Abstract
Objective
The aim of this study was to provide an interim safety analysis of the first 30 surgical procedures performed using the Versius Surgical System.
Background
Robot-assisted laparoscopy has been developed to overcome some of the important limitations of conventional laparoscopy. The new system is currently undergoing a first-in-human prospective clinical trial to confirm the safety and effectiveness of the device when performing minimal access surgery (MAS).
Methods
Procedures were performed using Versius by a lead surgeon supported by an operating room (OR) team. Male or female patients aged between 18 and 65 years old and requiring elective minor or intermediate gynaecological or general surgical procedures were enrolled. The primary endpoint was the rate of unplanned conversion of procedures to other MAS or open surgery.
Results
The procedures included nine cholecystectomies, six robot-assisted total laparoscopic hysterectomies, four appendectomies, five diagnostic laparoscopy cases, two oophorectomies, two fallopian tube recanalisation procedures, an ovarian cystectomy and a salpingo-oophorectomy procedure. All procedures were completed successfully without the need for conversion to MAS or open surgery. No patient returned to the OR within 24 h of surgery and readmittance rate at 30 and 90 days post-surgery was 1/30 (3.3%) and 2/30 (6.7%), respectively.
Conclusions
This first-in-human interim safety analysis demonstrates that the Versius Surgical System is safe and can be used to successfully perform minor or intermediate gynaecological and general surgery procedures. The cases presented here provide evidence that the Versius clinical trial can continue to extend recruitment and begin to include major procedures, in alignment with the IDEAL-D Framework Stage 2b: Exploration.
Electronic supplementary material
The online version of this article (10.1007/s00464-020-08014-4) contains supplementary material, which is available to authorised users.
Keywords: Clinical trial, Minimally invasive surgical procedure, Robotic surgical procedures, Gynaecologic surgical procedures, Hysterectomy, Cholecystectomy
Minimal access surgery (MAS) can help minimise blood loss, reduce post-operative complications and post-operative pain, shorten hospital stays and accelerate recovery times [1, 2]. However, MAS is associated with specific challenges; for example, the range of surgical movement is restricted, and the technique is associated with unfavourable ergonomics for the surgeon and bedside assistant. Surgeon competency is generally associated with a steep learning curve and longer training requirements. This has resulted in suboptimal MAS uptake and ultimately fewer patients benefitting from MAS [3–7].
Robot-assisted MAS has made progress in overcoming these challenges by providing a stable magnified three-dimensional view, tremor filtration, motion scaling and articulated or wristed instruments with greater degrees of movement, allowing for precise tissue dissection and suturing [8–12]. As a result, robotic surgery extends the feasibility of MAS to patients with more complex pathology and higher body mass indices (BMI), allowing a wider range of patients to benefit from the advantages of MAS [11, 13, 14]. Furthermore, surgical robots can be designed to improve the experience of the surgeon and surgical team, enhancing both career longevity and surgical outcomes [15].
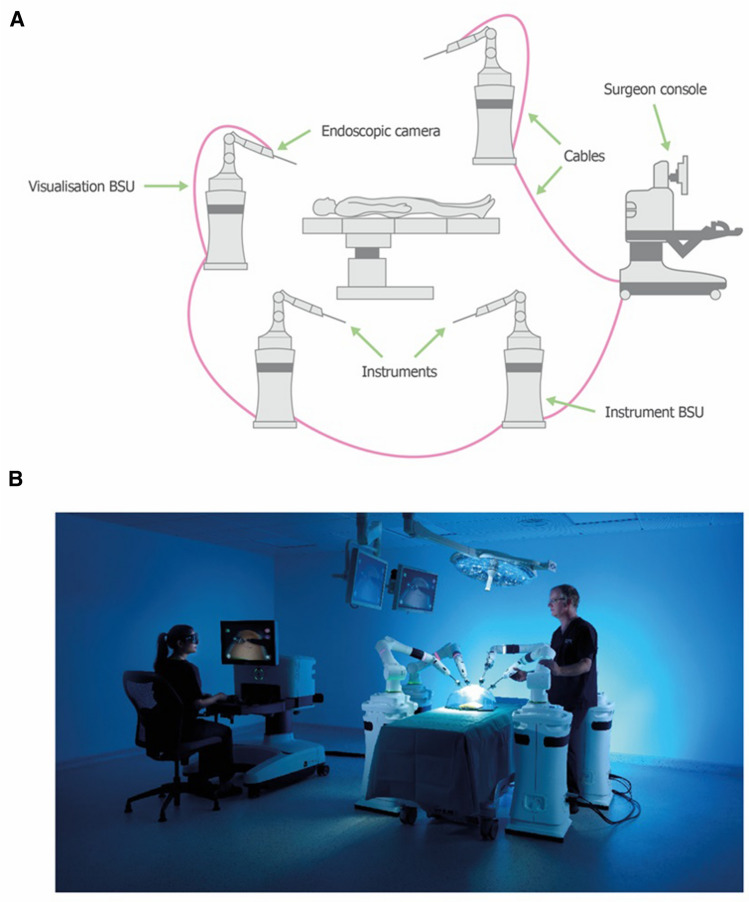
