Abstract
Introduction and aims
A case series of ten patients that received protocolized care for SARS-CoV-2 infection and developed severe gastrointestinal complications, is presented. The aim of our study was to contribute to the ongoing discussion regarding gastrointestinal complications related to SARS-CoV-2 infection. After reviewing the current literature, ours appears to be the first detailed case series on the topic.
Materials and methods
A retrospective filtered search of all patients admitted to our hospital for SARS-CoV-2 infection, who developed severe gastrointestinal complications, was performed. All relevant data on hospital patient management, before and after surgery, were collected from the medical records.
Results
Of the 905 patients admitted to our hospital due to SARS-CoV-2 infection, as of August 26, 2020, ten of them developed severe gastrointestinal complications. Seven of those patients were men. There were four cases of perforation of the proximal jejunum, three cases of perforations of the ascending colon, one case of concomitant perforation of the sigmoid colon and terminal ileum, one case of massive intestinal necrosis, and one preoperative death. Three right colectomies, four intestinal resections, one Hartmann’s procedure with bowel resection, and one primary repair of the small bowel were performed. The mortality rate of the patients analyzed was 50%.
Conclusion
Spontaneous bowel perforations and acute mesenteric ischemia are emerging as severe, life-threatening complications in hospitalized SARS-CoV-2 patients. More evidence is needed to identify risk factors, establish preventive measures, and analyze possible adverse effects of the current treatment protocols.
Keywords: COVID-19, Bowel perforation, Mesenteric ischemia, Mesenteric thrombosis
Abstract
Introducción y objetivos
Presentamos una serie de casos de diez pacientes recibiendo manejo protocolizado para infección por SARS-CoV-2 que desarrollaron complicaciones gastrointestinales severas. El objetivo del estudio es contribuir a la discusión sobre las complicaciones gastrointestinales de la infección por SARS-CoV-2. Hasta el momento y tras una revisión de la literatura, se trata de la primera serie de casos detallada sobre este tema.
Materiales y métodos
Se realizó una búsqueda retrospectiva y filtrada de todos los pacientes ingresados en nuestro hospital por infección por SARS-CoV-2 que desarrollaron complicaciones gastrointestinales severas. Se obtuvieron del expediente clínico todos los datos relevantes al manejo intrahospitalario antes y después de la cirugía.
Resultados
De 905 pacientes ingresados a la fecha del 26 de agosto de 2020 en nuestro hospital por infección por SARS-CoV-2, diez pacientes desarrollaron complicaciones gastrointestinales severas. Siete de ellos fueron hombres. Se presentaron cuatro perforaciones del yeyuno proximal, tres perforaciones del colon ascendente, una perforación concomitante del colon sigmoides e íleon terminal, una necrosis intestinal masiva y una defunción prequirúrgica. Se realizaron tres colectomías derechas, cuatro resecciones intestinales, un procedimiento de Hartmann con resección intestinal y una reparación primaria de intestino delgado. La mortalidad del grupo estudiado fue de 50%.
Conclusión
Las perforaciones intestinales espontáneas y la isquemia mesentérica aguda están surgiendo como complicaciones severas y que ponen en riesgo la vida de pacientes hospitalizados por SARS-CoV-2. Se requiere más evidencia para identificar factores de riesgo, establecer medidas preventivas y analizar los posibles efectos adversos de los protocolos de manejo actuales.
Palabras clave: COVID-19, Perforación intestinal, Isquemia mesentérica, Trombosis mesentérica
Introduction and aim
As of August 26, 2020, SARS-CoV-2 has affected 24.3 million patients worldwide, with 829,663 reported deaths, according to the World Health Organization. Numbers are still rising in several countries, and the exact pathologic mechanisms of this novel virus and its systemic implications remain unknown, despite the massive outpouring of published literature since the beginning of this global pandemic.
As has already been extensively reported, gastrointestinal symptoms can develop from SARS-CoV-2 infection in up to 50% of cases1, sometimes even before respiratory symptoms. The virus has been isolated in stool samples, and the confirmed mechanism of entry into gastrointestinal cells is the angiotensin converting enzyme 2 (ACE-2) receptor2. Xiao et al. reported abundant expression of the ACE-2 receptor in glandular cells of gastric, duodenal, and rectal epithelia, by immunofluorescence3.
