Abstract
Objective
Coronary heart disease (CHD) is a prevalent complication of type 2 diabetes mellitus (T2DM). The proatherogenic low-density lipoprotein (LDL) cholesterol is an established risk factor of cardiovascular disease, and evidence also suggests that postprandial plasma glucose (PPG) levels closely delineate CHD mortality in diabetes. The investigators hypothesized that the addition of telehealth consultation to standard antidiabetic therapy may help to reduce postprandial glucose variability and plasma LDL cholesterol levels in patients with T2DM.
Methods
This cross-sectional study enrolled patients with newly diagnosed T2DM who received standard antidiabetic therapy with or without additional telehealth consultation. Participants received blood tests for plasma lipid profile and glucose levels at the diagnosis of diabetes and after 1 month of therapeutic intervention. Laboratory results were compared between treatment groups to determine the efficacy of complementary telehealth consultation.
Results
In this study, 375 participants were enrolled. The standard treatment group had considerably greater levels of plasma LDL cholesterol than recipients of telehealth consultation (110 mg/dL vs 93.1 mg/dL, P < 0.001). Moreover, patients receiving standard treatment had greater levels of fasting plasma glucose (104 mg/dL vs 98.5 mg/dL, P = 0.027), 2-h PPG (169 mg/dL vs 111 mg/dL, P < 0.001), and postprandial glucose variability (65.4 mg/dL vs 12.8 mg/dL, P < 0.001) than participants under telehealth consultation.
Conclusions
Telemedicine in addition to standard antidiabetic therapy helped to reduce plasma LDL cholesterol levels and postprandial glucose variability in patients with newly diagnosed T2DM. Therefore, telehealth consultation is a suitable complement to pharmacologic therapy for diabetic patients to assist in the management of proatherogenic dyslipidemia and postprandial glucose variability.
Keywords: type 2 diabetes mellitus, telehealth, hyperlipidemia, glucose variability
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease that induces substantial morbidity in affected patients (1). Chronic hyperglycemia predisposes patients to microvascular complications including retinopathy, neuropathy, and kidney dysfunction (2). Importantly, diabetes is also linked to macrovascular diseases such as coronary heart disease (CHD) and peripheral arterial disease (3). Indeed, cardiovascular disease is a prevalent risk factor of mortality in T2DM (4), and prevention of diabetic CHD is an essential component of diabetes management guidelines (5, 6).
Diabetic dyslipidemia has several unique features. Insulin resistance increases the flux of free fatty acids to the liver, which leads to excessive plasma triglycerides (TG), reduced high-density lipoprotein (HDL) cholesterol, and oxidized low-density lipoprotein (LDL) cholesterol (7). Among these lipoprotein fractions, LDL cholesterol contributes to atheroma formation in blood vessels and is an established risk factor of CHD (8). Therefore, plasma LDL cholesterol is a suitable target for the prevention of diabetic cardiovascular disease (9).
Recently, postprandial glucose variability has garnered attention as a potential risk factor for cardiovascular complications. In the Whitehall study, the cardiovascular risk increased in line with postprandial glucose levels (10). Moreover, a European research consortium observed that 2-h postprandial plasma glucose (PPG) levels correlated with cardiovascular risk in T2DM (11). Therefore, investigators have proposed that postprandial hyperglycemia may be a risk factor of diabetic CHD (12).
Telemedicine is a complementary therapeutic modality in the treatment of T2DM. Conventional antidiabetic therapy based on drug prescriptions may lead to inadequate glycemic control (13). Importantly, individuals with newly diagnosed T2DM confront challenges including dietary choices and sick day management (14). Without adequate support from medical professionals, patients can experience diabetes-related distress that interferes with diabetes self-care (15). In contrast, telehealth consultation can provide timely and accurate information that leads to better compliance and clinical outcome (16).
Currently the effect of telemedicine on diabetic dyslipidemia and postprandial glucose variability remains uncertain. In this study, the investigators hypothesized that the addition of telehealth consultation to standard antidiabetic therapy may help to improve plasma lipid profile and postprandial glucose variability in patients with newly diagnosed T2DM.
