Abstract
In recent years, glucagon-like peptide 1 receptor agonists (GLP-1RAs) have become central in the treatment of type 2 diabetes (T2D). In addition to their glucose-lowering properties with low risk of hypoglycaemia, GLP-1RAs reduce body weight and show promising results in reducing cardiovascular risk and renal complications in high-risk individuals with T2D. These findings have changed guidelines on T2D management over the last years, and GLP-1RAs are now widely used in overweight patients with T2D as well as in patients with T2D and cardiovascular disease regardless of glycaemic control. The currently available GLP-1RAs have different pharmacokinetic profiles and differ in their ability to improve glycaemia, reduce body weight and in their cardio- and renal protective potentials. Understanding how these agents work, including insights into their pleiotropic effects on T2D pathophysiology, may improve their clinical utilisation and be useful for exploring other indications such as non-alcoholic steatohepatitis and neurodegenerative disorders. In this review, we provide an overview of approved GLP-1RAs, their clinical effects and mode of action, and we offer insights into the potential of GLP-1RAs for other indications than T2D. Finally, we will discuss the emerging data and therapeutic potential of using GLP-1RAs in combinations with other receptor agonists.
Keywords: diabetes, cardiovascular, metabolism, inflammation, obesity
Introduction
Glucagon-like peptide 1 (GLP-1) is a gut-derived glucoregulatory hormone secreted in response to food consumption. Human GLP-1 is synthesised from the proglucagon gene and is secreted from the enteroendocrine L cells (1). Some preproglucagon-expressing neurons in the nucleus tractus solitarius of the brain stem have also been shown to synthesise GLP-1 (2). Due to rapid breakdown by dipeptidyl peptidase 4 (DPP-4) and renal clearance, the half-life of circulating GLP-1 is only 1–2 min. Thus, only 10% of the native GLP-1 released from the enteroendocrine L cells reaches the systemic circulation (1). Activation of the GLP-1 receptor (GLP-1R) on the beta-cell enhances glucose-dependent secretion of insulin, thereby improving beta-cell sensitivity to glucose (1). In addition, GLP-1 exhibits a glucagonostatic effect through a glucose-dependent inhibition of glucagon release (3) which, compared to GLP-1-induced insulin secretion, may be equally important for the overall plasma glucose-lowering effect of GLP-1 (4). In normal physiology, GLP-1 modifies gastrointestinal function by decreasing motility of the stomach and intestine, thereby delaying gastric emptying and reducing postprandial plasma glucose excursions (5). Brain-derived GLP-1 acts as a neurotransmitter and can target multiple GLP-1Rs throughout the central nervous systemCNS, including effects on satiety (6). In addition, GLP-1R activation in the brain has been linked to neuroprotective properties in experimental neurodegenerative disease models (6). Deduced from both preclinical and human studies, GLP-1 has extra-pancreatic effects in the gut, kidney, nervous system, heart and immune system (Fig. 1) (2). Multiple GLP-1R agonists (GLP-1RAs) utilising the plasma glucose-lowering and body weight-lowering effects of GLP-1 have been developed, and today, we have more than 15 years of clinical experience with GLP-1RAs for the treatment of T2D and 6 years of clinical experience with GLP-1RA-based treatment of obesity. Despite the long clinical experience with GLP-1RAs, the exact mechanisms by which GLP-1RAs exert their effects are still debated (6).
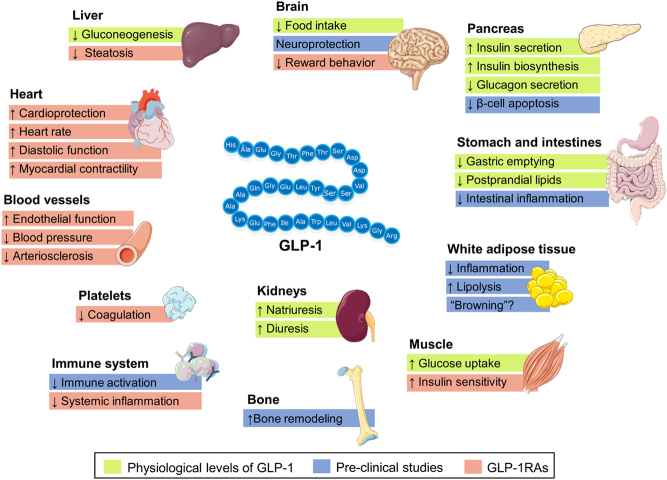
Figure 1.
Possible actions of glucagon-like peptide 1 (GLP-1) and the GLP-1 receptor agonists (GLP-1RAs) on various tissue. The applied colour code indicates whether the effect on the target tissue has been observed in preclinical studies (blue boxes), at physiological levels of GLP-1 in clinical studies (green boxes) or after treatment with GLP-1RAs (red boxes) (1, 2). The figure illustrates GLP-1 (7–36) amide.
Currently available GLP-1RAs
The first GLP-1RA for the treatment of T2D, exenatide twice daily, was approved by the United States Food and Drug Administration (FDA) in 2005. Since then, several GLP-1RAs have been developed and approved for the treatment of T2D, while albiglutide has been withdrawn due to commercial reasons. Until recently, all available GLP-1RAs were subcutaneously administrated; however, the GLP-1RA semaglutide (see below) is now available as an oral formulation. GLP-1RAs can be categorised based on molecular size, chemical structure and duration of action. As outlined below, the pharmacokinetic properties are important for the mode of action and the efficacy of the individual GLP-1RA. Short-acting GLP-1RAs are characterised by intermittent activation of the GLP-1R, whereas long-acting GLP-1RAs lead to continuous receptor activation over a 24-h period. Liraglutide, semaglutide and dulaglutide are – except for few modifications – highly similar to the structure of human GLP-1, while exenatide and lixisenatide are based on exendin-4, a naturally occurring peptide derived from the saliva of the Gila monster, and only share around 50% homology with the human GLP-1 molecule. Consequently, the exendin-based compounds cause a higher degree of antibody formation, but it is questionable whether this is important for efficacy and safety (7, 8). The pharmacokinetic properties of approved GLP-1RAs are listed in Table 1.
Table 1.
Pharmacokinetic properties of approved GLP-1RAs.
| GLP-1RAs | Approval year | Molecular weight (Da) | Pharmacokinetic components | Administration schedule | Dose | Pharmacokinetics (single-dose administration) | Antibody development (% of patients) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| FDA | EMA | Time to peak | Half-life | Elimination | ||||||
| Short-acting GLP-1RAs | ||||||||||
| Exenatide twice daily | 2005 | 2006 | 4187 | Peptide from the Gila lizard (53% homology with native GLP-1) | Twice daily | 5–10 µg | 2.1–2.2 h | 2.4 h | Mainly renal | 35 |
| Lixisenatide | 2016 | 2013 | 4859 | Exenatide plus poly-lysine tail | Once daily | 10–20 µg | ≈ 2 h | 3 h | Mainly renal | 56–70 |
| Long-acting GLP-1RAs | ||||||||||
| Liraglutide | 2010 | 2009 | 3751 | Modified GLP-1 with free fatty acid side chain attached (97% homology with native GLP-1) | Once daily | 0.6–3 mg | 11.0–13.8 h | 13 h | Peptide hydrolysis, renal (6%)+faecal (5%) | 8.6 |
| Exenatide once weekly | 2012 | 2011 | 4187 | See exenatide twice daily | Once weekly | 2 mg | Slow* | 2.4 h | Mainly renal | 57 |
| Dulaglutide | 2014 | 2014 | 59,671 | Modified GLP-1 with attached immunoglobulin (Fc) fragment (~90% homology with native GLP-1) | Once weekly | 0.75–1.5 mg | 3–5 days | 90 h | Peptidases and renal | 1.6 |
| Semaglutide once weekly | 2017 | 2019 | 4113 | Modified GLP-1 with free fatty acid side chain attached (94% homology to native GLP-1) | Once weekly | 0.5–1 mg | 24 h | 165 h | Peptidases and renal | 0.01–3.50 |
| Oral semaglutide | 2019 | 2020 | 4114 | See semaglutide once weekly | Once daily | 3–14 mg | 1–4 h | 165–185 h | Peptidases | 0.5 |
Has been withdrawn in July 2018 for commercial reasons. **Not formally assessed.
EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; GLP-1, glucagon-like peptide-1; GLP-1RAs, glucagon-like peptide-1 receptor agonists.
Short-acting GLP-1RAs
The short-acting exenatide is identical to the exendin-4 structure, while lixisenatide has been modified from exendin-4 (deletion of proline and an addition of six lysine amino acids at the carboxyl terminus) to make them resistant to degradation by DPP-4 without compromising their GLP-1R activating potencies (9). They are administrated once (lixisenatide) or twice daily (exenatide) in relation to meals and have plasma half-lives in the range of 2.4–3 h resulting in low plasma drug concentrations between injections. Because of their intermittent stimulation of the GLP-1R, short-acting GLP-1RAs retain their ability to delay gastric emptying leading to lower postprandial glucose excursions compared with the long-acting GLP-1RAs, which are associated with the development of tolerance (tachyphylaxis) to GLP-1’s effect on gastric emptying (10).
Long-acting GLP-1RAs
The long-acting GLP-1RAs are exenatide extended-release (once weekly), liraglutide (once daily), dulaglutide (once weekly) and semaglutide (once weekly injections or once daily oral administration). The long-acting GLP-1RAs have been altered in different ways to prolong plasma drug half-life. These modifications include attachment of a fatty acid chain that aids reversible binding to albumin (liraglutide and semaglutide) and covalent binding to carrier molecules such as antibody fragment (Fc) domains of immunoglobulin G (dulaglutide) (11, 12) (Table 1). Exenatide once weekly contains the same active drug as exenatide twice daily, but it is encapsulated in dissolvable microspheres (13). Oral semaglutide is a combination of semaglutide and the absorption enhancer sodium N-(8-(2-hydroxybenzoyl) amino) caprylate (SNAC), which protects semaglutide from degradation in the stomach and facilitates the absorption of semaglutide across the gastric mucosa (14).
Effects of GLP-1RAs in type 2 diabetes
Glycaemia and body weight
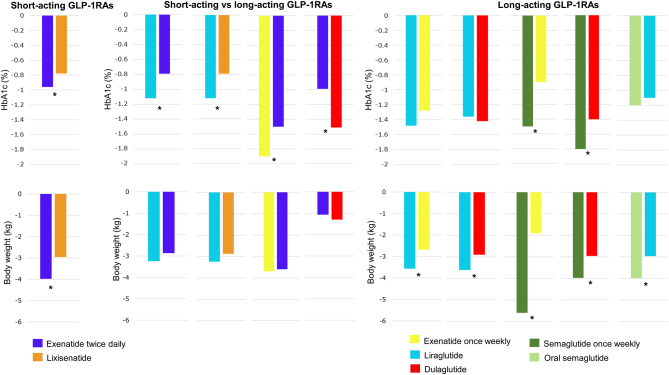
All available GLP-1RAs improve glycaemia and have the ability to reduce body weight (15). An overview of the published head-to-head trials that directly compare different pairs of GLP-1RA with the effects on glycaemia and body weight is provided in Fig. 2. The overall glucose-lowering effects, reflected by HbA1c, are generally more pronounced with long-acting GLP-1RAs compared to short-acting GLP-1RAs (Fig. 2). The reduction in HbA1c ranges between 0.8% and 1.8% across trials. As a class, the GLP-1RAs have all been shown to have a body weight-reducing effect without clinically significant differences in body weight reduction between short-acting and long-acting GLP-1RAs. However, there is a large variety in the effects of GLP-1RA treatment between individuals on body weight from weight gain (few) to more than 15% reduction in body weight (16). In most individuals, GLP-1RA-induced body weight loss is achieved within the first 3–6 months of treatment, and in most patients, the body weight loss is maintained if treatment is continued, while discontinuation will lead to some regain of lost body weight. The body weight-reducing effects of the GLP-1RAs in persons with T2D range between 0.3 and 6.3 kg, with the most marked effect observed with dulaglutide (17) and semaglutide (18).
Figure 2.
Results from clinical trials comparing the GLP-1 receptor agonists (GLP-1RAs) head-to-head in phase III trials. Left panels are trials comparing short-acting GLP-1RAs (97). Middle panels are trials comparing a short-acting GLP-1RA with a long-acting GLP-1RA (7, 98, 99, 100). Right panel are trials comparing long-acting GLP-1RAs (101, 102, 103, 104, 105, 106). Asterisk (*) represents significant differences when comparing the GLP-1RAs.
GLP-1RA-induced reduction in body weight is caused by the suppression of food intake (2). In preclinical studies, peripherally administrated GLP-1RA reaches and binds to GLP-1Rs in areas of the brain involved in the regulation of food intake in the hypothalamus and hindbrain (2). Peripherally administrated GLP-1RA may also indirectly act on the brain by binding to GLP-1Rs on vagal afferent parasympathetic nerve endings, thereby generating and transmitting satiety signals to the hypothalamus and hindbrain (19). In rodents, peripherally administrated semaglutide can modulate food preferences by modifying the reward system through dopamine changes in the brain (20). In line with this, a study including patients with obesity randomised to treatment with semaglutide had fewer food cravings and could endure food cravings better than during treatment with placebo (21). The multiple ways to affect the brain and modulate eating behaviour seem to explain the differences observed in body weight reductions between GLP-1RA compounds. Thus, smaller molecules have more accessible entree to penetrate the brain, and long-acting GLP-1RAs are capable of maintaining sufficient plasma levels throughout the day.
