Abstract
COVID-19’s high virus transmission rates have caused a pandemic that is exacerbated by the high rates of asymptomatic and presymptomatic infections. These factors suggest that face masks and social distance could be paramount in containing the pandemic. We examined the efficacy of each measure and the combination of both measures using an agent-based model within a closed space that approximated real-life interactions. By explicitly considering different fractions of asymptomatic individuals, as well as a realistic hypothesis of face masks protection during inhaling and exhaling, our simulations demonstrate that a synergistic use of face masks and social distancing is the most effective intervention to curb the infection spread. To control the pandemic, our models suggest that high adherence to social distance is necessary to curb the spread of the disease, and that wearing face masks provides optimal protection even if only a small portion of the population comply with social distance. Finally, the face mask effectiveness in curbing the viral spread is not reduced if a large fraction of population is asymptomatic. Our findings have important implications for policies that dictate the reopening of social gatherings.
Subject terms: Computational models, Epidemiology
Introduction
The COVID-19 outbreak has caused high levels of mortality and economic damage around the world. The causative agent of COVID-19, SARS-CoV-2, is an airborne pathogen that can be transmitted between humans through droplets and aerosols that can travel 1–8 m1. The virus is transmitted by both symptomatic and asymptomatic individuals. COVID-19 can cause severe symptoms that require hospitalization in 1–5% of cases, as well as severe long-term sequels and death. Accordingly, the outbreak has seriously impacted healthcare systems around the world2. One of the major difficulties to contain the COVID-19 pandemic has been detection of infected asymptomatic or pre-symptomatic individuals, who are estimated to be responsible for as much as 95% of all transmissions3,4. As these individuals carry and spread the virus without manifesting any sign of the disease, they represent a crucial variable in managing the outbreak.
In the absence of an effective vaccine or antiviral, most countries have implemented non-pharmaceutical interventions (NPIs) to curb the spread of COVID-195. These include closure of schools, workplaces, churches, offices, factories and other social venues, while encouraging preventative measures ranging from maintaining social distancing (SD, usually 2 m/6 ft. between individuals) to total quarantine and societal lockdowns. These measures aim to reduce the effective contact rate of the population, which in turn decreases the disease reproductive number Re. These NPIs limit the epidemic, but they present important drawbacks. Total lockdown can only be implemented for short periods, due to its severe impact on the social fabric and economy of a country. Meanwhile, essential workers remain vulnerable to infection and transmission due to the frequent encounters with infected, often asymptomatic, individuals. Because of their social and economic impact, lockdowns and SD measures have been lifted in some countries, leading to reactivation of virus spread and ensuing increased morbidity and mortality.
Face masks covering the nose and mouth area provide a level of filtration that blocks virus transmission to a certain extent6–8. Masks prevent the spread of droplets and aerosols generated by an infected individual1, and when correctly worn surgical masks can reduce viral transmission by 95%. Uninfected individuals wearing a surgical mask are about 85% protected against infection9. Masks may be more effective than restrictions in people’s interactions for controlling the spread of infectious virus because they prevent the larger expelled droplets from being converted into smaller droplets that can travel farther, rather than removing the interactions between individuals that cause droplets. Accordingly, face masks reduce the spread of influenza10 and coronaviruses11,12.
In the past, several papers have used theoretical models to study how efficacious mask wearing is in avoiding the spread of airborne viruses. In 2010 at least two studies were focused on the effect of face mask usage to contrast the diffusion of Novel Influenza A (H1N1)13,14. Although these works are not on COVID-19 or another Coronavirus disease, they underline the necessity of developing more accurate models to describe similar diseases and reinforce their results in the light of new diseases. Specifically on COVID-19, the efficacy of mask wearing has recently been studied using an ordinary differential equations (ODE) model15, which considered also a varying percentage of asymptomatic individuals, compliance with mandate to wear masks, and a different inward and outward efficacy of protection. However, the authors consider face masks as the sole preventative, excluding additional NPIs like SD or shelter in place1.
Additionally, the problem has been studied in several papers using agent-based models, each being focused on a specific part of the problem. In some cases, the presence of asymptomatic infected individuals was not considered16,17. In other studies, the efficacy of wearing masks was not analyzed when combined with other NPIs18. Finally, the difference in inward and outward protection given by a face mask was often neglected or parametrized with a single value19. A rather comprehensive data-driven investigation of all these effects has been performed by Hoertel et al20. However, their results are specific to the country of France, and the high dimensionality of the parameter space makes it difficult to disentangle the effect of the various interventions.
In our current study, we analyzed the relative efficacy of wearing face masks and/or exercising SD to reducing the spread of COVID-19 in the presence of asymptomatic individuals. This analysis may be particularly important in the current phase of the pandemic. As pharmaceutical interventions (e.g., vaccines) are deployed, people may experience a false sense of security, which may lead to unsafe behavior. This may allow the virus to circulate at the interface between immune and non-immune individuals, accelerating the emergence of variants resistant to vaccination. Face masks have distinct inward and outward protection, which we parameterized using a Gamma function (see Methods). Moreover, these parameters may depend on the material masks are made of. Through stochastic computer simulations of infection spread, we modeled realistic outbreak scenarios and found that SD, implemented as a sheltering in place with occasional contacts with non-SD agents, only yields beneficial effects if accompanied by a widespread population adherence to the practice. In contrast, wearing face masks is a highly effective strategy to reduce the spread of infection. Our results are general, and suggest that, even when a large fraction of infected individuals is asymptomatic, mask wearing is the most effective strategy to control virus spread and alleviate the impact of COVID-19 outbreak, particularly when combined with conditions of partial SD compatible with the function of society.
