Abstract
Introduction
Hepatic lymphorrhea (HL) is an uncommon but potentially life-threatening type of postoperative lymphatic leakage, especially following pancreaticoduodenectomy.
Case presentation
We herein report a case series of four patients with HL following pancreaticoduodenectomy that presented to the department with a severe clinical picture with the discovery in imaging and intraperitoneal fluid's tests. All our patients presented with a condition of Hepatic lymphorrhea secondary to pancreaticoduodenectomy, which were treated successfully with percutaneous hepatic lymphangiography (HLG).
Discussion
Hepatic lymphorrhea is an uncommon but potentially life-threatening complication following pancreaticoduodenectomy. Periportal lymphatic vessels, which was often isolated and dissected especially with extended lymphadenectomy, is potentially damaged and caused resistant chylous leakage. Newly techniques are updated and applied in diagnosis and treatment for this difficult-to-treat complication, one of them is percutaneous Hepatic Lymphangiography (HLG).
Conclusion
HLG with percutaneous access could be effective to identify and terminate the chylous fistula from periportal lymphatic vessels after pancreaticoduodenectomy.
Keywords: Pancreatic cancer, Extended lymphadenectomy, Hepatic lymphorrhea, Case series
Highlights
-
•
Hepatic lymphorrhea is a rare, life-threatening complication of pancreaticoduodenectomy.
-
•
Periportal lymphatic vessels was a potential location of lymphatic fistula and leakage.
-
•
This complication can be diagnosed with imaging and intraperitoneal fluid's tests.
-
•
Hepatic lymphangiography is a minor-invasive therapy to close the lymphatic fistula.
Abbreviations
- AJCC
American Joint Committee on Cancer
- HL
Hepatic lymphorrhea
- HLG
Hepatic Lymphangiography
- LD
Lymphadenectomy
- LL
Lymphatic leakage
- LN
Lymph node
- MCT
Medium-chain triglyceride
- MRL
Magnetic Resonance Lymphangiography
- PDAC
Pancreatic ductal adenocarcinoma
- PD
Pancreaticoduodenectomy
- PLL
Postoperative lymphatic leakage
- POD
postoperative day
- PV
Portal vein
- SMV
Superior mesenteric vein
- TPN
Total parenteral nutrition
1. Introduction
Lymphatic complications are rare, but well-known condition, and have been described in many researches, especially after in thoracic and head-and-neck surgery [1]. In general, These complications can be divided into two types: lymphatic leakage and lymphatic stasis [2]. In lymphatic leakage complication, many subtypes are presented, owing to different consequences and characteristics. A review by Shulan Lv et al. categorized this complication into five forms: lymphatic ascites, lymphocele, lymphatic fistula, lymphorrhea and special forms (chylous ascites or chyloperitoneum, chyloretroperitoneum and chylothorax) [2].
Hepatopancreatobiliary surgeries, and especially oncological pancreatic resections with lymphadenectomy, had usually seem to be associated with an increased risk of chyle leak compared with other major abdominal surgeries [3,4]. Disruption of the cisterna chyli or one of its major lymphatic tributaries in surgeries is the most likely cause of postoperative intra-abdominal chyle leak [5,6]. In contract, Hepatic lymphorrhea (HL) is an extremely rare complication, which was difficult to manage by surgery or intranodal Hepatic Lymphangiography [7]. Herein, we reported a case series of four patients with successful treatment of HL following pancreaticoduodenectomy by transhepatic lymphangiography. All our work has been reported in line with the PROCESS criteria and guidelines [8] and was registered in accordance with the declaration of Helsinki (ID: researchregistry6850, https://www.researchregistry.com/browse-the-registry#home/registrationdetails/60aa3094279723001e73bb94/).
2. Case presentation
2.1. Case 1
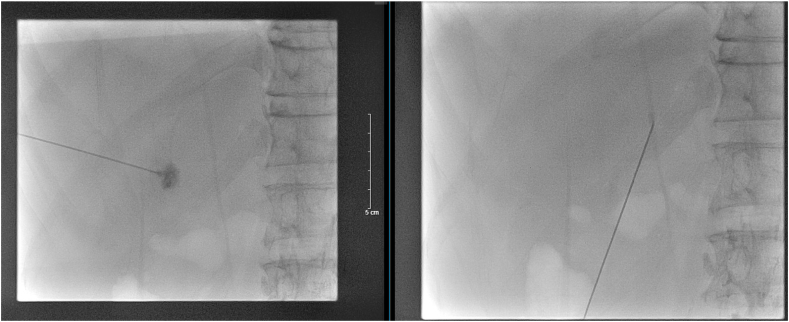
A 59-year-old man underwent Pancreaticoduodenectomy (PD) with extended lymphadenectomy and segmental Superior Mesenteric Vein (SMV) resection for pancreatic ductal adenocarcinoma (PDAC) with SMV involvement. On postoperative day (POD) 7, after starting refeeding, over 1000 mL of slightly milky ascites was discharged from the inserted drain. A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him but there were no improvements in 20 PODs. After that, he has been discharged to another hospital for further diagnosis and treatment. The first Magnetic Resonance Lymphangiography (MRL) with the intranodal injection of gadolinium-based contrast material into the bilateral inguinal lymph nodes were conducted, and the result showed abdominal effusions but no extravasation from central abdominal lymphatic channels (Fig. 1). This result made a hypothesis that the chylous extravasation may be located from the portal lymph system, which was not identified in Magnetic Resonance Lymphangiography. The interventional radiology service – HLG with percutaneous transhepatic access was consulted and performed on POD 24 (Fig. 2). Ultrasound guidance was used to access the right and left portal lymph nodes using 8 mm Chiba needles. A contrast agent was injected directly into the lymph nodes under intermittent fluoroscopic observation to confirm filling of lymphatic channels until the one feeding the leaking lymphatic channel was identified. A 1:3 mixture of Aetoxisclerol to air was formulated, and approximately 8 mL of this mixture was injected into the culprit lymph node to embolize the leaking lymphatic channels.
