Abstract
We report the functional characterization of two iron- and α-ketoglutarate-dependent dioxygenases that are capable of hydroxylating free-standing glutamine at its C3 and C4 position respectively. In particular, the C4 hydroxylase, Q4Ox, catalyzes the reaction with approximately 4,300 total turnover numbers, facilitating synthesis of a solid-phase compatible building block and stereochemical elucidation at the C4 position of the hydroxylated product. This work will enable the development of novel synthetic strategies to prepare useful glutamine derivatives and stimulate further discoveries of new amino acid hydroxylases with distinct substrate specificities.
Graphical Abstract
Introduction
Amino acids are some of the most indispensable chemicals in biological systems and modern drug discovery efforts.1 In addition to their incorporation into peptides and other biological macromolecules, amino acids also serve as precursors to amine-based primary metabolites and neurotransmitters. Their unique three-dimensionality, combined with the presence of useful and diverse functional groups, render amino acids highly desirable building blocks in combinatorial synthesis. Recent renaissance in peptide drug discovery2 has spurred the development of new methods to access novel amino acid structures that go beyond the twenty proteinogenic counterparts. Among the various possible modifications, C─H hydroxylation of proteinogenic amino acids (pAAs) can play an important role in modulating the biological and pharmacological properties of the resulting structures. For example, C3 hydroxylation of prolines in Gly-Pro-Pro repeats in collagen3 play a key role in regulating its tertiary structure and stability. Additionally, 4-hydroxy-L-isoleucine has been described to display antidiabetic and antiobesitic activities.4 Hydroxylation of pAAs can also act as a gateway for further modifications,5 as exemplified by the biogenesis of 4-methyl-L-proline from L-leucine6 and the recent discovery that the biosynthesis of ibotenic acid begins by selective C3-hydroxylation of glutamate.7 Synthetic chemistry similarly views the alcohol moiety on hydroxylated amino acids as a useful chemical handle for further structural diversification. Some of the methods commonly employed for such diversification include fluorine incorporation via nucleophilic substitution8 and C─C bond formation via oxidation/methylenation sequence.9
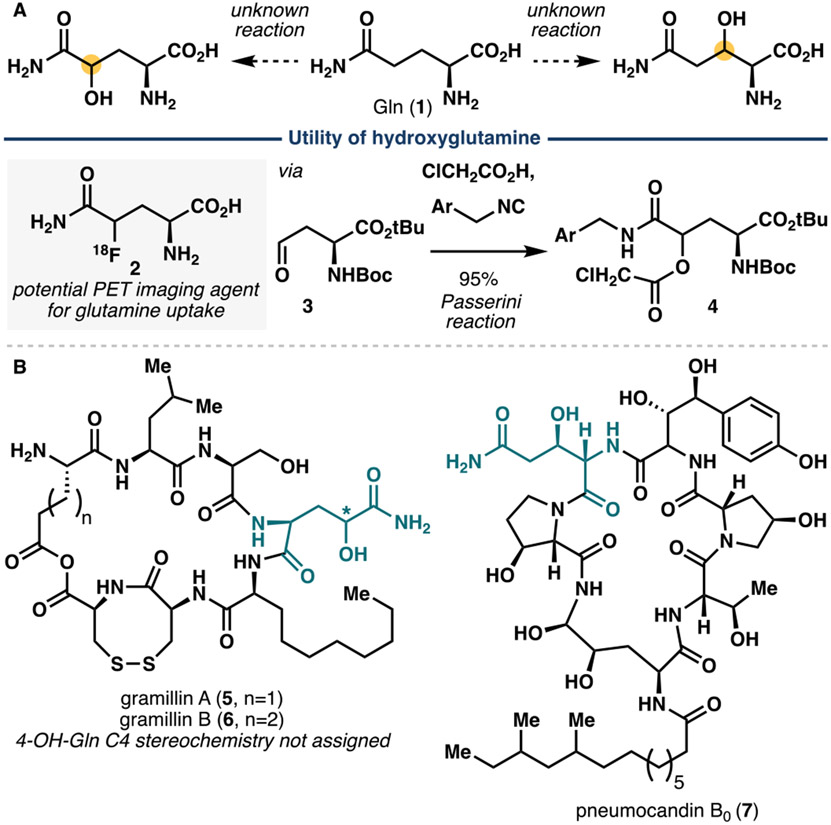
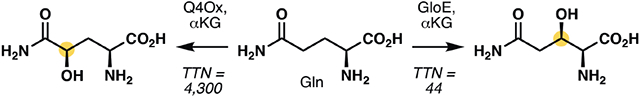
Given the importance of hydroxylated amino acids, our laboratory has developed a research program aimed at identifying useful oxygenases for regio- and stereoselective C─H hydroxylation of readily available amino acids. To date, our efforts have led to the discovery of a leucine 5-hydroxylase, GriE,10 capable of effecting selective C5 hydroxylation on a range of aliphatic amino acids and two additional hydroxylases, GlbB11 and GetI,12 that natively catalyze selective C4 hydroxylation on lysine and citrulline, respectively. Despite this success and efforts from other laboratories, no dedicated