI IEL count (Figure 4a,b; P = 0.024 and 0.036, respectively). No taxonomic associations were seen with IEL count in the FM. IBS status did not associate with any taxa in the MaM or FM.
Figure 4.
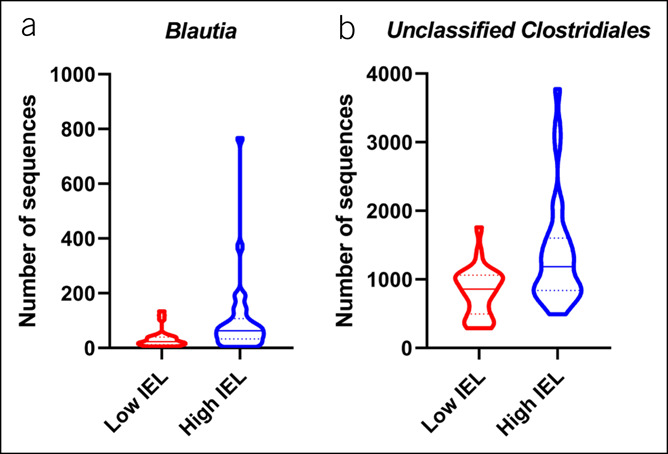
Violin plots of significant taxonomic differences in (a) Blautia spp. (P = 0.024) and (b) unclassified Clostridiales (P = 0.036) in the mucosa-associated microbiota between subjects with low and high intraepithelial lymphocyte (IEL) counts. Medians shown as full lines, and quartiles shown as dotted lines.
MaM and FM alterations according to cecal IEL count were also evaluated, and none were significant after P value correction (data not shown).
DISCUSSION
In the large PopCol study, we carefully assessed community subjects by quantitative histology and investigated whether there was an increase in inflammatory cells in subjects with IBS, defined by the Rome criteria, vs. IBS—free controls. All subjects were identified and selected from the same sampling frame minimizing any selection bias, a major strength of this nested case-control design. Blinded assessment of histology was undertaken to avoid measurement bias, and slides were dual read. Potential confounders were adjusted for in the multivariable analyses. We did observe a modest but significant increase in IELs in the terminal ileum in IBS vs. controls, and when IBS subgroups were analyzed, IBS—mixed was associated with increased IELs. In the cecum, similarly increased IELs in IBS—diarrhea were observed. Mast cells and eosinophils were not increased in IBS. Paneth cell numbers were not altered, but this is likely due to Paneth cells being largely absent in the large intestine. Subjects taking aspirin and NSAIDs were excluded, so these drugs do not explain the histological findings.
Age and female gender are risk factors for IBS, and smoking is a risk factor for postinfectious and IBS—diarrhea (21,26). It is notable that IELs were increased in the terminal ileum in IBS adjusting for age, sex, and smoking. This association remained significant when adjusted for mood in a sensitivity analysis, although this secondary analysis needs to be interpreted with caution as it was post hoc. Normal values for IELs in the terminal ileum have been uncertain. In a study of 61 asymptomatic patients undergoing colonoscopy and biopsy, counts of IELs per 100 enterocytes were low, with a median of 3 (range 0–9) (27), but this was not based on a random population sample in contrast to the PopCol study. Increased IELs in the small intestine are not specific, but may occur in celiac disease or with small intestinal bacterial overgrowth (SIBO) (28,29). All subjects in this study had a normal colonoscopy, and we excluded cases of celiac disease; however, measures of SIBO were not available, although most subjects had no other obvious risk factor for SIBO (30). PPI and NSAID users were excluded from the analyses, and drugs should not account for distal small intestinal pathology in this study. Chadwick et al. (13) reported increased colonic IELs in IBS in a New Zealand study, and we also observed significantly increased cecal IELs in IBS—diarrhea even when adjusting for mood. The finding of increased terminal ileum IELs is further supported by evidence of circulating CCR9+ small intestinal homing T cells in IBS, a marker of small intestinal inflammation (31). Increased IELs are seen in celiac disease, a gluten protein-driven immune response in a genetically primed host (23). Hence, another possibility is increased IELs in the terminal ileum may reflect a food antigen driven process, although the expected changes may often occur more proximally in the small intestine, as has been reported in rumination syndrome where increased IELs in the duodenum have been observed with eosinophils (and rumination syndrome strongly overlaps with FD in terms of gastroduodenal symptom profile) (32). Recent work has also found that in non-IgE food allergy in IBS, IELs become acutely increased in the duodenum after a food challenge consistent with our findings (33).
