Abstract
Aim
This study aimed to undertake a systematic review and meta-analysis of global prevalence and types of complementary and alternative medicine (CAM) use amongst adults with diabetes.
Methods
Nine databases, including MEDLINE and EMBASE, were searched for studies published between 2009 and 2019 which included extractable data for CAM use in adult patients with diabetes. Study characteristics, types of CAM, and overall and subgroup prevalence data in relation to CAM use were extracted. Meta-analysis of aggregate level data on prevalence and prevalence ratios (PRs) was performed using a random effects model.
Results
From the 38 studies included in the review, a total of 37 types of CAM and 223 types of herbs were identified. Pooled prevalence of CAM use was 51%. A wide variation in prevalence rates (predictive interval 8–93%) was observed. In the context of high heterogeneity, we found no evidence that CAM use was associated with gender, chronicity or type of diabetes. Approximately one third of patients did not disclose their use of CAM to healthcare professionals (95% PrI 25%, 97%). Herbal medicines, acupuncture, homoeopathy and spiritual healing were the common CAM types reported.
Conclusions
A wide variation in prevalence of CAM use by patients with diabetes was identified. Healthcare professionals should be aware of their patients’ use of CAM to ensure treatment optimization, avoid herb–drug interactions and promote medication adherence in diabetes. Diabetic reviews and clinical guidelines should incorporate exploration of patient use of CAM as many patients do not proactively disclose the use of CAM to their healthcare professionals.
Registration
The protocol for this study was registered with the Centre for Review and Dissemination (CRD). Protocol registration number CRD42019125036.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00228-021-03097-x.
Keywords: Prevalence, Complementary and alternative medicine, Diabetes, Systematic review
Introduction
The World Health Organization (WHO) estimates that over 400 million people have diabetes worldwide, and this is projected to increase to reach 592 million by 2035 [1]. Poorly managed diabetes can lead to serious and possibly fatal complications such as cardiovascular disease, renal failure, nerve damage and blindness [2–4].
Diabetes mellitus (DM) is a chronic metabolic disorder in which blood glucose levels are higher than normal for a long period of time. These high blood glucose levels are attributed to abnormal disturbances of insulin production and/or function [5]. Diabetes is caused by either lack of insulin production by the pancreas (type 1 diabetes, T1D), when the amount of insulin produced by the pancreas is insufficient to carry out all blood glucose regulation processes, or by decreased insulin sensitivity by the body cells (type 2 diabetes, T2D). Diabetes can also be caused by a combination of low insulin production as well as low insulin sensitivity or be due to hormonal dysregulation in pregnancy [5].
Self-care practices relevant to self-management of diabetes include adherence to prescribed treatment and clinical management plans, adopting a healthy lifestyle and having a balanced diet [6]. In addition, many patients also use complementary and alternative medicine (CAM) [7]. The WHO defines CAM as a ‘broad set of health care practices that are not part of that country’s own tradition or conventional medicine and are not fully integrated into the dominant health-care system’ [8].
CAM use is known to be prevalent in patients with diabetes as a supplement to their existing orthodox diabetes treatments, as a replacement, or for reasons that might not be directly related to diabetes such as using CAM for energy and general wellbeing [7]. Various factors may influence CAM use by patients with diabetes. A study of 3978 U.S. adults suggested that CAM use by patients who were diabetic for more than 10 years or patients who had a functional limitation caused by diabetes were more likely to use CAM compared to patients with less severe diabetes [9]. In addition, the study reported that 77% of patients who used CAM for the treatment of diabetes used CAM as a supplement to conventional treatment, while 23% used CAM as a replacement [9].
CAM users often perceive CAM to be an effective means of lowering blood glucose levels and treating side effects of prescribed diabetic medications [10–15]. However, adverse outcomes of CAM use have also been reported. For example, CAM can affect the management of diabetes by either direct herb–drug interaction with the use of herbal remedies or indirectly by affecting medication adherence when using herbal or any other CAM types [6].
There is a lack of an up-to-date systematic review that investigates the prevalence of CAM use by patients with diabetes. Patient sources of health-related information have changed immensely in the past decade [16]. In particular, increasing availability and use of web-based information sources, including social media and online health information in recent years, may encourage and inhibit CAM use in long term health conditions [17]. An up-to date systematic review on the prevalence of CAM use by patients with diabetes will help healthcare professionals to consider patient use of CAM when counselling patients, supporting adherence and identifying the risks of interactions and adverse effects when CAMs are used in conjunction with prescribed treatments.
The aim of this study was to systematically review the global prevalence of CAM use amongst adults with diabetes. Specific objectives were to identify the types of CAM that are used by the population with diabetes and to identify differences in CAM use amongst different populations with diabetes, including types of diabetes, demographic characteristics, duration of diabetes and presence or absence of diabetic complications.
Methods
This systematic review was informed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist [18]. A protocol was developed as per the PRISMA protocol guideline (protocol ID CRD42019125036).
Data sources and searches
Cochrane Library, MEDLINE, Embase, CINAHL, AMED, Web of Science, Google Scholar and PROSPERO databases were searched for the past 10 years covering 2009 to June 2019. Open Grey was searched for grey literature. Search terms and an example search strategy are listed in Supplementary Table 1. The review was restricted to studies published in English. Studies that recruited participants who are adult patients with diabetes, 18 years of age and older and reported partially or exclusively the prevalence and use of CAM amongst patients with diabetes were included. Studies which either focused on CAM use in conjunction with conventional treatments or as a replacement were considered.
Study selection
Screening and selection were performed independently by two review authors (AA, VP) and were carried out in three phases. Titles and abstracts were screened for inclusion of possible relevant studies followed by assessment of full texts for eligibility. Reference lists of included studies were screened. If a title was considered relevant; the study was manually searched and the abstract examined.
Data extraction and quality assessment
Data on study characteristics, prevalence of CAM use as well as types of CAM used by patients with diabetes were extracted. Two review authors (AA, VP) independently assessed the quality of included studies using the critical appraisal tool from the Joanna Briggs Institute (JBI) checklist [19]. Studies were classified into high, moderate and low quality based on the results of the JBI checklist (Supplementary Table 1). The quality assessment in included studies was focused on three fields: clarity of inclusion criteria and study setting and sampling, appropriateness of approaches to data collection and analysis, and outcome measurement (i.e. use of CAM). Included studies were judged to be of ‘high quality’ if quality criteria were satisfied by at least 7 items, ‘moderate quality’ for scores of 3–6 and ‘low quality’ for scores ≤2 [20]. All studies were included regardless of their quality.
Data synthesis and analysis
A quantitative synthesis of aggregate level data on prevalence was performed. Study specific results were reported as percentage prevalence with exact 95% confidence intervals (95%CI). When sufficient data were available for within-study comparisons of prevalence between dichotomous groups, e.g. sex, then relative prevalence ratios (PRs) together with 95% confidence intervals were calculated. Meta-analyses of proportions and PRs were performed using a random effects model fit using the method of Der Simonian & Laird [21]. Heterogeneity was assessed using the I2 statistic, the between study standard deviation and calculation of 95% prediction intervals (95%PrI) for the prevalence in a new study [22, 23]. Data are presented in forest plots which include pooled estimates where appropriate. All analyses were performed using STATA version 15.