Results
Stochastic model description and calibration
We developed an agent-based model (ABM) to examine the effectiveness of wearing masks and SD on the rate of infections and viral spread during the pandemic. Unlike ODE models of the spread of COVID-19 disease15,21, ABMs are stochastic models that allow the description of non-homogeneous distributions of agents that act individually12,22. In ABMs, each individual behaves dynamically and independently in response to environmental changes23 according to rules that describe their interactions. To model the COVID-19 pandemic, we used a SEAIR system in which each agent represents an individual who can be susceptible (S), exposed (E), asymptomatically infected (A), symptomatically infected (I), or recovered (R). Although important, we do not explicitly consider reinfection with the same or a different viral variant, as it is outside the scope of this work. To obtain a realistic model of virus spread, we chose parameters that describe the spread of SARS-CoV-2: transmission events occur through contacts made between susceptible and infectious individuals in close proximity (distance ≤ 2r, Fig. 1A), and exposed individuals undergo an incubation period of 5.1 days to become infectious (Fig. 1, spheres with red border). This incubation time represents the interval required to increase viral loads to levels sufficient for transmission3,4,24. We assume that recovered individuals (Fig. 1, spheres with black border), who resolve the infection, cannot be re-infected or infect others, which is a reasonable assumption for the duration of our models (45 days). Symptomatic agents are in the infected state for 7 days but are only infectious to other agents for 12 h. Asymptomatic agents are infectious for all 7 days but the probability of transmission is reduced by 33%. Presymptomatic agents are similar to susceptible agents in their interactions but have a countdown for 5.1 days until they become either asymptomatically or symptomatically Infected.
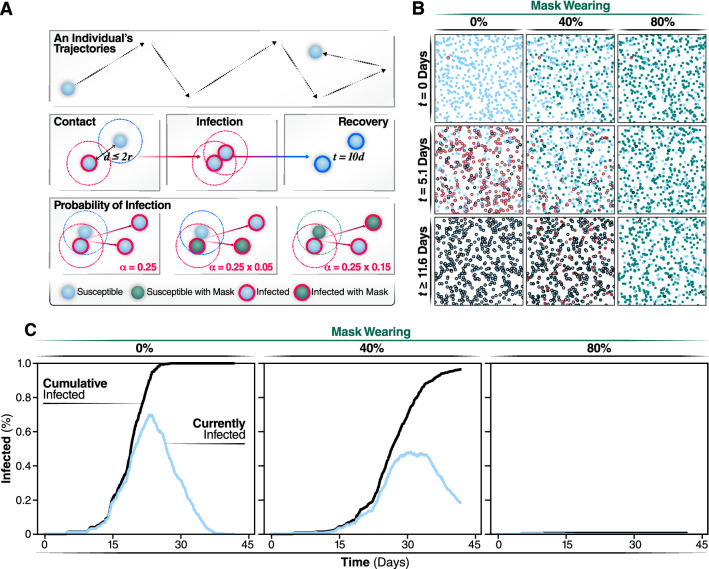
Figure 1.
Individual states and rules of interactions. Using the same coloring system used in animated simulations, we show the different states an individual can be in during a simulation. Individuals keep the masked or not masked attribute assigned at initialization during the course of the simulation. (A) Spread of infection is caused by interactions of overlapping trajectories between infected and susceptible individuals. If the distance between individuals, d, is less than the radius of the two individuals, r, then an interaction occurs. Interactions initiate the generation of a random number that determines if transmission occurs. The chance of infection is randomly generated, based on a gamma distribution with shape parameter, alpha, of 0.25. This probability is further modulated by which individuals in the interactions are wearing face masks. (B) Agents are shown at their positions and states during snapshots of the simulation. Representative simulations of are shown of 0, 40, or 80% of the population wearing face masks. Snapshots are collected at days 0, 20, and 40 of the simulations. (C) Progression of the outbreak, the trajectory of the number of new daily infected individuals (Currently infected) and cumulatively infected from a representative simulation are graphed in light blue and black, respectively.
To define the probabilities of infection, we used reported COVID-19 parameters24. The probability of transmission follows a Γ (gamma) distribution (see Methods) whose shape is described by a constant (α), estimated to be 0.25. Wearing masks reduces this probability (Fig. 1, probability of infection). To estimate the protective effect of masks, we used parameters determined for FDA-approved surgical masks, whose efficacy has been experimentally verified to inhibit virus transmission10. Based on previous studies (Leung et al.9), we assumed that, if an infectious individual wears a mask, the effective probability of transmission is reduced by 95%. If a susceptible individual wears a mask, α is reduced by 85% (Fig. 1A). Our assumption that wearing masks is more effective to reduce transmission than to prevent getting infected is supported by experimental data25. However, if both infectious and susceptible individuals wear masks the probability of transmission is the product of these probabilities (0.0075) and, thus, sufficiently low such that transmission is effectively null. We calibrated our model by running simulations without any individuals wearing a face mask or practicing SD and considering that 50% of infected individuals are asymptomatic at the beginning of the simulation, with each new infection throughout the epidemic having a 50% probability of being asymptomatic (Movie 1). In this way, we determined the simulation parameters, such as velocity and density of individuals, to obtain a value of R0 = 2.5, consistent with what was reported early on as the infection rate of the epidemic in Wuhan9.
Percentage of population wearing masks determines the daily infection incidence and cumulative number of cases
We started each simulation with one individual being infected and all others susceptible and assuming all subsequent infections have a 50% chance of being an asymptomatic. Non-infected and asymptomatic individuals circulate in the population without any restriction (i.e., they do not isolate themselves or become hospitalized) (see Methods). In contrast, symptomatic individuals no longer move after 12 h of the symptom onset, simulating hospitalization or self-isolation. Thus, symptoms are assumed to manifest after the incubation time of 5.1 days (Fig. 2B). Using these assumptions and model calibration, we carried out a set of simulations in which we gradually increased the percentage of individuals in the population that wear masks. Individuals, who are assigned randomly to wear a mask at the beginning of the simulation, keep on the mask for the entire duration of the simulation. Increasing the fraction of the population wearing face masks has a highly significant effect on the spread of the virus (Fig. 1B, C and Movie 2). Mask wearing reduces the cumulative number of infected individuals at the end of the simulation (Fig. 1B). As expected, we observed a negative correlation between the percentage of the population wearing masks and the overall number of cases (Fig. 1B). The description of the dynamics of infection generated by this model is consistent with previous clinical studies9,25 and highlights the benefit of wearing masks (Fig. 1C, Movie 1).
