Abstract
The present pandemic has posed a crisis to the economy of the world and the health sector. Therefore, the race to expand research to understand some good molecular targets for vaccine and therapeutic development for SARS-CoV-2 is inevitable. The newly discovered coronavirus 2019 (COVID-19) is a positive sense, single-stranded RNA, and enveloped virus, assigned to the beta CoV genus. The virus (SARS-CoV-2) is more infectious than the previously detected coronaviruses (MERS and SARS). Findings from many studies have revealed that S protein and RdRp are good targets for drug repositioning, novel therapeutic development (antibodies and small molecule drugs), and vaccine discovery. Therapeutics such as chloroquine, convalescent plasma, monoclonal antibodies, spike binding peptides, and small molecules could alter the ability of S protein to bind to the ACE-2 receptor, and drugs such as remdesivir (targeting SARS-CoV-2 RdRp), favipir, and emetine could prevent SASR-CoV-2 RNA synthesis. The novel vaccines such as mRNA1273 (Moderna), 3LNP-mRNAs (Pfizer/BioNTech), and ChAdOx1-S (University of Oxford/Astra Zeneca) targeting S protein have proven to be effective in combating the present pandemic. Further exploration of the potential of S protein and RdRp is crucial in fighting the present pandemic.
Keywords: SARS-CoV-2, Spike protein (S protein), RNA dependent RNA polymerase (RdRp), Drug repositioning, SARS-CoV-2-vaccines
1. Introduction
Coronaviruses (CoVs) such as middle east respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV) are diverse classes of virus that infect animals and cause severe/mild respiratory diseases such as sore throat, runny nose, headache, chest pain, pink eye, fever, cough, tiredness, shortness of breath or difficulty of breathing, muscle aches, and chills [1], the MERS-CoV was discovered in 2012 [2], and SARS-CoV was discovered in 2003 [3], [4], [5]. The newly discovered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped, single-stranded, and positively-sensed virus. Genomic sequencing studies showed that SARS-CoV-2 and bat coronaviruses have similar (89%) DNA sequence. The CoVs are classified into Alphacoronavirus, Betacoronavirus, and Gammacoronavirus. The recently discovered SARS-CoV-2, together with the previously discovered MERS-CoV and SARS-CoV, are classified under Betacoronavirus – further classified into Viruses; Riboviria; Orthornavirae; Pisuviricota; Pisoniviricetes; Nidovirales; Cornidovirineae; Coronaviridae; Orthocoronavirinae; Betacoronavirus; Embecovirus. Also, Embecovirus, Hibecovirus, Merbecovirus, Nobecovirus, and Sarbecovirus are subgenera assigned to Betacoronavirus [4], [5]. These viruses are highly pathogenic CoVs raising 21st-century public health concern [6]. The disease (COVID-19) can be transmitted from human to human and animal to human by a droplet from coughing, sneezing, and direct contact [3], [7]. Like other CoVs, studies have shown that SARS-CoV-2 is sensitive to ultraviolet rays (UV) and heat, and its functionality can be inhibited too using 70% ethanol, 75% ether, and chlorine [8].
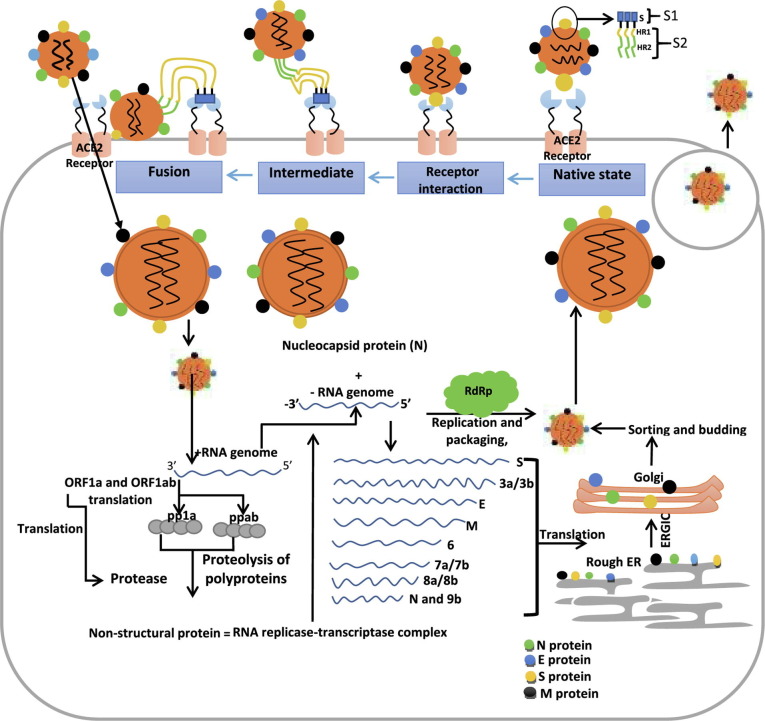
Interaction of SARS-CoV-2 with angiotensin-converting enzyme receptor 2 (ACE2) promotes host entry [9], then a cytoplasmic release of RNA, leading to translation of SARS-CoV-2 ORFab and ORF1a into polyprotein 1b (ppab) and polyprotein 1a (pp1a) respectively, then cleavage of its polyprotein to non-structural protein is carried out by an enzyme encoded by ORF1a (transcriptional complex driving the formation of negative RNA strand and structural protein synthesis) [10]. Through discontinuous transcription, the viral genomic DNA is fragmented into genome materials encoding S, M (membrane), E (envelope), and N (nucleocapsid) protein. The translation of the genomic material encoding the S protein is carried out in the rough endoplasmic reticulum to synthesize S protein. Again, in the endoplasmic reticulum, assembly of viral protein and genomic RNA is carried out to form virion [4], [5]. After the cellular invasion and pathogenic protein synthesis, the antigenic protein of SARS-CoV-2 binds to the central antiviral immunity called antigen-presenting cells. The major histocompatibility complex (MHC) presents the antigenic peptides followed by recognition by the cytotoxic T lymphocytes [11].
The RdRp and S protein are targets that could be explored to develop vaccines (some vaccines are currently developed encoding S protein) and repurpose existing antiviral drugs to fight the present pandemic. The S protein (relatively similar in both SARS-CoV-1 and SARS-CoV-2) allows SARS-CoV-2 to invade human cell after undergoing conformational changes leading from prefusion to post fusion conformation, resulting in binding and penetration into the human cell [9]. The RdRp of SARS-CoV-2 allows the virus to carry out RNA synthesis. A recent investigation by [12] has shown that the association of S protein with the ACE2 could be explored for drug and vaccine discoveries. Reported studies by [13] have shown that some of the investigated antiviral drugs such as Emetine (developed to inhibit RdRp) and umifenovir (developed to target viral entry) are proved to be safe and effective for the treatment of some viral infections, and they could be repurposed to target S protein and RdRp of SARS-CoV-2. To date, pharmaceutical companies and government are in the race of researching for effective drugs and vaccine development for COVID-19, and the World Health Organization (WHO) and many countries have encouraged the search for treatments to defeat the current pandemic [8]. However, this study reviewed research that is done on S protein and RdRp, towards vaccine, drug repurposing, and therapeutic development.
2. The role of S protein and RdRp in host infection
The S protein is the virulent target that mediates viral entry into the host and infection ( Fig. 1 ). This protein is a trimeric and transmembrane protein present in human coronaviruses, human immune deficiency virus (HIV), influenza virus, paramyxovirus, and Ebola virus. The virulent protein mediates attachment to the cell membrane, recognition of ACE2 receptor, and fusion during infection [13], [14]. Previous studies by [8], [9] reported that the S protein trimer forms the basic unit by which S protein binds to the receptor. Recently, [15], [16] highlighted that the S1 (mediating ACE2 recognition) and S2 (mediating fusion and entry) are division assigned to S protein (a glycosylated polypeptide existing in closed prefusion conformation), each playing a crucial role in SARS-CoV-2 infection. The S1 with the receptor-binding domain (RBD) participates in an interaction with the ACE2 receptor. While the S2 containing the HR domain (divided into HR1 and HR2) participates in the viral fusion [17]. An investigation by [18]revealed that the RdRp mediates the formation of replication machinery in the genome of SARS-CoV-2 by associating with replication factors. The elongation of the RNA strand is initiated and directed by RdRp through the addition of nucleotides. The RdRp using nucleotide analogue also catalyzes the halt of RNA elongation by inserting them into the new RNA chain that is being synthesized. The de novo (use of nucleotide by RdRp for RNA synthesis without the need of primers) and primer dependent synthesis (use of primers by RdRp to synthesize RNA by relying on short oligonucleotides) are the two modes of RNA synthesis used by SARS-CoV-2 [19]. Therefore, a better understanding of RdRp and S protein in the mediation of infection could lead to the development of novel vaccines and drugs for COVID-19.
Fig. 1.
An overview of the role of S protein and RdRp of SARS-CoV-2 in host infection.
3. SARS-CoV-2-S protein structure
The S protein of coronaviruses has a size of 180–200 kDa [12], [13] and a total length of 1,273 amino acids (in which signal peptide located in the N-terminus comprises of amino acids 1 to 13, S1 subunit comprises 14–685 amino acids, and 686–1,273 amino acids make up the S2 subunit) [16]. The S1 subunit is divided into domains such as the N-terminal domain (comprising of 14–305 amino acid residues) and RBD (with 319–541 amino acid residues); while within the S2 subunit, there are domains such as fusion peptides domain (comprising of 788–806 amino acid residues), heptapeptide repeated sequence 1 domain (with 912–984 amino residues), heptapeptide repeated sequence 2 domain (comprising of 1,163–1,213 amino acid residues), a transmembrane domain of 1,213–1,237 amino acid residues, and a cytoplasmic domain which starts with 1,237 amino acid residues and ends up with 1,273 residues [20]. Also, the 3D Receptor Binding Domain (RBD) of SARS-CoV-2 (located in the S1 subunit) has a size of ~ 30 kDa. Previous studies by [21] revealed that the S protein architecture consists of the extracellular domain localized in the N terminal, transmembrane domain, and a metastable and prefusion stage intracellular domain segment, localized in the C-terminal. Also, a report from [22] highlighted that a crown like a halo adjoining the SARS-CoV-2 particle is formed by the trimers of the S protein. In an investigation by [23], the viral interaction with a host receptor could lead to structural rearrangement of the S protein which mediates cell membrane fusion by SARS-CoV-2. Even though there is a mutation in the S protein of SARS-CoV-2, but there still exists an interaction between RBD of S protein and ACE2; this indicates that S protein is a target to prevents viral entry [24].
The S protein of SARS-CoV-2 is highly glycosylated, the 22 N-glycosites and 2 O-glycosites per monomer are glycosites found in the trimeric structure of the S protein, where the RBD region has the 2 O-glycosites and 2 N-glycosites, and the N-glycosites (N122 and N343) are conserved. Even though glycosylation is specific to cell, its role in host protein interaction and mediation of infection in the epithelial airways is not fully understood. However, other studies reveal that the coated polysaccharide (localized on S proteins) helps the virus to avoid the immune system.