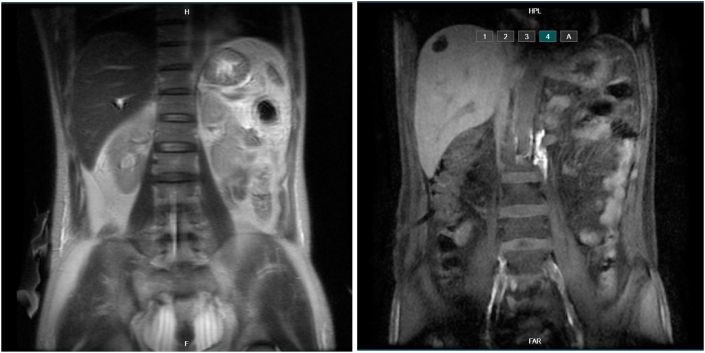
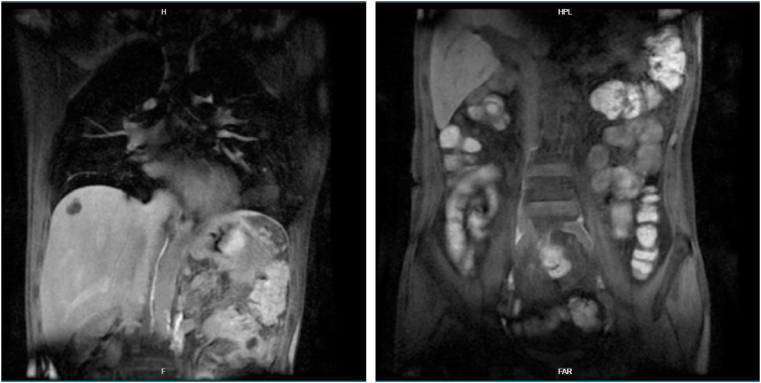
Fig. 1.
The first Magnetic Resonance Lymphangiography (MRL) result showed abdominal effusions but no extravasation from central abdominal lymphatic channels.
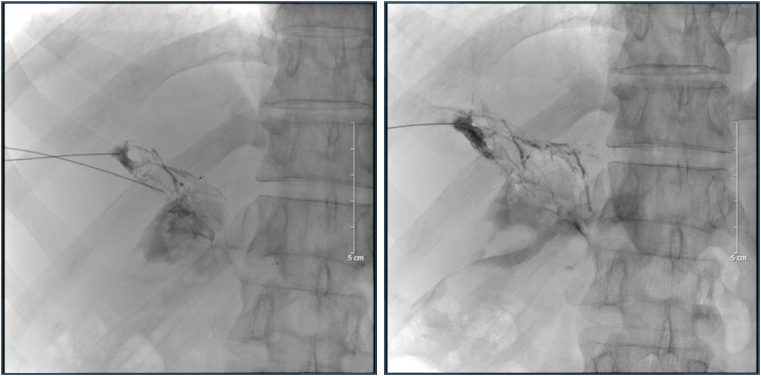
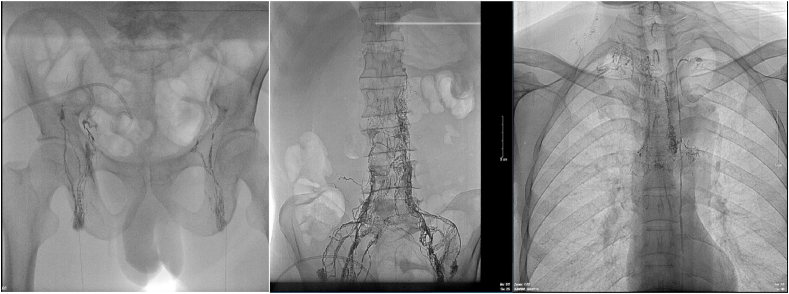
Fig. 2.