The Versius Surgical System is a new tele-operated robotic surgical system (CMR Surgical, Cambridge, UK) designed to assist surgeons in performing MAS. The surgical system was developed using feedback from surgeons and surgical teams, aiming to improve both end-user experience and surgical outcomes [15]. Specifically, Versius has been designed to mimic the articulation of the human arm, with the wristed instrument tip providing seven degrees of freedom inside the patient, allowing greater surgical access compared with standard laparoscopic surgery (Fig. 1). The open console design allows surgeons to sit or stand and enables easier communication between the surgeon and the team, facilitating both training and teaching. The surgeon interacts with the system through hand controls—which mirror the general design of video gaming controllers—and visual feedback on the surgeon console. The console’s head-up display (HUD) relays the three‑dimensional video from the endoscopic camera together with a display overlay. Each instrument and visualisation arm is attached to its own wheeled cart to form a compact and mobile bedside unit (BSU), providing maximum flexibility in the operating room (OR) [15].
Fig. 1.
Overview of the Versius Surgical System.
Adapted from Haig et al. [16]. A Schematic representation of the setup of Versius. B An image of the real-world setup of Versius. BSU: bedside unit
The operational safety and ease of use of the system was validated previously in a human cadaver study [16]. Likewise, the feasibility of Versius for transanal and mesorectal excision has also been evaluated [17]. Additional preclinical studies demonstrated that Versius can be used to successfully complete a range of gynaecological, urological, renal and general surgical procedures in both cadavers and live porcine studies [18–20].
These preclinical studies have fulfilled Stage 0 of the IDEAL-D (Idea, Development, Exploration, Assessment, Long-term follow-up) Framework—a set of recommendations for improving the evidence base from research at each stage of surgical innovation [21, 22]. The goal of the initial preclinical and Idea stages (Stage 0 and 1) is proof of concept—this was demonstrated through usability studies and procedure-specific assessment in cadavers and porcine models. [15, 16, 18–20]. These studies provided an opportunity to optimise the operational set up of the robot by confirming optimal BSU positions, port placings and procedural steps. Difficulties identified at this stage were corrected prior to proceeding to live clinical work.
The study reported herein aims to complete Stage 2a: Development. This stage seeks to refine the technique and report prospective development studies to demonstrate safety and success in performing the procedure in live humans [21, 22]. Versius is currently undergoing a prospective clinical trial to confirm the safety and effectiveness of the device when performing MAS procedures. This study reports the first-in-human use of Versius and aimed to (1) provide an initial safety analysis of 30 patients requiring minor or intermediate gynaecological or general surgery procedures, and (2) demonstrate system safety to support larger clinical trials (in line with the IDEAL-D Framework) [1, 22].
Methods
Ethical board review statement
Procedures were completed at the Deenanath Mangeshkar Hospital and Research Center, Erandwane, Pune, Maharashtra 411004, India, between 6 March and 2 April 2019. This study was reviewed and approved by the Institutional Ethics Committee, Deenanath Mangeshkar Hospital & Research Center. Approval for the study was received on 23 February 2019. This study has been registered on the Indian Clinical Trials Register (CTRI/2019/02/017872). All study activities were performed in compliance with ICH Good Clinical Practice Schedule Y, Indian Council of Medical Research and ISO14511 standards.
Study population
Potential patients were identified from Deenanath Mangeshkar Hospital surgical lists and approached directly by their surgeon or clinical team between the 4 March and 2 April 2019. Male or female patients aged between 18 and 65 years old and deemed suitable for at least one surgical procedure using Versius were enrolled. After being provided with relevant study information, patients provided written and audio-visual consent. Patients were again asked for confirmation of consent before the start of surgery. In keeping with the requirements of the Institutional Ethics Committee, Deenanath Mangeshkar Hospital & Research Center and in addition to the signed written consent, the consenting process was filmed and retained.