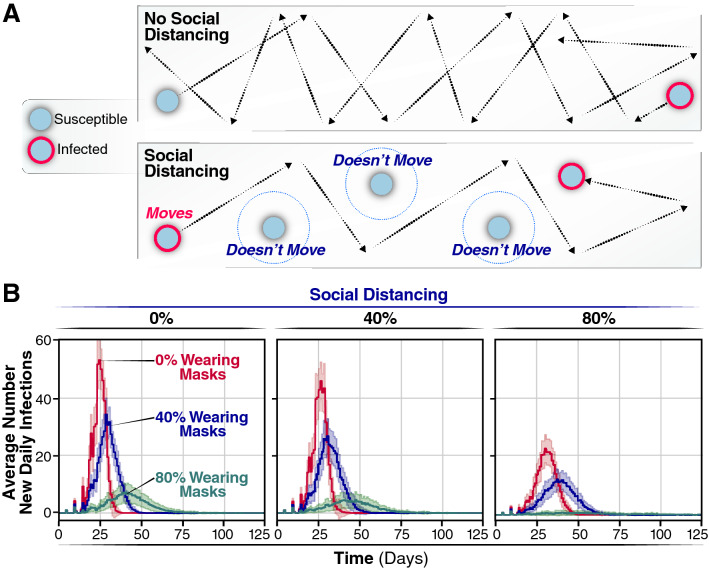
Figure 2.
Average new infections per day when varying the population wearing masks and practicing social distancing. (A) SD is implemented by assigning agents to not move along a trajectory during the simulation. This lack of movement reduces the number of trajectories overlaps between agents and subsequently reduces the number of transmission events. (B) The number of new infections per day for 0, 40, or 80% of a population wearing face-masks are displayed by red, blue, or green trajectories, respectively, for 0% of the population practicing SD, for the 40% and for the 80%. Simulations were repeated 100 times for each condition, the curves and the highlighted regions around the curves represent the mean value + /- one standard deviation.
If the daily incidence surpasses the treatment capacity, it will overwhelm the healthcare system with detrimental consequences for medical care of infected individuals and increased mortality and morbidity. Thus, we examined the effects of face masks on the daily incidence of infection over time (Fig. 2B). If all individuals move freely and randomly interact with others (i.e., 0% SD), the rate of daily infection through the population depends on the percentage of individuals wearing masks. The average number of new daily infection varies considerably, according to the number of individuals wearing face masks. When 0% of individuals wear face masks (Fig. 2B, red line), the number of daily infections peaks sharply at day 27 with a maximum of 54.8 (95% CI 51.2, 58.4) infections per day for a population of 500 individuals. No additional infections are observed after day 39, when the entire population (100%) has been infected. Thus, without any intervention, the infection quickly reaches every individual through an epidemic characterized by a very sharp infection peak. Note that, in these original models, we assume all individuals recover after ~ 11 days post-infection and cannot be re-infected. When 40% of all individuals wear masks, the number of individuals infected at any given day is reduced by approximately 30% (maximum of infected individuals 35.1 (95% CI 32.6, 37.7) Fig. 2A, blue line), both flattening the curve and extending the duration of the outbreak by more than 10 days, with a peak maximum at day 32. By day 52, an average of 472 individuals has been infected, and none of the individuals is infectious or exposed any longer. Even more significantly, if 80% of the population wear masks, we observed a significant flattening of the curve, with a substantial reduction in the maximum number of infected individuals per day, 5.9 (95% CI 4.6, 7.3) (Fig. 2A, green line), and the number of new infected individuals reached zero by day 57.8 ± 35.0. Thus, the shape of the outbreak changes from a curve characterized by a sharp peak when no intervention is considered to a broader peak when 80% of the individuals wear masks. By increasing the percentage of the individuals wearing masks, the number of newly infected individuals per day substantially decreases, which will reduce mortality and morbidity. Moreover, since the use of masks eliminates the sharp peak that characterizes the SARS-CoV-2 epidemic, the overall impact of the outbreak on the health system is alleviated. These results highlight the importance of widespread mask wearing as an effective intervention that can be implemented as soon as the first cases are reported.
Given the different levels of protection distinct fabrics exhibit for aerosol filtration11, we tested our model with the parameters for flannel cloth masks. The trend observed in the epidemic curves is maintained (Fig. SM7), although we observe a general increase in the number of daily cases.
Effect of social distancing on viral infection spread
Next, we evaluated the effect of SD in shaping the spread of infection. Practicing SD does not affect the probability of infection conditional on an encounter but reduces the chances of encounters leading to transmission. Thus, SD was introduced into the model by limiting the proportion of individuals in the population that move freely in the field. This simulates a scenario in which a given proportion of individuals in the population quarantines or shelters in place, thereby reducing the probability of contacts and transmission (Fig. 2A). As reported, if a percentage of individuals practices SD, even without any other non-pharmacological intervention, the number of infections and daily infection rate are reduced (compare Fig. 2B, SD 0, 40, and 80%, red line). However, if no one wears masks but 40% of individuals practice SD, we observed a very small effect in the shape of the infection curve (Fig. 2B, the peak maximum decreases from 51.0 (95% CI 48.3, 53.6) to 30.1 (95% CI 27.5, 32.7). When 80% of the population practices SD, a more significant reduction in the number of new daily infections was observed (Fig. 2B, 80%). Notably, the shape of the new daily infection curve broadens considerably upon increasing the percentage of individuals wearing face masks (Fig. 2B, compare blue and green curves with red curve). For instance, if 80% of the population wears masks with 40% SD, the peak maximum decreases to one-tenth [from 51.0 (95% CI 48.3, 53.6) to 5.7 (95% CI 4.5, 6.7)] and is slightly delayed (Fig. 2B). At 80% SD, the peak of new daily infections is no longer observed, and the number of new infected individuals per day averages 1.6 (95% CI 1.4, 1.9) (Fig. 2B). Thus, the effects of individuals wearing face masks and practicing SD are synergistic, with the most pronounced effects occurring when 60% or 80% of individuals wear masks.