The first HLG. Ultrasound guidance was used to access the right and left periportal lymphatic vessels using 8 mm Chiba needles. After identifying the contrast medium extravasation's location, A 1:3 mixture of Aetoxisclerol to air was formulated, and approximately 8 mL of this mixture was injected.
After the first embolization, drainage remained to approximately 1000–1500 mL of serous fluid per day in the following days. Thus, on the third day after the first invasion, a non-contrast post-lymphangiographic computed tomography (post-LG CT) is recommended and conducted, which can be carried out easily after iodized oil-based LAG, to illustrate better anatomical details of the lymphatic system and LL with three-dimensional spatial resolution, and the result was abdominal effusions concentrated at pouch of Douglas and root of the Mesentery (Fig. 3). On the fourth day after the first invasion, the second HLG with percutaneous transhepatic access was performed. After accessing the right portal lymph nodes and identifying the chylous extravasation's location, A 1:1 mixture of Aetoxisclerol to air was formulated, and approximately 2 mL of this mixture was injected (Fig. 4).
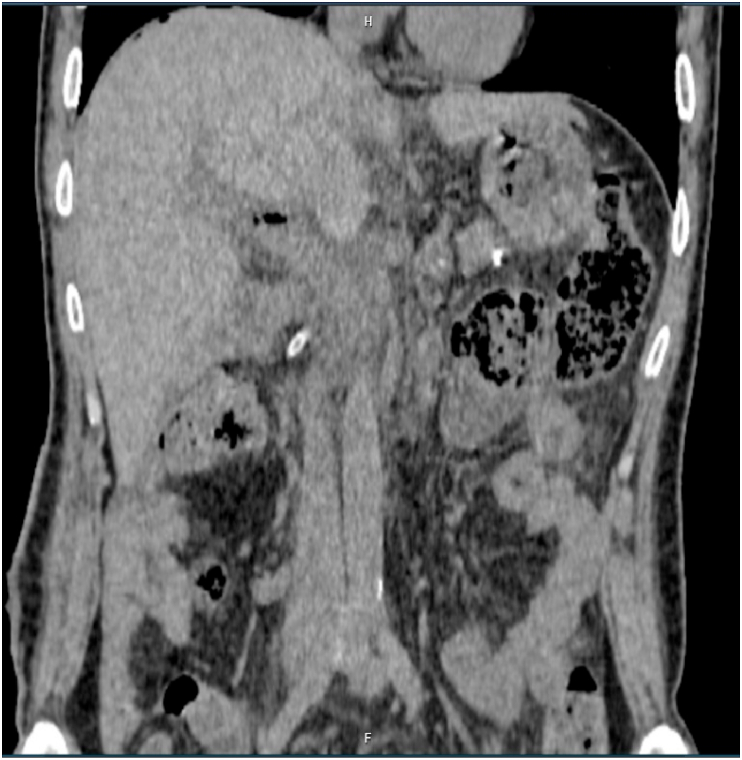
Fig. 3.
The post-lymphangiographic Computed tomography (post-LG CT) result was abdominal effusions concentrated at pouch of Douglas and root of the Mesentery.
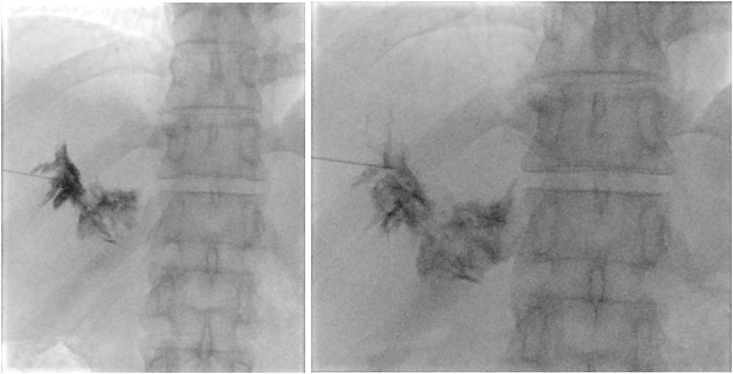
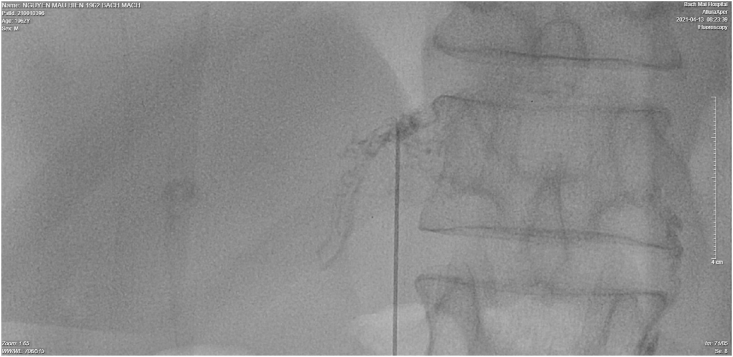
Fig. 4.
