Abstract
Background
A new syndrome of vaccine-induced immune thrombotic thrombocytopenia (VITT) has emerged as a rare side-effect of vaccination against COVID-19. Cerebral venous thrombosis is the most common manifestation of this syndrome but, to our knowledge, has not previously been described in detail. We aimed to document the features of post-vaccination cerebral venous thrombosis with and without VITT and to assess whether VITT is associated with poorer outcomes.
Methods
For this multicentre cohort study, clinicians were asked to submit all cases in which COVID-19 vaccination preceded the onset of cerebral venous thrombosis, regardless of the type of vaccine, interval between vaccine and onset of cerebral venous thrombosis symptoms, or blood test results. We collected clinical characteristics, laboratory results (including the results of tests for anti-platelet factor 4 antibodies where available), and radiological features at hospital admission of patients with cerebral venous thrombosis after vaccination against COVID-19, with no exclusion criteria. We defined cerebral venous thrombosis cases as VITT-associated if the lowest platelet count recorded during admission was below 150 × 109 per L and, if the D-dimer was measured, the highest value recorded was greater than 2000 μg/L. We compared the VITT and non-VITT groups for the proportion of patients who had died or were dependent on others to help them with their activities of daily living (modified Rankin score 3–6) at the end of hospital admission (the primary outcome of the study). The VITT group were also compared with a large cohort of patients with cerebral venous thrombosis described in the International Study on Cerebral Vein and Dural Sinus Thrombosis.
Findings
Between April 1 and May 20, 2021, we received data on 99 patients from collaborators in 43 hospitals across the UK. Four patients were excluded because they did not have definitive evidence of cerebral venous thrombosis on imaging. Of the remaining 95 patients, 70 had VITT and 25 did not. The median age of the VITT group (47 years, IQR 32–55) was lower than in the non-VITT group (57 years; 41–62; p=0·0045). Patients with VITT-associated cerebral venous thrombosis had more intracranial veins thrombosed (median three, IQR 2–4) than non-VITT patients (two, 2–3; p=0·041) and more frequently had extracranial thrombosis (31 [44%] of 70 patients) compared with non-VITT patients (one [4%] of 25 patients; p=0·0003). The primary outcome of death or dependency occurred more frequently in patients with VITT-associated cerebral venous thrombosis (33 [47%] of 70 patients) compared with the non-VITT control group (four [16%] of 25 patients; p=0·0061). This adverse outcome was less frequent in patients with VITT who received non-heparin anticoagulants (18 [36%] of 50 patients) compared with those who did not (15 [75%] of 20 patients; p=0·0031), and in those who received intravenous immunoglobulin (22 [40%] of 55 patients) compared with those who did not (11 [73%] of 15 patients; p=0·022).
Interpretation
Cerebral venous thrombosis is more severe in the context of VITT. Non-heparin anticoagulants and immunoglobulin treatment might improve outcomes of VITT-associated cerebral venous thrombosis. Since existing criteria excluded some patients with otherwise typical VITT-associated cerebral venous thrombosis, we propose new diagnostic criteria that are more appropriate.
Funding
None.
Introduction
Globally, more than 4·1 million people have died from COVID-19.1 In response to this public health emergency, several vaccines against COVID-19 have been developed, with more than 3·7 billion doses administered worldwide.2 After the introduction of the adenovirus vector vaccine ChAdOx1 (Oxford–AstraZeneca), five cases of severe venous thrombosis with thrombocytopenia were reported in Norway, each starting 7–10 days after administration of the first vaccine dose. Four of these cases had cerebral venous sinus thrombosis.3 This syndrome has since been termed vaccine-induced immune thrombotic thrombocytopenia (VITT).3, 4, 5 A similar condition has been described with another adenovirus vector vaccine, Ad26.COV2.S (Johnson & Johnson).6, 7 There are also case reports in which two mRNA vaccines, mRNA-1273 (Moderna)8, 9 and BNT162b2 (Pfizer–BioNTech),10 are associated with thrombocytopenia, although typically with purpura and mucosal bleeding8, 9, 10, 11 rather than thrombosis.11
Research in context.
Evidence before this study
We searched PubMed on May 26, 2021, for articles published in any language in 2021, with titles containing any of the following three search terms or their synonyms: “thrombosis”, “platelet”, or “PF4”, together with any of the following: “ChAdOx”, “AstraZeneca”, “Vaxzevria”, “Ad26.COV2.S”, “Janssen”, “Johnson”, “mRNA-1273”, “Moderna”, “BNT162b2”, “Pfizer”, “Comirnaty”, “COVID” and “vaccine”, or “SARS” and “vaccine”. 63 articles were identified, of which 29 were case reports or small case series (nine focused specifically on cerebral venous sinus thrombosis), six were summaries of drug side-effect reports submitted to surveillance agencies, six were consensus statements regarding guidelines for diagnosis or management, 19 were reviews, commentaries, or editorials, and three were relevant immunological studies in individuals who were vaccinated and remained healthy. Most case reports and small series were of vaccine-induced immune thrombotic thrombocytopenia (VITT) after vaccination with the adenovirus vector vaccine ChAdOx1 (Oxford–AstraZeneca), with the typical features of very low platelets, very high D-dimers, and, most commonly, cerebral venous sinus thrombosis or hepatic portal vein thrombosis. A similar syndrome has been reported following another adenovirus vector vaccine Ad26.COV2.S (Janssen/Johnson & Johnson). In both cases, anti-platelet factor 4 antibodies were found in most patients. The mRNA-based vaccines produced by Moderna (mRNA-1273) and Pfizer–BioNTech (BNT162b2) have also been associated with a syndrome of profound thrombocytopenia, but in this case the phenotype is typically idiopathic thrombocytopenic purpura, with a purpuric rash and mucosal bleeding as the most typical features. Although there have been occasional reports of thrombosis after mRNA vaccines, these did not have the characteristics of VITT and could have been incidental. Although cerebral venous thrombosis is the most severe manifestation of VITT, to date, to our knowledge, there have been no large studies focusing on this condition, and none of the reports so far have included a control group, making it difficult to draw inferences about how this condition differs from cerebral venous thrombosis without VITT.