Cryo-electron microscopy (Cryo-EM) revealed the trimeric structure of S protein, its functions, together with the open and closed conformation of the RBD [8], [25]. The S protein of CoVs exists as an inactive form precursor in its native state [17], [29]. Also, the S protein is cleaved by a protease enzyme into S1 and S2 during infection [27], which is essential in the activation of the domain responsible for fusion [28]. The cleavage of S protein into S1 and S2 is done through the action of cellular protease using TMPRSS2 serine protease as a primer [29]. Recently, Cryo-EM studies by [26] revealed that a breath of S1 domain was observed (using cryoSPARC v2) after a hinge-like movement of the RBD, which is attributed to the poor resolution of S1 domain in comparison to S2 domain, the observation of the same features during the structural characterization of MERS-CoV and SARS-CoV have shown that SARS-CoV-2-S protein has the same mechanism of triggering infection with that of MERS-CoV and SARS-CoV. However, [26] pointed that there is a difference in the structure of the RBD of SARS-CoV-2 and SARS-CoV, in which the position of the RBD of SARS-CoV-2 is in the down conformation, which forms an angle with a cavity, and that of SARS-CoV is opposite to the N-terminal of the neighbouring promoter which is packed in the down conformation. Both SARS-CoV-2 and SARS-CoV can bind to ACE2 receptor (as investigated using surface plasmon resonance) with different affinities, the binding affinity of SARS-CoV-2 (~15 nM) is higher than that of SARS-CoV, which accounts for its easy transmission from human to human [26]. Furthermore, [26] highlighted that high-resolution CryoEM revealed the formation of the complex by the ectodomain of SARS-CoV-2 with the human host ACE-2 receptor, which is the same as the complex formed by the previously discovered SARS-CoV. Reported studies by [8], [18] investigated that the S protein undergoes structural rearrangement before fusion with the ACE2 receptor. The rearrangement is turned on by the S1 domain binding to the ACE2 receptor, followed by the destabilization of the prefusion trimers; this leads to the formation of post-fusion conformation that is highly stable. Then upon passage of the RBD through a hinge-like movement, the ACE-2 receptor can either be accessible or inaccessible. No previous studies have investigated the design of new viral entry inhibitors based on the understanding of hairpin structure trimers. Little is known about exploring the RBD of the S protein for the development of monoclonal antibodies for SARS-CoV-2.
4. Structure of RdRp
The viral genome encodes RdRp that mediates replication and gene transcription in association with transcription factors. The folding of RdRP appears to be a right-handed thumb complete with palm and fingers domain. The catalytic motifs (five of seven) are localized in the palm domain, which are highly conserved, while the F and G catalytic motifs are localized in the finger's domain [10], [20], [21]. Studies by [30] revealed that the catalytic function of RdRp’s are mediated by RdRP core and related motifs. Therefore, no single studies have explored RdRp core and related motifs for therapeutic development. All RdRp’s have the same mechanism of action even though the substrate requirement differs. The structure of RdRp consists of the nsp7-nsp8 heterodimer, nsp8 subunit, and nsp12 core catalytic unit [31]. Interestingly, structural similarities exist among the RdRp of SARS-CoV-2 and SARS-CoV. Therefore, repurposing drugs that target RdRp of SARS-CoV can be useful in fighting the SARS-CoV-2 pandemic. Previous investigation by [32] disclosed that the b-hairpin nidovirus-specific extension domain is localized in the nsp12 N-terminal. The palm subdomain and nidovirus RdRp associated nucleotidyl transferase domain (NiRAN) sandwiches the b-hairpin, this configuration is not present in the structure of SARS-CoV-2 RdRp. The nsp12 C terminal catalytic domain is connected to NiRAN via interface subdomain [33].
The SARS-coV-2 RdRp active site forms A to G conserved catalytic motifs, in which the palm domain carries the A to E motifs, while F to G motifs are localized in the finger subdomain, the DX2-4D (with conserved aspartic acid) catalytic motif is housed in the catalytic motif A. [34], the hinge that mediates conformational arrangement in association with an RNA template and substrate binding is called flexible loop, which is located in Motif B [35], the catalytic motif SDD is located in the C motif, which plays a crucial role in iron-binding. Recently, the investigation of the RdRp structure revealed that the D760 and D761 are involved in magnesium iron coordination during catalysis [36]. The regulation of catalytic activity is mediated by the conserved catalytic motif which is in the SDD region. The interaction of phosphate from incoming NTP is mediated by the F motif. The G motif mediates interaction with the template strand and directs the achievement of the catalytic site by the RNA template [31].
Recent report by [37] revealed the in silico binding of some clinically approved drugs (ribavirin, tenofovir, hydroxychloroquine, sofosbuvir, remdesivir, galidesivir, cefuroxime, and favipiravir) to RdRp, using a dynamics simulations, docking, and molecular modelling. Investigation by [38] using a computational method revealed that the protein (RdRp) is a potential target for the repurposing of drugs that are either in the market or under clinical trial. Their studies showed that some drugs could bind to RdRp and effectively terminate its replication. Moreover, the computational methods for the identification of new possible drugs need to be validated in vitro (based on re-docking approaches). Furthermore, other computational studies investigate the potential of some antivirals and natural compounds in the inhibition of SARS-CoV-2-RdRp, where they found that adefovir, zanamivir, and adefovir antivirals are good potential candidates of RdRP inhibition, while natural compounds such as piperine, demethoxycurcumin, and curcumin could potentially bind to RdRp with low affinity [33], [35], [36], [37], [38]. These computational studies need to be considered with caution. Indeed, recent studies proved that remdesivir is not efficient as initially retained [32]. There has been little research in the investigation of the mechanism of RdRp inhibition by remdesivir, and this can be a new area of research [39].
5. Drugs repositioned to target SARS-CoV-2-S protein and RdRp
Many efforts are ongoing by several research groups of scientists to develop effective therapeutic interventions against the SARS-CoV-2 since the discovery of the virus in late December 2019. Previous medicinal chemistry studies on COVs have accelerated these efforts [27], [28]. Despite the diverse nature of CoV viruses, their genomic fundamentals are similar and thus essential for the identification of therapeutic targets [40]. Genetic sequencing has revealed that both SARS-CoV-2 and SARS-CoV belong to the same family of single-stranded enveloped RNA viruses [37], [38]. Owing to the many similarities between SARS-CoV, MERS-CoV, and SARS-CoV-2, it is only rational to try drugs that have shown some level of activity previously on SARS-CoV and MERS-CoV [41]. Key enzymes responsible for virulence, attachment to host cells, and replication are significant targets for developing new drugs [30], [31]. It has been shown that molecules that could inhibit the interaction between SARS-CoV-2-S protein and ACE2 receptor and other key viral enzymes essential for RNA synthesis and replication such as RdRp will serve as valuable targets in developing treatments for the novel virus [6], [32].
As for developing drugs for novel coronaviruses, scientists employ at least three different strategies [33], [42]. The first is to randomly test broad-spectrum antiviral agents on the novel pathogen. The second is the repurposing or repositioning of existing drugs meant for other therapeutic purposes and lastly is to develop drugs from scratch using available genomic information and pathological data of known coronaviruses [33], [35], [43]. With each method having its pros and cons, the combination of the three approaches is often utilized by scientists during CoVs outbreaks to identify potential drug candidates [40]. Different classes of drug agents that have displayed some level of potentials in silico, in vitro, or clinically against the novel coronavirus will be examined. Some of the drugs described have activity against other virus families or have already been used clinically against other viral pathogens, indicating potential broad-spectrum applications. These drugs can further be explored by other researchers owing to the potentials exhibited through prior studies.
5.1. Chloroquine
Drug repositioning has been initiated in recent years; drugs proven to be harmless with well-known optimal dosage and pharmacokinetics are important candidates for drug repurposing towards fighting the present pandemic [44]. Chloroquine synthesis was carried out in 1943, with the potential of treating malaria and rheumatoid arthritis. In 1955, hydroxychloroquine was developed and became a better candidate in place of chloroquine. The DNA interaction and heme polymerization inhibition are thought to be the mechanism of action of chloroquine and hydroxychloroquine [45]. The synthesis of hydroxychloroquine was done from chloroquine by introducing a hydroxyl group into a chloroquine structure, which is less toxic and safer than chloroquine [46]. Recently, [47] investigated the effect of chloroquine on primate cells infected with SARS-CoV. The effect of the drug was examined after and before infection with the virus (SARS-CoV-2), suggesting the possibility of both prophylactic and therapeutic potential. The terminal glycosylation of the ACE-2 receptor could be possibly terminated by chloroquine, which could possibly be the means of altering the viral entry. The elevation of endosomal pH by chloroquine could possibly contribute to affecting the growth of SARS-CoV. Recently, [47] investigated the potential of chloroquine to alter the replication of SARS by an in vitro study using indirect immunofluorescence. Their studies found that 10 µM of chloroquine could inhibit SARS-CoV replication. A recent report from [48] revealed the comparison of the effectiveness of chloroquine to hydroxychloroquine, with chloroquine being a more possible inhibitor of SARS-CoV than hydroxychloroquine in vitro in a Vero cell model. In contrast, the effectiveness of chloroquine in the inhibition of SARS-CoV-2 is still being debated. Recent studies by [49] reported that Vero cells expressing engineered TMPRSS2 (the protease enzyme involved in the activation of SARS-CoV-2 entry into the lung cells) makes the virus not sensitive to chloroquine. Hence, the target of chloroquine is the viral activation pathway, in which such pathway is inactive in lung cells. This accounts for its reduced ability to offer protection from the viral transmission.
5.2. Chlorpromazine
Chlorpromazine is a first-generation antipsychotic used for treating acute psychosis, schizophrenia, and bipolar disorder [50]. The idea to repurpose this drug to target SAR-CoV-2 stems from the observation relating a lower incidence of symptomatic COVID-19 disease in patients in psychiatric wards [51], leading to the hypothesis that psychotropic drugs administered to these patients may serve as prophylaxis for the SAR-CoV-2, granting immunity against virulent strains. Chlorpromazine has been recorded to have inhibitory activity against coronaviruses and could inhibit replication of MERS-CoV and SARS-CoV-1 [52], [53] by targeting clathrin-dependent endocytosis which is essential for viral invasion, replication, and colonization [54]. The genomic similarity of SAR-CoV-2 to the two afore-mentioned coronaviruses makes it plausible to consider chlorpromazine repurposing for the potential treatment of COVID-19. One major feature of advantage is the distribution channel of the drug within the body. Studies show that the highest concentrations are found in the lungs [55] and saliva [56] than in blood and as COVID-19 primarily manifests following initial lung infestation by the virus and mostly spreads due to high viral loads in body fluids like saliva, this biodistribution is important for reducing grave symptoms and limiting person-person transmission.