that catalyzes selective C─H hydroxylation on free-standing glutamine (Gln) has been identified to date (Figure 1A). The hydroxylated product from this transformation is a useful chemical, especially for the production of fluorine-18 labeled glutamine derivatives as positron emission tomography (PET) imaging agents. Seminal work from Qu and Kung in 201013 showed the viability of using derivative 2 to image glutamine uptake in SF188Bcl-xL tumor cells, which are glutamine addicted derivative of glioblastoma cells. Prior synthesis of 2 commenced from partially-reduced aspartate derivative 3, which was subjected to a three-component Passerini reaction to install the C5-amide of the target molecule (Figure 1A). An alternative approach to C4-hydroxylated Gln was reported by Brimble and co-workers in 2017, involving the use of C4-hydroxylated proline derivative, which was oxidized to the corresponding C5 amide and then opened to the linear form via ammonolysis.14 To identify a more direct access to hydroxylated glutamine derivatives, we sought to identify complementary biocatalytic methods for regioselective C─H oxidations of free-standing glutamine.
Figure 1.
A. Utility of glutamine derivatives (e.g., 2) and the current gap in knowledge for direct functionalization of glutamine (1). B. Representative examples of nonribosomal peptide natural products with hydroxylated glutamine motif.
Results and Discussion
To identify potential enzyme candidates, we noted the presence of hydroxylated glutamine motifs in several nonribosomal peptides, namely the gramillins (5, 6),15 pneumocandin B0 (7)16 and fellutamide A (Figure 1B).17 Among the biosynthetic gene clusters (BGCs) for these natural products, we observed that those corresponding to the gramillins and pneumocandin B0 contain genes encoding for iron- and α-ketoglutarate-dependent dioxygenases (Fe/αKGs). We first sought to characterize the gene product of FGSG_00048 (Uniprot ID: I1R9B5), the gene encoding for Fe/αKG from the gramillin BGC due to its potential implication in the production of 4-OH-Gln, which is more valuable synthetically than 3-OH-Gln, especially in light of recent discoveries of 2 as a viable PET imaging agent and IboH as an Fe/αKG glutamate C3 hydroxylase. At the outset, the role of the gene product of FGSG_00048 is unclear though Bahadoor and Harris had hypothesized that it might be involved in the biosynthesis of the 2-amino decanoic acid monomer and that a P450 might be responsible for the production of 4-OH-Gln. Furthermore, BLAST analysis of FGSG_00048 did not reveal any significant sequence similarity to any known amino acid hydroxylases. Finally, even if the gene product is indeed involved in C4 hydroxylation of the Gln monomer, there are still several biosynthetic scenarios to be considered, namely (a) hydroxylation might occur on the free-standing monomer prior to its loading to the NRPS module, (b) hydroxylation might occur on the peptidyl carrier protein-bound monomer after its loading and (c) hydroxylation might take place after the cyclic peptide is fully assembled by the NRPS module.