Patients requiring the following elective minor or intermediate gynaecological or general surgical procedures were eligible for the study: salpingectomy (unilateral or bilateral), salpingo-oophorectomy, oophorectomy (unilateral or bilateral), ovarian cystectomy for benign disease, robot-assisted total laparoscopic hysterectomy (RALH), appendectomy, cholecystectomy or diagnostic laparoscopic procedures.
All patients were suitable for MAS and baseline demographics were recorded at the time of screening. For a full list of inclusion/exclusion criteria see Supplemental Table 1.
Table 1.
Summary of patient clinical data
| Case/procedure | Primary diagnosis/indication | Gender | Age (years) | BMI (kg/m2) | Previous surgery?* Within the last year? |
|---|---|---|---|---|---|
| 1. Diagnostic laparoscopy | Primary infertility | Female | 34 | 18.5 | N |
| 2. Ovarian cystectomy and endometriosis (Grade IV)† | Ovarian cyst | Female | 23 | 22.4 | N |
| 3. Laparoscopic oophorectomy and endometriosis (Grade III)† | Left ovarian dermoid cyst | Female | 38 | 23.0 | N |
| 4. Hysteroscopy, laparoscopy cannulation, polycystic ovarian drilling | Secondary infertility with bilateral tubal block | Female | 30 | 29.9 | N |
| 5. Diagnostic laparoscopy sos cannulation | Primary infertility | Female | 28 | 23.2 | N |
| 6. Secondary infertility with left tubal block for recanalisation | Secondary infertility and left tubal block | Female | 37 | 20.2 | Y; not within the last year |
| 7. Diagnostic laparohysteroscopy | Secondary infertility for laparoscopy | Female | 30 | 28.6 | Y; not within the last year |
| 8. Diagnostic laparoscopy | Primary infertility | Female | 30 | 29.0 | N |
| 9. Appendectomy | Appendicitis | Male | 31 | 15.5 | N |
| 10. Bilateral oophorectomy | Breast cancer, advised oophorectomy | Female | 48 | 18.6 | N |
| 11. Cholecystectomy | Cholelithiasis | Female | 40 | 27.0 | N |
| 12. Appendectomy | Appendicitis | Female | 34 | 14.0 | N |
| 13. RALH | Uterine bleeding with adenomyosis | Female | 48 | 24.8 | N |
| 14. Diagnostic laparoscopy | Primary infertility | Female | 35 | 24.0 | N |
| 15. RALH | Abnormal uterine bleeding | Female | 48 | 22.6 | Y; within the last year |
| 16. RALH | Pelvic inflammatory disease | Female | 28 | 25.9 | Y; not within the last year |
| 17. Cholecystectomy | Cholelithiasis | Female | 41 | 33.4 | N |
| 18. Right salpingo-oophorectomy | Right ovarian complex cyst | Female | 40 | 28.0 | N |
| 19. Cholecystectomy | Cholelithiasis | Male | 53 | 23.8 | N |
| 20. Appendectomy | Appendicitis | Male | 30 | 34.3 | N |
| 21. Appendectomy | Appendicitis | Female | 27 | 19.2 | N |
| 22. Cholecystectomy | Cholelithiasis | Female | 33 | 27.4 | N |
| 23. RALH | Adenomyosis with fundal fibroid | Female | 44 | 29.0 | N |
| 24. Cholecystectomy | Cholelithiasis | Female | 42 | 24.0 | N |
| 25. Cholecystectomy | Cholelithiasis | Male | 38 | 28.3 | N |
| 26. Hysterectomy with salpingo-oophorectomy | Adenomyosis, endometriosis | Female | 38 | 33.5 | Y; not within the last year |
| 27. Cholecystectomy | Cholelithiasis | Female | 39 | 39.8 | Y; not within the last year |
| 28. Cholecystectomy | Pancreatitis with cholelithiasis | Male | 64 | 22.6 | N |
| 29. Cholecystectomy | Cholelithiasis | Female | 46 | 32.6 | Y; not within the last year |
| 30. RALH | Menorrhagia with adenomyosis with adenomyoma | Female | 37 | 25.0 | Y; not within the last year |
*Pelvic/abdominal surgery. †Endometriosis only discovered after insertion of endoscope
BMI: body mass index; N: no; RALH: robot-assisted total laparoscopic hysterectomy; Y: yes
Study design
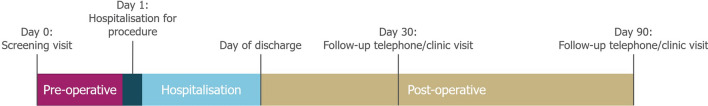
Following patient screening, hospitalisation and discharge, patients had follow-up clinical visits or telephone calls at 30 and 90 days post-operation (Fig. 2). Patients were under daily post-operative surveillance while an in-patient and the next case was not initiated until the surgical team and chief medical officer were satisfied that the preceding case was a success and the patient was not experiencing any adverse effects due to suboptimal performance of Versius. Accordingly, the first 10 cases were deliberately chosen to be minor or diagnostic procedures, before attempting more complex cases.
