Abstract
Neuroendocrine neoplasms (NENs) are a heterogeneous group of neoplastic proliferations showing different morphological features, immunophenotype, molecular background, clinical presentation, and outcome. They can virtually originate in every organ of the human body and their classification is not uniform among different sites. Indeed, as they have historically been classified according to the organ in which they primarily arise, the different nomenclature that has resulted have created some confusion among pathologists and clinicians. Although a uniform terminology to classify neuroendocrine neoplasms arising in different systems has recently been proposed by WHO/IARC, some issues remain unsolved or need to be clarified. In this review, we discuss the lights and shadows of the current WHO classifications used to define and characterize NENs of the pituitary gland, lung, breast and those of the head and neck region, and digestive and urogenital systems.
Keywords: Neuroendocrine neoplasm, Neuroendocrine tumor, Neuroendocrine carcinoma, Classification
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of epithelial neoplastic proliferations ranging from indolent well differentiated neuroendocrine tumors (NETs) to very aggressive poorly differentiated neuroendocrine carcinomas (NECs). They can arise virtually in any organ of the body and, although they show similar morphological and immunophenotypical features, they present some peculiar site-specific characteristics. The second decade of twenty-first century assisted to a terrific expansion of molecular technologies that has allowed an increasing insight into the pathogenetic mechanisms of NENs, as well as a greater understanding of their clinico-pathological relationships, and, last but not the least, the recognition of new prognostic (DAXX/ATRX, microsatellite instability, CD117 expression) and theranostic markers (somatostatin receptor subtype 2, deregulation in druggable pathways such as PI3K/AKT/mTOR and Notch signaling) [1]. In this context, several “hot topics” in the field of NENs classification have emerged. Synthetically, the most debated arguments have been: i) the conceptual separation between NETs and NECs, with the identification of distinct molecular pathogenetic pathways; ii) the existence of highly proliferating NETs, as well as their relationship and differential diagnosis with NECs; iii) the need to re-define the concept of mixed neuroendocrine/nonneuroendocrine neoplasms; iv) the growing exigence, from both the pathologists’ and the oncologists’ point of view, of a common framework for the nomenclature and classification of neoplasms arising in extra-GEP organs but showing overlapping morphological features with GEP NENs. These issues are better discussed, for each organ or system, in the following paragraphs. Here we present a general outline for each of the first four points, leaving the last one a specific discussion, later in the text.
Morphology represents the first cornerstone for the differential diagnosis between NET and NEC [2] and the combination of morphological features and Ki67 proliferative index improves the ability in this distinction, which has important clinical implications. Indeed, NETs and NECs should be considered as distinct clinico-pathological entities [2]. Molecular analysis has largely confirmed this assumption, showing that these two families of neoplasms recognize different pathogenetic pathways. In digestive NENs, the carcinogenesis of NECs seems to be strongly related to that of non-neuroendocrine carcinomas of the primary site in which they arise, with frequent inactivation of TP53 and RB1 [3–6]. In contrast, NETs of the GEP system exhibit unique molecular signatures, including, among other features, the inactivation of MEN1, VHL, TSC1/2 genes, and the hyperactivation of the PI3K/mTOR pathway [6, 7]. In the lung, a similar situation has been demonstrated, but progression from NETs to NECs has been suggested in a subset of cases, with distinct clinico-pathological features [8, 9].
The existence of morphologically well differentiated NETs with a high proliferation index was not included either in the WHO classification of digestive NETs published in 2010 [10] or in the last WHO classification of lung tumors [11]. In fact, such tumors were classified as NECs based on the mitotic count and/or Ki67 proliferation index, according to the classification schemes. However, starting from clinical observations [12], it soon became evident that NENs with high proliferation index were morphologically, clinically and biologically heterogeneous, both in digestive and in thoracic sites [13–15] and the concept of NET G3 was integrated in the classification of GEP organs, leaving the definition of NEC to NENs with poorly differentiated morphology [16, 17].
Mixed neoplasms with neuroendocrine and non-neuroendocrine components, although rare, have been a matter of speculation under both diagnostic and therapeutic points of view. Indeed, their morphological heterogeneity underlies an intrinsic difficulty in making a correct diagnosis on small biopsy samples, as well as in not adequately sampled surgical specimens [18]. On the other hand, their protean biological nature must be taken into account when choosing the proper treatment. In order to better convey the diversity of this group of neoplasms that can be composed of different combinations of NENs (NET or NEC) and non-NENs (adenocarcinoma, acinar cell carcinoma, squamous cell carcinoma, and others) we proposed the term Mixed neuroendocrine/non-neuroendocrine neoplasm (MiNEN) [19]. The term MiNEN has been accepted in the WHO classification of GEP NENs [16, 17].
The existence of NENs in extra-thoracic and extra-digestive organs is a well-known, albeit rare, event. Head and neck and genitourinary tract are the commonest sites, but any epithelial organ may virtually be affected by a NEN. The diagnostic and therapeutic challenges of these NENs are related to their rarity, as well as to the heterogeneous classification schemes, which are not uniform in the various organs. To address this issue, and with the aim to settle down the bases of a robust and clinically significant nomenclature of NENs, a panel of expert pathologists, under the aegis of WHO and IARC, proposed, in 2018, a common classification frame for NENs [20]. This represents a milestone in the history of NENs, as it underlies the concept of a category of neoplasms that, with important site- and grade-related variations, retains substantial identity under morphological, biological and clinical points of view.
Pituitary gland
Although the pituitary gland is small, it contains at least six neuroendocrine cells types secreting different hormones and bioactive peptides and this reflects the rather large number of different pituitary tumor types that can be found in the adenohypophysis. The pathological classification of anterior pituitary tumors has traditionally been bases on morphology, immunohistochemistry, and electron microscopy with the aim to correlate morphology and immunophenotype with function and clinical presentation [21]. With the advent of molecular techniques a large amount of new information has been obtained and the use of transcription factors involved in the lineage determination of different cell types has been proposed to classify anterior pituitary tumors [22]. This approach was the basis for the last WHO classification published in 2017 [16] that included two main tumor types: pituitary adenoma and pituitary carcinoma. Although this approach has the advantage to better characterize different pituitary tumor types based on cell lineage, it is not able to predict patients’ outcome. Indeed, this classification does not allow identifying with certitude those cases that will behave in an indolent manner and distinguishing them from those that will locally recur and need additional treatment, with a great impact on quality of life. In addition, this approach does not identify tumors that will give metastatic dissemination during follow-up, therefore deferring the diagnosis of pituitary carcinoma a posteriori, only when the presence of meningeal dissemination or metastatic spread will become clinically and/or radiologically evident (Fig. 1). Moreover, in the general attempt to conceptually unify NENs arising in the different anatomical sites, the need was felt to include also anterior pituitary tumors in the NENs family, to which they belong for morphological and functional reasons. Taken together, these considerations prompted a multidisciplinary group of experts in pituitary pathology to propose the new terminology “pituitary neuroendocrine tumor (PitNET)” instead of “pituitary adenoma” (Table 1) [23]. Thus, all anterior pituitary tumors are considered as lesions showing a potential clinical impact that can be additionally evaluated and better defined in terms of prognosis using a multiparametric approach including the Ki67 proliferative index and radiology appearance (Table 2) [24, 25]. Although this new approach appears appropriate and clinically useful since it reflects the real biology of these tumors, it is still matter of debate [26–28]. It is worth noting that the actual efficacy of this terminology has been supported by the WHO/IARC, which has recently proposed a common classification framework for neuroendocrine tumors to be used for all body sites [20].
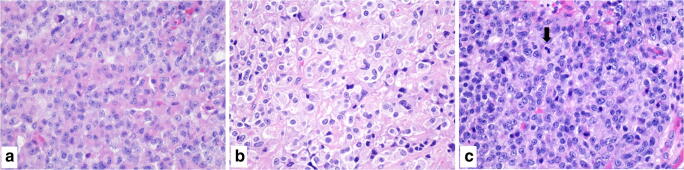
Fig. 1.