5.3. Hormone receptor antagonists
The attachment of SAR-CoV-2 spike protein with cells is accelerated by transmembrane protease serine 2 (TMPRSS2) through the androgen receptor signalling pathway [57] and this has been linked to variations in individual susceptibility, especially along the lines of age, as well as gendered disparity in mortality [58]. Compounds that inhibit androgen receptors are, therefore potentially beneficial for treating COVID-19. Malignant tumours are often characterized by high concentrations of androgen receptors and certain hormone receptor-amplified types of breast cancer tend to possess higher concentrations [59]. Tamoxifen is a drug used to treat hormone receptor-positive breast cancers as it prevents the binding of androgens to their receptor by binding there itself [60] and this makes it a potential drug candidate for the management of COVID-19. Toremifene is another drug candidate for SAR-CoV-2 treatment. It is a first-generation selective estrogen receptor modulator (SERM) and is currently used for the treatment of breast cancer [61]. While, unlike Tamoxifen, it does not appear to have direct receptor inhibitory properties, recent research has found that Toremifene has the potential to interact with SAR-CoV-2 spike glycoprotein in a manner that distorts the secondary structure, thus inhibiting its activity [62] and a related study found it capable of SARS-CoV-2 inhibition with an EC50 02.5 µM [63].
5.4. Remdesivir
Remdesivir (a broad-spectrum drug targeting viral replication) is currently under investigation towards repositioning. Gilead is the first pharmaceutical company that synthesized remdesivir for the treatment of Ebola. The drug is metabolized in its active form, which can lead to a reduction in the synthesis of viral RNA. The drug delayed chain cessation of nascent viral RNA as a means of terminating viral replication. The investigation of the antiviral activity of remdesivir using cell-based assay has revealed the activity of the drug on a rhesus monkey model infected with the Ebola virus [64]. Also, the possibility of in vitro inhibitory effect of remdesivir was observed in primary human epithelial cell culture infected with SARS-CoV-2. The drug has shown potential in the inhibition of human and zoonotic coronaviruses such as SARS-CoV, related bat coronaviruses, MERS-CoV, HCoVOC43, mouse hepatitis virus, coronavirus HKU5, HCoV-229E, and HCoV-NL63 [64]. An investigation has shown that intravenous treatment of rhesus monkey with 10 mg/kg of remdesivir (with an intracellular half-life of 14 h) can lead to 24 h presence of an active form of remdesivir (trisphosphate form) in the peripheral blood mononuclear cells and this could be applicable in SARS-CoV-2 infected animal species. According to [65], randomization of 1,062 patients (521 placebo and 541 remdesivir treated for 10 days), remdesivir treated patients recovered in 10 days, while placebo-treated group recovered in 15 days. In May 2020, the drug remdesivir was given emergency approval by the FDA. In the same month, two randomized clinical trials (one by Wang and Colleague and the other one by the National Institutes of Health-sponsored Adaptive COVID-19 Treatment Trial) took place to compare remdesivir with a placebo. The trial of Wang and his colleague with 237 patients failed to show any efficacy. While the trial of the institute recruited 1,063 patients (treated with remdesivir for 10 days) and observed a recovery period that is shorter by 4 days compared to the placebo-treated group. After the approval of remdesivir by the FDA, there have been considerable debates to ensure adequate, equitable, and affordable access to remdesivir as a result of the huge demand by patients and physicians [66]. However, a recent report by [67] highlighted against the use of remdesivir, as there is controversy in whether it improves survival and other outcomes (such as improving mortality rate, reduction in the need of ventilators etc.) in COVID-19 patients. This is a conditional recommendation against its use by WHO. Furthermore, recent news in BMJ [68] reported that the largest trial of remdesivir showed that it is not beneficial in reducing the disease course or mortality rate. The drug is too expensive to afford (costing $2,600 for 10 days intravenous infusion) and the disease is not rare. Therefore, many nations cannot afford the therapy. According to WHO, there is a need for the continued search for new antivirals, anti-SARS-CoV-2 monoclonal antibodies, and immune modulators.
5.5. Favipiravir
Favipiravir is another broad-spectrum antiviral that has demonstrated in vitro activity against many RNA viruses. It also acts as an inhibitor of RdRp [69], affecting the replication of a significant number of RNA viruses such as influenza A virus, flaviviruses, noroviruses, yellow fever virus, Ebola virus, and Lassa virus [70]. The drug inhibits replication moderately in Vero E6 cells when its efficacy against SARS-CoV-2 was tested [71]. Recently, Wang and colleagues highlighted the efficacy of favipiravir in reducing SARS-CoV-2 infection with a half-maximal effective concentration (EC50) of 61.88 mM, which similar to its EC50 against Ebola Virus Disease (67 mM)[72]. Overall, from clinical studies in COVID-19, when compared to lopinavir/ritonavir (LPV/RTV), favipiravir has shown a faster viral clearance rate and higher recovery rate when compared to umifenovir [72].
5.6. Emetine
The treatment of amebiasis can be done with Emetine (an FDA approved drug). This drug has the potential of causing toxicity of the heart, which restricted its utilization for the treatment of amebiasis. According to [73], some phytocompounds and antiviral drugs approved by FDA were tested for binding to RdRp, in which drugs such as Paritaprevir, Rilpinvirine, Simeprevir show affinity to RdRp with the binding energy ranging from −8.08 kcal/mol to −10.46 kcal/mol. Among the top phytocompounds, drug candidates that show affinity to RdRp are Oleanolic acid, 7,4-di-O-galloyltricetifavan, and Emetine, with a binding energy of −7.81 kcal/mol to −8.17 kcal/mol. The interaction between these drugs and RdRp is mediated by hydrogen and hydrophobic interaction. Therefore, investigation in vitro has shown the potential of Emetine to halt the replication of SARS-CoV-2, hCoV-OC43, MERS-CoV, hCoV-NL43, and MHV-A59 [74], [75]. Upon the emergence of SARS-CoV-2 infection, the therapeutic potential of Emetine was examined in vitro, which shows that at cytotoxic concentration 50 (CC50) of 1,603.8 nM and at 50 (EC50) of 0.147 nM, Emetine has a therapeutic index of 10910.4. Treatment with Emetine could decrease the replication of virus by decreasing the synthesis of RNA and protein. Emetine could disrupt the binding of the eukaryotic translation initiation factor (a cap-binding protein needed for translation) with SARS-CoV-2 RNA in an investigation using chromatin immunoprecipitation assay [73], [76].
5.7. Simeprevir
This drug is approved by FDA for the treatment of patients with Hepatitis C Virus (HCV) genotype 1, the drug is designed to inhibit the protease, and it is regarded as a second-generation macrocyclic inhibitor of protease. Simeprevir binds specifically to NS3 protease; this is an advantage over the first-generation protease inhibitor [77], [78], [79], [80]. Interestingly, remdesivir could act synergistically with simeprevir, and it could inhibit the activity of Mpro protease and RdRp based on evidence from molecular and biochemical characterization studies [81].
5.8. Lumacaftor
Lumacaftor is an orphan drug used in combination with ivacaftor to manage cystic fibrosis (CF) cases [82], [83]. CF, an autosomal recessive disorder, is caused by genetic mutation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein causes an autosomal recessive disorder known as cystic fibrosis [82], [84]. Lumacaftor corrects mutation in the gene of CFTR protein by trafficking mutant protein to the plasma membrane in cystic fibrosis patients [84]. With a half-life of about 26 h in CF patients and reaching peak plasma concentrations 4 h after multiple oral ingestions, lumacaftor is well absorbed from the gastrointestinal tract with a 99% plasma-protein binding. It acts as a protein-folding chaperone, increasing the processing and trafficking of mature protein to the cell surface by improving F508del-CFTR conformational stability. Lumacaftor is poorly metabolized, with more than half (over 50%) of it excreted unchanged [85], [86], [87]. In search of therapeutics to curtail COVID-19, repositioning of existing drugs is a prime lead focus for the search. Lumacaftor has been reported to inhibit SARS-CoV-2 by targeting main protease (3CLpro) and Nsp13 helicase [88], [89], [90]. It is also reported to have a high-affinity binding to the receptor-binding domain (RBD) of Spike (S) protein, thereby preventing SARS-CoV-2 interaction with angiotensin-converting enzyme-2 (ACE-2) [91], [92]. In particular, lumacaftor forms hydrophobic bond to Leu455 and H-bonds to Lys417 and Gln493 – which are responsible for SARS-CoV-2 high affinity to ACE2 – thereby inhibiting S glycoprotein binding to ACE-2 [93].
5.8. Phytochemicals and natural products
The glycosylation process of the S protein could be inhibited by α-glucosidase 1 and α-glucosidase 2, resulting in the incorrect formation of glycoform, which will lead to ER-associated degradation, altering of protein–protein interaction, maturation inhibition. The unavailability of α-Glucosidase in the pneumocytes is evidence of its potential as an anti-SARS-CoV-2. An investigation using deep mutational scanning revealed that N343 glycosylation is important in the expression of RBD [91]. Also, evidence from computational simulation revealed that N-glycans (N165 and N234) in mediating the binding of S protein to ACE2 receptor [24]. Recent investigation revealed that monoterpenes such as 1,8-cineole and camphor, together with diterpenes (patchouli and carnosic) and triterpenes (ursolic acid), could potentially inhibit the entry and replication of SASR-CoV-2. The mechanisms used by these metabolites in the inhibition of SARS-CoV-2 infection are still unknown [94]. An alkaloid extracted from Stephania called Cepharanthine (used for snake bites, alopecia, and leukopenia) have shown to possessed antiviral efficacy [94], [95]. The cancer and autoimmune drug called Thioguanine (with IC50 of 1.7 μM), after transformation to thioguanosine triphosphate, could potentially inhibit the GTP-binding protein (related to replication and DNA synthesis) and RdRp. Molecular docking investigation revealed that thioguanosine trisphosphate binds well with viral RdRp, but in vitro studies have shown that thioguanosine triphosphate did not exhibit the ability of RdRP inhibition. However, other studies revealed that thioguanosine triphosphate binds slowly and reversibly to PLpro of SARS-CoV-2 and SARS-CoV-2. Therefore, the potential of thioguanine in the inhibition of SARS-CoV-2 replication is a new area of research [96]. Until recently, there has been no reliable evidence that plant metabolite can alter the activity of S protein, there is a need for researchers to fully explore plant metabolites (Alkaloids, diterpneoid, anthraquinones, glycosides, flavonoids, Natural lupane triterpenoids, saponins, phenols, Tannins, gallic acid, flavonoids like quercetin and quercitrin, phenolics, Tannins, diterpenes, Saponins, triterpenes, terpenoids, sugar bearing compound, protein, thiols, etc. [96]) that can block the viral entry, especially from a plant with proof of antiviral properties for a potential chemical model for a cure against the novel SARS CoV-2 (Table 1 ).
Table 1.