Results
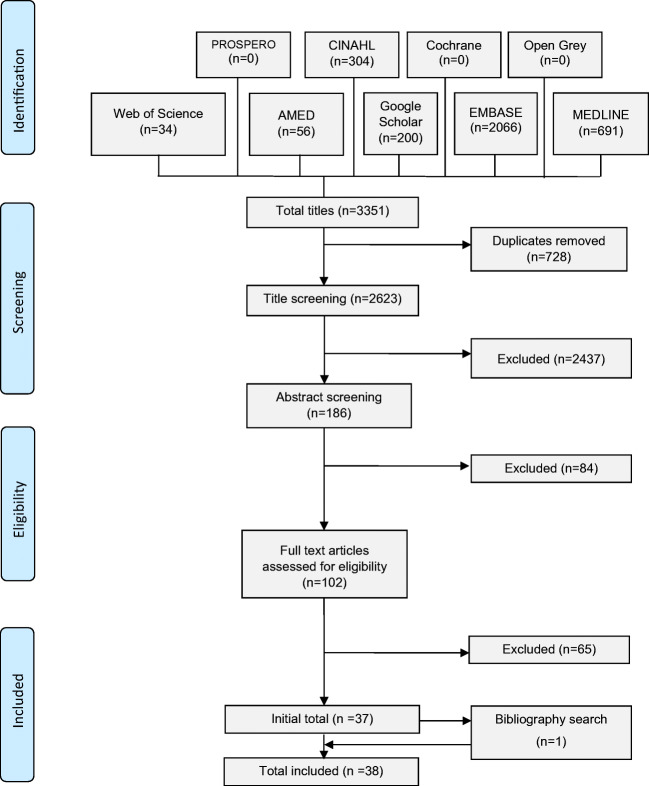
A total of 2623 unique titles were screened of which 38 articles met the inclusion criteria (Fig. 1). After applying quality assessment, studies fell into these categories (8 high quality studies, 30 moderate quality studies and no low quality studies). Details of critical appraisal results are available in Supplementary Table 2.
Fig. 1.
Prisma flow diagram
Study characteristics
Included studies originated from 25 different countries. Participants were mostly recruited from diabetes clinics and healthcare centres (Table 1). Fifteen of the studies enrolled participants with either T1D or T2D, and 23 studies only included patients with T2D. Out of the included 38 studies, 37 were cross-sectional surveys and one analysed data from another cohort study (Table 1).
Table 1.
Study characteristics
| Author and year | Country of study | Focus of the study | Study settings and recruitment of participants | Study design | Data collection method | Study participants |
|---|---|---|---|---|---|---|
| Alami et al. 2015 [24] | Morocco | Herbal supplements only | Mohammad VI university hospital,Oujda | Cross-sectional | Face-to-face interview using a semi-structured questionnaire | T1D and T2D patients |
| Al-Eidi et al. 2016 [25] | Saudi | Any CAM type | Diabetic Centre of King Salman bin Abdul-Aziz Hospital, in Riyadh city | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
| Al-garni et al. 2017 [26] | Saudi | Herbal supplements only | Jeddah Diabetic Centre (JDC) | Cross-sectional | Interviewer-administered semi-structured questionnaire | T2D patients |
| Ali-Shtayehet et al. 2012 [14] | Palestine | Any CAM type | Patients attending outpatient departments at Governmental Hospitals in 7 towns in the Palestinian territories (Jenin, Nablus, Tulkarm, Qalqilia, Tubas, Ramalla, and Hebron) | Cross-sectional | Structured questionnaires | T1D and T2D patients |
| Amaeze et al. 2018 [27] | Nigeria | Herbal supplements only | 5 secondary healthcare facilities across Lagos State | Cross-sectional | Interviewer-administered questionnaires | T2D patients |
| Andrews et al. 2018 [28] | Guatemala | Any CAM type | Interview three groups in the San Lucas Tolimán area | Cross-sectional | Semi-structured questionnaires | T2D patientsHealth promotersTraditional healers |
| Ashur et al. 2017 [29] | Libya | Any CAM type | National Centre for Diabetes and Endocrinology in Tripoli | Cross-sectional | Self-administered structured questionnaire | T2D patients |
| Avci et al. 2018 [30] | Turkey | Any CAM type | Van Yuzuncu Yil University, Van | Cross-sectional | Semi-structured questionnaires | T1D and T2D patients |
| Azizi-Fini et al. 2016 [11] | Iran | Herbal supplements only | Golabchi and Naqavi diabetes clinics in the Kashan city | Cross-sectional | Interviewer-administered structured questionnaires | T2D patients |
| Baharom et al. 2016 [31] | Malaysia | Any CAM type | 45 government health clinics across Nigeria Sembilan | Cross-sectional | Interviewer-administered structured questionnaires | T2D patients |
| Bradley et al. 2012 [13] | USA | Any CAM type | Patients with moderately to poorly controlled T2D who receive care from Group Health Cooperative (GHC), a large non-profit, integrated health care system in Washington State | Cross-sectional | Telephone-administered questionnaires. | T2D patients |
| Candar et al. 2018 [32] | Turkey | Any CAM type | Patients registered with the Bursa Yuksek Ihtisas Training and Research Hospital Education Family Health Centre | Cross-sectional | Questionnaires | T1D and T2D patients |
| Chao et al. 2014 [33] | USA | Any CAM type | Patients who received primary care at one of four publicly funded clinics in the Community Health Network of San Francisco | Cross-sectional | Data collected for the Self-Management Automated and Real-Time Telephonic Support (SMART Steps) Study | T2D patients |
| Ching et al. 2013 [34] | Malaysia | Any CAM type | Primary health care clinic at Salak in Sepang | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
| Damnjanovic et al. 2015 [35] | Serbia | Herbal supplements only | 6 Remedia Pharmacy HealthFacilities in the territory of Nis | Cross-sectional | Structured questionnaires | T2D patients |
| Devi et al. 2015 [36] | India | Any CAM type | Diabetes Health camp conducted by VS micro lab, Madurai | Cross-sectional | Structured questionnaires | T2D patients |
| Fabian et al. 2011 [37] | Austria | Herbal supplements only | Diabetes Centre of the Division of Endocrinology and Metabolism, Department of Internal Medicine, Medical University of Graz | Cross-sectional | Face-to-face interview using a structured questionnaire | T1D and T2D patients |
| Fan et al. 2013 [38] | Singapore | Any CAM type | Single centre study conducted in an outpatient diabetes Centre with an average load of 2500 patients a month | Cross-sectional | Self-administered questionnaires. | T2D patients |
| Hashempur et al. 2015 [39] | Iran | Any CAM type | Two outpatient diabetes clinics affiliated with the Shiraz University of Medical Sciences, Shiraz | Cross-sectional | Face-to-face interview using semi-structured questionnaire | T1D and T2D patients |
| Kamel et al. 2017 [40] | Saudi | Herbal supplements only | King Abdul-Aziz University and King Fahad General Hospitals in Jeddah | Cross-sectional | Interviewer-administered structured questionnaires | T1D and T2D patients |
| Karaman et al. 2018 [10] | Turkey | Herbal supplements only | Endocrinology clinics of two hospitals in Izmir | Cross-sectional | Face-to-face interview using a structured questionnaire | T1D and T2D patients |
| Khalaf and Whitford 2010 [41] | Bahrain | Any CAM type | Patients attending two hospital diabetes clinics | Cross-sectional | Questionnaires (administration not detailed) | T1D and T2D patients |
| Khalil et al. 2013 [42] | Egypt | Herbal supplements only | Outpatient clinics of Alexandria University Hospital, from seven health insurance centres, six MOH hospitals, and one private healthcare facility. | Cross-sectional | Questionnaires (administration method not reported) | T2D patients |
| Koren et al. 2015 [43] | Israel | Herbal supplements only | Internal medicine department at Assaf Harofeh Medical Centre, Zerifin | Cross-sectional | Interviewer-administered structured questionnaires | T2D patients |
| Lui et al. 2012 [44] | Australia | Any CAM type | Data reported here are taken from the Living with Diabetes Study (LWDS), a five-year, prospective cohort study being conducted in the State of Queensland | Data from cohort study | Questionnaires (administration not detailed) | T1D and T2D patients |