Morphology alone is not able to identify pituitary neuroendocrine tumors (PitNETs) that will behave in an indolent manner (a) or that will locally recur with signs of aggressiveness (b) or that will give metastatic dissemination (c), although in this latter case high cellularity and mitoses (arrow) are more frequently observed
Table 1.
Specific pituitary neuroendocrine tumors (PitNETs) types*, transcription factor and hormone expression
| Cell type | TS | Hormone | Tumor type(s) |
|---|---|---|---|
| Corticotroph | TPIT, NeuroD1 | ACTH, β-endorphin, MSH |
•DG corticotroph PitNET •SG corticotroph PitNET •Crooke cell PitNET |
| Somatotroph | PIT-1 | GH |
•DG somatotroph PitNET •SG somatotroph PitNET |
| Mammosomatotroph | PIT-1, ERα | GH, prolactin |
•Mammosomatotroph PitNET •Mixed somatotroph/lactotroph PitNET |
| Lactotroph | PIT-1, ERα | Prolactin |
•SG lactotroph PitNET •DG lactotroph PitNET •Acidophil stem cell PitNET |
| Thyrotroph | PIT-1, TEF, GATA2 | TSH | •Thyrotroph PitNET |
| Gonadotroph | SF-1, ERα, GATA2 | FSH, LH | •Gonadotroph PitNET |
| Null cells | None | None | •Null cell PitNET |
*: in the current WHO classification [32], the term adenoma is used instead of PitNET; TS: transcription factor; DG: densely granulated; SG: sparsely granulated; ERα: estrogen receptor α
Table 2.
Prognostic classification of pituitary neuroendocrine tumors Modified from Trouillas et al. 2013 [24]
| Grade 1a: non-invasive PitNET | |
| Grade 1b: non-invasive and proliferative PitNET | |
| Grade 2a: invasive PitNET | |
| Grade 2b: invasive and proliferative PitNET | |
| Grade 3: metastatic PitNET (pituitary carcinoma) |
Invasion is defined as histological and/or radiological (MRI) signs of cavernous or sphenoid sinus invasion; Proliferation is considered on the presence of at least one of two criteria: Ki67 > 3% or mitoses >2/10HPF
Head and neck
NENs of the head and neck are a group of heterogeneous epithelial neoplastic proliferations arising in virtually all the different organs of this region, including the nasal cavity, paranasal sinuses, nasopharynx, larynx, salivary glands, and middle ear. Their morphological and clinical features mainly depend on the degree of differentiation and on the site of origin and for these reasons they will be discussed separately in the following paragraphs.
In this region, the larynx is the commonest site of occurrence of NENs and both NETs and NECs (of small and large cell types) have been reported. The terminology used over the last years to define NENs of the larynx has been matter of debate [29]. In the WHO classification published in 2005, in analogy with NENs of the lung, they were subdivided into typical carcinoid, atypical carcinoid, and neuroendocrine carcinoma (small and large cell subtype) [30]. Unfortunately, the last edition of the WHO classification of tumors of the head and neck published in 2017 [31] changed this terminology resulting in a problematic and rather confusing scheme, as it misses several entities and is not in line with the terminology used for thoracic or digestive NENs. In this context, the most relevant issue regards the use of the term neuroendocrine carcinoma as a synonym for NEN, under the heading of which both morphologically well- poorly differentiated neoplasms are included [31]. This leads to a non-realistic framework, in which a three-tiered grading of so called “neuroendocrine carcinoma”, including well-differentiated, moderately differentiated and poorly differentiated neoplasms [31] introduces a continuum from very indolent to very aggressive neoplasms that is not supported by biological and genetic evidences [32, 33]. Consequently, the terminology used in the 2017 WHO classification to define neuroendocrine neoplasms clearly appears not appropriate and, most important for the clinical impact on the patient’s management, the use of the term neuroendocrine carcinoma to define a NET is dangerous because can be confounding for clinicians, who can be encouraged to use platinum-based chemotherapy to treat patients who would not benefit from it and would only experience severe collateral effects. Thus, we strongly recommend the use of the common classification framework for NENs also in this site, with NETs corresponding to typical and atypical carcinoids of the 2005 classification, and the term NEC reserved for morphologically poorly differentiated and clinically aggressive neoplasms [20, 29]. In addition, it is worth to be noted that in the 2017 WHO classification there is no mention on mixed neoplasms, which, on the contrary, have been described in the literature and may represent a diagnostic challenge for pathologists [19].
In the nasal cavity, the WHO classification only includes NECs [31], although the existence of NETs and mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs) has been well documented [19, 29, 34]. These two entities, although rare, need to be recognized because they show distinct prognosis and deserve a specific therapeutic approach.
A dedicated chapter on salivary gland NENs is not included in the 2017 WHO classification of head and neck tumors and they seem to be included in the chapter of poorly differentiated carcinoma, which also includes cases without a neuroendocrine differentiation [31]. Although the spectrum of the salivary gland NENs is almost totally covered by NECs of the small cell and large cell subtypes, a few cases of NETs have been reported and need to be considered among the possible differential diagnoses [29].
The last entity to be considered among NENs of the head and neck is the so-called middle ear adenoma. Several studies have demonstrated that this tumor type is composed of both a glandular (exocrine) and solid (neuroendocrine) component, making the neuroendocrine tumor of the middle ear a mixed neoplasm (Fig. 2), for which the term MiNENs may be more appropriate [29].
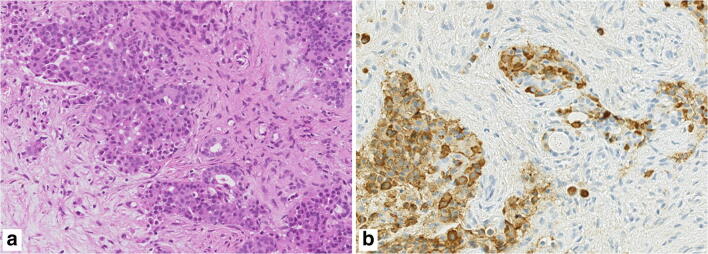
Fig. 2.
The so-called middle ear adenoma should be considered as a mixed neuroendocrine/nonneuroendocrine neoplasm, since it is composed of both a glandular (exocrine) and a solid (neuroendocrine) component (a), the latter positive for neuroendocrine markers including chromogranin (b)
In line with the recent classification framework supported by the WHO/IARC [20] the terminology used to define head and neck NEN needs to be revised and in Table 3 a classification scheme is proposed.
Table 3.
Classification of epithelial head and neck neuroendocrine neoplasms
| 2005 WHO classification [30] | Current (2017) WHO classification [31] | Proposed new WHO classification [20] |
|---|---|---|
| Typical carcinoid | Well-differentiated neuroendocrine carcinoma, grade I | NET G1 |
| Atypical carcinoid | Moderately differentiated neuroendocrine carcinoma, grade II | NET G2 |
| Small-cell NEC and large cell NEC | Poorly differentiated neuroendocrine carcinoma, grade III | NEC (small and large cell types) |
| Combined small cell NEC | Neuroendocrine carcinoma with non small cell component (squamous cell carcinoma, adenocarcinoma, etc.) | MiNEN |
Lung
NENs of the lung are currently classified in four main categories, including typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SmCLC) [11]. This classification is meant to be applied on surgical samples, and its mainstays are represented by morphological parameters: mitotic index, the presence of necrosis, and cell size, whereas Ki67 proliferation index is not included in the assessment for classification purposes. Although this terminology is not in line with the already mentioned common classification framework proposed by WHO/IARC [20], a substantial overlapping exists. In fact, TC and AC are considered well differentiated NENs (i.e., NETs), whereas LCNEC and SmCLC are regarded as poorly differentiated NENs (i.e., NECs). Besides these semantic issues, the practicing pathologist has experienced cases in which the clear-cut separation between, for example, TC and AC, or AC and LCNEC is not affordably allowed using the classical morphological parameters and additional workup is needed to reach a clinically meaningful diagnosis. In fact, Ki67 proliferation index has proven to be a useful parameter, at least in two different practical settings. First, on small biopsies with crash artifacts impairing the morphological evaluation, Ki67 may have paramount diagnostic value in distinguishing a NET (carcinoid) from a NEC (LCNEC or SmCLC) [35–39]. Second, Ki67 proliferation index has been shown to be a relevant prognostic factor in lung NETs (carcinoids) and its evaluation should be added to the pathological report, even if no agreement has been reached, until now, neither on the cut-off levels, nor on the possible integration with morphological parameters in a grading system similar to that of digestive NENs [40]. In addition, and importantly, NET (carcinoids) with high Ki67 proliferation index (between 10% and 20%) have been reported to have peculiar morphological and clinical features, that resemble those of digestive NET G3, and may represent a distinct type of aggressive well differentiated pulmonary NEN [41, 42].