Added value of this study
To our knowledge, our report describes the largest study of cerebral venous thrombosis after vaccination against COVID-19. We can make the first direct comparison between 70 patients with VITT-associated cerebral venous thrombosis and 25 patients who developed cerebral venous thrombosis after vaccination but did not have VITT, in addition to secondary comparisons with a large historical cohort with cerebral venous thrombosis. Our results show, for the first time to our knowledge, that when they are compared with those without VITT, patients with VITT-associated cerebral venous thrombosis were younger, had fewer venous thrombosis risk factors, and were more likely to have been given the ChAdOx1 vaccine. They developed more extensive cerebral venous thrombosis with more veins or sinuses thrombosed, and multiple intracerebral haemorrhage was more common. They were more likely to have concurrent extracranial venous or arterial thromboses. Their outcomes at the end of hospital admission were worse, with higher rates of death and disability. Although the response of patients with VITT-associated cerebral venous thrombosis to treatment is difficult to assess in a purely observational study, non-heparin anticoagulants and intravenous immunoglobulin were both associated with better outcomes. The starting criteria for VITT, based on low platelets and high D-dimers, appeared to miss two patients who had typical features for this condition.
Implications of all the available evidence
VITT is specifically associated with adenovirus vector vaccines against COVID-19 and urgent work is needed to elucidate the trigger for this reaction, in the hope that future vaccines can be designed to avoid this. Clinicians need to be aware of the clinical, laboratory, and radiological markers of this condition, as without prompt treatment the outcome is very poor. Adoption of our proposed definition of VITT-associated cerebral venous thrombosis should make it less likely that atypical cases will be missed, but these diagnostic criteria will need to be tested as more data accumulate.
Scully and colleagues4 proposed the following definition for VITT: patients presenting with acute thrombosis and thrombocytopenia with elevated D-dimers, using a D-dimer threshold of <2000 μg/L for VITT unlikely and >4000 μg/L for VITT suspected. They showed that 22 (96%) of 23 patients with VITT had antibodies against platelet factor 4 (PF4). Similar observations were made in other smaller case series.3, 5
We aimed to document the clinical features, laboratory and imaging results, and outcomes in a large cohort of patients with VITT-associated cerebral venous thrombosis, and to compare these with patients with cerebral venous thrombosis without VITT, and with historical data from the 624 patients in the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) cohort.12
Methods
Study design and participants
For this multicentre cohort study, clinicians involved in the care of patients with cerebral venous thrombosis after vaccination against COVID-19 were identified through existing networks of communication among UK doctors, advertisement through the Association of British Neurologists and the British Association of Stroke Physicians, and via reports submitted to the UK Medicines and Healthcare products Regulatory Agency (MHRA). Clinicians were asked to submit all cases in which COVID-19 vaccination preceded the onset of cerebral venous sinus thrombosis or cortical vein thrombosis, regardless of the type of vaccine, interval between vaccine and onset of cerebral venous thrombosis symptoms, or blood test results. There were no exclusion criteria for the study. Clinicians were encouraged to report their cases to the MHRA, the UK Expert Haematology Panel, and Public Health England, so data from those sources will include most of our cases. The study includes a combination of retrospective and prospective cases.
Data were extracted from clinical notes, discharge summaries, results systems, and radiology reports, by consultants (56 patients), specialist trainees (29 patients), other clinicians involved in patient care (four patients), or trained stroke research practitioners (six patients). We included details of exposure to COVID-19 vaccines in patients with cerebral venous thrombosis, for a case-control comparison between those with and without VITT. We collected baseline data on demographics, venous thrombosis risk factors (including cerebral venous thrombosis risk factors identified in ISCVT12), clinical features, laboratory results, radiological findings, and treatments given, with death or dependency (modified Rankin score13 3–6) at the end of hospital admission as the primary outcome. Data were checked centrally for omissions, duplications, or inconsistencies, and data queries were sent back to the submitting clinicians until these were resolved. Case report forms were received between April 1 and May 20, 2021. The UK Health Research Authority confirmed that this surveillance study, using routine patient data in anonymised form, could proceed without the need for patient consent or review by an ethics committee.
Defining VITT-associated cerebral venous thrombosis
We defined cerebral venous thrombosis cases as VITT-associated if the lowest platelet count recorded during admission was below 150 × 109 per L and, if the D-dimer was measured, the highest value recorded was greater than 2000 μg/L, the lower of the two thresholds suggested by Scully and colleagues.4 These criteria are referred to as the starting criteria (different from the proposed criteria in the panel). Before proceeding with any comparisons between groups, we examined the frequency distributions of the minimum platelet count and maximum D-dimers recorded during admission across the whole study population, to confirm the appropriateness of these diagnostic thresholds in a population of patients with cerebral venous thrombosis.
We then compared the characteristics of patients with VITT-associated cerebral venous thrombosis with the patients in our study who did not satisfy our starting criteria for VITT. The VITT group was also compared with the historical ISCVT cohort.12
Anti-PF4 antibody assays
Anti-PF4 antibody tests used were as follows: automated chemiluminescent heparin-induced thrombocytopenia assay (HemosIL Acustar HIT-IgG assay; Instrumentation Laboratory; Milan, Italy), ELISA (Asserachrom HPIA-IgG; Diagnostica Stago; Reading, UK; Lifecodes PF4 IgG; Immucor; Norcross, GA, USA; and Zymutest HIA IgG; Hyphen Biomed; Neuville-sur-Oise, France), flow cytometry platelet activation assay (HITAlert; Diapharma Group; West Chester Township, OH, USA), or gel agglutination assay (Diamed ID-PaGIA Heparin/PF4 Antibody Test; Bio-Rad Laboratories; Hercules, CA, USA).
Statistical analysis
We compared categorical variables between groups using χ2 tests, unless the expected number of patients in any one category was less than five, in which case Fisher's exact test was used. The age distribution of VITT-associated cerebral venous thrombosis was compared with a single value representing the median age of patients in the ISCVT cohort,12 using the one-sample Wilcoxon signed rank test. All other continuous variables were compared using the Mann-Whitney U test.
The frequency of cases submitted was calculated for each 5-year interval between the ages of 15 years and 70 years. The frequency was then corrected for the number of patients vaccinated in each age group, using a bin width of 10 years to match with national data from OpenSAFELY.14
Statistical analysis was done using Microsoft Excel for Microsoft 365 MSO with the Real Statistics Resource Pack plugin.
Role of the funding source
There was no funding source for this study.