| Lunyera et al. 2016 [45] | Tanzania | Herbal supplements only | Kilimanjaro Region of Tanzania | Cross-sectional | Verbally administered structured questionnaire | T1D and T2D patients |
| Medagama et al. 2014 [46] | Sri Lanka | Herbal supplements only | Diabetes clinic at Teaching Hospital Peradeniya | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
| Mekuria et al. 2018 [47] | Ethiopia | Herbal supplements only | Diabetes care clinic of University of Gondar comprehensive specialized hospital | Cross-sectional | Interviewer-administered questionnaires | T2D patients |
| Mohamed Ali and Mahfouz 2014 [48] | Sudan | Herbal supplements only | 125 primary health care centres in Khartoum | Cross-sectional | Interviewer-administered questionnaires | T2D patients |
| Naja et al. 2014 [49] | Lebanon | Any CAM type | Patients recruited from two major referral centres in Beirut- a public hospital and a private academic medical Centre | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
| Nguyen et al. 2014 [50] | USA | Any CAM type | Patients recruited from seven primary care or endocrinology clinics affiliated with an academic medical centre in Southern California | Cross-sectional | Self-administered structured questionnaire | T2D patients |
| Putthapiban et al. 2017 [51] | Thailand | Herbal supplements only | At the Endocrine Clinic in Ramathibodi Hospital, Bangkok | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
| Rhee et al. 2018 [52] | USA | Any CAM type | Non-institutionalized civilians in US | Cross-sectional | Data were from the 2012 NHIS, which was administrated by the National Centre for Health Statistics of the Centers for Disease Control and Prevention | T1D and T2D patients |
| Sethi et al. 2011 [12] | India | Any CAM type | Tertiary care Centre in Delhi | Cross-sectional | Face-to-face interview using a Semi-structured questionnaire | T1D and T2D patients |
| Vishnu et al. 2017 [53] | India | Any CAM type | Rural Kollam district of the Indian state of Kerala (community based) | Cross-sectional | Interviewer-administered structured questionnaires | T1D and T2D patients |
| Wanchai and Phrompayak 2016 [54] | Thailand | Any CAM type | Four primary healthcare unitsand two secondary hospitals in the north of Thailand | Cross-sectional | Semi-structured questionnaire | T2D patients |
| Wazaify et al. 2011 [15] | Jordan | Herbal supplements only | Outpatient departments at The National Centre for Diabetes, Endocrine and Genetics (NCDEG. | Cross-sectional | Face-to-face interview using a Semi-structured questionnaire | T1D and T2D patients |
| Yildirim and Marakoglu 2018 [55] | Turkey | Any CAM type | Outpatient diabetes from Selçuk University Family Medicine Diabetes Education Clinic | Cross-sectional | Face-to-face interview using a structured questionnaire | T2D patients |
CAM Complementary and alternative medicine
Types of CAM
Sixteen studies focused exclusively on herbal and nutritional supplement use by patients with diabetes (Table 1). The remaining 22 studies discussed other CAM types. Fourteen of those 22 studies that investigated other CAM types also reported the use of herbal and nutritional supplements as a form of CAM [12–14, 25, 32, 34, 36, 38, 39, 41, 49, 52–54]. A total of 35 different CAM types were reported in at least one study. CAM types used by patients with diabetes and mentioned in the most studies were acupuncture (n = 6 studies), Mind–body therapies (n = 6 studies) religious and spiritual healing (n = 5 studies) and homoeopathy (n = 4 studies) (Table 2).
Table 2.
List of complementary and alternative medicine types as cited by included studies
| CAM forms (other than herbal supplements) |
Studies cited the CAM form | CAM forms (other than herbal supplements) |
Studies cited the CAM form |
|---|---|---|---|
| Acupuncture | [25, 29, 36, 39, 50, 52] | Ruqyah (recitation) with the Quran | [25, 29] |
| Mind–body therapies | [32, 34, 36, 39, 41, 52] | Ruqyah water or oil | [25, 29] |
| Religious and spiritual healing | [29, 32, 49, 50, 54] | Balneotherapy | [32] |
| Homoeopathy | [12, 36, 52, 53] | Biofeedback | [52] |
| Meditation | [13, 36, 52, 54] | Chelation | [52] |
| Massage | [13, 25, 38, 49] | Chinese medicine | [49] |
| Ayurveda | [36, 52, 53] | Curandero | [50] |
| Chiropractic Massage | [13, 50, 52] | Daode Xinxi | [54] |
| Energy therapies | [34, 41, 52] | Deep breathing exercises | [13] |
| Specific diet | [13, 25, 36] | Leech (Hirudotherapy) | [32] |
| Yoga | [13, 36, 52] | Music therapy | [36] |
| Al-hijama (wet cupping) | [25, 29] | Prayer by religion person (imam) | [30] |
| Biologically based therapies | [36, 52] | Progressive muscle relaxation | [13] |
| Cupping | [32, 39] | Qi gong | [52] |
| Folk medicine | [13, 49] | Sugar therapy | [53] |
| Honey | [14, 25] | Tai chi | [52] |
| Movement therapies | [36, 52] | Traditional healers | [52] |
| Naturopathy | [50, 52] | ||
CAM Complementary and alternative medicine
Within the 31 studies which reported the use of herbal and nutritional supplements by patients with diabetes, a total of 223 different herbal and nutritional supplements were reported (Supplementary Table 3). The five herbs that were mentioned in the most studies were, cinnamon (Cinnamomum verum) and fenugreek (Trigonella foenum-graecum) each reported in 18 different studies, garlic (Allium sativum) reported in 17 studies, aloe vera (Aloe Vera) reported in 14 studies and black seed (Nigella sativa) reported in 12 studies.
Prevalence of CAM use
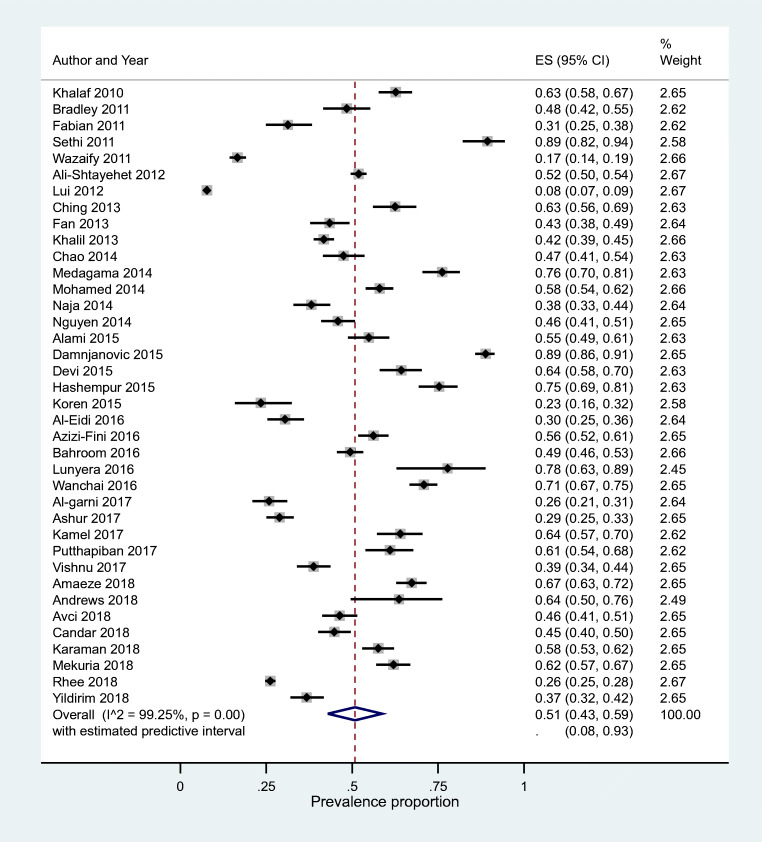
The highest prevalence of CAM (all types) use was reported at 89% by two studies, one each from India and Jordan followed by studies in Tanzania (78%), Sri Lanka (76%) and Iran (75%) (Table 3) [12, 35, 39, 45, 46]. The lowest prevalence of CAM use was 17% as reported by a study conducted in Jordan [15]. A study in Australia reported a prevalence of 8%, but the study gathered data from patients about their visits to CAM practitioners only and did not include data on CAM use in general by patients with diabetes [44]. Other studies reporting the lowest prevalence of CAM use included studies in Libya (29%), Saudi Arabia (26%), USA (26%), Israel (23%) and Jordan (17%) [15, 26, 29, 43, 52]. Pooled prevalence of CAM use was 51% (95%CI 43%, 59%). However, heterogeneity was very high (I2 = 99%) with the predictive interval ranging from 8% to 93%. (Fig. 2).
