Abstract
Background
Inhibition of the programmed death ligand 1, programmed death 1 pathway has been successfully used for treatment of multiple advanced adult cancers. However, its use in pediatric osteosarcoma is still in its infancy. In this study, we investigated programmed death ligand 1 and other checkpoint molecules' expression to determine the potential usefulness as targets for drug therapy.
Methods
We incubated human wild-type osteosarcoma cells with incremental concentrations of doxorubicin to create a doxorubicin-resistant cell line. Matrigel in vitro invasion assays were used to compare invasiveness. Comparative programmed death ligand 1 expression was evaluated by Western blot assays. An immuno-oncology checkpoint protein panel was used to compare concentrations of 16 other checkpoint molecules. Chi-square tests and Wilcoxon rank-sum tests were used to determine significant differences.
Results
A doxorubicin-resistant cell line was successfully created and was significantly more invasive than wild-type cells (0.47 vs 0.07, P < .001). On Western blot assay, doxorubicin-resistant but not wild-type cells expressed programmed death ligand 1. Doxorubicin-resistant cells had significantly higher levels of T-cell immunoglobulin-3 and cluster of differentiation 86 and higher cluster of differentiation 27, cluster of differentiation 40, lymphocyte-activation gene-3, cluster of differentiation 80, programmed death ligand 1, programmed death ligand 2, and inducible T-cell costimulatory expression than wild-type cells. Both lines expressed B- and T-lymphocyte attenuator, cluster of differentiation 28, herpesvirus entry mediator, and programmed death 1. Herpesvirus entry mediator, cluster of differentiation 40, and programmed death ligand 2 were also present in the culture media of both cell lines.
Conclusion
Doxorubicin-resistant osteosarcoma seems to express higher programmed death ligand 1 than nonresistant wild-type cells. Benchmarking checkpoint molecules may provide the basis for future studies that elucidate pathways of drug resistance and tumor metastasis, biomarkers for cancer prognosis or recurrence, and future targets for directed drug therapy.
Abbreviations: PD-1, programmed death 1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; LAG-3, lymphocyte-activation gene-3; TIM-3, T-cell immunoglobulin-3; BTLA, B- and T-lymphocyte attenuator; CD28, cluster of differentiation 28; WT, wild type; DoxR, doxorubicin resistant; CD27, cluster of differentiation 27; CD40, cluster of differentiation 40; CD80, cluster of differentiation 80; CD86, cluster of differentiation 86; HVEM, herpesvirus entry mediator; ICOS, inducible T-cell costimulatory (ICOS); GITR, glucocorticoid-induced TNFR-related protein; GITRL, ligand for receptor TNFRSF18/AITR/GITR; TLR-2, Toll like receptor 2; FDA, Food and Drug Administration
INTRODUCTION
Osteosarcoma is the most common primary bone cancer in childhood, with an estimated incidence of 3.1 cases per million, and is often lethal [1]. When possible, complete resection is the most important component of treatment, often accompanied with doxorubicin and cisplatin or high-dose methotrexate, cisplatin, and doxorubicin as neoadjuvant or adjuvant therapies for better outcomes [[1], [2], [3]]. Nevertheless, resistance to these agents is a major barrier to cure, with 5-year survival rates less than 30% when recurrent or metastatic [1,4]. Therefore, novel, effective therapies for advanced disease are urgently needed.
Inhibition of immunomodulatory checkpoint molecules, inhibitory cell receptors that dampen the unwanted imune response against healthy cells, has been a successful novel treatment for chemoresistant and metastatic cancer in adults [[5], [6], [7]]. Cancer can hijack these regulatory pathways to prevent immune recognition and destruction [7]. One of the most well-known pathways is the interaction between programmed death 1 (PD-1) and its ligands. PD-1 is a tyrosine-kinase receptor protein expressed by B- and T-lymphocytes that inhibits T-cell proliferation when activated by either of its ligands: programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) [[8], [9], [10]]. This is an interaction of particular interest because multiple drugs that block this pathway are already on the market for treatment of advanced melanoma, non–small cell lung cancer, and Hodgkin's lymphoma, among others [[11], [12], [13], [14], [15]]. However, many novel immunotherapies that inhibit other checkpoint pathways have been, or are being, developed and have the potential to be effective cancer treatments too, such as inhibition of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte-activation gene-3 (LAG-3), T-cell immunoglobulin-3 (TIM-3), and B- and T-lymphocyte attenuator (BTLA), to name a few [7,[16], [17], [18], [19], [20]].
