Abstract
Copper-catalyzed decarboxylative coupling reactions of conjugated β,γ-unsaturated carboxylic acids have been achieved for allylic amination, alkylation, sulfonylation, and phosphinoylation. This approach was effective for a broad scope of amino, alkyl, sulfonyl, and phosphinoyl radical precursors as well as various conjugated β,γ-unsaturated carboxylic acids. These reactions also feature high regioselectivity, good functional group tolerance, and simple operation procedure. Mechanistic studies show that the reaction proceeds via copper-catalyzed electrophilic addition onto an olefin followed by decarboxylation, with radical intermediates involved. These insights present a modular and powerful strategy to access versatilely functionalized allyl-containing skeletons from readily available and stable carboxylic acids.
Keywords: decarboxylation, amination, alkylation, sulfonylation, radical reaction, copper catalysis
Graphical Abstract

Carboxylic acids represent an important class of building blocks in organic synthesis.1 Developing decarboxylative functionalization strategies that utilize carboxylic acids as stable and highly prevalent starting materials has received great attention, offering an attractive approach to introduce versatile functionalities and to assemble complex skeletons.2 In comparison to numerous examples in sp- and sp2-decarboxylative functionalization,3 fewer have been reported on sp3-decarboxylative functionalization and usually required the use of a stoichiometric strong oxidant (e.g., Hunsdiecker reaction).4 Not until recently, remarkable advances in sp3-decarboxylative functionalization have been achieved with different transition-metal catalysts such as silver, copper, nickel, palladium, and even visible-light catalysis systems under environmentally friendly conditions.5
Decarboxylative coupling reactions of β,γ-unsaturated carboxylic acids under mild conditions will offer an appealing approach to construct allylic functionalized skeletons that are of great value in organic synthesis. Among various decarboxylative functionalizations, the Hu group reported copper-catalyzed decarboxylative fluoroalkylations using Togni-type fluoroalkylating agents (Scheme 1a).6 The Minakata group reported decarboxylative amidation and oxygenation using hypervalent iodine reagents or N-iodo-N-chloroamides generated in situ from chloramine salts and I2 (Scheme 1b).7 Interestingly, the reactions reported by the Hu and Minakata groups were mechanistically distinct from classic decarboxylative ipso-functionalization as they were initiated by the addition to the olefin by an electrophile followed by decarboxylation. Such an unusual decarboxylation pathway presents an efficient strategy for regiospecific γ-functionalization (Scheme 1). So far, only hypervalent iodine(III) agents have been used as the precursors to introduce the functional groups, while greater potential remains to be explored to enable the installation of different functionalities. Herein, we report the development of copper-catalyzed decarboxylative coupling reactions of conjugated β,γ-unsaturated carboxylic acids with different precursors to achieve regioselective allylic amination, alkylation, sulfonylation, and phosphinoylation (Scheme 1c). These transformations offer a direct approach to access a diverse range of allyl-containing skeletons.
Scheme 1. Decarboxylation-Assisted γ-Functionalization of Conjugated β,γ-Unsaturated Carboxylic Acids.
(a) Hu's work: Cu-catalyzed decarboxylase fluoroalkylation
(b) Minakata's work: decarboxylative amidation and oxygenation
(c) This work: Cu-catalyzed decarboxylative functionalization including amination, alkylation, sulfonylation, and phosphinoylation
Our group has recently demonstrated that O-acyl-N-hydroxylamines are effective in engaging in the copper-catalyzed electrophilic amination onto an olefin.8 Thus, we envisioned that O-acylzoyl-N-hydroxylamines could be used as the amino precursors for developing a decarboxylative allylic amination reaction of conjugated β,γ-unsaturated carboxylic acids. Such an allylic amination transformation will greatly expand beyond the use of chloramine salts as the nitrogen precursors in previous work.7b With allylic amines as highly valuable building blocks in organic synthesis,9 the development of such a direct and regioselective synthesis of allylic amines is desired. Our studies began with the decarboxylative amination of 3-phenylbut-3-enoic acid 1a and O-benzoyl-N-hydroxylmorpholine 2a (Table 1). With Cu(OTf)2 as the catalyst and Na2CO3 as the base, the reaction in DCE afforded 4-(2-phenylallyl)morpholine 3a in 62% yield (Table 1, entry 1). In comparison, 3a was not formed without a copper catalyst (entry 2). The absence of Na2CO3 decreased the formation of 3a to 52% yield, suggesting that the carboxylate form may facilitate the decarboxylation (entry 3). Various solvents (entries 4–6) and ligands (entries 7–9) were examined, none of which gave further improvement. Among the copper catalysts tested, Cu(OTf)2 was the most effective one (entries 10–14). Finally, the yield of 3a was increased to 76% when the reaction was run with 2a as the limiting reagent and reversing the ratio of 1a:2a (entry 15), which was subsequently chosen as standard conditions to examine the generality of the decarboxylative allylic amination reaction.
