Abstract
The aim of the current study was to assess the structural centrality and microstructural integrity of the cortical hubs of the salience network, the anterior insular cortex (AIC) subregions and anterior cingulate cortex (ACC), and their relationship to cognitive and affective impairment in PD. MRI of 53 PD patients and 15 age-matched controls included 3D-T1 for anatomical registration, and diffusion tensor imaging for probabilistic tractography. Network topological measures of eigenvector and betweenness centrality were calculated for ventral (vAI) and dorsal (dAI) AIC. Microstructural tract integrity between vAI, dAI and the ACC was quantified with fractional anisotrophy (FA) and mean diffusivity (MD). Structural integrity and connectivity were related to cognitive and affective scores. The dAI had significantly higher eigenvector centrality in PD than controls (p < 0.01), associated with higher depression scores (left dAI only, rs = 0.28, p < 0.05). Tracts between dAI and ACC showed lower FA and higher MD in PD (p < 0.05), and associated with lower semantic fluency, working memory and executive functioning, and higher anxiety scores (range 0.002 < p < 0.05). This study provides evidence for clinically relevant structural damage to the cortical hubs of the salience network in PD, possibly due to extensive local neuropathology and loss of interconnecting AIC-ACC tracts.
Subject terms: Parkinson's disease, Neuroscience
Introduction
Parkinson’s disease (PD) is one of the most common and debilitating neurodegenerative disorders in which motor, cognitive, and neuropsychiatric deficits are prevalent1,2. Although symptomatic treatments are available for motor symptoms, non-motor deficits remain poorly understood. About 83% of PD patients will develop some level of cognitive impairment3, the domains specifically affected include visuospatial, attention, executive, and even memory. In fact, about 10–20% of PD patients have MCI at time of diagnosis and up to 80% develop dementia provided they survive the disease for more than 10 years4. As such, these deficits have a huge impact on patients’ quality of life and social and mental well-being. However, to date, effective symptomatic or disease modifying treatments for cognitive impairment remain lacking. To meet the urgent need to find treatments delaying the onset or slowing cognitive impairment, more emphasis is needed on the substrates of cognitive impairment including but not limited to imaging biomarkers.
In PD, neuropathological lesions affect cortical and subcortical brain areas, as well as the integrity of interconnecting white matter tracts5–7. By this very nature, PD can be seen as a ‘disconnection syndrome’8,9. Critically, disconnection in PD was shown to be associated with cognitive and affective impairment10–12. Regional evaluations remain rare, but of interest.
The salience network, in which the insula and anterior cingulate cortex (ACC) play a key role, is involved in a myriad of cognitive, affective and viscero-sensory functions13,14. Several MRI studies indicate widespread involvement of the insular cortex in PD-related cognitive and affective decline, although in-vivo quantification of this damage remains difficult15–19. This difficulty partly lies in the heterogeneous nature of the insula, with a distinct cytoarchitecture of variably differentiated cortical layers, and widespread connectivity, which can be captured with diffusion MRI. The anterior insular cortex (AIC) consists of a transitional allocortical-neocortical dorsal anterior insula (dAI), and an allocortical ventral anterior insula (vAI)20. While the dAI is important in goal-oriented cognitive and executive control, the vAI plays a role in affective functions, in part due to its connections with limbic regions21,22. In turn, the posterior insula largely shows a granular 6-layer architecture20, and is involved in the integration of autonomic and interoceptive signals22,23.
Within the salience network, connections between the AIC and ACC are important for rapid orientation of attention and adaptive switching of cognitive control13,21,24–26. There is strong evidence that functional changes in the salience network are related to cognitive impairment and depression in PD, but structurally this network remains understudied27,28. Therefore, the aim of the current study is to address the structural centrality of the dAI and vAI, as well as the microstructural integrity of tracts between the dAI, vAI and ACC, in relation to cognitive and affective functions in PD patients and healthy controls. Revealing structural damage in these circuits would allow a more integrative view of non-moter deficits in PD, with a better understanding of the central role of the insula, its subregions and its connections.
Results
Subject demographics and clinical characteristics
The demographics and functional test scores are summarized in Table 1. PD patients had a mean age of 67.3 years (± standard deviation [SD] 6 years) with a moderate disease duration (mean 11.3 ± 3.6 years). Based on clinical scores, patients had mild to moderate disease severity (Unified Parkinson’s Disease Rating Scale [UPDRS] III median score of 31 (range 14–56) and Hoehn and Yahr scale [H&Y] median score of 2.5 (range 2–3)). There were no significant differences in age, sex distribution, or educational levels between PD patients and controls. With regards to cognitive assessment, Cambridge Cognitive Examination (CAMCOG) scores were significantly lower in PD patients compared to controls (t(63) = 4.54, p < 0.001). Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI) test scores, as well as performance on additional cognitive tests, were significantly worse in PD compared to controls (p < 0.01). The Mini Mental Status Exam (MMSE) score was not significantly different between groups (control median = 28 (range 27–30); PD median = 28 (range 19–30), t(65) = 0.91, p = 0.336), although four PD patients fell below the MMSE score of 24.
Table 1.