The second HLG. After accessing the right portal lymph nodes and identifying the lymphatic extravasation's location, A 1:1 mixture of Aetoxisclerol to air was formulated, and approximately 2 mL of this mixture was injected.
After the second embolization, A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him, and drainage decreased to approximately 150 mL of serous fluid in the following days. The drain output remained low, and after 5 weeks until the surgery, the drainage was <20 mL daily and all drains were removed. Follow-up ultrasound and Magnetic Resonance Lymphangiography (MRL) demonstrated no residual fluid collection (Fig. 5).
Fig. 5.
The second Magnetic Resonance Lymphangiography (MRL) result demonstrated no residual fluid collection and no fistulas of the main lymphatic channels.
2.2. Case 2
A 59-year-old man underwent Pancreaticoduodenectomy (PD) with extended lymphadenectomy and segmental Portal Vein (PV) resection for pancreatic ductal adenocarcinoma (PDAC) with PV involvement (pT4N2M0 or Stage III according to AJCC Staging 2017) [9]. After one month and a half conservative treatment with dietary therapy and drainage, he was discharged with an abdominal drainage with an average of 500 mL fluid per day, because of nontypical symptoms (slightly clear abdominal fluid with a low content of triglyceride level).
On postoperative day (POD) 61, his drainage slipped off and he had to hospitalize to further examinations. Intranodal lymphangiography was consulted and performed on POD 62. The intranodal HLG to access the main lymphatic system resulted no abnormalities (Fig. 6), and percutaneous HLG with ultrasound guidance using 21G Chiba needles was not able to access the right and left portal lymph nodes after 4 times access. After 4 days of the first HLG, the second one was proceeded and at that time the portal lymph plexus around the PV were accessed. A contrast agent was injected directly into the lymph nodes under fluoroscopic observation to confirm filling of lymphatic channels, however no contrast medium extravastion was identified. Though, A 1:3 mixture of Aetoxisclerol to air was formulated, and approximately 8 mL of this mixture was injected into the lymphatic vessels (Fig. 7).
Fig. 6.
The intranodal HLG identified of no abnormalities in the main lymphatic system.
Fig. 7.
The second HLG. After no identifying the chylous extravasation's location, we decided injected a 1:3 mixture of Aetoxisclerol to air, and approximately 8 mL of this mixture was injected.
After the hepatic lymphatic sclerotherapy, a low-fat, middle-chain triglyceride (MCT) diet was prescribed for him, and drainage decreased from 700 mL to approximately 300 mL of serous fluid in the following days. The drain output remained low, and after 2 weeks of the second discharge, the drainage was <20 mL daily and all drains were removed.
2.3. Case 3
A 73-year-old man underwent Pancreaticoduodenectomy (PD) and total pancreatectomy with extended lymphadenectomy and segmental Portal Vein (PV) resection for pancreatic ductal adenocarcinoma (PDAC) with PV involvement (pT4N2M0 or Stage III according to AJCC Staging 2017) [9]. On postoperative day (POD) 5, after starting refeeding, over 1000 mL of slightly milky ascites was discharged from the inserted drain. A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him but there were no improvements in 10 PODs.
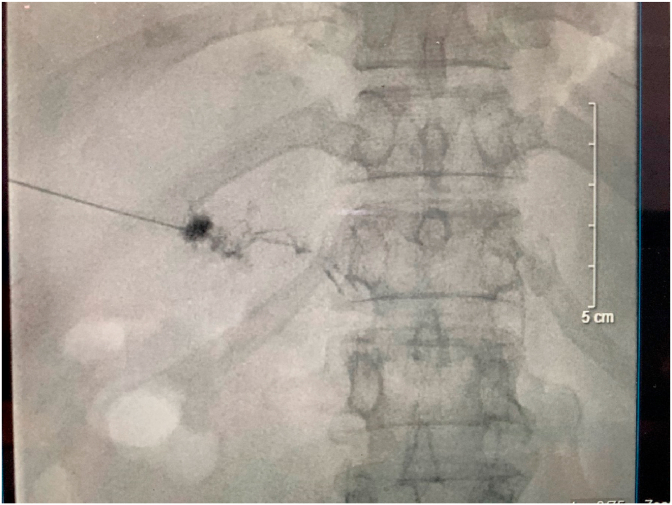
On postoperative day (POD) 11, intranodal lymphangiography was consulted and performed. The intervention resulted no abnormalities (Fig. 8), and percutaneous HLG with ultrasound guidance using 21G Chiba needles was not able to access the right and left periportal lymphatic vessels. We decided injected a 1:3 mixture of Aetoxisclerol to air was formulated, and approximately 8 mL of this mixture was injected around the right and left portal veins (Fig. 9).