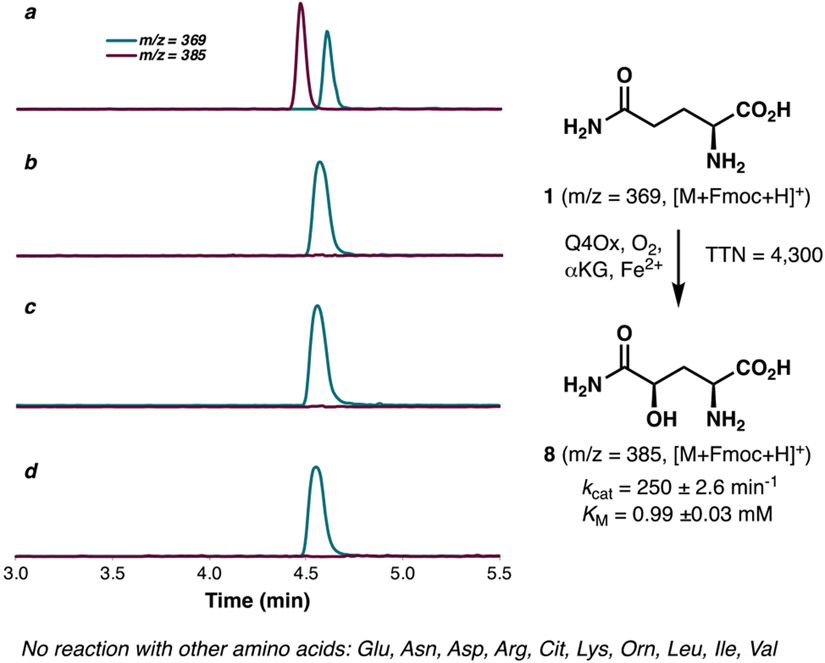
To facilitate functional characterization, FGSG_00048 was codon optimized and inserted into pET28 expression vector. Heterologous expression of its gene product, hereby named Q4Ox, produced soluble enzyme, which upon in vitro assay in the presence of Fe2+, αKG and O2, validated its function as glutamine C4 hydroxylase (Figure 2). No hydroxylated product could be observed when Fe2+ or αKG was omitted from the reaction, which confirmed that Q4Ox belongs to the iron- and α-ketoglutarate-dependent dioxygenase superfamily. Further analysis showed that the hydroxylation reaction proceeded in a highly efficient fashion (4,300 total turnover number) and with complete diastereoselectivity. This observation was validated with steady-state kinetic analysis, which afforded a kcat of 250 ± 2.6 min−1 and a KM of 0.99 ±0.03 mM. These parameters are within the range of those obtained from other amino acid hydroxylases characterized in our lab in recent years,10-12 suggesting that Q4Ox natively acts on free-standing L-glutamine. Testing of other pAAs in the reaction revealed a highly narrow substrate specificity as no other amino acids were accepted as substrate in the reaction. This discovery also suggests that hydroxylation of Gln in gramillin biosynthesis occurs early in the pathway, prior to its loading to the NRPS module and macrocyclization.
Figure 2.
LCMS profile of glutamine hydroxylation with Q4Ox following Fmoc derivatization; (a) in the presence of FeSO4, αKG, ascorbic acid and enzyme; (b) in the absence of enzyme; (c) in the absence of FeSO4; (d) in the absence of αKG.