Materials and methods
Study population
This cross-sectional study was conducted at a medical center in Taiwan. Candidates visiting the Endocrinology clinic between October 2014 and September 2016 were screened for eligibility. Inclusion criteria were as follows: (i) patients over 21 years of age, (ii) newly diagnosed T2DM according to the diagnostic criteria of the American Diabetes Association, namely, serum glycosylated hemoglobin A1c (HbA1c) level ≥ 6.5%, fasting plasma glucose (FPG) ≥ 126 mg/dL, 2-h PPG after 75-g oral glucose tolerance test ≥ 200 mg/dL, or random plasma glucose levels ≥ 200 mg/dL with classic symptoms of diabetes, (iii) no concomitant prescription of antidiabetic or lipid-lowering medications, and (iv) compliance with blood tests and dietary instructions.
Exclusion criteria were as follows: (i) patients with familial hypercholesterolemia, chronic kidney disease, hypothyroidism, or hemoglobin disorders, (ii) chronic alcoholism, or (iii) recipients of estrogen replacement or antidepressants.
Study protocol
Demographic data including age, sex, body weight, and height were recorded at the initial clinic visit. Thereafter, two treatment groups were established according to shared decision-making between patients and their attending physicians. The control group received metformin prescription and medical nutrition consultation at disease diagnosis and during monthly clinic appointments. The telemedicine group received telehealth consultation during workdays in addition to metformin prescription. Participants maintained their therapeutic regimens for 1 month prior to follow-up laboratory tests.
Medical nutrition consultation
Participants received one session of medical nutrition consultation at disease diagnosis and during monthly clinic appointments. Certified diabetes educators provided 30 min of comprehensive consultation according to current guidelines (5). Specifically, patients were instructed to limit saturated fat to less than 5% of total calories and increase whole grain intake. Furthermore, participants learned the carbohydrate counting technique and their daily recommended carbohydrate intake.
Telehealth consultation
For the group receiving telemedicine, each participant was assigned to a diabetes educator who provides consultation via telephone or messaging software during workdays. The messaging software was provided by the Health2Sync developer that allows participants to upload photographs of daily meals and plasma glucose readings from finger-stick glucose meters. Diabetes educators assess data collected by Health2Sync and contact their corresponding participants once a week to monitor dietary habits and medication use.
Laboratory evaluation
Participants received blood tests for serum creatinine, serum alanine transaminase, HbA1c, and plasma lipid profile after a 12-h fast at the initial clinic visit. These patients consumed a meal consisting of 75 g of carbohydrates, 5 g of fat, and 10 g of protein, and PPG levels were measured 2 h afterward. Participants could modify the content of their meals, for instance, forgoing fruit juice or snacks, according to their dietary choices.
Patients maintained their therapeutic regimens and returned for follow-up after 1 month. At the follow-up appointment, participants fasted for 12 h before venous sampling for plasma TG, LDL cholesterol, HDL cholesterol, and FPG. Subsequently, patients consumed a meal with the previously mentioned nutritional composition. Once again, participants were given an option to alter the content of the meal, and venous sampling for PPG level was performed 2 h afterward.
Blood samples were delivered to the central laboratory within 1 h of venous sampling and assayed by Beckman Coulter UniCel DxC 800 Synchron Clinical Systems. The analytical precision was within 1.7 mg/dL for HDL cholesterol, within 3.0 mg/dL for LDL cholesterol, within 7.5 mg/dL for TG, and within 2.0 mg/dL for plasma glucose level. The plasma LDL cholesterol level was measured by the timed endpoint method using a commercial polyanion solution.
Ethics approval
This study was conducted in accordance to the World Medical Association’s Declaration of Helsinki. The protocol was approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB No. Y_107_0195). Participants provided written informed consent to take part in the study.
Statistical analysis
Study outcomes including age, gender, mean serum HbA1c, plasma glucose levels, and lipid profile were compared between therapeutic groups using independent t-test for continuous variables and Pearson’s χ2-test for categorical variables. Statistical analysis was performed using Statistical Package for the Social Sciences (version 22.0, SPSS, Chicago, IL), with a two-sided P value of less than 0.05 interpreted as statistically significant.