Mast cells have been implicated in the pathogenesis of IBS. Miner et al. in the United States were the first to report increased mast cells in the terminal ileum in IBS (34). We have observed increased duodenal mast cells in those with overlapping IBS and FD in another random Swedish population-based cohort who underwent endoscopy (29). However, it is controversial whether increased mast cells are a marker for the disorder (8–12,35). Mast cell activation has been demonstrated in IBS in a number of studies of referred patients through measurement of mast cell mediators in supernatants (36). However, protocols for handling the biopsies and undertaking the supernatant studies have varied, and potential confounders such as current stress or infection may have impacted the results (36). We conclude if increased ileocolonic mast cells occur in IBS, it is likely only in a subgroup of selected referral cases with presumably more severe symptoms, a hypothesis needing to be tested.
Paneth cells have an important role in microbe-host interactions (15,16) but have not been investigated in IBS to the best of our knowledge. In this study, Paneth cells were not altered in the terminal ileum in IBS in contrast to observations in Crohn disease (15). As we did not examine other small intestinal sites, the conclusion there is no change is incomplete. As no dysbiosis was found in this population-based study (24), this may account for the negative findings. As there was no change in Paneth cells in IBS, we did not look for a microbiota Paneth cell link.
Increased colonic eosinophils have been identified in patients with colonic spirochetosis identified in the PopCol study as previously reported (7); those with colonic spirochetes were excluded from the current study. These novel pathological observations have been confirmed in an Australian study (37), and an association between colonic spirochetes and IBS—diarrhea has been observed (7). Furthermore, increased duodenal eosinophilia is found in FD, most notably those with postprandial distress syndrome, observations that have been confirmed worldwide (5,38,39), and FD overlaps with IBS more than expected by chance (1). Because of these previous striking associations, we carefully evaluated for increased ileocolonic eosinophilia in IBS, but no significant changes (or even trends) were observed. Nonceliac gluten sensitivity (NCGS) has also been identified in a subset with IBS (40), and rectal eosinophilia has been observed in NCGS in an Italian study (41). Although these observations of increased rectal eosinophils in NCGS are yet to be confirmed, we did not detect any such changes in the present investigation and suggest this will be uncommon in IBS if it occurs.
Our results suggest that there are modest, discernible differences in the MaM (but not the FM) in the presence of an expanded IEL compartment in the terminal ileum, but this was not significantly associated with IBS status. In particular, we found that the genus Blautia and unclassified Clostridiales were overexpressed in the MaM of participants with high IEL counts in the terminal ileum. Blautia was a prominent genus in the MaM relative to the FM in the wider PopCol study. Although Blautia was not associated with IBS in the PopCol study (24), an association was seen in a previous smaller study (42). It is therefore notable that, in our analysis, IELs are increased in the presence of Blautia, raising the possibility that there may be transmucosal interaction between these bacteria and the host immune system. Blautia species and many other Clostridiales are producers of short-chain fatty acids, which are increasingly recognized to be immunomodulatory in nature (43). Bile acid–induced diarrhea is likely an important mechanism in IBS (44), but we did not evaluate bile acids in this study and cannot comment on whether this relates to the microbiome alterations observed; unclassified Clostridiales (associated with high IEL count) might well include Clostridia with bile acid metabolizing activity (e.g., Clostridium scindens), but this is speculative.
The novel finding of a distinct microbiome profile in the setting of an expanded IEL compartment in the TI is plausible in the context of emerging evidence of subtle intestinal inflammation in IBS. Nonetheless, this outcome should be interpreted with caution, given the modest numbers of participants included in the current analysis. Our results merit further study and validation in independent cohorts.