Potential inhibitors of SARS-CoV-2 virus.
| Drug | Class and point of inhibition | Antiviral effects exhibited in studies | Type of study | Reported MOA | Ref |
|---|---|---|---|---|---|
| Antibodies | Biologic; Cellular entry | SARS-CoV; SARS-CoV-2; MERS-CoV | In vivo | Neutralizes viruses by preventing the binding of viral spike protein to ACE2 on host cells | [71], [103], [115], [116] |
| Chloroquine | Antimalarial; Cellular entry | SARS-CoV; SARS-CoV-2; MERS-CoV | In vitro/In vivo | RdRp and ACE2 cellular receptor | [117] |
| Hydroxychloroquine | Antimalarial; Cellular entry | SARS-CoV; SARS-CoV-2; MERS-CoV | In vitro/In vivo | RdRp inhibitor also targets endosomes and cause pH elevation | [70] |
| Remdesivir | Antiviral; Replication | SARS-CoV; SARS-CoV-2; MERS-CoV; EVD, | In vitro/In vivo | Binds to RdRp which affects RNA production and ultimately putting a halt to viral replication | [118], [119] |
| Favipiravir | Antiviral; Replication | SARS-CoV; SARS-CoV-2; MERS-CoV; EVD, Norovirus; | In vitro/In vivo | RdRp Inhibitor | [70] |
| Chlorpromazine | Anti-psychotic; Viral entry | SARS-CoV; SARS-CoV-2; MERS-CoV | In vivo/in vitro | Inhibition of clathrin-mediated endocytosis | [120] |
| Curcumin | Natural compound; Replication | SARS-CoV-2 | In silico | Acts by interfering with vital steps in replication cycles such as viral attachment and genome replication | [121], [122] |
| Emodin | Natural compound; Cellular entry | Hinders the interaction of SARS-CoV S protein with ACE2 which is essential for entry into the host cell | [70] | ||
| Theaflavin | Natural compound; Replication | SARS-CoV; MERS-CoV; SARS-CoV-2 | In silico/In vitro | May inhibit RdRp | [123] |
| Emetine | Natural compound; Replication | SARS-CoV-2; Zika virus; EBOV; Dengue virus; Human CMV | In vitro/In vivo | Acts through viral RdRp inhibition | [41] |
| Simeprevir | Antiviral; Cellular entry | MERS-CoV; SARS-CoV-2 | In silico | Binds RDB with high affinity and prevent ACE2 interaction | [93] |
| Hormone receptor antagonists | Anti-cancer; Cellular entry | SARS-CoV-2 | Interaction with S protein and distort it secondary structure | ||
| Lumacaftor | CFTR corrector; Cellular entry | SARS-CoV-2 | In silico | Attaches to the RBD of CoV with high affinity and prevent ACE2 interaction | [93] |
| Phytochemicals and natural products | Natural compounds; Cellular entry | SARS-CoV-2, SARS-CoV, MERS-CoV | In silico | Altering the interaction of ACE2 receptor with S protein | [96] |
6. Vaccines encoding spike protein
With the current outbreak of COVID-19, there is a strong need for the synthesis, distribution, and administration of the SARS-CoV-2 vaccines to safeguard public health. The scientific efforts to make this a reality has witnessed tremendous contributions from over 40 pharmaceutical companies and academic institutions globally. The development of not less than 321 vaccines has been recorded to be in progress as of October 2020, approximately more than twice as of April, indicating a significant advancement [97]. A total of 56 vaccines has undergone clinical trials (clinical trial phase 1 to 3) as of November 2020. However, the WHO reports a total of 280 vaccine candidates, with 97 COVID-19 vaccine candidates currently at different phases of clinical trials and 183 vaccine candidates at preclinical trials as of May 7, 2021 [98].
From preclinical studies to vaccine development against SARS-CoV-2, the antigenic target for coronavirus vaccines has become clear [99]. The S (host entry mediator) protein appears to be the target for a vast number of coronaviruses [29], [100]. The viral genome is synthesized into the cytoplasm after entry [101]. Antibodies binding to the S protein serves as a building block for vaccine development [102]. The S protein-host-cell interface also serves as the main target of neutralizing antibodies and the focus of therapeutic and vaccine design. Vaccine development based on the S1 subunit of SARS-CoV-2 appears to be a promising approach [103]. The rigorous process of vaccine development takes a reasonable amount of effort and time before its approval for mass production, distribution, and administration. The availability of information on existing vaccine candidates in addition to the animal models from SARS and MERS expedited the regulatory processes owing to the inordinate and urgent need for efficient vaccines throughout the world. The protective ability of the vaccine is dependent upon the vaccine target and platform. In the last decade, there has been a significant evolution in the area of vaccine technology [104]. Several platform technologies (Table 2 ) including RNA replicons, plasmid-based DNA vaccines, subunit vaccines, live-attenuated vaccines, inactivated viral vaccines, killed whole virus vaccines, and the virus-like particle has been produced to provide immunity against diseases [45].
Table 2.
Some of the recently developed vaccines for COVID-19.
| Vaccine platform acronym | Vaccine platform description | Type of candidate vaccine | No. of doses | Schedule | Route of administration | Developers | Phase |
|---|---|---|---|---|---|---|---|
| IV | Inactivated virus | CoronaVac; SARS-CoV-2 vaccine (inactivated) | 2 | Day 0 + 14 | IM | Sinovac Research and Development Co., Ltd | Phase 4 |
| IV | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 | Day 0 + 21 | IM | Sinopharm + China National Biotec Group Co., + Wuhan Institute of Biological Products | Phase 3 |
| IV | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell), vaccine name BBIBP-CorV | 2 | Day 0 + 21 | IM | Sinopharm + China National Biotec Group Co., + Beijing Institute of Biological Products | Phase 3 |
| VVnr | Viral vector (Non-replicating) | ChAdOx1-S - (AZD1222) (Covishield) | 1–2 | Day 0 + 28 | IM | AstraZeneca + University of Oxford | Phase 4 |
| 1–2 | Day 0 + 28 | IN | University of Oxford | Phase 1 | |||
| VVnr | Viral vector (Non-replicating) | Recombinant novel coronavirus vaccine (Adenovirus type 5 vector) | 1 | Day 0 | IM | CanSino Biological Inc./Beijing Institute of Biotechnology | Phase 3 |
| VVnr | Viral vector (Non-replicating) | Gam-COVID-Vac Adeno-based (rAd26-S + rAd5-S) | 2 | Day 0 + 21 | IM | Gamaleya Research Institute; Health Ministry of the Russian Federation | Phase 3 |
| VVnr | Viral vector (Non-replicating) | Ad26.COV2.S | 1–2 | Day 0 or Day 0 + 56 | IM | Janssen Pharmaceutical | Phase 3 |
| PS | Protein subunit | SARS-CoV-2 rS/Matrix M1-Adjuvant (Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M) | 2 | Day 0 + 21 | IM | Novavax | Phase 3 |
| RNA | RNA based vaccine | mRNA −1273 | 2 | Day 0 + 28 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 4 |
| RNA | RNA based vaccine | BNT162b2 (3 LNP-mRNAs), also known as “Comirnaty” | 2 | Day 0 + 21 | IM | Pfizer/BioNTech + Fosun Pharma | Phase 4 |
| PS | Protein subunit | Recombinant SARS-CoV-2 vaccine (CHO Cell) | 2–3 | Day 0 + 28 or Day 0 + 28 + 56 | IM | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Phase 3 |
| RNA | RNA based vaccine | CVnCoV Vaccine | 2 | Day 0 + 28 | IM | CureVac AG | Phase 3 |
| IV | Inactivated virus | SARS-CoV-2 vaccine (vero cells) | 2 | Day 0 + 28 | IM | Institute of Medical Biology + Chinese Academy of Medical Sciences | Phase 3 |
| IV | Inactivated virus | QazCovid-in® - COVID-19 inactivated vaccine | 2 | Day 0 + 21 | IM | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Phase 3 |
| DNA | DNA based vaccine | INO-4800 + electroporation | 2 | Day 0 + 28 | ID | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | Phase 2/3 |
| DNA | DNA based vaccine | AG0301-COVID19 | 2 | Day 0 + 14 | IM | AnGes + Takara Bio + Osaka University | Phase 2/3 |
| DNA | DNA based vaccine | nCov vaccine | 3 | Day 0 + 28 + 56 | ID | Zydus Cadila | Phase 3 |
| DNA | DNA based vaccine | GX-19N | 2 | Day 0 + 28 | IM | Genexine Consortium | Phase 1/2 |
| IV | Inactivated virus | Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) | 2 | Day 0 + 14 | IM | Bharat Biotech International Limited | Phase 3 |
| PS | Protein subunit | KBP-COVID-19 (RBD-based) | 2 | Day 0 + 21 | IM | Kentucky Bioprocessing Inc. | Phase 1/2 |
| PS | Protein subunit | VAT00002: SARS-CoV-2 S protein with adjuvant | 2 | Day 0 + 21 | IM | Sanofi Pasteur + GSK | Phase 3 |
| RNA | RNA based vaccine | ARCT-021 | ND | ND | IM | Arcturus Therapeutics | Phase 2 |
| VLP | Virus like particle | RBD SARS-CoV-2 HBsAg VLP vaccine | 2 | Day 0 + 28 | IM | Serum Institute of India + Accelagen Pty + SpyBiotech | Phase 1/2 |
| IV | Inactivated virus | Inactivated SARS-CoV-2 vaccine (Vero cell) | 1,2 or 3 | ND | IM | Beijing Minhai Biotechnology Co., | Phase 3 |
| VVnr | Viral vector (Non-replicating) | GRAd-COV2 (Replication defective Simian Adenovirus (GRAd) encoding S) | 1 | Day 0 | IM | ReiThera + Leukocare + Univercells | Phase 2/3 |
| VVnr | Viral vector (Non-replicating) | VXA-CoV2-1 Ad5 adjuvanted Oral Vaccine platform | 2 | Day 0 + 28 | Oral | Vaxart | Phase 1 |
| VVnr | Viral vector (Non-replicating) | MVA-SARS-2-S | 2 | Day 0 + 28 | IM | University of Munich (Ludwig-Maximilians) | Phase 1 |
| PS | Protein subunit | SCB-2019 + AS03 or CpG 1018 adjuvant plus Alum adjuvant (Native like Trimeric subunit Spike Protein vaccine) | 2 | Day 0 + 21 | IM | Clover Biopharmaceuticals Inc./GSK/Dynavax | Phase 2/3 |
| PS | Protein subunit | COVAX-19® Recombinant spike protein + adjuvant | 1 | Day 0 + 21 | IM | Vaxine Pty Ltd. | Phase 1 |
| PS | Protein subunit | MVC-COV1901 (Spike-2P protein + adjuvant CpG 1018) | 2 | Day 0 + 28 | IM | Medigen Vaccine Biologics + Dynavax + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 |
| PS | Protein subunit | FINLAY-FR1 anti-SARS-CoV-2 Vaccine (RBD + adjuvant) | 2 | Day 0 + 28 | IM | Instituto Finlay de Vacunas | Phase 1/2 |
| PS | Protein subunit | FINLAY-FR-2 anti-SARS-CoV-2 Vaccine (RBD chemically conjugated to tetanus toxoid plus adjuvant) | 2 | Day 0 + 28 | IM | Instituto Finlay de Vacunas | Phase 3 |
| PS | Protein subunit | EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | 2 | Day 0 + 21 | IM | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | Phase 3 |
| PS | Protein subunit | RBD (baculovirus production expressed in Sf9 cells) Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | 2 | Day 0 + 28 | IM | West China Hospital + Sichuan University | Phase 2 |
| PS | Protein subunit | IMP CoVac-1 (SARS-CoV-2 HLA-DR peptides) | 1 | Day 0 | SC | University Hospital Tuebingen | Phase 1 |
| PS | Protein subunit | UB-612 (Multitope peptide based S1-RBD-protein based vaccine) | 2 | Day 0 + 28 | IM | Vaxxinity | Phase 2/3 |