The use of checkpoint molecule inhibition in pediatric solid tumors is still in its infancy, with only a few phase I/II trials underway and conflicting evidence as to which checkpoint molecules are expressed across the myriad of different tumor types [18,21,22]. Therefore, our primary goal was to investigate the expression of PD-L1 with a doxorubicin-resistant (DoxR) osteosarcoma cell line to determine the potential usefulness as a target for drug therapy. Second, we sought to identify and benchmark additional checkpoint proteins that may be expressed to guide future studies on the mechanisms of drug resistance and metastasis, potential use as biomarkers for prognosis, and evaluation of targeted drug treatments as novel immunotherapies are continuing to be developed. We hypothesized that PD-L1 expression and select checkpoint molecule expression would be greater in the drug-resistant cell line and, therefore, potentially serve as mechanisms for drug resistance or markers of advanced disease.
MATERIALS AND METHODS
Reagents
The SJSA-1 osteosarcoma cell line was purchased from the American Type Culture Collection (ATCC). Dulbecco's modified Eagle's medium (DMEM) and heat-inactivated fetal bovine serum (FBS) were obtained from Cellgro, whereas penicillin and streptomycin were obtained from HyClone. Doxorubicin and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Rabbit monoclonal antibodies for PD-L1 (clone E1L3N) were obtained from Cell Signaling Technology, and monoclonal mouse anti–β-actin (clone AC-15) was from Sigma-Aldrich. Secondary goat anti-rabbit immunoglobin G (IgG)-HRP (W401B) and goat anti-mouse IgG-HRP (W402B) monoclonal antibodies were purchased from Promega. Enhanced chemiluminescence reagents were obtained from Thermo Fisher Scientific (32106).
Cell culture, drug treatment, and cytotoxicity assay
Cells were cultured in a humidified incubator maintained at 37°C and 5% CO2 environment. Cells were cultured in complete medium (DMEM supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin). DoxR cells were generated by incubating parental WT cells with incremental concentrations of doxorubicin ranging from 1 nM to 1 μM over a 6-month period. Treatment began with 1 nM and was increased to the next 10-fold increment after surviving 5 consecutive passages. Cells were considered to be resistant after surviving 5 consecutive passages in 1 μM doxorubicin. Cell viability was determined by the quantitative colorimetric MTT assay according to Boehringer Mannheim (Cat. No. 1465007) as previously described [23].
In vitro invasion assay
Cell invasion was determined and analyzed using a membrane invasion culture system (BD BioCoat Growth Factor Reduced BD Matrigel; BD Biosciences). The number of cells able to invade through a membrane coated with the defined Matrigel extracellular matrix during a 24-hour period was compared to the number counted using a control insert with no Matrigel. Cells were seeded at 2.5 × 104 and incubated for 24 hours. Cells that migrated through the membrane were fixed and stained with a Diff-Quik staining kit (Allegiance Catalog #B4132-1A). Three fields at 40 × magnification were counted by light microscopy. All experiments were repeated in triplicate by different researchers and reported as the number of cells on the membrane divided by the number on the control membrane (mean ± standard error). The cells were also counted by 3 separate researchers with similar results that were averaged. Statistical difference in invasion was determined using χ2 tests, SPSS 26 (Armonk, NY).