Subject demographics and functional test scores.
| Demographics and functional tests | Controls (n = 15) | PD (n = 53) | p values | |||
|---|---|---|---|---|---|---|
| N/count (%) | Median (range) | N/count (%) | Median (range) | |||
| Age (Years)^ | 15 | 66.9 (52–81) | 53 | 67.3 (54–81) | p = 0.85 | |
| Education | 2 | 2 (13,3%) | 5 (2–6) | 16 (30,2%) | 3 (2–6) | p = 0.07 |
| 3 | 3 (20%) | 15 (28,3%) | ||||
| 4 | 1 (6,7%) | 2 (3,8%) | ||||
| 5 | 8 (53,3%) | 19 (35,8%) | ||||
| 6 | 1 (6,7%) | 1 (1,9%) | ||||
| Gender (Males) | 66% | – | 58% | – | p = 0.57 | |
| Disease duration (years)^ | NA | NA | 53 | 11.3 (4–21) | NA | |
| UPDRS_III | NA | NA | 53 | 31 (14–56) | NA | |
| HY | 2,0 | NA | NA | 21 (39,6%) | 2.5 (2–3) | NA |
| 2,5 | NA | NA | 18 (34%) | NA | ||
| 3,0 | NA | NA | 14 (26,4%) | NA | ||
| Mini mental status exam | 14 | 28 (27–30) | 53 | 28 (19–30) | p = 0.34 | |
| CAMCOG_Total | 14 | 99.5 (95–104) | 53 | 96 (71–103)** | p < 0.001 | |
| Semantic fluency | 14 | 23.5 (16–40) | 53 | 19 (5–32)* | p = 0.03 | |
| Pattern recognition memory | 14 | 23 (21–24) | 52 | 21.5 (13–24)** | p < 0.001 | |
| Spatial span length | 14 | 5 (5–7) | 52 | 5 (2–8)* | p = 0.01 | |
| Spatial working memory | 14 | 22 (0–57) | 52 | 38 (2–106)* | p = 0.015 | |
| Intra-extra dimension shift | 14 | 9 (7–9) | 51 | 9 (0–9)** | p = 0.003 | |
| Vienna perseveration test | 14 | 18 (14–27) | 52 | 21 (10–63)* | p = 0.001 | |
| Beck depression inventory | 14 | 3.5 (0–23) | 52 | 11 (0–31)** | p = 0.001 | |
| Beck anxiety inventory | 12 | 24 (21–31) | 31 | 32 (24–57)** | p < 0.001 | |
| LEDD | 0 | 48 | 900 (225–2600) | NA | ||
Education is based on ISCED system ranging from 0 to 6. CAMCOG_Total: Cambridge Cognitive Battery Total score, H&Y: Hoehn and Yahr stage, ISCED: International Standard Classification of Education, LEDD: Levodopa equivalent daily dose, PD: Parkinson’s disease, SD: Standard Deviation, UPDRS III: Unified Parkinson’s Disease Rating Scale part III, LEDD: L-DOPA equivalent daily dose.. ^mean and range is reported for these variables.. * p < 0.05, ** p < 0.01. Table adapted from Fathy et al., 202028.
Network topological differences of insula sub-regions
Eigenvector centrality
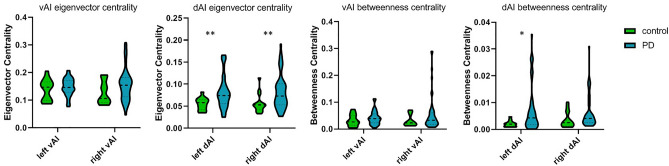
The left dAI showed a significantly higher eigenvector centrality in PD than in controls (median = 0.07, interquartile range (IQR) = 0.056–0.096 vs median = 0.058, IQR = 0.042–0.065; U = 201 p = 0.004). The right dAI also showed a significantly higher eigenvector centrality in PD than controls (median = 0.07, IQR = 0.05–0.10 vs median = 0.05, IQR = 0.04–0.06, U = 217 p = 0.008). This was not significant for the left vAI (median = 0.14, IQR = 0.13–0.17 vs median = 0.14, IQR = 0.099–0.166, U = 344.5 p = 0.43) or right vAI (median = 0.15, IQR = 0.112–0.182 vs median = 0.11, IQR = 0.091–0.168, U = 291 p = 0.115) (Fig. 1).
Figure 1.
Group differences in eigenvector centrality and betweenness centrality. PD patients showed significantly higher left and right dAI eigenvector centrality and higher left dAI betweenness centrality than controls. vAI = ventral anterior insula; dAI = dorsal anterior insula. *p < 0.05 **p < 0.01 after Bonferroni correction.
Associating these significant increases in dAI eigenvector centrality to cognitive and affective measures in PD patients, an increase in left dAI centrality associated with higher scores on the BDI depression scale (rs = 0.277; p = 0.047; Table 2). No significant associations were observed with any of the other clinical measures of cognitive and affective functions.
Table 2.