Table 1.
Optimization for Decarboxylative Aminationa
 | ||||
|---|---|---|---|---|
| entry | catalyst | solvent | ligand | yield (%)b |
| 1 | Cu(OTf)2 | DCE | none | 60 (62)c |
| 2 | none | DCE | none | ND |
| 3d | Cu(OTf)2 | DCE | none | 52 |
| 4 | Cu(OTf)2 | dioxane | none | 53 |
| 5 | Cu(OTf)2 | toluene | none | 47 |
| 6 | Cu(OTf)2 | MeCN | none | 43 |
| 7 | Cu(OTf)2 | DCE | bpy | 43 |
| 8 | Cu(OTf)2 | DCE | BINAP | 55 |
| 9 | Cu(OTf)2 | DCE | diamine | 53 |
| 10 | Cu(OAc)2 | DCE | none | 56c |
| 11 | CuI | DCE | none | 47 |
| 12 | Cu(MeCN)4BF4 | DCE | none | 45 |
| 13 | CuTC | DCE | none | 52 |
| 14 | Cu(acac)2 | DCE | none | 55 |
| 15e | Cu(OTf)2 | DCE | none | 76c |
Conditions: 1a (0.2 mmol), 2a (0.4 mmol), catalyst (0.02 mmol), ligand (0.02 mmol), Na2CO3 (0.24 mmol), solvent (3.0 mL).
Yields determined by 1H-NMR with dibromomethane as an internal standard.
Isolation yield.
Without Na2CO3.
Reaction using 1a (0.4 mmol), 2a (0.2 mmol) instead of 1a (0.2 mmol), 2a (0.4 mmol). bpy = 1,1′-dipyridine. BINAP = (±)-2,2′-bis(diphenylphosphino)-1,1′-binaphthalene. Diamine = trans-N,N′-dimethylcyclohexane-1,2-diamine. ND = Not detected.
With established decarboxylative amination conditions, we studied the scope of both hydroxylamines and carboxylic acids in this reaction (Table 2). A diverse range of piperidine and piperazine-derived allylic amines were all formed in good yields (3b–3f). Besides six-membered cyclic amines, five-membered pyrrolidine 3g and seven-membered azepane derivatives 3h were also obtained, albeit in lower yields. Acyclic O-benzoyl-N-hydroxylamines were compatible in the formation of 3i and 3j. The scope of carboxylic acids was also examined. Substrates bearing a methyl group at the ortho-, meta-, and para-positions on the phenyl ring effectively produced allylic amines 3k–3m, respectively. Both electron-donating (3n, 3o) and electron-withdrawing (3p–3r) substitutions were well tolerated, with no significant impact on the outcomes. Even the free hydroxyl group was compatible (3s). The formation of thienyl-substituted product 3t showed the applicability of this reaction with heteroarenes. Yet, aliphatic-substituted substrates did not participate in the reaction, with 3u undetected. Encouragingly, γ-methyl- and γ,γ-dimethyl-substituted carboxylic acids afforded 3v and 3w, with lower yields resulting from the increased steric hindrance, respectively. The cyclic dialin-containing substrate also delivered 3x. Furthermore, α-methyl-, α,α-dimethyl- and even α,α,γ-trimethyl-substituted substrates afforded 3y, 3z, and 3aa successfully, respectively. Results from α- and γ-substituted carboxylic acids suggested that the formation of the allylic amine products was initiated by Cu-catalyzed amination of the olefin at the γ-position of carboxylic acid followed by the decarboxylation step to regenerate the double bond in the products. Correspondingly, the steric bulkiness at the γ-position hindered the efficiency of the reactions, as seen in the poor formation of 3w. Excitingly, when β-alkynyl- and β-vinyl-substituted carboxylic acids were tested for this reaction, all led to the selective formation of desired allylic amine products 3ab–3ad while other unsaturated carbon–carbon bonds remained intact.
Table 2.
Decarboxylative Amination of Conjugated β,γ-Unsaturated Carboxylic Acidsa
 |
Condition A: 1 (2.0 equiv.), 2 (0.2 mmol, 1.0 equiv.), Cu(OTf)2 (10 mol %), Na2CO3 (1.2 equiv.), DCE, 90 °C. Isolation yields.
Z/E ratio determined by 1H-NMR of the crude reaction.