Western blotting
WT and DoxR cells were seeded in complete medium and cultured for 48 hours. Cells were lysed using NP40 Cell Lysis Buffer (Thermo Fisher Scientific) with Protease Inhibitor Cocktail (Sigma-Aldrich P8340). Total protein concentration was determined using the bicinchoninic acid assay (BCA) assay (Thermo Fisher Scientific) using the supplied albumin as the analytical standard. Equal amounts of protein were reduced in 1 × sample buffer (Laemmli, Bio-Rad, #161-0737, with 5% β-mercaptoethanol) boiled for 5 minutes, separated by electrophoresis on 4%–20% SDS-PAGE gels (Bio-Rad), and transferred onto Immobilon-P membranes (Millipore). Proteins of interest were identified with specific primary antibodies followed by HRP-conjugated secondary antibodies. Immunoreactive bands were detected by chemiluminescence with image capture on an iBright CL 1500 Imaging System (Thermo Fischer Scientific).
Human Immuno-Oncology Checkpoint Protein Panel
Proteins from cell lysates and conditioned media were collected in a 10% protease inhibitors cocktail (Sigma-Aldrich) with RIPA buffer and DMEM, respectfully, and then tested with the Human Immuno-Oncology Checkpoint Protein Panel (MilliporeSigma). This panel consists of 17 checkpoint molecules, which include PD-1, PD-L1, PD-L2, CTLA-4, LAG-3, TIM-3, BTLA, cluster of differentiation (CD) 27 (CD27), CD28, CD40, CD80, CD86, herpesvirus entry mediator (HVEM), inducible T-cell costimulatory (ICOS), glucocorticoid-induced TNFR-related protein (GITR), ligand for receptor TNFRSF18/AITR/GITR (GITRL), and toll-like receptor 2 (TLR-2). All primary data points were collected via the Luminex FLEXMAP 3D system, and protein concentrations were calculated using a 5-parametric fit algorithm (xPONENT v4.0.3 Luminex Corp, Austin, TX). All samples were run in triplicate using lysates or media from different passages. Statistical differences were determined using Wilcoxon rank-sum tests, SPSS 26 (Armonk, NY).
RESULTS
Doxorubicin-resistant cells are more invasive than their parental WT cells
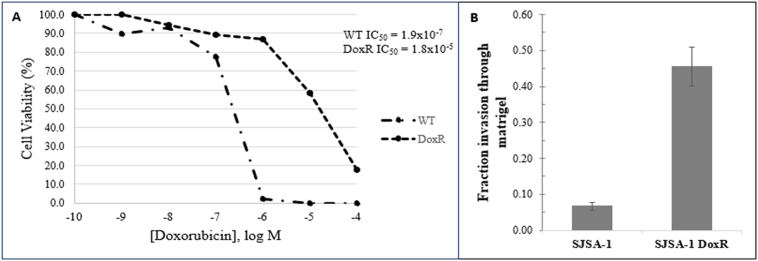
DoxR cells were determined to be resistant by MTT assay after surviving 5 consecutive passages in 1 μM doxorubicin and had half-maximal inhibitory concentrations 2 log greater than their parental, doxorubicin-sensitive, WT cells. Cell viability was determined by the quantitative colorimetric MTT assay (Fig 1, A). The Matrigel in vitro invasion assays were used to compare the invasiveness of human osteosarcoma SJSA-1 DoxR cells to their parental WT cell lines and demonstrated that DoxR cell lines were significantly more invasive than parental cells (fraction of invasion 0.455 vs 0.056, P < .001) (Fig 1, B).
Fig 1.
Cell viability assay demonstrating the doxorubicin resistant cell line is resistant compared to wild-type cells (A) and Matrigel in vitro invasion assay (mean and standard error) demonstrating that doxorubicin-resistant cells are more invasive compared to their parental WT cells (B). Data are representative of 3 independent experiments.