Associations between centrality measures and cognitive and affective measures in PD.
| Left dAI eigenvector centrality | Right dAI eigenvector centrality | Left dAI betweenness centrality | Right dAI betweenness centrality | |
|---|---|---|---|---|
| CAMCOG | ||||
| rs | − 0.081 | 0.098 | − 0.045 | 0.199 |
| p-value | 0.564 | 0.487 | 0.749 | 0.153 |
| N | 53 | 53 | 53 | 53 |
| Fluency | ||||
| rs | − 0.183 | 0.024 | − 0.160 | 0.172 |
| p-value | 0.189 | 0.866 | 0.254 | 0.219 |
| N | 53 | 53 | 53 | 53 |
| PRM | ||||
| rs | − 0.101 | − 0.067 | − 0.110 | 0.005 |
| p-value | 0.478 | 0.635 | 0.436 | 0.972 |
| N | 52 | 52 | 52 | 52 |
| SSP | ||||
| rs | − 0.057 | − 0.022 | 0.006 | − 0.118 |
| p-value | 0.688 | 0.878 | 0.964 | 0.405 |
| N | 52 | 52 | 52 | 52 |
| SWM | ||||
| rs | 0.077 | 0.136 | 0.033 | 0.218 |
| p-value | 0.585 | 0.336 | 0.817 | 0.120 |
| N | 52 | 52 | 52 | 52 |
| IED | ||||
| rs | − 0.059 | − 0.156 | − 0.076 | − 0.055 |
| p-value | 0.682 | 0.274 | 0.595 | 0.700 |
| N | 51 | 51 | 51 | 51 |
| VPT | ||||
| rs | 0.262 | − 0.112 | 0.223 | − 0.078 |
| p-value | 0.061 | 0.431 | 0.111 | 0.584 |
| N | 52 | 52 | 52 | 52 |
| BDI | ||||
| rs | 0.277* | − 0.057 | 0.177 | − 0.035 |
| p-value | 0.047 | 0.687 | 0.210 | 0.805 |
| N | 52 | 52 | 52 | 52 |
| BAI | ||||
| rs | − 0.111 | − 0.208 | − 0.163 | − 0.286 |
| p-value | 0.552 | 0.261 | 0.380 | 0.119 |
| N | 31 | 31 | 31 | 31 |
rs = Spearman correlation; vAI = ventral anterior insula; dAI = dorsal anterior insula. CAMCOG = Cambridge Cognitive Examination revised test battery; PRM = pattern recognition memory; SSP = spatial span; SWM = spatial working memory; IED = the intra-extra dimensional set shift test; VPT = Vienna perseveration test; BAI = Beck Anxiety Inventory; BDI = the Beck Depression Inventory. *p < 0.05.
Normalized betweenness centrality
The left dAI showed a significantly higher betweenness centrality in PD than in controls (median = 0.004, IQR = 0.0018–0.0088 vs median = 0.002, IQR = 0.001–0.0026, U = 223 p = 0.01). The right dAI also showed a significantly higher betweenness centrality in PD than in controls (median = 0.004, IQR = 0.0025–0.0077 vs median = 0.0025, IQR = 0.0013–0.0066, U = 247 p = 0.026), but this did not survive multiple comparisons. No difference in betweenness centrality was found for the left vAI (median = 0.038, IQR = 0.023–0.056, U = 316 p = 0.228) or right vAI (median = 0.033, IQR = 0.018–0.061, U = 310 p = 0.193) (Fig. 1). There were no significant correlations between dAI betweenness centrality and cognitive and affective measures in PD patients. (Table 2).
Microstructural alterations between dorsal anterior insula and ACC
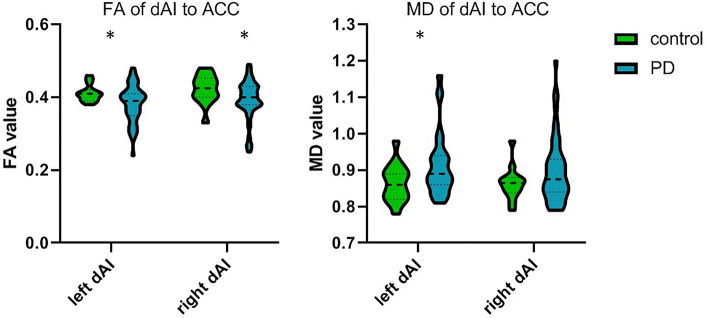
The anterior insula has strong connections with the ACC13, and is associated with cognitive control in PD28. Since only the dAI, not the vAI, showed network topological alterations of eigenvector and betweenness centrality in PD, we assessed microstructural alteration in the connections between the dAI and ACC only. A significant lower FA was observed in PD patients within the tract between left dAI and left ACC compared to controls (median = 0.31, IQR = 0.035–0.041 vs median = 0.41, IQR = 0.39–0.042, U = 244 p = 0.040), as well as a significant higher MD (median = 0.89, IQR = 0.86–0.94 vs median = 0.86, IQR = 0.82–0.89, U = 238 p = 0.040) Lastly, a significantly lower FA was observed in the tract between right dAI and right ACC in PD compared to controls (median = 0.40, IQR 0.38–0.4, U = 235 p = 0.034), but here MD was not significant (median = 0.88, IQR = 0.84–0.93, U = 292 p = 0.254) (Fig. 2).
Figure 2.