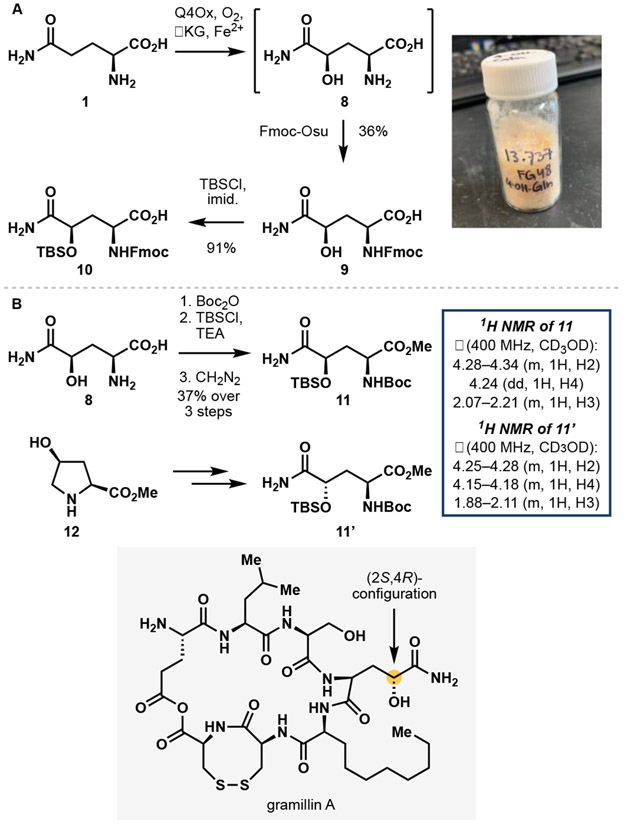
We next sought to demonstrate the practicality of this reaction in a preparative scale biotransformation (Figure 3A). On 100 mg scale, C─H hydroxylation of Gln in the presence of purified Q4Ox proceeded with complete conversion and the desired product could be isolated cleanly through ion-exchange purification. The same reaction could also be performed with lysates of Escherichia coli (E. coli) expressing Q4Ox with similar levels of efficiency, thereby bypassing the need for tedious protein purification. It is worth noting that reaction with lysates proved sensitive to reaction scale, lysis conditions and headspace volume and that minor amounts of transamination products between Gln and αKG, likely catalyzed by endogenous transaminases in E. coli lysates, could be observed when small deviations to these parameters were introduced. Initial efforts to derivatize 8 were hampered by solubility issues. Furthermore, the ion-exchange purification procedure consistently led to only 80–85% mass recovery. To circumvent these issues, we developed a telescoped protocol to generate Fmoc-protected 4-OH-Gln (9) which bypassed the isolation of the free amino acid product. Upon completion of the enzymatic hydroxylation step, a solution of 9-fluorenylmethyl-succinimidyl carbonate (Fmoc-OSu) in acetonitrile could simply be added to effect Fmoc protection under biphasic conditions, providing 9 in moderate yield on preparative scale (100 mg). In our previous work, we found that several C4- and C5-hydroxylated amino acids displayed high propensity towards self-lactonization10,11 during protection of their α-amino group. In contrast, 9 did not possess such tendencies and was observed to remain as the open-chain form in organic solvent or upon storage at ambient temperature. This unique feature of 9 was leveraged in the synthesis of a solid-phase peptide synthesis-compatible building block 10 via TBS protection of the secondary alcohol. To elucidate the stereochemical configuration at C4 of the hydroxylated Gln product, we converted 8 to protected derivative 11 and compared its 1H and 13C NMR spectra to a related compound 11’, which was used by Brimble and co-workers as a key intermediate towards hemerocallisamine I (Figure 3B).14 Several key differences in chemical shifts and 1H splitting patterns were noted between the two structures, suggesting that they exist in a diastereomeric relationship. Based on this observation, we propose that 11 exists in a (2S,4R)-configuration and as a corollary, the modified Gln motif in the gramillins likely exists in a (2S,4R)-configuration as well.
Figure 3.