We next tested the dependence of our results on the way SD is implemented in the model. We compared the effect on virus spread when considering three types of SD, without any use of masks. First, we limited the proportion of individuals in the population that move freely in the field, thus reducing the probability of encounter and transmission (Fig. SM6, first row). Next, we simulated epidemics assuming that SD is best modeled by removing individuals from the population, effectively reducing population density (Fig. SM6, second row). Finally we also examine a condition in which individuals who practice social distancing can not longer get infected (akin to moving them to the Recovered compartment, R) (Fig. SM6, four row). The latter condition typically reflects a modeling choice of standard ODE-based models with isolation5. Interestingly, removing individuals from the simulation yields lower density, indicating that ~ 60–80% of the population must practice SD to effectively slow the rate of infection, even when SD reduces the probability of transmission to zero. On the other hand, moving individuals in the R compartment is as effective as using face masks alone to prevent an epidemic. Therefore, the predictions of the tradeoffs between the two control strategies depend significantly on the modeling choice that is made. Our choice of SD is motivated by the observation that even high adherence to SD practices may trigger community spread through, for example, individuals unable to shelter in place or work from home who become asymptomatically infected and spread the virus through their own community26. This becomes an emergent property of our simulations instead of being implemented directly in the model.
Effectiveness of combining mask wearing and social distancing to control infection in populations with high proportions of pre-symptomatic and asymptomatic cases
Asymptomatic or pre-symptomatic SARS-CoV-2 infection is emerging as possibly the most common clinical manifestation of COVID-1927–31. This finding could only be revealed once mass testing campaigns were performed, regardless of symptoms (e.g., universal testing campaigns). One of the earliest studies documenting clinical manifestations in a testing campaign (which still focused testing mainly on symptomatic individuals) was on the Diamond Princess cruise ship, where the rate of asymptomatic infection was 18%32,33. In a mass testing campaign in Iceland, where testing was offered to a segment of the general population (regardless of symptoms), 43% of individuals were asymptomatic at the time of testing26,34. Thus, the actual percentage of pre-symptomatic and asymptomatic cases is currently unknown, but it is clear that a large number of SARS-CoV-2 new infections derive from undetected infections. Controlling the outbreaks by isolation or even by increased population testing is a big challenge and may be difficult to implement. We thus determined the efficacy of mask wearing and SD in the context of different proportions of asymptomatic incidence. We assume that asymptomatic individuals are less infectious than symptomatic individuals: asymptomatic individuals may have lower viral loads and reduced coughing, sneezing, and nasal secretions, all of which may facilitate transmission35–37. The model considers that symptomatic infected individuals isolated themselves 12 h after the onset of symptoms, because they get hospitalized or self-isolated. In contrast, asymptomatic individuals remain infectious, circulate and transmit for a period of 7 days (Fig. 3A). Because some individuals can be pre-symptomatic [i.e., symptoms emerge later after the initial incubation time (Fig. 2B, t = 5.1 days)], we modelled infectivity of asymptomatic/pre-symptomatic as linearly declining until the individual is no longer infectious, after 7 days38. Using these assumptions, we performed a sensitivity analysis by carrying out simulations, varying the percent of asymptomatic/pre-symptomatic cases to determine the efficiency of non-pharmacological interventions in populations with 25, 50, and 75% pre-symptomatic and asymptomatic infected individuals (Fig. 3).
Figure 3.
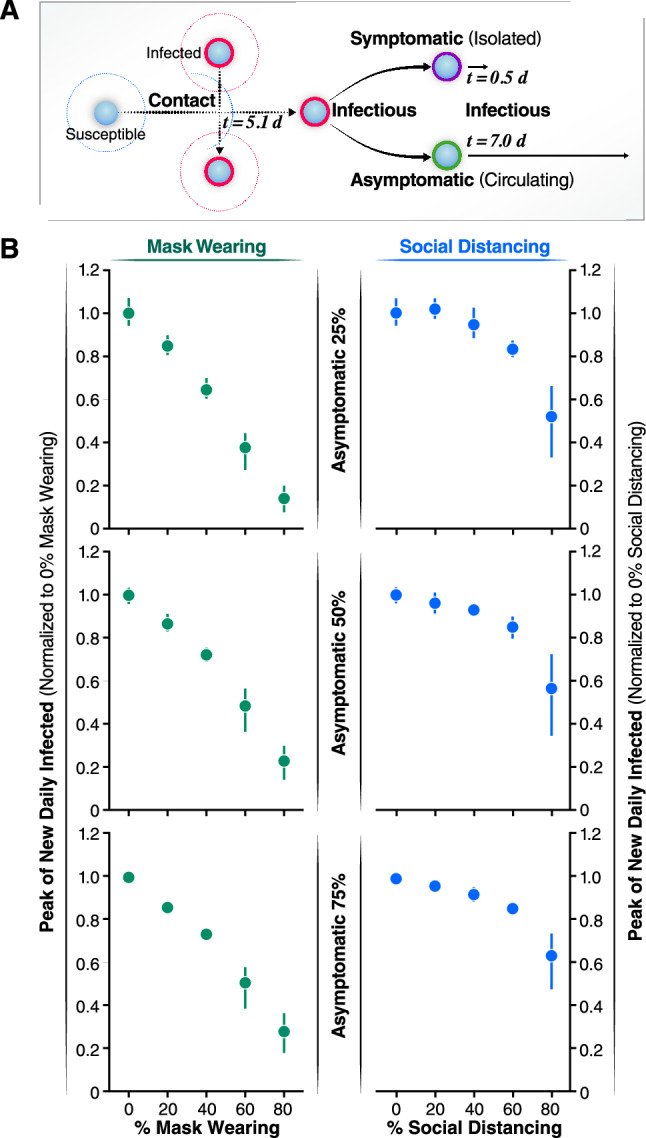
Effects of the asymptomatic population on the infected peak number. (A) Schematic representation of the asymptomatic transmission during the simulations. After 5.1 days of coming into contact with an infected individual, infectious agents can develop symptoms and after 12 h will be isolated. On the contrary, asymptomatic individuals don’t develop symptoms of the disease so they are not isolated but keep circulating thus contributing to spread the infection for 7 more days after the initial contact with an infectious agent. (B) The normalized peak of new daily infected is represented as a function of the percentage of the population wearing mask (left panels, green solid circles) and as a function of increasing the percentage of asymptomatic individuals (25% top left panel, 50% central left panel, 75% bottom left panel). The normalized peak of new daily infected is represented as a function of the percentage of the population practicing social distance (right panels, blue solid circles) and as a function of increasing the percentage of asymptomatic individuals (25% top right panel, 50% central right panel, 75% bottom right panel). Error bars represent 95% confidence intervals calculated.