Group differences in FA and MD of dAI to ACC tract. PD patients showed lower FA in dAI to ACC tracts than controls, and higher MD in left dAI to ACC tract. FA = fractional anisotrophy; MD = mean diffusivity; vAI = ventral anterior insula; dAI = dorsal anterior insula. *p < 0.05.
Associating microstructural alterations of the tracts between dAI and ACC to cognitive and affective measures in PD, significant associations for increased MD between the left and right dAI and ACC were found for semantic fluency (left rs = -0.31, p = 0.028; right rs = -0.28, p = 0.04), spatial span length (SSP; left rs = -0.30, p = 0.036; right rs = -0.28, p = 0.042) and spatial working memory (SWM; left rs = 0.33, p = 0.02; right rs = 0.43, p = 0.002). In addition, a significant association was observed between increased MD of the left dAI and ACC tract and increased BAI (rs = 0.39, p = 0.03) (Table 3).
Table 3.
dAI-ACC WM tract microstructural feature association with cognitive and affective measures in PD.
| FA of left dAI to ACC | FA of right dAI to ACC | MD of left dAI to ACC | MD of right dAI to ACC | |
|---|---|---|---|---|
| CAMCOG | ||||
| rs | 0.135 | 0.007 | − 0.277 | − 0.267 |
| p-value | 0.350 | 0.959 | 0.054 | 0.056 |
| N | 50 | 53 | 49 | 52 |
| Fluency | ||||
| rs | 0.137 | − 0.057 | − 0.314* | − 0.280* |
| p-value | 0.341 | 0.687 | 0.028 | 0.044 |
| N | 50 | 53 | 49 | 52 |
| PRM | ||||
| rs | − 0.160 | − 0.182 | − 0.107 | − 0.007 |
| p-value | 0.271 | 0.196 | 0.468 | 0.963 |
| N | 49 | 52 | 48 | 51 |
| SSP | ||||
| rs | 0.168 | 0.184 | − 0.304* | − 0.285* |
| p-value | 0.249 | 0.192 | 0.036 | 0.042 |
| N | 49 | 52 | 48 | 51 |
| SWM | ||||
| rs | − 0.144 | − 0.083 | 0.329* | 0.429** |
| p-value | 0.324 | 0.556 | 0.022 | 0.002 |
| N | 49 | 52 | 48 | 51 |
| IED | ||||
| rs | 0.027 | 0.164 | − 0.144 | − 0.129 |
| p-value | 0.858 | 0.252 | 0.335 | 0.371 |
| N | 48 | 51 | 47 | 50 |
| VPT | ||||
| rs | − 0.014 | − 0.060 | 0.089 | 0.104 |
| p-value | 0.925 | 0.671 | 0.548 | 0.468 |
| N | 49 | 52 | 48 | 51 |
| BDI | ||||
| rs | − 0.212 | − 0.110 | 0.214 | 0.263 |
| p-value | 0.144 | 0.436 | 0.143 | 0.062 |
| N | 49 | 52 | 48 | 51 |
| BAI | ||||
| rs | − 0.298 | 0.014 | 0.392* | 0.289 |
| p-value | 0.104 | 0.940 | 0.032 | 0.121 |
| N | 31 | 31 | 30 | 30 |
rs = Spearman correlation; vAI = ventral anterior insula; dAI = dorsal anterior insula. CAMCOG = CAMbridge COGnitive examination revised test battery; PRM = pattern recognition memory; SSP = spatial span; SWM = spatial working memory; IED = the intra-extra dimensional set shift test; VPT = Vienna perseveration test; BAI = Beck Anxiety Inventory; BDI = the Beck Depression Inventory. *p < 0.05, **p < 0.01.
Discussion
The salience network, with the insula and ACC as its main cortical hubs26, shows structural and functional alterations associated with non-motor deficits in PD15,27,29,30. In these studies, the (anterior) insula is mostly studied as one region, although the cytoarchitecture of the anterior insula reveals a sub-regional division into dorsal and ventral anterior insula (dAI and vAI), associated with cognitive control and affective functions, respectively20,21,23. Our study shows that the dAI has a higher betweenness and eigenvector centrality in PD, the latter being associated with depression. In addition, white matter tracts between the dAI and ACC showed microstructural damage in PD, associated with cognitive impairments in a range of domains related to semantic fluency, working memory capacity, executive functioning and with symptoms of anxiety.
Other nodes in the salience network include the amygdala, hypothalamus, ventral striatium, thalamus and several brainstem nuclei21. However, it is the anterior insula and ACC within this network that receive the most attention in PD. The anterior insula and ACC are strikingly similar in their difference to other cortical regions; both regions contain subregions featuring agranular cortices (without a visible layer 4), and both regions contain von Economo neurons, unique cells thought to play a role in awareness31. In addition, the (anterior) insula and ACC are severely affected by Lewy body pathology in advanced stages of PD, during which patients often suffer from cognitive impairment32.
Network topological measures, specifically of centrality, have consistently shown alterations suggesting network disintergration in PD, and associate with (onset of mild) cognitive impairment33–35. This is in line with our results showing higher eigenvector and betweenness centrality of the dAI in PD, indicating a more central role for these regions, possibly due to a loss of connections with non-hub regions36. Furthermore, altered eigenvector centrality of the dAI shows an association with depressive symptoms, which ranged from mild to moderate in our PD cohort. This association is consistent with the literature in which disrupted anterior insula connectivity and white matter atrophy have been shown to contribute to depression associated with PD30,37.