Results
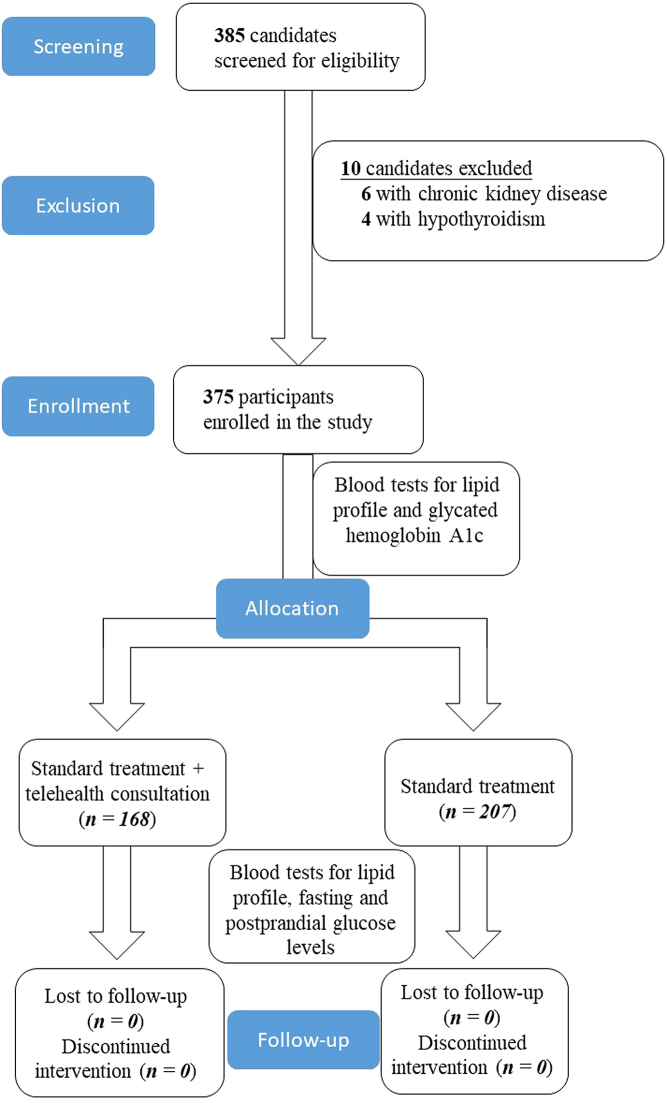
This study screened 385 patients visiting the endocrinology clinic for eligibility. Six individuals were excluded due to chronic kidney disease, and four patients were ineligible due to coexisting hypothyroidism. The enrollment protocol is illustrated in Fig. 1.
Figure 1.
Enrollment protocol of the study.
Demographic characteristics of the participants at diagnosis
The study enrolled 375 participants who were divided into two therapeutic groups according to shared decision making with their attending physicians. As demonstrated in Table 1, demographic characteristics of the participants including age, sex, serum HbA1c, BMI, blood pressure, serum creatinine, and serum alanine transaminase were similar between treatment groups. Patients in each group also had similar plasma metabolic profile and received comparable dose of metformin therapy.
Table 1.
Demographic features of the participants at diagnosis of diabetes.
| Parameters | Standard treatment + telemedicine (n = 168) | Standard treatment (n = 207) | P value |
|---|---|---|---|
| Age (years) | 66.9 ± 12.0 | 64.9 ± 13.1 | 0.116 |
| Sex (Female) | 92 (54.8%) | 108 (52.2%) | 0.617 |
| Serum HbA1c (%) | 9.1 ± 2.2 | 9.3 ± 2.3 | 0.334 |
| Fasting plasma glucose level (mg/dL) | 169 ± 6.3 | 170 ± 6.1 | 0.132 |
| 2-h postprandial plasma glucose level (mg/dL) | 243 ± 34.6 | 239 ± 35.5 | 0.446 |
| Body weight (kg) | 54.9 ± 10.7 | 54.6 ± 11.2 | 0.766 |
| Body height (m) | 1.45 ± 0.07 | 1.46 ± 0.20 | 0.795 |
| Body mass index (kg/m2) | 25.9 ± 4.2 | 25.8 ± 4.8 | 0.837 |
| Systolic blood pressure (mm Hg) | 157 ± 19.5 | 158 ± 19.4 | 0.786 |
| Diastolic blood pressure (mm Hg) | 63.0 ± 9.2 | 63.2 ± 8.1 | 0.817 |
| Serum creatinine (mg/dL) | 0.67 ± 0.19 | 0.68 ± 0.19 | 0.583 |
| Serum alanine transaminase (U/L) | 42.5 ± 29.5 | 41.6 ± 29.8 | 0.788 |
| Plasma triglycerides (mg/dL) | 230 ± 124 | 216 ± 108 | 0.267 |
| Plasma high-density lipoprotein cholesterol (mg/dL) | 57.8 ± 23.3 | 58.5 ± 16.3 | 0.744 |
| Plasma low-density lipoprotein cholesterol (mg/dL) | 124 ± 47.4 | 127 ± 46.3 | 0.575 |
| Metformin dose (mg per day) | 1313 ± 470 | 1345 ± 488 | 0.508 |
Data are expressed as means with standard deviation of the mean for continuous variables and number (percentage) for categorical variables. Variables are compared between groups using independent t-test for continuous variables and Pearson’s χ2-test for categorical variables.
