Abstract
Purpose
Methamphetamine (MA) is associated with adverse health effects, including the rampant tooth decay condition called “Meth Mouth.” However, the impact of MA use on oral health-related quality of life (OHRQOL) is unknown. This study assessed the relationship between MA use and self-reported OHRQOL.
Methods
This cross-sectional study uses information from 545 MA-using participants recruited from Los Angeles County, California. Dental examinations were performed by three calibrated dentists using National Health and Nutrition Examination Survey (NHANES) protocols. Data on socio-demographic, behavioral, and drug-use history were recorded using questionnaires. Participants were categorized as ‘light’ or ‘moderate/heavy’ users based on reported frequency of MA use in the past 30 days. Route of MA administration was categorized as ‘smoking’ or ‘other.’ Self-reported OHRQOL was based on the Oral Health Impact Profile scale.
Results
Majority of the participants were male (80.9%). Median age was 45.0 years (IQR-13.0). Median number of days of MA use was 10.0 (IQR-12.0). Smoking was the preferred route of MA use (70.2%). Root caries in ≥ 3 teeth were reported in 78% of MA users. More than half of the participants reported having painful aching in mouth, avoidance of particular food items, feeling embarrassed, and discomfort while eating in the last 12 months. In unadjusted logistic models, moderate/heavy MA users were more likely to report an affected sense of taste [OR = 1.58, 95% CI (1.10–2.27)] and avoidance of particular foods [OR = 1.45, 95% CI (1.02–2.01)] than light users. Among individuals preferring other MA administration routes, moderate/heavy MA users were 3.09 times as likely to report an affected sense of taste than light users [OR = 3.09, 95% CI (1.52–6.27)].
Conclusion
Oral health and OHRQOL appear to be worse among Methamphetamine users than in the US general population.
Keywords: Methamphetamine, Oral health, Quality of life, Illicit substance use, Dental public health, Oral epidemiology
Introduction
Methamphetamine (MA) is a central nervous system (CNS) stimulant that gained popularity in the 1990s. Originally used in nasal decongestants and bronchial inhalers, MA is similar to its parent drug amphetamine in promoting increased activity, talkativeness, decreased appetite, and a pleasurable sense of well-being or euphoria. However, at comparable doses to amphetamine, MA is a more potent stimulant and its effects are longer-lasting and more harmful on the CNS [1]. Methamphetamine, acts by altering the CNS neurotransmitter levels. It stimulates release and blocks reuptake of dopamine, norepinephrine, and serotonin leading to neurodegeneration and neurotoxicity [2-5]. Methamphetamine was recognized by the U.S. Food and Drug Administration (FDA) as a Schedule II amphetamine because it has a high potential for misuse and psychological or physical dependence. According to 2016 National Survey on Drug Use and Health (NSDUH), 14,533,000 people (5.4% of the U.S. population in 2016) had used MA in their lifetime, and 1,391,000 (0.5 percent of the U.S. population) had used MA in the past year [6]. Methamphetamine is known by a number of “street” names such as ‘crank,’ ‘super ice,’ ‘LA glass,’ ‘crystal meth,’ ‘meth,’ ‘wash,’ ‘chicken feed,’ ‘trash,’ and others. The common routes of MA administration are oral, intranasal, smoking, and injection [7].
Methamphetamine has both short-term and long-term effects on the body. Short-term effects include hyperactivity, talkativeness, teeth-grinding, euphoria, insomnia, and loss of appetite. Long-term use can lead to dependence, immunomodulation, weight-loss, hypertension, stroke, skin lesions, anxiety, and several psychological abnormalities [8, 9]. Methamphetamine is known to have deleterious effects on oral health as well. Higher incidences and severity of caries, periodontal problems, xerostomia, and tooth loss have been reported in MA users [10-12]. The term ‘meth mouth’ has been ascribed to MA users who have rampant tooth decay, which resembles early childhood caries (ECC). Like ECC, it involves the interproximal and facial surfaces of teeth, especially anterior teeth, giving the teeth a blackened, stained, rotting, crumbling, or falling apart appearance [11, 12]. Methamphetamine use has also been shown to be associated with xerostomia, clenching, and bruxism, which indirectly contribute to severe tooth decay and demineralization [7, 13]. Poor oral hygiene and high intake of calorie-rich carbonated beverages reported in MA users also increase the likelihood of tooth decay [7, 12-14].
In a large community study of MA users, Shetty and colleagues observed a significant association between MA use and the number of missing teeth [10]. They also found that users who administered MA intravenously have significantly higher number of missing teeth than MA smokers [15]. More recently, other investigators have reaffirmed the observed significant association of MA use with number of missing teeth, caries, and periodontal problems [16, 17]. Studies on MA use and oral habits showed significantly lower frequency of tooth-brushing in MA users when compared to non-users [18, 19]. Despite extreme dental consequences, only one study has investigated the effect of MA on Oral Health-Related Quality of Life (OHRQOL) to date [20]. Using selected questions from the Oral Health Impact Profile (OHIP 14), Truong and colleagues measured OHRQOL in Australian illicit drug users and reported that users had a poor OHRQOL when compared to the general population. However, this study included participants who used a wide range of illicit drugs including MA and heroin, and MA users comprised only 11.2% of the studied population [20].
Studies conducted to assess general quality of life (QOL) suggest that severity and duration of drug use, socio-demographic factors, HIV status, behavioral factors, economic status, and lack of access to care are some of the most important factors contributing to poor QOL in illicit drug users. Oral health-related QOL is also a multidimensional concept that captures people’s perception of oral health on their quality of daily life. Instead of focusing on oral cavity alone, OHRQOL shifts the focus to the patient as a whole [21]. According to the Surgeon General’s Report on Oral Health [22], OHRQOL reflects people’s contentment with eating, sleeping, and engaging in social interaction; their self-esteem; and their satisfaction with respect to oral health [22]. Because poor oral health status has been reported in MA users, understanding how MA use impacts OHRQOL is important in assessing factors affecting unmet dental needs and in developing policies to address disproportionate disease burden in this substance abusing population. Our objective was to assess the association between frequency and route of administration of MA use and self-reported OHRQOL among urban MA users from a large community sample in the United States, who had used MA in the past 30 days. Our study was based on the following alternate hypothesis: the frequency of MA use and route of MA administration are associated with OHRQOL among urban MA users.