SARS-CoV-2 infection has also been shown to cause coagulopathies, and hypercoagulability in the critically ill has been demonstrated through thromboelastography4. Thrombosis secondary to a hypercoagulation state can lead to both pulmonary embolisms and intestinal ischemia, which is why parenteral anticoagulation is being used in the treatment of the disease.
In Mexico, as of August 26, 2020, 573,888 confirmed cases of SARS-CoV-2 have been reported, along with 62,076 deaths, and there are no indications of a flattening of the curve. The Hospital San Jose Tec de Monterrey is an advanced specialty hospital in Monterrey, one of the largest cities in the country. It has been designated as a COVID-only hospital, and patients have been admitted and treated in its 150 beds since May 2020. According to our search of the literature, few reports have been published regarding bowel ischemia or perforation in patients with SARS-CoV-2 infection5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18.
The aim of our study was to present a fully comprehensive case series on the subject, with as many preoperative and postoperative details as possible.
Materials and methods
We performed a retrospective filtered search of all patients hospitalized at the Hospital San Jose Tec de Monterrey since it was established as a SARS-CoV-2 referral center in May 2020, with the following inclusion criteria: (1) a positive PCR test for SARS-CoV-2, (2) patients hospitalized under protocolized care for COVID pneumonia, and (3) the development of either spontaneous bowel perforation or acute mesenteric ischemia.
We collected the demographic characteristics, as well as all relevant admission, perioperative, surgical, and postoperative data. Relevant admission data included SARS-CoV-2 viral load and serum markers. Relevant preoperative data before symptom onset included time interval from admission, time interval from the first immunomodulator dose, modality of supplementary oxygen required, admission to the intensive care unit (ICU), and hemodynamic instability. Relevant perioperative data included imaging findings, surgical procedure performed, surgical findings, and presence or absence of bowel necrosis or perforation. Relevant postoperative data included all-cause mortality rate and pathology report.
Baseline characteristics were reported as frequencies and percentages. Because of extreme outliers in the proinflammatory marker results, median and range were used. For normally distributed parameters, the arithmetic mean was utilized. Data were presented in a descriptive fashion, with measures of central tendency calculated to express the baseline characteristics. No statistical analysis was performed.
All patients included in the present study signed an informed consent form, accepting in-hospital standardized care for SARS-CoV-2 infection, as well as an informed consent form regarding the use of their personal clinical files for investigation purposes. The study complies with current bioethical standards and was approved by our institution’s ethics committee. The study and signed informed consent forms did not constitute an experimental treatment protocol, but rather an empirical treatment based on continuously emerging available data on the subject. No identifying data or images were included in the present study.
Results
All patients hospitalized at our center during the study period were actively sick from SARS-CoV-2 pneumonia. Of the 905 hospital records analyzed, 10 patients met the inclusion criteria, with an incidence of 1.1%. All hospitalized patients received the same standardized care, which included supportive measures and oxygen therapy, ranging from nasal cannulae to mechanical ventilation, as needed, in addition to the following standardized treatment: methylprednisolone 40 mg QD or dexamethasone 6 mg QD, azithromycin 250 mg QD, ceftaroline 600 mg QD, lopinavir/ritonavir 200/50 mg BID, ribavirin 400 mg BID, enoxaparin 40−60 mg QD. Immunomodulators were used as follows: all patients were started on baricitinib 4 mg QD; if at any point a patient had ferritin levels >1,000 ng/ml and IL-6 > 80 pg/ml, two doses of tocilizumab 8 mg/kg were administered 12 h apart, and baricitinib was stopped.
The rationale for the use of each of those drugs was based on continuously emerging data that became available as the pandemic evolved. Namely, steroids have been the mainstay of treatment worldwide; antibiotics were used to prevent or treat superimposed infections by bacterial organisms; antivirals and immunomodulators were used, as they were emerging and promising treatments during the second quarter of 2020; and low molecular weight heparin was used as thromboprophylaxis. Remdesivir and ivermectin were not used. Recommendations on the medical treatment of SARS-CoV-2 infection have been continuously changing, and treatment protocols around the world must either adhere to the emerging recommendations or establish their own experimental protocol. The former has been the norm at our institution, which constitutes empirical treatment based on ongoing and emerging data from around the world.