We next explored the decarboxylation-assisted allylic functionalization by using other radical species besides O-acyl-N-hydroxylamines. Under modified copper-catalyzed conditions,10 we have established a decarboxylative alkylation by using alkyl radicals derived from α-bromocarbonyl derivatives or α-bromonitrile.11 We evaluated the scope in a diverse range of alkyl bromides and carboxylic acids (Table 3). When different alkyl bromides were examined in this reaction, all successfully formed allylic alkylation products, demonstrating the generality of this decarboxylative alkylation reaction from various α-carbonyl alkyl radical precursors, ranging from tertiary and secondary α-bromoacetates (5a–5g), primary α-bromonitrile (5h), α-bromoacetamide (5i), and even α-bromoketone (5j). A range of carboxylic acids bearing different substitutions on the phenyl ring were also investigated, including ortho-, meta-, and para-methyl groups (5k–5m), various electron-withdrawing groups (5n–5p), and electron-donating groups (5q–5s). Naphthyl-substituted carboxylic acid afforded product 5t in 52% yield, while thienyl-containing carboxylic acid formed 5u in only 17% yield. Substitution effects on the α- and γ-positions were also investigated in the reactions. Although α-monomethyl-substituted acid provided 5v in 76% yield, both α,α-dimethyl-substituted acid and γ-methyl-substituted substrates failed to deliver 5w and 5x effectively, respectively, suggesting that steric bulkiness significantly hampered the decarboxylative alkylation. Finally, the reactions of β-alkynyl and β-vinyl substitutions successfully afforded the alkylation products 5y–5aa.
Table 3.
Decarboxylative Alkylation of Conjugated β,γ-Unsaturated Carboxylic Acidsa
 |
Condition B: 1 (1.5 equiv.), 4 (0.3 mmol, 1.0 equiv.), Cu(OTf)2 (10 mol %), bpy (10 mol %), Na2CO3 (1.1 equiv.), dioxane, 90 °C. Isolation yields.
Z/E ratio determined by 1H-NMR of the crude reaction mixture.
We have also developed an analogous sulfur radical-initiated decarboxylative sulfonylation reaction of conjugated β,γ-unsaturated carboxylic acid (Table 4). The copper-catalyzed decarboxylative conditions were found to be effective,10 promoting the formation of aromatic and aliphatic allylic sulfone products 7a–7e using different sulfonyl chlorides.12 Carboxylic acids bearing various functional groups on the phenyl ring all gave the desired allylic sulfones (7f–7l). Thienyl-substituted acid gave sulfone 7m in only 29% yield. An α-monomethyl-substituted acid smoothly formed 7n, while a γ-monomethyl-substituted acid failed to deliver the desired product 7o. Interestingly, the dialin substrate formed 7p, with the opposite regioselectivity to the amination product 3x, possibly resulting from a direct decarboxylation pathway.13 Carboxylic acids bearing β-alkynyl and β-vinyl groups worked effectively, generating desired allylic sulfones 7q–7s.
Table 4.
Decarboxylative Sulfonylation of Conjugated β,γ-Unsaturated Carboxylic Acidsa
 |
Condition B: 1 (1.5 equiv.), 6 (0.3 mmol, 1.0 equiv.), Cu(OTf)2 (10 mol %), bpy (10 mol %), Na2CO3 (1.1 equiv.), dioxane, 90 °C. Isolation yields.
Z/E ratio determined by 1H-NMR of the crude reaction mixture.
Our development of the copper-catalyzed decarboxylative functionalization began with the hypothesis that the reactions would be initiated by electrophile addition to the olefin followed by subsequent decarboxylation. To examine this hypothesis, we ran a series of control experiments (Scheme 2). First, comparative experiments were performed using β,γ-unsaturated carboxylic acid 1a, ester 8, and simple olefin 9 under the standard conditions for decarboxylative amination, alkylation, and sulfonylation (Scheme 2a). The results from these experiments demonstrated that the free carboxyl group is critical in the decarboxylation step. To probe the presence of radical intermediates, control experiments were run with the addition of radical inhibitors, such as TEMPO (Scheme 2b) and butylated hydroxytoluene (BHT) (Scheme 2c). In all cases, the presence of a radical scavenger suppressed the formation of desired products. The observation of BHT-trapped products 10–12 further supports the formation of radical intermediates, which initiate the reaction by adding onto the double bond in these copper-catalyzed reactions. Note that the stereochemical outcomes observed in these decarboxylative transformations also indicated the involvement of radical intermediates. For example, when α-methyl-substituted β,γ-unsaturated carboxylic acid was used, allylic amine 3y was obtained as a mixture of E/Z isomers. Similarly, allylic alkylation product 5v and allylic sulfone 7n were also formed as a mixture of E/Z isomers.