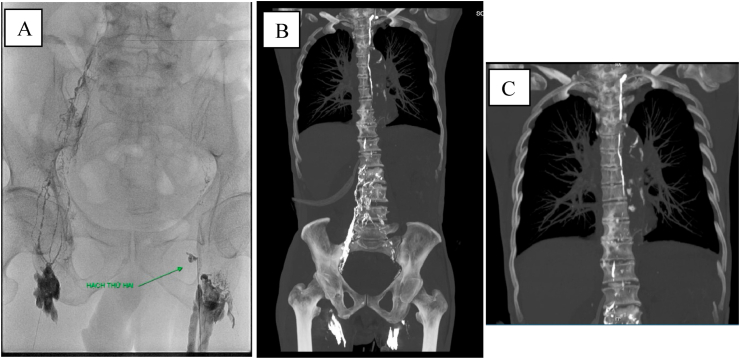
Fig. 8.
A: The intranodal lymphangiography identified of no abnormalities in the main lymphatic system. B–C: Post-procedural CT scanner confirmed the intact of central lymphatic system.
Fig. 9.
The Hepatic Lymphangiography was attempted to access the periportal lymphatic vessels, we decided injected a 1:3 mixture of Aetoxisclerol to air around the right and left portal veins.
After the HLG, A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him, and drainage decreased to 700 mL on the following day and to approximately 300 mL of serous fluid in the following seventh day. The drain output remained low, and after 10 days of the invasion, the drainage was 50 mL daily and all drains were removed.
2.4. Case 4
A 71-year-old woman underwent Pancreaticoduodenectomy (PD) with standard lymphadenectomy for pancreatic ductal adenocarcinoma (PDAC) (pT2N0M0 or Stage Ib according to AJCC Staging 2017) [9]. On postoperative day (POD) 5, after starting refeeding, over 1000 mL of slightly milky ascites was discharged from the inserted drainage (Fig. 10). A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him but there were no improvements in 20 PODs.
Fig. 10.

The slightly milky ascites was discharged from the inserted drainage.
On postoperative day (POD) 21, he has been discharged to another hospital for further diagnosis and treatment. The triglyceride level of abdominal fluid was 4,56 mmol/L. The HLG was consulted and performed. The percutaneous transhepatic HLG with ultrasound guidance using 8 mm Chiba needles to access the right and left portal lymph systems, but we did not find any lymphatic extravasations. (Fig. 11).
Fig. 11.
The Hepatic Lymphangiography with no extravasation was found.
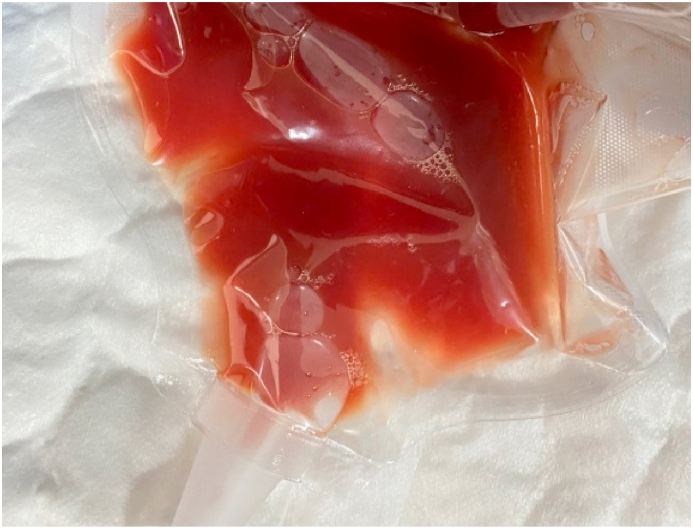
Though, after the HLG, A low-fat, middle-chain triglyceride (MCT) diet was prescribed for him, and drainage decreased significantly from about 1000 mL to approximately 100 mL of blood-like fluid in the following day (Fig. 12). The drain output remained low, and at the second day after the invasion, the drainage was 50 mL daily and all drains were removed.
Fig. 12.
The abdominal fluid in the following after invasion.
3. Discussion
Generally, there are three main sources of retroperitoneal lymphatic pathways, which is terminated in Cisterna chyli. They are two lumbar lymphatic trunks - lead to lower limb lymphoid and lower body part; intestinal lymphatic trunk - receive the lymphatic fluid and chyle from the stomach, intestine, pancreas, and spleen; and the hepatic lymphatic vessels – receive lymphatic fluid from the liver. Hepatic Lymphatic Vascular System, like in other organs, hepatic lymphatic vessels work as an immunological control as well as a tissue drainage system [10]. Three are totally three categories that the hepatic lymphatic vessels flow into, depending on their locations: portal, sub-lobular, and superficial (or capsular) lymphatic vessels [11]. About 80 % of hepatic lymph flow into portal lymph system, while the remainder drains through sub-lobular and capsular lymph system [12]. After draining toward the portal pedicle, much of hepatic lymphatic fluid goes through the coeliac region (LNs group no.9 according to Japanese gastric lymphatic stations [13]) and then to nodes located between the aorta and inferior vena cava under the renal vein (LNs group no.16a2 according to Japanese gastric lymphatic stations [13]), before reaching the thoracic duct [10].