Methods
Data collection and management
Data were collected from MA users, at two large community health centers in Los Angeles County: AIDS Project, Los Angeles (APLA) and Mission Community Hospital in the San Fernando Valley (Mission), using snowball sampling approaches. A combination of street outreach methods like newspaper advertisements, posters, flyers, Craigslist postings were used to recruit participants. To participate in the parent study, individuals had to be 18 years or older, speak either English or Spanish, had used MA in the past 30 days, and had to express willingness to undergo a detailed dental exam, to complete a psychological assessment, and to provide a urine sample. Between February 2011 and August 2013, 1793 potential participants contacted the research team. A cohort of 1120 individuals were identified as being eligible based on the inclusion criteria; 571 completed at least some of the planned assessments. In this crosssectional analysis, we included 545 participants who had provided information related to OHRQOL (Fig. 1). Each participant received a one-time participation fee of $60 for completing the required questionnaires and examinations. Written informed consent was obtained from all the study participants. The University of California, Los Angeles (UCLA) Institutional Review Board approved the informed consent process, data collection, and study design protocol [10]. Additional information related to study design, data collection, and data quality has been published elsewhere [10, 23].
Fig. 1.
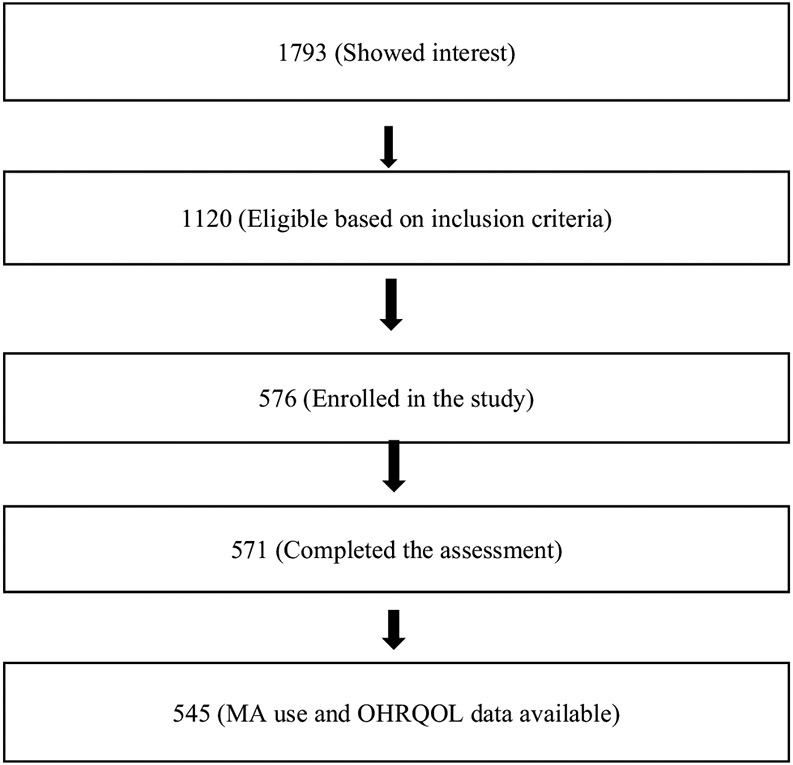
Flow chart showing study population in the study assessing OHRQOL in methamphetamine users
Outcome variables
We assessed self-reported OHRQOL using a shortened/modified version of the OHIP-14. The OHIP-14 is the most commonly used OHRQOL measure used in the literature, and has been reported to be valid, reliable, and precise [24, 25]. The 7 key indicators of OHIP-14 scale are functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap. The shortened version of OHIP-14 uses 7 questions to capture impact across the 7 key indicators of OHIP-14 and has been shown to be valid [25] and has been used on the National Health and Nutrition Examination Survey (NHANES). Because of the length of the overall questionnaire, the shortened version of the OHIP-14 was used to address respondent burden concerns. The 7 OHRQOL questions are as follows:
How often during the last year have you had painful aching anywhere in your mouth?
How often during the last year have you felt that life in general was less satisfying because of problems with your teeth, mouth, or dentures?
How often during the last year you have had difficulty doing your usual jobs or going to school because of problems with your teeth, mouth, or dentures?
How often during the last year has your sense of taste been affected by problems with your teeth, mouth, or dentures?
How often during the last year have you avoided particular foods because of problems with your teeth, mouth, or dentures?
How often during the last year have you found it uncomfortable to eat any food because of problems with your teeth, mouth, or dentures?
How often during the last year have you been self-conscious or embarrassed because of your teeth, mouth, or dentures?
Responses for each of the indicators included ‘very often,’ ‘fairly often,’ ‘occasionally,’ ‘hardly ever,’ and ‘never,’ respectively. We created dichotomous variables for ‘unfavorable OHRQOL’ (yes/no) for each of the seven indicators. Oral health-related Quality of Life was determined to be unfavorable if the responses were ‘very often’ or ‘fairly often’ or ‘occasionally.’ We assessed overall unfavorable OHRQOL by using all unfavorable OHRQOL indicators. If participants responded ‘yes’ to all 7 unfavorable OHRQOL indicators, they were categorized as having an overall unfavorable OHRQOL.
Independent variables
Our key independent variable was MA use. We categorized MA users as ‘light’ and ‘moderate/heavy’ users based on their frequency of MA use in the past 30 days. We used median split method to dichotomize our exposure of interest. Participants who used MA for less than 10 days in the past 30 days fell in the ‘light’ category, and participants who used MA for 10 or more days in the past 30 days were considered as ‘moderate/heavy’ users. Route of MA administration was categorized into a dichotomous variable based on how participants reported their preferred use: either ‘smoking’ or ‘other.’ If participants used MA by inhalation, injection, oral, or any way other than by smoking, their route of administration was considered to be ‘other.’
Participants were grouped into four age categories initially: less than 30 years of age, 30 to < 45 years of age, 45 to < 60 years of age, and 60 years and over. Because there were few participants in age groups less than 30 years and 60 years and over, we decided to dichotomize the age categories into less than 45 years of age and 45 years and above. Race/ethnicity was categorized as White, Black, Hispanic, and Other races. Education was categorized into three groups based on high school graduation: ‘less than high school,’ ‘high school,’ and ‘more than high school.’ Marital status was based on these valid responses: married, widowed, divorced, separated, never married, and living with partner. Participants who were married or widowed or divorced or who lived with partners were considered ‘ever married/partner’ with the remaining classified as ‘never married.’ Dichotomous variables were used for country of birth (United States/other) and HIV-positive status (yes/no). Language spoken at home had three categories: English, Spanish, and both. Information on cigarette smoking was categorized as current smokers, past smokers, and never smokers. Symptoms of depression or anxiety were recoded as a dichotomized variable (yes/no), depending on whether the participants had reported feeling blue, hopeless, or tense. Clinical dental status was evaluated using derived dichotomous variables (yes/no) based on the presence or absence of three dental conditions: anterior caries in 5 or more teeth surfaces, root caries in 3 or more teeth, and severe periodontitis. Periodontitis was measured following the CDC/AAP case definition recommendations [26]. Participant information on experiencing any painful aching or sores/irritation in the mouth in the past 30 days were also analyzed as a dichotomized variable (yes/no), based on participants’ responses.
