Abstract
Background: Guillain-Barré Syndrome (GBS) is a rapidly progressive immune-mediated polyneuropathy often associated with an antecedent infectious illness or vaccination. The classic presentation of GBS is characterized by ascending limb weakness and numbness with loss of reflexes. However, atypical variants involving the face and arms or with purely sensory symptoms also exist. In up to 30% of cases, GBS progresses to respiratory failure, with patients requiring mechanical ventilation.
Case Report: We report a case of atypical GBS occurring after Coronavirus disease 2019 (COVID-19) vaccination in an otherwise healthy 38-year-old man. The patient's clinical presentation was characterized by bilateral hand and foot paresthesias, dysarthria, bilateral facial weakness, and an absence of classic ascending limb weakness. Albuminocytological dissociation within the cerebrospinal fluid was suggestive of GBS. The patient received intravenous immunoglobulin therapy, with modest improvement in his symptoms at the time of his discharge from the hospital.
Why Should an Emergency PhysicianBe Aware of This? Patients with GBS are at risk for life-threatening complications, including respiratory failure requiring mechanical ventilation. It is critical for emergency physicians to be aware of the manifold presentations of GBS for early recognition and treatment. This may be of particular importance in the context of a worldwide vaccination campaign in response to the COVID-19 pandemic.
Keywords: Guillain-Barré Syndrome (GBS), facial diplegia, CN-VII palsy, COVID-19 vaccination, dysarthria
Introduction
Guillain-Barré Syndrome (GBS) is a rapidly progressive immune-mediated polyneuropathy often associated with an antecedent infectious illness or vaccination. Classically, the syndrome is characterized by symmetric paresthesias, weakness of the extremities, or diminished or absent reflexes. Atypical variants involving the face and arms, or with purely sensory symptoms, have also been identified [1]. In up to 30% of cases, GBS progresses to respiratory failure, with patients requiring mechanical ventilation [2]. Therefore, it is critical for emergency physicians to rapidly recognize the signs and symptoms of this disorder for timely diagnosis and initiation of treatment. Most patients will require admission for expedited workup, initiation of immune-modulatory therapy, and monitoring for respiratory compromise. We report a case of an atypical presentation of GBS presenting with extremity paresthesias, dysarthria, and bilateral facial weakness in the absence of classic ascending limb weakness, and discuss differential diagnosis, triage, and management.
Case Report
A 38-year-old man presented to the Emergency Department (ED) with facial weakness, slurred speech, and bilateral hand and foot paresthesias. The patient had been in a state of good health upon receipt of the Johnson & Johnson Coronavirus disease 2019 (COVID-19) vaccine 32 days prior. Approximately 2 weeks after vaccination, the patient developed numbness and tingling in his tongue, lips, and bilateral hands and feet. Two days prior to presentation the patient began to develop difficulty moving his mouth and forming words, as well as difficulty drinking from a straw and controlling his lips, cheeks, and tongue while eating. Review of systems was notable for headaches, mild gait unsteadiness, generalized fatigue, and dyspnea on exertion. The patient denied diplopia, dysphagia, or dysphonia; chest pain or palpitations; lightheadedness; fevers or chills; nausea, vomiting, or diarrhea; urinary or bowel incontinence; and falls or other trauma. Past medical history was notable for anxiety and depression, for which the patient takes bupropion. The patient reported drinking, on average, 6–8 beers per day and using marijuana daily.
The patient presented in clinically good condition, not in acute distress and ambulating independently. Initial vital signs were as follows; blood pressure 218/80 mm Hg, heart rate 85 beats/min, respiratory rate 16 breaths/min, and oxygen saturation of 100%. The physical examination was notable for significant dysarthria and bilateral facial weakness in a lower-motor-neuron pattern. The patient exhibited weak eye closure bilaterally and inability to smile or puff cheeks against resistance. Extraocular movements were intact, and tongue was midline without fasciculations. Strength in the upper and lower extremities was intact (5/5), as was sensation to light touch throughout. There was no dysmetria or gait ataxia. Deep tendon reflexes were reduced, 1+ in the lower extremities and only faintly present in the bilateral upper extremities.