Hepatic lymphatic leakage is an extremely type of abdominal lymphatic ascites, which was represented in a few case reports in English literature [7,14]. Normally, the liver produces a large volume of lymphatic fluid, accounted for about 25–50 % of lymph flowing through the thoracic duct [12], associated with a typical lymphatic flow of 0.25 mL/min [7]. So that hepatic lymphatic disruption caused a massive and difficult-to-treat condition of abdominal ascites.
In definition, according the statement by the International Study Group on Pancreatic Surgery (ISGPS), Chyle leak (CL) was considered if there was an output of milky-colored fluid from a drain, drain site, or wound on or after POD 3, with a high content of triglyceride level (≥110 mg/dL or ≥1.2 mmol/L) [15] and the cut-off values of volume of drainage range between ≥100 mL and >600 mL per day. In our research, only two of four patients had the triglyceride level of abdominal fluid over this range. This can be explained by the anatomical characteristics of the retroperitoneal lymphatic pathways which is mentioned below: the milky appearance of chyle is caused by fat-soluble vitamins and long-chain triglycerides (TG) incorporated in chylomicrons, which is absorbed to intestinal lymphatic trunk, which is not related directly to the hepatic lymphatic plexus. So that, and from the four cases, we suggested that the disruption of hepatic lymphatic plexus may lead to an atypical chylous leakage condition with a normal triglyceride level and clear appearance of abdominal fluid.
Following the severity as well as the management needed, there are three different grades were defined: grade A, which may be treated with oral dietary restrictions and no specific interventions; grade B, which may be lead to prolong of hospital stay, and need more interventions such as nasoenteral nutrition with dietary restriction, total parenteral nutrition, octreotide, maintenance of surgical drains, or placement of new percutaneous drains; and grade C, with the need of other invasive treatments, intensive care unit admission, or mortality [15]. All our four patients had the volume of all intra-abdominal drainages ranged between 1000 mL and 1500 mL per day from POD 15, which were classified as grade C, and they also had a fat – limited diet after surgery but it is failed to manage the symptoms. So that, more invasive therapies must proceed.
In incidence, a report by Singh H. et al. showed that the incidence of CL after pancreaticoduodenectomy was about 1.8–11.0 % [16]. Several studies revealed that the risk factors of CL including radical lymphadenectomy, concomitant vascular resection as well as associated with several complications like pancreatic fistula, and intra-abdominal abscess [17,18]. Assumpcao et al. suggested that the number of lymph nodes harvested, and vascular resection were independent risk factors for chylous ascites after pancreatic resection [19] and two other studies showed that early enteral feeding was an another independent risk factor [20,21]. In other studies, dissection of the paraaortic area, as well as some factors such that the extent of lymph node dissection, the presence of retroperitoneal invasion, chronic pancreatitis, and total number of lymph nodes resected were identified as risk factors for development of CL following hepatopancreatobiliary surgeries [19,[21], [22], [23], [24]], but only a review by van der Gaag et al. resumed that the lymphatic extravasation and fistula were often located from intestinal lymphatics, cisterna chyli, and the peritoneal cavity due to the related anatomy with the pancreatic head [24]. Two of our patients had the condition of venous invasion, and this leaded to a higher extended lymphadenectomy as well as vascular resection and reconstruction, all of them caused to improve the risk of CL, especially hepatic lymphatic disruption.
In diagnosis, sampling peritoneal fluid by paracentesis is gold standard for diagnosis of CL. Hypoalbuminemia and lymphocytopenia may be suggested as a progressive condition and reflecting a poor prognosis. On computed tomography, chylous fluid has a low attenuation, so that this imaging examination can be a useful tool to distinguished from acute hemorrhage. MR lymphangiography and intranodal lymphangiography have become feseable and safety examinations which can evaluate central lymphatic systems. But they cannot reveal the hepatic lymphatic systems. But they cannot reveal the hepatic lymphatic systems. Percutaneous HLG remains the gold standard in defining and treatment of cases of lymphatic obstruction and fistula.
Beside the use for diagnosis, lymphangiography is still considered as a novel therapeutic procedure and an alternative to conservative or surgical treatment of PLL. Recently, a systematic review by Sommer C.M. et al. summarized all data about HLG for treatment performed up to September 2019 in the PubMed database. The results were optimistic, with the technical success rate is 75–100%, and the inability to inject the contrast material into the lymphatic system selectively is one of the most reasons for technical failure [25]. Percutaneous transhepatic HLG is a newly developed technique which has many advantages in invasion of the intrahepatic and portal lymphatic systems. Technically, intrahepatic lymphatic vessels are non-visualized by ultrasound and difficult to access, however base on the anatomical characteristic that lymphatic vessels run into Glisson's sheath, so that they can be accessed parallel to portal vein by the Chiba needle [7]. In our three of four patients, we successfully accessed through intrahepatic lymphatic vessels percutaneously and, but in two cases, we didn't find any lymphatic fistula: in the 2nd case, we decided injected a 1:3 mixture of Aetoxisclerol to air into the culprit portal lymph plexus and successfully terminated the fistula, and in the 4th case, right after the invasion, blood-liked fluid discharged from the inserted drain and in following day, the patient's hepatic lymphorrhea was successfully treated, we hypothesized that our invasion caused the intrahepatic hemorrhage and embolize the lymphatic vessels and so on, terminated the lymphatic fistula's condition. In 3rd case, we were failure to access the intrahepatic and portal lymphatic vessels, so that we decided to inject fibral materials around the right and left portal veins, and this technique was successfully terminated the hepatic lymphorrhea's condition.