A1c, mm Hg, millimeters of mercury; HbA1c, glycosylated haemoglobin; kg, kilograms; m, meters; mg, milligrams.
Effect of telemedicine and standard treatment on plasma lipid profile after 1 month
As observed in the study, participants in both groups had similar levels of plasma TG (177 mg/dL vs 179 mg/dL, P = 0.824) and HDL cholesterol (56.1 mg/dL vs 53.9 mg/dL, P = 0.133). However, patients in the standard treatment group had considerably greater levels of plasma LDL cholesterol than recipients of additional telemedicine (110 mg/dL vs 93.1 mg/dL, P < 0.001). These findings are summarized in Table 2.
Table 2.
Effect of telemedicine and standard treatment on plasma lipid profile.
| Parameters | Standard treatment + telemedicine (n = 168) | Standard treatment (n = 207) | P value |
|---|---|---|---|
| Plasma triglycerides (mg/dL) | 179 ± 97.7 | 177 ± 88.7 | 0.824 |
| Plasma high-density lipoprotein cholesterol (mg/dL) | 53.9 ± 14.5 | 56.1 ± 13.9 | 0.133 |
| Plasma low-density lipoprotein cholesterol (mg/dL) | 93.1 ± 29.0 | 110 ± 27.1 | <0.001 |
Data are expressed as means with standard deviation of the mean for continuous variables. Variables are compared between groups using independent t-test.
mg/dL, milligrams per decilitre.
Effect of telemedicine and standard treatment on plasma glucose levels after 1 month of treatment
As shown in Table 3, recipients of standard treatment had greater levels of FPG than those receiving additional telehealth consultation (104 mg/dL vs 98.5 mg/dL, P = 0.027). Furthermore, participants receiving standard treatment had higher 2-h PPG relative to those under telemedicine (169 mg/dL vs 111 mg/dL, P < 0.001). Postprandial plasma glucose variability, defined as the difference between 2-h PPG and FPG levels, was also higher in standard treatment compared to the telemedicine group (65.4 mg/dL vs 12.8 mg/dL, P < 0.001).
Table 3.
Effect of telemedicine and standard treatment on plasma glucose levels.
| Parameters | Standard treatment + telemedicine (n = 168) | Standard treatment (n = 207) | P value |
|---|---|---|---|
| Fasting plasma glucose level (mg/dL) | 98.5 ± 21.1 | 104 ± 26.1 | 0.027 |
| 2-h postprandial plasma glucose level (mg/dL) | 111 ± 22.8 | 169 ± 44.7 | <0.001 |
| Glucose variability (mg/dL) | 12.8 ± 7.3 | 65.4 ± 35.1 | <0.001 |
Glucose variability is defined as the difference between 2-h postprandial and fasting plasma glucose levels.
kg, kilograms; m, meters; mg, milligrams; mg/dL, milligrams per decilitre; mm Hg, millimeters of mercury.
Discussion
This study observed that recipients of standard antidiabetic therapy had greater plasma LDL cholesterol levels than patients under telemedicine. Moreover, patients receiving standard therapy harbored higher levels of FPG, 2-h PPG, and postprandial glucose variability compared to recipients of a telehealth consultation.
Telemedicine may help to improve diabetic dyslipidemia and glucose variability by providing accurate information about dietary choices (17). Indeed, clinic appointments rarely provide diabetic patients with adequate self-care skills due to inexperienced medical staff (18). Moreover, common misconceptions about T2DM warrant discussion with diabetes educators (19). For example, herbal medicine may adversely influence glycemic control, which can be promptly discussed with the patients during telemedicine sessions (20).