Fig. 2.
Schematic overview of the study design
The primary endpoint was the rate of unplanned conversion of procedures to other MAS techniques or open surgery. Secondary endpoints included intra-operative complications, complications occurring during hospital stay or within 90 days after discharge. All post-operative complications were graded according to the Clavien–Dindo classification [23]. Additional secondary endpoints included: intra‑operative blood transfusion, estimated intra‑operative blood loss, return to the OR within 24 h, return to the OR after 24 h, readmission to hospital within 30 and/or 90 days, operative time (from incision to skin closure), length of hospital stay and 90‑day mortality. BSU and port positions in relation to anatomical landmarks were also recorded.
Surgical team and system setup
Procedures were performed by a lead surgeon supported by an OR team. The lead surgeon performed the surgical steps for the procedure from the surgeon console. The bedside assistant manipulated the robotic arms and carried out any additional manual tasks as instructed by the lead surgeon. All members of the surgical team completed and passed the validated 3.5-day Versius training programme prior to the start of the study, as per the Versius training protocol [24]. The six lead surgeons who performed the procedures were accredited, practising, high-volume gynaecological or general consultant surgeons.
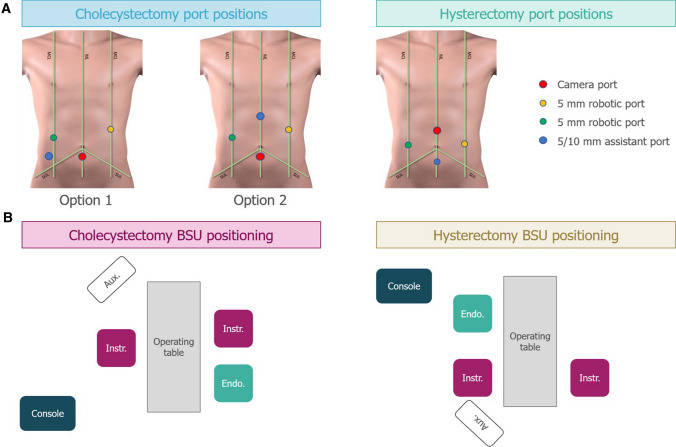
The port placement for cholecystectomy and RALH procedures are shown in Fig. 3a. For cholecystectomy procedures, the camera port was positioned up to 2 cm below the umbilicus on the midline, with a 5 mm robotic port on the right and left midclavicular line (MCL). Either a 5 mm or 10 mm assistant port was positioned either superior to the iliac crest (option 1) or in the epigastrium (Fig. 3a; option 2). For high BMI patients, the camera port was positioned above the umbilicus. For RALH procedures, the camera port was positioned up to 2 cm above the umbilicus on the midline, with a 5 mm robotic port on the right and left MCL, at the level of the umbilicus. A 5 mm assistant port was positioned below the umbilicus at the midline. For high BMI patients, the camera port was positioned below the umbilicus. The most frequent operational setup for cholecystectomy and RALH is represented in Fig. 3b.
Fig. 3.
Common operative setup for cholecystectomy and hysterectomy procedures. A Common port positions for cholecystectomy and hysterectomy procedures with corresponding BSU positions shown below in B. The assistant port was for nonrobotic laparoscopic instruments. Umbilicus is where the ML crosses the SUL. Aux: auxiliary monitor; BSU: bedside unit; Console: surgeon console; Endo: endoscope; Instr: instrument; MCL: midclavicular line; ML: midline; SUL: supine-umbilical line
Results
Patient demographics and procedures
Of the 30 patients included in the analysis, the majority were female (83.3% female, 16.7% male), with a median age of 37.5 years (range: 23–64 years; Table 1). Median BMI was 24.9 kg/m2 (range: 14.0–39.8 kg/m2) and all but one patient (Case 15) had not undergone pelvic or abdominal surgery within the last year.