Table 3.
Included studies’ overall and subgroups prevalence of CAM use
| Country | Study | Sample size | Prevalence of CAM use | All participants | Female | Male | T1D | T2D | Had diabetes for ≤5y | Had diabetes for >5y | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| users | non users | users | non users | % | users | non users | % | users | non users | % | users | non users | % | users | non users | % | users | non users | % | ||||
| India | Sethi et al. 2011 [12] | 113 | 89.38% | 101 | 12 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Serbia | Damnjanovic et al. 2015 [35] | 519 | 88.82% | 461 | 58 | 261 | 15 | 95% | 200 | 43 | 82% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Tanzania | Lunyera et al. 2016 [45] | 45 | 77.78% | 35 | 10 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sri Lanka | Medagama et al. 2014 [46] | 252 | 76.19% | 192 | 60 | 139 | 28 | 83% | 53 | 32 | 62% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Iran | Hashempur et al. 2015 [39] | 239 | 75.31% | 180 | 59 | 124 | 37 | 77% | 56 | 22 | 72% | 10 | 7 | 59% | 170 | 52 | 77% | 80 | 25 | 76% | 100 | 34 | 75% |
| Thailand | Wanchai and Phrompayak 2016 [54] | 508 | 70.87% | 360 | 148 | 282 | 102 | 73% | 78 | 46 | 63% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Nigeria | Amaeze et al. 2018 [27] | 453 | 67.33% | 305 | 148 | 98 | 45 | 69% | 207 | 103 | 67% | NR | NR | NR | NR | NR | NR | 96 | 66 | 59% | 209 | 82 | 72% |
| India | Devi et al. 2015 [36] | 252 | 64.29% | 162 | 90 | 98 | 41 | 71% | 64 | 49 | 57% | NR | NR | NR | NR | NR | NR | 55 | 40 | 58% | 107 | 50 | 68% |
| Saudi | Kamel et al. 2017 [40] | 214 | 64.02% | 137 | 77 | 84 | 44 | 66% | 53 | 33 | 62% | 50 | 20 | 71% | 87 | 57 | 60% | NR | NR | NR | NR | NR | NR |
| Guatemala | Andrews et al. 2018 [28] | 55 | 63.64% | 35 | 20 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Bahrain | Khalaf and Whitford 2010 [41] | 402 | 62.69% | 252 | 150 | 149 | 69 | 68% | 103 | 81 | 56% | NR | NR | NR | NR | NR | NR | 48 | 51 | 48% | 204 | 99 | 67% |
| Malaysia | Ching et al. 2013 [34] | 240 | 62.50% | 150 | 90 | 96 | 49 | 66% | 54 | 41 | 57% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ethiopia | Mekuria et al. 2018 [47] | 387 | 62.02% | 240 | 147 | 149 | 73 | 67% | 91 | 74 | 55% | NR | NR | NR | NR | NR | NR | 168 | 60 | 74% | 72 | 87 | 45% |
| Thailand | Putthapiban et al. 2017 [51] | 200 | 61.00% | 122 | 78 | 76 | 42 | 64% | 46 | 36 | 56% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sudan | Mohamed Ali and Mahfouz 2014 [48] | 600 | 58.00% | 348 | 252 | 206 | 167 | 55% | 142 | 85 | 63% | NR | NR | NR | NR | NR | NR | 67 | 76 | 47% | 281 | 176 | 61% |
| Turkey | Karaman et al. 2018 [10] | 455 | 57.58% | 262 | 193 | 225 | 148 | 60% | 37 | 45 | 45% | 53 | 49 | 52% | 209 | 114 | 65% | 51 | 62 | 45% | 211 | 131 | 62% |
| Iran | Azizi-Fini et al. 2016 [11] | 500 | 56.20% | 281 | 219 | 203 | 153 | 57% | 78 | 66 | 54% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Morocco | Alami et al. 2015 [24] | 279 | 54.84% | 153 | 126 | 117 | 83 | 59% | 36 | 43 | 46% | 36 | 43 | 46% | 117 | 83 | 59% | NR | NR | NR | NR | NR | NR |
| Palestine | Ali-Shtayehet et al. 2012 [14] | 1883 | 51.89% | 977 | 906 | 519 | 470 | 52% | 458 | 436 | 51% | 114 | 84 | 58% | 863 | 822 | 51% | 341 | 325 | 51% | 636 | 581 | 52% |
| Malaysia | Baharom et al. 2016 [31] | 680 | 49.41% | 336 | 344 | 224 | 175 | 56% | 112 | 169 | 40% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| USA | Bradley et al. 2012 [13] | 219 | 48.40% | 106 | 113 | 47 | 50 | 48% | 59 | 63 | 48% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| USA | Chao et al. 2014 [33] | 278 | 47.48% | 132 | 146 | 101 | 38 | 73% | 31 | 108 | 22% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Turkey | Avci., 2018 [30] | 386 | 46.37% | 179 | 207 | 95 | 122 | 44% | 84 | 85 | 50% | 29 | 16 | 64% | 150 | 191 | 44% | 68 | 107 | 39% | 111 | 100 | 53% |
| USA | Nguyen et al. 2014 [50] | 410 | 45.85% | 188 | 222 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Turkey | Candar et al. 2018 [32] | 442 | 44.80% | 198 | 244 | 137 | 134 | 51% | 61 | 110 | 36% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Singapore | Fan et al., 2013 [38] | 304 | 43.42% | 132 | 172 | 67 | 69 | 49% | 65 | 103 | 39% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Egypt | Khalil et al. 2013 [42] | 1100 | 41.73% | 459 | 641 | 252 | 359 | 41% | 207 | 282 | 42% | NR | NR | NR | NR | NR | NR | 87 | 202 | 30% | 372 | 439 | 46% |
| India | Vishnu et al. 2017 [53] | 400 | 38.75% | 155 | 245 | 73 | 109 | 40% | 82 | 136 | 38% | NR | NR | NR | NR | NR | NR | 95 | 142 | 40% | 60 | 103 | 37% |
| Lebanon | Naja et al. 2014 [56] | 333 | 38.14% | 127 | 206 | 51 | 98 | 34% | 76 | 108 | 41% | NR | NR | NR | NR | NR | NR | 34 | 77 | 31% | 93 | 129 | 42% |
| Turkey | Yildirim and Marakoglu 2018 [55] | 400 | 36.75% | 147 | 253 | 91 | 115 | 44% | 56 | 138 | 29% | NR | NR | NR | NR | NR | NR | 77 | 149 | 34% | 70 | 104 | 40% |
| Austria | Fabian et al. 2011 [37] | 198 | 31.31% | 62 | 136 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Saudi | Al-Eidi et al. 2016 [25] | 302 | 30.46% | 92 | 210 | 50 | 121 | 29% | 42 | 89 | 32% | NR | NR | NR | NR | NR | NR | 24 | 93 | 21% | 68 | 117 | 37% |
| Libya | Ashur et al. 2017 [29] | 523 | 28.87% | 151 | 372 | 102 | 206 | 33% | 49 | 166 | 23% | NR | NR | NR | NR | NR | NR | 86 | 226 | 28% | 65 | 146 | 31% |
| USA | Rhee et al. 2018 [52] | 3386 | 26.17% | 886 | 2500 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Saudi | Al-garni et al. 2017 [26] | 310 | 25.81% | 80 | 230 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Israel | Koren et al. 2015 [43] | 111 | 23.42% | 26 | 85 | 12 | 37 | 24% | 14 | 48 | 23% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Jordan | Wazaify et al. 2011 [15] | 1000 | 16.60% | 166 | 834 | 99 | 432 | 19% | 67 | 402 | 14% | 8 | 44 | 15% | 158 | 790 | 17% | NR | NR | NR | NR | NR | NR |
| Australia | Lui et al. 2012 [44] | 3337 | 7.73% | 258 | 3079 | 157 | 1727 | 8% | 101 | 1352 | 7% | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
CAM Complementary and alternative medicine, NR Not reported
Fig. 2.