A. Derivatization of 4-OH-Gln (8) to a solid-phase peptide synthesis-compatible building block 10. B. Determination of stereochemical configuration of 8 via derivatization and 1H NMR comparison with known compound 11’.
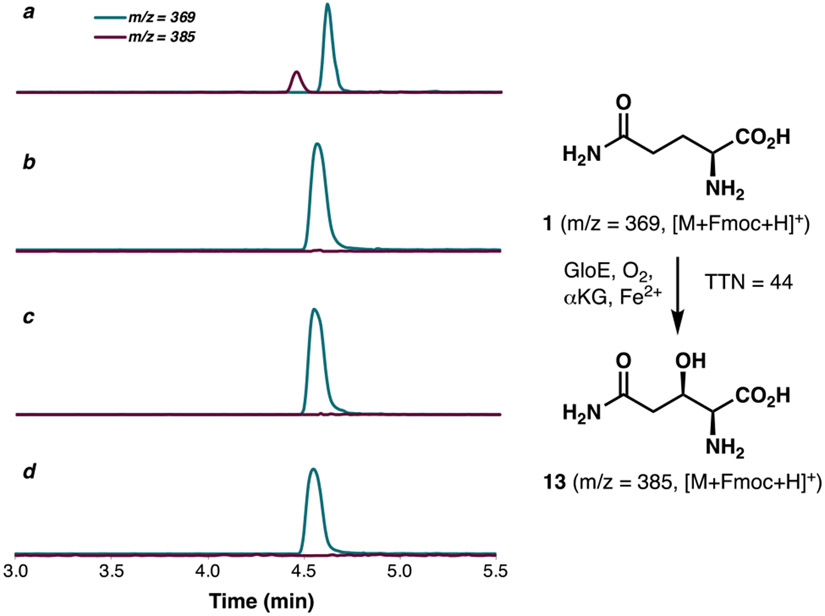
Following the above work, we turned our focus to the functional characterization of GloE (Uniprot ID: S3E7Q2). Our effort in this area was greatly enabled by pioneering report from Tang and Walsh, who had previously characterized several Fe/αKGs from echinocandin B biosynthesis.18 While the BGC of pneumocandin B0 also contains multiple Fe/αKG-encoding genes, no homologs of GloE could be found in the BGC of echinocandin B, which differs from the pneumocandins by the substitution of the 3-OH-Gln unit with Thr. Based on this observation, we hypothesized that GloE is responsible for the production of the 3-OH-Gln monomer in pneumocandin B0. At this stage, however, it was unclear whether GloE would act on a free-standing amino acid or one that is bound to a peptidyl carrier protein. GloE could be solubly expressed in E. coli as an N-His6-tagged protein. In vitro assay of GloE in the presence of Gln, αKG, Fe2+ and molecular oxygen, followed by Fmoc derivatization revealed the presence of a new species with an m/z corresponding to hydroxylated Gln (Figure 4). Though only modest total turnover number (TTN = 44) could be observed, the reaction could be scaled up sufficiently to support 1H NMR analysis, which provides conclusive evidence for hydroxylation of Gln at C3.
Figure 4.
LCMS profile of glutamine hydroxylation with GloE following Fmoc derivatization; (a) in the presence of FeSO4, αKG, ascorbic acid and enzyme; (b) in the absence of enzyme; (c) in the absence of FeSO4; (d) in the absence of αKG.