GLP-1RAs and CVOTs
Available GLP-1RAs (except for exenatide twice daily) have been examined in multicentre, double-blinded, randomised, placebo-controlled cardiovascular (CV) outcomes trials (CVOTs). In these trials, participants were randomised to the GLP-1RA in question or placebo, and all participants were treated with standard of care with the possibility of adding non-incretin-based diabetes therapies. Currently, seven CVOTs investigating the CV safety of GLP-1RAs in patients with or without established CVD have been published. As mentioned, albiglutide is no longer available, and thus, details on the HARMONY trial are not included in this clinical review. Baseline participant characteristics and primary composite CV endpoints of the remaining six completed CVOTs for GLP-1RA are listed in Table 2. In the majority of the trials, the primary composite CV endpoint was a three-component major adverse CV event (MACE) outcome including CV-related mortality, non-fatal myocardial infarction (MI), and non-fatal stroke (the CVOT investigating lixisenatide, the ELIXA trial, also included time to the first occurrence of hospitalisation for unstable angina pectoris). The CVOTs investigating liraglutide (LEADER), s.c. semaglutide (SUSTAIN-6) and dulaglutide (REWIND) have shown significant GLP-1RA-induced reductions in MACE. In the ELIXA trial, no effect of lixisenatide on MACE was observed; perhaps explained by lixisenatide’s short half-life compared with the long-acting GLP-1RAs being tested in the other CVOTs (Table 1), the short follow-up period of 2 years and the highest percentage of participants on statin therapy (Table 2). The EXSCEL trial did not find any significant difference in the primary outcome between treatment with the long-acting GLP-1RA exenatide once weekly and placebo. This may be explained by a relatively low trial drug exposure (76%) compared to other CVOTs, and that the trial had no run-in period and, therefore, had one of the highest discontinuation rates. Also, the PIONEER-6 showed no treatment effect with oral semaglutide on the primary composite outcome, which seems to be explained by the low power and short duration of PIONEER-6 (1.3 years). Oral semaglutide demonstrated a hazard ratio (HR) of 0.79, which is like what was observed with s.c. semaglutide in SUSTAIN-6 (HR 0.74). Of note, both PIONEER-6 and SUSTAIN-6 were non-inferiority CV safety studies and, thus, not powered to demonstrate superiority. The larger and longer-lasting (5 years) SOUL trial is ongoing and will determine if oral semaglutide has CV benefits in patients with T2D and CVD or chronic kidney disease (22). The REWIND study found CV benefits of treatment with dulaglutide in participants where only 32% had underlying CVD and who had low baseline HbA1c levels, thereby classifying the study population as a low-risk population compared to the other GLP-1RA CVOTs. A recent meta-analysis including all seven GLP-1RA CVOTs, that is, 42,920 patients, showed a 12% relative risk reduction in MACE during GLP-1RA treatment compared to placebo. Also, significant reductions in all of the separate components of MACE were observed (15). In addition, treatment with GLP-1RAs reduced all-cause mortality by 12% and hospital admission for heart failure (HF) by 9% compared with placebo. Treatment with GLP-1RA also led to reductions in systolic blood pressure and body weight as well as a slight, sustained increase in heart rate compared to the control arm (15). Another meta-analysis, including 35 trials with GLP-1RAs investigating changes in lipid profile, found that treatment with GLP-1RAs leads to minor improvements in lipid profile and a modest effect on lipid metabolism (23).
Table 2.
Summary of baseline characteristics and primary composite cardiovascular outcomes of the completed CVOTs for GLP-1RAs.
| Trial name | Trial drug | Year of completion | N | Median follow-up (years) | Dose/interval | Exposition of drug in trial (%) | Risk factors at baseline | Main outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | HbA1c (%) | Baseline BMI | % with CVD | % with heart failure | % of statin use | MACE (HR (95% CI)) | All–cause mortality (HR(95% CI)) | CV mortality (HR(95% CI)) | Non-fatal MI (HR(95% CI)) | Non-fatal stroke (HR (95% CI)) | Time to first occurrence of hospitalisation for UAP (HR (95% CI)) | |||||||
| ELIXA (107) | Lixisenatideonce daily | 2015 | 6068 | 2.1 | 20 µg/day | 90.5 | 60 | 7.7 | 30.1 | 100 | 22.5 | 93 | 1.02 (0.89–1.17) | 0.94 (0.78–1.13) | 0.98 (0.78–1.22) | 1.03 (0.87–1.22) | 1.12 (0.79–1.58) | 1.11 (0.47–2.62) |
| LEADER (108) | Liraglutideonce daily | 2015 | 9340 | 3.8 | 1.8 mg/day | 84 | 64 | 8.7 | 32.5 | 81.3 | 17.9 | 72 | 0.89 (0.78–0.97) | 0.85 (0.74–0.97) | 0.78 (0.66–0.93) | 0.86 (0.73–1.00) | 0.86 (0.71–1.06) | |
| SUSTAIN-6 (49) | Semaglutide once weekly | 2016 | 3297 | 2.1 | 0.5/1.0 mg/week | 86.5 | 65 | 8.7 | 32.8 | 80 | 23.1 | 73 | 0.74 (0.58–0.95) | 1.05 (0.74–1.50) | 0.98 (0.65–1.48) | 0.81 (0.57–1.16) | 0.65 (0.41–1.03) | |
| EXSCEL (109) | Exenatideonce weekly | 2017 | 14,752 | 3.2 | 2 mg/week | 76 | 62 | 8 | 31.8 | 73.1 | 15.8 | 74 | 0.91 (0.83–1.00) | 0.86 (0.77–0.97) | 0.88 (0.76–1.02) | 0.97 (0.85–1.10) | 0.85 (0.70–1.03) | |
| REWIND (110) | Dulaglutideonce weekly | 2019 | 9901 | 5.5 | 1.5 mg/week | 82.2 | 66 | 7.3 | 32.3 | 31.4 | 8.6 | 66 | 0.88 (0.79–0.99) | 0.90 (0.80–1.01) | 0.91 (0.78–1.06) | 0.96 (0.79–1.15) | 0.76 (0.62–0.94) | |
| PIONEER-6 (111) | Oral semaglutide | 2019 | 3183 | 1.3 | 14 mg/day | 75 | 66 | 8.2 | 32.3 | 84.6 | NR | 85 | 0.79 (0.47–1.11) | 0.51 (0.31–0.84) | 0.49 (0.27–0.92) | 1.04 (0.66–1.66 | 0.76 (0.37–1.56) | |
CVD, cardiovascular disease; GLP-1, glucagon-like peptide-1; GLP-1RAs, glucagon-like peptide-1 receptor agonists; HR, hazard ratio; N, number; NR, not reported; UAP, unstable angina pectoris.