Doxorubicin-resistant cells express PD-L1, and osteosarcoma cells express multiple checkpoint molecules
The PD-L1 protein level from whole cell lysates was upregulated in DoxR cells compared to WT SJSA-1 cells (Fig 2). Cell lysates of both WT and DoxR cell lines expressed 13 out of the 17 checkpoint molecules that include BTLA, CD27, CD28, TIM-3, HVEM, CD40, LAG-3, PD-1, CD80, CD86, PD-L1, PD-L2, and ICOS, whereas GITR, TLR-2, and GITRL were below the assay detection limit and CTLA-4 was only expressed in the WT cell line. DoxR cells had significantly higher levels of TIM-3 and CD86, although CD40, LAG-3, and PD-L1 approached significance with higher levels in the DoxR cells (Table 1). Conditioned media from culture of both WT and DoxR cells also contained 3 out of the 17 checkpoint molecules, which included HVEM, CD40, and PD-L2 (Table 1).
Fig 2.
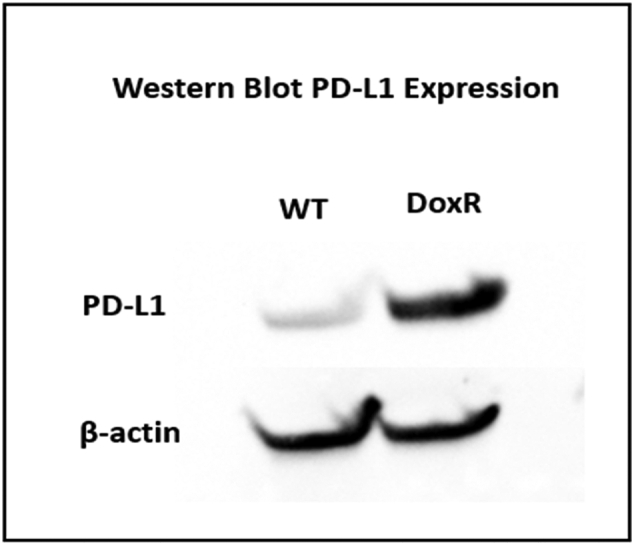
Western blot demonstrating increased PD-L1 expression in SJSA-1 DoxR cells compared to WT.
Table 1.
Summary of immune checkpoint proteins present in wild-type versus doxorubicin-resistant (DoxR) osteosarcoma cell lysates (cellular) and media (soluble)
|
Cellular |
Soluble |
|||||
|---|---|---|---|---|---|---|
| Target | Wild type | DoxR | P value | Wild type | DoxR | P value |
| BTLA | 490.0 | 246.5 | .127 | < LLoQ | < LLoQ | N/A |
| CD27 | 2.2 | 3.7 | .275 | < LLoQ | < LLoQ | N/A |
| CD28 | 6.5 | 6.1 | .564 | < LLoQ | < LLoQ | N/A |
| TIM-3 | 3.4 | 7.3 | .050 | < LLoQ | < LLoQ | N/A |
| HVEM | 38.8 | 26.4 | .275 | 8.6 | 1.7 | .121 |
| CD40 | 2310.3 | 3263.1 | .127 | 48.8 | 29.6 | .121 |
| GITR | < LLoQ | < LLoQ | N/A | < LLoQ | < LLoQ | N/A |
| LAG-3 | 46.3 | 62.9 | .127 | < LLoQ | < LLoQ | N/A |
| TLR-2 | < LLoQ | < LLoQ | N/A | < LLoQ | < LLoQ | N/A |
| GITRL | < LLoQ | < LLoQ | N/A | < LLoQ | < LLoQ | N/A |
| PD-1 | 2.3 | 1.8 | .180 | < LLoQ | < LLoQ | N/A |
| CTLA-4 | 0.6 | < LLoQ | N/A | < LLoQ | < LLoQ | N/A |
| CD80 | 2.2 | 3.9 | .513 | < LLoQ | < LLoQ | N/A |
| CD86 | 3.3 | 460.6 | .050 | < LLoQ | < LLoQ | N/A |
| PD-L1 | 19.9 | 50.4 | .127 | < LLoQ | < LLoQ | N/A |
| PD-L2 | 290.0 | 342.9 | .827 | 109.6 | 53.6 | 1 |
| ICOS | 250.7 | 404.3 | .275 | < LLoQ | < LLoQ | N/A |
Values are reported as medians in pg/mL/mg of cellular protein.