In addition to a change in insular network topology, we specifically addressed microstructural alterations of the white matter tracts between the dAI and ACC. The dAI is strongly connected to other regions important for cognitive control, most notably the ACC21,26,38. Our results show microstructural damage within this tract, specifically a decrease in FA and increase in MD, which may relate to the increasing pathological load in both regions. It has been shown that within the insular cortex, especially the anterior insula subregion is susceptible to severe Lewy body pathology in PD39. Furthermore, the increased MD of the dAI to ACC bundles was associated with a decline in semantic fluency, working memory, executive functioning, and an increase in symptoms of anxiety, reflecting the wide array of cognitive and affective domains associated with insular (dys)function15,40.
The main strength of our study is the region-specific approach, based on functional associations within the salience network, specifically the anterior insula and ACC28. There are however, several limitations that need to be addressed. First, our retrospective cohort consisted of a unbalanced and limited sample size, particularly for controls, and although comparable in size to other insula studies, this limits the generalizability of our data, specifically in terms of effects of normal aging on structural integrity. Larger cohorts should further explore these effects as well as compare with dementia syndromes. Second, future endeavors should investigate the impact of covariates in group differences and association with cognitive outcome measures. Especially sex has recently shown to have a differential effect on patterns of cognitive decline in PD41. Furthermore, because the anterior insula is involved in such a myriad of cognitive and affective functions, including speech, attention, saliency, and emotional processing13, we included all available cognitive tests, limiting our ability to correct for multiple comparisons in the associations. All in all, further research in a large cohort of PD patients is needed to further establish structural connectivity alterations of the (anterior) insular cortex with ACC, and other subcortical brain regions in the salience network, associated with specific cognitive domains in PD.
Conclusions
In this study, we found alterations in network topology and microstructural integrity of white matter tracts for the anterior insula, primarily centered around the dAI, which showed disconnection from the ACC. These alterations within the cortical hubs of the salience network were associated with PD related cognitive impairment and affective symptoms. Considering the two regions’ functional importance in cognitive control and their susceptibility to neuropathological change, the changes in network topological and microstructural integrity found in the current study adds to an integrative view of these regions, and towards a more precise understanding of cognitive impairment in PD.
Material and methods
Standard protocol approvals, registrations, and donor consents
The research protocol was approved by the local institutional ethics review board of Amsterdam UMC, location VUmc (Amsterdam, the Netherlands), in adherence to the Helsinki declaration. All participants provided written informed consent to participate in the study. Clinical datasets and MRI imaging of both PD patients and healthy individuals were collected in a standardized manner at the outpatient clinic of Amsterdam UMC, location VUmc, as previously described42.
Inclusion of participants
PD patients fulfilled the UK Parkinson’s Disease Brain Bank Clinical diagnostic criteria43. Age-of-onset and disease duration was calculated based on the first occurrence of PD motor symptoms. Educational levels were classified according to the International Standard Classification of Education with scores ranging from 0–6 (0 = no primary education & 6 = university education). Exclusion criteria for the study included previous stereotactic surgery, extensive white matter lesions (Fazekas > 2)44, as well as other abnormalities (e.g. space occupying lesions) seen on structural MRI images. Subjects were examined by a trained neurologist with specific experience in movement disorders. Healthy volunteers were recruited as either spouses of patients or non-neurological participants within the same age range. After excluding two patients with insular hypo-intensities on T1 MRI (suspected of being infarcts), we included data of 53 sporadic PD patients and 15 age-matched controls for whom complete clinical, neuropsychological, and imaging data were available28. Clinical and imaging evaluations, as previously described42,45, were performed on the same day in the “ON” medication state to avoid unnecessary discomfort.
Clinical, cognitive, and neuropsychiatric assessment
The Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) was used to assess the severity of motor symptoms46. Hoehn and Yahr (H&Y) staging was used to provide information on patients’ disease stages47. PD patients were on combinations of anti-parkinsonian medications, An L-DOPA equivalent daily dose (LEDD) was calculated for each patient48 (Table 1). Cognitive performance was evaluated using the Mini Mental Status Examination (MMSE) and the CAMbridge COGnitive examination revised test battery (CAMCOG). This latter assesses orientation (maximum score: 10), language (maximum score: 30), memory (maximum score: 27), attention and calculation (maximum score: 9), praxis (maximum score: 12), abstract thinking (maximum score: 8), and perception (maximum score: 9)49. Other cognitive tests included in the study to assess working memory, visuospatial memory, attention and executive functions consist of semantic fluency, pattern recognition memory (PRM), spatial span (SSP), spatial working memory (SWM), the intra-extra dimensional set shift test (IED), and the Vienna perseveration test (VPT). As the insular cortex is not only involved in cognition, but also in affective alterations in PD50–52, symptoms of anxiety and depression were assessed through the Beck Anxiety inventory (BAI) and Beck Depression Inventory (BDI), respectively.