In total, 13 general surgical procedures were performed: 9 cholecystectomies with a primary diagnosis of cholelithiasis and 4 appendectomies with a primary diagnosis of appendicitis. The majority of the gynaecology cases required RALH (six cases) or diagnostic laparoscopy (five cases); the remaining cases required oophorectomy (two cases), fallopian tube recanalisation (two cases), ovarian cystectomy (one case), or salpingo-oophorectomy (one case). The primary indication for RALH was bleeding abnormalities resistant to conservative management. A complete list of specific diagnoses and procedures is shown in Table 1.
Patient outcomes
No procedure required conversion to conventional MAS or open surgery, all procedures were completed successfully and there were no intra-operative complications. The procedures were performed according to the procedural steps described in the preclinical studies, therefore there was no need to modify steps during this clinical phase [18, 19]. Intra-operative blood loss was estimated as negligible (< 5 mL) for 19/30 (63.3%) procedures or minimal (< 500 mL) for 11/30 (36.7%) procedures; only one case (3.3%; Case 15) required the use of blood transfusion products (required post-operatively and not related to intra-operative blood loss reported). No patient returned to the OR within or after 24 h of surgery, and readmittance rate at 30 and 90 days was 1/30 (3.3%) and 2/30 (6.7%), respectively. Both cases (Cases 24 and 25) were acute gastroenteritis of Clavien–Dindo Grade I and were not related to the surgical device. Patients were treated symptomatically with pain killers and antiemetics and made a full recovery. Median operative time was 120 min (range: 35–306 min; Table 2); extended operating times reflect the degree of caution taken by the surgical team as they gained familiarity with the system and instrumentation. Median length of hospital stay was 3 days (range: 2–10 days) and 90-day mortality was 0% with all patients completing the study.
Table 2.
Summary of patient outcome data
| Case/procedure | Operative time (mins) | Conversion? | Estimated intra-operative blood loss (mL); blood transfusion required? | Return to OR within 24 h? | Length of hospital stay (days) | Intra-and post-operative complications | Readmitted to hospital |
|---|---|---|---|---|---|---|---|
| 1. Diagnostic laparoscopy | 75 | N | < 5; N | N | 2 | N | N |
| 2. Ovarian cystectomy and endometriosis (Grade IV)† | 165 | N | < 500; N | N | 2 | N | N |
| 3. Laparoscopic oophorectomy and endometriosis (Grade III)† | 75 | N | < 5; N | N | 2 | N | N |
| 4. Hysteroscopy laparoscopy sos cannulation sos polycystic ovarian drilling | 60 | N | < 5; N | N | 2 | N | N |
| 5. Diagnostic laparoscopy sos cannulation | 45 | N | < 5; N | N | 2 | N | N |
| 6. Secondary infertility with left tubal block for recanalisation | 35 | N | < 5; N | N | 2 | N | N |
| 7. Diagnostic laparohysteroscopy | 60 | N | < 5; N | N | 2 | N | N |
| 8. Diagnostic laparoscopy | 90 | N | < 5; N | N | 2 | N | N |
| 9. Appendectomy | 90 | N | < 5; N | N | 4 | N | N |
| 10. Bilateral oophorectomy | 60 | N | < 500; N | N | 3 | N | N |
| 11. Cholecystectomy | 150 | N | < 500; N | N | 2 | N | N |
| 12. Appendectomy | 80 | N | < 5; N | N | 3 | N | N |
| 13. RALH | 210 | N | < 5; N | N | 4 | N | N |
| 14. Diagnostic laparoscopy | 45 | N | < 500; N | N | 3 | N | N |
| 15. RALH | 120 | N | < 5; Y | N | 7 | N | N |
| 16. RALH | 120 | N | < 5; N | N | 3 | N | N |
| 17. Cholecystectomy | 60 | N | < 5; N | N | 2 | N | N |
| 18. Cholecystectomy | 120 | N | < 5; N | N | 3 | N | N |
| 19. Cholecystectomy | 150 | N | < 500; N | N | 3 | N | N |
| 20. Appendectomy | 120 | N | < 5; N | N | 7 | N | N |
| 21. Appendectomy | 135 | N | < 5; N | N | 2 | N | N |
| 22. Cholecystectomy | 150 | N | < 5; N | N | 3 | N | N |
| 23. RALH | 210 | N | < 5; N | N | 4 | N | N |
| 24. Cholecystectomy | 135 | N | < 500; N | N | 2 | Y;POa | Y;a within 30 days |
| 25. Cholecystectomy | 230b | N | < 500; N | N | 10 | Y;POa | Y;a within 90 days |
| 26. Hysterectomy with salpingo-oophorectomy | 306c | N | < 500; N | N | 4 | N | N |
| 27. Cholecystectomy | 120 | N | < 500; N | N | 3 | N | N |
| 28. Cholecystectomy | 275 | N | < 500; N | N | 7 | N | N |
| 29. Cholecystectomy | 195 | N | < 500; N | N | 2 | N | N |
| 30. RALH | 140 | N | < 5; N | N | 6 | N | N |
N: no; OR: operating room; PO: post-operative; RALH: robot-assisted laparoscopic hysterectomy; Y: yes
aReadmitted due to acute gastroenteritis, not related to the device