About the outcome of this complication, because chylous fluid consists of intestinal lymphatic fluid feeding with chylomicrons which was incorporated with fat-soluble vitamins and long-chain triglycerides (TG), the loss of chyle could lead to the condition of malnutrition and hypoproteinemia [2]. Similarly, while containing lymphocytes and immunoglobulins, loss of the lymphatic fluid results in lymphocytopenia, which make patients to be more susceptible to infection-related complications [24]. A retrospective study by Mohammed A.H. et al. showed that CL was associated with a significantly increased incidence of portal or mesenteric venous thrombosis [22]. A prospective study by Lia Assumpcao et al. reported other associated complications of CL: abscess (4.3 %), concomitant pancreatic fistula (4.3 %), malnutrition (e.g., albumin <3.5 mg/dL) (91.5 %), peritonitis (6.4 %), and sepsis (12.8 %) [19]. Hospital stay was considered as the main factor to assess the impact of CL on post – op result [17]. The grade A of isolated CL was not led to increase the length of hospital stay, while grade B or C on the other hand led to an increased hospital stay [24]. However, with coincidental CL, only grade C prolonged the hospital stay significantly [24]. In long – term outcome, few studies saw the significantly different results between groups of patients with or without CL complications [19]. The cohort study by O. Strobel et al. showed that the development of isolated CL and its grade or a condition of drainage volume at least 2000 mL per day were not associated with postoperative overall survival (OS) in the entire group of pancreatic-cancer patients, as well as in separate analyses of subgroups who had given potentially curative resection, bypass procedures or palliative exploration [17]. However, failure to manage the chyle leaks' condition in 14 days was associated with worse survival in patients who underwent palliative procedures significantly (in patients with failed treatment, the median OS was 5,2 months versus 16,4 months in patients whose chyle leak resolved, P = 0.016), but not be significant in patients who had curative resections (median OS 20,5 versus 23,8 months respectively, P = 0.877) [17]. So that, it is important to control this complication as soon as possible, especially in group of patients in advanced stage of malignancy diseases. Lymphangiography itself has several complications including the tissue necrosis, fat embolism and contrast agents’ hypersensitivity relating to the volume and type [2].
4. Conclusions
Hepatic lymphorrhea is a rare but difficult-to-treat and potentially life-threatening type of postoperative lymphatic leakage, especially following pancreaticoduodenectomy. Periportal lymphatic vessels was a potential location of lymphatic fistula and leakage, due to the regularity of isolation and dissection in pancreaticoduodenectomy, especially with extended lymphadenectomy. Percutaneous transhepatic (HLG) is a newly developed technique which has many advantages in intervention for treatment of hepatic lymphorrhea.
Ethical approval
The study was approved by the Research Ethics Committee of Hanoi Medical University. The procedures used in this study adhere to the tenets of the Declarations of Helsinki.
Sources of funding for your research
The authors declare no funding for this study.
Author contribution
Thanh Khiem NGUYEN: the main doctor conceived the original idea and operated the patients, wrote manuscript. Tuan Hiep LUONG: followed up, wrote manuscript. Ngoc Cuong NGUYEN: assessed the protocol, summed up, revised manuscript. Ham Hoi NGUYEN: operated the patients, summed up, revised manuscript. Van Khang LE: assessed the protocol, summed up, revised manuscript. Hong Son TRINH: summed up, revised manuscript. Hai Dang DO: operated the patients, summed up, revised manuscript. Van Duy LE: operated the patients, summed up, revised manuscript. Ngoc Hung NGUYEN: operated the patients, summed up, revised manuscript. All authors contributed to the interpretation of the results, discussed the results. All authors read and approved the final manuscript to submit.
Trial registry number
-
1.
Name of the registry:
-
2.
Unique identifying number or registration ID:
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Thanh Khiem NGUYEN, MD. PhD.
Department of Gastrointestinal and Hepato-pancreato-biliary surgery, Bach Mai Hospital, Hanoi, Vietnam.
Email: khiemnguyenthanh@yahoo.com.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102648.
Contributor Information
Thanh Khiem Nguyen, Email: khiemnguyenthanh@yahoo.com.
Tuan Hiep Luong, Email: Hiep1995hsgs@gmail.com.
Ngoc Cuong Nguyen, Email: cuongcdha@hmuh.vn.
Ham Hoi Nguyen, Email: Hamhoint30@gmail.com.
Van Khang Le, Email: drkhang2006@gmail.com.