Cardioprotective mechanisms of action
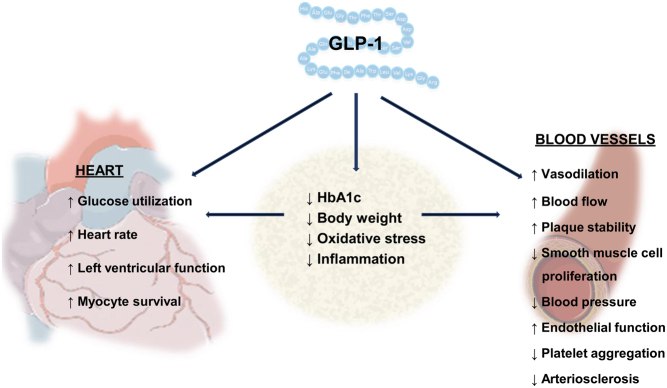
The mechanisms behind the cardioprotective properties of GLP-1RAs are still unclear. Studies have demonstrated that GLP-1RAs exert a direct effect on the heart and blood vessels (6) and an indirect effect via improved glycaemia (24), a reduction in blood pressure and postprandial rise in triglycerides (25), a reduction in markers of oxidative stress and systemic inflammation (26, 27) (Fig. 3). The reduction in MACE observed in treatment with GLP-1RAs is mainly driven by a significant reduction in fatal or non-fatal stroke, but also a significant reduction in risks for CV death and fatal or non-fatal myocardial infarction (15), suggesting that GLP-1RAs may exert their cardioprotective effects through anti-atherosclerotic mechanisms. The atherosclerotic process is complex and strongly associated with inflammation. Treatment with oral semaglutide led to a greater reduction in the inflammatory marker C-reactive protein compared to the sodium-glucose co-transporter-2 inhibitor (SGLT-2i) empagliflozin, without any difference in reduction in body weight between compounds (28). Findings from animal studies suggest that GLP-1R activation may promote anti-arteriosclerotic mechanisms inducing plaque stabilisation and preventing plaque progression (29, 30, 31, 32, 33). Interestingly, GLP-1RA treatment is associated with small reductions in systolic blood pressure, which seem independent of GLP-1RA-induced weight loss (34). This effect is thought to be mediated by increasing natriuresis (35) and vasorelaxation in the renal vasculature (36). In addition, GLP-1RAs increase heart rate in humans, most likely via the GLP-1R located in the sinoatrial node of the heart (37) and perhaps also via activation of the sympathetic nervous system and inhibition of the parasympathetic nervous system as shown in animals studies (38, 39). The increase in heart rate observed in patients treated with GLP-1RAs has led to cautious use of GLP-1RAs in patients with congestive HF New York Heart Associations (NYHA) class IV. In the abovementioned meta-analysis by Kristensen et al. (15), a sub-analysis found that GLP-1RAs are safe in patients with HF and in fact reduces their risk of hospitalisation for HF.
Figure 3.
Possible cardiovascular actions of glucagon-like peptide 1 (GLP-1) and GLP-1 receptor agonists (GLP-1RAs).
Renal complications
Diabetic kidney disease is a complication of diabetes that leads to increased mortality (40). Most GLP-1RA CVOTs incorporated secondary exploratory renal endpoints. In their meta-analysis, Kristensen et al. found that treatment with GLP-1RA leads to a 17% reduction in a broad composite kidney endpoint (development of new-onset macroalbuminuria, doubling of serum creatinine or 40% greater decline in estimated glomerular filtration rate (eGFR), progression to end-stage kidney disease, or kidney-related death), mainly as a result of a reduction in new-onset macroalbuminuria (15). Ongoing trials will determine if liraglutide (41), lixisenatide (ELIXIRS trial) (42) and semaglutide (FLOW trial) (43) reduce dialysis rates and reduce worsening kidney function in people with diabetic kidney disease. The potential nephroprotective mechanisms of GLP-1RAs likely involve GLP-1RA-mediated improvements of conventional risk factors for diabetic kidney disease (control of glycaemia, postprandial lipid level, body weight and blood pressure), but perhaps also direct effects on the kidneys. The exact anatomical location of the GLP-1RA in the human kidney is debated, but the consent is that the GLP-1R is expressed in the renal afferent arterioles, whereas the presence of GLP-1Rs in the renal glomeruli and proximal tubuli remains uncertain (44, 45). Chronic administration of GLP-1RAs have been suggested to affect renal haemodynamic by decreasing eGFR and ameliorating glomerular hyperfiltration in patients with T2D. In addition, GLP-1RAs may have anti-albuminuria actions by increasing natriuresis and decreasing plasma renin activity (46). In addition, the positive effects of GLP-1RA on oxidative stress and inflammation may also contribute to the nephroprotective mechanisms of GLP-1RAs (47, 48).
Ophthalmic complications
The ELIXA, EXSCEL, HARMONY outcomes and PIONEER-6 trials did not include retinopathy as an outcome. In the SUSTAIN-6 trial, there was a significantly higher event rate of diabetic retinopathy in the semaglutide-treated group compared with the control (49). During the first month of the trial, most events coincided with a rapid and massive drop in HbA1c in dysregulated patients with pre-existing retinopathy. A recent CVOT meta-analysis showed that the magnitude of HbA1c reduction was correlated with retinopathy risk and was not associated with GLP-1RA treatment per se (50). However, a direct and unknown action of GLP-1RAs cannot be excluded. The ongoing FOCUS trial investigates the long-term effects of semaglutide compared with placebo on diabetic retinopathy, including relatively well-regulated (HbA1c 7–10%) patients with T2D (51).
Treatment with GLP-1RAs for other indications than T2D and obesity
Liver diseases
Non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH) is highly prevalent in patients with T2D and/or obesity. Currently, there are no FDA-approved treatments for NASH. In a recent phase II trial, patients with liver biopsy-confirmed NASH and liver fibrosis were treated once daily with 0.4 mg s.c. semaglutide for 72 weeks. A higher percentage of patients treated with semaglutide had resolution of NASH than with placebo; however, no improvement in fibrosis was found (52). These results are in line with previous findings on the effect of GLP-1RAs on NASH (53, 54). As the GLP-1R is not expressed on human hepatocytes, the GLP-1RA-mediated effects on fatty liver are probably indirectly mediated through GLP-1RA-induced weight loss and ensuing improvement in insulin resistance, and, potentially, anti-inflammatory effects associated with GLP-1RA treatment (29). Integrating the actions of GLP-1 with other receptor agonists is emerging as a potential new drug class with pharmaceutical potential in treating, for example, liver diseases. The potential of GLP-1/glucagon dual receptor agonist on steatohepatitis, fibrosis and liver regeneration has also shown some promising results in clinical studies (55) and may have a therapeutic potential in NAFLD. In addition, combining semaglutide with cilofexor, a farnesoid X receptor agonist, and/or firsocostat, an acetyl-CoA carboxylase inhibitor, has also shown potential in preclinical studies. Unpublished results from the first phase II trial with the triple combination of semaglutide, cilofexor and/or firsocostat treatment for 24 weeks in 108 patients with NASH showed significant improvements in hepatic steatosis (measured with MRI) and liver injury (measured by serum alanine aminotransferase) in the combination treatment arm vs semaglutide monotherapy (56).
Type 1 diabetes
By exploiting GLP-1’s glucose-dependent glucagonostatic effect and its inhibiting effect on gastric emptying, treatment with GLP-1RAs could supplement insulin treatment in patients with type 1 diabetes (T1D). Liraglutide (57, 58, 59) and short-acting exenatide (60) have been studied in placebo-controlled trials as an add-on to treatment with insulin in patients with T1D. Liraglutide significantly reduced HbA1c and body weight but caused more hypoglycaemic incidents and a slight increase in ketoacidosis in one trial (57). Treatment with short-acting exenatide leads to a minor reduction in HbA1c and significantly reduced body weight without increasing hypoglycaemia or ketoacidosis (60). Thus, no convincing data support the general use of GLP-1RAs as adjuvant therapy in patients with T1D. Still, with the right combination and timing with insulin, treatment with GLP-1RAs may have the potential of reducing HbA1c, postprandial glycaemic excursions, insulin dose, body weight, and frequency of hypoglycaemic episodes, especially in obese patients with T1D (61).