Scheme 2. Control Experiments and Radical Capture Experimentsa.
a Isolation yields. Condition A shown in Table 2. Condition B shown in Tables 3 and 4. ND = Not detected. bYields determined by 1H-NMR with dibromomethane as an internal standard.
Based on these experimental observations and related studies,8,11,12 we propose that the decarboxylation-assisted amination, sulfonylation, and alkylation of conjugated β,γ-unsaturated carboxylic acids may involve a similar pathway as shown in Scheme 3. First, the Cu(II) precatalyst would generate the active Cu(I) catalyst via disproportionation. Then the oxidative addition of radical precursors X–Z (i.e., 2, 4, or 6) to Cu(I) would generate a copper complex II, which possibly exists in an equilibrium with a Cu(II) species III. The resultant radical intermediates can be trapped by BHT, as shown in Scheme 2c. Subsequent addition to olefin would occur at the γ-position of β,γ-unsaturated carboxylic acid 1a. The resulting intermediates IV–VI would undergo decarboxylation by either a one-electron or two-electron pathway to regenerate the Cu(I) catalyst and afford the allylic-functionalized product (i.e., 3, 5, or 7). The control experiments with compound 9 in Scheme 2a suggest that C─H allylic functionalization pathways unlikely contribute to the formation of products (i.e., 3, 5, or 7) under our reaction conditions, although allylic functionalization of α-methyl styrene has been reported for allylic alkylation using α-bromoesters11f–g or allylic sulfonylation using sulfonyl chlorides.12
Scheme 3.
Proposed Reaction Pathways for Decarboxylative Amination, Alkylation, and Sulfonylation
With these mechanistic insights, we examined the potential of this decarboxylative functionalization pathway further on the diene system (Scheme 4). Encouragingly, the reactions of 13 successfully delivered products 15, under standard conditions for the decarboxylative amination, alkylation, and sulfonylation, presumably through the intermediate 14 via selective addition onto the terminal double bond. The poor yield of 15a resulted in part from the competing amino lactonization pathway (see the Supporting Information).
Scheme 4. Decarboxylative Functionalization of (E)-Hexa-3,5-dienoic Acida.
aIsolation yields. bRun under condition A shown in Table 2, see the Supporting Information for by-products. cRun under condition B shown in Tables 3 and 4. dE/Z ratio determined by 1H-NMR of the crude reaction mixture.
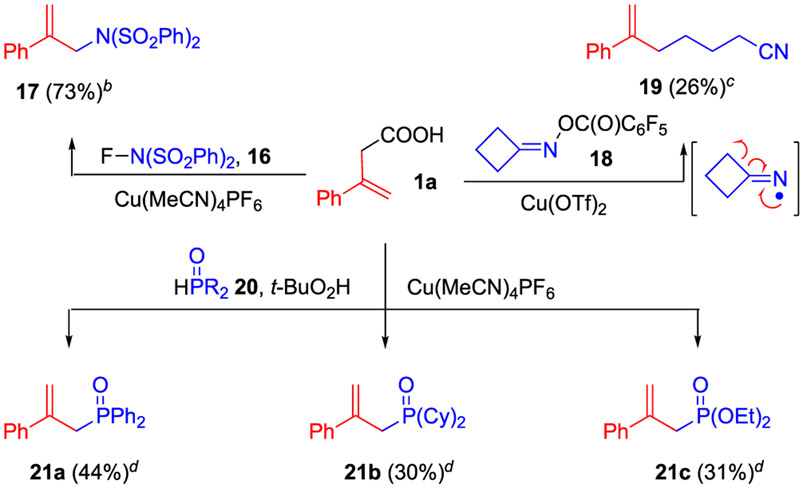
Finally, we examined the applicability of this decarboxylation-assisted functionalization strategy on other radical precursors (Scheme 5). Using N-fluorobenzenesulfonimide (NFSI) 16 as a different type of nitrogen precursor, the reaction of carboxylic acid 1a readily formed the allylic sulfonimide product 17 in 73% yield under modified conditions. The reaction of cyclobutanone O-acyloxime 18 led to the decarboxylative alkylation product 19 via a ring-opening generation of a primary alkyl radical. We also investigated a phosphine-centered radical generated under copper-catalyzed oxidative conditions in this decarboxylative approach.14 Excitingly, the decarboxylative allylic phosphinoylation and phosphonylation of 1a were viable, affording allylphosphine oxides 21a and 21b and allylphosphonate product 21c.