Furthermore, contact with healthcare professionals can improve adherence to a healthy lifestyle and antidiabetic medications (21, 22). This study has shown that telemedicine can promote appropriate dietary choices, leading to lower postprandial glucose variability. Indeed, a preceding study has shown that nutrition consultation at clinic visits rarely provides sufficient self-care skills to diabetic patients (23).
Postprandial glucose variability can exacerbate diabetic CHD through several mechanisms. Transient hyperglycemia induces proinflammatory micro-RNA particles that disrupt the endothelial function of coronary arteries (24). Furthermore, glucose variability is positively correlated with an arterial intimal thickness (25), a measure of intravascular atheroma burden. This study demonstrated that telemedicine can help to lower postprandial glucose variability and maybe a complementary therapeutic option for diabetic CHD.
Since LDL cholesterol is an established risk factor of diabetic CHD (26), even modest lowering of this lipoprotein by telemedicine may help to attenuate cardiovascular mortality. Moreover, this study observed that telemedicine has a complementary role in alleviating plasma glucose variability. Considering that hyperglycemia is central to diabetic microvascular complications (27), telemedicine in addition to pharmacologic intervention may lead to better diabetes care.
The strength of this study includes a sizeable population enrolled from the endocrinology clinic, which renders the findings applicable to general practice. Diabetes educators provided medical nutrition therapy to participants to ensure similar levels of dietary lipid intake. Participants received metformin prescription, which can reduce the confounding effect of different antidiabetic medications on plasma glucose levels.
Nonetheless, this study has several limitations. First, 2-h PPG was assessed by consuming a standard meal and may not reflect a patient’s daily dietary habits. Moreover, additional factors that influence postprandial glucose levels, such as the metabolic syndrome or insulin resistance, were unaccounted for in the study. Finally, the insulin secretory reserve of the pancreas can modify postprandial glucose levels but was not quantified in the study.
In conclusion, recipients of standard antidiabetic therapy had greater plasma LDL cholesterol levels and postprandial glucose variability than participants under telemedicine. Therefore, telemedicine may be a complementary option to assist in the management of proatherogenic dyslipidemia and glucose variability in diabetes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, Georgia: Centers for Disease Control and Prevention, United States Department of Health and Human Services, 2020. [Google Scholar]
- 2.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DWet al. Diabetic microvascular disease: an endocrine society scientific statement. Journal of Clinical Endocrinology and Metabolism 2017. 102 4343–4410. ( 10.1210/jc.2017-01922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, Watada H, Shimomura I, Chen H, Cid-Ruzafa Jet al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCover study program). Cardiovascular Diabetology 2018. 17 150. ( 10.1186/s12933-018-0787-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnethon MR, Biggs ML, Barzilay J, Kuller LH, Mozaffarian D, Mukamal K, Smith NL, Siscovick D. Diabetes and coronary heart disease as risk factors for mortality in older adults. American Journal of Medicine 2010. 123 556.e1–556.e9. ( 10.1016/j.amjmed.2009.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Introduction: Standards of medical care in diabetes-2020. Diabetes Care 2020. 43 S1–S2. ( 10.2337/dc20-Sint) [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels, Belgium: International Diabetes Federation, 2017. [Google Scholar]
- 7.Hirano T.Pathophysiology of diabetic dyslipidemia. Journal of Atherosclerosis and Thrombosis 2018. 25 771–782. ( 10.5551/jat.RV17023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda Aet al. Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk In Communities Study (CIRCS). Preventive Medicine 2011. 52 381–386. ( 10.1016/j.ypmed.2011.02.019) [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE.et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2019. 73 3168–3209. ( 10.1016/j.jacc.2018.11.002) [DOI] [PubMed] [Google Scholar]
- 10.Fuller JH, McCartney P, Jarrett RJ, Keen H, Rose G, Shipley MJ, Hamilton PJ. Hyperglycaemia and coronary heart disease: the Whitehall study. Journal of Chronic Diseases 1979. 32 721–728. ( 10.1016/0021-9681(7990051-1) [DOI] [PubMed] [Google Scholar]