Results
Between April 1 and May 20, 2021, we received data on 99 patients from collaborators in 43 hospitals across the UK. Four patients were excluded because they did not have definitive evidence of cerebral venous thrombosis on imaging (appendix p 9). In 83 (87%) of 95 patients, the modality on which cerebral venous thrombosis was shown was CT venography (figure 1 ). The lowest platelet count during admission was available for all 95 patients and the highest D-dimer was available in 62 (89%) of 70 patients with VITT and 20 (80%) of 25 patients without VITT.
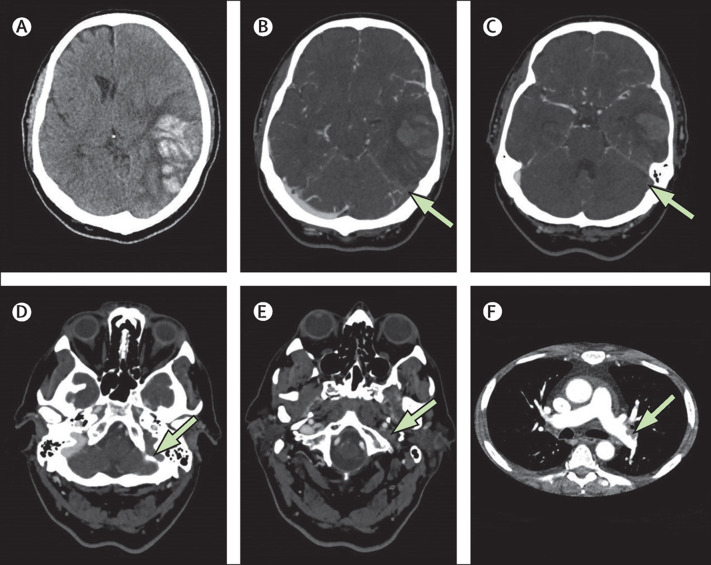
Figure 1.
Imaging from patient A, who had typical vaccine-induced immune thrombotic thrombocytopenia-associated cerebral venous thrombosis
This man in his 50s was well before vaccination with the ChAdOx1 (AstraZeneca) vaccine, but 17 days later developed a headache, abdominal pain, vomiting, dysphasia and confusion. (A) Axial CT without contrast showing a large haemorrhagic venous infarct in the left temporal lobe. (B–E). Axial CT venogram. Arrows indicate voids left by thrombus in the left transverse sinus (B, C) and the left sigmoid sinus (D) and lack of opacification of the left internal jugular vein (E). Each structure can be compared with its well-opacified counterpart on the right side. (F) CT pulmonary angiogram showing thrombus in the left pulmonary artery.
76 (80%) of 95 patients were investigated for anti-PF4 antibodies on one or more anti-PF4 antibody tests. 74 patients were tested with at least one ELISA. 17 of these patients were additionally tested with an automated chemiluminescent HIT assay (Acustar HIT-IgG assay), of whom nine patients were positive on ELISA but negative on Acustar. No patients were positive on Acustar and negative on ELISA (appendix p 2). Six patients were tested on a flow cytometry platelet activation assay (Diapharma HITAlert assay) and one patient on a gel agglutination assay (Diamed ID-PaGIA Heparin/PF4 Antibody test). Patients were counted as anti-PF4 positive if the result by any method was positive.
We examined the study population for evidence from their platelet counts and D-dimer results that there might be two subgroups, postulated to be those with VITT and those without VITT. Given existing evidence that anti-PF4 antibodies are a reliable diagnostic marker for VITT,3, 4, 5 we also classified patients by anti-PF4 status, as follows: positive on any test, negative in all tests used always including at least one ELISA test, or not tested.
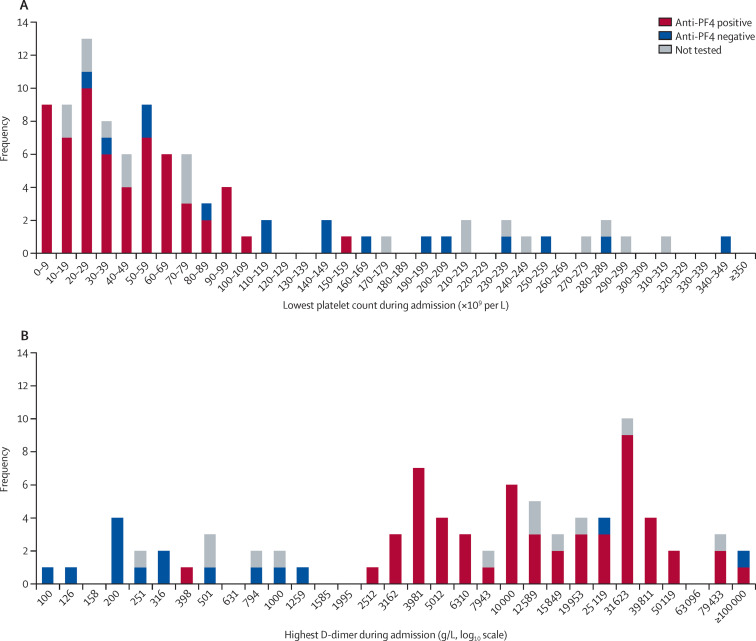
We found evidence to support the hypothesis that there was a distinct subgroup of patients with platelet counts below 150 × 109 per L who, when tested, tended to be positive for anti-PF4 antibodies, as predicted for the VITT group (figure 2 A). However, one patient with evidence of anti-PF4 antibodies on two ELISA assays (Stago Asserachrom and Immucor Lifecodes) had a lowest platelet count of 158 × 109 per L (patient B; appendix p 3).
Figure 2.
Distributions of lowest platelet counts (A) and highest D-dimers (B) recorded during admission, in patients with anti-PF4 antibodies, without PF4 antibodies, or not tested
The x-axis labels represent the lowest limit of the bin, so that the label 100 refers to the range 100–125, the label 126 refers to the range 126–157 and so on. Patients with atypical anti-PF4 results are described in the appendix (p 3) as follows: the patient with a normal platelet count and positive anti-PF4 antibodies is patient B; the patient with normal D-dimers and positive anti-PF4 antibodies is patient C; the two patients with high D-dimers and negative anti-PF4 antibodies are patients E and F. PF4=platelet factor 4.