Scheme 5. Decarboxylative Sulfonimidation, Alkylation, Phosphinoylation, and Phosphonylationa.
aIsolation yields. bConditions: 1a (2.0 equiv.), 16 (0.1 mmol, 1.0 equiv.), Cu(MeCN)4PF6 (10 mol %), DCE, 60 °C, 3 h. cConditions: 1a (2.0 equiv.), 2 (0.1 mmol, 1.0 equiv.), Cu(OTf)2 (10 mol%), Na2CO3 (1.2 equiv.), DCE, 90 °C. dConditions: 1a (2.0 equiv.), 20 (0.1 mmol, 1.0 equiv.), Cu(MeCN)4PF6 (10 mol %), t-BuO2H (2.0 equiv.), MeCN, 90 °C, 1 h.
In conclusion, we have developed a copper-catalyzed decarboxylative redox-neutral and traceless approach for the preparation of allylic amines, homoallylic carbonyl derivatives, and allylic sulfones from conjugated β,γ-unsaturated carboxylic acids. Decarboxylative allylic phosphinoylation and phosphonylation have also been achieved under oxidative conditions. These reactions feature high regioselectivity, remarkable functional group tolerance, and simple operation procedure. Mechanistic studies suggest that the reaction is initiated by copper-catalyzed addition of radicals onto the olefin followed by decarboxylation. These insights support a general allylic functionalization strategy to rapidly construct diverse allyl-containing skeletons.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support by Duke University and the National Institutes of Health (GM118786). We thank Dr. Peter Silinski for the assistance with high-resolution mass spectrometry data.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.0c03621.
Condition optimizations, experimental procedures, compound characterization, and NMR spectra (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).((a)) Hudlick M Oxidation in Organic Chemistry; American Chemical Society: Washington, 1990, p. 105–109. [Google Scholar]; ((b)) March J Advanced Organic Chemistry; 6th edn, Wiley: New York, 1992, p. 1768–1773. [Google Scholar]; ((c)) Vollhardt KPC; Schore NE Organische Chemie; 3. Aufl., Wiley-VCH: Weinheim, 2000, p. 893. [Google Scholar]; (d) Gooßen LJ; Rodríguez N; Gooßen K Carboxylic Acids as Substrates in Homogeneous Catalysis. Angew. Chem., Int. Ed 2008, 47, 3100–3120. [DOI] [PubMed] [Google Scholar]; (e) Gooßen LJ; Gooßen K; Rodríguez N; Blanchot M; Linder C; Zimmermann B New catalytic transformations of carboxylic acids. Pure Appl. Chem 2008, 80, 1725–1733. [Google Scholar]
- (2).(a) For selected reviews, see: (Goossen LJ; Collet F; Goossen K Decarboxylative Coupling Reactions. Isr. J. Chem 2010, 50, 617–629. [Google Scholar]; (b) Rodríguez N; Goossen LJ Decarboxylative coupling reactions: a modern strategy for C─C-bond formation. Chem. Soc. Rev 2011, 40, 5030–5048. [DOI] [PubMed] [Google Scholar]; (c) Weaver JD; Recio A III; Grenning AJ; Tunge JA Transition Metal-Catalyzed Decarboxylative Allylation and Benzylation Reactions. Chem. Rev 2011, 111, 1846–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xuan J; Zhang Z-G; Xiao W-J Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem., Int. Ed 2015, 54, 15632–15641. [DOI] [PubMed] [Google Scholar]; (e) Patra T; Maiti D Decarboxylation as the Key Step in C─C Bond-Forming Reactions. Chem. – Eur. J 2017, 23, 7382–7401. [DOI] [PubMed] [Google Scholar]; (f) Wei Y; Hu P; Zhang M; Su W Metal-Catalyzed Decarboxylative C─H Functionalization. Chem. Rev 2017, 117, 886–8907. [DOI] [PubMed] [Google Scholar]; (g) Arshadi S; Ebrahimiasl S; Hosseinian A; Monfared A; Vessally E Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N─H compounds. RSC Adv. 2019, 9, 8964–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) For selected reviews and examples, see: Baudoin O New Approaches for Decarboxylative Biaryl Coupling. Angew. Chem., Int. Ed 2007, 46, 1373–1375. [DOI] [PubMed] [Google Scholar]; (b) Zhao D; Gao C; Su X; He Y; You J; Xue Y Copper-catalyzed decarboxylative cross-coupling of alkynyl carboxylic acids with aryl halides. Chem. Commun 2010, 46, 9049–9051. [DOI] [PubMed] [Google Scholar]; (c) Borah AJ; Yan G Decarboxylative functionalization of cinnamic acids. Org. Biomol. Chem 2015, 13, 8094–8115. [DOI] [PubMed] [Google Scholar]