Neurodegenerative diseases
T2D is a risk factor for cognitive dysfunction and for developing Alzheimer’s disease (62). In rodents, modulation of GLP-1R activity can influence amyloid-beta peptide aggregation in Alzheimer’s disease (63) and dopamine levels in Parkinson's disease (64). Impaired insulin signalling has been proposed to contribute to the development of Alzheimer’s disease (65). GLP-1RAs have shown some neuroprotective effects in rodents with neurogenerative diseases (65). In a pilot study, treatment with liraglutide increased glucose utilisation in the brain as detected by (18)F-fluorodeoxyglucose(18FDG)-positron emission tomographyPET (PET) scans (66). However, the study was underpowered to make any conclusions on amyloid plaques and cognitive outcome. A phase II study investigating the efficacy and safety of liraglutide in 200 patients with Alzheimer’s disease has recently been completed (67) but remains unpublished. Recently, it was announced that a phase III trial investigating whether oral semaglutide can prevent progression in patients with early Alzheimer’s disease is to be initiated in 2021 (68). In Parkinson’s disease, treatment with exenatide once weekly for 12 months has improved motor symptoms (69). Currently, two ongoing phase II trials investigate the effect of semaglutide (70) and liraglutide (71) on motor symptom progression. Also, one study is evaluating the effect of exenatide once weekly on Parkinson’s disease progression by MRI (72). Even though clinical trials have shown some positive results on motor function in patients with Parkinson's disease treated with exenatide (73), a recent Cochrane review found 'low-certainty evidence that exenatide improves motor impairment' in patients with Parkinson’s disease (74). Further research is needed to elucidate the role of GLP-1RAs in the treatment of neurodegenerative diseases.
Safety
The most frequently reported side effects of GLP-1RAs are of gastrointestinal (GI) origin, including nausea, vomiting, diarrhoea and obstipation and may be more common in patients with higher age, lower body weight, renal impairment, and if there is a history or currently smoking (75). GI adverse events are usually mild or moderate, dose-dependent, decline with continued treatment and do not affect glycaemic control. GLP-1RA therapy has previously been linked to acute pancreatitis and pancreatic cancer. However, meta-analyses do not support such an association (76, 77), and an increase in amylase and/or lipase levels may be detected (78). When using GLP-1RAs in patients with risk factors for pancreatitis, such as hypertriglyceridaemia or excessive alcohol use, or in persons with previously diagnosed pancreatitis, great caution is recommended. Since the incidence of cholelithiasis and cholecystitis were higher in patients treated with liraglutide (79), concerns about GLP-1RA and gallbladder adverse events have been raised. In the recent STEP 1 trial investigating the body weight-lowering effect of s.c. semaglutide 2.4 mg once weekly in patients with obesity, gallbladder-related disorders (mostly cholelithiasis) were reported in 2.6% and 1.2% of participants in the semaglutide and placebo groups, respectively (80). Treatment with GLP-1RAs may affect gallbladder motility and thereby prolong gallbladder refilling (81), which perhaps explains the increase in gallbladder adverse events associated with GLP-1RAs.
Perspectives
Enthused by the results after bariatric surgery where multiple gut hormones are increased (82), peptide multi-agonists are emerging and have shown promising results. By targeting appetite reduction and energy expenditure with dual or triple agonists, it may be possible to pharmacologically mimic the effects seen with bariatric surgery to produce 30–40% body weight reduction, diabetes remission and CV benefits (83, 84). The dual GLP-1R and GIP receptor agonist tirzepatide is administrated once weekly and under development for the treatment of T2D, obesity (85) and NASH (86). In a phase II trial, tirzepatide once weekly was superior to dulaglutide in terms of HbA1c reduction and body weight reduction in patients with T2D with safety data for tirzepatide similar to that of GLP-1RAs (87). Results from the phase III trial (SURPASS 2) investigating the effects of 40 weeks of treatment with tirzepatide vs s.c. semaglutide in patients with T2D have recently been announced, with tirzepatide 15 mg reducing HbA1c levels by 2.46% and body weight reduction by 12.4 kg (13.1%), double the body weight reduction compared to those taking semaglutide 1 mg (88). Tirzepatide has been superior in reducing glycaemia and body weight compared to the GLP-1RA drug class, appearing that GIP adds effectiveness to GLP-1 agonism. CV safety of treatment with tirzepatide in patients with T2D compared to dulaglutide is to be investigated in the SURPASS-CVOT phase III clinical trial scheduled to finish in 2024 (89).
In addition, several dual GLP-1R-glucagon receptor co-agonists are under development, and their therapeutic potential for the treatment of T2D and obesity is being investigated in clinical trials (55, 90, 91, 92). Also, the combination of GLP-1RA with cholecystokinin (CCK) and fibroblast growth factor 21 (FGF21) has been studied in preclinical settings (93, 94). CCK has shown potential in reducing body weight in combination with GLP-1RAs in the mouse models (93). FGF21 is a newly discovered metabolic hormone produced in multiple tissues and secreted in the fasting state and regulates metabolic responses in the pancreas, liver and adipose tissues (95). FGF21 has thermogenic and insulin-sensitising effects when combined with a GLP-1RA with elastin-like polypeptide (ELP) as a flexible linker (94). In a diabetic mouse model, administration of GLP-1RA-ELP-FGF21 reduced glycaemia without incidences of hypoglycaemia and greater body weight reductions compared with the monotherapy or an equimolar mixture of GLP1-ELP and ELP-FGF21 (94). In summary, the combination of GLP-1RAs with other GI peptide receptor agonists appears to amplify the effects of GLP-1R activation, and these combinations are currently being investigated in preclinical and clinical studies, not only for the treatment of T2D but also for the treatment of other metabolic disorders.
Conclusion
Over the last decades, extensive preclinical and clinical research have revealed the complexity of the (patho-)physiology of GLP-1 in health and disease. This research and knowledge related to the mode of action have been successfully translated into clinical pharmacology and have been and are a game-changer in T2D and, to some extent, in obesity. GLP-1RAs represent an important drug class for the treatment of T2D and offer marked reductions in plasma glucose levels with a low risk of hypoglycaemia, body weight reductions and a reduction in CVD in patients with high CVD risk. Based on the CVOTs, the treatment algorithm of T2D has changed to include GLP-1RAs independent of glycaemia in patients with established CVD or high-risk (96). Ongoing studies explore the potential of CV, renal and neuroprotective benefits of GLP-1RAs and the potential of using GLP-1RAs for other indications, including NASH, neurodegenerative diseases, and T1D. In addition, emerging dual agonists combing the effects of GLP-1RAs with other peptides have shown a huge potential of superior effects on glycaemia and body weight in patients with T2D compared to currently available peptides. Investigating the physiological and cellular interactions of multi-peptide receptor agonist combinations could further elucidate the therapeutic potential as well as minimising unwanted action or adverse events. Future multi-peptide receptor agonist compounds could potentially target selected organ tissues offering an individualised therapy for a variety of diseases.