The “molecular revolution” of the last decade has involved pulmonary NENs, as well. Based on the systematic review of compelling molecular evidences reported in literature, it has recently been proposed a molecular classification, which recognizes three different types of lung NENs, showing distinct molecular signatures and clinical behavior [43, 44]. In detail, they listed: 1) primary high grade NENs, which are the most frequent pulmonary NENs (70–75%), are diagnosed on small biopsies of heavy smokers, arise de novo with no recognizable precursor lesions, show classic SmCLC or LCNEC morphology, have low intra- and inter-tumor genetic heterogeneity with consistent inactivation of TP53 and RB1, a high mutation burden, an extremely high Ki67 index, and a very aggressive clinical behavior, with no role for radical surgery; 2) secondary high grade NENs, which represent 20% to 25% of pulmonary NENs, arise in heavy smoker men, have variable morphology (AC, LCNEC, SmCLC), may show the presence of precursor lesions (neuroendocrine cell hyperplasia/DIPNECH, neuroepithelial bodies, carcinoids, non-small cell lung carcinoma), have high intra- and inter-tumor genetic heterogeneity with involvement of a variety of different pathways (inactivation of TP53, RB1, and NOTCH, KRAS/LKB1/MEN1 mutation, MYC, TERT, SDHA, RICTOR amplification and epithelial-mesenchymal transition), suggesting a multistep pathogenesis, present a heterogeneous Ki67 index, and behave less aggressively than the previous type, being diagnosed mainly on surgical specimen after oncologically radical intervention; 3) indolent low grade NENs, which are the rarest type (5% of lung NENs), are diagnosed in non-smoker women, have well differentiated morphology (TC or AC), are often accompanied by precursor lesions (DIPNECH), may arise in MEN1 or other familial syndromes, show low mutation burden with involvement of chromatin remodeling genes, have an evenly low Ki67 index (10% or less), behave indolently and are successfully treated with surgery. In addition, a growing burden of evidence has been accumulating in support of the hypothesis that at least a subset of high grade NENs (NECs) in this site may arise from the progression of pre-existent NETs (carcinoids). In a recently published integrative analyses on 257 lung neuroendocrine neoplasms, it was possible to stratify atypical carcinoids into two prognostic groups with significantly different 10-year overall survival. Interestingly, a third group of neoplasms with carcinoid-like morphology but molecular profile closer to that of large cell NEC, defined supra-carcinoid, suggests a molecular link between carcinoids and large cell NECs also suggesting the possibility that a subset of large cell NEC may derive from pre-existing carcinoids [45].
The combination of morphological, proliferation and molecular parameters has leaded to the proposal of a comprehensive classification of lung NENs, which follows the common classification framework for NENs and has important clinical and therapeutic correlates [46].
Breast
The issue of neuroendocrine differentiation in breast neoplasms has been a matter of debate since its first description in 1963 [47]. Indeed, although neuroendocrine phenotype has been demonstrated in a number of breast tumors using both immunohistochemical and ultrastructural methods [48–50], several morphological and genetic considerations prevent the full inclusion of so-called breast NENs in the common classification framework of NENs [51]. First, as the current classification of NENs is primarily based on morphological criteria that allow the distinction between NETs and NECs, a careful revision of the so-called breast NETs, as they are defined in the last WHO classification of breast tumors [52], shows that a definite and recognizable morphology is not identified, and the diagnosis, in practice, relies on immunostains for synaptophysin and chromogranin A, which are not enough to define a NEN [51]. Second, the genetic and expression profiles of so-called breast NETs is, in fact, overlapping with that of luminal A breast carcinomas and shares no similarities with those described for well differentiated NENs of other sites [53]. Third, the grading system for these neoplasms relies on the Elston-Ellis criteria and not on the proliferation rates as in NETs of other anatomical sites [52]. Fourth, which derives from the previous points, treatment strategies for the so-called breast NETs do not take in account the presence of neuroendocrine differentiation but follow standard protocols for the carcinomas of the breast of special and no special types [54]. Fifth, and last, there is no significant difference in the outcome of patients with so-called breast NETs, when compared to patients with non-neuroendocrine breast carcinomas of the same grade, stage and molecular profile [52]. Indeed, the therapeutic choices for so-called breast NET rely on predictive factors traditionally used for non-neuroendocrine breast cancer (hormone receptor expression, HER2 hyperexpression and amplification, and proliferative index) and targeted therapies for NETs of other sites (somatostatin analogues, mTOR inhibitors) have not proved to be effective on Br-NETs [55]. Indeed, although the expression of somatostatin receptors (SSRs) has been demonstrated in a subgroup of mammary NETs [56], several studies have demonstrated that SSRs, are also frequently expressed in breast carcinomas of luminal A type, removing any specificity of somatostatin analogues in the management of breast NET [57, 58].
A separate discussion is needed for breast NECs, which, albeit rare, represent a well-defined entity, showing morphological and clinical analogies with pulmonary and extra-pulmonary NECs. In these neoplasms, molecular studies have demonstrated the presence, in early pathogenetic steps, of alterations overlapping those of the non-endocrine breast carcinoma, similarly to what happens in other sites [59]. Indeed, these findings are paralleled by the morphological observation that, in many of these cases, the NEC component is associated with an in situ or invasive non-endocrine breast carcinoma [60]. For these reasons, breast NECs may be entitled to be included in the NENs category [51].
Digestive system
The classification of digestive NENs has undergone a significant evolution over the last 20 years, reflecting the increasing knowledge on pathogenesis and molecular background of this heterogeneous group of neoplasms. The last WHO classification published in 2019 recapitulates these changes and integrates the most recent clinico-pathologic and molecular findings [17]. It is worth noting that, due to its easy application, good reproducibility, and great clinical significance, the classification approach for digestive NENs has been employed as the model for the common classification framework for NENs originating in different organs [20]. The basis of the classification includes the integration of both morphological (histological differentiation) and proliferative (grade) features and identifies three main groups (Table 4 and Fig. 3): well differentiated neuroendocrine tumor (NET), poorly differentiated neuroendocrine carcinoma (NEC), and mixed neuroendocrine/non-neuroendocrine neoplasm (MiNEN). The distinction between NET and NEC relies on morphology and includes specific cellular and architectural criteria [2]. NETs are then graded based on the proliferation index (mitotic count and Ki67-related proliferation index) and are divided in three groups (NET G1, NET G2, and NET G3), while NECs are by definition high grade neoplasms, and the specification G3 has been removed to avoid confusion with NET G3 (Table 4). This approach has proven to be of great help for the prognostic stratification of patients and it is particularly useful for the distinction, among the group of G3 neoplasms (Ki67 > 20%), between NET G3 and NEC, two entities showing distinct molecular background, clinical outcome, and therapeutic approach. This classification, based on the combination of both morphology and proliferation, appears as an important evolution of the WHO classification published in 2010, in which the distinction of NETs from NECs was mainly based on the proliferative index [10].
Table 4.