- (4).(a) Hunsdiecker H; Hunsdiecker C Über den Abbau der Salze aliphatischer Säuren durch Brom. Ber. Dtsch. Chem. Ges. B 1942, 75, 291–297. [Google Scholar]; (b) Johnson RG; Ingham RK The Degradation Of Carboxylic Acid Salts By Means Of Halogen - The Hunsdiecker Reaction. Chem. Rev 1956, 56, 219–269. [Google Scholar]; (c) Cristol S; Firth W Jr. Communications. A Convenient Synthesis of Alkyl Halides from Carboxylic Acids. J. Org. Chem 1961, 26, 280–280. [Google Scholar]
- (5).(a) For selected examples of silver-catalyzed decarboxylative functionalization of aliphatic carboxylic acids: Yin F; Wang Z; Li Z; Li C Silver-Catalyzed Decarboxylative Fluorination of Aliphatic Carboxylic Acids in Aqueous Solution. J. Am. Chem. Soc 2012, 134, 10401–10404. [DOI] [PubMed] [Google Scholar]; (b) Hu F; Shao X; Zhu D; Lu L; Shen Q Silver-Catalyzed Decarboxylative Trifluoromethylthiolation of Aliphatic Carboxylic Acids in Aqueous Emulsion. Angew. Chem., Int. Ed 2014, 53, 6105–6109. [DOI] [PubMed] [Google Scholar]; (c) Liu C; Wang X; Li Z; Cui L; Li C Silver-Catalyzed Decarboxylative Radical Azidation of Aliphatic Carboxylic Acids in Aqueous Solution. J. Am. Chem. Soc 2015, 137, 9820–9823. [DOI] [PubMed] [Google Scholar]; (d) For selected examples of copper-catalyzed decarboxylative functionalization of aliphatic carboxylic acids: Zhang Y; Patel S; Mainolfi N Copper-catalyzed decarboxylative C─N coupling for N-arylation. Chem. Sci 2012, 3, 3196–3199. [Google Scholar]; (e) Liu Z-J; Lu X; Wang G; Li L; Jiang W-T; Wang Y-D; Xiao B; Fu Y Directing Group in Decarboxylative Cross-Coupling: Copper-Catalyzed Site-Selective C─N Bond Formation from Nonactivated Aliphatic Carboxylic Acids. J. Am. Chem. Soc 2016, 138, 9714–9719. [DOI] [PubMed] [Google Scholar]; (f) Xue W; Oestreich M Copper-Catalyzed Decarboxylative Radical Silylation of Redox-Active Aliphatic Carboxylic Acid Derivatives. Angew. Chem., Int. Ed 2017, 56, 11649–11652. [DOI] [PubMed] [Google Scholar]; (g) Kong D; Moon PJ; Bsharat O; Lundgren RJ Direct Catalytic Decarboxylative Amination of Aryl Acetic Acids. Angew. Chem., Int. Ed 2020, 59, 1313–1319. [DOI] [PubMed] [Google Scholar]; (h) For selected examples of nickel-catalyzed decarboxylative functionalization of aliphatic carboxylic acids: Weaver JD; Ka BJ; Morris DK; Thompson W; Tunge JA Stereospecific Decarboxylative Allylation of Sulfones. J. Am. Chem. Soc 2010, 132, 12179–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Shang R; Yang Z-W; Wang Y; Zhang S-L; Liu L Palladium-Catalyzed Decarboxylative Couplings of 2-(2-Azaaryl)acetates with Aryl Halides and Triflates. J. Am. Chem. Soc 2010, 132, 14391–14393. [DOI] [PubMed] [Google Scholar]; (j) Edwards JT; Merchant RR; McClymont KS; Knouse KW; Qin T; Malins LR; Vokits B; Shaw SA; Bao D-H; Wei F-L; Zhou T; Eastgate MD; Baran PS Decarboxylative alkenylation. Nature 2017, 545, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Li C; Wang J; Barton LM; Yu S; Tian M; Peters DS; Kumar M; Yu AW; Johnson KA; Chatterjee AK; Yan M; Baran PS Decarboxylative borylation. Science 2017, 356, eaam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) For selected examples of visible-light-catalyzed decarboxylative functionalization of aliphatic carboxylic acids: Ventre S; Petronijevic FR; MacMillan DWC Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis. J. Am. Chem. Soc 2015, 137, 5654–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhou Q-Q; Guo W; Ding W; Wu X; Chen X; Lu L-Q; Xiao W-J Decarboxylative Alkynylation and Carbonylative Alkynylation of Carboxylic Acids Enabled by Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed 2015, 54, 11196–11199. [DOI] [PubMed] [Google Scholar]; (n) Zhao W; Wurz RP; Peters JC; Fu GC Photoinduced, Copper-Catalyzed Decarboxylative C─N Coupling to Generate Protected Amines: An Alternative to the Curtius Rearrangement. J. Am. Chem. Soc 2017, 139, 12153–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Mao R; Frey A; Balon J; Hu X Decarboxylative C(sp3)–N Cross-Coupling via Synergetic Photoredox and Copper Catalysis. Nat. Catal 2018, 1, 120–126. [Google Scholar]; (p) Liang Y; Zhang X; MacMillan DWC Decarboxylative sp3 C─N