Ten patients were included, 7 of whom were men (70%), with a mean age of 61.6 years and a mean BMI of 29.2 kg/m2. Three patients (30%) had diabetes mellitus, 4 patients had arterial hypertension (40%), and 3 patients were smokers (30%). Table 1 shows their baseline characteristics.
Table 1.
Baseline characteristics.
| Demographics | (n = 10) |
|---|---|
| Age, years (mean) | 61.6 |
| Sex, % male | 70% |
| BMI, kg/m2 (mean) | 29.2 |
| Comorbidities | |
| Diabetes mellitus, frequency (%) | 3 (30%) |
| Arterial hypertension, frequency (%) | 4 (40%) |
| Smoking, frequency (%) | 3 (30%) |
| Preoperative variables | |
| CORADS score (mean) | 4.7/5 |
| Time from admission to onset, days (mean) | 10.6 |
| Admission to ICU before onset, frequency (%) | 9 (90%) |
| Use of pressors before onset, frequency (%) | 5 (50%) |
| Mechanical ventilation before onset, frequency (%) | 8 (80%) |
| # Baricitinib doses (mean) | 6.6 |
Of the 10 patients studied, 9 (90%) were in the ICU at symptom onset, 8 of whom were under invasive mechanical ventilation. Five of those 9 critically ill patients were hemodynamically unstable, receiving vasopressors. Table 2 shows the viral load and biomarkers at admission.
Table 2.
Admission biomarkers.
| Biomarker | Median | Range |
|---|---|---|
| Viral Load (copies/ml) | 3383 | (45.2–35060) |
| Leu (×103/ml) | 11.2 | (4.6−15.5) |
| LDH (U/l) | 376 | (190–684) |
| CRP (mg/l) | 17.6 | (3.42−45.10) |
| D-Dimer (ng/l) | 353 | (8–2117) |
| Procalcitonin (ng/ml) | 0.2 | (0.06−1.28) |
| Ferritin (ng/ml) | 1098 | (16.3–4011) |
| IL-6 (pg/ml) | 63.2 | (3.71–1624) |
| BNP (pg/ml) | 35.3 | (10–342) |
| Troponin (ng/l) | 16.6 | (1.5–383) |
| Neutrophils (%) | 88.1 | (69.6−92.8) |
| Platelets (×103/ml) | 280 | (105–520) |
| Lactate (mmol/l) | 2.1 | (1−3.71) |
| Creatinine (mg/dl) | 0.8 | (0.6−6.6) |
| Fibrinogen (mg/dl) | 438.5 | (65–643) |
BNP: brain natriuretic peptide; CRP: C-reactive protein; LDH: lactate dehydrogenase; Leu: leukocytes.
Regarding immunomodulator use, all patients received baricitinib, as well as concomitant steroids (dexamethasone, methylprednisolone, etc.). No dose adjustments were necessary in relation to lymphopenia, as it was not present in any of the patients in our case series. Baricitinib dosage was not adjusted for renal function. Tocilizumab was used in 2 (20%) patients.
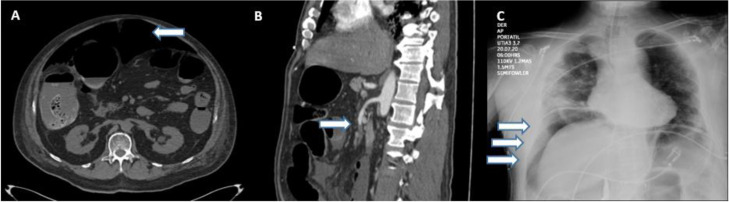
Eight patients were diagnosed through an abdominal CT scan, 6 of whom presented with signs of pneumoperitoneum, suggesting bowel perforation (Fig. 1 A). One patient presented with thrombosis of the superior mesenteric artery (Fig. 1B), with no free air or signs of perforation. The other patient presented with an enterocutaneous fistula, connecting a previous (14-year) jejunostomy site with the skin, as shown on the CT. Two patients were diagnosed through a plain chest X-ray that revealed subdiaphragmatic air consistent with bowel perforation (Fig. 1C), one of whom (M, 74) died 7 h after diagnosis, during preoperative resuscitation and attempts at hemodynamic stabilization as preparation for emergent surgery. All the other patients underwent laparotomy.