Hong Son Trinh, Email: thsonvd@yahoo.com.
Hai Dang Do, Email: dangsp94@gmail.com.
Van Duy Le, Email: leduydr.2010@gmail.com.
Ngoc Hung Nguyen, Email: nnh@bachmai.edu.vn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Misthos P., Kanakis M.A., Lioulias A.G. Chylothorax complicating thoracic surgery: conservative or early surgical management? Updates Surg. 2012;64(1):5–11. doi: 10.1007/s13304-012-0133-8. [DOI] [PubMed] [Google Scholar]
- 2.Lv S. A review of the postoperative lymphatic leakage. Oncotarget. 2017;8(40):69062–69075. doi: 10.18632/oncotarget.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strobel O. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann. Surg. 2015;261(5):961–969. doi: 10.1097/SLA.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 4.Tol J.A. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156(3):591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans J.G. Chylous ascites after post-chemotherapy retroperitoneal lymph node dissection: review of the M. D. Anderson experience. J. Urol. 2006;176(4 Pt 1):1463–1467. doi: 10.1016/j.juro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Ablan C.J., Littooy F.N., Freeark R.J. Postoperative chylous ascites: diagnosis and treatment. A series report and literature review. Arch. Surg. 1990;125(2):270–273. doi: 10.1001/archsurg.1990.01410140148027. [DOI] [PubMed] [Google Scholar]
- 7.Kojima M. Successful treatment of hepatic lymphorrhea by percutaneous transhepatic lymphangiography followed by sclerotherapy using OK-432. Surg Case Rep. 2019;5(1):203. doi: 10.1186/s40792-019-0761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N for the PROCESS Group The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Zins M., Matos C., Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology. 2018;287(2):374–390. doi: 10.1148/radiol.2018171670. [DOI] [PubMed] [Google Scholar]
- 10.Moszkowicz D. Routine pedicular lymphadenectomy for colorectal liver metastases. J. Am. Coll. Surg. 2012;214(6):e39–45. doi: 10.1016/j.jamcollsurg.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Lupinacci R.M. Lymphatic drainage of the liver and its implications in the management of colorectal cancer liver metastases. Updates Surg. 2014;66(4):239–245. doi: 10.1007/s13304-014-0265-0. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani O., Ohtani Y. Lymph circulation in the liver. Anat. Rec. 2008;291(6):643–652. doi: 10.1002/ar.20681. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guez D. Transhepatic lymphatic embolization of intractable hepatic lymphorrhea. J. Vasc. Intervent. Radiol. 2014;25(1):149–150. doi: 10.1016/j.jvir.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Besselink M.G. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365–372. doi: 10.1016/j.surg.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Singh H. Management of chylous ascites following pancreaticobiliary surgery. JGH Open. 2019;3(5):425–428. doi: 10.1002/jgh3.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobel O. Incidence, risk factors and clinical implications of chyle leak after pancreatic surgery. Br. J. Surg. 2017;104(1):108–117. doi: 10.1002/bjs.10316. [DOI] [PubMed] [Google Scholar]
- 18.Koga C. [A case of successful management of postoperative chylous ascites by combination therapy with octreotide and etilefrine] Gan To Kagaku Ryoho. 2018;45(3):572–574. [PubMed] [Google Scholar]
- 19.Assumpcao L. Incidence and management of chyle leaks following pancreatic resection: a high volume single-center institutional experience. J. Gastrointest. Surg. 2008;12(11):1915–1923. doi: 10.1007/s11605-008-0619-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.K. Drainage volume After pancreaticoduodenectomy is a warning sign of chyle leakage that inversely correlates with a diagnosis of pancreatic fistula. World J. Surg. 2013;37(4):854–862. doi: 10.1007/s00268-013-1919-7. [DOI] [PubMed] [Google Scholar]
- 21.Kuboki S. Chylous ascites after hepatopancreatobiliary surgery. Br. J. Surg. 2013;100(4):522–527. doi: 10.1002/bjs.9013. [DOI] [PubMed] [Google Scholar]
- 22.Abu Hilal M. Postoperative chyle leak after major pancreatic resections in patients who receive enteral feed: risk factors and management options. World J. Surg. 2013;37(12):2918–2926. doi: 10.1007/s00268-013-2171-x. [DOI] [PubMed] [Google Scholar]
- 23.Pan W. Incidence and risk factors of chylous ascites after pancreatic resection. Int. J. Clin. Exp. Med. 2015;8(3):4494–4500. [PMC free article] [PubMed] [Google Scholar]
- 24.van der Gaag N.A. Chylous ascites after pancreaticoduodenectomy: introduction of a grading system. J. Am. Coll. Surg. 2008;207(5):751–757. doi: 10.1016/j.jamcollsurg.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Sommer C.M. Conventional lymphangiography (CL) in the management of postoperative lymphatic leakage (PLL): a systematic review. Röfo. 2020;192(11):1025–1035. doi: 10.1055/a-1131-7889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.