WHO classification of digestive neuroendocrine neoplasms
| Morphological differentiation | Mitotic count/2mm2 | Ki67 index | |
|---|---|---|---|
| NET G1 | well-differentiated | <2 | <3% |
| NET G2 | well-differentiated | 2–20 | 3–20% |
| NET G3 | well-differentiated | >20 | >20% |
| NEC | poorly differentiated | >20 | >20% |
| MiNENs | well or poorly differentiated | variable | variable |
NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma: MiNEN: mixed neuroendocrine/non-neuroendocrine neoplasm
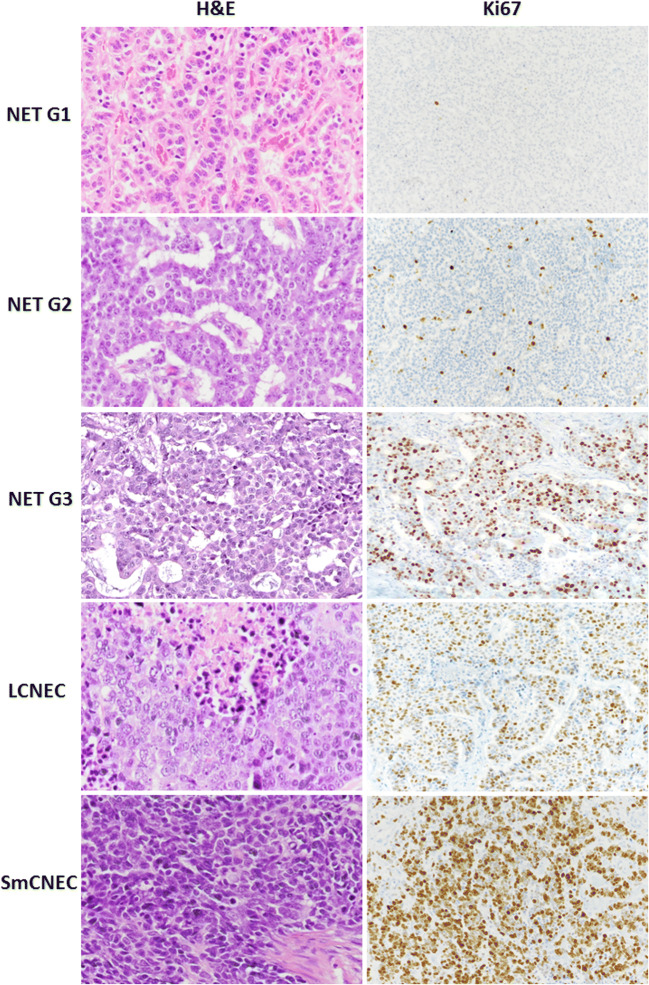
Fig. 3.
Morphology and Ki67 proliferation index of different NEN types of the digestive system. Neuroendocrine tumors (NETs) show a well differentiated morphology and on the basis of Ki67 labelling index they can be divided into G1, G2, and G3 category. Neuroendocrine carcinomas, both of large cell (LCNEC) and small cell (SmNEC) subtype, show poorly differentiated morphology and high Ki67 proliferative index. H&E: hematoxylin and eosin
In the current classification scheme, the identification of NEC is rather easy by combining morphology and proliferation, and its clinical and prognostic features seem to be similar, independently of the site of origin. Conversely, NETs show peculiar site-specific characteristics to be kept in mind and, for a better prognostic classification of tumors, the WHO classification needs to be integrated with other parameters, depending on the anatomical site. In this context, the prognostic role of Ki67 proliferative index deserves a discussion. Although the Ki67 proliferative index is a well-known prognostic marker, its prognostic role may vary depending on the tumor type and the site of origin [61].
In gastric NETs, the best prognostic stratification of patients is achieved by combining the clinico-pathologic subtype (type1, type 2, and type 3 gastric NETs), which is per se of prognostic value, with the Ki67 proliferative index, especially in patients with type 3 NETs [62].
In duodenal NETs, Ki67 has been demonstrated to be a predictor of lymph node metastasis and, although, associated with disease-specific survival at univariate analysis, it failed to be an independent prognostic factor discriminating survival between G1 and G2 tumors [63]. For this reason, a multiparametric approach including tumor size, site (ampulla versus other duodenal sites), and proliferative activity seems the most accurate to classify duodenal NETs.
Ileal NETs are peculiar tumors due to their ability to metastasize early to regional lymph nodes and/or the liver despite a low proliferation index (most tumors are G1). Consequently, it is conceivable that tumor grading fails to predict the metastatic potential of these tumors. However, Ki67 proliferative index is correlated with prognosis and, interestingly, it has been demonstrated that the increasing risk for tumor progression and tumor death for each increasing Ki67 unit was 14% and 18%, respectively [64]. This underlines the biological concept that Ki67 should be considered as a continuous variable and the calculation of the prognostic risk for each increasing Ki67 unit may be superior to the separation of tumors using fixed Ki67 categories, which, however, are useful to give a general classification approach.
Among digestive NETs, appendiceal ones are the most peculiar. Indeed, despite frequent infiltrative growth into the muscular layer and subserosa, lymph node metastases are rare and distant metastases are virtually absent [65]. Patients with these tumors, who often are young, have an excellent outcome after appendectomy. The Ki67 labeling index, which is generally low, has recently been demonstrated to predict nodal metastases together with size >1.5 cm and lympho-vascular invasion. However, the prognostic role of lymph node metastases is still matter of debate since no statistically different prognosis has been observed between patient with or without lymph node metastases [65–67]. For this reason, the survival benefit of right hemicolectomy is still unclear, and its choice needs to be demanded to multidisciplinary tumor boards in expert referral centers [66, 68].
About 90% of the rectal NETs are G1 which show better survival than G2 NETs [69], demonstrating the prognostic role of tumor grade and consequently of Ki67 proliferative index in these tumors. However, although tumor grade has been proved to be a prognostic marker in univariate analysis, it was not found as an independent factor at the multivariate analysis [69, 70]. For this reason, it has therefore been suggested that the best approach for stratifying patients into different prognostic categories seems to be multiparametric considering tumor grade together with tumor size, lympho-vascular invasion, level of wall infiltration, and immunophenotype (L-cell versus EC-cell NET) [69, 70], which have been demonstrated to have a prognostic role [71]. G1 rectal NETs with a size less than 10 mm, absence of lympho-vascular and muscular layer infiltration, and L-cell phenotype require only endoscopic resection, while larger G2 NETS, especially when of EC-cell type and deeply infiltrating the rectal wall in the presence of lympho-vascular invasion, need surgical resection.
Pancreatic NENs (PanNENs) are the tumors where the prognostic role of Ki67 proliferative index has been most investigated during the last years [72–75] and it represents an important prognostic marker together with stage [75]. The current WHO classification is very useful to stratify patients with PanNENs in different prognostic categories and its use is strongly recommended. It is worth noting that PanNETs less than 1 cm with a low proliferation index have been considered for a long time as benign tumors and, for this reason, the term pancreatic microadenoma has been proposed. However, as all other neuroendocrine tumors of the body, small and low proliferative PanNETs should be also considered malignant because they can give lymph node metastases [76].
In addition to NET and NEC, the WHO classification includes mixed neuroendocrine/ non-neuroendocrine neoplasms (MiNENs). The introduction of the term MiNEN, that we proposed for the first time in 2016 [19], represents an evolution in the definition of mixed neoplasms. Indeed, the term MANEC (mixed adenoneuroendocrine carcinoma) included in the previous WHO classification [10] did not convey the real spectrum of digestive mixed neoplasms creating confusion among pathologists and clinician [18]. The advantage of the term MiNEN resides in the fact that all different entities resulting from the different combinations of various neoplastic components can be included under this term, which represents an umbrella covering all different entities. Consequently, MiNEN should be regarded as a conceptual category, rather than a specific diagnosis. Indeed, in the pathology report, a diagnosis of MiNEN needs to be better specified including the correct identification and categorization of each component [18]. Despite this improvement, a main issue remains unsolved. By definition, the two components of a MiNEN should represent at least 30% of the tumor burden, but this cut-off was arbitrarily chosen presumably to assure that each component was quantitatively enough to influence the natural history of the disease and patient’s outcome. However, no systematic study has been performed, to date, to confirm the biological validity of this cut-off. The 30% cut-off seems a dangerous criterion, especially when a minor tumor component (<30%) is represented by a high grade NEC, which can drive patient’s prognosis independently on its percentage. Since in the last WHO classifications to define a MiNEN the two components are to be “morphologically recognizable” based on well-established criteria, the maintenance of the 30% cut off is probably not useful and/or essential. For this reason, further studies may help to solve this issue.