Figure 1.
(A) CT scan showing pneumoperitoneum (arrow). (B) CT scan showing superior mesenteric artery thrombosis (arrow). (C) Chest X-ray showing subdiaphragmatic air (arrows).
Three patients presented with small anterior perforations in the ascending colon and were managed with right colectomy. One of them (M, 69) was re-intervened due to signs of hemodynamic shock; an inferior vena cava laceration, resulting from a challenging dissection of the ascending colon due to localized phlegmon during the initial surgery, was identified and repaired, and the patient died 12 h later. The other two patients were discharged (M, 34; F, 54). None of those 3 patients underwent primary anastomosis, and none had signs of thrombosis in the histopathologic review.
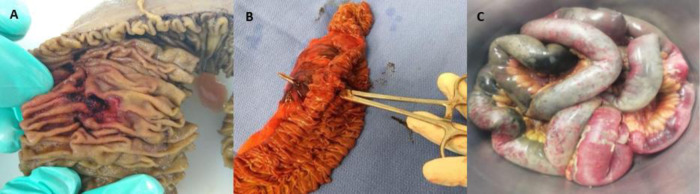
Four patients presented with small perforations in the proximal small bowel (jejunum) (Fig. 2 A), all on their mesenteric attachment. The first patient (M, 51) presented with a perforated jejunal diverticulum. The second patient (F, 79) presented with 4 jejunal diverticula. They were both managed with resection of the affected segment (Fig. 2B) and primary end-to-end anastomosis. The pathology study of the first patient reported mesenteric small bowel thrombosis, and the other study reported only perforated diverticula with local inflammation. The first patient was discharged; the second patient died 12 days after surgery. The third patient (M, 67) presented with a 2 mm jejunal perforation at a distance of 10 cm from the duodenojejunal junction. The injury was managed with primary repair and gastrojejunostomy bypass; no surgical specimen was studied. The patient died 48 h after surgery. The last patient (M, 73) developed an enterocutaneous fistula connecting a previous jejunostomy site with the abdominal wall skin, while hospitalized for SARS-CoV-2 infection under our treatment protocol. He underwent bowel resection and primary end-to-end anastomosis. The pathology study reported no mesenteric thrombosis, and the patient was discharged after a successful recovery.
Figure 2.
(A) Surgical specimen of jejunum resection showing full thickness perforation. (B) Resected small bowel, same patient, showing full thickness perforation and hematoma in the mesentery. (C) Resected small bowel; patchy areas of necrosis can be seen.
One patient (M, 55) presented with bowel necrosis affecting the most distal 250 cm of the small bowel, requiring resection of the affected portion (Fig. 2C), with the creation of an end jejunostomy. Because the viability of his ascending colon was uncertain, an intraperitoneal negative pressure device (ABThera®, KCI, San Antonio, TX, USA) was used, planning a second-look. The patient was re-intervened 48 h later, finding a viable ascending colon; the ABThera system was removed, and his abdominal wall closed. The pathology study reported wall necrosis and mesenteric ischemia and thrombosis. The patient died 28 days after surgery.
One patient (F, 60) presented with both a sigmoid colon perforation and a terminal ileum perforation, which were managed with a Hartmann’s procedure and concomitant small bowel resection, with primary end-to-end anastomosis. The pathology study reported a perforated colon, with no signs of thrombosis, and the patient was discharged after recovery.
The mean time interval from admission to abdominal symptom onset was 10.6 days (range 7–16). Overall inpatient mortality due to SARS-CoV-2 infection at our hospital is 10.05%. In our study group, five patients survived, resulting in a 50% mortality rate and a relative risk of death of 4.97, compared with the rest of our hospital’s SARS-CoV-2 population. The full details of each of our study patients are included in Table 3 .
Table 3.