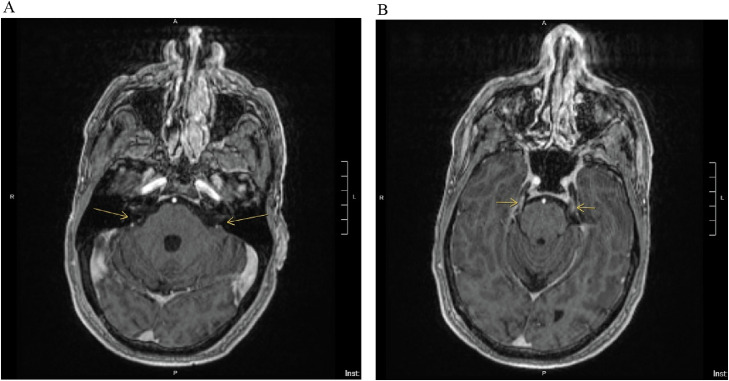
Laboratory studies were notable for a white blood cell count of 12.25 × 103/mL with a normal differential, a hemoglobin of 16.7 g/dL, and a hematocrit of 49.5 L/L. Serum chemistries including tests of liver function, thyroid-stimulating hormone, folate, and B12, were all within normal limits. Urine toxicology was positive for tetrahydrocannabinol, and COVID-19 polymerase chain reaction was negative. A Neurology consultation was obtained. Magnetic resonance imaging (MRI) of the brain with and without contrast and constructive interference in steady state (CISS) sequencing revealed focal enhancement of the bilateral internal auditory canal fundi and the bilateral cisternal segments of the trigeminal nerves (Figure 1 ). Cerebrospinal fluid (CSF) analysis revealed glucose of 73 mg/dL, an elevated protein to 181 mg/dL, and 7 nucleated cells/high-power field (HPF; corrected for 2987 red blood cells/HPF), constituting albuminocytological dissociation.
Figure 1.
The brain magnetic resonance imaging (MRI) constructive interference in steady state (CISS) sequence is useful for evaluating structures surrounded by cerebrospinal fluid, such as cranial nerves. In this brain MRI with and without contrast on the day of presentation, there is (A) evidence of focal enhancement (arrows) of the bilateral internal auditory canal fundi, which carry CN-VII and CN-VIII. There is also (B) bilateral enhancement of the cisternal segments of the trigeminal nerves (CN-V; arrows), suggesting focal inflammation, which may be seen in Guillain-Barré syndrome and other inflammatory or infectious conditions.
The patient was admitted and received intravenous immunoglobulin (IVIG) over 2 days to a total dose of 2 g/kg, with mild improvement of his symptoms. Measurements of negative inspiratory force were performed daily, and the patient did not develop any signs or symptoms of respiratory compromise. On hospital day 1, the patient developed new hyponatremia, which reached a nadir of 124 mEq/L during admission. This was concluded to be multifactorial, attributed to pseudohyponatremia in the setting of IVIG, as well as syndrome of inappropriate antidiuretic hormone in the setting of GBS. The patient was treated with fluid restriction and salt tabs, with improvement in sodium to 134 mEq/L on discharge. The patient's symptoms showed no further progression and he was discharged home on hospital day 4. The discharge examination was notable for stable-to-improving bilateral facial weakness and dysarthria with generalized areflexia.
Discussion
We describe the case of a 38-year-old man who developed subacute, rapidly progressive, bilateral hand and foot paresthesias, dysarthria, and bilateral facial weakness in the setting of recent COVID-19 vaccination. This was thought to represent an atypical variant of GBS, with absence of classic ascending limb weakness but presence of diminished reflexes on examination. Facial weakness is present as an associated symptom in as many as 70% of cases of GBS and is usually bilateral [2,3]. Atypical variants of GBS differ in presentation and severity and include pure sensory, pure motor, or autonomic variants [2]. The Miller-Fisher variant includes ophthalmoplegia, ataxia, and areflexia with minimal weakness. The pharyngeal-cervical-brachial variant causes dysphagia, cervical weakness, and proximal arm weakness. Our patient presented with the facial diplegia with acral paresthesias variant, which is characterized by isolated bilateral facial weakness and minimal, if any, extremity weakness [4,5].
Bilateral facial nerve palsy is an uncommon presentation with a limited differential diagnosis, including GBS, Lyme disease, Varicella-zoster virus, acute human immunodeficiency virus infection, or neurosarcoidosis [6]. Inflammatory or infiltrative disease involving the leptomeninges at the level of the brainstem (e.g., leptomeningeal carcinomatosis or tuberculous meningitis) can also present with facial diplegia, although more often in association with additional cranial neuropathies. A vascular etiology (e.g., pontine stroke involving bilateral cranial nerve [CN]-VII nuclei or fascicles) is very unlikely to cause isolated bilateral facial paralysis without other brainstem symptoms and signs [7]. Toxic or metabolic derangements including excessive alcohol use, as well as vitamin deficiencies, are on the differential for peripheral neuropathy, but also are very unlikely to present as isolated bilateral facial weakness.