Statistical analysis
SAS software, version 9.4 (SAS Institute Inc.), was used for all data analyses. We conducted bivariate analyses of participants’ socio-economic status, clinical dental status, mental status, and MA use patterns by OHRQOL. Logistic regression analyses (including unadjusted and multivariable variations) were used to assess the association of MA use with OHRQOL. Alpha (α) value was set at 0.05 (two-sided) and odds ratios (OR) and 95% confidence intervals (CI) were noted in all logistic regression models. In early model building, we included variables with a Wald p value of 0.1 or less identified in the unadjusted logistic regression models, as well as the variables of interest pertaining to level of MA use and route of administration. We then used backwards selection to remove less significant variables to produce reduced models that included only those variables with p values less than or equal to 0.05. We also evaluated for interactions during modeling, and found a significant interaction between frequency of MA use and route of MA administration for outcome variable affected sense of taste (p value < 0.05). Based on statistical significance of the interaction variables, we decided to stratify by route of administration to complete the regression modeling. In our sensitivity analysis, we used simple random sampling to create smaller subsets of data, and checked for internal validity.
Results
Of the 545 MA users included in this study, most were male (80.9%) (Table 1). The median age of participants was 45.0 years (data not shown) and nearly 54% of the participants were 45 years or older. A substantial proportion (68.3%) of the participants smoked cigarettes and a quarter of the participants were HIV positive. Symptoms of depression or anxiety were reported in 48.6% of the participants at the time of study. Most individuals (78%) had root caries that affected 3 or more teeth and severe periodontitis was detected in 21.1% of the participants. The median number of days of MA use in the study population was 10.0 (data not shown) and 56% of the participants were identified as moderate/heavy MA users. Smoking was the preferred route of MA administration (70.2%) with the rest administering MA via other routes such as oral, injection, inhalation, or other. More than half of the participants reported painful aching in mouth (59.5%), avoidance of particular foods (56.5%), discomfort while eating (63.5%), and feeling embarrassed (60.7%) in the last 12 months. Less satisfying life and affected sense of taste was reported in 43.9 and 33.2% of the participants, respectively.
Table 1.
Distribution of socio-demographic, behavioral, oral health status, and methamphetamine use indicators by unfavorable oral health-related quality of life outcomes in the last year
| Characteristics | Study metrics | Painful aching in mouth |
Less satisfying life |
Difficulty doing usual work/ activity |
Affected sense of taste |
Particular food avoided |
Uncomfortable to eat |
Felt embarrassed |
|---|---|---|---|---|---|---|---|---|
| N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 545 | 324 (59.5) | 239 (43.9) | 164 (30.1) | 181 (33.2) | 308 (56.5) | 346 (63.5) | 331 (60.7) |
| Age | ||||||||
| < 45 years | 253 (46.4) | 141 (55.7) | 93 (36.8) | 67 (26.5) | 68 (26.9) | 134 (53.0) | 151 (59.7) | 140 (55.3) |
| ≥ 45 years | 292 (53.6) | 183 (62.7) | 146 (50.0) | 97 (33.2) | 113 (38.7) | 174 (59.6) | 195 (66.8) | 191 (65.4) |
| Sex | ||||||||
| Male | 441 (80.9) | 258 (58.5) | 192 (43.5) | 128 (29.0) | 146 (33.1) | 240 (54.4) | 274 (62.1) | 266 (60.3) |
| Female | 104 (19.1) | 66 (63.5) | 47 (45.2) | 36 (34.6) | 35 (33.7) | 68 (65.4) | 72 (69.2) | 65 (62.5) |
| Race/ethnicity | ||||||||
| Hispanic | 174 (31.0) | 111 (63.8) | 61 (35.1) | 49 (28.2) | 62 (35.6) | 93 (53.5) | 107 (61.5) | 107 (61.5) |
| African-American | 233 (42.8) | 142 (61.0) | 114 (49.0) | 76 (32.6) | 80 (34.3) | 144 (61.8) | 159 (68.2) | 146 (62.7) |
| White | 102 (18.7) | 51 (50.0) | 49 (48.0) | 29 (28.4) | 27 (26.5) | 48 (47.1) | 58 (56.9) | 58 (56.9) |
| Other | 36 (6.7) | 20 (55.6) | 15 (41.7) | 10 (27.8) | 12 (33.3) | 23 (63.9) | 22 (61.1) | 20 (55.6) |
| Education | ||||||||
| Less than HS | 158 (28.0) | 106 (67.1) | 79 (50.0) | 64 (40.5) | 65 (41.1) | 100 (63.3) | 107 (67.7) | 102 (64.6) |
| High school | 194 (35.6) | 117 (60.3) | 79 (40.1) | 54 (27.8) | 67 (34.5) | 114 (58.8) | 138 (71.1) | 122 (62.9) |
| More than HS | 193 (35.4) | 101 (52.3) | 81 (42.0) | 46 (23.8) | 49 (25.4) | 94 (48.7) | 101 (52.3) | 107 (55.4) |
| Marital status | ||||||||
| Ever married/partner | 155 (28.4) | 92 (59.4) | 76 (49.0) | 48 (31.0) | 55 (35.5) | 93 (60.0) | 103 (66.5) | 94 (60.7) |