Results.
| Patient | Viral load | Days since admission | Findings | Procedure | Primary anastomosis | Pathology report | Final result |
|---|---|---|---|---|---|---|---|
| 1 | 35060 | 7 | Ascending colon perforation | Right colectomy | No | Perforated colon | Discharge |
| 2 | 47.77 | 16 | Ascending colon perforation | Right colectomy | No | Transmural necrosis | Death |
| 3 | 1668 | 12 | Perforated jejunal diverticulum | Bowel resection | Yes | Mesenteric thrombosis | Discharge |
| 4 | 395548 | 7 | Jejunal perforation | Bowel repair | – | – | Death |
| 5 | 12515 | 7 | Small bowel necrosis | Bowel resection | No | Mesenteric thrombosis | Death |
| 6 | 45.2 | 13 | – | – | – | – | Death |
| 7 | 2480 | 14 | Enterocutaneous fistula with abscess | Bowel Resection | Yes | Chronic peritonitis | Discharge |
| 8 | 3338 | 12 | Sigmoid perforation + terminal ileum perforation | Hartmann’s procedure + bowel resection | Yes (small bowel) | Chronic peritonitis | Discharge |
| 9 | 7531 | 10 | Perforated jejunal diverticula (3) | Bowel resection | Yes | Perforated diverticula | Death |
| 10 | 3428 | 8 | Ascending colon perforation | Right colectomy | No | Perforated colon | Discharge |
| Patient | # Baricitinib doses | # Tocilizumab doses | Vasopressin dose* | Norepinephrine dose* | Weight | LMWH dose |
|---|---|---|---|---|---|---|
| 1 | 3 | 0 | – | – | 90 | 60 mg QD |
| 2 | 3 | 0 | .06 U/min | 0.8 mcg/kg/min | 91 | 80 mg QD |
| 3 | 8 | 0 | – | – | 100 | 60 mg QD |
| 4 | 0 | 0 | .04 U/min | .4 mcg/kg/min | 85 | 60 mg QD |
| 5 | 3 | 0 | – | .06 mcg/kg/min | 89 | 80 mg QD |
| 6 | 7 | 0 | .06 U/min | .5 mcg/kg/min | 84 | 60 mg QD |
| 7 | 6 | 0 | – | – | 91 | 80 mg QD |
| 8 | 7 | 2 | .04 U/min | .05 mcg/kg/min | 99 | 80 mg QD |
| 9 | 10 | 0 | .04 U/min | .11 mcg/kg/min | 62 | 40 mg QD |
| 10 | 8 | 2 | .04 U/min | .08 mcg/kg/min | 94 | 60 mg QD |
Initial dose; continually titrated to achieve adequate mean arterial pressures.
Discussion
With an incidence of 1.1%, and a relative risk of death of 4.97, as reported in our study, severe bowel complications in hospitalized patients with SARS-CoV-2 infection are emerging as a serious, life-threatening phenomenon. Interestingly, the outcome of those patients accounts for 5.49% of our hospital’s SARS-CoV-2 deaths. The proposed mechanisms of viral injury to the gastrointestinal tract have already been discussed. At present, 8 single case reports have been published worldwide5, 6, 7, 8, 9, 10, 11, 12, 13. One case series with 7 patients was also published by Norsa et al.14, but it provides little information on diagnosis, surgical findings, and previous treatment. In a case series presented by Gao et al.15, they described 4 patients with acute abdomen, all of whom were initially suspected of having SARS-CoV-2 infection, but ultimately tested negative. Ignat et al.16 reported a case series of 3 patients that developed bowel ischemia, while hospitalized for SARS-CoV-2 infection in France. However, no mention was made of the time interval from admission to the development of acute abdomen, or of the prior care received while hospitalized. Kaafarani et al.17 presented a case series of 141 critically ill patients, describing gastrointestinal complications related to SARS-CoV-2 infection, in which 5 patients (3.54%) presented with bowel ischemia. Lastly, Bhayana et al.18 presented a radiology-oriented case series that included radiologic and surgical details of 4 SARS-CoV-2 patients with mesenteric thrombosis. All the published literature and available details appear in Table 4 , except for the study by Kaafarani et al. Their case series was excluded from the table because it lacked individual patient details and did not specify the survival results. To the best of our knowledge, ours is the first fully comprehensive case series on the subject.
Table 4.