Urogenital system
Neuroendocrine neoplasms (NENs) of the genitourinary tract are rare, but it is important to be aware of their existence as the correct diagnosis drives their treatment and prognosis. NENs have been described in the kidney, urinary bladder, prostate, testes, uterine cervix, uterine corpus, and ovaries. In all these organs, except for ovaries and testes, in which NETs are more frequent, the commonest NEN type is NEC. In the kidney and in the bladder, non-epithelial NENs (i.e. paragangliomas) have been described as well. The current classifications of tumors of the urinary tract and genital organs [77, 78] are partially in line with the common classification framework proposed by WHO/IARC [20]. Indeed, NENs of the kidney, of the urinary bladder and of the uterus are subdivided in NETs and NECs (with site-related variations), whereas in the prostate and in the gonads the nomenclature is still confusing and should be revised to adhere to the new criteria [77, 78]. In addition, the current classifications do not adequately recognize that most of poorly differentiated NENs of genitourinary tract are, in fact, combined with non-neuroendocrine components, and the category of MiNEN should be included in the classification scheme. Here, we will focus on bladder and prostatic NENs, which represent the main hot topic in the urogenital region, both because of their frequency and because of issue related to the nomenclature.
In the urinary bladder most NEN are small cell NECs, while the large cell subtype is exceedingly rare. Urinary bladder NECs are frequently associated with other carcinomatous components (urothelial and squamous carcinomas and adenocarcinoma), constituting bladder MiNENs. Since the poorly differentiated neuroendocrine component seems to drive the prognosis in MiNENs, its proper recognition is important for patients’ management. Immunohistochemical stains for synaptophysin and chromogranin A help to confirm the neuroendocrine differentiation of neoplastic cells, but additional markers can be used in discriminating and quantifying neuroendocrine versus non-neuroendocrine components. It has been demonstrated that NECs of the urinary bladder are consistently p16-positive, CK20-negative, GATA3-negative, and p63-negative, whereas high grade urothelial carcinomas show an opposite profile (p16-, p63+, GATA3+, and CK20+) [79, 80].
In the prostate, the presence of neuroendocrine differentiation, in the sense of general neuroendocrine markers expression, is relatively frequent and a significant subset of prostatic adenocarcinomas show immunoreactivity for synaptophysin, despite the absence of a true neuroendocrine morphology. Although these cases are listed by the last WHO classification as belonging to neuroendocrine neoplasms [77], they are not entitled to be part of the NENs category, as it is currently defined. Another entity that is included among prostatic neuroendocrine neoplasms is the so-called adenocarcinoma with Paneth cell-like neuroendocrine differentiation, which is defined as a prostatic adenocarcinoma with morphologically recognizable well-differentiated neuroendocrine cells showing cytoplasm stippled with brightly eosinophilic granules. The designation “Paneth cell-like” is a misnomer, as these granules do not contain lysozyme, as Paneth cells do, but are rather functionally and morphologically similar to neuroendocrine cells interspersed in the intestinal mucosa. Therefore, we prefer the designation of adenocarcinoma with well differentiated neuroendocrine cells for this entity and we consider that, when neuroendocrine cells are clustered in organoid structures that represent a significant proportion of the tumor mass, it should be regarded to as a MiNEN [81]. In this case, the presence of solid architecture in the neuroendocrine component should not be graded as Gleason pattern 5 [82]. Real NENs of the prostate are mainly represented by NECs. Prostatic NECs are uncommon neoplasms that may present in pure neuroendocrine form or as MiNENs, in association with prostatic adenocarcinoma. About half of cases occur in patients with a previous diagnosis of prostatic adenocarcinoma treated with androgen deprivation therapy, which has become castration-resistant. However, prostatic NEC may also occur de novo [81]. NETs are exceedingly rare in the prostate, and their existence itself is questioned. They may be diagnosed only when all the following criteria are satisfied: 1- presence of well differentiated neuroendocrine morphology; 2- absence of adenocarcinomatous component; 3- immunohistochemical expression of general neuroendocrine markers; 4- negativity of immunostainings for AR and PSA; 5- exclusion of prostatic metastasis or infiltration from a primary NET of another site. The main diagnostic problem on small biopsies is to distinguish prostatic NET from NEC, which has completely different prognostic implications [81]. The spectrum of prostatic neoplasms with neuroendocrine differentiation has been recently expanded by an entity called prostatic carcinoma with amphicrine features, an aggressive variant of prostatic carcinoma in which the totality of neoplastic cells present both a neuroendocrine and an exocrine phenotype [83]. However, it is important to recall that amphicrine neoplasms do not belong to the category of NENs, as they are currently defined [18].
NENs of unknown primary origin
Virtually all NENs have metastatic potential and up to 20% of the cases present as metastasis from an occult primary [84–86]. The identification of the primary site is an important step towards the correct management of the patient, particularly when dealing with a NET since the therapeutic approach may vary depending on the site and cell type. In contrast, NECs, independent of the primary site, are currently treated with platinum-based regimens [87]. In this context, the role of the pathologist may be limited to the distinction between a visceral NEC and a Merkel cell carcinoma of the skin, because the latter requires wide local excision, sentinel node biopsy and, possibly, radiotherapy [88]. In contrast, thorough morphological and immunohistochemical analyses combined with imaging techniques are expected to give important clues to the recognition of the site of origin of a metastatic NET [89].
Among NETs, the tendency to metastasize is highest for those of pancreatic origin, followed by small intestinal, colonic, pulmonary and gastric neoplasms [87]. Irrespective of the primary site, the liver represents the most frequent location of metastatic NENs; lymph nodes, peritoneum, bone and lung represent further usual secondary sites [86]. However, virtually any body organ including those that can give rise to primary NENs may host metastatic NENs, including breast, ovary, thyroid, pancreas and pituitary [89]. Thus, it becomes evident that the diagnosis of a metastatic NEN gives rise to two orders of problems: (i) the identification of the occult primary site, and (ii) the distinction from a putative primary NEN of the organ in which the lesion is present. Both challenges are of crucial importance in the management of patients and the pathologist should be aware of the diagnostic tools to approach them and of the entities, which enter in the differential diagnosis. To address these issues, a coordinated and comprehensive clinical and pathological investigation is required. A diagnostic algorithm to identify the primary site of neuroendocrine neoplasms of unknown origin by using radiological and endoscopic methods along with radionuclear markers has been proposed [87]. From a pathological point of view, the critical employment of immunohistochemical stains for transcription factors and hormonal products has proved to be effective in identify the primary site of a metastatic NET, and the reader is referred to specific papers for detailed dissertation and diagnostic flowcharts [1]. Importantly, after the primary site has been identified, a careful grading and site-specific staging of the NET is crucial. In this context, the application of the common framework for the nomenclature and classification of NENs [20] shows, in our opinion its great value in guiding the physician hand in the management of these neoplasms, as the subdivision in NET, NEC and MiNEN families, independently of the primary site, provides an important initial characterization of the disease. Then, for the best treatment, this has to be obviously placed in the context of the specific site of origin of the NET.