Forest plot showing pooled prevalence of complementary and alternative medicine in diabetes
Subgroup analysis
Study level factors
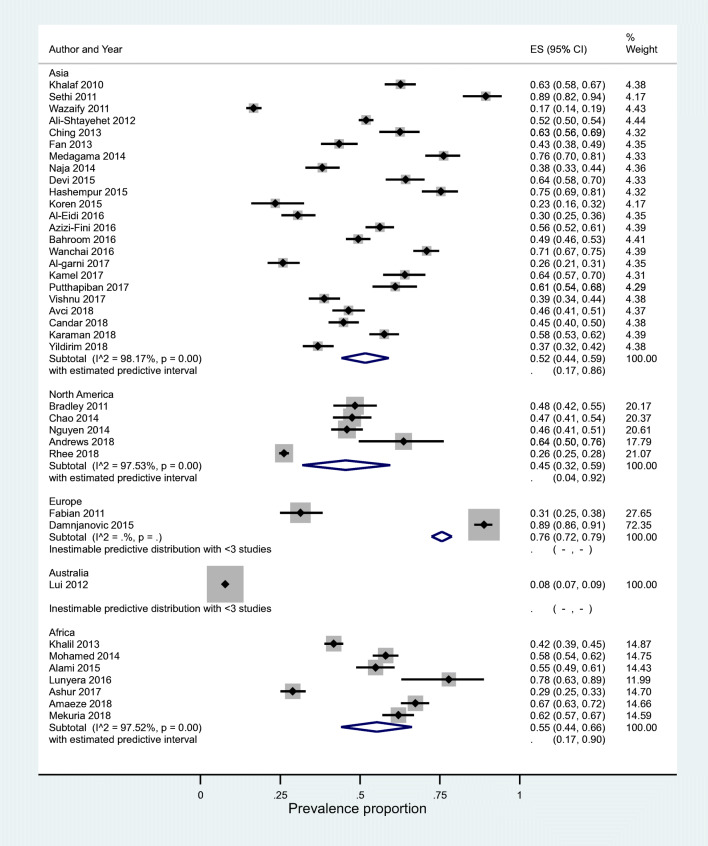
Meta-analysis was conducted for results stratified at the study level by continent. I2 was 97.5% and predictive intervals were found to be wide. The highest prevalence rates of 76% were observed in Europe (PrI inestimable), followed by Africa 55%, (95%PrI 0.17, 0.90) from seven studies. The lowest prevalence rates were observed in North America 45%, (95%PrI 0.04, 0.92) from five studies (Fig. 3).
Fig. 3.
Meta-analysis of study level factors in relation to CAM use (prevalence proportion by continent). CAM, complementary and alternative medicine
Patient level factors
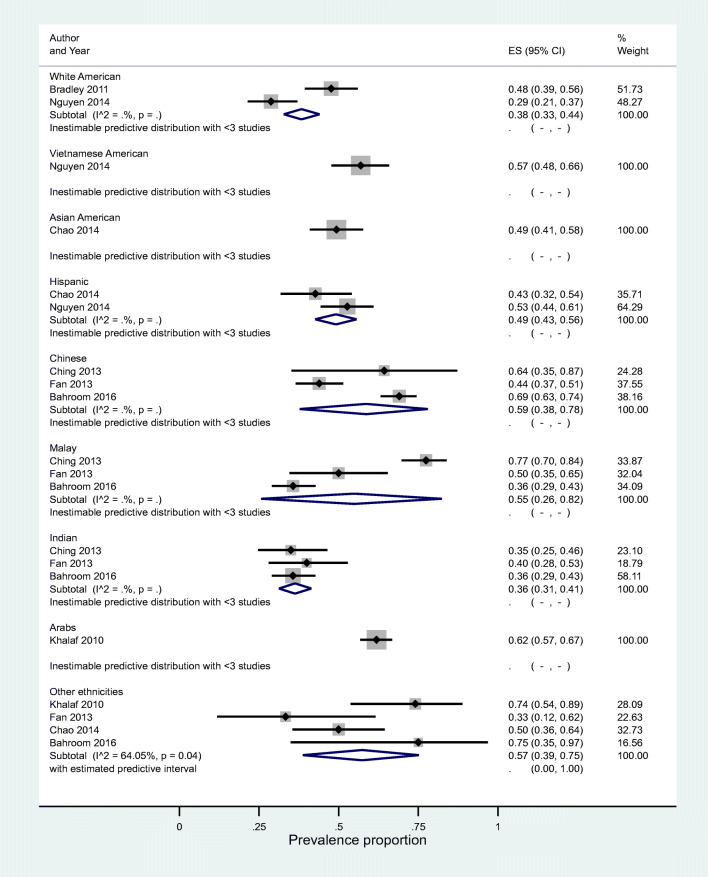
Subgroup analyses were conducted across ethnicity (reported in eight studies). All meta-analyses at subgroup level also showed high levels of heterogeneity. Results were as follows: for the ethnicity subgroup, no predictive interval could be estimated other than PrI for the group of ‘other ethnicities’ prevalence ratio 0.57 (95%CI 0.39–0.75); the estimated predictive intervals ranged between 0.00 and 1.00, I2 = 64.05%) (Fig. 4).
Fig. 4.
Ethnicity subgroup forest plot showing prevalence of CAM use. CAM, Complementary and alternative medicine
For analysis stratified by binary subgroups within-study comparative data were extractable for sex (31 studies), type of diabetes (7 studies), duration of diabetes (15 studies) and presence or absence of diabetic complications (10 studies). Within study pooled estimates PRs for patients with no diabetic complications versus patients with diabetic complications gave a prevalence ratio (PR) 0.81 (95%CI 0.66, 0.99), (95%PrI 0.39–1.67) (I2 = 89%) (Supplementary Fig. 1). For patients who had had diabetes for more than 5 years versus less than 5 years pooled PR was 1.71 (95%CI 1.04, 1.32), (95%PrI 0.73, 1.88) (I2 of 83%) (Supplementary Fig. 2). For male versus female participants, pooled PR was 0.86 (95%CI 0.81, 0.91), (95%PrI 0.64, 1.16) (I2 of 72%) (Supplementary Fig. 3). Pooled PR for patients with T2D versus T1D patients was 1.00 (95%CI 0.83, 1.20), 95%(PrI 0.56, 1.77) (I2 = 75%) (Supplementary Fig. 4).