bRequired additional time to remove extensive port site adhesions and adhesions covering Calot’s Triangle
cExtensive endometriosis was surgically treated before performing the hysterectomy. †Endometriosis only discovered after insertion of endoscope
There were two cases (Cases 2 and 3) of Grade III–IV endometriosis, only identified on insertion of the endoscope. The operating surgeon and the chief medical officer decided to proceed, on the proviso that the cases were being performed as safely as they would be with conventional surgery. The diagnoses did, however, influence operative times; 75 min for laparoscopic oophorectomy with Grade III endometriosis (Case 3) and 165 min for ovarian cystectomy with Grade IV endometriosis (Case 2). In addition, two other cases had extended operating times, a cholecystectomy (case 25) and hysterectomy (case 26) respectively. Case 25 required additional time to remove extensive port site adhesions and adhesions covering Calot’s Triangle. While extensive endometriosis was surgically treated before performing the hysterectomy in case 26. All cases were completed successfully.
Discussion
Overall, this first-in-human interim safety analysis demonstrates that Versius is safe and feasible for use in performing minor and intermediate gynaecological and general surgery procedures. No intra-operative complications were recorded and none of the procedures required conversion to open surgery or conventional MAS. Furthermore, estimated intra-operative blood loss was negligible for 63.3% (19/30) of cases, while the remaining cases reported minimal blood loss. There was no return to the OR within or after 24 h and most patients were discharged after 3 days; however, this ranged between 2 and 10 days. A hospital stay of longer than three days is a standard precaution taken at the study institution for all RALH and cholecystectomy cases (robotic or otherwise), due to the distance between a patient’s home and the hospital and the affordability of repeated travel. It is not related to additional post-operative complications or safety of surgery with Versius.
The versatility of the system enabled procedures to be successfully completed in a wide range of patient BMIs. Over the course of the study, only two patients (6.7%) were readmitted to hospital due to acute gastroenteritis which was not device‑related. The successful completion of the first 30 cases justifies continuation of the clinical trial.
All robot-assisted surgical devices can potentially fail during procedures and cause harm or damage to internal structures or organs [25]. Versius had first undergone rigorous preclinical testing and surgical teams had been extensively trained in the use of the system to minimise the risk in this study [15–18, 20, 24]. Moreover, device safety was continuously monitored throughout surgery and each case was considered individually. Accordingly, minor cases were selected first to allow the surgeon and OR teams to gain live-surgery experience using Versius in the least stressful environment possible. However, two cases of endometriosis, only identified on insertion of the endoscope, were beyond the case complexity intended. A decision was made to proceed as the surgeon felt confident that they were within the reach of the system. These procedures were completed in the presence of the chief medical officer, who continually monitored the safe continuation of the surgery. Both cases were completed successfully and demonstrated the ability of the system to deal with advanced dissection required for treating Grade IV endometriosis, a key umbrella and indicated procedure. In addition, several more complex procedures such as RALH and cholecystectomy were successfully performed using Versius with no intra-operative complications.
Performing a procedure with a new complex surgical device is expected to be associated with a slower time of surgery as the teams gain familiarity with the system and instrumentation (e.g. not only the surgeon but also the bedside team moving Versius during the procedure). Care was being taken to ensure patient safety, hence longer operating times were recorded early in the cohorts. As hospital teams become increasingly familiar and confident, it is anticipated that operative times will decrease. However, as safety is of paramount importance, conclusions drawn from metrics such as operative times should be moderated and not taken as an authoritative measure of patient outcome.