Our simulations demonstrate that the total number of infected individuals increases linearly with the increase of the percentage of asymptomatic individuals in the population (Fig. 3B). To compare each condition, we normalized the peak of new daily infected individuals to conditions in which no one practiced SD or wore masks. Strikingly, increasing the percentage of individuals wearing masks linearly reduces the normalized peak number of infected individuals per day (Fig. 3B). For instance, when 40% of the individuals wear face masks, the peak number of infected decreases similarly independently of the percent of asymptomatic cases considered (to 0.6–0.7, Fig. 3B). Furthermore, the linear relationship between the decrease in peak number of newly infected individuals per day and increase in percent individuals wearing masks is independent of the proportion of asymptomatic individuals in the population (Fig. 3B). This finding indicates that, when the fraction of asymptomatic cases is high, as in the case for COVID-19, wearing face masks is as effective to reduce the peak number of infected as when a low percentage of individuals are asymptomatically infected.
In contrast, SD was only effective in populations with high incidence of asymptomatic infections when a very high fraction of the population practice SD (more than 60%) (Fig. 3B). Furthermore, the low efficacy of SD as a containment strategy is more pronounced when the proportion of asymptomatic individuals increase (compare 25, 50, and 75%). Of note, if the number of individuals wearing face masks is high, increasing the number of individuals practicing SD has negligible effects on the daily number of infected individuals (see Supplementary Figs. SM4 and SM5). One noteworthy observation from our model is that, at high rates of SD (e.g., 80%), there is more variability.
Interplay of face masks and social distancing for controlling infection spread and protecting from COVID-19
Next, we determined the average cumulative incidence as a function of the percentage of individuals wearing masks and practicing SD (standard deviation described in Fig. SM3-A). When neither SD nor masks were used, our simulations indicate that up to 99% of the population will end up infected, leading to unacceptable levels of mortality and morbidity. When the proportion of the population wearing masks was increased, no significant effect on the total number of infected people was noted until more than 40% of the population wore masks. However, if 80% of people use masks, the average cumulative incidence of infection decreased to around 35%. In contrast, increasing SD compliance to 80% only reduced total infections by 8%, and 87% of the population was eventually infected. Importantly, the combination of wearing masks and practicing SD by a high proportion of the population dramatically reduces the total number of infected individuals to 10% of the population (Fig. 4A; and Fig. SM2-C).
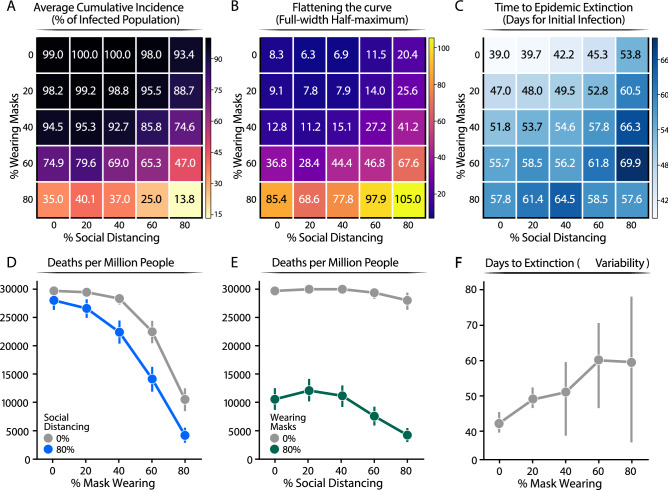
Figure 4.
Summary values from 2500 simulations of varying percentages of population wearing masks or social distancing. (A) Proportion of infected population. The average cumulative incidence is represented as a function of the population practicing SD or wearing a mask, which are given by the x and y axis, respectively. (B) The shape of the daily new infection curve. Full-width half-maximum (FWHM), denoting the number of days between the first day and last day of cases that have half the peak number of infected individuals. (C) Extinction rate of the epidemic estimated as the average number of days for which the simulation reports no new infected individuals. In the figure, the numbers represent the mean value calculated over 100 of simulations carried out for each condition. For clarity, we reported the standard deviation of the mean for each value reported in this figure in Fig. SM5. (D, E) Number of death people. Extrapolated for one million individuals from the cumulative incidence of infections estimated from our model at a mortality rate of 3%. (F) Number of days from the start of infection spread to the last infected agent recovering. Error bars represent 95% confidence intervals calculated for (D, E, F).
To gain additional insights into the characteristics of the epidemic in response to these mitigation strategies, we studied the shape of the epidemic curve by calculating the peak and full-width half-maximum (FWHM) of the peak of the new daily infections (Methods). While the peak reports on the maximum number of daily infections at the height of the epidemic, the FWHM represents the duration of the characteristic peak of new daily infections. Indeed, FWHM reports on the extinction time of the infection within the population (number of days until no individual is still infected) (Fig. 4A, and Fig. SM3-B standard deviation). A larger FWHM corresponds to a flatter but longer epidemic curve. A flatter epidemic curve enables better management of the epidemic, as the healthcare system is not overwhelmed by the number of cases at a given time. Initially, the FWHM was calculated assuming no individual practice SD. FWHM was ~ 8.3 days if none of the individuals wears masks, ~ 12.8 if 40% of individuals wear masks, and but increases dramatically to 85.4 when 80% of the population wears masks (Fig. 2B). Our simulations indicate that, with our SD modeling assumptions, wearing masks has a more profound effect than SD on flattening the epidemic curve. For instance, when 80% of the population wears masks, the epidemic curve is eight times flatter than without any non-pharmacological intervention (Fig. 4B). In contrast, if 80% of the population practices SD, the flattening of the curve is less than threefold. The most dramatic effect on flattening of the curve is observed when wearing masks is combined with SD; for instance, if 80% of the population wears masks and 80% practices SD, the curve is flattened over tenfold, compared to no intervention.