This study had limitations. We acknowledge that this is an exploratory analysis of IELs in IBS, and based on the available data, we do not claim “proof” of association; the findings need independent corroboration with prospective follow-up to identify whether there is a temporal relationship which would suggest causality. However, our results were consistent on adjusting for potential confounders. No correction for multiple hypothesis testing was made because of the exploratory nature of the study. Taking the minimum clinically important effect size to be RRR = 2.0, statistical power is approximately 0.9 for the contrast of overall IBS and controls given the sample size available; however, for the smallest IBS subtype, statistical power is approximately 0.4 under the same conditions. The study was constrained by the available sample size. With regard to the paired IEL—microbiome analysis, although our focus was on IEL count in the terminal ileum, samples for microbiome analysis were available only from the sigmoid colon and feces. The histopathology was performed blinded to case-control status, but we could not assess for mast cell activation, which may still play a key role in the pathogenesis of IBS even if absolute mast cells numbers are not increased, and we did not assess enterochromaffin cells and IBS. The ASQ asks for abdominal pain and discomfort, but the current Rome IV criteria focus on abdominal pain, not discomfort (2). However, the ASQ includes 11 modalities of abdominal pain and discomfort in Swedish and only 2 of the IBS subject did not specify a pain modality, suggesting that most cases had pain-predominant symptoms consistent with Rome IV criteria (18). Notably, a representative community sample was obtained, and selection bias was minimal as previously reported (18).
In conclusion, in a novel nested case-control study from a representative population, we conclude there is no significant increase in ileocolonic mast cells or eosinophils or changes in Paneth cells in subjects with IBS vs. IBS-free subjects in a Northern European community. Mast cell counts alone seem to be unhelpful as a biomarker for IBS in this part of the world in the terminal ileum or colon. Although increased IELs in the terminal ileum and cecum were identified in IBS, their clinical and pathological significance is unclear and requires further investigation.
CONFLICTS OF INTEREST
Guarantor of the article: Nicholas J. Talley, MD, PhD.
Specific author contributions: A.A., M.M.W., N.P., and N.J.T.: study concept and design. L.E., L.W.H., M.M.W., and L.A.: acquisition of data. A.A., M.P.J., J.L.A., N.P., L.E., L.W.H., and N.J.T.: analysis and interpretation of data. N.J.T., J.L.A., and A.A.: drafting of the manuscript. All authors: critical revision of the manuscript for important intellectual content. A.A. and M.P.J.: statistical analysis. L.A.: obtained funding; administrative, technical, or material support; study supervision.
Financial support: This work was supported in part by grants from NHMRC to N. J. Talley, and the Richard Julins and Nanna Svartz foundations to A. Andreasson. J. L. Alexander and N. Powell are supported by the NIHR Imperial Biomedical Research Center (BRC) and the Freed Foundation. N. J. Talley acknowledges funding from the National Health and Medical Research Council (NHMRC) for the Center for Research Excellence in Digestive Health and holds an NHMRC Investigator grant.
Potential competing interests: N. J. Talley reports grants from Abbott Pharmaceuticals, grants from Commonwealth Diagnostics, nonfinancial support from HVN National Science Challenge NZ, grants and personal fees from GI therapies, grants from Viscera USA, personal fees from Adelphi values, personal fees from Allergens PLC, personal fees from Takeda, personal fees from Ampligent, personal fees from Progenity, personal fees from Sanofi-aventis, personal fees from IM Health Sciences, personal fees from Napo Pharmaceutical, personal fees from Outpost Medicine, personal fees from Samsung Bioepis, personal fees from Synergy, personal fees from Theravance, personal fees from Yuhan, grants from Prometheus (IBS), grants from Pfizer, grants from Rome Foundation, grants from Salix, personal fees from Aviro Health (Digestive health), personal fees from ARENA Pharmaceuticals, personal fees from Anatara Life Sciences, personal fees from Allakos, personal fees from Censa, personal fees from Cadila Pharmaceuticals, personal fees from Danone, personal fees from Dr. Reddy's Laboratories, personal fees from Planet Innovation, personal fees from twoXAR, personal fees from Theravance, from Jorveza, from Bayer, from Glutagen outside the submitted work; In addition, N. J. Talley has a patent Biomarkers of IBS licensed, a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued. Committees: Australian Medical Council (AMC) [Council Member]; Australian Telehealth Integration Programme; MBS Review Taskforce; NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors. Boards: GESA Board Member, Sax Institute, Committees of the Presidents of Medical Colleges. Community group: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). Miscellaneous: Avant Foundation (judging of research grants); N. J. Talley holds an Investigator Grant from the NHMRC and is the director for the CRE in Digestive Health.