Funding
Open access funding provided by University of Lausanne.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uccella 2021, Uccella S, La Rosa S, Volante M, Papotti M. Immunohistochemical biomarkers of gastrointestinal, pancreatic, pulmonary, and thymic neuroendocrine neoplasms. Endocr Pathol. 2018;29:150–68. [DOI] [PubMed]
- 2.Uccella S, Sessa F, La Rosa S. Diagnostic approach to neuroendocrine neoplasms of the gastrointestinal tract and pancreas. Turk Patoloji Derg. 2015;31(Suppl 1):113–127. doi: 10.5146/tjpath.2015.01319. [DOI] [PubMed] [Google Scholar]
- 3.Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, Penzel R, Endris V, Arsenic R, Hermann G, Stenzinger A, Weichert W, Pfarr N, Klöppel G. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30:610–619. doi: 10.1038/modpathol.2016.220. [DOI] [PubMed] [Google Scholar]
- 4.Woischke C, Schaaf CW, Yang HM, Vieth M, Veits L, Geddert H, Märkl B, Stömmer P, Schaeffer DF, Frölich M, Blum H, Vosberg S, Greif PA, Jung A, Kirchner T, Horst D. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod Pathol. 2017;30:95–103. doi: 10.1038/modpathol.2016.150. [DOI] [PubMed] [Google Scholar]
- 5.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, DeMarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpa A. The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann Endocrinol. 2019;80:153–158. doi: 10.1016/j.ando.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 8.George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, Maas L, Müller C, Dahmen I, Delhomme TM, Ardin M, Leblay N, Byrnes G, Sun R, de Reynies A, McLeer-Florin A, Bosco G, Malchers F, Menon R, Altmüller J, Becker C, Nürnberg P, Achter V, Lang U, Schneider PM, Bogus M, Soloway MG, Wilkerson MD, Cun Y, McKay JD, Moro-Sibilot D, Brambilla CG, Lantuejoul S, Lemaitre N, Soltermann A, Weder W, Tischler V, Brustugun OT, Lund-Iversen M, Helland Å, Solberg S, Ansén S, Wright G, Solomon B, Roz L, Pastorino U, Petersen I, Clement JH, Sänger J, Wolf J, Vingron M, Zander T, Perner S, Travis WD, Haas SA, Olivier M, Foll M, Büttner R, Hayes DN, Brambilla E, Fernandez-Cuesta L, Thomas RK. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9:1048. doi: 10.1038/s41467-018-03099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, Sperandio N, Corbo V, Rusev B, Mastracci L, Grillo F, Pilotto S, Pelosi G, Pelliccioni S, Lawlor RT, Tortora G, Fontanini G, Volante M, Scarpa A, Bria E. Gene expression profiling of lung atypical carcinoids and large cell neuroendocrine carcinomas identifies three Transcriptomic subtypes with specific genomic alterations. J Thorac Oncol. 2019;14:1651–1661. doi: 10.1016/j.jtho.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Kloppel G, Komminoth P, Solcia E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. Lyon: IARC; 2010. [Google Scholar]
- 11.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: IARC; 2015. [DOI] [PubMed] [Google Scholar]
- 12.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 13.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogeneous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, Pisa E, Barberis M, Vanoli A, Buzzoni R, Pusceddu S, Concas L, Sessa F, Solcia E, Capella C, Fazio N, la Rosa S. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104:85–93. doi: 10.1159/000445165. [DOI] [PubMed] [Google Scholar]
- 15.Pelosi G, Bianchi F, Hofman P, Pattini L, Ströbel P, Calabrese F, Naheed S, Holden C, Cave J, Bohnenberger H, Dinter H, Harari S, Albini A, Sonzogni A, Papotti M, Volante M, Ottensmeier CH. Recent advances in the molecular landscape of lung neuroendocrine tumors. Expert Rev Mol Diagn. 2019;19:281–297. doi: 10.1080/14737159.2019.1595593. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. Lyon: IARC; 2017. [Google Scholar]
- 17.Klimstra DS, Klöppel G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board editors. WHO classification of tumours, 5th edn. Digestive system tumours. Lyon: IARC; 2019. pp. 16–9.
- 18.Uccella S, La Rosa S. Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: subtypes, prognosis, and predictive factors. Histopathology. 2020;77:700–717. doi: 10.1111/his.14178. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016;27:284–311. doi: 10.1007/s12022-016-9432-9. [DOI] [PubMed] [Google Scholar]
- 20.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, el-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770–1786. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solcia E, Klöppel G, Sobin LH (in collaboration with 9 pathologist from 9 countries) Histological typing of endocrine tumors. WHO international histological classification of tumors2nd ed. Heidelberg: Springer-Verlag; 2000. [Google Scholar]
- 22.Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998;19:798–827. doi: 10.1210/edrv.19.6.0350. [DOI] [PubMed] [Google Scholar]
- 23.Asa SL, Casar-Borota O, Chanson P, Delgrange E, Earls P, Ezzat S, Grossman A, Ikeda H, Inoshita N, Karavitaki N, Korbonits M, Laws ER, Lopes MB, Maartens N, McCutcheon IE, Mete O, Nishioka H, Raverot G, Roncaroli F, Saeger W, Syro LV, Vasiljevic A, Villa C, Wierinckx A, Trouillas J, _ _. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an international pituitary pathology Club proposal. Endocr Relat Cancer. 2017;24:C5–C8. doi: 10.1530/ERC-17-0004. [DOI] [PubMed] [Google Scholar]
- 24.Trouillas J, Roy P, Sturm N, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126:123–135. doi: 10.1007/s00401-013-1084-y. [DOI] [PubMed] [Google Scholar]
- 25.Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). 2020;12:514. [DOI] [PMC free article] [PubMed]
- 26.Ho KKY, Fleseriu M, Wass J, van der Lely A, Barkan A, Giustina A, Casanueva FF, Heaney AP, Biermasz N, Strasburger C, Melmed S. A tale of pituitary adenomas: to NET or not to NET: pituitary society position statement. Pituitary. 2019;22:569–573. doi: 10.1007/s11102-019-00988-2. [DOI] [PubMed] [Google Scholar]
- 27.Asa SL, Asioli S, Bozkurt S, Casar-Borota O, Chinezu L, Comunoglu N, Cossu G, Cusimano M, Delgrange E, Earls P, Ezzat S, Gazioglu N, Grossman A, Guaraldi F, Hickman RA, Ikeda H, Jaffrain-Rea ML, Karavitaki N, Kraljević I, la Rosa S, Manojlović-Gačić E, Maartens N, McCutcheon IE, Messerer M, Mete O, Nishioka H, Oz B, Pakbaz S, Pekmezci M, Perry A, Reiniger L, Roncaroli F, Saeger W, Söylemezoğlu F, Tachibana O, Trouillas J, Turchini J, Uccella S, Villa C, Yamada S, Yarman S. Pituitary neuroendocrine tumors (PitNETs): nomenclature evolution, not clinical revolution. Pituitary. 2020;23:322–325. doi: 10.1007/s11102-019-01015-0. [DOI] [PubMed] [Google Scholar]
- 28.Ho KKY, Fleseriu M, Wass J, et al. The tale in evolution: clarity, consistency and consultation, not contradiction and confusion [published online ahead of print, 2020 Jan 7]. Pituitary. doi:10.1007/s11102-019-01027-w, 23, 476, 477. [DOI] [PubMed]