Additional outcomes
CAM as a complementary or alternative treatment
Eight of the 38 included studies assessed whether CAM was used as an additional treatment or as an alternative treatment to conventional medicines. Prevalence of CAM use as an additional treatment to prescribed medicine was 78% (95%CI 56%, 94%) with 95% PrI (4%, 1.00%) (I2 = 98%), and the percentage of patients who used CAM as an alternative to their prescribed medicine was 21% (95%CI 12%, 31%) with 95% PrI (0.%, 63%) (I2 = 89%) (Supplementary Figs. 5, 6).
Patients’ disclosure of CAM use to healthcare professionals
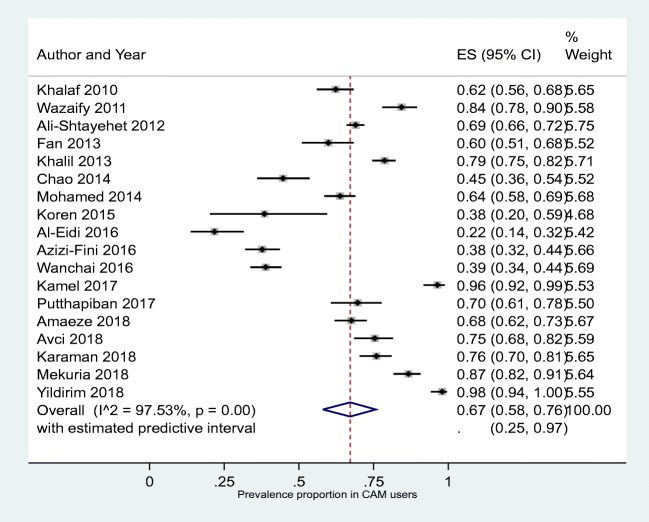
The percentage of patients who do not disclose their CAM use to healthcare professionals was 67% (95%CI 58%, 76%) with 95% PrI (25%, 97%) (I2 = 98%) (Fig. 5).
Fig. 5.
Patient who do not disclose the use of CAM to the healthcare professionals. CAM, complementary and alternative medicine
Discussion
This study provides up-to-date data on the global prevalence of CAM use by patients with diabetes as reported in the peer reviewed research literature. The last literature review on CAM use of patients with diabetes was published in 2007 [7] which reviewed studies conducted in nine countries and reported prevalence ranging from 17% to 73%. A similarly wide variation in prevalence rate of 8–89% was observed in our updated review that included studies from 25 countries.
According to the included studies, CAM use is common amongst patients with diabetes for the purpose of diabetes management. Most of the studies showed that the participants they recruited used CAM as an additional approach to conventional treatment, while in other studies, the reason for CAM use (additional or alternative) was not specified. Only seven studies reported that some patients with diabetes used CAM as the sole means of managing their diabetes. Most of the included studies were conducted in healthcare settings. Therefore, patients who do not use conventional treatments for diabetes may not have been included. The prevalence of patients with diabetes who use CAM in the general population with diabetes is hence likely to be higher than the estimates provided by the included studies.
The meta-analysis of the prevalence data demonstrated extreme variation in prevalence of CAM use amongst patients with diabetes across studies.
Strengths and limitations
This systematic review was a protocol driven review with a pre-specified aim, objectives and methodology. A range of relevant databases were used which covered the prevalence of CAM used by patients with diabetes globally. Data collection methods varied amongst studies. Some studies used structured questionnaires while other studies used semi-structured questionnaires. In addition there was a wide variation in the nature of the study settings. The content of the questionnaires used to collect the data on prevalence and nature of CAMs are likely to influence patient response. Therefore, the included studies may have underreported the nature and extent of CAM use by study participants. In addition, our study only included studies published in the English language.
Implications for practice and research
This systematic review shows that CAM use amongst patients with diabetes is prevalent in many populations. This review suggests that healthcare professionals should consider use of CAM by patients with diabetes when advising them about using their prescribed treatments and monitor their medication adherence while using any forms of CAM. They should also be aware of patients’ use of herbal supplements as some forms of herbal medicine can lead to herb–drug interactions [57]. For example, the most frequently mentioned herbal supplement used by patients with diabetes was cinnamon. It is reported that cinnamon has a potentiating effect on diabetic drugs increasing the risk of hypoglycaemia [58]. For example, cinnamon shows an inhibitory effect on the CYP3A4 enzyme in rabbits which potentiates the effect of pioglitazone if combined with cinnamon, leading to a hypoglycaemic effect [59]. Aloe vera, which was the most frequently reported CAM by the studies included in our review, has been linked to potential interaction with 45 different drugs, including diabetic drugs such as glimepiride [60]. Concomitant use of Aloe vera and glimepiride can produce hypoglycaemic effects as Aloe vera has an inhibiting effect on ATP sensitive potassium channels in pancreatic β cells leading to additional release of insulin [61].
Understanding CAM use patterns and considering any possible interactions between them and other potential medications could help healthcare professionals to appropriately minimize drug-related problems or herb–drug interactions. It could help them to encourage their patient to discuss their CAM use and offer the opportunity to provide better advice for patients with diabetes.
The observed prevalence of CAM, and the many varieties of CAM that are used by patients with diabetes, call for revision of diabetes management guidelines. The National Institute for Health and Care Excellence (NICE) guideline on management of diabetes does not explicitly advise healthcare professionals to discuss patient use of herbal medicines or CAM in their consultation [6]. Guidelines should enable healthcare professionals to counsel patients with diabetes, their families and carers, who should all be educated about the safe use of CAM in conjunction with prescribed medicines.
Owing to the variable and often high prevalence of CAM use amongst patients with diabetes worldwide, research that generates evidence-based information about CAM is needed. This includes effectiveness and safety profiles of commonly used CAMs, including herbal medicines as identified in this systematic review.
This systematic review has identified that on average up to two-thirds of patients who use CAM do not disclose this to their healthcare professionals. Use of CAM such as herbal medicines could be incorporated as part of the comprehensive medication review services offered at community pharmacies and primary care [62].
Future studies need to consider the perspectives of patients with diabetes who do not visit conventional healthcare facilities for the management of diabetes to provide a better estimate of prevalence rates. In addition, there is a need to gather evidence on the factors that affect use and non-use of CAM by patients with diabetes.
Conclusion
A wide variation in prevalence rate of CAM use in diabetes (8–89%) was observed, and pooled prevalence of CAM use was 51%. Our findings show that CAM use by patients with diabetes is common. Healthcare professionals should be aware of the use of CAM by patients with diabetes to ensure treatment optimization and medication adherence. Future studies should incorporate patient and healthcare professionals’ perspectives of CAM use in diabetes, evaluate patient outcomes through the use of healthcare databases and carefully designed prospective studies, and identify opportunities to promote rational use of CAM through evidence-based guidelines and patient-centred approaches.
Supplementary Information
(DOCX 170 kb)
Acknowledgments
This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham in relation to MJP involvement. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. We would like to thank University of Birmingham Library Services for assisting with the literature search.
Authors contributions
This study relates to AA’s PhD. AA, SG and VP designed the study. VP and SG supervised AA’s PhD. MJP led the statistical analysis and presentation of quantitative results. AA led the drafting of the manuscript to which all authors contributed through editing and revision. All authors had access to the data sets and agreed to the final version of the manuscript.
Funding
This study was funded by University of Birmingham. AA was supported for his PhD studies by the Government of Saudi Arabia.
Data availability
All data in relation to this study are presented in this manuscript.
Code availability
Not applicable.
Declarations
Ethics approval statement
Not applicable.