Coupling via Dual Copper and Photoredox Catalysis. Nature 2018, 559, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Sakakibara Y; Ito E; Fukushima T; Murakami K; Itami K Late-Stage Functionalization of Arylacetic Acids by Photoredox-Catalyzed Decarboxylative Carbon–Heteroatom Bond Formation. Chem. – Eur. J 2018, 24, 9254–9258. [DOI] [PubMed] [Google Scholar]; (r) Mao R; Balon J; Hu X Decarboxylative C(sp3)-O Cross-Coupling. Angew. Chem., Int. Ed 2018, 57, 13624–13628. [DOI] [PubMed] [Google Scholar]; (s) Fu M-C; Shang R; Zhao B; Wang B; Fu Y Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 2019, 363, 1429–1434. [DOI] [PubMed] [Google Scholar]; (t) Li Z; Wang X; Xia S; Jin J Ligand-Accelerated Iron Photocatalysis Enabling Decarboxylative Alkylation of Heteroarenes. Org. Lett 2019, 21, 4259–4265. [DOI] [PubMed] [Google Scholar]; (u) Na CG; Ravelli D; Alexanian EJ Direct Decarboxylative Functionalization of Carboxylic Acids via O─H Hydrogen Atom Transfer. J. Am. Chem. Soc 2020, 142, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) He Z; Hu M; Luo T; Li L; Hu J Copper-Catalyzed Difluoromethylation of β,γ-Unsaturated Carboxylic Acids: An Efficient Allylic Difluoromethylation. Angew. Chem., Int. Ed 2012, 51, 11545–11547. [DOI] [PubMed] [Google Scholar]; (b) He Z; Tan P; Hu J Copper-Catalyzed Trifluoromethylation of Polysubstituted Alkenes Assisted by Decarboxylation. Org. Lett 2016, 18, 72–75. [DOI] [PubMed] [Google Scholar]
- (7).(a) Kiyokawa K; Yahata S; Kojima T; Minakata S Hypervalent Iodine(III)-Mediated Oxidative Decarboxylation of β,γ-Unsaturated Carboxylic Acids. Org. Lett 2014, 16, 4646–4649. [DOI] [PubMed] [Google Scholar]; (b) Kiyokawa K; Kojima T; Hishikawa Y; Minakata S Iodine-Catalyzed Decarboxylative Amidation of β,γ-Unsaturated Carboxylic Acids with Chloramine Salts Leading to Allylic Amides. Chem. – Eur. J 2015, 21, 15548–15552. [DOI] [PubMed] [Google Scholar]
- (8).(a) Shen K; Wang Q Copper-catalyzed diamination of unactivated alkenes with hydroxylamines. Chem. Sci 2015, 6, 4279–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hemric BN; Wang Q Copper-catalyzed intermolecular oxyamination of olefins using carboxylic acids and O-benzoylhydroxylamines. Beilstein J. Org. Chem 2016, 12, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hemric BN; Shen K; Wang Q Copper-Catalyzed Amino Lactonization and Amino Oxygenation of Alkenes Using O-Benzoylhydroxylamines. J. Am. Chem. Soc 2016, 138, 5813–5816. [DOI] [PubMed] [Google Scholar]; (d) Hemric BN; Chen AW; Wang Q Copper-Catalyzed Modular Amino Oxygenation of Alkenes: Access to Diverse 1,2-Amino Oxygen-Containing Skeletons. J. Org. Chem 2019, 84, 1468–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hemric BN; Chen AW; Wang Q Copper-Catalyzed 1,2-Amino Oxygenation of 1,3-Dienes: A Chemo-, Regio-, and Site-Selective Three-Component Reaction with O-Acylhydroxylamines and Carboxylic Acids. ACS Catal. 2019, 9, 10070–10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Cheikh RB; Chaabouni R; Laurent A; Mison P; Nafti A Synthesis of Primary Allylic Amines. Synthesis 1983, 685–700. [Google Scholar]; (b) Stütz A Allylamine Derivatives–a New Class of Active Substances in Antifungal Chemotherapy. Angew. Chem., Int. Ed 1987, 26, 320–328. [Google Scholar]; (c) Birnbaum JE Pharmacology of the allylamines. J. Am. Acad. Dermatol 1990, 23, 782–785. [DOI] [PubMed] [Google Scholar]; (d) Nussbaumer P; Ryder NS; Stütz A Allylamine Antimycotics: Recent Trends in Structure-Activity Relationships and Syntheses. Pestic. Sci 1991, 31, 437–455. [Google Scholar]; (e) Johannsen M; Jørgensen KA Benzotriazole-Mediated Stereoselective Olefination of Carboxylic Esters: Transformation of α-Amino Acid Esters into Chiral Allylamines. Allylic Amination. Chem. Rev 1998, 98, 1689–1708. [DOI] [PubMed] [Google Scholar]; (f) Skoda EM; Davis GC; Wipf P Allylic Amines as Key Building Blocks in the Synthesis of (E)-Alkene Peptide Isosteres. Org. Process Res. Dev 2012, 16, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).See the Supporting Information for more details about condition optimizations.