Among the 75 patients found to be thrombocytopenic on their lowest platelet count, seven were negative for anti-PF4 antibodies on ELISA tests. Two of these patients satisfied the starting criteria for VITT, with thrombocytopenia and peak D-dimers greater than 2000 μg/L but were negative on two different ELISA assays (Stago Asserachrom and Hyphen Zymutest; patients E and F; appendix p 3).
We plotted a histogram for the highest D-dimer on a logarithmic scale (figure 2B). The distribution was bimodal. The value separating the two empty bars near the centre of the chart, the lower of which is labelled 1585, was log10(D-dimer) 3·3, equivalent to D-dimer of 1995 μg/L. Therefore, this distribution supports the incorporation of a D-dimer threshold of 2000 μg/L into the criteria for diagnosing VITT-associated cerebral venous thrombosis.
The median time interval between vaccination and cerebral venous thrombosis symptom onset was 9 days (IQR 7–12) in patients with VITT and 11 days (6–21) in those without VITT (table 1 ; appendix p 10). One patient with VITT developed clumsiness of the left arm 40 days after the first and only dose of ChAdOx1 vaccine, the first manifestation of a cortical vein thrombosis. However, the patient had developed a deep vein thrombosis, their first manifestation of VITT, 21 days after vaccination. The deep vein thrombosis was initially treated with tinzaparin, but the patient was found to be thrombocytopenic before this treatment was started. This patient was the only individual in the whole study to receive any form of heparin within the 2 weeks preceding the cerebral venous thrombosis.
Table 1.
Demographics, vaccine details, and blood results on admission in patients with VITT-associated cerebral venous thrombosis and those with non-VITT cerebral venous thrombosis
| VITT (n=70) | Non-VITT (n=25) | p value (VITT vs non-VITT) | ISCVT cohort (n=624) | p value (VITT vs ISCVT) | ||
|---|---|---|---|---|---|---|
| Age, years | 47 (32–55) | 57 (41–62) | 0·0045 | 37 | 0·0001 | |
| Sex | 0·31 | 0·0007 | ||||
| Female | 39 (56%) | 11 (44%) | 465 (75%) | |||
| Male | 31 (44%) | 14 (56%) | .. | 159 (25%) | ||
| Ethnicity | ||||||
| White | 61 (87%) | 21 (84%) | 0·74 | 550/621 (89%) | 0·72 | |
| Asian | 7 (10%) | 2 (8%) | 1·00 | 21/621 (3%) | 0·017 | |
| Black | 0 | 1 (4%) | 0·26 | 31/621 (5%) | 0·063 | |
| Other or mixed | 2 (3%) | 1 (4%) | 1·00 | 19/621 (3%) | 1·00 | |
| Vaccine details | ||||||
| Proportion given AstraZeneca vaccine | 70 (100%) | 21 (84%) | 0·0040 | .. | .. | |
| Time from vaccine to cerebral venous thrombosis, days | 9 (7–12) | 11 (6–21) | 0·10 | .. | .. | |
| Venous risk factors | ||||||
| Patients with no venous risk factors | 46 (66%) | 11 (44%) | 0·057 | .. | .. | |
| Patients with no ISCVT risk factors | 61 (87%) | 20 (80%) | 0·51 | 78 (13%) | <0·0001 | |
| Fibrinogen, g/L | 2·0 (1·3–2·8)* | 3·3 (2·9–4·1)† | 0·0001 | .. | .. | |
| Prothrombin time, s | 13·0 (11·9–14·8)‡ | 11·5 (10·8–12·6)§ | 0·0005 | .. | .. | |
| Activated partial thrombloplastin time, s | 28·8 (25·1–34·8)¶ | 26·9 (24·4–32·7)‖ | 0·030 | .. | .. | |
| Anti-platelet factor 4 antibodies | ||||||
| Positive on ELISA | 56/58 (97%) | 2/16 (13%) | <0·0001 | .. | .. | |
| Positive on Acustar HIT-IgG assay | 3/13 (23%) | 0 | 0·52 | .. | .. | |
Data are median (IQR), n (%), or n/N (%). Blood results were the closest available to the admission date. Normal ranges are typically fibrinogen 1·9–4·3 g/L, prothrombin time 10–13 s, and activated partial thromboplastin time 23–30 s. VITT=vaccine-induced immune thrombotic thrombocytopenia. ISCVT=International Study on Cerebral Venous Vein and Dural Sinus Thrombosis.
n=59.
n=15.
n=69.
n=24.
n=67.
n=24.
The age distribution of patients with VITT-associated cerebral venous thrombosis showed an abrupt increase in the frequency of cases in patients older than 45 years, in keeping with the UK COVID-19 vaccination strategy (appendix p 10). The patients in this study were all vaccinated on or before April 30, 2021, and before this date most individuals vaccinated in the UK were aged 45 years or older (appendix p 1). When adjusted for the UK rate of vaccination per age group, using data from OpenSAFELY,14 the step-change in frequency above age 45 years was no longer apparent (appendix p 10).
We compared the 70 patients with VITT-associated cerebral venous thrombosis with the 25 patients who developed cerebral venous thrombosis without evidence of VITT after vaccination, as well as with historical data from the 624 patients with cerebral venous thrombosis in the ISCVT cohort (table 1).12 Patients with VITT were significantly younger than patients who did not have VITT (table 1). All 70 cases of VITT-associated cerebral venous thrombosis occurred after a first dose of the ChAdOx1 (Oxford–AstraZeneca) vaccine, compared with 21 (85%) of 25 patients with non-VITT cerebral venous thrombosis, in whom the remaining four patients had been given their first dose (three patients) or second dose (one patient) of BNT162b2 (Pfizer–BioNTech) vaccine. The clinical features of cerebral venous thrombosis were similar in the VITT and non-VITT groups (appendix p 4).
Patients with VITT-associated cerebral venous thrombosis had lower levels of fibrinogen at hospital admission than the non-VITT group (table 1; appendix p 11), although both medians were within the normal range (1·9–4·3 g/L). 56 (97%) of 58 patients with VITT who were investigated for anti-PF4 antibodies using an ELISA assay tested positive; the characteristics of the other two patients are given in the appendix (p 3; patients E and F). Two patients with anti-PF4 antibodies on ELISA were classified as non-VITT using the current criteria, one because her platelet count never fell below 150 × 109 per L (patient B) and the other because her D-dimers never rose above 2000 μg/L (patient C, appendix p 3).