- 11.DECODE Study Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Archives of Internal Medicine 2001. 161 397–405. ( 10.1001/archinte.161.3.397) [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Zhao L, Lin L, Gui M, Aleteng Q, Wu B, Wang S, Pan B, Ling Y, Gao X. Postprandial blood glucose outweighs fasting blood glucose and HbA1c in screening coronary heart disease. Scientific Reports 2017. 7 14212. ( 10.1038/s41598-017-14152-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousefzadeh G, Shokoohi M, Najafipour H. Inadequate control of diabetes and metabolic indices among diabetic patients: a population based study from the Kerman Coronary Artery Disease Risk Study (KERCADRS). International Journal of Health Policy and Management 2014. 4 271–277. ( 10.15171/ijhpm.2015.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardsten C, Blomqvist K, Rask M, Larsson Å, Lindberg A, Olsson G. Challenges in everyday life among recently diagnosed and more experienced adults with type 2 diabetes: a multistage focus group study. Journal of Clinical Nursing 2018. 27 3666–3678. ( 10.1111/jocn.14330) [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Zhu J, Liu L, Li F, Fish AF, Chen T, Lou Q. Diabetes-related distress and its associated factors among patients with type 2 diabetes mellitus in China. Psychiatry Research 2017. 252 45–50. ( 10.1016/j.psychres.2017.02.049) [DOI] [PubMed] [Google Scholar]
- 16.Wu C, Wu Z, Yang L, Zhu W, Zhang M, Zhu Q, Chen X, Pan Y. Evaluation of the clinical outcomes of telehealth for managing diabetes: a PRISMA-compliant meta-analysis. Medicine 2018. 97 e12962. ( 10.1097/MD.0000000000012962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashshur RL, Shannon GW, Smith BR, Woodward MA. The empirical evidence for the telemedicine intervention in diabetes management. Telemedicine Journal and e-Health 2015. 21 321–354. ( 10.1089/tmj.2015.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritz E.Limitations and future treatment options in type 2 diabetes with renal impairment. Diabetes Care 2011. 34 (Supplement 2) S330–S334. ( 10.2337/dc11-s242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil R, Nasrin N, Datta SS, Boratne AV, Lokeshmaran. Popular misconceptions regarding the diabetes management: where should we focus our attention? Journal of Clinical and Diagnostic Research 2013. 7 287–291. ( 10.7860/JCDR/2013/4416.2749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CC, Chen CL, Ko Y. The misconceptions and determinants of diabetes knowledge in patients with diabetes in Taiwan. Journal of Diabetes Research 2020. 2020 2953521. ( 10.1155/2020/2953521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welch G, Balder A, Zagarins S. Telehealth program for type 2 diabetes: usability, satisfaction, and clinical usefulness in an urban community health center. Telemedicine Journal and e-Health 2015. 21 395–403. ( 10.1089/tmj.2014.0069) [DOI] [PubMed] [Google Scholar]
- 22.Vadheim LM, Patch K, Brokaw SM, Carpenedo D, Butcher MK, Helgerson SD, Harwell TS. Telehealth delivery of the diabetes prevention program to rural communities. Translational Behavioral Medicine 2017. 7 286–291. ( 10.1007/s13142-017-0496-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adu MD, Malabu UH, Malau-Aduli AEO, Malau-Aduli BS. Enablers and barriers to effective diabetes self-management: a multi-national investigation. PLoS ONE 2019. 14 e0217771. ( 10.1371/journal.pone.0217771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melak T, Baynes HW. Circulating microRNAs as possible biomarkers for coronary artery disease: a narrative review. EJIFCC 2019. 30 179–194. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Xu X, Jiao X, Wu J, Zhou S, Lv X. The effects of glucose fluctuation on the severity of coronary artery disease in type 2 diabetes mellitus. Journal of Diabetes Research 2013. 2013 576916. ( 10.1155/2013/576916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storey BC, Staplin N, Haynes R, Reith C, Emberson J, Herrington WG, Wheeler DC, Walker R, Fellström B, Wanner C.SHARP Collaborative Group, et al. Lowering LDL cholesterol reduces cardiovascular risk independently of presence of inflammation. Kidney International 2018. 93 1000–1007. ( 10.1016/j.kint.2017.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Cao Y, Liu W, Wang Q, Qian Y, Lu P. Correlations among diabetic microvascular complications: a systematic review and meta-analysis. Scientific Reports 2019. 9 3137. ( 10.1038/s41598-019-40049-z) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a