| VVr | Viral vector (Replicating) | DelNS1-2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD) | 2 | Day 0 + 28 | IN | University of Hong Kong, Xiamen University, and Beijing Wantai Biological Pharmacy | Phase 2 |
| RNA | RNA based vaccine | LNP-nCoVsaRNA | 2 | ND | IM | Imperial College London | Phase 1 |
| RNA | RNA based vaccine | SARS-CoV-2 mRNA vaccine (ARCoV) | 2 | Day 0 + 14 orDay 0 + 28 | IM | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | Phase 3 |
| VLP | Virus like particle | Coronavirus-Like Particle COVID-19 (CoVLP) | 2 | Day 0 + 21 | IM | Medicago Inc. | Phase 2/3 |
| VVr + APC | Viral vector (Replicating) + APC | COVID-19/aAPC vaccine. The COVID-19/aAPC vaccine is prepared by applying lentivirus modification with immune modulatory genes and the viral minigenes to the artificial antigen presenting cells (aAPCs). | 3 | Day 0 + 14 + 28 | SC | Shenzhen Geno-Immune Medical Institute | Phase 1 |
| VVnr + APC | Viral vector (Non-replicating) + APC | LV-SMENP-DC vaccine. Dendritic cells are modified with lentivirus vectors expressing COVID-19 minigene SMENP and immune modulatory genes. CTLs are activated by LV-DC presenting COVID-19 specific antigens. | 1 | Day 0 | SC & IV | Shenzhen Geno-Immune Medical Institute | Phase 1/2 |
| PS | Protein subunit | AdimrSC-2f (recombinant RBD +/− Aluminium) | ND | ND | ND | Adimmune Corporation | Phase 1 |
| DNA | DNA based vaccine | Covigenix VAX-001 - DNA vaccines + proteo-lipid vehicle (PLV) formulation | 2 | Day 0 + 14 | IM | Entos Pharmaceuticals Inc. | Phase 1 |
| DNA | DNA based vaccine | CORVax - Spike (S) Protein Plasmid DNA Vaccine | 2 | Day 0 + 14 | ID | Providence Health & Services | Phase 1 |
| RNA | RNA based vaccine | ChulaCov19 mRNA vaccine | 2 | Day 0 + 21 | IM | Chulalongkorn University | Phase 1 |
| DNA | DNA based vaccine | bacTRL-Spike oral DNA vaccine | 1 | Day 0 | Oral | Symvivo Corporation | Phase 1 |
| VVnr | Viral vector (Non-replicating) | Human Adenovirus Type 5: hAd5 S + N bivalent vaccine (S-Fusion + N-ETSD). E2b- Deleted Adeno. | 1–2 | Day 0 + 21 | SC Oral, or SL | ImmunityBio, Inc. | Phase 1/2 |
| VVnr | Viral vector (Non-replicating) | COH04S1 (MVA-SARS-2-S) - Modified vaccinia ankara (sMVA) platform + synthetic SARS-CoV-2 | 1–2 | Day 0 + 28 | IM | City of Hope Medical Center + National Cancer Institute | Phase 1 |
| VVr | Viral vector (Replicating) | rVSV-SARS-CoV-2-S Vaccine | 1 | Day 0 | IM | Israel Institute for Biological Research | Phase 1/2 |
| VVr + APC | Viral vector (Replicating) + APC | Dendritic cell vaccine AV-COVID-19. A vaccine consisting of autologous dendritic cells loaded with antigens from SARS-CoV-2, with or without GM-CSF | 1 | Day 0 | IM | Aivita Biomedical, Inc., National Institute of Health Research and Development, Ministry of Health Republic of Indonesia | Phase 1/2 |
| LAV | Live attenuated virus | COVI-VAC | 1–2 | Day 0 or Day 0 + 28 | IN | Codagenix/Serum Institute of India | Phase 1 |
| PS | Protein subunit | CIGB-669 (RBD + AgnHB) | 3 | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IN | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 1/2 |
| PS | Protein subunit | CIGB-66 (RBD + aluminium hydroxide) | 3 | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IM | Center for Genetic Engineering and Biotechnology (CIGB) | Phase 3 |
| IV | Inactivated Virus | VLA2001 | 2 | Day 0 + 21 | IM | Valneva, National Institute for Health Research, United Kingdom | Phase 1/2 |
| PS | Protein subunit | BECOV2 | 2 | Day 0 + 28 | IM | Biological E. Limited | Phase 1/2 |
| VVr | Viral vector (Replicating) | AdCLD-CoV19 (adenovirus vector) | 1 | Day 0 | IM | Cellid Co., Ltd. | Phase 1/2 |
| DNA | DNA based vaccine | GLS-5310 | 2 | Day 0 + 56, or Day 0 + 84 | ID | GeneOne Life Science, Inc. | Phase 1/2 |
| PS | Protein subunit | Recombinant SARS-CoV-2 Spike protein, Aluminum adjuvanted | 2 | Day 0 + 21 | IM | Nanogen Pharmaceutical Biotechnology | Phase 1/2 |
| PS | Protein subunit | Recombinant protein vaccine S-268019 (using Baculovirus expression vector system) | 2 | Day 0 + 21 | IM | Shionogi | Phase 1/2 |
| VVnr | Viral vector (Non-replicating) | AdCOVID, Adenovirus-based platform expresses the receptor-binding domain (RBD) of the SARS-Cov-2 spike protein | 1–2 | Day 0 | IN | Altimmune, Inc. | Phase 1 |
| PS | Protein subunit | SARS-CoV-2-RBD-Fc fusion protein | N/A | N/A | SC or IM | University Medical Center Groningen + Akston Biosciences Inc. | Phase 1/2 |
| IV | Inactivated Virus | ERUCOV-VAC, inactivated virus | 2 | Day 0 + 21 | IM | Erciyes University | Phase 2 |
| PS | Protein subunit | COVAC-1 and COVAC-2 sub-unit vaccine (spike protein) + SWE adjuvant | 2 | Day 0 + 28 | IM | University of Saskatchewan | Phase 1/2 |
| PS | Protein subunit | GBP510, a recombinant surface protein vaccine with adjuvant AS03 (aluminium hydroxide) | 2 | Day 0 + 28 | IM | SK Bioscience Co., Ltd. and CEPI | Phase 1/2 |
| PS | Protein subunit | Razi Cov Pars, recombinant spike protein | 3 | Day 0 + 21 + 51 | IM and IN | Razi Vaccine and Serum Research Institute | Phase 2 |
| IV | Inactivated Virus | COVID-19 inactivated vaccine | 2 | Day 0 + 14 | IM | Shifa Pharmed Industrial Co., | Phase 2/3 |
| PS | Protein subunit | MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | 2 | Day 0 + 28 | IM | The University of Queensland | Phase 1 |
| DNA | DNA based vaccine | COVIGEN | 2 | Day 0 + 28 | ID or IM | University of Sydney, Bionet Co., Ltd., Technovalia | Phase 1 |
| DNA | DNA based vaccine | COVID-eVax, a candidate plasmid DNA vaccine of the Spike protein | N/A | N/A | IM or IM + electroporation | Takis + Rottapharm Biotech | Phase 1/2 |
| VVnr | Viral vector (Non-replicating) | BBV154, Adenoviral vector COVID-19 vaccine | 1 | Day 0 | IN | Bharat Biotech International Limited | Phase 1 |
| RNA | RNA based vaccine | PTX-COVID19-B, mRNA vaccine | 2 | Day 0 + 28 | IM | Providence Therapeutics | Phase 1 |
| IV | Inactivated virus | Inactivated (NDV-based) chimeric vaccine with or without the adjuvant CpG 1018 | 2 | Day 0 + 28 | IM | The Government Pharmaceutical Organization (GPO); PATH; Dynavax | Phase 1/2 |
| RNA | RNA based vaccine | CoV2 SAM (LNP) vaccine. A self-amplifying mRNA (SAM) lipid nanoparticle (LNP) platform + Spike antigen | Day 0 + 28 | IM | GlaxoSmithKline | Phase 1 | |
| VLP | Virus like particle | VBI-2902a. An enveloped virus-like particle (eVLP) of SARS-CoV-2 spike (S) glycoprotein and aluminum phosphate adjuvant. | 2 | Day 0 + 28 | IM | VBI Vaccines Inc. | Phase 1/2 |
| PS | Protein subunit | SK SARS-CoV-2 recombinant surface antigen protein subunit (NBP2001) + adjuvanted with alum. | 2 | Day 0 + 28 | IM | SK Bioscience Co., Ltd. | Phase 1 |
| VVnr | Viral vector (Non-replicating) | Chimpanzee Adenovirus serotype 68 (ChAd) and self-amplifying mRNA (SAM) vectors expressing spike alone, or spike plus additional SARS-CoV-2 T cell epitopes. | 2–3 | Day 0 + 14 + 28 or Day 0 + 28 + 56 or Day 0 + 112 | IM | Gritstone Oncology | Phase 1 |
| RNA | RNA based vaccine | mRNA-1273.351. A lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized S protein of the SARS-CoV-2B.1.351 variant. | 3 | Day 0 or Day 0 + 28 or Day 56 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 |
| PS | Protein subunit | SpFN (spike ferritin nanoparticle) uses spike proteins with a liposomal formulation QS21 (ALFQ) adjuvant. | 2–3 | Day 0 + 28 + 180 | IM | Walter Reed Army Institute of Research (WRAIR) | Phase 1 |
| PS | Protein subunit | EuCorVac-19; A spike protein using the recombinant protein technology and with an adjuvant. | 2 | Day 0 + 21 | IM | POP Biotechnologies and EuBiologics Co.,Ltd | Phase 1/2 |
| IV | Inactivated virus | Inactivated SARS-CoV-2 vaccine FAKHRAVAC (MIVAC) | 2 | Day 0 + 14 +/− 21 | IM | Organization of Defensive Innovation and Research | Phase 1 |
| LAV | Live attenuated virus | MV-014–212, a live attenuated vaccine that expresses the spike (S) protein of SARS-CoV-2 | 3 | Day 0 +/− 35 | IN | Meissa Vaccines, Inc. | Phase 1 |
| RNA | RNA based vaccine | MRT5500, an mRNA vaccine candidate | 2 | Day 0 + 21 | IM | Sanofi Pasteur and Translate Bio | Phase 1/2 |
| VLP | Virus like particle | SARS-CoV-2 VLP Vaccine | 2 | Day 0 | SC | The Scientific and Technological Research Council of Turkey | Phase 1 |
| PS | Protein subunit | ReCOV: Recombinant two-component spike and RBD protein COVID-19 vaccine (CHO cell). | 2 | Day 0 + 21 | IM | Jiangsu Rec-Biotechnology | Phase 1 |
| RNA | RNA based vaccine | DS-5670a, mRNA vaccine | 2 | IM | Daiichi Sankyo Co., Ltd. | Phase 1/2 | |
| IV | Inactivated Virus | Inactivated COVID-19 vaccine | 2 | Day 0 + 21 | IM | Kocak Farma | Phase 1 |
| VVnr | Viral vector (Non-replicating) | COVIVAC. Newcastle disease virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein +/− adjuvant CpG 1018 | 2 | Day 0 + 28 | IM | Institute of Vaccines and Medical Biologicals, Vietnam | Phase 1/2 |
| VVnr | Viral vector (Non-replicating) | SC-Ad6-1, Adneviral vector vaccine | 1–2 | Day 0 +/− 21 | IM | Tetherex Pharmaceuticals Corporation | Phase 1 |
| VLP | Virus like particle | ABNCoV2 capsid virus-like particle (cVLP) +/− adjuvant MF59 | 2 | Day 0 + 28 | IM | Radboud University | Phase 1 |
| PS | Protein subunit | Recombinant SARS-CoV-2 fusion protein vaccine (V-01) | 2 | Day 0 + 21 | IM | Guangdong Provincial Center for Disease Control and Prevention/Gaozhou Center for Disease Control and Prevention | Phase 2 |
| RNA | RNA based vaccine | HDT-301: Self-replicating mRNA vaccine formulated as a lipid nanoparticle. | 2 | Day 0 + 28 | IM | SENAI CIMATEC | Phase 1 |
| IV | Inactivated Virus | Adjuvanted inactivated vaccine against SARS-CoV-2 | 2 | Day 0 + 21 | SC | The Scientific and Technological Research Council of Turkey (TÜBITAK) | Phase 1 |
| RNA | RNA based vaccine | mRNA-1283 | 2 | Day 0 + 28 | IM | ModernaTX, Inc. | Phase 1 |
| PS | Protein subunit | Recombinant SARS-CoV-2 vaccine (CHO cell) | 2 | Day 0 | IM | National Vaccine and Serum Institute, China | Phase 1/2 |
| RNA | RNA based vaccine | EXG-5003; a temperature-sensitive self-replicating RNA vaccine expressing the receptor-binding domain of the SARS-CoV-2 spike protein. | 1 | Day 0 | ID | Elixirgen Therapeutics, Inc. | Phase 1/2 |
| IV | Inactivated Virus | Inactivated COVID-19 vaccine | 2 | Day 0 + 28 | IM | KM Biologics Co., Ltd. | Phase 1/2 |
Source – WHO [98] (updated on April 1, 2021). ND: No Data; IM: Intramuscular; SC: Subcutaneous; ID: Intrademal; IN: Intranasal; SL: Sublingual; IV: Intravenous.