The number of veins thrombosed on the first venogram was higher in the VITT group (median 3, IQR 2–4) than in the non-VITT group (2, 2–3; p=0·041; appendix pp 5, 11). On neuroimaging at the time of admission, patients with VITT were more likely to have evidence of multiple venous infarction (10 [14%] of 70 patients) than those without VITT (0 of 25 patients; p=0·046), and more likely to have multiple intracerebral haemorrhages (23 [33%] of 70 patients) than non-VITT patients (three [12%] of 25 patients; p=0·045; appendix p 5).
31 (44%) of 70 patients with VITT-associated cerebral venous thrombosis had evidence of extracranial venous thrombosis, arterial thrombosis, or both, with pulmonary embolism and hepatic portal vein thrombosis being particularly common (appendix p 5). By contrast, extracranial thrombosis was only seen in one (4%) of 25 patients classified as non-VITT. This patient (patient D; appendix p 3) had pulmonary embolism and hepatic vein thrombosis in addition to cerebral venous thrombosis and presented with a platelet count of 57 × 109 per L. Even though the patient was not classified as having VITT in this study, because her highest D-dimer was only 822 μg/L, the clinical team treated her for VITT.
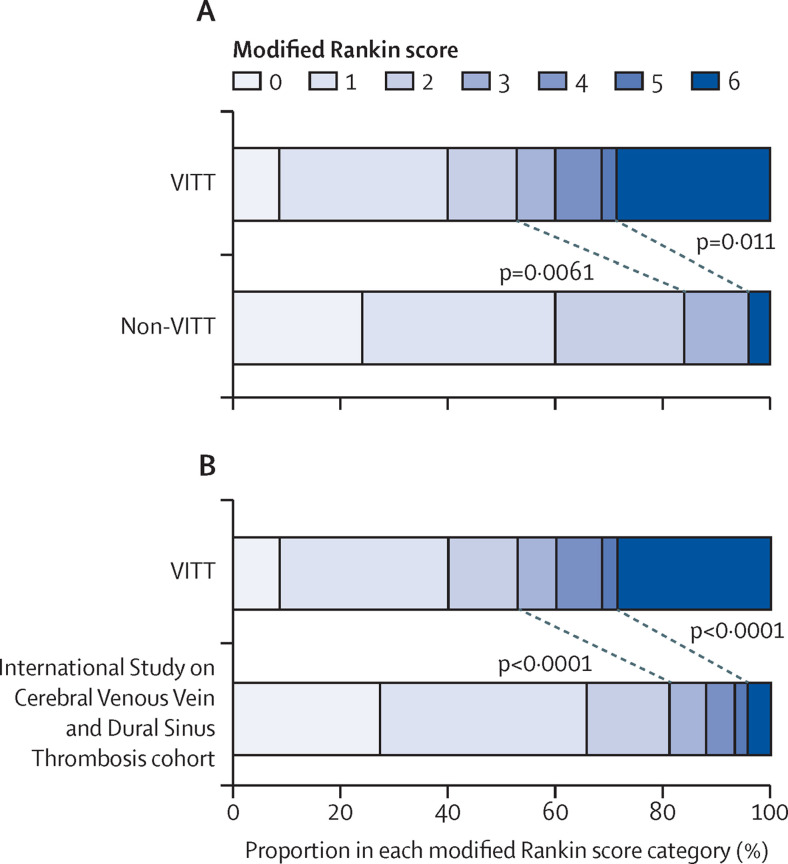
We compared the modified Rankin scale13 at discharge for patients with VITT compared with the non-VITT group (figure 3 A) and the ISCVT cohort (figure 3B). The primary outcome, death or dependency on others for care (modified Rankin score 3–6), was significantly more common in patients with VITT-associated cerebral venous thrombosis (33 [47%] of 70 patients) than in patients without VITT (four [16%] of 25 patients; p=0·0061). More patients died during admission in the VITT-associated cerebral venous thrombosis group (20 [29%] of 70 patients) than in the non-VITT group (one [4%] of 25 patients; p=0·011). Low Glasgow Coma Scale15 on admission and cerebral haemorrhage were the strongest predictors of death or dependency, as expected in patients with cerebral venous thrombosis (appendix p 6).12
Figure 3.
Comparison of disability on discharge
(A) Comparison of the outcomes from cerebral venous thrombosis in patients with VITT versus patients without VITT. (B) Comparison between VITT-associated cerebral venous thrombosis and historical data from the International Study on Cerebral Vein and Dural Sinus Thrombosis cohort. Each horizontal bar represents the percentage of patients in each modified Rankin scale category, which varies from 0 (no symptoms) through to 5 (severe disability). 6 represents death during this hospital admission. Diagonal lines and p values show comparisons of death and dependency (modified Rankin score 3–6) or death (modified Rankin score 6). VITT=vaccine-induced immune thrombotic thrombocytopenia.
The proportion of patients with VITT who had died or were dependent on others for their care at the end of admission was significantly lower in those given non-heparin parenteral anticoagulation (18 [36%] of 50 patients) compared with those who were not (15 [75%] of 20 patients; p=0·0031), in those who were given a direct oral anticoagulant (four [18%] of 22 patients) compared with those who were not (29 [60%] of 48 patients; p=0·0016), and in those who were given intravenous immunoglobulin (22 [40%] of 55 patients) compared with those who were not (11 [73%] of 15 patients; p=0·022; table 2 ).
Table 2.