We also find that the reduction in the number of infected individuals per day correlates with a lengthening of the outbreak. Broadening of the peak affects the extinction time of the infection (Fig. 4C). For instance, when the percentage of individuals wearing face masks rises from 0 to 80% of the population, the extinction time increases from ~ 39.0 to ~ 58 days (Fig. 4C). Similarly, as a higher proportion of individuals practice SD, the time to extinction of the infection also increases (Fig. 4C, ~ 39 to ~ 54 days for 0–80% of SD). Importantly, even though the time to epidemic extinction is extended, the total number of infected individuals dramatically decreases (Fig. 4A). If a higher proportion of the population (80%) wears face masks and practices SD, the time to epidemic extinction is reduced (Fig. 4C) because the total number of infected individuals is dramatically reduced (Fig. 4A). Thus, stochastic effects dominate, and this can be appreciated by the wider error bars (Fig. 4F).
To relate the impact of these interventions to their societal impact, we determined the number of deaths per million after each mitigation strategy, assuming a mortality rate of ~ 3%39. This analysis illustrates the heavy cost of lives of the virus, but also demonstrates that according to our model a high level of mask wearing compliance is the most effective non-pharmacological approach to protect human lives, particularly when combined with even moderate SD measures (Fig. 4D). In contrast, SD, without masks wearing is not effective to reduce mortality (Fig. 4E). Finally, our simulations predict that increasing the proportion of the population wearing masks will increase the time to outbreak extinction (from ~ 40 to ~ 60 days) (Fig. 4F). Together with the broadening of the peak (Fig. 4C), this shows an effective flattening of the curve. Importantly, with 80% mask wearing, we observed an increase in the statistical distribution from the average time to extinction (Fig. 4F, see 95% confidence intervals). Thus, a generally low disease incidence triggers stochastic events leading to extinction of the infection. Our simulations represent real outbreak scenarios and reveal that as the outbreak approaches its extinction there is an increase in the uncertainty of whether or not the infection has been completed eliminated, which argue to be prudent before society reopening can be done safety.
Discussion
Here we use realistic simulations rooted in experimentally measured parameters of SARS-CoV-2 spread, contagion mode and mortality, to evaluate two available NPIs that reduce the spread of a respiratory infection, such as COVID-19. In our simulation, we assumed proper use of FDA-approved surgical face masks, as well as widely available cloth masks (flannel). We showed that a high degree of compliance in the use of masks, regardless of whether the wearer displays symptoms, slows the spread of infection. Face masks substantially reduce the transmission of respiratory droplets and aerosols containing viral particles9–11. Increasing the fraction of the population wearing face masks reduces the number of new infected individuals per day and flattened the curve of total individuals infected (Figs. 2A, 4A). These two effects should reduce mortality and morbidity, alleviate the current stress on healthcare systems, and enable a more effective management of severe cases. However, solely wearing masks cannot entirely prevent an outbreak from occurring. It cannot by itself extinguish the virus, since as long as a small fraction of the population is non-compliant, the virus can persist in the population. Our results show that combining proper use of masks with SD indisputably decreases the number of new infected individuals per day (Fig. 2). In addition, our model was demonstrated to be scalable and able to describe realistic situations such as interactions between 5000 agents (see Supplementary Fig. SM2).
Our analysis can be used as guidance for policies to protect the population from COVID-19 or similar airborne diseases. Optimizing the use of masks with SD practices effectively limits the virus spread and reduces several parameters in the epidemic, including cumulative incidence, shape of the peak, and the extinction rate (Fig. 3). In particular, our specific modeling assumptions result in the emergence of a complex relationship between face masks and SD. Even in a population with a high number of asymptomatic infections, increasing the use of masks up to 80% results in a significant reduction in infection (Fig. 4A). Meanwhile, even 80% of individuals practicing SD has only a marginal effect (Fig. 4D). This result can be understood, within our modeling assumptions, in terms of contact rate, since we assume that asymptomatic infectious individuals have higher mobility than symptomatic infectious ones. If the vast majority of the population is asymptomatic, then high compliance with face mask use is a key factor for curbing the epidemic. When our SD assumptions are made more stringent and individuals isolating are not allowed to interact with infected agents, we see SD and face masks contributing similarly to curbing the infection. We justify our assumption in terms of a more realistic view of SD being an imperfect control method. Furthermore, we believe that our methods are general, and they may inform policies against other respiratory infections like influenza.
Our simulations also provide insights into how enforcing different mitigation practices affects the length of the epidemic. Assuming a homogenous population, the trajectory of epidemic extinction lasts 50–60 days, when 80% of the population either wears masks or practices SD (Fig. 4C). However, when 80% of the population is wearing masks and 0% of the population is practicing SD, the cumulative incidence is reduced three times (~ 35%), and the peak is very broad (FWHM of ~ 85 days). In contrast, when the population solely practices SD (80%), the majority of the population (93%) will end up infected, and the peak of daily infected individuals will be sharper (FWHM of ~ 20 days).
Our model indicates that the synergistic utilization of face mask wearing and social distancing practice is most effective in controlling SARS-CoV-2 spread. We observed that wearing masks in combination with some degree of SD relaxes the need for a complete lockdown, leading to a potential suggestion for an intervention policy based on a mix of the two measures. The effectiveness of mask wearing to control virus spread is not reduced if a large fraction of the population is asymptomatic. This suggests that, in the absence of universal testing, widespread use of face masks is necessary and sufficient to prevent a large outbreak. Our results are supported by the real data of Korea26 and Taiwan35, where an early mandate to requiring face mask usage, in combination with SD, severely limited the spread of the virus. While more work is necessary to specifically assess the impact of other variables shaping COVID-19 outbreaks, such as increased mobility, age stratification, testing a fraction of the population, our study can accurately inform strategies to reduce the spread of the virus. In particular, our results may be highly relevant toward informing specific realistic situations, such as the spread of the disease in a confined space, where effective SD may not be easily achievable (e.g., schools, essential businesses, correctional facilities, public transportation, hospitals).. According to our model, if the United States (330 million people) does not implement any NPIs, then ~ 627,000 people are expected to die. Contrastingly, if 80% of the population wear face masks, that number would significantly shrink to about 250,000. If both face masks and SD were practiced by 80% of the population from the start of a pandemic, the mortality rate decreases to 65,600 people.