6.1. Oxford/AstraZeneca vaccine
One of the most prominent vaccines developed against COVID-19 is the chimpanzee adenovirus vectored vaccine called ChAdOx1 nCoV-19. The ChAdOx1 nCoV-19 (encoding spike protein) triggers humoral and cell-mediated response. The T helper cell is the agent that triggers this response; this is from IgG sub subclass profiling and cytokine expression evidence. Induction of a balanced cellular and humoral immune response is experienced upon vaccination with ChAdOx1 nCoV-19 [105]. A clinical trial of the vaccine revealed the vaccine was 62 to 90 percent effective, depending on the initial dosage. After phase 1 results supported a two-dose regimen, the trial protocols were adjusted two standard doses (SD/SD cohort) of approximately 5 × 1010 viral particles per dose administered 28 days apart, but a subset (LD/SD cohort) in one of the UK trials inadvertently received a half-dose of the vaccine (low dose) as the first dose before a change in dosage quantification [106]. One of the good features of this vaccine is that it can be stored, transported, and handled at normal refrigerated conditions of about 36 °F–46 °F for at least six months and administered within existing healthcare settings. The vaccine uses technology from an Oxford spinout company, Vaccitech. It deploys a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (Adenovirus) that causes infections in chimpanzees. It contains the genetic materials of the spike protein. After vaccination, the cells produce the spike protein, stimulating the immune system to attack the SARS-CoV-2 virus [107]. Serious adverse events were evaluated in ChAdOx1 nCoV-19 recipients and control recipients. No serious adverse events or deaths that were treatment associated occurred in ChAdOx1 nCoV-19 recipients [106].
6.2. Pfizer/BioNTech vaccine
Another promising COVID-19 vaccine of much notable attention is the Pfizer-BioNTech's vaccine which was sent to the FDA for possible Emergency Use Authorization (EUA) on November and was authorized on December 11, 2020. It is an mRNA vaccine that codes for the virus's spike protein and is encapsulated in a lipid nanoparticle. Once injected, the cells churn out the spike protein, triggering the body's immune system to recognize the virus. In phase 3 trials, it demonstrated 95% efficacy. One of the major setbacks of the Pfizer-BioNTech vaccine is that it requires a cryo-storage of about −94 °F, which requires specialized freezers and therefore can affect the distribution chain [107].
6.3. Moderna vaccine
The vaccine (mRNA 1273) targeting spike protein developed by Moderna is also among the most promising vaccines. The mRNA 1273 vaccine contains RNA that codes for S protein. After its injection into the human system, the RNA migrates to cells and get translated to S protein; any cell containing the cell protein is targeted by the antibodies specific for S protein. The vaccine has good features of prompt modification of the encoded immunogen and quick manufacturing process [108]. Vaccine derived from RNA is safe and immunogenic from clinical trial evidence [78], [79]. The United States is the first place where the clinical trial phase 1 of mRNA1273 (novel lipid nanoparticle encapsulated mRNA-based vaccine) vaccine took place in March 2020. The vaccine encodes the S protein with propitious production [109]. The clinical trial of the recently developed mRNA1273 in a mouse model has shown neutralizing antibody induction with promising protection against the current pandemic. Therefore, the vaccine can prevent the infection of lower and upper airways in non-human primates [110]. According to [111], the clinical trial phase 1 of mRNA1273 using three doses of the vaccine (25 µg, 100 µg, or 250 µg), where each dose was tested on 15 adults (aged between 18 and 55) for 28 days apart, after the first immunization, antibody level elevated, and after the second immunization, neutralizing activity in the serum were detected. But there are some adverse effects (chills, headache, pain associated with the location of injection, fatigue, myalgia) detected in most of the participants (half), but the mRNA1273 induced immunity in all adults that participated. Also, [112] investigates the immunogenicity and safety of the mRNA1273 vaccine and found that the effect can range from mild to severe, which is mostly after the second dose treatment.
6.4. Johnson and Johnson's vaccine
A biopharma called Johnson and Johnson's is involved too in the COVID-19 vaccine project. The vaccine is designed with a DNA encoding S protein, the DNA is inserted into Adenovirus (virus causing flu-like symptoms), as the vaccine was made with a DNA, it can be stored for up to 3 months at 2 °C - 8 °C, this is because DNA is more stable than RNA. The S protein produced by the cell migrate outside the cell and stick to the cell surface. Upon vaccination, the vaccine breaks up SARS-CoV-2 S protein into fragments and released to the cell surface. The immune system then recognizes the protruding S protein and S protein fragment. Immunity is also provoked by the Adenovirus through switching the alarm system of the cell, which results in activation of nearby immune cells. Hence, resulting in the vaccine evoking a strong immunity against the S protein [113]. Clinical trial (phase 1 and 2) of Johnson and Johnson’s vaccine in the US and Japan measures the immunogenicity, safety, reactogenicity upon intramuscular injection in 1 or 2 doses, while phase 3 trial of the vaccine measures the efficacy of the vaccine using 60,000 participants. The effect of the vaccine was assessed based on viral load, the occurrence of COVID-19, titres of neutralizing/binding antibodies, systemic and local adverse effects. However, the trial stopped as a result of illness observed in participants that are unexplained. During the phase 3 clinical trial, 30,000 participants were used to investigate the co-morbidities and molecular confirmation. Interestingly, the US (100 million doses) and EU (400 million doses) have signed a contract for the production of Johnson and Johnson’s vaccine [114].
7. Conclusion
COVID-19 pandemic has drawn great attention across multiple disciplines, including economics, finance, epidemiology, virology, and pharmacology, as a result of its widespread and life-threatening nature. The scientific efforts to provide an everlasting solution to this health menace have witnessed the production, prescription, and administration of a myriad of drugs and vaccines, some of which are still in the pipeline undergoing pharmacological studies to safeguard public health. The S protein essential in host interaction and entry and RdRP that mediates viral RNA synthesis are good molecular targets that researchers and scientists can explore to develop novel therapeutics. Currently, efforts have been made to develop mRNA1273 (Moderna), 3LNP-mRNAs (Pfizer/BioNTech), and ChAdOx1-S (University of Oxford/Astra Zeneca) vaccines targeting the S protein, but there is no novel drug developed to fight the present pandemic. The trial on the repurposing of existing antiviral drugs (such as chloroquine targeting spike protein and remdesivir targeting RdRP) is ongoing now. However, the safety of those drugs is still under investigation. Therefore, there is no known repurposed drug that is officially approved and recommended for use in fighting the present pandemic. Hence, a better understanding of the structure of S protein and RdRP toward novel therapeutic development and drug repurposing is highly recommended.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Yusuf Muhammed: Conceptualization, Project Administration, Software, Writing – Original Draft, Visualization, Supervision. Abduljalal Yusuf Nadabo: Writing – Original Draft, Visualization. Mkpouto Pius: Writing – Original Draft. Bashiru Sani: Writing – Original Draft. Jafar Usman: Writing – Original Draft. Nasir Anka Garba: Writing – Review & Editing. Jaafaru Mohammed Sani: Writing – Review & Editing. Basit Opeyemi Olayanju: Writing – Review & Editing. Sunday Zeal Bala: Writing – Original Draft. Musa Garba Abdullahi: Writing – Review & Editing. Misbahu Sambo: Writing – Review & Editing.
References
- 1.MAYO CLINIC, Coronavirus disease 2019 (COVID-19). https://www.mayoclinic.org/diseases-conditions/coronavirus/symptoms-causes/syc-20479963, 2020 (accessed 10 January 2021).
- 2.WHO, MERS-CoV. https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov), 2019 (accessed 19 January 2021).
- 3.WHO, Severe Acute Respiratory Syndrome (SARS), https://www.who.int/westernpacific/health-topics/severe-acute-respiratory-syndrome, 2020 (accessed 19 January 2021).