Patient consent statement
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Global Report on Diabetes. https://www.who.int/publications/i/item/9789241565257. Accessed 26 Nov 2020
- 2.Moneta G. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Yearbook Vasc Surg. 2011;17(10):49–51. doi: 10.1016/j.yvas.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim A. Diabetic nephropathy; complications and treatment. Int J Nephrol Renov Dis. 2014;2014:361–381. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon S, Chew E, Duh E, Sobrin L, Sun J, VanderBeek B, Wykoff C, Gardner T. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner DG, Shoback DM. Greenspan’s basic and clinical endocrinology. New York: McGraw-Hill Education; 2017. [Google Scholar]
- 6.National Institute of Clinical Excellence. NICE guideline: type 2 diabetes in adults: management NG28 2015. https://www.nice.org.uk/guidance/ng28. Accessed 26 Nov 2020
- 7.Chang H, Wallis M, Tiralongo E. Use of complementary and alternative medicine among people living with diabetes: literature review. J Adv Nurs. 2007;58(4):307–319. doi: 10.1111/j.1365-2648.2007.04291.x. [DOI] [PubMed] [Google Scholar]
- 8.World_Health_Orgnization (2018) Traditional, complementary and integrative medicine. https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1. Accessed 26 Nov 2020
- 9.Nahin RL, Byrd-Clark D, Stussman BJ, Kalyanaraman N. Disease severity is associated with the use of complementary medicine to treat or manage type-2 diabetes: data from the 2002 and 2007 National Health Interview Survey. BMC Complement Altern Med. 2012;12:193. doi: 10.1186/1472-6882-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaman E, Erkin O, Senman S, Yildirim Y. The use of herbal supplements by individuals with diabetes mellitus. J Pak Med Assoc. 2018;68(4):587–594. [PubMed] [Google Scholar]
- 11.Azizi-Fini I, Adib-Hajbaghery M, Gharehboghlou Z. Herbal medicine use among patients with type 2 diabetes in Kashan, Iran, 2015. European J Integrat Med. 2016;8(4):570–575. doi: 10.1016/j.eujim.2016.04.003. [DOI] [Google Scholar]
- 12.Sethi A, Srivastava S, Madhu SV. Prevalence and pattern of use of indigenous medicines in diabetic patients attending a tertiary care Centre. J Indian Med Assoc. 2011;109(7):469–471. [PubMed] [Google Scholar]
- 13.Bradley R, Sherman K, Catz S, Calabrese C, Oberg E, Cherkin D (2012) Self-care, use of CAM and satisfaction with health care in people with inadequately controlled type 2 diabetes. BMC complementary and alternative medicine. International Research Congress on Integrative Medicine and Health 12(SUPPL.1)
- 14.Ali-Shtayeh MS, Jamous RM, Jamous RM. Complementary and alternative medicine use amongst Palestinian diabetic patients. Complement Ther Clin Pract. 2012;18(1):16–21. doi: 10.1016/j.ctcp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Wazaify M, Afifi FU, El-Khateeb M, Ajlouni K. Complementary and alternative medicine use among Jordanian patients with diabetes. Complement Ther Clin Pract. 2011;17(2):71–75. doi: 10.1016/j.ctcp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Amante D, Hogan T, Pagoto S, English T, Lapane K. Access to care and use of the internet to search for health information: results from the US National Health Interview Survey. J Med Internet Res. 2015;17(4):e106. doi: 10.2196/jmir.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma V, Holmes JH. And S. IN., identifying complementary and alternative medicine usage information from internet resources. A systematic review. Methods Inf Med. 2016;55(4):322–332. doi: 10.3414/ME15-01-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA group, preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4) [PMC free article] [PubMed]
- 19.Janna Briggs Institute (2019) Critical appraisal tools, The Joanna Briggs Institute The University of Adelaide, Australia. https://joannabriggs.org/critical-appraisal-tools. Accessed 26 Nov 2020
- 20.Janna Briggs Institute (2017) Joanna Briggs Institute reviewer’s manual. The Joanna Briggs Institute. https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Scoping-.pdf. Accessed 26 Nov 2020
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J, Thompson S, Spiegelhalter D. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alami Z, Aynaou H, Alami B, Hdidou Y, Latrech H. Herbal medicines use among diabetic patients in oriental Morocco. J Pharmacogn Phytother. 2015;7(2):9–17. doi: 10.5897/JPP2014.0338. [DOI] [Google Scholar]
- 25.Al-Eidi S, Tayel S, Al-Slail F, Qureshi NA, Sohaibani I, Khalil M, Al-Bedah AM. Knowledge, attitude and practice of patients with type 2 diabetes mellitus towards complementary and alternative medicine. J Integrat Med. 2016;14(3):187–196. doi: 10.1016/S2095-4964(16)60244-3. [DOI] [PubMed] [Google Scholar]
- 26.Al-Garni AM, Al-Raddadi RM, Al-Amri TA. Patterns and determinants of complementary and alternative medicine use among type 2 diabetic patients in a diabetic center in Saudi Arabia: herbal alternative use in type 2 diabetes. J Fund Appl Sci. 2017;9(1):1738–1748. [Google Scholar]
- 27.Amaeze OU, Aderemi-Williams RI, Ayo-Vaughan MA, Ogundemuren DA, Ogunmola DS, Anyika EN. Herbal medicine use among type 2 diabetes mellitus patients in Nigeria: understanding the magnitude and predictors of use. Int J Clin Pharm. 2018;40(3):580–588. doi: 10.1007/s11096-018-0648-2. [DOI] [PubMed] [Google Scholar]
- 28.Andrews CM, Wyne K, Svenson JE. The use of traditional and complementary medicine for diabetes in rural Guatemala. J Health Care Poor Underserved. 2018;29(4):1188–1208. doi: 10.1353/hpu.2018.0092. [DOI] [PubMed] [Google Scholar]
- 29.Ashur ST, Shah SA, Bosseri S, Shamsuddin K. Use of traditional medicine among type 2 diabetic Libyans. East Mediterr Health J. 2017;23(5):375–382. doi: 10.26719/2017.23.5.375. [DOI] [PubMed] [Google Scholar]
- 30.Avci DK. The use of traditional and complementary medicine among diabetes patients, and the awareness and attitudes of physicians. J Pak Med Assoc. 2018;68(11):1650–1654. [PubMed] [Google Scholar]
- 31.Baharom N, Shah SA, Rotina AB. Prevalence of complementary alternative medicine use among patients with type II diabetes in Negeri Sembilan, Malaysia. Med Health (Universiti Kebangsaan Malaysia) 2016;11(2):257–266. [Google Scholar]
- 32.Candar A, Demirci H, Baran AK, Akpinar Y. The association between quality of life and complementary and alternative medicine use in patients with diabetes mellitus. Complement Ther Clin Pract. 2018;31:1–6. doi: 10.1016/j.ctcp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Chao MT, Handley M, Quan J, Sarkar U, Ratanawongsa N, Schillinger D. Disclosure of complementary and alternative medicine use among diverse safety net patients with diabetes. J Altern Complement Med. 2014;20(5):A126. doi: 10.1089/acm.2014.5335.abstract. [DOI] [Google Scholar]
- 34.Ching SM, Zakaria ZA, Paimin F, Jalalian M. Complementary alternative medicine use among patients with type 2 diabetes mellitus in the primary care setting: a cross-sectional study in Malaysia. BMC Complement Altern Med. 2013;13:148. doi: 10.1186/1472-6882-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damnjanovic I, Kitic D, Stefanovic N, Zlatkovic-Guberinic S, Catic-Djordjevic A, Velickovic-Radovanovic R. Herbal self-medication use in patients with diabetes mellitus type 2. Turkish J Med Sci. 2015;45(4):964–971. doi: 10.3906/sag-1410-60. [DOI] [PubMed] [Google Scholar]