MRI acquisition
All participants underwent structural whole-brain 3 T MR imaging (Sigma HDXT, V15M; GE Healthcare) using a sagittal three-dimensional T1-weighted fast spoiled gradient-echo sequence (repetition time [TR] = 7.8 ms, echo time [TE] = 3.0 ms, inversion time [TI] = 450 ms; flip angle 12°; resolution 1.0 × 0.9 × 0.9 mm). In addition, DTI acquisition consisted of five volumes without directional weighting, and 30 volumes with non-collinear gradient directions (b-value 1000 s/mm2, TR = 13,275 ms, TE = 91 ms, slice thickness 2.4 mm; in-plane resolution 2 × 2 mm).
Image pre-processing
Data preprocessing was performed using tools from the FMRIB (Functional Magnetic Resonance Imaging of the Brain) software library (FSL 5.0.9, http://www.fmrib.ox.ac.uk/fsl). For 3D-T1 images, brain extraction (bet functionality) and grey and white tissue-type segmentation with partial volume estimation was carried out (SIENAX functionality)53. The diffusion-weighted images were corrected for motion and eddy current distortion using FMRIB’s Diffusion Toolbox (FSL-FDT; part of FSL 5.0.9). Non-linear registration (FNIRT) was used to calculate registration parameters to standard space, which were inverted to bring our anatomical atlas (see below) into subject anatomical space, using nearest neighbor interpolation. The full atlas was then multiplied with the SIENAX grey matter mask, to remove CSF and eliminate displacement due to grey matter atrophy.
The transformation between 3DT1 and diffusion-weighted images was derived by using Boundary-Based registration (BBR; part of FSL). Bedpostx was run to build up diffusion parameter distributions at each voxel.
Construction of an anatomical brain atlas with insula sub-regions
A surface‐based version of the automated anatomical labeling (AAL) atlas was used to parcellate the cortex into 78 areas54 and FIRST (part of FSL) was used to delineate deep gray matter structures. We did not use the insular cortex from this atlas, as it did not provide an anterior insula sub-division. Instead, delineation of the insular cortex was based on the Hammersmith brain atlas55. Based on the anatomical features of the insular cortex as described in Naidich et al.56 and shown in a previous publication by our group28, the three short gyri regions were merged into a single region designated as the dorsal anterior insula (dAI). The anterior inferior cortex will be referred to as the ventral anterior insula (vAI). As our focus is on cognition rather than autonomic dysfunction and interception, we only included the anterior and not posterior insula in our atlas. In summary, the constructed anatomical atlas consisted of a total of 94 nodes: 90 (sub)cortical areas (excluding insula), as well as the left and right dAI and vAI.
Construction of structural connectomes and computation of graph theory measures
Probabilistic tractography was conducted (probtrackx2 wit standard settings, 5000 streamlines per voxel) to obtain probabilistic maps of WM connections running between all pairs of nodes (regions of interests (ROIs)) of the anatomical atlas. Each ROI was overlaid on the probalistic map to obtain the number of fibers at a node, subsequently corrected for node volume, resulting in a non-weighted 94 × 94 structural network matrix for each subject.
The structural network matrices were analyzed in Matlab (MATLAB R2012a, The MathWorks Inc., Natick, MA, 2000). The matrix was symmetrized by computing the average between the original matrix and its transpose, and subsequently thresholded to reduce the number of false-positive connections; the top 20% strongest links were retained57. From the binarized matrix, graph theoretical characteristics were computed using the Brain Connectivity Toolbox as described previously58.
In the current study, we included eigenvector centrality and normalized betweenness centrality of the dAI and vAI. Eigenvector centrality is defined as a self-referential measure of centrality; nodes (e.g. dAI or vAI) have a high eigenvector centrality if they connect to other nodes in the network that have a high eigenvector centrality58. Normalized betweenness centrality (BC) is defined as the fraction of all shortest paths in the network that contain the dAI or vAI, divided by the number of nodes (N) in the network; BC/[(N-1)(N-2)]. This normalizes the Betweenness centrality to the range [0,1], with larger values corresponding to greater centrality58.
Assessment of microstructural tract integrity between anterior insula and ACC
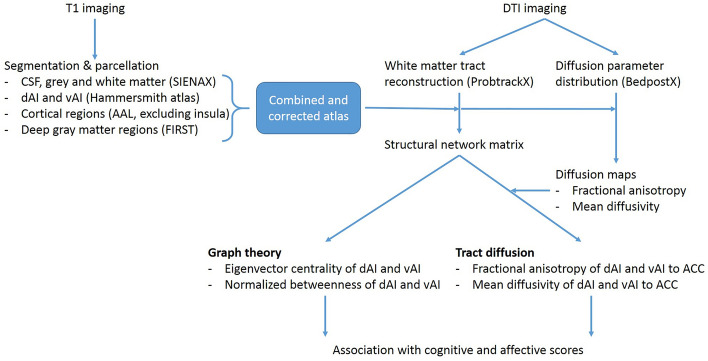
Aside from obtaining graph theoretical measures of the AIC sub-divisions, a restricted structural connectivity analysis was performed between the dAI, vAI and the ACC, to obtain the microstructural integrity of the tracts connecting these regions. The dAI and vAI were entered as a way-point, and the ACC as an end-point, in order to provide a map of all white-matter voxels included in the tract. These connectivity maps were subsequently thresholded and binarized, and used to calculate the mean fractional anisotropy (FA) and mean diffusivity (MD) of the tract by overlaying the tracts onto the diffusion maps. Tracts and diffusion overlays were visually inspected, which resulted in the exclusion of one PD patient for this analysis, which showed erroneous tracts going through the CSF. For an overview of our processing pipeline, see Fig. 3.