| Never married | 390 (71.6) | 232 (59.5) | 163 (41.2) | 116 (29.7) | 126 (32.6) | 215 (55.1) | 243 (62.3) | 237 (60.8) |
| Country of birth | ||||||||
| US | 458 (84.0) | 264 (57.6) | 206 (45.0) | 135 (29.5) | 151 (33.0) | 265 (57.9) | 292 (63.8) | 279 (60.9) |
| Other | 87 (16.0) | 60 (69.0) | 33 (38.0) | 29 (33.3) | 30 (34.5) | 43 (49.4) | 54 (62.1) | 52 (59.8) |
| Language at home | ||||||||
| Spanish | 52 (9.5) | 39 (75.0) | 18 (34.6) | 17 (32.7) | 21 (40.4) | 30 (57.7) | 37 (71.2) | 34 (65.4) |
| English | 459 (84.2) | 262 (57.1) | 211 (46.0) | 139 (30.3) | 150 (32.7) | 263 (57.3) | 292 (63.6) | 279 (60.8) |
| Both | 34 (6.2) | 23 (67.7) | 10 (29.4) | 8 (23.5) | 10 (29.4) | 15 (44.1) | 17 (50.0) | 18 (52.9) |
| Cigarette smoking history | ||||||||
| Current smoker | 372 (68.3) | 222 (59.7) | 177 (47.6) | 123 (33.1) | 132 (35.5) | 220 (59.1) | 248 (66.7) | 232 (62.4) |
| Former smoker | 52 (9.5) | 32 (61.5) | 21 (40.4) | 15 (28.9) | 14 (26.9) | 34 (65.4) | 36 (69.2) | 31 (59.6) |
| Never smoked | 121 (22.2) | 70 (57.9) | 41 (33.9) | 26 (21.5) | 35 (28.9) | 54 (44.6) | 62 (51.2) | 68 (56.2) |
| HIV status | ||||||||
| Positive | 140 (25.7) | 92 (65.7) | 64 (45.7) | 43 (30.8) | 55 (39.3) | 79 (56.4) | 93 (66.4) | 89 (63.6) |
| Negative | 405 (74.3) | 232 (57.3) | 175 (43.2) | 121 (29.9) | 126 (31.1) | 229 (56.5) | 253 (62.5) | 242 (59.8) |
| Depression/anxiety | ||||||||
| Yes | 265 (48.6) | 173 (65.3) | 138 (52.1) | 99 (37.4) | 120 (45.3) | 173 (65.3) | 191 (72.1) | 180 (67.9) |
| No | 280 (51.4) | 151 (53.9) | 101 (36.1) | 65 (923.2) | 61 (21.8) | 135 (48.2) | 155 (55.4) | 151 (53.9) |
| Severe periodontitis | ||||||||
| Yes | 115 (21.1) | 69 (60.0) | 53 (46.1) | 37 (32.2) | 38 (33.0) | 79 (69.0) | 75 (65.2) | 77 (67.0) |
| No | 430 (78.9) | 255 (59.3) | 186 (43.3) | 127 (29.5) | 143 (33.3) | 239 (53.6) | 271 (63.0) | 254 (59.1) |
| Anterior caries ≥ 5 | ||||||||
| Yes | 100 (18.4) | 68 (68.0) | 60 (60.0) | 40 (40.0) | 45 (45.0) | 69 (69.0) | 79 (79.0) | 80 (80.0) |
| No | 445 (81.6) | 256 (57.5) | 179 (40.2) | 124 (27.9) | 136 (30.6) | 239 (53.7) | 267 (60.0) | 251 (56.4) |
| Root caries ≥ 3 | ||||||||
| Yes | 425 (78.0) | 268 (63.1) | 209 (49.2) | 147 (34.6) | 152 (35.8) | 253 (59.5) | 295 (69.4) | 271 (63.8) |
| No | 120 (22.0) | 56 (46.7) | 30 (25.0) | 17 (14.2) | 29 (24.2) | 55 (45.8) | 51 (42.5) | 60 (50.0) |
| Experienced painful tooth/ sores in past 30 days | ||||||||
| Yes | 273 (50.1) | 217 (79.5) | 152 (55.7) | 129 (47.3) | 126 (46.2) | 202 (74.0) | 219 (50.2) | 192 (70.3) |
| No | 272 (49.9) | 107 (39.3) | 87 (32.0) | 35 (12.9) | 55 (20.2) | 106 (39.0) | 127 (46.7) | 139 (51.1) |
| Route of MAa | ||||||||
| Smoking | 382 (70.2) | 223 (58.4) | 165 (43.2) | 112 (29.3) | 113 (29.6) | 209 (54.7) | 236 (61.8) | 224 (58.6) |
| Other | 162 (29.8) | 101 (62.0) | 74 (45.4) | 52 (31.9) | 68 (41.7) | 99 (60.7) | 111 (67.7) | 107 (65.6) |
| MA use | ||||||||
| Light | 242 (44.4) | 136 (56.2) | 104 (43.0) | 66 (27.3) | 67 (27.7) | 125 (51.7) | 143 (59.1) | 145 (60.0) |
| Moderate/heavy | 303 (55.6) | 188 (62.1) | 135 (44.6) | 98 (32.3) | 114 (37.6) | 183 (60.4) | 203 (67.0) | 186 (61.4) |
N total study population; n participants in each group; % frequency percentage (row)
Missing frequency (1); all subjects in Table 1 have valid dental exam records and responses to the OHRQOL indicators
In unadjusted logistic models, moderate/heavy users were more likely than light users to report an affected sense of taste [OR 1.58; 95% CI (1.10, 2.27)] and avoidance of particular foods [OR 1.45; 95% CI (1.02, 2.01)] due to dental problems (Table 2). Participants smoking MA were less likely to report an unfavorable response to affected sense of taste compared to participants using other routes [OR 0.58; 95% CI 0.40–0.85]. Users aged 45 and above were more likely to report less that satisfying life [OR 1.72; 95% CI (1.22, 2.43)], affected sense of taste [OR 1.72; 95% CI (1.19, 2.47)], and embarrassment [OR 1.53; 95% CI (1.08, 2.16)] because of dental problems than their younger counterparts. Participants with more than high school education were less likely to report discomfort while eating [OR 0.45; 95% CI (0.29, 0.67)] compared to high school graduates. Cigarette smoking was found to be associated with 4 of the 7 OHRQOL indicators. Symptoms of depression or anxiety were found to be significantly associated with all seven unfavorable OHRQOL responses in the unadjusted regression models. Having anterior caries in more than five teeth surfaces, root caries in more than 3 teeth, and experiencing painful tooth/sores in mouth were significantly associated with unfavorable responses to all 7 OHRQOL indicators as well.
Table 2.