New users of any surgical robotic system will undergo a learning curve during the training period in which they develop the skills required to safely and effectively operate the device during surgical procedures [26, 27]. To aid effective training, all study participants completed a 3.5-day-long residential training programme, representative of commercial training. The Versius training programme was developed to include both didactic and practical, hands-on training and incorporate tasks designed to develop the motor and cognitive skills required to achieve competency in using Versius. The successful completion of all procedures undertaken demonstrates the high level of competency achieved. Additionally, completion of the more complex endometriosis cases, demonstrate that the operating surgeon (who routinely operates on advanced grades of endometriosis) was confident the system provided the same surgical ability as a conventional, straight stick system.
Study limitations
The cases presented in this study represent the first-in-human use of Versius and, as such, place additional pressures on the lead surgeons and their surgical teams. Consequently, extreme care and caution was taken throughout the procedures and may not be entirely representative of how they would be performed on a routine basis. It is anticipated that with more experience, surgical outcomes such as operative time will decrease.
Conclusion and future perspectives
This study shows promising first-in-human clinical trial data that support previous preclinical study results [18–20]. All 30 cases presented in this analysis were completed successfully and provide evidence supporting continued recruitment into the trial and inclusion of major surgical procedures. Continuation and expansion of this trial will ensure continued alignment with the IDEAL-D Framework and aim to demonstrate evidence of framework Stage 2b: Exploration. The goal of this stage is to build upon the technique established and expand the patient base to > 100 [21, 22]. Increasing the number of operations completed with Versius will demonstrate the safety and efficacy of the robotic system and allow for comparison with other available robotic systems.
Disclosures
DK: None declared; MB: None declared; GG: None declared; UK: None declared; MS: Chief Medical Officer and founder of CMR Surgical.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Marc Lynch, PhD, Emma Phillips, PhD, and Simon Foulcer, PhD, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Author contributions
Substantial contributions to study conception and design: MS; substantial contributions to analysis and interpretation of the data: DK, MB, GG, UK, MS; drafting the article or revising it critically for important intellectual content: DK, MB, GG, UK, MS; final approval of the version of the article to be published: DK, MB, GG, UK, MS.
Funding
This study was sponsored by CMR Surgical. This article was based on the original studies TR-0732, TR-0913, TR-1326, TR-1518 and TR-0883 sponsored by CMR Surgical. Support for third-party writing assistance for this article, was funded by CMR Surgical in accordance with Good Publication Practice (GPP3) guidelines (https://www.ismpp.org/gpp3).
Compliance with ethical standards
Conflict of interest
Dr. Slack is a founding board member and Chief Medical Officer of CMR Surgical. Drs. Kelkar, Borse, Godbole and Kurlekar have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He H, Zeng D, Ou H, Tang Y, Li J, Zhong H. Laparoscopic treatment of endometrial cancer: systematic review. J Minim Invasive Gynecol. 2013;20:413–423. doi: 10.1016/j.jmig.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y-z, Deng L, Xu H-c, Zhang Y, Liang Z-q. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. doi: 10.1186/s12885-015-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Gazala M, Wexner SD. Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol Rep. 2017;5:1–10. doi: 10.1093/gastro/gox001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chand M, Bhoday J, Brown G, Moran B, Parvaiz A. Laparoscopic surgery for rectal cancer. J R Soc Med. 2012;105:429–435. doi: 10.1258/jrsm.2012.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 6.Cooper MA, Hutfless S, Segev DL, Ibrahim A, Lyu H, Makary MA. Hospital level under-utilization of minimally invasive surgery in the United States: retrospective review. BMJ (Clin Res ed) 2014;349:g4198. doi: 10.1136/bmj.g4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins AT, Ford MM, Benjamin Hopkins M, Muldoon RL, Wanderer JP, Parikh AA, Geiger TM. Barriers to laparoscopic colon resection for cancer: a national analysis. Surg Endosc. 2018;32:1035–1042. doi: 10.1007/s00464-017-5782-8. [DOI] [PubMed] [Google Scholar]