Outcomes in patients with cerebral venous sinus thrombosis associated with vaccine-induced immune thrombotic thrombocytopenia, by treatment modality
| Patients treated or not treated | Patients who had died or were dependent | p value | ||
|---|---|---|---|---|
| Pharmacological | ||||
| Any anticoagulation | .. | .. | 0·0047 | |
| Yes | 60 | 24 (40%) | .. | |
| No | 10 | 9 (90%) | .. | |
| Heparin or low-molecular-weight heparin | .. | .. | 1·0 | |
| Yes | 16 | 8 (50%) | .. | |
| No | 54 | 25 (46%) | .. | |
| Non-heparin parenteral anticoagulant | .. | .. | 0·0031 | |
| Yes | 50 | 18 (36%) | .. | |
| No | 20 | 15 (75%) | .. | |
| Direct oral anticoagulant | .. | .. | 0·0016 | |
| Yes | 22 | 4 (18%) | .. | |
| No | 48 | 29 (60%) | .. | |
| Corticosteroid | .. | .. | 0·27 | |
| Yes | 51 | 22 (43%) | .. | |
| No | 19 | 11 (58%) | .. | |
| Anticonvulsant | .. | .. | 0·71 | |
| Yes | 26 | 13 (50%) | .. | |
| No | 44 | 24 (55%) | .. | |
| Fibrinogen replacement | .. | .. | 1·00 | |
| Yes | 15 | 7 (47%) | .. | |
| No | 55 | 26 (47%) | .. | |
| Intravenous immunoglobulin | .. | .. | 0·022 | |
| Yes | 55 | 22 (40%) | .. | |
| No | 15 | 11 (73%) | .. | |
| Plasma exchange | .. | .. | 0·78 | |
| Yes | 16 | 7 (44%) | .. | |
| No | 54 | 26 (48%) | .. | |
| Platelet transfusion | .. | .. | <0·0001 | |
| Yes | 25 | 21 (84%) | .. | |
| No | 45 | 12 (27%) | .. | |
| Invasive | ||||
| Endovascular management | .. | .. | 0·73 | |
| Yes | 9 | 5 (56%) | .. | |
| No | 61 | 28 (46%) | .. | |
| Intracranial pressure monitor | .. | .. | <0·0001 | |
| Yes | 13 | 13 (100%) | .. | |
| No | 57 | 20 (35%) | .. | |
| Decompressive hemicraniectomy | .. | .. | <0·0001 | |
| Yes | 13 | 13 (100%) | .. | |
| No | 57 | 20 (35%) | .. | |
Data are n or n (%). p values are for χ2 tests comparing the proportion of patients who died or were dependent on others for help with their activities of daily living (modified Rankin score 3–6) at the end of their admission in patients treated compared with those not treated.
Among patients treated with parenteral anticoagulants, 52 were given just one of the two options of heparin (low-molecular-weight or unfractionated) or a non-heparin parenteral alternative (argatroban or fondaparinux). This choice appears to have been determined mainly by the treatment date rather than patient characteristics—among patients with VITT, up to March 12, 2021, heparins were used, between March 13 and March 18, 2021, there was a mixture of heparin and non-heparin parenteral anticoagulants, and from March 19, 2021, onwards only non-heparin intravenous agents were used (except for one patient who was given unfractionated heparin briefly before being switched to argatroban later on the same day). Six (67%) of nine patients with VITT-associated cerebral venous thrombosis who received some form of heparin as their only parenteral anticoagulant had died or were dependent on others for their care at the end of their hospital admission, whereas 16 (37%) of 43 patients given a non-heparin alternative as their only parenteral anticoagulant had this poor outcome, although this difference was not significant (p=0·14).
Discussion
To our knowledge, our study provides the most detailed information reported to date on the clinical and radiological characteristics of VITT-associated cerebral venous thrombosis. The age distribution of our patient population was skewed towards older ages because of the UK policy of vaccinating older patients first, but patients with VITT-associated cerebral venous thrombosis were younger than those without VITT. Other key findings were that, compared with non-VITT patients, those with VITT-associated cerebral venous thrombosis had more extensive venous thrombosis and higher rates of multiple infarcts, multiple intracerebral haemorrhages, and extracranial thrombosis. VITT was associated with significantly more death or dependency at the end of hospital admission, but both the use of non-heparin anticoagulants and of intravenous immunoglobulin were associated with an improved outcome. As these treatments become better established, the outcomes after VITT-associated cerebral venous thrombosis might improve.
The ratio of patients with VITT to patients without VITT was 2·8:1, as expected from the estimated incidence of VITT-associated cerebral venous thrombosis in individuals receiving a first dose of the ChAdOx2 vaccine (12·3 per million16) and the expected background incidence of cerebral venous thrombosis in the same subpopulation during the 4-month study period (4·4 per million17), suggesting that cerebral venous thrombosis was probably unrelated to vaccination in most or all of our non-VITT cases and that there was no significant bias towards reporting of VITT cases.
A normal platelet count (conventionally ≥150 × 109 per L) is regarded as ruling out VITT in existing peer-reviewed published guidelines,18, 19 but adopting a platelet count threshold of less than 150 × 109 per L as a criterion for VITT-associated cerebral venous thrombosis in the present study could have been a weakness. First, defining thrombocytopenia as a fall to less than 50% of a known baseline platelet count is recommended in the analogous condition of heparin-induced thrombocytopenia.20 Second, patient B (appendix p 3), who was excluded from our VITT group because her platelet count did not fall below 150 × 109 per L, was treated as having VITT because of positive anti-PF4 antibodies and very high D-dimer of 4985 μg/L. Although we regard thrombocytopenia as the hallmark for VITT, adopting a hard threshold of 150 × 109 per L for defining thrombocytopenia risks excluding patients who have good evidence of VITT.
Additionally, making D-dimer greater than 2000 μg/L an absolute requirement for diagnosis of VITT-associated cerebral venous thrombosis might have been suboptimal. Patient C (appendix p 3) had cerebral venous sinus thrombosis, a platelet count of 110 × 109 per L, and positive anti-PF4 antibodies, which is strong evidence for VITT, but even after repeated testing her D-dimer was never higher than 410 μg/L. Patient D (appendix p 3) had a lowest platelet count of 37 × 109 per L and in addition to her cerebral venous sinus thrombosis had evidence of hepatic vein thrombosis, suspicious for VITT even though her anti-PF4 antibody was negative, yet the highest D-dimer was only 822 μg/L. Neither patient met the criteria for VITT-associated cerebral venous thrombosis used in this study, yet both were judged to have VITT by their treating clinicians.