To probe the possibility that GloE might act on peptidyl-carrier-bound amino acid substrate, we also attempted in vitro assay with N-acetylcysteamine derivative of Gln (Gln-SNAc, 14) as a substrate mimic19 of carrier-protein-bound Gln (Figure 5). However, no reaction could be observed with this substrate, suggesting that free Gln is the likely native substrate of GloE. For benchmarking purposes, 14 was also tested for in vitro hydroxylation reaction with Q4Ox. No reaction could be observed in this assay, further validating our hypothesis that Q4Ox accepts only free-standing L-glutamine in its reaction. Unfortunately, due to the low activity of GloE, accurate determination of its steady-state kinetic parameters was not possible. Though the low activity of GloE might seem surprising for a secondary metabolite biosynthetic enzyme, it is worth noting that several Fe/αKGs from echinocandin B biosynthesis also exhibited low TTNs on their native substrates before undergoing self-inactivation.18 Finally, reaction with lysates of E. coli expressing GloE was attempted, but due to the modest activity of GloE, the competing transamination pathway, previously described in the reaction with Q4Ox, predominated.
Figure 5.
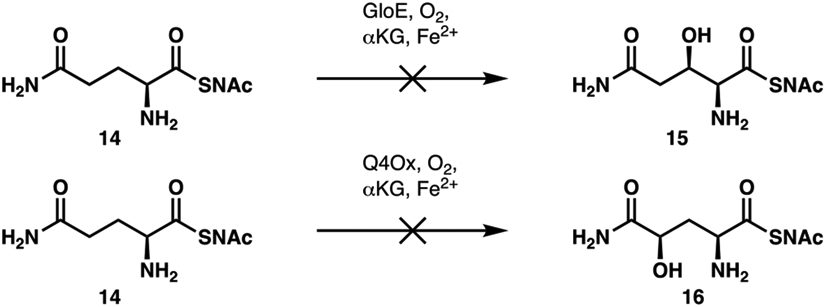
Attempted hydroxylations of N-acetylcysteamine derivative of Gln (Gln-SNAc, 14) with GloE and Q4Ox.
Conclusion
In this work, we described the functional characterization of two new glutamine hydroxylases, Q4Ox and GloE, belonging to the gramillins and pneumocandin B0 biosynthetic pathways, respectively. Both enzymes exhibited strict substrate specificity towards Gln and while the hydroxylation activity of GloE is only modest, Q4Ox is able to catalyze the conversion of Gln to 4-OH-Gln in a highly efficient fashion. Biocatalytic hydroxylation with Q4Ox could be performed on preparative scale, allowing sufficient material throughput for further product derivatization to afford a building block that is anticipated to be compatible with solid-phase peptide synthesis. This work further expands the biocatalytic toolbox for selective C─H functionalization of amino acids and paves the way for the development of a biocatalytic-centered synthetic strategy to access 18F derivative of Gln for PET tracer studies.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (Grant R35 GM128895). We thank Prof. Margaret Brimble for sharing the NMR spectra of compound 11’. We acknowledge the Kodadek and Shen labs for generous access to their instrumentations.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaskovich MAT Unusual Amino Acids in Medicinal Chemistry. J. Med. Chem 2016, 59, 10807–10836. [DOI] [PubMed] [Google Scholar]
- 2.Henninot A; Collins JC; Nuss JM The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem 2018, 61, 1382–1414. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins CL; Bretscher LE; Guzei IA; Raines RT Effect of 3-Hydroxyproline Residues on Collagen Stability. J. Am. Chem. Soc 2003, 125, 6422–6427. [DOI] [PubMed] [Google Scholar]
- 4.Sauvaire Y; Petit P; Broca C; Manteghetti M; Baissac Y; Fernandez-Alvarez J; Gross R; Roye M; Leconte A; Gomis R; Ribes G 4-Hydroxyisoleucine: A Novel Amino Acid Potentiator of Insulin Secretion. Diabetes 1998, 47, 206–210. [DOI] [PubMed] [Google Scholar]
- 5.Hedges JB; Ryan KS Biosynthetic Pathways to Nonproteinogenic α-Amino Acids. Chem. Rev 2020, 120, 3161–3209. [DOI] [PubMed] [Google Scholar]