- 36.Devi K, Santhini E, Manikandan R, Prabhu NM. The prevalence, awareness and potential of complementary alternative medicine in type 2 diabetics living in Madurai, India. European J Integrat Med. 2015;7(5):469–473. doi: 10.1016/j.eujim.2015.04.003. [DOI] [Google Scholar]
- 37.Fabian E, Toscher S, Elmadfa I, Pieber TR. Use of complementary and alternative medicine supplements in patients with diabetes mellitus. Ann Nutr Metab. 2011;58(2):101–108. doi: 10.1159/000326765. [DOI] [PubMed] [Google Scholar]
- 38.Fan PE, Chan MF, Chan YL, Koh SL. Patterns of complementary and alternative medicine use among a group of patients with type 2 diabetes receiving outpatient care in Singapore. Int J Nurs Pract. 2013;19(Suppl 3):44–55. doi: 10.1111/ijn.12173. [DOI] [PubMed] [Google Scholar]
- 39.Hashempur MH, Heydari M, Mosavat SH, Heydari ST, Shams M. Complementary and alternative medicine use in Iranian patients with diabetes mellitus. J Integrat Med. 2015;13(5):319–325. doi: 10.1016/S2095-4964(15)60196-0. [DOI] [PubMed] [Google Scholar]
- 40.Kamel FO, Magadmi RM, Hagras MM, Magadmi B, AlAhmad RA. Knowledge, attitude, and beliefs toward traditional herbal medicine use among diabetics in Jeddah Saudi Arabia. Complement Ther Clin Pract. 2017;29:207–212. doi: 10.1016/j.ctcp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Khalaf AJ, Whitford DL. The use of complementary and alternative medicine by patients with diabetes mellitus in Bahrain: a cross-sectional study. BMC Complement Altern Med. 2010;10:35. doi: 10.1186/1472-6882-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalil SH, Zaki A, Ibrahim AM, El-Moughazi AM, Khater AM, Youssef AM, El-Sa'ed AT, Rashed EM. Pattern of use of complementary and alternative medicine among type 2 diabetes mellitus patients in Alexandria, Egypt. J Egypt Public Health Assoc. 2013;88(3):137–142. doi: 10.1097/01.EPX.0000440994.89503.45. [DOI] [PubMed] [Google Scholar]
- 43.Koren R, Lerner A, Tirosh A, Zaidenstein R, Ziv-Baran T, Golik A, Koren S. The use of complementary and alternative medicine in hospitalized patients with type 2 diabetes mellitus in Israel. J Altern Complement Med. 2015;21(7):395–400. doi: 10.1089/acm.2015.0019. [DOI] [PubMed] [Google Scholar]
- 44.Lui C-W, Dower J, Donald M, Coll JR (2012) Patterns and determinants of complementary and alternative medicine practitioner use among adults with diabetes in Queensland, Australia. Evid Based Complement Alternat Med 2012:659419 [DOI] [PMC free article] [PubMed]
- 45.Lunyera J, Wang D, Maro V, Karia F, Boyd D, Omolo J, Patel UD, Stanifer JW. Traditional medicine practices among community members with diabetes mellitus in northern Tanzania: an ethnomedical survey. BMC Complement Altern Med. 2016;16:1–12. doi: 10.1186/s12906-016-1262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medagama AB, Bandara R, Abeysekera RA, Imbulpitiya B, Pushpakumari T. Use of complementary and alternative medicines (CAMs) among type 2 diabetes patients in Sri Lanka: a cross sectional survey. BMC Complement Altern Med. 2014;14:374. doi: 10.1186/1472-6882-14-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mekuria AB, Belachew SA, Tegegn HG, Ali DS, Netere AK, Lemlemu E, Erku DA. Prevalence and correlates of herbal medicine use among type 2 diabetic patients in teaching Hospital in Ethiopia: a cross-sectional study. BMC Complement Altern Med. 2018;18(1):85. doi: 10.1186/s12906-018-2147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohamed Ali BA, Mahfouz MS. Herbal medicine use among patients with type 2 diabetes in North Sudan. Annual Res Rev Biol. 2014;4(11):1827–1838. doi: 10.9734/ARRB/2014/8015. [DOI] [Google Scholar]
- 49.Naja F, Alameddine M. Prevalence and correlates of complementary and alternative medicine use among diabetic patients in Lebanon: a cross sectional study. Clin Nutr. 2014;1:S179–S180. doi: 10.1016/S0261-5614(14)50469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen H, Sorkin DH, Billimek J, Kaplan SH, Greenfield S, Ngo-Metzger Q. Complementary and alternative medicine (CAM) use among non-Hispanic white, Mexican American, and Vietnamese American patients with type 2 diabetes. J Health Care Poor Underserved. 2014;25(4):1941–1955. doi: 10.1353/hpu.2014.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Putthapiban P, Sukhumthammarat W, Sriphrapradang C (2017) Concealed use of herbal and dietary supplements among Thai patients with type 2 diabetes mellitus. J Diab Metabol Dis 16:36 [DOI] [PMC free article] [PubMed]
- 52.Rhee TG, Westberg SM, Harris IM. Complementary and alternative medicine in US adults with diabetes: reasons for use and perceived benefits. J Diab. 2018;10(4):310–319. doi: 10.1111/1753-0407.12607. [DOI] [PubMed] [Google Scholar]
- 53.Vishnu N, Mini GK, Thankappan KR. Complementary and alternative medicine use by diabetes patients in Kerala, India. Glob Health Epidemiol Genom. 2017;2(e6):1–7. doi: 10.1017/gheg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanchai A, Phrompayak D. Use of complementary and alternative medicine among Thai patients with type 2 diabetes mellitus. J Integrat Med. 2016;14(4):297–305. doi: 10.1016/S2095-4964(16)60263-7. [DOI] [PubMed] [Google Scholar]
- 55.Yildirim DI, Marakoglu K. Complementary and alternative medicine use amongst Turkish type 2 diabetic patients: a cross-sectional study. Complement Ther Med. 2018;41:41–46. doi: 10.1016/j.ctim.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Naja F, Mousa D, Alameddine M, Shoaib H, Itani L, Mourad Y (2014) Prevalence and correlates of complementary and alternative medicine use among diabetic patients in Beirut, Lebanon: a cross-sectional study. BMC Complement Altern Med 14:185. 10.1186/1472-6882-14-185 [DOI] [PMC free article] [PubMed]
- 57.Brantley SJ, Argikar AA, Lin YS, Nagar S, Paine MF. Herb-drug interactions: challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;42(3):301–317. doi: 10.1124/dmd.113.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11(4):452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mamindla S, Koganti VSRGP, Ravouru N, Koganti B. Effect of Cinnamomum cassia on the pharmacokinetics and pharmacodynamics of pioglitazone. Curr Clin Pharmacol. 2017;12(1):41–49. doi: 10.2174/1574884712666170207152020. [DOI] [PubMed] [Google Scholar]
- 60.Drugs.com (2020) Aloe vera drug interactions. 4 May 2020. https://www.drugs.com/drug-interactions/aloe-vera-index.html. Accessed 26 Nov 2020
- 61.Gupta RC, Chang D, Nammi S, Bensoussan A, Bilinski K, Roufogalis BD. Interactions between antidiabetic drugs and herbs: an overview of mechanisms of action and clinical implications. Diabetol Metab Syndr. 2017;9:59. doi: 10.1186/s13098-017-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodgers RM, Gammie SM, Loo RL, Corlett SA, Krska J (2016) Comparison of pharmacist and public views and experiences of community pharmacy medicines-related services in England. Patient Pref Adher 10:1749–1758 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 170 kb)
Data Availability Statement
All data in relation to this study are presented in this manuscript.
Not applicable.