Aside from the lowest platelet count and highest D-dimer that were used to make the diagnosis of VITT-associated cerebral venous thrombosis, three other features showed a significant association with the diagnosis: anti-PF4 antibodies, fibrinogen, and extracranial venous thromboses. The specificity of anti-PF4 antibodies was probably underestimated in our study, as the only two patients who were positive for the antibody but were classified as non-VITT using current criteria were patients B and C (appendix p 3)—ie, patients with probable VITT who were most likely misclassified. However, patients E and F (appendix p 3) had evidence for VITT but both were negative for anti-PF4 antibodies on two different ELISA assays, suggesting that a negative ELISA result should not be used to define VITT as unlikely19 or to cease further investigation,18 as is recommended in existing guidelines.18, 19
These observations lead us to propose a new set of diagnostic criteria for VITT-associated cerebral venous thrombosis (panel ). A diagnosis of possible VITT-associated cerebral venous thrombosis will alert clinicians to an urgent need for further investigation for this condition and they are likely to avoid the use of heparins or platelet transfusions if possible. A diagnosis of probable VITT constitutes sufficient evidence to offer a patient full treatment for this condition, including intravenous immunoglobulin or plasma exchange. A definite diagnosis will be useful for defining a population for future research studies on this condition. According to these criteria it is possible to make a diagnosis of probable VITT in patients with a normal platelet count (≥150 × 109 per L), a normal D-dimer, or a negative anti-PF4 antibody test, provided other evidence strongly supports the diagnosis.
Panel. Diagnostic criteria for VITT-associated cerebral venous thrombosis.
Definite VITT-associated cerebral venous thrombosis
-
•
Post-vaccine cerebral venous thrombosis (proven on neuroimaging and with first symptom of venous thrombosis within 28 days of vaccination against COVID-19)
and
-
•
Thrombocytopenia (lowest recorded platelet count <150 × 109 per L or documented platelet count decrease to less than 50% of baseline)
and
-
•
Anti-PF4 antibodies (detected on ELISA or functional assay)
Probable VITT-associated cerebral venous thrombosis
-
•
Post-vaccine cerebral venous thrombosis
and
-
•
Either thrombocytopenia or anti-PF4 antibodies
and
-
•
Coagulopathy (D-dimer >2000 μg/L or fibrinogen <2·0 g/L with no other explanation such as severe sepsis, malignancy, or recent trauma or surgery) or extracranial venous thrombosis (clinical or imaging evidence with onset since vaccination against COVID-19)
Possible VITT-associated cerebral venous thrombosis
-
•
Post-vaccine cerebral venous thrombosis
and
-
•
Either thrombocytopenia or anti-PF4 antibodiesIn assessing the interval since vaccination, the date of the first symptom of venous thrombosis should be used, even if this was a symptom of an extracranial thrombosis. The retrospective time window within which a pre-cerebral venous thrombosis baseline platelet count can be used to define a fall of greater than 50% has not been defined, as this will depend on what medical events have occurred in the interim.
PF4=platelet factor 4. VITT=vaccine-induced immune thrombotic thrombocytopenia.
In patients with cerebral venous thrombosis following COVID-19 vaccination, anti-PF4 testing should not be reserved for patients with admission platelet counts below 150 × 109 per L. This strategy would risk missing patients with VITT. A patient with a low-normal platelet count may still have anti-PF4 antibodies, as was the case for patient B (appendix pp 3–4), and a diagnosis of VITT should still be considered while further diagnostic tests are undertaken, including further full blood counts.
Clinicians should be aware that patients with VITT-associated cerebral venous thrombosis are more likely to have extracranial thrombosis than other patients with cerebral venous thrombosis. Some patients, such as patient A (figure 1; appendix p 3), might be dysphasic and have difficulty reporting their symptoms.
Anticoagulation and treatment with intravenous immunoglobulin were associated with a lower probability of death or dependency at the end of hospital admission, but this finding is difficult to interpret, as the most unwell patients might have died before these treatments could be offered, biasing the results. Similarly, the association between decompressive hemicraniectomy and poor outcome probably reflects selection of patients with the most severe cerebral venous thrombosis for this invasive procedure. However, the mortality rate of 54% after decompressive hemicraniectomy for VITT-associated cerebral venous thrombosis is high compared with a historical mortality of 16% after this procedure in cerebral venous thrombosis.21
The relationship between platelet transfusion and poor outcome in VITT-associated cerebral venous thrombosis appears to support concerns about the safety of this treatment,4 but the findings are difficult to interpret; in 12 (48%) of 25 patients offered this treatment, the indication was to support decompressive hemicraniectomy, which was only offered to patients with severe cerebral venous thrombosis.
We present the largest and most detailed study of VITT-associated cerebral venous thrombosis to date, with a well-matched control group consisting of patients presenting to UK hospitals with cerebral venous thrombosis after vaccination against COVID-19 but without evidence of VITT. However, our study has some limitations. The number of patients in each group in our study was small, because of the rarity of these conditions. The study was underpowered for some of the comparisons made between the VITT and non-VITT groups. Although our study will generate important hypotheses for future research, we cannot draw inferences about other populations of patients with cerebral venous thrombosis after COVID-19 vaccination. Comparison of our patients with the much larger historical ISCVT cohort12 might have been confounded by the higher age of our patients, attributable to COVID-19 vaccination policy in the UK, rather than to VITT. The median interval between vaccination and symptom onset could be an underestimate; in some cases in which the first symptom of cerebral venous thrombosis was reported as headache, this symptom might initially have been caused by mechanisms other than cerebral venous thrombosis, and also patients with a shorter interval might have been preferentially reported. We were dependent on local radiology reports for interpretation of scans, and on routine clinical observations, laboratory tests, and radiology, which might have led to indication bias. For example, we found only one patient with anti-PF4 antibodies but normal platelets (patient B; appendix p 3), but nine (45%) of 20 patients with normal platelets were not checked for anti-PF4 antibodies, so other cases with this combination might have been missed. We were unable to draw firm conclusions about treatments for VITT-associated cerebral venous thrombosis because we could not control for differences in the baseline characteristics between patients offered or not offered those treatments.
In conclusion, we have described the clinical features of VITT-associated cerebral venous thrombosis in detail, allowing us to propose diagnostic criteria for this condition. We recommend that all patients presenting with cerebral venous thrombosis within 28 days of COVID-19 vaccination should be checked for anti-PF4 antibodies, whatever their platelet count, until there are sufficient data to set an upper limit on the platelet count with which VITT-associated cerebral venous thrombosis might occur. We have shown that VITT-associated cerebral venous thrombosis has poorer outcomes than other forms of cerebral venous thrombosis and our data suggest that non-heparin anticoagulants and immunoglobulin might improve outcomes. However, VITT appears to be a very rare side-effect of vaccination with the ChAdOx1 (Oxford–AstraZeneca) vaccine, the risk of which is likely to be greatly outweighed by the benefit of vaccination against COVID-19 for most people.22
Data sharing
After publication, anonymised individual patient data will be made available on any reasonable request made to the corresponding author, subject to a data sharing agreement and the constraints imposed by UK data control and research governance regulations.