- 4.Chan J.-W., Kok K.-H., Zhu Z., Chu H., To K.-W., Yuan S., Yuen K.-Y. novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassenaar T.M., Zou Y. 2019_nCoV/SARS-CoV-2: rapid classification of betacoronaviruses and identification of Traditional Chinese Medicine as potential origin of zoonotic coronaviruses. Lett. Appl. Microbiol. 2020;70:342–348. doi: 10.1111/lam.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.T. Li, D. Liu, Y. Yang, J. Guo, Y. Feng, X. Zhang, S. Cheng, J. Feng, Phylogenetic supertree reveals detailed evolution of SARS-CoV-2, Sci. Rep. (2020) 1–9, 10.21203/rs.3.rs-33194/v1. [DOI] [PMC free article] [PubMed]
- 8.Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect. Genet. Evol. 2020;83:104327. doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2021;78(4):1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang R.-D., Liu M.-Q., Chen Y., Shan C., Zhou Y.-W., Shen X.-R., Li Q., Zhang L., Zhu Y., Si H.-R., Wang Q.i., Min J., Wang X.i., Zhang W., Li B., Zhang H.-J., Baric R.S., Zhou P., Yang X.-L., Shi Z.-L. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182(1):50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammed Y. Molecular targets for COVID-19 drug development: Enlightening Nigerians about the pandemic and future treatment. Biosaf. Heal. 2020;2(4):210–216. doi: 10.1016/j.bsheal.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.S. Liu, C. Lien, P. Selvaraj, T. Wang, Evaluation of 19 antiviral drugs against SARS-CoV-2 infection, BioRxiv (2020), 10.1101/2020.04.29.067983. [DOI]
- 13.Senanayake S.L. Drug repurposing strategies for COVID-19. Futur. Drug Discov. 2020;2:6–8. doi: 10.4155/fdd-2020-0010. [DOI] [Google Scholar]
- 14.Z. Li, X. Li, Y.Y. Huang, Y. Wu, R. Liu, L. Zhou, Y. Lin, D. Wu, L. Zhang, H. Liu, X. Xu, K. Yu, Y. Zhang, J. Cui, C.G. Zhan, X. Wang, H. Bin Luo, Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs, Proc. Natl. Acad. Sci. U. S. A. 117 (2020) 27381–27387, 10.1073/pnas.2010470117. [DOI] [PMC free article] [PubMed]
- 15.B. Turoňová, M. Sikora, C. Schürmann, W.J.H. Hagen, S. Welsch, F.E.C. Blanc, S. von Bülow, M. Gecht, K. Bagola, C. Hörner, G. van Zandbergen, J. Landry, N.T.D. de Azevedo, S. Mosalaganti, A. Schwarz, R. Covino, M.D. Mühlebach, G. Hummer, J.K. Locker, M. Beck, In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges, Science 370 (2020) 203–208, 10.1126/science.abd5223. [DOI] [PMC free article] [PubMed]
- 16.Huang Y., Yang C., Xu X.-F., Xu W., Liu S.-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.P. Ahlquist, RNA-dependent RNA polymerases, viruses, and RNA silencing, Science (80-.). 296 (2002) 1270–1273, 10.1126/science.1069132. [DOI] [PubMed]
- 19.Ranjith-Kumar C.T., Gutshall L., Kim M.-J., Sarisky R.T., Kao C.C. Requirements for De Novo initiation of RNA synthesis by recombinant flaviviral RNA-dependent RNA polymerases. J. Virol. 2002;76:12526–12536. doi: 10.1128/JVI.76.24.12526-12536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L.u. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The Coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Y. Watanabe, J.D. Allen, D. Wrapp, J.S. McLellan, M. Crispin, Site-specific glycan analysis of the SARS-CoV-2 spike, Science 369 (2020) 330–333, 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed]
- 24.J.-T. Jan, T.-J.R. Cheng, Y.-P. Juang, H.-H. Ma, Y.-T. Wu, W.-B. Yang, C.-W. Cheng, X. Chen, T.-H. Chou, J.-J. Shie, W.-C. Cheng, R.-J. Chein, S.-S. Mao, P.-H. Liang, C. Ma, S.-C. Hung, C.-H. Wong, Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection, Proc. Natl. Acad. Sci. 118 (2021), e2021579118. 10.1073/pnas.2021579118. [DOI] [PMC free article] [PubMed]
- 25.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D. Wrapp, N. Wang, K.S. Corbett, J.A. Goldsmith, C.L. Hsieh, O. Abiona, B.S. Graham, J.S. McLellan, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science 367 (2020) 1260–1263, 10.1126/science.aax0902. [DOI] [PMC free article] [PubMed]
- 27.Bertram S., Dijkman R., Habjan M., Heurich A., Gierer S., Glowacka I., Welsch K., Winkler M., Schneider H., Hofmann-Winkler H., Thiel V., Pohlmann S. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J. Virol. 2013;87:6150–6160. doi: 10.1128/JVI.03372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with novel coronavirus in Wuhan, China. Lancet. 2019;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu B., Gong P. Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E4005–E4014. doi: 10.1073/pnas.1602591113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y., Yin W., Xu H.E. RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19. Biochem. Biophys. Res. Commun. 2021;538:47–53. doi: 10.1016/j.bbrc.2020.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A. Dwivedy, R. Mariadasse, M. Ahmed, D. Kar, J. Jeyakanthan, B.K. Biswal, In silico characterization of the NiRAN domain of RNA-dependent RNA polymerase provides insights into a potential therapeutic target against SARS-CoV2, OFS Preprints (2020), 10.31219/osf.io/wd6zu. [DOI] [PMC free article] [PubMed]
- 33.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P., Masciovecchio C., Angeletti S., Ciccozzi M., Gallo R.C., Zella D., Ippodrino R. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020;18:179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong P., Peersen O.B. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22505–22510. doi: 10.1073/pnas.1007626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas-Lemus M., Minasov G., Shuvalova L., Inniss N.L., Kiryukhina O., Wiersum G., Kim Y., Jedrzejczak R., Maltseva N.I., Endres M., Jaroszewski L., Godzik A., Joachimiak A., Satchell K.J.F. The crystal structure of nsp10-nsp16 heterodimer from SARS CoV-2 in complex with S-adenosylmethionine. BioRxiv. 2020 doi: 10.1101/2020.04.17.047498. [DOI] [Google Scholar]
- 36.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for the inhibition of the RNA-Dependent RNA polymerase from SARS-CoV-2 by Remdesivir. BioRxiv. 2020;1560 doi: 10.1101/2020.04.08.032763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2020;39:3204–3212. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aftab S.O., Ghouri M.Z., Masood M.U., Haider Z., Khan Z., Ahmad A., Munawar N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020;18:1–15. doi: 10.1186/s12967-020-02439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 40.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses-drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shyr Z.A., Gorshkov K., Chen C.Z., Zheng W. Drug discovery strategies for sars-cov-2. J. Pharmacol. Exp. Ther. 2020;375(1):127–138. doi: 10.1124/jpet.120.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R. Yan, Y. Zhang, Y. Li, L. Xia, Y. Guo, Q. Zhou, Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2, Science 367 (2020) 1444–1448, 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed]
- 43.Gorbalenya A.E., Pringle F.M., Zeddam J.-L., Luke B.T., Cameron C.E., Kalmakoff J., Hanzlik T.N., Gordon K.H.J., Ward V.K. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 2002;324(1):47–62. doi: 10.1016/S0022-2836(02)01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55(4):105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B., Poehlmann S. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D., De Clercq E. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49(9):2845–2849. doi: 10.1021/jm0601856. https://doi.org/10.1021/jm060185610.1021/jm0601856.s001. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann M., Mösbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Krüger N., Gassen N.C., Müller M.A., Drosten C., Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585(7826):588–590. doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 50.R. Mann, S.K., Marwaha, Chlorpromazine, 2021.
- 51.Plaze M., Attali D., Petit A.-C., Blatzer M., Simon-Loriere E., Vinckier F., Cachia A., Chrétien F., Gaillard R. Repurposing chlorpromazine to treat COVID-19: The reCoVery study. Encephale. 2020;46:169–172. doi: 10.1016/j.encep.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cong Y., Hart B.J., Gross R., Zhou H., Frieman M., Bollinger L., Wada J., Hensley L.E., Jahrling P.B., Dyall J., Holbrook M.R., Pöhlmann S. MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells. PLoS One. 2018;13(3):e0194868. doi: 10.1371/journal.pone.0194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J.M., Bosch B.J., de Haan C.A.M., Perlman S. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10(11):e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forrest I.S., Bolt A.G., Serra M.T. Distribution of chlorpromazine metabolites in selected organs of psychiatric patients chronically dosed up to the time of death. Biochem. Pharmacol. 1968;17:2061–2070. doi: 10.1016/0006-2952(68)90180-9. [DOI] [PubMed] [Google Scholar]
- 56.S.R. Marder, D. Ames, W.C. Wirshing, T.V. Putten, Schizophrenia, Psychiatr. Clin. North Am. 16 (1993) 567–587. [PubMed]
- 57.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D., Morrissey C., Corey E., Montgomery B., Mostaghel E., Clegg N., Coleman I., Brown C.M., Schneider E.L., Craik C., Simon J.A., Bedalov A., Nelson P.S. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.S. Bravaccini, E. Fonzi, M. Tebaldi, D. Angeli, G. Martinelli, F. Nicolini, P. Parrella, M. Mazza, Estrogen and androgen receptor inhibitors: unexpected allies in the fight against COVID-19, Cell Transplant. 30 (2021), 096368972199147. 10.1177/0963689721991477. [DOI] [PMC free article] [PubMed]
- 59.Chia K., O’Brien M., Brown M., Lim E. Targeting the androgen receptor in breast cancer. Curr. Oncol. Rep. 2015;17:4. doi: 10.1007/s11912-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 60.Karamouzis M.V., Papavassiliou K.A., Adamopoulos C., Papavassiliou A.G. Targeting androgen/estrogen receptors crosstalk in cancer. Trends Cancer. 2016;2:35–48. doi: 10.1016/j.trecan.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Mustonen M.V., Pyrhönen S., Kellokumpu-Lehtinen P.L. Toremifene in the treatment of breast cancer. World J. Clin. Oncol. 2014;5:393–405. doi: 10.5306/wjco.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin W.R., Cheng F. Repurposing of FDA-approved toremifene to treat COVID-19 by blocking the spike glycoprotein and NSP14 of SARS-CoV-2. J. Proteome Res. 2020;19:4670–4677. doi: 10.1021/acs.jproteome.0c00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L., Pei R.-juan., Li H., Ma X.-na., Zhou Y., Zhu F.-hua., He P.-lan., Tang W., Zhang Y.-cheng., Xiong J., Xiao S.-qi., Tong X.-kun., Zhang B., Zuo J.-ping. Identification of SARS-CoV-2 entry inhibitors among already approved drugs. Acta Pharmacol. Sin. 2021;42:1347–1353. doi: 10.1038/s41401-020-00556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Tawfiq J.A., Petersen E., Memish Z.A., Perlman S., Zumla A. Middle East respiratory syndrome coronavirus – The need for global proactive surveillance, sequencing and modeling. Travel Med. Infect. Dis. 2021;43:102118. doi: 10.1016/j.tmaid.2021.102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Lane H.C., et al. Remdesivir for the treatment of Covid-19 — Final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.-Y., Subramanian A., et al. Remdesivir for 5 or 10 days in patients with severe covid-19. N. Engl. J. Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.W.H.O. (WHO), WHO recommends against the use of remdesivir in COVID-19 patients, 2020.