- 6.Luesch H; Hoffmann D; Hevel JM; Becker JE; Golakoti T; Moore RE Biosynthesis of 4-Methylproline in Cyanobacteria: Cloning of nosE and nosF Genes and Biochemical Characterization of the Encoded Dehydrogenase and Reductase Activities. J. Org. Chem 2003, 68, 83–91. [DOI] [PubMed] [Google Scholar]
- 7.Obermaier S; Müller M Ibotenic Acid Biosynthesis in the Fly Agaric Is Initiated by Glutamate Hydroxylation. Angew. Chem. Int. Ed 2020, 59, 12432–12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rentmeister A; Arnold FH; Fasan R Chemo-Enzymatic Fluorination of Unactivated Organic Compounds. Nat. Chem. Biol 2009, 5, 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy AC; Mitova MI; Blunt JW; Munro MHG Concise, Stereoselective Route to the Four Diastereomers of 4-Methylproline. J. Nat. Prod 2008, 71, 806–809. [DOI] [PubMed] [Google Scholar]
- 10.Zwick CR III; Renata H Remote C─H Hydroxylation by an a-Ketoglutarate-Dependent Dioxygenase Enables Efficient Chemoenzymatic Synthesis of Manzacidin C and Proline Analogs. J. Am. Chem. Soc 2018, 140, 1165–1169. [DOI] [PubMed] [Google Scholar]
- 11.Amatuni A; Renata H Identification of a Lysine 4-Hydroxylase from the Glidobactin Biosynthesis and Evaluation of Its Biocatalytic Potential. Org. Biomol. Chem 2019, 17, 1736–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwick CR III; Sosa MB; Renata H Characterization of a Citrulline 4-Hydroxylase from GE81112 Biosynthesis and Engineering of Its Substrate Specificity for the Chemoenzymatic Synthesis of Enduracididine. Angew. Chem. Int. Ed 2019, 58, 18854–18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu W; Zha Z; Ploessl K; Lieberman BP; Zhu L; Wise DR; Thompson CB; Kung HF Synthesis of Optically Pure 4-Fluoro-Glutamines as Potential Metabolic Imaging Agents for Tumors. J. Am. Chem. Soc 2011, 133, 1122–1133. [DOI] [PubMed] [Google Scholar]
- 14.Wood JM; Furkert DP; Brimble MA Total Synthesis and Stereochemical Revision of the 2-Formylpyrrole Alkaloid Hemerocallisamine I. J. Nat. Prod 2017, 80, 1926–1929. [DOI] [PubMed] [Google Scholar]
- 15.Bahadoor A; Brauer EK; Bosnich W; Schneiderman D; Johnston A; Aubin Y; Blackwell B; Melanson JE; Harris LJ Gramillin A and B: Cyclic Lipopeptides Identified as the Nonribosomal Biosynthetic Products of Fusarium graminearum. J. Am. Chem. Soc 2019, 140, 16783–16791. [DOI] [PubMed] [Google Scholar]
- 16.Li Y; Lan N; Xu L; Yue Q Biosynthesis of Pneumocandin Lipopeptides and Perspectives for Its Production and Related Echinocandins. Appl. Microbiol. Biotechnol 2018, 102, 9881–9891. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K; Tsuji T; Wakuri S; Yazawa K; Kondo K; Shigemori H; Kobayashi J Stimulation of Nerve Growth Factor Synthesis and Secretion by Fellutamide A in Vitro. Biosci. Biotech. Biochem 1993, 57, 195–199. [DOI] [PubMed] [Google Scholar]
- 18.Cacho RA; Jiang W; Chooi Y-H; Walsh CT; Tang Y Identification and Characterization of the Echinocandin B Biosynthetic Gene Cluster from Emericella rugulosa NRRL 11440. J. Am. Chem. Soc 2012, 134, 16781–16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke J; Hertweck C Biomimetic Thioesters as Probes for Enzymatic Assembly Lines: Synthesis, Applications, and Challenges. Cell Chem. Biol 2016, 23, 1179–1192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.