Study Highlights.
WHAT IS KNOWN
✓ Reports on the presence of colonic mucosal immune cells (e.g., mast cells) in IBS have been inconsistent but have been based on case-control studies in selected patients.
✓ The normal ileocolonic cell counts are unclear because of a lack of population-based studies.
✓ No random population-based colonoscopy studies have investigated ileocolonic mucosal immune cells in IBS and healthy subjects.
WHAT IS NEW HERE
✓ Normal values for mast cells, IELs, eosinophils, and Paneth cells in the ileum and colon in healthy subjects and IBS are described.
✓ Subtle increased numbers of IELs (n/100 epithelial cells) in the terminal ileum in IBS (TI, median 17) and cecum in IBS-diarrhea (median 12) were observed, compared with healthy controls (TI median 15, cecum 9).
✓ IELs may be associated with mucosal-associated microbiome alterations, but these alterations are not related to the presence of IBS.
TRANSLATIONAL IMPACT
✓ Knowledge of normal cutoffs for immune cell counts in the terminal ileum and colon is likely to assist in identifying subtle low-grade inflammatory diseases currently overlooked.
✓ In IBS, IELs may be a marker of disease.
ACKNOWLEDGMENT
We thank Lars Kjellström who made a major contribution to the colonoscopies performed and Jeanie Chui and Alasdair Warwick who contributed to histological quantification.
Contributor Information
James L. Alexander, Email: jamesalexander@doctors.org.uk.
Marjorie M. Walker, Email: marjorie.walker@newcastle.edu.au.
Michael P. Jones, Email: mike.jones@mq.edu.au.
Luisa W. Hugerth, Email: luisa.hugerth@scilifelab.se.
Lars Engstrand, Email: lars.engstrand@ki.se.
Lars Agréus, Email: lars.agreus@ki.se.
Nicholas Powell, Email: nicholas.powell@imperial.ac.uk.
Anna Andreasson, Email: anna.andreasson@su.se.
REFERENCES
- 1.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017;376(26):2566–78. [DOI] [PubMed] [Google Scholar]
- 2.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150(6):1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 3.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2016;1(2):133–46. [DOI] [PubMed] [Google Scholar]
- 4.Burns G, Carroll G, Mathe A, et al. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: A systematic review. Am J Gastroenterol 2019;114(3):429–36. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: An adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol 2007;5(10):1175–83. [DOI] [PubMed] [Google Scholar]
- 6.Keely S, Walker MM, Marks E, et al. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest 2015;45(12):1350–9. [DOI] [PubMed] [Google Scholar]
- 7.Walker MM, Talley NJ, Inganäs L, et al. Colonic spirochetosis is associated with colonic eosinophilia and irritable bowel syndrome in a general population in Sweden. Hum Pathol 2015;46(2):277–83. [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132(1):26–37. [DOI] [PubMed] [Google Scholar]
- 9.Lobo B, Ramos L, Martínez C, et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United European Gastroenterol J 2017;5(6):887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: Is there any relationship? Am J Gastroenterol 2012;107(5):715–26. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Adeyemo M, Karagiannides I, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol 2012;107(2):262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil 2018;30(1):e13192. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122(7):1778–83. [DOI] [PubMed] [Google Scholar]
- 14.Martínez C, Rodiño-Janeiro BK, Lobo B, et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017;66(9):1537–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu TC, Gurram B, Baldridge MT, et al. Paneth cell defects in Crohn's disease patients promote dysbiosis. JCI Insight 2016;1(8):e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature 2017;551:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamdar K, Johnson AMF, Chac D, et al. Innate recognition of the microbiota by TLR1 promotes epithelial homeostasis and prevents chronic inflammation. J Immunol 2018;201(1):230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjellström L, Molinder H, Agréus L, et al. A randomly selected population sample undergoing colonoscopy: Prevalence of the irritable bowel syndrome and the impact of selection factors. Eur J Gastroenterol Hepatol 2014;26(3):268–75. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys K, Grankvist A, Leu M, et al. The genetic structure of the Swedish population. PLoS One 2011;6(8):e22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liljebo T, Störsrud S, Andreasson A. Presence of fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs) in commonly eaten foods: Extension of a database to indicate dietary FODMAP content and calculation of intake in the general population from food diary data. BMC Nutr 2020;6(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry SD, Barton JR, Welfare MR. Factors associated with the development of post-infectious functional gastrointestinal diseases: Does smoking play a role? Eur J Gastroenterol Hepatol 2005;17(10):1071–5. [DOI] [PubMed] [Google Scholar]