Materials and methods
We developed and used our python codes using the NumPy library version 1.15.440 to carry out ABM and describe the dynamic evolution of a SEAIR system affected by COVID-19 disease, in which each individual can be in a susceptible (S), exposed (E), asymptomatic infected (A), symptomatic infected (I), or recovered (R) status. In addition, each individual can wear a face mask (M) or can practice social distancing (SD), where wearing face masks or practicing SD are independent binary values of an individual. In the simulations, each individual was represented by a position in a 2-D lattice of 21 × 21 dimension with periodic boundary conditions. Individual initial positions are assigned randomly by NumPy’s random module, and the simulations start with all susceptible individuals and only one exposed. During the simulation, each individual move along a randomly oriented trajectory at a constant velocity, moves the same distance between each time step of the simulation, and interacts with individuals whose position is within a fixed diameter of another individual. The data from each individual are saved as vectors of attributes, including position, velocity, state (S, E, A, I, R, M, SD), and number of individuals they come into contact with at every simulation time step. A time step corresponds to 1 h. We implemented the state of M by defining sub-routine during the interaction between two individuals where the probability of infection was reduced by an amount corresponding to which of the two individuals is wearing a mask. For the SD state, we assigned the individual to be stationary and not follow along a randomly assigned trajectory.
We refer to Ferguson et al. for defining the rules governing the interactions among individuals24. For the interaction between symptomatic infected and susceptible individuals, the infection probability was randomly sampled from a gamma distribution with a mean of 1 and a shape of 2.5, whereas for that between asymptomatic infected and susceptible individuals, the infection probability is reduced by 33%. We assume this reduction of infectivity for the asymptomatic, based on the absence of transmission-aiding symptoms, such as coughing, sneezing, and a runny nose24. The time between a susceptible individual being exposed to being in the infectious infected state is 5.1 days24. Pre-symptomatic agents are defined as infected agents during the incubation period. Symptomatic infected individuals are infectious for 12 h before self-isolating. After this time, we assume that these individuals no longer infect those around them because they are hospitalized or self-isolating. Asymptomatic infected individuals are infectious for 7 days, and the infectivity linearly declines until the individual is no longer infectious on day 738. If an infected, a susceptible, or both individuals are wearing a mask their probability of another individual being infected is reduced to 5, 15, or 0%, respectively, from the original gamma distribution.
First, we carried out a simulation with 500 individuals with 0% of individuals wearing face masks and 0% practicing SD to optimize parameters as velocities and individual density to obtain a basic reproduction number, R0, equal to 2.5, based on reported values. The basic reproduction number was computed from the mean number of symptomatic cases, resulting from a single symptomatic individual. Then, we carried out four more sets of simulations in which we increased the percentage of individuals wearing masks by the 20, 40, 60, and 80%. Finally, for each of these simulation sets we carried out four more sets in which we increased the percentage of individuals practicing SD by the 20, 40, 60, and 80%. Simulations are stochastic, and we simulated each condition 100 times to increase the statistical power of the analysis. In total, we simulated 25 different conditions for a total of 2500 simulations, 100 for each condition. In all these simulations, the probability of a new infection being asymptomatic was 50%. Simulations were run until there were no individuals either infected or exposed. Summary values were computed by methods in the NumPy module. Error bars were calculated by the standard deviation method in NumPy and for Figs. 3 and 4 the error bars were calculated by 95% confidence interval. The full-width half-maximum (FWHM) was calculated for each simulation from the newly infected per day data by taking the maximum and using it to find the days that intersect the value of half the maximum value. The first and last days of this intersection were then used to calculate the number of days of the FWHM. From the set of FWHM for each simulation, an average and standard deviation were calculated using the NumPy module. FWHM error bars are large due to a large variation in how flat the curves are. Graphs were created by using the Matplotlib41 and Seaborn42 modules in python.
We followed the same protocol described above to perform simulations, varying the probability of a new infection being asymptomatic from 25 to 75%. Finally, to monitor the reproducibility of our results with a larger population and contrast with real data, we ran four sets of 30 simulations with 5000 individuals in the combinations of 0 or 80% SD and 0 or 80% wearing masks. All 5000 individual simulations were done with 50% asymptomatic infection rates.
As the position, trajectory, and state of each individual in the simulation are explicitly known, using the matplotlib library’s animation package, we converted each time step of the simulation to an image in a movie, representing 1 day as a second spanning the length of the simulation.
For each simulation, we saved in a .csv in tidy data format the summary values of the simulations, which we used for the analysis. The simulation was written in python and all scripts are available on GitHub at https://github.com/adamcatching/SARS_SEIR_Simulation, simulations were generated using script mask_single_sim.py.
Supplementary Information
Acknowledgements
We acknowledge the use of the UCSF Computing Cluster Wynton (https://wynton.ucsf.edu/hpc/index.html). We thank Judith Frydman, Alma Andino-Frydman, and Patrick Dolan for their suggestions on the manuscript.
Author contributions
A.C., S.C., and M.T.Y. conceived the study. A.C. and S.C. designed the simulations. A.C. wrote and executed the simulations. A.C., S.C., and S.B. analyzed the data. S.B. and R.A. provided guidance and support. All authors wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Adam Catching and Sara Capponi.
Contributor Information
Simone Bianco, Email: sbianco@us.ibm.com.
Raul Andino, Email: Raul.Andino@ucsf.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94960-5.