In each of the infectious or inflammatory conditions on the differential above, CN-VII enhancement may be seen on MRI of the brain [8]. This finding was demonstrated on our patient's MRI CISS sequence, with enhancement of the bilateral internal auditory canal fundi, which carry CN-VII and CN-VIII (Figure 1). The CISS sequence can be particularly useful for evaluating structures surrounded by cerebrospinal fluid, such as cranial nerves [9,10]. The presence of albuminocytological dissociation within the CSF supports a diagnosis of GBS, and can be seen in 90% of cases, although it is not always present at the time of initial diagnosis [2]. CSF pleocytosis would point more toward alternative infectious or inflammatory etiologies. Nerve conduction studies can help confirm a diagnosis of GBS, demonstrating typical features of a demyelinating polyneuropathy. In this case, given the strongly supportive clinical presentation and CSF profile, electrodiagnostic evaluation was not indicated.
The most feared complication of GBS is respiratory failure [11]. Respiratory evaluation is of the highest importance when determining disposition in the ED, as patients who may require intubation or mechanical ventilation should be triaged to the intensive care unit [12]. Risk factors for mechanical ventilation include presence of facial nerve palsy, short interval from onset of symptoms to admission, and glossopharyngeal or vagal nerve deficits (i.e., poor cough and gag reflexes) [13]. Although pulse oximetry may be normal at initial presentation, it is insufficient to rule out respiratory compromise, as respiratory weakness may lead to hypercarbia without significant hypoxia. Instead, patients should be evaluated by measuring lung mechanics and negative inspiratory force, which can be serially monitored. Additional complications include hyponatremia, which our patient developed, as well as autonomic dysfunction. Hyponatremia is associated with GBS and is thought to be secondary to the syndrome of inappropriate antidiuretic hormone; it correlates with the severity of GBS and is an indicator of poor prognosis [14]. In the case of facial weakness, corneal inflammation from inability to close the eye(s) may be an additional complication.
Initial management of GBS includes rapid initiation of IVIG or plasmapheresis, which seem to be equivalent in efficacy and hasten the time to recovery [15]. Treatment should be in the inpatient setting with frequent monitoring, as progression of disease may require intensive care [11,12]. Most symptoms peak within 4–8 weeks. Weakness and paresthesias typically begin to recover after about 1 month, but it may take several months for patients to return to their premorbid baseline. Some patients will continue to experience sensorimotor deficits long term, and may benefit from physical, occupational, and speech language pathology therapy [2,16].
GBS is classically preceded by an acute diarrheal or respiratory illness in the weeks prior to presentation, but has also been associated with recent vaccination [17]. To date, there has been a small but statistically significant association found between influenza vaccination and GBS [18]. The risk of GBS is estimated to be in the range of one to two additional GBS cases per 1 million influenza vaccine doses administered [18]. Concerns have been raised linking GBS with the measles/mumps/rubella, human papillomavirus, and quadrivalent conjugated meningococcal vaccines; however, the association between vaccine administration and an increased incidence above chance of GBS has only been proven with the influenza vaccine [19].
In this case report, the patient received the Johnson & Johnson vaccine against the COVID-19 virus 32 days prior to presentation. In the Johnson & Johnson COVID-19 vaccine trial, one case of GBS in the post-vaccination arm was reported, however, there was also a case of GBS in the placebo arm. This does not imply a causal association between the Johnson & Johnson COVID-19 vaccine and GBS [20]. We cannot say definitively whether our patient's prior vaccination contributed to his development of GBS or was incidental to it. The risk of developing vaccine-associated GBS remains very low when viewed across the general population, and cases of GBS with a temporal association to vaccination are not necessarily caused by a vaccine-mediated immune response [20,21]. Overall, the benefits of vaccination to prevent COVID-19 infection likely outweigh the risks of any potential adverse effects, including GBS, as stated in recent guidelines from the Centers for Disease Control and World Health Organization 22, 23, 24.
Why Should an Emergency Physician Be Aware of This?