- 22.Ljótsson B, Jones M, Talley NJ, et al. Discriminant and convergent validity of the GSRS-IBS symptom severity measure for irritable bowel syndrome: A population study. United European Gastroenterol J 2020;8(3):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology 2010;139(1):112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugerth LW, Andreasson A, Talley NJ, et al. No distinct microbiome signature of irritable bowel syndrome found in a Swedish random population. Gut 2020;69(6):1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks DH, Tyson GW, Hugenholtz P, et al. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014;30(21):3123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 2014;40(3):288–97. [DOI] [PubMed] [Google Scholar]
- 27.Istvanic S, Yantiss RK, Baker SP, et al. Normal variation in intraepithelial lymphocytes of the terminal ileum. Am J Clin Pathol 2007;127(5):816–9. [DOI] [PubMed] [Google Scholar]
- 28.Walker MM, Talley NJ. Clinical value of duodenal biopsies: Beyond the diagnosis of coeliac disease. Pathol Res Pract 2011;207(9):538–44. [DOI] [PubMed] [Google Scholar]
- 29.Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 2009;29(7):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am J Gastroenterol 2017;112(5):775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132(3):913–20. [DOI] [PubMed] [Google Scholar]
- 32.Halland M, Talley NJ, Jones M, et al. Duodenal pathology in patients with rumination syndrome: Duodenal eosinophilia and increased intraepithelial lymphocytes. Dig Dis Sci 2019;64(3):832–7. [DOI] [PubMed] [Google Scholar]
- 33.Fritscher-Ravens A, Pflaum T, Mosinger M, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019;157(1):109–18.e5. [DOI] [PubMed] [Google Scholar]
- 34.Weston AP, Biddle WL, Bhatia PS, et al. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci 1993;38(9):1590–5. [DOI] [PubMed] [Google Scholar]
- 35.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59(9):1213. [DOI] [PubMed] [Google Scholar]
- 36.Lee KN, Lee OY. The role of mast cells in irritable bowel syndrome. Gastroenterol Res Pract 2016;2016:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodsall TM, Talley NJ, Rassam L, et al. Unique pathology of colonic spirochaetosis characterised by mucosal eosinophilia is linked to diarrhoea and IBS. Gut 2017;66(5):978. [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, Jung H-K, Lee KE, et al. Degranulated eosinophils contain more fine nerve fibers in the duodenal mucosa of patients with functional dyspepsia. J Neurogastroenterol Motil 2019;25(2):212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taki M, Oshima T, Li M, et al. Duodenal low-grade inflammation and expression of tight junction proteins in functional dyspepsia. Neurogastroenterol Motil 2019;31(10):e13576. [DOI] [PubMed] [Google Scholar]
- 40.Potter MDE, Walker MM, Jones MP, et al. Wheat intolerance and chronic gastrointestinal symptoms in an Australian population-based study: Association between wheat sensitivity, celiac disease and functional gastrointestinal disorders. Am J Gastroenterol 2018;113(7):1036–44. [DOI] [PubMed] [Google Scholar]
- 41.Carroccio A, Giannone G, Mansueto P, et al. Duodenal and rectal mucosa inflammation in patients with non-celiac wheat sensitivity. Clin Gastroenterol Hepatol 2019;17(4):682–90.e3. [DOI] [PubMed] [Google Scholar]
- 42.Labus JS, Hollister EB, Jacobs J, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017;5(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiha MG, Ashgar Z, Fraser EM, et al. High prevalence of primary bile acid diarrhoea in patients with functional diarrhoea and irritable bowel syndrome-diarrhoea, based on Rome III and Rome IV criteria. EClinicalMedicine 2020;25:100465. [DOI] [PMC free article] [PubMed] [Google Scholar]