Unadjusted logistic regression models showing relationships between socio-demographic, behavioral, oral health status, and methamphetamine use indicators and unfavorable oral health-related quality of life outcomes in the last year
| Indicators | Painful aching in mouth |
Less satisfying life because of dental problem |
Difficulty doing usual work/activity |
Affected sense of taste | Particular food avoided |
Uncomfortable to eat | Felt embarrassed |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age | |||||||
| < 45 yearsR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥ 45 years | 1.33 (0.95–1.88) | 1.72 (1.22–2.43) | 1.38 (0.95–2.00) | 1.72 (1.19–2.47) | 1.31 (0.93–1.84) | 1.36 (0.96–1.93) | 1.53 (1.08–2.16) |
| Sex | |||||||
| FemaleR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 0.81 (0.52–1.26) | 0.94 (0.67–1.42) | 0.77 (0.49–1.22) | 0.98 (0.62–1.53) | 0.63 (0.41–.99) | 0.73 (0.46–1.15) | 0.91 (0.56–1.42) |
| Race/ethnicity | |||||||
| WhiteR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 1.76 (1.07–2.89) | 0.58 (0.36–0.96) | 0.99 (0.57–1.70) | 1.54 (0.90–2.63) | 1.29 (0.79–2.11) | 1.21 (0.74–2.00) | 1.21 (0.74–1.99) |
| African-American | 1.56 (0.98–2.29) | 1.04 (0.65–1.65) | 1.22 (0.73–2.23) | 1.45 (0.87–2.43) | 1.82 (1.14–2.91) | 1.63 (1.01–2.63) | 1.27 (0.79–2.04) |
| Other | 1.25 (0.58–2.68) | 0.77 (0.36–1.67) | 0.97 (0.42–2.26) | 1.40 (0.61–3.16) | 1.99 (0.91–4.36) | 1.19 (0.55–2.60) | 0.95 (0.44–2.04) |
| Education | |||||||
| High schoolR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Less than HS | 1.34 (0.87–2.08) | 1.46 (0.93–2.22) | 1.77 (1.13–2.76) | 1.0.6533 (0.86–2.04) | 1.21 (0.79–1.86) | 0.85 (0.54–1.34) | 1.08 (0.69–1.66) |
| More than HS | 0.72 (0.48–1.08) | 1.05 (0.70–1.58) | 0.81 (0.51–1.28) | 0.65 (0.42–1.00) | 0.67 (0.45–1.00) | 0.45 (0.29-.67) | 0.73 (0.49–1.10) |
| Marital status | |||||||
| Ever married/partnerR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Never married | 1.01 (0.69–1.47) | 0.75 (0.51–1.09) | 0.94 (0.63–1.41) | 0.87 (0.59–1.28) | 0.82 (0.56–1.20) | 0.84 (0.56–1.23) | 1.01 (0.69–1.47) |
| Country of birth | |||||||
| OtherR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| United States | 0.61 (0.38–1.00) | 1.34 (0.84–2.14) | 0.84 (0.51–1.36) | 0.94 (0.58–1.52) | 1.41 (0.89–2.22) | 1.08 (0.67–1.73) | 1.05 (0.66–1.68) |
| Language used at home | |||||||
| EnglishR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Spanish | 2.26 (1.17–4.34) | 0.62 (0.34–1.13) | 1.12 (0.61–2.06) | 1.40 (0.78–2.51) | 1.02 (0.57–1.82) | 1.41 (0.75–2.65) | 1.22 (0.67–2.22) |
| Both | 1.57 (0.75–3.30) | 0.49 (0.23–1.05) | 0.71 (0.31–1.60) | 0.86 (0.40–1.84) | 0.59 (0.29–1.19) | 0.57 (0.28–1.15) | 0.73 (0.36–1.46) |
| Cigarette smoking | |||||||
| Never smokedR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current smoker | 1.08 (0.71–1.64) | 1.77 (1.15–2.72) | 1.81 (1.11–2.93) | 1.35 (0.87–2.11) | 1.80 (1.19–2.72) | 1.90 (1.26–2.89) | 1.29 (0.85–1.96) |
| Former smoker | 1.17 (0.60–2.27) | 1.32 (0.68–2.58) | 1.48 (0.71–3.12) | 0.91 (0.44–1.88) | 2.34 (1.19–4.60) | 2.14 (1.08–4.26) | 1.15 (0.60–2.23) |
| HIV status | |||||||
| NegativeR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 1.43 (0.96–2.13) | 1.11 (0.75–1.63) | 1.04 (0.67–1.58) | 1.43 (0.96–2.14) | 1.00 (0.68–1.47) | 1.19 (0.80–1.78) | 1.18 (0.79–1.75) |
| Depression/anxiety | |||||||
| NoR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.61 (1.14–2.27) | 1.93 (1.37–2.71) | 1.97 (1.36–2.80) | 2.97 (2.05–4.31) | 2.02 (1.43–2.85) | 2.08 (1.46–2.98) | 1.81 (1.28–2.56) |
| Severe periodontitis | |||||||
| NoR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.03 (0.68–1.57) | 1.12 (0.74–1.70) | 1.13 (0.73–1.76) | 0.99 (0.64–1.53) | 1.20 (0.79–1.82) | 1.10 (0.72–1.69) | 1.40 (0.91–2.17) |
| Anterior caries ≥ 5 | |||||||
| NoR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.57 (0.99–2.49) | 2.23 (1.43–3.47) | 1.73 (1.10–2.71) | 1.86 (1.19–2.89) | 1.92 (1.21–3.05) | 2.51 (1.50–4.21) | 3.09 (1.83–5.22) |
| Root caries ≥ 3 | |||||||
| NoR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.95 (1.30–2.93) | 2.90 (1.82–4.58) | 3.20 (1.85–5.56) | 1.75 (1.10–2.77) | 1.74 (1.16–2.61) | 3.07 (2.02–4.66) | 1.76 (1.17–2.65) |
| Painful tooth/ sores in past 30 days | |||||||
| NoR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 5.98 (4.08–8.75) | 2.67 (1.89–3.79) | 6.07 (4.96–9.30) | 3.38 (2.31–4.94) | 4.46 (3.10–6.41) | 4.63 (3.16–6.78) | 2.27 (1.60–3.23) |
| Route of MA# | |||||||
| Not smokingR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Smoking | 0.86 (0.59–1.25) | 0.91 (0.63–1.32) | 0.89 (0.60–1.32) | 0.58 (0.40–.85) | 0.78 (0.54–1.13) | 0.78 (0.53–1.15) | 0.74 (0.51–1.09) |
| MA use | |||||||
| LightR | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate/heavy | 1.27 (0.90–1.80) | 1.07 (0.76–1.50) | 1.28 (0.88–1.85) | 1.58 (1.10–2.27) | 1.45 (1.02–2.01) | 1.41 (0.99–2.00) | 1.06 (0.75–1.50) |
OR in bold suggest p-values < 0.05
OR odds ratio; 95% CI 95% confidence interval; R reference group
Results of multivariable modeling for all 7 unfavorable OHRQOL responses indicated that only an unfavorable response to affected sense of taste was associated with MA use and these results are shown in Table 3. In the multivariable model (all covariates with p value < 0.1 identified from the unadjusted models), there was a weak association between frequency of MA use and unfavorable response to affected sense of taste as moderate/heavy users were nearly 50% more likely to report altered taste sensation than light users [OR 1.49; 95% CI (1.00, 2.23)]. Participants smoking MA were less likely to report an unfavorable response to affected sense of taste [OR 0.62; 95% CI (0.41, 0.96)] compared to participants using other routes. Participants aged 45 and over were 57% more likely to report an unfavorable response to affected sense of taste than their younger counterparts [OR 1.57; 95% CI (1.04, 2.38)]. Having more than high school education, symptoms of depression or anxiety, and painful tooth/sores in the past 30 days were also significantly associated with altered taste sensation. In the reduced/parsimonious model, moderate/heavy MA users were 1.53 times as likely to report an altered sense of taste compared to light users [OR 1.53; 95% CI (1.03, 2.28)]. Participants who smoked MA were 38% less likely to report an altered sense of taste than participants who used other routes [OR 0.62; 95% CI (0.41, 0.94)]. Participants aged 45 and over had higher odds of reporting an unfavorable response to affected sense of taste compared to participants aged less than 45 years [OR 1.73; 95% CI (1.17, 2.58)]. Experiencing painful tooth/sores in the past 30 days [OR 3.11; 95% CI (2.08, 4.64)] was associated with greater likelihood of reporting affected sense of taste when compared to their counterparts. Higher educational attainment was inversely associated with affected sense of taste (p value 0.02).