- 68.Dyer O. Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371 doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 69.T. Smith, J. Bushek, A. Leclaire, T. Prosser, COVID-19 Drug Therapy, (2020).
- 70.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elshabrawy H.A. SARS-CoV-2: An update on potential antivirals in light of SARS-CoV antiviral drug discoveries. Vaccines. 2020;8:1–33. doi: 10.3390/vaccines8020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., Patil S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gurung A.B., Ali M.A., Lee J., Farah M.A., Al-Anazi K.M. The potential of Paritaprevir and Emetine as inhibitors of SARS-CoV-2 RdRp. Saudi J. Biol. Sci. 2021;28:1426–1432. doi: 10.1016/j.sjbs.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreani J., Le M., Du I., Jardot P., Rolland C., Lisi L., Miguel P., Luisa M., Graziani G., Huang X., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bleasel M.D., Peterson G.M. Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals. 2020;13 doi: 10.3390/ph13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar R., Khandelwal N., Chander Y., Riyesh T., Gulati B.R., Pal Y., Tripathi B.N., Barua S., Kumar N. Emetine as an antiviral agent suppresses SARS-CoV-2 replication by inhibitinginteraction of viral mRNAwith eIF4E: An in vitro study. BioRxiv. 2020 doi: 10.1101/2020.11.29.401984. [DOI] [Google Scholar]
- 77.Takehara T. Simeprevir for the treatment of chronic hepatitis C genotype 1 infection. Expert Rev. Anti. Infect. Ther. 2014;12(8):909–917. doi: 10.1586/14787210.2014.925800. [DOI] [PubMed] [Google Scholar]
- 78.Sekar V., Verloes R., Meyvisch P., Spittaels K., Akuma S.H., De Smedt G. 1076 evaluation of metabolic interactions for Tmc435 via cytochrome P450 (Cyp) enzymes in healthy volunteers. J. Hepatol. 2010;52:S416. doi: 10.1016/s0168-8278(10)61077-x. [DOI] [Google Scholar]
- 79.Alric L., Bonnet D. Grazoprevir + elbasvir for the treatment of hepatitis C virus infection. Expert Opin. Pharmacother. 2016;17(5):735–742. doi: 10.1517/14656566.2016.1161028. [DOI] [PubMed] [Google Scholar]
- 80.Rosenquist Å., Samuelsson B., Johansson P.-O., Cummings M.D., Lenz O., Raboisson P., Simmen K., Vendeville S., de Kock H., Nilsson M., Horvath A., Kalmeijer R., de la Rosa G., Beumont-Mauviel M. Discovery and development of simeprevir (TMC435), a HCV NS3/4A protease inhibitor. J. Med. Chem. 2014;57(5):1673–1693. doi: 10.1021/jm401507s. [DOI] [PubMed] [Google Scholar]
- 81.Lo H.S., Hui K.P.Y., Lai H.-M., Khan K.S., Kaur S., Huang J., Li Z., Chan A., Cheung H.H.-Y., Ng K.-C., Wang Ho J.C., et al. Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. BioRxiv. 2020 doi: 10.1101/2020.05.26.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Canadian Agency for Drugs and Technologies in Health, Lumacaftor/Ivacaftor (Orkambi): (Vertex Pharmaceuticals (Canada) Incorporated): Indication: For the treatment of cystic fibrosis in patients aged six years and older who are homozygous for the F508del mutation in the cystic fibrosis transmembrane conducta, 2018. [PubMed]
- 83.Connett G.J. Lumacaftor-ivacaftor in the treatment of cystic fibrosis: Design, development and place in therapy. Drug Des. Devel. Ther. 2019;13:2405–2412. doi: 10.2147/DDDT.S153719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng X., Wang Y., Wang X., Wrennall J.A., Rimington T.L., Li H., Cai Z., Ford R.C., Sheppard D.N. Two small molecules restore stability to a subpopulation of the cystic fibrosis transmembrane conductance regulator with the predominant disease-causing mutation. J. Biol. Chem. 2017;292:3706–3719. doi: 10.1074/jbc.M116.751537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Q., Sabirzhanova I., Anne E., Bergbower S., Yanda M., William G. Trafficking mutants: a potential treatment for Stargardt disease. Cell Physiol Biochem. 2020;53:400–412. doi: 10.33594/000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Talamo Guevara M., McColley S.A. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Opin. Drug Saf. 2017;16:1305–1311. doi: 10.1080/14740338.2017.1372419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li G., De Clercq E., Therapeutic options for the novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2019;19(2020):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 88.Alamri M.A., Tahir ul Qamar M., Mirza M.U., Bhadane R., Alqahtani S.M., Muneer I., Froeyen M., Salo-Ahen O.M.H. Pharmacoinformatics and molecular dynamics simulation studies reveal potential covalent and FDA-approved inhibitors of SARS-CoV-2 main protease 3CLpro. J. Biomol. Struct. Dyn. 2020;39:4936–4948. doi: 10.1080/07391102.2020.1782768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White M.A., Lin W., Cheng X. Discovery of COVID-19 inhibitors targeting the SARS-CoV-2 Nsp13 helicase. J. Phys. Chem. Lett. 2020;11:9144–9151. doi: 10.1021/acs.jpclett.0c02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.A.S. Brierley, P.G. Fernandes, M.A. Brandon, F. Armstrong, N.W. Millard, S.D. McPhail, P. Stevenson, M. Pebody, J. Perrett, M. Squires, D.G. Bone, G. Griffiths, Antarctic krill under sea ice: Elevated abundance in a narrow band just south of ice edge, Science 295 (2002) 1890–1892, 10.1126/science.1068574. [DOI] [PubMed]
- 91.Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., King N.P., Veesler D., Bloom J.D. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeong G.U., Song H., Yoon G.Y., Kim D., Kwon Y.C. Therapeutic strategies against COVID-19 and structural characterization of SARS-CoV-2: A review. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trezza A., Iovinelli D., Santucci A., Prischi F., Spiga O. An integrated drug repurposing strategy for the rapid identification of potential SARS-CoV-2 viral inhibitors. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-70863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szlávik L., Gyuris Á,, Minárovits J., Forgo P., Molnár J., Hohmann J. Alkaloids from Leucojum vernum and antiretroviral activity of amaryllidaceae alkaloids. Planta Med. 2004;70:871–873. doi: 10.1055/s-2004-827239. [DOI] [PubMed] [Google Scholar]
- 95.Andleeb R., Ashraf A., Muzammil S., Naz S., Asad F., Ali T., Rafi R., Al-Ghanim K.A., Al-Misned F., Ahmed Z., Mahboob S. Analysis of bioactive composites and antiviral activity of Iresine herbstii extracts against Newcastle disease virus in ovo. Saudi J. Biol. Sci. 2020;27:335–340. doi: 10.1016/j.sjbs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhuiyan F.R., Howlader S., Raihan T., Hasan M. Plants metabolites: possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 98.WHO, COVID-19 vaccine tracker and landscape, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines, 2021 (accessed 01 April 2021).
- 99.Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.M.A. Tortorici, D. Veesler, Structural insights into coronavirus entry, 1st ed., Elsevier Inc., 2019. 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed]
- 101.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., Kong W.-P., Andres E.L., Kettenbach A.N., Denison M.R., Chappell J.D., Graham B.S., Ward A.B., McLellan J.S. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Depfenhart M., de Villiers D., Lemperle G., Meyer M., Di Somma S. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern. Emerg. Med. 2020;15:801–812. doi: 10.1007/s11739-020-02383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knoll M.D., Wonodi C. Oxford – AstraZeneca COVID-19 vaccine efficacy. Lancet Reg. Heal. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.M. Terry, UPDATED comparing COVID-19 vaccines: timelines, types and prices, https://www.biospace.com/article/comparing-covid-19-vaccines-pfizer-biontech-moderna-astrazeneca-oxford-j-and-j-russia-s-sputnik-v/, 2021 (accessed 10 July 2021).
- 108.C.P. Verschoor, P. Singh, M.L. Russell, D.M.E. Bowdish, A. Brewer, L. Cyr, B.J. Ward, M. Loeb, Correction: Microneutralization assay titres correlate with protection against seasonal Influenza H1N1 and H3N2 in children (PLoS ONE (2016) 10:6 (e0131531) DOI: 10.1371/journal.pone.0131531), PLoS One. 11 (2016), e0131531. 10.1371/journal.pone.0163830. [DOI] [PMC free article] [PubMed]
- 109.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARScov-2 vaccine development. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383(16):1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.J. Corum, C. Zimmer, How the Johnson & Johnson Vaccine Works. https://www.nytimes.com/interactive/2020/health/johnson-johnson-covid-19-vaccine.html, 2021 (accessed 15 July 2021).
- 114.Prüβ B.M. Current state of the first covid-19 vaccines. Vaccines. 2021;9 doi: 10.3390/vaccines9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.M. Yuan, N.C. Wu, X. Zhu, C.C.D. Lee, R.T.Y. So, H. Lv, C.K.P. Mok, I.A. Wilson, A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV, Science 368 (2020) 630–633, 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed]
- 116.Hoffmann M., Kleine-Weber H., Schroeder S., Muller M., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Front. Oncol. 2020;10:271–280. doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chellapandi P., Saranya S. Genomics insights of SARS-CoV-2 (COVID-19) into target-based drug discovery. Med. Chem. Res. 2020;29:1777–1791. doi: 10.1007/s00044-020-02610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hendaus M. Remdesivir in the treatment of coronavirus disease (COVID-19): a simplified summary. J. Biomol. Struct. Dyn. 2019;39:3787–3791. doi: 10.1080/07391102.2020.1767691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santos I.de.A., Grosche V.R., Bergamini F.R.G., Sabino-Silva R., Jardim A.C.G. Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stip E., Rizvi T.A., Mustafa F., Javaid S., Aburuz S., Ahmed N.N., Abdel Aziz K., Arnone D., Subbarayan A., Al Mugaddam F., Khan G. The large action of chlorpromazine: translational and transdisciplinary considerations in the face of COVID-19. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.577678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Emirik M. Potential therapeutic effect of turmeric contents against SARS-CoV-2 compared with experimental COVID-19 therapies: in silico study. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1835719. [DOI] [PubMed] [Google Scholar]
- 122.Tsuji M. Potential anti-SARS-CoV-2 drug candidates identified through virtual screening of the ChEMBL database for compounds that target the main coronavirus protease. FEBS Open Bio. 2020;10:995–1004. doi: 10.1002/2211-5463.12875. https://doi.org/10.1002/feb4.v10.610.1002/2211-5463.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lung J., Lin Y., Yang Y., Chou Y., Shu L., Cheng Y., Liu H.T., Wu C. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020;92:693–697. doi: 10.1002/jmv.25761. https://doi.org/10.1002/jmv.v92.610.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]