Figure 3.
Flowchart of MRI methods and outcome measures. From the T1 images an atlas is constructed which includes the (dorsal and ventral) anterior insula sub-regions from the Hammersmith atlas, as well as other cortical and subcortical regions from the AAL atlas, together corrected for CSF and displacement due to gray matter atrophy. From the DTI images, the diffusion parameters were built, resulting in an FA and MD map. In addition, white matter tracts were reconstructed from the DTI images, and combined with the constructed atlas, the structural network matrix was created. From this, the graph theoretical outcome measures (EC and BC) could be calculated, as well as the microstructural diffusion (FA and MD) outcome measures of specific anterior insula to anterior cingulate tracts. These outcome measures were then compared between PD patients and controls, as well as associated with cognitive and affective scores in PD patients. DTI = diffusion tensor imaging; EC = eigenvector centrality; BC = betweenness centrality; FA = fractional anisotropy; MD = mean diffusivity.
Statistical analysis
Descriptive and statistical analyses were performed using IBM SPSS 22.0 for Windows (SPSS, Inc., Chicago, IL, USA). Normally distributed continuous demographic data were assessed using an independent two-sample t-test. Categorical variables were analyzed using Chi-square tests. Most MRI outcome measures, cognitive and affective scores, as well as their residuals, were not normally distributed. Therefore, we continued our analysis with non-parametric tests, corrected for multiple comparisons. Differences between PD and controls for network topology (e.g. eigenvector centrality and normalized betweenness centrality), were assessed with non-parametric Mann–Whitney U test and Bonferroni corrected for multiple (four) comparisons; left as well as right dAI and vAI. Similarly, microstructural tract integrity of insular sub-regions to the ACC, were assessed with non-parametric Mann–Whitney U test and Bonferroni corrected for multiple (four) comparisons; left as well as right dAI and vAI to ACC. Correlations of microstructural integrity of tracts with cognitive and affective test scores in PD patients were done using non-parametric Spearman’s correlation coefficients.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors wish to express their gratitude to the patients in this study. This study was supported by the Michael J. Fox Foundation for Parkinson’s Research (MJFF-009210), and Stichting Parkinson Fonds (SPF 38000, project number: 105475).
Author contributions
L.E.J. design of the study, analysis of data, writing of the manuscript, and provided study funding. Y.Y.F. design of the study, analysis of data, writing of the manuscript. H.B. design of the study and provided critical revisions to the manuscript. M.M.S. design of the study and provided critical revisions to the manuscript. W.D.J.vdB. conceptualization and design of the study, provided critical revisions to the manuscript, and provided study funding. All authors reviewed the manuscript.
Competing interst
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: L. E. Jonkman and Y. Y. Fathy.
References
- 1.Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat. Disord. 2016;22(Suppl 1):S144–S148. doi: 10.1016/j.parkreldis.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 2010;20:640–645. doi: 10.1111/j.1750-3639.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;187(18):435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, et al. Cognitive decline in Parkinson disease. Nat. Rev. Neurosci. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.McCann H, Stevens CH, Cartwright H, Halliday G. M. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014;20:S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 7.Henderson MX, et al. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat. Neurosci. 2019;22:1248–1257. doi: 10.1038/s41593-019-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronin-Golomb A. Parkinson’s disease as a disconnection syndrome. Neuropsychol. Rev. 2010;20:191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminiti SP, et al. Axonal damage and loss of connectivity in nigrostriatal and mesolimbic dopamine pathways in early Parkinson’s disease. NeuroImage. Clin. 2017;14:734–740. doi: 10.1016/j.nicl.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vriend C, van den Heuvel OA, Berendse HW, van der Werf YD, Douw L. Global and subnetwork changes of the structural Connectome in de novo Parkinson’s disease. Neuroscience. 2018;386:295–308. doi: 10.1016/j.neuroscience.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 11.Galantucci S, et al. Structural Brain Connectome and Cognitive Impairment in Parkinson Disease. Radiology. 2017;283:515–525. doi: 10.1148/radiol.2016160274. [DOI] [PubMed] [Google Scholar]
- 12.Koshimori Y, et al. Disrupted nodal and hub organization account for brain network abnormalities in Parkinson’s disease. Front. Aging Neurosci. 2016;8:259. doi: 10.3389/fnagi.2016.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017;34:300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fathy YY, et al. Differential insular cortex sub-regional atrophy in neurodegenerative diseases: a systematic review and meta-analysis. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monchi O, et al. Neural bases of set-shifting deficits in Parkinson’s disease. J. Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shine JM, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum. Brain Mapp. 2014;35:2206–2219. doi: 10.1002/hbm.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain. 2014;137:2143–2154. doi: 10.1093/brain/awu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou Y, et al. Altered brain network centrality in depressed Parkinson’s disease patients. Mov. Disord. 2015;30:1777–1784. doi: 10.1002/mds.26321. [DOI] [PubMed] [Google Scholar]