Table 3.
Associations of methamphetamine use and key socio-demographic, behavioral, and oral health covariates with unfavorable response to affected sense of taste
| Independent variables | Unfavorable response to affected sense of taste |
||
|---|---|---|---|
| Unadjusted models OR (95% CI) |
Multivariable modela OR (95% CI) |
Parsimonious modelb OR (95% CI) |
|
| MA use | |||
| LightR | 1.00 | 1.00 | 1.00 |
| Moderate/heavy | 1.58 (1.10–2.27) | 1.49 (1.00–2.23) | 1.53 (1.03–2.28) |
| Route of MA | |||
| Not smokingR | 1.00 | 1.00 | 1.00 |
| Smoking | 0.58 (0.40–0.85) | 0.62 (0.41–0.96) | 0.62 (0.41–0.94) |
| Age | |||
| < 45 yearsR | 1.00 | 1.00 | 1.00 |
| ≥ 45 years | 1.72 (1.19–2.47) | 1.57 (1.04–2.38) | 1.73 (1.17–2.58) |
| Education | |||
| High schoolr | 1.00 | 1.00 | 1.00 |
| Less than HS | 1.33 (0.86–2.04) | 1.17 (0.72–1.89) | 1.18 (0.73–1.89) |
| More than HS | 0.65 (0.42–1.00) | 0.62 (0.38–1.00) | 0.59 (0.37–0.96) |
| HIV status | |||
| NegativeR | 1.00 | 1.00 | |
| Positive | 1.43 (0.96–2.14) | 1.51 (0.97–2.35) | |
| Depression/anxiety | |||
| NoR | 1.00 | 1.00 | 1.00 |
| Yes | 2.97 (2.05–4.31) | 2.64 (1.77–3.94) | 2.60 (1.75–3.87) |
| Anterior caries ≥ 5 | |||
| NoR | 1.00 | 1.00 | |
| Yes | 1.86 (1.19–2.89) | 1.46 (0.88.–2.43) | |
| Root caries ≥ 3 | |||
| NoR | 1.00 | 1.00 | |
| Yes | 1.75 (1.10–2.77) | 1.12 (0.66–1.90) | |
| Painful tooth/sores in past 30 days | |||
| NoR | 1.00 | 1.00 | 1.00 |
| Yes | 3.38 (2.31–3.4.94) | 3.14 (2.09–4.72) | 3.11 (2.08–4.64) |
OR in bold suggest p-values < 0.05
OR odds ratio; 95% CI 95% confidence interval; R reference group
Adjusted for: MAuse, Route of MA, Age, Education, HIV status, Anterior caries, Root caries, Depression/anxiety, and pain/sores in past 30 days
Fitted model after running backwards selection on Pre–Planned Multiple–Predictor Model; order of variables removed: Root caries, Anterior caries, HIV status
Further analyses identified a significant interaction between route of MA administration and frequency of MA use (p value 0.03). For participants who smoked MA, no significant association was observed between frequency of MA use and affected sense of taste when stratified by route of MA administration (Table 4). However, in participants preferring other routes of MA administration, moderate/heavy users were more than 3 times as likely to report affected sense of taste than light users [OR = 3.09, 95% CI (1.52, 6.27)] in the parsimonious model. Symptoms of depression or anxiety and presence of a painful tooth/sores in the past 30 days were significantly associated with affected sense of taste in both groups (MA smokers and other routes) in all the models.
Table 4.
Associations of methamphetamine use and key socio-demographic, behavioral, and oral health covariates with affected sense of taste, stratified by route of methamphetamine administration
| Unfavorable response to affected sense of taste |
||||||||
|---|---|---|---|---|---|---|---|---|
| People who smoke MA (n = 382) |
Other routes of use (n = 162) |
|||||||
| (n) | Unadjusted model OR (95% CI) |
Multivariable model OR (95% CI) |
Parsimonious model OR (95% CI) |
(n) | Unadjusted model OR (95% CI) |
Multivariable model OR (95% CI) |
Parsimonious model OR (95% CI) |
|
| MA use | ||||||||
| LightR | 172 | 1.00 | 1.00 | 1.00 | 70 | 1.00 | 1.00 | 1.00 |
| Mod/heavy | 210 | 1.22 (0.78–1.90) | 1.07 (0.65–1.77) | 1.12 (0.69–1.83) | 92 | 2.73 (1.41–5.28) | 3.05 (1.47–6.32) | 3.09 (1.52–6.27) |
| Age | ||||||||
| < 45 yearsR | 184 | 1.00 | 1.00 | 1.00 | 68 | 1.00 | 1.00 | |
| ≥ 45 years | 198 | 1.89 (1.21–2.97) | 1.70 (1.02–2.82) | 1.87 (1.15–3.04) | 94 | 1.31 (0.69–2.47) | 1.20 (0.57–2.54) | |
| Education | ||||||||
| HSR | 132 | 1.00 | 1.00 | 1.00 | 62 | 1.00 | 1.00 | |
| < HS | 117 | 1.39 (0.82–2.35) | 1.19 (0.66–2.13) | 1.18 (0.66–2.10) | 41 | 1.36 (0.62–3.01) | 1.03 (0.43–2.44) | |
| > HS | 133 | 0.64 (0.37–1.12) | 0.54 (0.29–0.99) | 0.52 (0.28–0.95) | 59 | 0.67 (0.32–1.39) | 0.79 (0.35–1.80) | |
| HIV status | ||||||||
| NegativeR | 285 | 1.00 | 1.00 | 119 | 1.00 | 1.00 | ||
| Positive | 97 | 1.59 (0.98–2.60) | 1.57 (0.91–2.72) | 43 | 1.13 (0.56–2.29) | 1.29 (0.59–2.84) | ||
| Depression/anxiety | ||||||||
| NoR | 208 | 1.00 | 1.00 | 1.00 | 72 | 1.00 | 1.00 | 1.00 |
| Yes | 174 | 3.04 (1.92–4.80) | 2.69 (1.65–4.39) | 2.69 (1.66–4.37) | 92 | 2.65 (1.38–5.11) | 2.67 (1.29–5.51) | 2.48 (1.23–5.01) |
| Anterior caries ≥ 5 | ||||||||
| NoR | 317 | 1.00 | 1.00 | 127 | 1.00 | 1.00 | ||
| Yes | 65 | 2.24 (1.30–3.87) | 1.76 (0.94–3.30) | 35 | 1.22 (0.57–2.58) | 1.11 (0.45–2.72) | ||
| Root caries ≥ 3 | ||||||||
| NoR | 91 | 1.00 | 1.00 | 28 | 1.00 | 1.00 | ||
| Yes | 291 | 1.43 (0.83–2.45) | 0.98 (0.53–1.82) | 134 | 2.51 (1.00–6.29) | 2.14 (0.75–6.12) | ||
| Painful tooth/ sores or irritation in past 30 days | ||||||||
| NoR | 193 | 1.00 | 1.00 | 1.00 | 78 | 1.00 | 1.00 | 1.00 |
| Yes | 189 | 3.77 (2.34–6.08) | 3.70 (2.22–6.15) | 3.69 (2.23–6.11) | 84 | 2.76 (1.44–5.28) | 2.38 (1.17–4.87) | 2.58 (1.29–5.14) |
OR in bold suggest p-values < 0.05
OR odds ratio; 95% CI 95% confidence interval; R reference group
Discussion
In a group of MA users from Los Angeles County, unfavorable responses to painful aching in the mouth, avoidance of particular food items, discomfort while eating, and feeling embarrassed in the last 12 months were reported by more than half of the study participants. At least 30% of the participants reported unfavorably to less satisfying life, difficulty doing usual work, and affected sense of taste. Increased frequency of MA use and smoking as the preferred route of MA administration was found to be significantly associated with affected sense of taste. Having symptoms of depression or anxiety and painful tooth/sores in the past 30 days were also found to be associated with unfavorable responses in all 7 indicators of OHRQOL, in the OHIP scale.