Declaration of interests
RJP receives grants from Randox Laboratories on an unrelated subject and from The Stroke Association for work on COVID-19 and stroke, not related to vaccination. PA-F receives grants from the Wellcome Trust for work on an unrelated subject. EH receives grants from MND Scotland and the National Institute for Health Research (NIHR) for work on an unrelated subject. TS sits on the Medicines and Healthcare products Regulatory Agency Vaccine Benefit Versus Risk Expert Working Group and was on the Data Safety Monitoring Committee of the GSK study to evaluate the safety and immunogenicity of a candidate Ebola vaccine in children GSK3390107A (ChAd3 EBO-Z) vaccine. MS receives grants from Shire and Novartis, and has received personal fees from Takeda, Novartis, Octapharma, and Sanofi for work on unrelated subjects. BS received a grant from the Medical Research Council, via the UK Research Institutes/NIHR Global Effort on COVID-19 Research to study neurological disease in relation to COVID-19, and has been a case management consultant to WHO-South-East Asia via the Global Outbreak Alert and Response Network since April, 2020, but vaccination against the infection is not the focus in either case. CR receives grants from the NIHR for work on an unrelated subject and is also collaborating with FirstKind Medical on a grant on an unrelated subject. CR is chair of the NIHR Hyperacute Stroke Research Oversight Group and is a member of the European Stroke Organization board of directors. DJW has received personal fees from Bayer, Alnylam, and Portola, unrelated to the work presented here. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the wider group of Cerebral venous thrombosis After Vaccination Against COVID-19 (CAIAC) collaborators who submitted cases. We also thank the British Association of Stroke Physicians and the Association of British Neurologists for promoting the study. This work was undertaken at UCL Hospitals/UCL, which receives a proportion of funding from the UK Department of Health NIHR Biomedical Research Centre funding scheme. RJP is supported by The Stroke Association for his work on COVID-19 and stroke. TS is supported by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections (grant number NIHR200907), NIHR Global Health Research Group on Brain Infections (17/63/110), and the UK Medical Research Council Global Effort on COVID-19 Programme (MR/V033441/1) for his work on COVID-19 and neurological disease, including stroke. TS and BS are supported by the NIHR Global Health Research Group on Brain Infections (17/63/110).
Contributors
CR and RJP conceived the study. The Steering Committee comprised RJP, AW, TS, MS, DJW, and CR. RJP wrote the protocol and clarified the regulatory framework of the study. TS, AT, and BS independently initiated a similar study that was amalgamated into this one. RJP designed the case report form and AT, BS, PF, AW, MS, DJW, and CR provided critical review of the content. RJP designed, implemented, and maintained the database and uploaded the data. RJP, AT, and BS continuously reviewed the data to ensure its validity and submitted data queries where there were errors or omissions. BC provided data on where some of the cases had been seen. RJP, AT, RM, PA-F, JMY, LZ, MJ, EH, DWh, PF, and AW coordinated data collection in their sites and submitted case report forms. GH-S, CH, and DWa submitted case report forms. RJP performed the statistical analysis and wrote the manuscript. All authors critically reviewed the manuscript, had full access to the data in the study, and shared responsibility for the decision to submit for publication.
Contributor Information
CVT After Immunisation Against COVID-19 (CAIAC) collaborators:
Sara Al-izzi, Aravindhan Baheerathan, Soma Banerjee, Gary Benson, Claudia Boshier, Sandeep Buddha, Nathan Burley, Ruaridh Cameron Smail, Arvind Chandratheva, Pavel Chudakou, Philip Clatworthy, Alasdair Coles, Thomas Cox, Ranjit Dasgupta, Richard Davenport, Darrell Devine, Stephen Fenlon, Carolyn Gabriel, Rita Ghatala, Claire Hall, Milan Hargovan, Kirsty Harkness, Ian Harvey, Lucy Hicken, Laura Howaniec, Abubaker Ibnouf, Luis Idrovo, Gordon Ingle, Yong Kyan Lee, Ailidh Lang, Simon McBride, Malcolm McLeod, Ruth Medlock, Puja Mehta, Ian Morrison, Girish Muddegowda, Sharon Muzerengi, Donald Pang, Gopinath Periyasamy, Gavin Preston, Naomi Priestley, Lydia Revicka, Sadia Saber, Elliott Smith, Youssef Sorour, Oliver Spooner, Jon Stone, Laszlo Sztriha, Narmathey Thambirajah, Rhys Thomas, David Veale, Jasmine Wall, Sarah White, James White, Syarah Yusoff, and Laura Zambreanu
Supplementary Material
References
- 1.Our World in Data Coronavirus (COVID-19) deaths. https://ourworldindata.org/covid-deaths
- 2.Our World in Data Coronarivus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms JM, Ansteatt KT, Roberts JC, et al. Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine. J Blood Med. 2021;12:221–224. doi: 10.2147/JBM.S307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric rash and thrombocytopenia after the mRNA-1273 (Moderna) COVID-19 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E-J, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smadja DM, Yue Q-Y, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58 doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 13.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 14.The OpenSAFELY Collaborative OpenSAFELY NHS COVID-19 Vaccine Coverage. https://www.opensafely.org/research/2021/covid-vaccine-coverage
- 15.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 16.GOV.UK; Medicines and Healthcare products Regulatory Agency Coronavirus vaccine—weekly summary of yellow card reporting. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- 17.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43:3375–3377. doi: 10.1161/STROKEAHA.112.671453. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184–189. doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 19.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19:1585–1588. doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warkentin TE, Roberts RS, Hirsh J, Kelton JG. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch Intern Med. 2003;163:2518–2524. doi: 10.1001/archinte.163.20.2518. [DOI] [PubMed] [Google Scholar]
- 21.Avanali R, Gopalakrishnan MS, Devi BI, Bhat DI, Shukla DP, Shanbhag NC. Role of decompressive craniectomy in the management of cerebral venous sinus thrombosis. Front Neurol. 2019;10:511. doi: 10.3389/fneur.2019.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537 913 COVID-19 cases. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After publication, anonymised individual patient data will be made available on any reasonable request made to the corresponding author, subject to a data sharing agreement and the constraints imposed by UK data control and research governance regulations.