Literature search for other case reports involving bowel ischemia, thrombosis, or perforation in hospitalized SARS-CoV-2 patients.
| Author | n | Sex | Age | Diagnostic tool | Free air image | Thrombosis (CT) | Surgery | Affected site | Necrosis present | Perforation | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Norsa | 1 | M | 62 | CT | No | Yes | Bowel resection | Small bowel | Yes | No | No |
| Bhayana | 4 | – | – | CT | No | Yes | Bowel resection | Small bowel | Yes | No | – |
| – | – | CT | No | Yes | Laparotomy | Small bowel | Yes | No | – | ||
| – | – | CT | No | Yes | Laparotomy | Small bowel | No | No | – | ||
| – | – | CT | No | Yes | Laparotomy | Colon/stomach | No | No | – | ||
| Farinaa | 1 | M | 70 | CT | No | Yes | No | Small bowel | – | – | No |
| Azouz | 1 | M | 56 | CT | No | No | Bowel resection | Small bowel | Yes | – | – |
| Norsa | 7 | M | 85 | CT |
Information not provided as per-patient Two patients had marked colon wall edema Four patients had pneumatosis intestinalis One patient had inferior vena cava and superior mesenteric vein thrombosis Information on surgery or surgical findings not provided |
Yes | |||||
| F | 71 | Endoscopy | Yes | ||||||||
| M | 69 | CT | No | ||||||||
| F | 79 | CT | Yes | ||||||||
| M | 63 | CT | No | ||||||||
| M | 62 | CT | No | ||||||||
| F | 83 | CT | No | ||||||||
| Chan | 1 | M | 73 | CT | No | Yes | No | Sigmoid colon | – | – | No |
| De Nardi | 1 | M | 53 | CT | Yes | Yes (PE) | Right colectomy | Ascending colon | No | Yes | Yes |
| Neto | 1 | F | 80 | CT | Yes | No | Sigmoidectomy | Sigmoid colon | No | Yes | No |
| Winnicka | 1 | M | 73 | Radiography | Yes | – | No | – | – | – | Yes |
| Guardiola | 1 | M | 66 | CT | Yes | No | Right colectomy | Ascending colon | No | Yes | – |
| Gaob | 4 | F | 71 | – | – | – | Bowel resection | Small bowel | No | – | Yes |
| M | 41 | – | – | – | Bowel resection | Small bowel | Yes | – | Yes | ||
| M | 52 | – | – | – | Bowel repair | Small bowel | No | Yes | Yes | ||
| M | 63 | – | – | – | Bowel repair | Small bowel | No | Yes | Yes | ||
| Bianco | 1 | M | 59 | CT | No | No | Bowel resection | Small bowel | Yes | No | No |
CT: computed tomography; F: female; M: male; PE: pulmonary embolism.
Farina et al. reported a case of a suspected COVID patient with negative PCR.
All patients reported by Gao et al. were initially suspected of having COVID, but ultimately tested negative.
Our rationale for using baricitinib and tocilizumab to treat SARS-CoV-2 pneumonia was based on available literature at the inception of our treatment protocol, all of which was observational. No protocol changes were made during our study period. The first prospective trial on tocilizumab (Phase III COVACTA Study), which was being carried out by the Roche Group, has been halted because the interim analysis showed it did not meet its intended endpoints, according to a recent publication on their website. However, that occurred after our data collection.
Several hypotheses have been proposed as to why SARS-CoV-2 appears to cause gastrointestinal injury, as explained by Pan et al. First, the virus can upregulate the expression of ACE-2 receptors, which allows further entry of the virus into digestive cells. Similarly, it can directly or indirectly damage the gastrointestinal system through an inflammatory response. Furthermore, given that the intestine is a large organ of the immune system, the virus can disrupt the so-called “gut-lung axis” by causing changes in the composition and function of the gut microbiota. At the same time, a coagulation disorder is induced by systemic inflammation, and hypoxia and immobilization may also play a role. Elevated levels of von Willebrand factor have been reported to be produced in response to damage to the vascular endothelium, which expresses ACE-2 receptors19. Both of those mechanisms may contribute to mesenteric thrombosis, which can lead to intestinal ischemia. Finally, mesenteric ischemia may simply be a consequence of hemodynamic shock because of SARS-CoV-2 pneumonia20, 21.