References
- 1.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 2.Miller, I. F., Becker, A. D., Grenfell, B. T. & Metcalf, C. J. E. (2020) Disease and healthcare burden of COVID-19 in the United States. Nat. Med.10.1038/s41591-020-0952-y [DOI] [PubMed]
- 3.Bai Y, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfel R, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz, I. B., Kaufman, J., Hu, K. & Bianco, S. Predicting the impact of asymptomatic transmission, non-pharmaceutical intervention and testing on the spread of COVID19. BMJ. 10.1101/2020.04.16.20068387
- 6.Feng S, et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020;8:434. doi: 10.1016/s2213-2600(20)30134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng K, et al. COVID-19 and the risk to health care workers: A case report. Ann. Intern. Med. 2020 doi: 10.7326/l20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suess T, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: Results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect. Dis. 2012;12:1. doi: 10.1186/1471-2334-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung NHL, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: A systematic review and meta-analysis. Clin. Infect. Dis. 2017;65:1934. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konda A, et al. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 12.Howard, J. et al. Face masks against COVID-19: An evidence review. 10.20944/preprints202004.0203.v1 (2020)
- 13.Tracht, S. M., Valle, S. Y. D. & Hyman, J. M. Mathematical Modeling of the Effectiveness of Facemasks in Reducing the Spread of Novel Influenza A (H1N1). Plos One. 10.1371/journal.pone.0009018 (2010). [DOI] [PMC free article] [PubMed]
- 14.Brienen NCJ, Timen A, Wallinga J, Steenbergen JEV, Teunis PFM. The effect of mask use on the spread of influenza during a pandemic. Risk Anal. 2010;30:1210. doi: 10.1111/j.1539-6924.2010.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngonghala, C. N. et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math. Biosci.10.1016/j.mbs.2020.108364 (2020). [DOI] [PMC free article] [PubMed]
- 16.Mahdizadeh Gharakhanlou, N. & Hooshangi, N. Spatio-temporal simulation of the novel coronavirus (COVID-19) outbreak using the agent-based modeling approach (case study: Urmia, Iran). Inform. Med. Unlocked. 10.1016/j.imu.2020.100403 (2020). [DOI] [PMC free article] [PubMed]
- 17.Chang, S. L., Harding, N., Zachreson, C., Cliff, O. M. & Prokopenko, M. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat. Commun.11, 1–13 (2020). [DOI] [PMC free article] [PubMed]
- 18.Kai, D., Goldstein, G.-P., Morgunov, A., Nangalia, V. & Rotkirch, A. Universal Masking is urgent in the COVID-19 pandemic: SEIR and agent based models, empirical validation, policy recommendations. arXiv:2004.13553 [physics, q-bio] (2020).
- 19.Silva, P. C. L. et al. COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos Solitons Fract.10.1016/j.chaos.2020.110088 (2020). [DOI] [PMC free article] [PubMed]
- 20.Hoertel N, et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat. Med. 2020;26:1417. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 21.Kuniya, T. Prediction of the epidemic peak of coronavirus disease in Japan, 2020. J. Clin. Med. 10.3390/jcm9030789 (2020). [DOI] [PMC free article] [PubMed]
- 22.Bauer AL, Beauchemin CAA, Perelson AS. Agent-based modeling of host–pathogen systems: The successes and challenges. Inf. Sci. 2009;179:1379. doi: 10.1016/j.ins.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmandad H, Hu K, Tebbens RJD, Thompson KM. Development of an individual-based model for polioviruses: Implications of the selection of network type and outcome metrics. Epidemiol. Infect. 2011;139:836. doi: 10.1017/s0950268810001676. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson, N. et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College, London (2020). [DOI] [PMC free article] [PubMed]
- 25.Liang M, et al. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heck, T. G. et al. Insufficient social distancing may contribute to COVID-19 outbreak: The case of Ijuí city in Brazil. Plos One16, e0246520 (2021). [DOI] [PMC free article] [PubMed]
- 27.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection. Ann. Intern. Med. 2020 doi: 10.7326/m20-3012. [DOI] [PubMed] [Google Scholar]
- 28.Ing AJ, Cocks C, Green JP. COVID-19: In the footsteps of Ernest Shackleton. Thorax. 2020;75:693. doi: 10.1136/thoraxjnl-2020-215091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arons MM, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day, M. Covid-19: Four fifths of cases are asymptomatic, China figures indicate. BMJ. 10.1136/bmj.m1375 (2020). [DOI] [PubMed]
- 31.Cline, S. Cases at seafood plant cause spike in Oregon COVID numbers | News Break. https://www.newsbreak.com/oregon/salem/news/1581086288035/cases-at-seafood-plant-cause-spike-in-oregon-covid-numbers.
- 32.Moriarty, L. F. Public health responses to COVID-19 outbreaks on cruise ships—worldwide, February–March 2020. MMWR. Morbid. Mortal. Wkly Rept.10.15585/mmwr.mm6912e3 (2020). [DOI] [PMC free article] [PubMed]
- 33.Mizumoto, K., Kagaya, K., Zarebski, A. & Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess Cruise ship, Yokohama, Japan, 2020. Euro Surveillance: Bulletin European Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 10.2807/1560-7917.es.2020.25.10.2000180 (2020). [DOI] [PMC free article] [PubMed]
- 34.Gudbjartsson DF, et al. Spread of SARS-CoV-2 in the icelandic population. N. Engl. J. Med. 2020;382:2302. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buitrago-Garcia, D. C. et al. Asymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. medRxiv. 10.1101/2020.04.25.20079103 (2020). [DOI] [PMC free article] [PubMed]
- 36.Sakurai A, et al. Natural history of asymptomatic SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez, M. WHO: Data suggests it's “very rare” for coronavirus to spread through asymptomatics. https://www.axios.com/who-coronavirus-asymptomatic-patients-08d84e31-1846-44d8-8fe1-2343ff850764.html
- 38.He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med.10.1038/s41591-020-0869-5 (2020). [DOI] [PubMed]
- 39.Mortality Risk of COVID-19—Statistics and Research. https://ourworldindata.org/mortality-risk-covid.
- 40.Van Der Walt S, Colbert SC, Varoquaux G. The NumPy array: A structure for efficient numerical computation. Comput. Sci. Eng. 2011;13:22. doi: 10.1109/mcse.2011.37. [DOI] [Google Scholar]
- 41.Matplotlib: A 2D Graphics Environment—IEEE Journals & Magazine. https://ieeexplore.ieee.org/document/4160265.
- 42.mwaskom/seaborn: v0.8.1 (September 2017) | Zenodo. https://zenodo.org/record/883859.
- 43.Kai, D., Goldstein, G.-P., Morgunov, A., Nangalia, V. & Rotkirch, A. Universal Masking is urgent in the COVID-19 pandemic: SEIR and agent based models, empirical validation, policy recommendations (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.