Methamphetamine use is generally thought to have a negative impact on physical health and oral health status of individuals and MA users have reported poor quality of life in general [27]. Our study shows that OHRQOL is not very different from QOL in general. Information is sparse regarding OHRQOL in illicit drug users. Truong and colleagues have shown that a group of illicit drug users in Australia have poorer OHRQOL when compared to non-users with similar socio-demographic characteristics [20]. Findings from our study, which focused entirely on MA users, were consistent with Truong’s findings. Due to the lack of a suitable non-MA-using control group, we could not compare MA users from non-MA users in our analysis. However, when comparing findings from a separate study using the OHRQOL instrument from NHANES, which was based on the shortened version of the OHIP, Seirawan and colleagues observed that 18.9% of the US general population reported unfavorably to painful aching in last 12 months, compared to 59.5% among MA users in our study [28]. Discomfort while eating was reported by 17.2% in the US population compared to 63.5% among MA users; 12.6% reported feeling embarrassed due to dental problems in the US overall, whereas 60.7% of MA users reported feeling embarrassed in this study. For affected sense of taste and for avoidance of particular foods, the difference was substantial between the US population and MA users in this study (4.4 vs. 33.2% and 16.3 vs. 56.5%, respectively) [28]. In general, these comparisons indicate that MA users have poorer OHRQOL than the general population.
Affected sense of taste was found to be significantly associated with increased frequency of MA use, as well as with route of MA administration. Like other MA-using population, majority of the MA users in this study preferred smoking as the principle route of MA administration [29]. We found route of administration to be an effect modifier for affected sense of taste. Unlike participants who smoked MA, moderate/heavy users who administered MA by routes other than smoking were three times as likely to report unfavorable OHRQOL compared to light users. Methamphetamine, when administered intravenously, reaches high plasma concentration rapidly and this is followed by a period of relaxation and marked feeling of confidence and well-being. This immediate sense of euphoria encourages individuals to administer MA in higher doses and thus eventually they develop abuse-related problems [30]. Continued presence of MA is more likely to aggravate xerostomia and reduced salivary functions which might result in affected sense of taste. This suggests the possibility of a dose-response relationship in participants who administer MA by routes other than smoking like oral, intravenous, intranasal, and snorting. However, this finding contrasts with Truong’s findings where he reported absence of any dose–response relation in heroin users [20].
The association between poor QOL and depression is well documented. Prior research has shown that individuals with depression were more likely to report poor QOL in general [31]. The same association persists for OHRQOL as well. Having symptoms of depression or anxiety was associated with unfavorable response to all aspects of self-reported OHRQOL. Similar to poor general QOL, MA users who reported of being HIV positive had poor OHRQOL. Education, employment, and economic status are some of the factors known to be associated with individuals’ perception of health and quality of life. OHRQOL is not much different from QOL in general. In this study, greater educational attainment was found to be associated with better OHRQOL.
In their OHRQOL model, Sischo and Broder proposed ‘oral symptoms’ as one of the contributing factors affecting OHRQOL [32]. Our study supports that rationale. Methamphetamine users who experienced dental problems and painful tooth/oral sores in the past 30 days were significantly more likely to report unfavorable OHRQOL. In our study, 78.9% of the participants had root caries in more than 3 teeth, and severe periodontitis was detected in 21.1% of the participants, reflecting higher prevalence than estimated in the US general population. Using NHANES data, Kim and colleagues reported root caries in 10% and periodontitis in 7% of the US general population [33]. This suggests that oral health is a highly neglected issue in the MA-using population. Increased root caries, severe periodontitis, painful teeth, and presence of sores along with symptoms of depression and anxiety raise questions of health care priorities and access to care in this population.
There are some limitations with our study. As mentioned in the OHIP, we did not have information on MA use for the last 12 months; instead, we used information for the past 30 days. That might have resulted in an under-estimation of the actual association between MA use and OHRQOL. Sufficient information on employment and economic status of the study participants could not be obtained. As socioeconomic status is known to be related with both quality of life and illicit drug use, it would have been interesting to measure how employment and economic status could affect OHRQOL in MA users. Because of the absence of a randomized study population and a suitable non-MA-using comparison group, as well as of the cross-sectional design of this study, the direction of the relationships observed could not be determined. We did not have any information on other illicit substances used by the study participants. Drug–drug interactions often play a significant role in participants’ physical and mental health, perception of health status, and quality of life. We could not assess if this was true in case of OHRQOL as well. Self-reported nature of our outcome of interest and symptoms of depression/anxiety status might have biased the true association between these two variables. Finally, the majority of our study participants used smoking as the route of MA administration; we did not have enough data to assess how differently other routes of MA administration like oral, intranasal, and injection affected OHRQOL in this population. Nevertheless, this is the first study assessing OHRQOL in a large sample of MA users. Measuring OHRQOL by the widely used OHIP addresses issues of internal validity and reliability of our outcome measure and the diverse nature of our study population suggests that our findings are generalizable to other MA-using populations in the US. Understanding how MA use impacts individuals’ perceptions of oral health and satisfaction beyond basic health status can help guide future public health research involving MA abuse and identify areas where behavioral health interventions could be explored.