- 20.Morel A, Gallay MN, Baechler A, Wyss M, Gallay DS. The human insula: architectonic organization and postmortem MRI registration. Neuroscience. 2013;236:117–135. doi: 10.1016/j.neuroscience.2012.12.076. [DOI] [PubMed] [Google Scholar]
- 21.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurth F, et al. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb. Cortex. 2010;20:1448–1461. doi: 10.1093/cercor/bhp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai W, Ryali S, Chen T, Li C-SR, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci. 2014;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y-T, et al. Salience network and depressive severities in parkinson’s disease with mild cognitive impairment: a structural covariance network analysis. Front. Aging Neurosci. 2018;9:417. doi: 10.3389/fnagi.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fathy YY, et al. Anterior insular network disconnection and cognitive impairment in Parkinson’s disease. NeuroImage Clin. 2020;28:102364. doi: 10.1016/j.nicl.2020.102364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putcha D, Ross RS, Cronin-Golomb A, Janes AC, Stern CE. Salience and default mode network coupling predicts cognition in aging and Parkinson’s disease. J. Int. Neuropsychol. Soc. 2016;22:205–215. doi: 10.1017/S1355617715000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang P, et al. Damaged Insula Network Contributes to Depression in Parkinson’s Disease. Front. Psychiatry. 2020;11:119. doi: 10.3389/fpsyt.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allman JM, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- 32.Del Tredici K, Braak H. Review: Sporadic Parkinson’s disease: development and distribution of α -synuclein pathology. Neuropathol. Appl. Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 33.Nigro S, et al. Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum. Brain Mapp. 2016;37:4500–4510. doi: 10.1002/hbm.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Schipper LJ, et al. Altered whole-brain and network-based functional connectivity in Parkinson’s disease. Front. Neurol. 2018;9:419. doi: 10.3389/fneur.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezdicek O, et al. Mild cognitive impairment disrupts attention network connectivity in Parkinson’s disease: a combined multimodal MRI and meta-analytical study. Neuropsychologia. 2018;112:105–115. doi: 10.1016/j.neuropsychologia.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Tuovinen N, et al. The reorganization of functional architecture in the early-stages of Parkinson’s disease. Parkinsonism Relat. Disord. 2018;50:61–68. doi: 10.1016/j.parkreldis.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Huang P, et al. Cortical abnormalities in Parkinson’s disease patients and relationship to depression: a surface-based morphometry study. Psychiatry Res. Neuroimaging. 2016;250:24–28. doi: 10.1016/j.pscychresns.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Menon, V. et al. Microstructural organization of human insula is linked to its macrofunctional circuitry and predicts cognitive control. Elife9, (2020). [DOI] [PMC free article] [PubMed]
- 39.Fathy YY, et al. Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol. Appl. Neurobiol. 2019;45:262–277. doi: 10.1111/nan.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MC, B. et al. Differential effects of sex on longitudinal patterns of cognitive decline in Parkinson’s disease. J. Neurol.268, 1903–1912 (2021). [DOI] [PMC free article] [PubMed]
- 42.Olde Dubbelink, K. T. E. et al. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology83, 2046–2053 (2014). [DOI] [PubMed]
- 43.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazekas F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/WNL.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 45.Hepp DH, et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology. 2017;285:896–903. doi: 10.1148/radiol.2017170438. [DOI] [PubMed] [Google Scholar]
- 46.Fahn, S. Recent developments in Parkinson’s disease. (New York Raven Press, 1986).
- 47.Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression, and mortality. 1967. Neurology57, S11–26 (2001). [PubMed]
- 48.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 49.Huppert FA, Brayne C, Gill C, Paykel ES, Beardsall L. CAMCOG–a concise neuropsychological test to assist dementia diagnosis: socio-demographic determinants in an elderly population sample. Br. J. Clin. Psychol. 1995;34:529–541. doi: 10.1111/j.2044-8260.1995.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, et al. The relationship of anxious and depressive symptoms in Parkinson’s disease with voxel-based neuroanatomical and functional connectivity measures. J. Affect. Disord. 2019;245:580–588. doi: 10.1016/j.jad.2018.10.364. [DOI] [PubMed] [Google Scholar]
- 51.Maillet A, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain. 2016;139:2486–2502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- 52.Alzahrani H, Venneri A. Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson’s disease: a systematic review. J. Neurol. Sci. 2015;356:32–44. doi: 10.1016/j.jns.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 54.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Faillenot I, Heckemann RA, Frot M, Hammers A. Macroanatomy and 3D probabilistic atlas of the human insula. Neuroimage. 2017;150:88–98. doi: 10.1016/j.neuroimage.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 56.Naidich TP, et al. The insula: anatomic study and MR imaging display at 1.5 T. AJNR. Am. J. Neuroradiol. 2004;25:222–232. [PMC free article] [PubMed] [Google Scholar]
- 57.Achard S, Bullmore E, et al. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.