Declaration of interest
C R A and A A declare no conflicts of interest. F K K has served on scientific advisory panels and/or been part of speaker’s bureaus, served as a consultant to and/or received research support from Amgen, AstraZeneca, Bayer Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi and Zealand Pharma. M M R is a minority shareholder of and consultant for Antag Therapeutics, Bainan Biotech and Synklino. T V has served on scientific advisory panels, been part of speaker’s bureaus, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharmaceuticals. All authors declare that the study was conducted in the absence of any financial or conflicts of interest.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Holst JJ.The physiology of glucagon-like peptide 1. Physiological Reviews 2007. 87 1409–1439. ( 10.1152/physrev.00034.2006) [DOI] [PubMed] [Google Scholar]
- 2.Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JFet al. Glucagon-like peptide 1 (GLP-1). Molecular Metabolism 2019. 30 72–130. ( 10.1016/j.molmet.2019.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. Journal of Clinical Endocrinology and Metabolism 2002. 87 1239–1246. ( 10.1210/jcem.87.3.8355) [DOI] [PubMed] [Google Scholar]
- 4.Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010. 59 1765–1770. ( 10.2337/db09-1414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006. 55 243–251. ( 10.1136/gut.2004.059741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocrine Reviews 2021. 42 101–132. ( 10.1210/endrev/bnaa032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. & DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008. 372 1240–1250. ( 10.1016/S0140-6736(0861206-4) [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. & Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004. 27 2628–2635. ( 10.2337/diacare.27.11.2628) [DOI] [PubMed] [Google Scholar]
- 9.Bhavsar S, Mudaliar S, Cherrington A. Evolution of exenatide as a diabetes therapeutic. Current Diabetes Reviews 2013. 9 161–193. ( 10.2174/1573399811309020007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011. 60 1561–1565. ( 10.2337/db10-0474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ.GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Reviews: Endocrinology 2012. 8 728–742. ( 10.1038/nrendo.2012.140) [DOI] [PubMed] [Google Scholar]
- 12.Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nature Reviews: Endocrinology 2018. 14 390–403. ( 10.1038/s41574-018-0016-2) [DOI] [PubMed] [Google Scholar]
- 13.Wysham C, Grimm M, Chen S. Once weekly exenatide: efficacy, tolerability and place in therapy. Diabetes, Obesity and Metabolism 2013. 15 871–881. ( 10.1111/dom.12084) [DOI] [PubMed] [Google Scholar]
- 14.Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clinical Pharmacokinetics 2019. 58 1193–1203. ( 10.1007/s40262-019-00756-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet: Diabetes and Endocrinology 2019. 7 776–785. ( 10.1016/S2213-8587(1930249-9) [DOI] [PubMed] [Google Scholar]
- 16.Fujioka K, O’Neil PM, Davies M, Greenway F, C W Lau D, Claudius B, Skjøth TV, Bjørn Jensen C, P H Wilding J. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity 2016. 24 2278–2288. ( 10.1002/oby.21629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frias JP, Bonora E, Nevarez Ruiz L, Li YG, Yu Z, Milicevic Z, Malik R, Bethel MA, Cox DA. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care 2021. 44 765–773. ( 10.2337/dc20-1473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingvay I, Hansen T, MacUra S, Marre M, Nauck MA, De La Rosa R, Woo V, Yildirim E, Wilding J. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Research and Care 2020. 8 1–9. ( 10.1136/bmjdrc-2020-001706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999. 140 1687–1694. ( 10.1210/endo.140.4.6643) [DOI] [PubMed] [Google Scholar]
- 20.Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KSet al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020. 5 e133429. ( 10.1172/jci.insight.133429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, Hjerpsted JB. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obesity and Metabolism 2017. 19 1242–1251. ( 10.1111/dom.12932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A heart disease study of semaglutide in patients with type 2 diabetes (SOUL). (available at: https://clinicaltrials.gov/ct2/show/NCT03914326) [Google Scholar]
- 23.Sun F, Wu S, Wang J, Guo S, Chai S, Yang Z, Li S, Zhang Y, Ji L, Zhan S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clinical Therapeutics 2015. 37 225.e8–241.e8. ( 10.1016/j.clinthera.2014.11.008) [DOI] [PubMed] [Google Scholar]
- 24.Xiao C, Bandsma RHJ, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arteriosclerosis, Thrombosis, and Vascular Biology 2012. 32 1513–1519. ( 10.1161/ATVBAHA.112.246207) [DOI] [PubMed] [Google Scholar]
- 25.Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006. 49 452–458. ( 10.1007/s00125-005-0126-y) [DOI] [PubMed] [Google Scholar]
- 26.Ceriello A, Novials A, Canivell S, Sala L, La, Pujadas G, Esposito K, Testa R, Bucciarelli L, Rondinelli M, Genovese S. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinf lammatory and antioxidant action in type 2 diabetes. Diabetes Care 2014. 37 1938–1943.( 10.2337/dc13-2618) [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Esposito K, Giugliano D, Genovese S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013. 36 2346–2350. ( 10.2337/dc12-2469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya Eet al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the Pioneer 2 trial. Diabetes Care 2019. 42 2272–2281. ( 10.2337/dc19-0883) [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Tuo X, Li B, Deng Z, Qiu Y, Xie H. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sciences 2020. 250 117531. ( 10.1016/j.lfs.2020.117531) [DOI] [PubMed] [Google Scholar]
- 30.Sudo M, Li Y, Hiro T, Takayama T, Mitsumata M, Shiomi M, Sugitani M, Matsumoto T, Hao H, Hirayama A. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2017. 265 283–291. ( 10.1016/j.atherosclerosis.2017.06.920) [DOI] [PubMed] [Google Scholar]
- 31.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012. 61 888–896. ( 10.2337/db11-1073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010. 59 1030–1037. ( 10.2337/db09-1694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, Hecksher-Sørensen J, Ingvorsen C, Polex-Wolf J, Knudsen LB. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE −/− and LDLr −/− mice by a mechanism that includes inflammatory pathways. JACC: Basic to Translational Science 2018. 3 844–857. ( 10.1016/j.jacbts.2018.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circulation Research 2014. 114 1788–1803. ( 10.1161/CIRCRESAHA.114.301958) [DOI] [PubMed] [Google Scholar]
- 35.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015. 38 132–139. ( 10.2337/dc14-1958) [DOI] [PubMed] [Google Scholar]
- 36.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. American Journal of Physiology: Renal Physiology 2015. 308 F867–F877. ( 10.1152/ajprenal.00527.2014) [DOI] [PubMed] [Google Scholar]
- 37.Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, Cappola TP, Margulies KB, Drucker DJ. GLP-1 receptor expression within the human heart. Endocrinology 2018. 159 1570–1584. ( 10.1210/en.2018-00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto H, Lee CE, Marcus JN, Williams TD, Michael Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJet al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. Journal of Clinical Investigation 2002. 110 43–52. ( 10.1172/JCI15595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, Mighiu AS, Zhang H, Ludwig A, Seeley RJet al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Molecular Metabolism 2017. 6 1339–1349. ( 10.1016/j.molmet.2017.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afkarian M, Katz R, Bansal N, Correa A, Kestenbaum B, Himmelfarb J, De Boer IH, Young B. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson heart study. Clinical Journal of the American Society of Nephrology 2016. 11 1384–1391. ( 10.2215/CJN.13111215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The effect of glucagon-like-peptide 1 (GLP-1) receptor agonism on diabetic kidney disease. (available at: https://clinicaltrials.gov/ct2/show/NCT01847313) [Google Scholar]