Guillain-Barré Syndrome is a rapidly progressive inflammatory polyneuropathy characterized by varying degrees of usually symmetric sensory or motor impairment and diminished or absent reflexes. Complications of GBS include respiratory failure requiring mechanical ventilation. It is important to recognize atypical presentations of GBS, such as our patient's case of paresthesias, dysarthria, and isolated bilateral facial weakness. GBS may occur without correlation to antecedent infectious illness or vaccination, although both remain important risk factors to elicit on history. Particularly in the context of a worldwide increase in vaccination, the emergency physician should keep atypical variants of GBS in mind when evaluating, triaging, and caring for patients with new neurologic symptoms or deficits.
References
- 1.Sheridan JM, Smith D. Atypical Guillain-Barré in the emergency department. West J Emerg Med. 2010;11:80–82. [PMC free article] [PubMed] [Google Scholar]
- 2.Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31:491–510. doi: 10.1016/j.ncl.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P, Charaniya R, Bahl A, Ghosh A, Dixit J. Facial diplegia with paresthesia: an uncommon variant of Guillain-Barre syndrome. J Clin Diagn Res. 2016;10:OD01–OD02. doi: 10.7860/JCDR/2016/19951.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susuki K, Koga M, Hirata K, Isogai E, Yuki N. A Guillain-Barré syndrome variant with prominent facial diplegia. J Neurol. 2009;256:1899–1905. doi: 10.1007/s00415-009-5254-8. [DOI] [PubMed] [Google Scholar]
- 6.Wakerley BR, Yuki N. Isolated facial diplegia in Guillain-Barré syndrome: bifacial weakness with paresthesias. Muscle Nerve. 2015;52:927–932. doi: 10.1002/mus.24887. [DOI] [PubMed] [Google Scholar]
- 7.Kim JK, Oh SY, Sohn EH, Hong YH, Jun SM, Bae JS. When is facial diplegia regarded as a variant of Guillain-Barré syndrome? J Peripher Nerv Syst. 2015;20:32–36. doi: 10.1111/jns.12115. [DOI] [PubMed] [Google Scholar]
- 8.Fulbright R, Erdum E, Sze G, Byrne T. Cranial nerve enhancement in Guillain-Barré syndrome. AJNR Am J Neuroradiol. 1995;16:923–925. [PMC free article] [PubMed] [Google Scholar]
- 9.Besta R, Shankar YU, Kumar A, Rajasekhar E, Prakash SB. MRI 3D CISS – A novel imaging modality in diagnosing trigeminal neuralgia – a review. J Clin Diagn Res. 2016;10:ZE01–ZE03. doi: 10.7860/JCDR/2016/14011.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romano N, Federici M, Castaldi A. Imaging of cranial nerves: a pictorial overview. Insights Imaging. 2019;10:33. doi: 10.1186/s13244-019-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang HL, Wu X, Zhu J. Complications of Guillain-Barré syndrome. Expert Rev Clin Immunol. 2016;12:439–448. doi: 10.1586/1744666X.2016.1128829. [DOI] [PubMed] [Google Scholar]
- 12.Shang P, Feng J, Wu W, Zhang HL. Intensive care and treatment of severe Guillain-Barré syndrome. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Li C, Zhang B, et al. Predictors for mechanical ventilation and short-term prognosis in patients with Guillain-Barré syndrome. Crit Care. 2015;19:310. doi: 10.1186/s13054-015-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saifudheen K, Jose J, Gafoor A. Guillain-Barré syndrome and SIADH. Neurology. 2011;76:701–704. doi: 10.1212/WNL.0b013e31820d8b40. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RAC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2012;(7) doi: 10.1002/14651858.CD002063.pub5. [DOI] [PubMed] [Google Scholar]
- 16.Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 17.Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58:1149–1155. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 18.Tokars JI, Lewis P, DeStefano F, et al. The risk of Guillain-Barré syndrome associated with influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines: results from self-controlled analyses. Pharmacoepidemiol Drug Saf. 2012;21:546–552. doi: 10.1002/pds.3220. [DOI] [PubMed] [Google Scholar]
- 19.Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain-Barre' syndrome. Vaccine. 2019;37:5544–5550. doi: 10.1016/j.vaccine.2018.05.119. [DOI] [PubMed] [Google Scholar]
- 20.Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021 April 6 doi: 10.1212/WNL.0000000000011881. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourdette D, Killestein J. Quelling public fears about Guillain-Barré syndrome and COVID-19 vaccination. Neurology. 2021;96:1021–1022. doi: 10.1212/WNL.0000000000011882. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Vaccines for COVID-19. Updated. May 23, 2021 https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html Available at. Accessed June 32021. [Google Scholar]
- 24.World Health Organization. COVID-19 advice for the public: getting vaccinated. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. Accessed June 3, 2021.