- 29.Uccella S, Ottini G, Facco C, Maragliano R, Asioli S, Sessa F, La Rosa S. Neuroendocrine neoplasms of the head and neck and olfactory neuroblastoma. Diagnosis and classification Pathologica. 2017;109:14–30. [PubMed] [Google Scholar]
- 30.Barnes L, Eveson JW, Reichart P, Reichart P, Sidransky D. World Health Organization classification of Tumours. Pathology & Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 31.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. [Google Scholar]
- 32.Yan J, Yu S, Jia C, Li M, Chen J. Molecular subtyping in pancreatic neuroendocrine neoplasms: new insights into clinical, pathological unmet needs and challenges [published online ahead of print. Biochim Biophys Acta Rev Cancer. 2020;2020. [DOI] [PubMed]
- 33.Rindi G, Inzani F. Neuroendocrine neoplasm update: toward universal nomenclature. Endocr Relat Cancer. 2020;27:R211–R218. doi: 10.1530/ERC-20-0036. [DOI] [PubMed] [Google Scholar]
- 34.Turri-Zanoni M, Maragliano R, Battaglia P, Giovannardi M, Antognoni P, Lombardi D, Morassi ML, Pasquini E, Tarchini P, Asioli S, Foschini MP, Sessa F, Nicolai P, Castelnuovo P, la Rosa S. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, Travis WD. ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 36.Fabbri A, Cossa M, Sonzogni A, Papotti M, Righi L, Gatti G, Maisonneuve P, Valeri B, Pastorino U, Pelosi G. Ki-67 labeling index of neuroendocrine tumors of the lung has a high level of correspondence between biopsy samples and surgical specimens when strict counting guidelines are applied. Virchows Arch. 2017;470:153–164. doi: 10.1007/s00428-016-2062-2. [DOI] [PubMed] [Google Scholar]
- 37.Naheed S, Holden C, Tanno L, Jaynes E, Cave J, Ottensmeier CH, Pelosi G. The utility of Ki-67 as a prognostic biomarker in pulmonary neuroendocrine tumours: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e031531. doi: 10.1136/bmjopen-2019-031531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelosi G, Massa F, Gatti G, Righi L, Volante M, Birocco N, Maisonneuve P, Sonzogni A, Harari S, Albini A, Papotti M. Ki-67 evaluation for clinical decision in metastatic lung carcinoids: a proof of concept. Clin Pathol. 2019;12:2632010X19829259. [DOI] [PMC free article] [PubMed]
- 39.Milione M, Maisonneuve P, Grillo F, Mangogna A, Centonze G, Prinzi N, et al. Ki-67 index of 55% distinguishes two groups of bronchopulmonary pure and composite large cell neuroendocrine carcinomas with distinct prognosis. Neuroendocrinology. 2020. 10.1159/000508376. [DOI] [PubMed]
- 40.Marchevsky AM, Hendifar A, Walts AE. The use of Ki-67 labeling index to grade pulmonary well-differentiated neuroendocrine neoplasms: current best evidence. Mod Pathol. 2018;31:1523–1531. doi: 10.1038/s41379-018-0076-9. [DOI] [PubMed] [Google Scholar]
- 41.Marchiò C, Gatti G, Massa F, Bertero L, Filosso P, Pelosi G, Cassoni P, Volante M, Papotti M. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch. 2017;471:713–720. doi: 10.1007/s00428-017-2177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasajima A, Konukiewitz B, Oka N, Suzuki H, Sakurada A, Okada Y, Kameya T, Ishikawa Y, Sasano H, Weichert W, Klöppel G. Clinicopathological profiling of lung carcinoids with a Ki67 index > 20. Neuroendocrinology. 2019;108:109–120. doi: 10.1159/000495806. [DOI] [PubMed] [Google Scholar]
- 43.Pelosi G, Bianchi F, Dama E, Simbolo M, Mafficini A, Sonzogni A, Pilotto S, Harari S, Papotti M, Volante M, Fontanini G, Mastracci L, Albini A, Bria E, Calabrese F, Scarpa A. Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: innovative findings skipping the current pathogenesis paradigm. Virchows Arch. 2018;472:567–577. doi: 10.1007/s00428-018-2307-3. [DOI] [PubMed] [Google Scholar]
- 44.Cros J, Théou-Anton N, Gounant V, Nicolle R, Reyes C, Humez S, et al. Specific genomic alterations in high grade pulmonary neuroendocrine tumours with carcinoid morphology. Neuroendocrinology. 2020. 10.1159/000506292. [DOI] [PubMed]
- 45.Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, Sertier AS, Ferrari A, Derks J, Ghantous A, Delhomme TM, Chabrier A, Cuenin C, Abedi-Ardekani B, Boland A, Olaso R, Meyer V, Altmuller J, le Calvez-Kelm F, Durand G, Voegele C, Boyault S, Moonen L, Lemaitre N, Lorimier P, Toffart AC, Soltermann A, Clement JH, Saenger J, Field JK, Brevet M, Blanc-Fournier C, Galateau-Salle F, le Stang N, Russell PA, Wright G, Sozzi G, Pastorino U, Lacomme S, Vignaud JM, Hofman V, Hofman P, Brustugun OT, Lund-Iversen M, Thomas de Montpreville V, Muscarella LA, Graziano P, Popper H, Stojsic J, Deleuze JF, Herceg Z, Viari A, Nuernberg P, Pelosi G, Dingemans AMC, Milione M, Roz L, Brcic L, Volante M, Papotti MG, Caux C, Sandoval J, Hernandez-Vargas H, Brambilla E, Speel EJM, Girard N, Lantuejoul S, McKay JD, Foll M, Fernandez-Cuesta L. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun. 2019;10:3407. doi: 10.1038/s41467-019-11276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelosi G, Massa F, Gatti G, Righi L, Volante M, Birocco N, Maisonneuve P, Sonzogni A, Harari S, Albini A, Papotti M. Ki-67 evaluation for clinical decision in metastatic lung carcinoids: a proof of concept. Clin Pathol. 2019;12:263. doi: 10.1177/2632010X19829259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feyrter F, Hartmann G. Uber die carcinoide Wuchsform der Carcinoma mammae, insbesondere das Carcinoma Solidum (gelatinosum) mammae. Frankf Z Pathol. 1963;73:24–39. [PubMed] [Google Scholar]
- 48.Bussolati G, Gugliotta P, Sapino A, Eusebi V. Lloyd RV Chromogranin reactive endocrine cells in argyrophilic carcinomas (“carcinoids”) and normal tissue of the breast. Am J Pathol. 1985;120:186–192. [PMC free article] [PubMed] [Google Scholar]
- 49.Papotti M, Macri L, Finzi G, Capella C, Eusebi V, Bussolati G. Neuroendocrine differentiation in carcinomas of the breast: a study of 51 cases. Semin Diagn Pathol. 1989;61:174–188. [PubMed] [Google Scholar]
- 50.Capella C, Usellini L, Papotti M, Macri L, Finzi G, Eusebi V. Bussolati G Ultrastructural features of neuroendocrine differentiated carcinomas of the breast. Ultrastruct Pathol. 1990;14:321–334. doi: 10.3109/01913129009032247. [DOI] [PubMed] [Google Scholar]
- 51.Uccella S, Finzi G, Sessa F, La Rosa S. On the endless dilemma of neuroendocrine neoplasms of the breast: a journey through concepts and entities [published online ahead of print, 2020 Jul 2]. Endocr Pathol. 2020. 10.1007/s12022-020-09637-y. [DOI] [PubMed]
- 52.Rakha EA, Reis-Filho JS, Sasano H, Wu. Neuroendocrine neoplasms: introduction. In: WHO Classification of Tumours Editorial Board editors. Breast tumours, 5th Ed, vol. 2. Lyon: IARC; 2019. pp. 155.