- 42.Effect of LIXIsenatide on the renal system (ELIXIRS). (available at: https://clinicaltrials.gov/ct2/show/NCT02276196) [Google Scholar]
- 43.A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). (available at: https://clinicaltrials.gov/ct2/show/NCT03819153) [Google Scholar]
- 44.Tsimihodimos V, Elisaf M. Effects of incretin-based therapies on renal function. European Journal of Pharmacology 2018. 818 103–109. ( 10.1016/j.ejphar.2017.10.049) [DOI] [PubMed] [Google Scholar]
- 45.Muskiet MHA, Tonneijck L, Smits MM, Van Baar MJB, Kramer MHH, Hoorn EJ, Joles JA, Van Raalte DH. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nature Reviews: Nephrology 2017. 13 605–628. ( 10.1038/nrneph.2017.123) [DOI] [PubMed] [Google Scholar]
- 46.Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 receptor agonists and kidney protection. Medicina 2019. 55 1–14. ( 10.3390/medicina55060233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helmstädter J, Frenis K, Filippou K, Grill A, Dib M, Kalinovic S, Pawelke F, Kus K, Kröller-Schön S, Oelze Met al. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology 2020. 40 145–158. ( 10.1161/atv.0000615456.97862.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care 2010. 33 1028–1030. ( 10.2337/dc09-1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren MLet al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. New England Journal of Medicine 2016. 375 1834–1844. ( 10.1056/NEJMoa1607141) [DOI] [PubMed] [Google Scholar]
- 50.Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care 2021. 44 290–296. ( 10.2337/dc20-1815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.A research study to look at how semaglutide compared to placebo affects diabetic eye disease in people with Type 2 diabetes (Focus). (available at: https://clinicaltrials.gov/ct2/show/NCT03811561) [Google Scholar]
- 52.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling A-S, Harrison SA. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. New England Journal of Medicine 2021. 384 1113–1124. ( 10.1056/NEJMoa2028395) [DOI] [PubMed] [Google Scholar]
- 53.Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Journal of Gastroenterology and Hepatology 2016. 31 23–31. ( 10.1111/jgh.13026) [DOI] [PubMed] [Google Scholar]
- 54.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K.LEAN Trial Team, Abouda Get al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016. 387 679–690. ( 10.1016/S0140-6736(1500803-X) [DOI] [PubMed] [Google Scholar]
- 55.Valdecantos MP, Pardo V, Ruiz L, Castro-Sánchez L, Lanzón B, Fernández-Millán E, García-Monzón C, Arroba AI, González-Rodríguez Á, Escrivá Fet al. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology 2017. 65 950–968. ( 10.1002/hep.28962) [DOI] [PubMed] [Google Scholar]
- 56.Gilead and Novo Nordisk present new data from proof-of-concept trial in NASH. (available at: http://investors.gilead.com/node/37711/pdf) [Google Scholar]
- 57.Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, Linder M, Bode B. & ADJUNCT ONE Investigators. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the adjunct one treat-to-target randomized trial. Diabetes Care 2016. 39 1702–1710. ( 10.2337/dc16-0691) [DOI] [PubMed] [Google Scholar]
- 58.Ahren B, Hirsch IB, Pieber TR, Mathieu C, Gomez-Peralta F, Hansen TK, Philotheou A, Birch S, Christiansen E, Jensen TJet al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the adjunct two randomized trial. Diabetes Care 2016. 39 1693–1701. ( 10.2337/dc16-0690) [DOI] [PubMed] [Google Scholar]
- 59.Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow Let al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet: Diabetes and Endocrinology 2016. 4 221–232. ( 10.1016/S2213-8587(1500436-2) [DOI] [PubMed] [Google Scholar]
- 60.Johansen NJ, Dejgaard TF, Lund A, Schlüntz C, Frandsen CS, Forman JL, Wewer Albrechtsen NJ, Holst JJ, Pedersen-Bjergaard U, Madsbad Set al. Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet: Diabetes and Endocrinology 2020. 8 313–324. ( 10.1016/S2213-8587(2030030-9) [DOI] [PubMed] [Google Scholar]
- 61.Nauck MA, Meier JJ. GLP-1 receptor agonists in type 1 diabetes: a MAG1C bullet? Lancet: Diabetes and Endocrinology 2020. 8 262–264. ( 10.1016/S2213-8587(2030043-7) [DOI] [PubMed] [Google Scholar]
- 62.Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neuroscience and Biobehavioral Reviews 2007. 31 1046–1063. ( 10.1016/j.neubiorev.2007.04.004) [DOI] [PubMed] [Google Scholar]
- 63.Batista AF, Forny-Germano L, Clarke JR, Lyra e Silva NM, Brito-Moreira J, Boehnke SE, Winterborn A, Coe BC, Lablans A, Vital JFet al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. Journal of Pathology 2018. 245 85–100. ( 10.1002/path.5056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park Het al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine 2018. 24 931–938. ( 10.1038/s41591-018-0051-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taouis M, Lambrecht N, Lizard G, Vieira MNN, De Felice FG, Ferreira LSS, Fernandes CS. Insulin resistance in Alzheimer’s disease. Frontiers in Neuroscience 2018. 12 830. ( 10.3389/fnins.2018.00830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht Aet al. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Frontiers in Aging Neuroscience 2016. 8 108. ( 10.3389/fnagi.2016.00108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evaluating liraglutide in Alzheimer’s disease (ELAD). (Available at: https://clinicaltrials.gov/ct2/show/NCT01843075) [Google Scholar]
- 68.Novo Nordisk to enter phase 3 development in Alzheimer’s disease with oral semaglutide. (available at: https://www.globenewswire.com/news-release/2020/12/16/2146164/0/en/Novo-Nordisk-to-enter-phase-3-development-in-Alzheimer-s-disease-with-oral-semaglutide.html) [Google Scholar]
- 69.Athauda D, Gulyani S, Karnati HK, Li Y, Tweedie D, Mustapic M, Chawla S, Chowdhury K, Skene SS, Greig NHet al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD Trial. JAMA Neurology 2019. 76 420–429. ( 10.1001/jamaneurol.2018.4304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.GLP1R in Parkinson’s disease (GIPD). (available at: https://clinicaltrials.gov/ct2/show/NCT03659682) [Google Scholar]
- 71.Safety and efficacy of liraglutide in Parkinson’s disease. (available at: https://clinicaltrials.gov/ct2/show/NCT02953665) [Google Scholar]
- 72.Effects of exenatide on motor function and the brain. (available at: https://clinicaltrials.gov/ct2/show/NCT03456687) [Google Scholar]
- 73.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson Jet al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017. 390 1664–1675. ( 10.1016/S0140-6736(1731585-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mulvaney CA, Duarte GS, Handley J, Evans DJW, Menon S, Wyse R, Emsley HCA. GLP-1 receptor agonists for Parkinson’s disease. Cochrane Database of Systematic Reviews 2020. 7 CD012990. ( 10.1002/14651858.CD012990.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gastrointestinal adverse events with once-weekly semaglutide: risk predictors and effect on semaglutide response – Virtual Meeting|EASD. (available at: https://www.easd.org/virtualmeeting/home.html#)!resources/gastrointestinal-adverse-events-with-once-weekly-semaglutide-risk-predictors-and-effect-on-semaglutide-response) [Google Scholar]
- 76.Storgaard H, Cold F, Gluud LL, Vilsbøll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 2017. 19 906–908. ( 10.1111/dom.12885) [DOI] [PubMed] [Google Scholar]