Acute mesenteric ischemia secondary to thrombosis can be explained by the hypercoagulation state already attributed to SARS-CoV-2 infection, as was previously explained. However, spontaneous perforations, such as those described in our patients, raise the question of whether the mechanism is simply the ACE-2 receptor-mediated direct injury mentioned above, or if there are other factors at play, possibly including an aspect of the protocolized care received. Interestingly, the spontaneous perforations seem to occur predominantly in either the cecum or the proximal jejunum. Furthermore, both Kaafarani et al. and Bhayana et al. noticed a patchy, yellowish discoloration of the bowel serosa in the ischemic lesions they observed, as opposed to the usual purple or black color of necrotic bowel. We observed no unusual discoloration in our patients.
Limitations of the present case series include its retrospective nature and the lack of a control group, serving as the motivation for a case-control study that is already underway. An important limitation of the new case-control study will be the impossibility of analyzing the use of immune-based drugs and corticosteroids as a risk factor, since all of our patients receive them, and so both the cases and controls will have the same exposure. Nevertheless, it will be interesting to compare those patients with the rest of the population that did not develop bowel complications, comparing their viral loads and other markers of disease severity. Inclusion in the present study of the patient with an enterocutaneous fistula was discussed thoroughly among the authors and we decided against exclusion for two reasons. First, the patient met all the inclusion criteria. Second, regardless of the previous jejunostomy, which was successfully closed 14 years earlier, and taking into consideration the patient’s uneventful recovery since then, we believe it is unlikely that the reopening at the jejunostomy site would have occurred, had the patient not been hospitalized for SARS-CoV-2 pneumonia. Furthermore, all enterocutaneous fistulae occur in the context of bowel perforation or loss of integrity of the bowel wall, which is why that patient is discussed in the small bowel perforation section.
Because no correlation or association can be expected from a small case series, no definitive conclusions could be made. However, the authors of the present article recommend the use of prophylactic anticoagulation in any treatment regimen for SARS-CoV-2 infection, as well as the constant evaluation of hospitalized patients for the development of abdominal symptoms. Studies on the safety profile of medications already being used to treat SARS-CoV-2 must also be performed, as immune-based drugs, such as tocilizumab and baricitinib, have already been associated with a risk of spontaneous bowel perforation6, 22, 23, 24. Corticosteroid use and its relation to gastrointestinal perforation have been discussed as early as 198025, 26, and a more recent cohort study in 2017 found that corticosteroid use greatly increased mortality (i.e., doubled it), in the context of bowel perforation27. As the current pandemic advances and more cases with gastrointestinal complications present, further studies may elucidate the mechanism of said phenomenon, determine the risk factors involved, and establish preventive measures. Therefore, we urge all authors contributing to the discussion of this topic to provide full details on hospital admission and the perioperative period, describing the complete treatment protocol for patients with SARS-CoV-2 infection.
Because no correlation or association can be expected from a small case series, no definitive conclusions could be made. However, the authors of the present article recommend the use of prophylactic anticoagulation in any treatment regimen for SARS-CoV-2 infection, as well as the constant evaluation of hospitalized patients for the development of abdominal symptoms. Studies on the safety profile of medications already being used to treat SARS-CoV-2 must also be performed, as immune-based drugs, such as tocilizumab and baricitinib, have already been associated with a risk of spontaneous bowel perforation6, 22, 23, 24. Corticosteroid use and its relation to gastrointestinal perforation have been discussed as early as 198025, 26, and a more recent cohort study in 2017 found that corticosteroid use greatly increased mortality (i.e., doubled it), in the context of bowel perforation27. As the current pandemic advances and more cases with gastrointestinal complications present, further studies may elucidate the mechanism of said phenomenon, determine the risk factors involved, and establish preventive measures. Therefore, we urge all authors contributing to the discussion of this topic to provide full details on hospital admission and the perioperative period, describing the complete treatment protocol for patients with SARS-CoV-2 infection.
Financial disclosure
No financial support was received in relation to this article.
Conflict of interest
All the authors declare that there is no conflict of interest.