- (11).(a) Eckenhoff WT; Pintauer T Copper Catalyzed Atom Transfer Radical Addition (ATRA) and Cyclization (ATRC) Reactions in the Presence of Reducing Agents. Catal. Rev.: Sci. Eng 2010, 52, 1–59. [DOI] [PubMed] [Google Scholar]; (b) Liu C; Tang S; Liu D; Yuan J; Zheng L; Meng L; Lei A Nickel-Catalyzed Heck-Type Alkenylation of Secondary and Tertiary α-Carbonyl Alkyl Bromides. Angew. Chem., Int. Ed 2012, 51, 3638–3641. [DOI] [PubMed] [Google Scholar]; (c) Nishikata T; Noda Y; Fujimoto R; Sakashita T An Efficient Generation of a Functionalized Tertiary-Alkyl Radical for Copper-catalyzed Tertiary-Alkylative Mizoroki-Heck type Reaction. J. Am. Chem. Soc 2013, 135, 16372–16375. [DOI] [PubMed] [Google Scholar]; (d) Zhu K; Dunne J; Shaver MP; Thomas SP Iron-Catalyzed Heck-Type Alkenylation of Functionalized Alkyl Bromides. ACS Catal. 2017, 7, 2353–2356. [Google Scholar]; (e) Tang C; Zhang R; Zhu B; Fu J; Deng Y; Tian L; Guan W; Bi X Directed Copper-Catalyzed Intermolecular Heck-Type Reaction of Unactivated Olefins and Alkyl Halides. J. Am. Chem. Soc 2018, 140, 16929–16935. [DOI] [PubMed] [Google Scholar]; (f) Nishikata T; Nakamura K; Itonaga K; Ishikawa S General and Facile Method for exo-Methlyene Synthesis via Regioselective C─C Double-Bond Formation Using a Copper-Amine Catalyst System. Org. Lett 2014, 16, 5816–5819. [DOI] [PubMed] [Google Scholar]; (g) Noda Y; Nishikata T A highly efficient Cu catalyst system for the radical reactions of α-bromocarbonyls. Chem. Commun 2017, 53, 5017–5019. [DOI] [PubMed] [Google Scholar]; (h) Gockel SN; Buchanan TL; Hull KL Cu-Catalyzed Three-Component Carboamination of Alkenes. J. Am. Chem. Soc 2018, 140, 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ye Z; Cai X; Li J; Dai M Catalytic Cyclopropanol Ring Opening for Divergent Syntheses of γ-Butyrolactones and δ-Ketoesters Containing All-Carbon Quaternary. ACS Catal. 2018, 8, 5907–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Pudikova AA; Gerasimova NP; Moskvichev YA; Alov EM; Danilova AS; Kozlova OS Cascade synthesis of new aryl 2-phenylallyl sulfones from α-methylstyrene and aromatic mono- and bis-sulfonyl chlorides. Russ. J. Org. Chem 2010, 46, 352–354. [Google Scholar]; (b) Hossain A; Engl S; Lutsker E; Reiser O Visible-Light-Mediated Regioselective Chlorosulfonylation of Alkenes and Alkynes: Introducing the Cu(II) Complex [Cu(dap)Cl2] to Photochemical ATRA Reactions. ACS Catal. 2019, 9, 1103–1109. [Google Scholar]
- (13).Similar results were reported in ref 7b where the α-amidation product was observed on the same carboxylic acid.
- (14).Zhang G; Fu L; Chen P; Zou J; Liu G Proton-Coupled Electron Transfer Enables Tandem Radical Relay for Asymmetric Copper-Catalyzed Phosphinoylcyanation of Styrenes. Org. Lett 2019, 21, 5015–5020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.