- 77.Pinto LC, Falcetta MR, Rados DV, Leitão CB, Gross JL. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Scientific Reports 2019. 9 2375. ( 10.1038/s41598-019-38956-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinberg WM, Buse JB, Ghorbani MLM, Ørsted DD, Nauck MA.LEADER Steering Committee & LEADER Trial Investigators. Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treatedwith liraglutide: results from the LEADER randomized trial. Diabetes Care 2017. 40 966–972. ( 10.2337/dc16-2747) [DOI] [PubMed] [Google Scholar]
- 79.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA. & NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015. 314 687–699. ( 10.1001/jama.2015.9676) [DOI] [PubMed] [Google Scholar]
- 80.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TAet al. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine 2021. 384 989. ( 10.1056/NEJMoa2032183) [DOI] [PubMed] [Google Scholar]
- 81.Gether IM, Nexøe-Larsen C, Knop FK. New avenues in the regulation of gallbladder motility – implications for the use of glucagon-like peptide-derived drugs. Journal of Clinical Endocrinology and Metabolism 2019. 104 2463–2472. ( 10.1210/jc.2018-01008) [DOI] [PubMed] [Google Scholar]
- 82.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet: Diabetes and Endocrinology 2014. 2 152–164. ( 10.1016/S2213-8587(1370218-3) [DOI] [PubMed] [Google Scholar]
- 83.Holst JJ, Madsbad S, Bojsen-Møller KN, Svane MS, Jørgensen NB, Dirksen C, Martinussen C. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surgery for Obesity and Related Diseases 2018. 14 708–714. ( 10.1016/j.soard.2018.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasib A.Multiagonist unimolecular peptides for obesity and Type 2 diabetes: current advances and future directions. Clinical Medicine Insights: Endocrinology and Diabetes 2020. 13 1179551420905844. ( 10.1177/1179551420905844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.A study of Tirzepatide (LY3298176) in participants with obesity or overweight (SURMOUNT-1). (available at: https://clinicaltrials.gov/ct2/show/NCT04184622) [Google Scholar]
- 86.A study of Tirzepatide (LY3298176) in participants with nonalcoholic steatohepatitis (NASH) (SYNERGY-NASH). (available at: https://clinicaltrials.gov/ct2/show/NCT04166773) [Google Scholar]
- 87.Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 receptor agonist Tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. Journal of Clinical Endocrinology and Metabolism 2021. 106 388–396. ( 10.1210/clinem/dgaa863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tirzepatide achieved superior A1C and body weight reductions across all three doses compared to injectable semaglutide in adults with type 2 diabetes|Eli Lilly and Company. (available at: https://investor.lilly.com/news-releases/news-release-details/tirzepatide-achieved-superior-a1c-and-body-weight-reductions) [Google Scholar]
- 89.A study of Tirzepatide (LY3298176) compared with dulaglutide on major cardiovascular events in participants with Type 2 diabetes (SurPASS-CVOT). (available at: https://clinicaltrials.gov/ct2/show/NCT04255433) [DOI] [PubMed] [Google Scholar]
- 90.Visentin R, Schiavon M, Göbel B, Riz M, Cobelli C, Klabunde T, Dalla Man C. Dual glucagon-like peptide-1 receptor/glucagon receptor agonist SAR425899 improves beta-cell function in type 2 diabetes. Diabetes, Obesity and Metabolism 2020. 22 640–647. ( 10.1111/dom.13939) [DOI] [PubMed] [Google Scholar]
- 91.Parker VER, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, Posch MG, Heise T, Plum-Moerschel L, Schlichthaar Het al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. Journal of Clinical Endocrinology and Metabolism 2020. 105 803–820. ( 10.1210/clinem/dgz047) [DOI] [PubMed] [Google Scholar]
- 92.A study to evaluate the efficacy and safety of MEDI0382 in the treatment of overweight and obese subjects with type 2 diabetes. (available at: https://clinicaltrials.gov/ct2/show/results/NCT03235050) [Google Scholar]
- 93.Hornigold DC, Roth E, Howard V, Will S, Oldham S, Coghlan MP, Blouet C, Trevaskis JL. A GLP-1:CCK fusion peptide harnesses the synergistic effects on metabolism of CCK-1 and GLP-1 receptor agonism in mice. Appetite 2018. 127 334–340. ( 10.1016/j.appet.2018.05.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilroy CA, Capozzi ME, Varanko AK, Tong J, D’Alessio DA, Campbell JE, Chilkoti A. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Science Advances 2020. 6 eaaz9890. ( 10.1126/sciadv.aaz9890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metabolism 2019. 29 246–253. ( 10.1016/j.cmet.2019.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2020. Diabetes Care 2020. 43 (Supplement 1) S98–S110. ( 10.2337/dc20-S009) [DOI] [PubMed] [Google Scholar]
- 97.Rosenstock J, Raccah D, Koranyi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type2diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013. 36 2945–2951. ( 10.2337/dc12-2709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. & LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009. 374 39–47. ( 10.1016/S0140-6736(0960659-0) [DOI] [PubMed] [Google Scholar]
- 99.Nauck M, Rizzo M, Johnson A, Bosch-Traberg H, Madsen J, Cariou B. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care 2016. 39 1501–1509. ( 10.2337/dc15-2479) [DOI] [PubMed] [Google Scholar]
- 100.Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, Kuhstoss D, Lakshmanan M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014. 37 2159–2167. ( 10.2337/dc13-2760) [DOI] [PubMed] [Google Scholar]
- 101.Buse JB, Nauck M, Forst T, Sheu WHH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman Met al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013. 381 117–124. ( 10.1016/S0140-6736(1261267-7) [DOI] [PubMed] [Google Scholar]
- 102.Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart Met al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet: Diabetes and Endocrinology 2014. 2 289–297. ( 10.1016/S2213-8587(1370214-6) [DOI] [PubMed] [Google Scholar]
- 103.Dungan KM, Povedano ST, Forst T, González JGG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 2014. 384 1349–1357. ( 10.1016/S0140-6736(1460976-4) [DOI] [PubMed] [Google Scholar]
- 104.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 2018. 41 258–266. ( 10.2337/dc17-0417) [DOI] [PubMed] [Google Scholar]
- 105.Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A. & SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet: Diabetes and Endocrinology 2018. 6 275–286. ( 10.1016/S2213-8587(1830024-X) [DOI] [PubMed] [Google Scholar]
- 106.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ. & PIONEER 4 Investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (Pioneer 4): a randomised, double-blind, phase 3a trial. Lancet 2019. 394 39–50. ( 10.1016/S0140-6736(1931271-1) [DOI] [PubMed] [Google Scholar]
- 107.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EFet al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. New England Journal of Medicine 2015. 373 2247–2257. ( 10.1056/NEJMoa1509225) [DOI] [PubMed] [Google Scholar]
- 108.Marso SP, Daniels GH, Frandsen KB, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LSet al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2016. 375 311–322. ( 10.1056/NEJMoa1603827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal Net al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2017. 377 1228–1239. ( 10.1056/NEJMoa1612917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén Let al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019. 394 121–130. ( 10.1016/S0140-6736(1931149-3) [DOI] [PubMed] [Google Scholar]
- 111.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SDet al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2019. 381 841–851. ( 10.1056/NEJMoa1901118) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a