Footnotes
Please cite this article as: Estevez-Cerda SC, Saldaña-Rodríguez JA, Alam-Gidi AG, Riojas-Garza A, Rodarte-Shade M, Velazco-de la Garza J, et al. Complicaciones intestinales graves en pacientes SARS-CoV-2 recibiendo manejo protocolizado. Revista de Gastroenterología de México. 2021;86:378–386.
References
- 1.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotfis K., Skonieczna-Żydecka K. COVID-19: gastrointestinal symptoms and potential sources of SARS-CoV-2 transmission. Anaesthesiol Intensive Ther. 2020;52:171–172. doi: 10.5114/ait.2020.93867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norsa L., Valle C., Morotti D. Intestinal ischemia in the COVID-19 era. Dig Liver Dis. 2020;52:1090–1091. doi: 10.1016/j.dld.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guardiola P.G., Ares J.A., Tomás N.P. Perforación intestinal en paciente COVID-19 en tratamiento con tocilizumab y corticoides. A propósito de un caso. Cir Esp. 2020;99:156–157. doi: 10.1016/j.ciresp.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Nardi P., Parolini D.C., Ripa M. Bowel perforation in a Covid-19 patient: Case report. Int J Colorectal Dis. 2020 doi: 10.1007/s00384-020-03627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azouz E., Yang S., Monnier-Cholley L., Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan K.H., Lim S.L., Damati A. Coronavirus disease 2019 (COVID-19) and ischemic colitis: an under-recognized complication. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neto I.J., Viana K.F., Silva M.B. Perforated acute abdomen in a patient with COVID-19: an atypical manifestation of the disease. J Coloproctol. 2020 doi: 10.1016/j.jcol.2020.05.011. [DOI] [Google Scholar]
- 11.Farina D., Rondi P., Botturi E. Gastrointestinal: bowel ischemia in a suspected coronavirus disease (COVID‐19) patient. J Gastroenterol Hepatol. 2020;36:41. doi: 10.1111/jgh.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winnicka L.M., Shenoy M.A. Gastrointestinal Perforation in a COVID-19 Patient. Infect Dis Clin Pract. 2020 [Google Scholar]
- 13.Bianco F., Ranieri Aj, Paterniti G. Acute intestinal ischemia in a patient with COVID-19. Tech Coloproctol. 2020 doi: 10.1007/s10151-020-02255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norsa L., Pietro B., Indriolo A. Poor outcome of intestinal ischemic manifestations of COVID 19. Gastroenterology. 2020;159:1595–1597. doi: 10.1053/j.gastro.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Xi H., Chen L. Emergency Surgery in Suspected COVID-19 Patients With Acute Abdomen. Ann Surg. 2020;272:e38–e39. doi: 10.1097/SLA.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignat M., Philouze G., Aussenac-Belle L. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery. 2020;168:14–16. doi: 10.1016/j.surg.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaafarani H.M., Moheb M.E., Hwabejire J.O. Gastrointestinal complications in critically ill patients with COVID 19. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhayana R., Som A., Li M.D. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020 doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parry A.H., Wani A.H., Yaseen M. Acute mesenteric ischemia in severe coronavirus-19 (COVID-19): possible mechanisms and diagnostic pathway. Acad Radiol. 2020;27:1190. doi: 10.1016/j.acra.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology. 2019;58:i34–i42. doi: 10.1093/rheumatology/key287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smolen J.S., Genovese M.C., Takeuchi T. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46:7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 24.Xie F., Yun H., Bernatsky S. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 2016;68:2612–2617. doi: 10.1002/art.39761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arsura E.L. Corticosteroid-associated perforation of colonic diverticula. Arch Intern Med. 1990;150:1337–1338. doi: 10.1001/archinte.1990.00390180139026. [DOI] [PubMed] [Google Scholar]
- 26.Remine S.G., Mcilrath D.C. Bowel perforation in steroid-treated patients. Ann Surg. 1980;192:581–586. doi: 10.1097/00000658-198010000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broersen L.H., Horváth-Puhó E., Pereira A.M. Corticosteroid use and mortality risk in patients with perforated colonic diverticular disease: a population-based cohort study. BMJ Open Gastroenterol. 2017;4 doi: 10.1136/bmjgast-2017-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]