- 8.Barrera RJO. The surgical robot: applications and advantages in general surgery. Surgical robotics. London: IntechOpen; 2017. [Google Scholar]
- 9.Bouquet de Joliniere J, Librino A, Dubuisson J-B, Khomsi F, BenAli N, Fadhlaoui A, Ayoubi JM, Feki A. Robotic surgery in gynecology. Front Surg. 2016 doi: 10.3389/fsurg.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayaraman S, Davies W, Schlachta CM. Getting started with robotics in general surgery with cholecystectomy: the Canadian experience. Canad J Surg J Canad Chirurgie. 2009;52:374–378. [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrie TA, Liu H, Lu D, Dowswell T, Song H, Wang L, Shi G. Robot-assisted surgery in gynaecology. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD011422.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orady M, Hrynewych A, Nawfal AK, Wegienka G. Comparison of robotic-assisted hysterectomy to other minimally invasive approaches. J Soc Laparoendosc Surg. 2012;16:542. doi: 10.4293/108680812X13462882736899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buderath P, Aktas B, Heubner M, Kimmig R. Robot-assisted hysterectomy: a critical evaluation. Robot Surg Res Rev. 2015;2:51–58. [Google Scholar]
- 14.Morris B. Robotic surgery: applications, limitations, and impact on surgical education. Medscape Gen Med. 2005;7:72. [PMC free article] [PubMed] [Google Scholar]
- 15.Hares L, Roberts P, Marshall K, Slack M. Using end-user feedback to optimize the design of the versius surgical system, a new robot-assisted device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2019;1:e000019. doi: 10.1136/bmjsit-2019-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haig F, Chitty K, Medeiros A, Slack M. Usability assessment of versius, a minimally invasive robot-assisted surgical device. BMJ Surg Interv Health Technol. 2020;2:e000028. doi: 10.1136/bmjsit-2019-000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atallah S, Parra-Davila E, Melani AGF. Assessment of the versius surgical robotic system for dual-field synchronous transanal total mesorectal excision (taTME) in a preclinical model: will tomorrow's surgical robots promise newfound options? Tech Coloproctol. 2019;23:471–477. doi: 10.1007/s10151-019-01992-1. [DOI] [PubMed] [Google Scholar]
- 18.Carey M, Bali A, Pandeva I, Pradhan A, Slack M. Preclinical evaluation of a new obot-assisted surgical system for use in gynecology minimal access surgery. Gynecol Surg. 2020;17:2. doi: 10.1186/s10397-020-01069-0. [DOI] [Google Scholar]
- 19.Morton J, Hardwick RH, Tilney HS, Gudgeon AM, Jah A, Stevens L, Marecik S, Slack M. Preclinical evaluation of versius, a new robotic-assisted surgical device for use in minimal access general and colorectal procedures. Surg Endosc. 2020 doi: 10.1007/s00464-020-07622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas BC, Slack M, Hussain M, Barber N, Pradhan A, Dinneen E, Stewart GD. Preclinical evaluation of a new robot-assisted surgical system for use in renal and prostate minimal access surgery. Eur Urol Focus. 2020 doi: 10.1007/s00464-020-07622-4. [DOI] [PubMed] [Google Scholar]
- 21.Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J, Rovers M, Blencowe N, Pennell C, Quinn T, Rogers W, Cook J, Kolias AG, Agha R, Dahm P, Sedrakyan A, McCulloch P. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg. 2019;269:211–220. doi: 10.1097/SLA.0000000000002794. [DOI] [PubMed] [Google Scholar]
- 22.McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterworth J, Sadry M, Julian D, Haig F (2020) Assessment of the training programme for versius, a new innovative robot-assisted surgical device for use in minimal access surgery. Manuscript submitted
- 25.Alemzadeh H, Raman J, Leveson N, Kalbarczyk Z, Iyer RK. Adverse events in robotic surgery: a retrospective study of 14 years of FDA data. PLoS ONE. 2016;11:e0151470. doi: 10.1371/journal.pone.0151470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayn MH, Hussain A, Mansour AM, Andrews PE, Carpentier P, Castle E, Dasgupta P, Rimington P, Thomas R, Khan S, Kibel A, Kim H, Manoharan M, Menon M, Mottrie A, Ornstein D, Peabody J, Pruthi R, Palou Redorta J, Richstone L, Schanne F, Stricker H, Wiklund P, Chandrasekhar R, Wilding GE, Guru KA. The learning curve of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2010;58:197–202. doi: 10.1016/j.eururo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology. 2011;78:813–819. doi: 10.1016/j.urology.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.