This research represents the first MA-specific study to assess the association between MA use and self-reported OHRQOL in a largest sample of users. Findings from this study have important practical implications for public health and dental practice by highlighting factors that affect perception and value of oral health in an illegal substance using population. Information from this study could assist public health and social service workers, health care providers and policymakers in creating screening, drug prevention, education and treatment interventions, as well as in improving access to oral care in this underserved, high-risk population.
Funding
Parent study Grant Number: R01 DA025680. Granting agency: National Institute on Drug Abuse, National Institutes of Health (Recipient: Dr. Vivek Shetty).
Footnotes
Conflict of interest The authors declare they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.NIDA (2018). Methamphetamine. 2018, June 6 [cited 2018 June 26]; Retrieved from https://www.drugabuse.gov/publications/drugfacts/methamphetamine. [Google Scholar]
- 2.Baumgarten HG, & Lachenmayer L (2004). Serotonin neurotoxins–past and present. Neurotox Research, 6(7–8), 589–614. [DOI] [PubMed] [Google Scholar]
- 3.Cadet JL, Jayanthi S, & Deng X (2003). Speed kills: Cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 17(13), 1775–1788. [DOI] [PubMed] [Google Scholar]
- 4.Itzhak Y, & Achat-Mendes C (2004). Methamphetamine and MDMA (ecstasy) neurotoxicity: ‘of mice and men’. IUBMB Life, 56(5), 249–255. [DOI] [PubMed] [Google Scholar]
- 5.Sulzer D, et al. (2005). Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol, 75(6), 406–433. [DOI] [PubMed] [Google Scholar]
- 6.NSDUH (2016). 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration. 2017 June 6, 2018.]; Retrieved from https://nsduhweb.rti.org/respweb/homepage.cfm. [PubMed] [Google Scholar]
- 7.Klasser GD, & Epstein J (2005). Methamphetamine and its impact on dental care. J Can Dent Assoc, 71(10), 759–762. [PubMed] [Google Scholar]
- 8.Beebe DK, & Walley E (1995). Smokable methamphetamine (‘ice’): An old drug in a different form. American Family Physician, 51(2), 449–453. [PubMed] [Google Scholar]
- 9.Yu Q, Larson DF, & Watson RR (2003). Heart disease, methamphetamine and AIDS. Life Science, 73(2), 129–140. [DOI] [PubMed] [Google Scholar]
- 10.Shetty V, et al. (2015). Dental disease patterns in methamphetamine users: Findings in a large urban sample. Journal of the American Dental Association, 146(12), 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Communications, & ADADo, Journal of the American Dental Association, Affairs ADADoS (2005). For the dental patient … methamphetamine use and oral health. Journal of the American Dental Association, 136(10), 1491. [DOI] [PubMed] [Google Scholar]
- 12.Shaner JW (2002). Caries associated with methamphetamine abuse. Journal Michigan Dental Association, 84(9), 42–47. [PubMed] [Google Scholar]
- 13.McGrath C, & Chan B (2005). Oral health sensations associated with illicit drug abuse. British Dental Journal, 198(3): p. 159 – 62; dicussion 147; quiz 174. [DOI] [PubMed] [Google Scholar]
- 14.Morio KA, et al. (2008). Comparing diet, oral hygiene and caries status of adult methamphetamine users and nonusers: A pilot study. Journal of the American Dental Association, 139(2), 171–176. [DOI] [PubMed] [Google Scholar]
- 15.Shetty V, et al. (2010). The relationship between methamphetamine use and increased dental disease. Journal of the American Dental Association, 141(3), 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravenel MC, et al. (2012). Methamphetamine abuse and oral health: A pilot study of “meth mouth”. Quintessence International, 43(3), 229–237. [PubMed] [Google Scholar]
- 17.Rommel N, et al. (2016). The impact of the new scene drug “crystal meth” on oral health: A case-control study. Clinical Oral Investigations, 20(3), 469–475. [DOI] [PubMed] [Google Scholar]
- 18.Boyer EM, et al. (2015). The relationship between methamphetamine use and dental caries and missing teeth. The Journal of Dental Hygiene, 89(2), 119–131. [PubMed] [Google Scholar]
- 19.Smit DA, & Naidoo S (2015). Oral health effects, brushing habits and management of methamphetamine users for the general dental practitioner. British Dental Journal, 218(9), 531–536. [DOI] [PubMed] [Google Scholar]
- 20.Truong A, et al. (2015). Oral health-related quality of life among an Australian sample of people who inject drugs. Journal of Public Health Dentistry, 75(3), 218–224. [DOI] [PubMed] [Google Scholar]
- 21.Inglehart MR (2002). Oral Health-Related Quality of Life. Oral health-related quality of life: An introduction, (p. 244). Chicago, IL: Quintessence Publishing. [Google Scholar]
- 22.USHHS (2000). Oral Health in America: A Report of the Surgeon General. 2000 [cited 2016 December 30]; Retrieved from https://www.nidcr.nih.gov/DataStatistics/SurgeonGeneral/. [Google Scholar]
- 23.Dye BA, et al. (2015). Performance of a quality assurance program for assessing dental health in methamphetamine users. BMC Oral Health, 15, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slade G (1997). Derivation and validation of a short-form oral health impact profile. Community Dentistry and Oral Epidemiology, 25(4), 284–290. [DOI] [PubMed] [Google Scholar]
- 25.Sanders AE, et al. (2009). Impact of oral disease on quality of life in the US and Australian populations. Community Dentistry and Oral Epidemiology, 37(2), 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eke PI, et al. (2012). Update of the case definitions for population-based surveillance of periodontitis. Journal of Periodontology, 83(12), 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzales R, et al. (2011). Quality of life among treatment seeking methamphetamine-dependent individuals. The American Journal on Addictions, 20(4), 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seirawan H, Sundaresan S, & Mulligan R (2011). Oral health-related quality of life and perceived dental needs in the United States. Journal of Public Health Dentistry, 71(3), 194–201. [PubMed] [Google Scholar]
- 29.Gonzales R, et al. (2009). Health-related quality of life trajectories of methamphetamine-dependent individuals as a function of treatment completion and continued care over a 1-year period. The Journal of Substance Abuse Treatment, 37(4), 353–361. [DOI] [PubMed] [Google Scholar]
- 30.McLellan AT, et al. (2000). Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA, 284(13), 1689–1695. [DOI] [PubMed] [Google Scholar]
- 31.Jones DR, et al. (2004). Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatric Services, 55(11), 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sischo L, & Broder HL (2011). Oral health-related quality of life: What, why, how, and future implications. Journal of Dental Research, 90(11), 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JK, et al. (2012). Prevalence of oral health problems in U.S. adults, NHANES 1999–2004: Exploring differences by age, education, and race/ethnicity. Special Care Dentistry, 32(6), 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