- 53.Marchiò C, Geyer FC, Ng CKY, Piscuoglio S, de Filippo MR, Cupo M, Schultheis AM, Lim RS, Burke KA, Guerini-Rocco E, Papotti M, Norton L, Sapino A, Weigelt B, Reis-Filho JS. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J Pathol. 2017;241:405–419. doi: 10.1002/path.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, Zamboni G, Carbognin G, Alongi F, Salgarello M, Gori S. Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist. 2016;21:28–32. doi: 10.1634/theoncologist.2015-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosen LE, Gattuso P. Neuroendocrine tumors of the breast. Arch Pathol Lab Med. 2017;141:1577–1581. doi: 10.5858/arpa.2016-0364-RS. [DOI] [PubMed] [Google Scholar]
- 56.Terlević R, Perić Balja M, Tomas D, Skenderi F, Krušlin B, Vranic S, Demirović A. Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrine breast cancer. Ann Diagn Pathol. 2019;38:62–66. doi: 10.1016/j.anndiagpath.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Kumar U, Grigorakis SI, Watt HL, Sasi R, Snell L, Watson P, Chaudhari S. Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1–5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res Treat. 2005;92:175–186. doi: 10.1007/s10549-005-2414-0. [DOI] [PubMed] [Google Scholar]
- 58.Frati A, Rouzier R, Lesieur B, Werkoff G, Antoine M, Rodenas A, et al. Expression of somatostatin type-2 and -4 receptor and correlation with histological type in breast cancer. Anticancer Res. 2014;34:3997–4003. [PubMed] [Google Scholar]
- 59.McCullar B, Pandey M, Yaghmour G, Hare F, Patel K, Stein K, Feldman R, Chandler JC, Martin MG. Genomic landscape of small cell carcinoma of the breast contrasted to small cell carcinoma of the lung. Breast Cancer Res Treat. 2016;158:195–202. doi: 10.1007/s10549-016-3867-z. [DOI] [PubMed] [Google Scholar]
- 60.Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000;24:1231–1238. doi: 10.1097/00000478-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms [published correction appears in Virchows arch. 2017 Dec 26] Virchows Arch. 2018;472:341–349. doi: 10.1007/s00428-017-2258-0. [DOI] [PubMed] [Google Scholar]
- 62.Vanoli A, La Rosa S, Miceli E, et al. Prognostic evaluations tailored to specific gastric neuroendocrine neoplasms: analysis of 200 cases with extended follow-up. Neuroendocrinology. 2018;107:114–126. doi: 10.1159/000489902. [DOI] [PubMed] [Google Scholar]
- 63.Vanoli A, La Rosa S, Klersy C, et al. Four neuroendocrine tumor types and neuroendocrine carcinoma of the duodenum: analysis of 203 cases. Neuroendocrinology. 2017;104:112–125. doi: 10.1159/000444803. [DOI] [PubMed] [Google Scholar]
- 64.Panzuto F, Campana D, Fazio N, Brizzi MP, Boninsegna L, Nori F, di Meglio G, Capurso G, Scarpa A, Dogliotti L, de Braud F, Tomassetti P, Delle Fave G, Falconi M. Risk factors for disease progression in advanced jejunoileal neuroendocrine tumors. Neuroendocrinology. 2012;96:32–40. doi: 10.1159/000334038. [DOI] [PubMed] [Google Scholar]
- 65.Brighi N, La Rosa S, Rossi G, et al. Morphological factors related to nodal metastases in neuroendocrine tumors of the appendix: a multicentric retrospective study. Ann Surg. 2020;271:527533. doi: 10.1097/SLA.0000000000002939. [DOI] [PubMed] [Google Scholar]
- 66.de Lambert G, Lardy H, Martelli H, Orbach D, Gauthier F, Guérin F. Surgical management of neuroendocrine tumors of the appendix in children and adolescents: a retrospective French multicenter study of 114 cases. Pediatr Blood Cancer. 2016;63:598–603. doi: 10.1002/pbc.25823. [DOI] [PubMed] [Google Scholar]
- 67.Rault-Petit B, Do Cao C, Guyétant S, Guimbaud R, Rohmer V, Julié C, Baudin E, Goichot B, Coriat R, Tabarin A, Ramos J, Goudet P, Hervieu V, Scoazec JY, Walter T. Current management and predictive factors of lymph node metastasis of appendix neuroendocrine tumors: a National Study from the French Group of Endocrine Tumors (GTE) Ann Surg. 2019;270:165–171. doi: 10.1097/SLA.0000000000002736. [DOI] [PubMed] [Google Scholar]
- 68.Mehrvarz Sarshekeh A, Advani S, Halperin DM, Conrad C, Shen C, Yao JC. Dasari a regional lymph node involvement and outcomes in appendiceal neuroendocrine tumors: a SEER database analysis. Oncotarget. 2017;8:99541–99551. doi: 10.18632/oncotarget.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jernman J, Valimaki MJ, Louhimo J, Haglund C, Arola J. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology. 2012;95:317–324. doi: 10.1159/000333035. [DOI] [PubMed] [Google Scholar]
- 70.Sohn JH, Cho MY, Park Y, et al. Prognostic significance of defining l-cell type on the biologic behavior of rectal neuroendocrine tumors in relation with pathological parameters. Cancer Res Treat. 2015;47:813–822. doi: 10.4143/crt.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramage JK, De Herder WW, Delle Fave G, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103:139–143. doi: 10.1159/000443166. [DOI] [PubMed] [Google Scholar]
- 72.La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, Rindi G. Solcia E. Prognostic criteria in nonfunctioning pancreatic endocrine tumours Virchows Arch. 1996;429:323–333. doi: 10.1007/BF00198436. [DOI] [PubMed] [Google Scholar]
- 73.Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol. 1996;27:1124–1134. doi: 10.1016/S0046-8177(96)90303-2. [DOI] [PubMed] [Google Scholar]
- 74.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, la Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Nat Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 75.Rindi G, Klersy C, Albarello L, Baudin E, Bianchi A, Buchler MW, Caplin M, Couvelard A, Cros J, de Herder WW, Delle Fave G, Doglioni C, Federspiel B, Fischer L, Fusai G, Gavazzi F, Hansen CP, Inzani F, Jann H, Komminoth P, Knigge UP, Landoni L, la Rosa S, Lawlor RT, Luong TV, Marinoni I, Panzuto F, Pape UF, Partelli S, Perren A, Rinzivillo M, Rubini C, Ruszniewski P, Scarpa A, Schmitt A, Schinzari G, Scoazec JY, Sessa F, Solcia E, Spaggiari P, Toumpanakis C, Vanoli A, Wiedenmann B, Zamboni G, Zandee WT, Zerbi A, Falconi M. Competitive testing the WHO 2010 vs the WHO 2017 grading of pancreas neuroendocrine neoplasia: data from a large international cohort study. Neuroendocrinology. 2018;107:375–386. doi: 10.1159/000494355. [DOI] [PubMed] [Google Scholar]
- 76.Kwon J, Kim HJ, Park DH, et al. Incidentally detected pancreatic neuroendocrine microadenoma with lymph node metastasis. Virchows Arch. 2018;473:649–653. doi: 10.1007/s00428-018-2407-0. [DOI] [PubMed] [Google Scholar]
- 77.Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2016. [DOI] [PubMed] [Google Scholar]
- 78.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of the female reproductive organs. Lyon: IARC Press; 2014. [Google Scholar]
- 79.Buza N, Cohen PJ, Hui P, Parkash V. Inverse p16 and p63 expression in small cell carcinoma and high-grade urothelial cell carcinoma of the urinary bladder. Int J Surg Pathol. 2010;18:94–102. doi: 10.1177/1066896909359914. [DOI] [PubMed] [Google Scholar]
- 80.Verduin L, Mentrikoski MJ, Heitz CT, Wick MR. The utility of GATA3 in the diagnosis of urothelial carcinomas with variant morphologic patterns. Appl Immunohistochem Mol Morphol. 2016;24:509–513. doi: 10.1097/PAI.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 81.Uccella S, Mathias-Guiu X, La Rosa S. Genitourinary neuroendocrine neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The spectrum of neuroendocrine neoplasia. Practical approach to diagnosis, classification and therapy. New York: Springer Cham; 2020. pp. 301-333
- 82.Tamas EF. Epstein JI prognostic significance of Paneth cell-like neuroendocrine differentiation in adenocarcinoma of the prostate. Am J Surg Pathol. 2006;30:980–985. doi: 10.1097/00000478-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Prendeville S, Al-Bozom I, Compérat E, Sweet J, Evans AJ, Ben-Gashir M, Mete O, van der Kwast TH, Downes MR. Prostate carcinoma with amphicrine features: further refining the spectrum of neuroendocrine differentiation in tumours of primary prostatic origin? Histopathology. 2017;71:926–933. doi: 10.1111/his.13330. [DOI] [PubMed] [Google Scholar]
- 84.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol. 2013;20:285–314. doi: 10.1097/PAP.0b013e3182a2dc67. [DOI] [PubMed] [Google Scholar]
- 85.Koo J, Dhall D. Problems with the diagnosis of metastatic neuroendocrine neoplasms. Which diagnostic criteria should we use to determine tumor origin and help guide therapy? Semin Diagn Pathol. 2015;32:456–468. doi: 10.1053/j.semdp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 86.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139:2679–2686. doi: 10.1002/ijc.30400. [DOI] [PubMed] [Google Scholar]
- 87.Alexandraki KI, Tsoli M, Kyriakopoulos G, Angelousi A, Nikolopoulos G, Kolomodi D, Kaltsas GA. Current concepts in the diagnosis and management of neuroendocrine neoplasms of unknown primary origin. Minerva Endocrinol. 2019;44:378–386. doi: 10.23736/S0391-1977.19.03012-8. [DOI] [PubMed] [Google Scholar]
- 88.Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445–454. doi: 10.1016/j.jaad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Uccella S, Asa SL, Mete O. Metastatic neuroendocrine neoplasms of unknown primary site. In: Asa SL, La Rosa S, Mete O, editors. The spectrum of neuroendocrine neoplasia. Practical approach to diagnosis, classification and therapy. New York: Springer Cham; 2021. pp. 357-387.