DISCUSSION
This is one of the first studies using a chemotherapy-resistant osteosarcoma cell line to evaluate the expression of checkpoint molecules in both the cellular and soluble microenvironments. First, we confirmed the expression of PD-L1 in this osteosarcoma cell line and demonstrated that it is upregulated with drug resistance. Second, we identified 12 additional checkpoint molecules that are cellularly expressed by osteosarcoma, 3 of which were also expressed in soluble form. We also identified multiple differences between the WT and DoxR cell lines. This study successfully benchmarked the checkpoint molecule expression of one osteosarcoma cell line and lays the groundwork for further exploration of checkpoint molecule studies.
The expression of PD-L1 in both WT and DoxR cell lines suggests that PD-1 and PD-L1 pathway inhibition may be a promising target for drug therapy. A plethora of recent studies have demonstrated varying levels of PD-L1 expression in osteosarcoma cell lines, in vivo models, and patient tumor samples [[24], [25], [26], [27]]. In particular, upregulation appears to coincide with metastatic and drug-resistant tumors [4,[24], [25], [26],28]. Pathway blockade has even been added to a few small phase I and II trials of pediatric solid tumors, albeit the osteosarcoma cohorts have all been small [18,[29], [30], [31], [32]]. The upregulation of PD-L1 in this study, particularly in the DoxR cell line, is consistent with prior histopathologic evaluation of patient tumor samples and suggests that this pathway may play a role in drug resistance and metastasis and that blockade may be a beneficial treatment. However, this study alone only provides preliminary evidence and is not sufficient to draw definitive conclusions. Our laboratory is currently investigating the T-cell response of PD-L1 inhibition and plans to expand to an in vivo model while further studying our own institutional biorepository.
The success of the PD-1 and PD-L1 pathway inhibition in multiple adult cancers has also led us to explore and benchmark the expression of other checkpoint molecules in osteosarcoma. Understanding the tumor characteristics that modulate immune suppression may expand the patient population known to respond to immune checkpoint inhibitors. Using a wild-type and drug-resistant cell line provides multiple major advantages. First, it enabled us to conduct a large exploratory study without depleting our biorepository. Second, it directs future study of checkpoint pathways by providing meaningful preliminary data. Third, identifying differences between the wild-type and chemotherapy-resistant cell line in this study may provide more insight into the pathways leading to drug resistance and metastasis, as our more invasive DoxR cell line also serves as a proxy for metastatic disease. In this study, 14 of 17 checkpoint molecules were measurable, 13 in both the WT and DoxR cell lines. As of this study, there are currently Food and Drug Administration (FDA)–approved drugs to inhibit CTLA-4, PD-1, and PD-L1, with many novel agents under investigation [33]. A better understanding of the checkpoint molecule activity of osteosarcoma in both the WT and DoxR cell lines provides clinically relevant information to direct further in vitro and in vivo studies of checkpoint molecules previously unknown to be expressed by osteosarcoma, especially as new checkpoint inhibiting agents become commercially available.
The DoxR cells had significantly or near significantly higher levels of 5 checkpoint molecules including TIM-3, CD86, CD40, LAG-3, and PD-L1. In this study, as DoxR cells were more invasive than WT cells, the DoxR cell line serves to model both drug resistance and, to an extent, metastasis—with invasion as a surrogate marker for metastatic disease. Identifying different checkpoint molecule profiles between cell lines may offer insight into mechanisms of drug resistance and metastasis, provide prognostic value, and again provide potential targets for drug treatment. For instance, PD-L1 and TIM-3 have been associated with epithelial–mesenchymal transition in lung adenocarcinoma and may be implicated here in osteosarcoma [34]. Although future studies are needed, perhaps upregulation of these varying checkpoint molecules in the DoxR compared to WT cell lines may be biomarkers with negative prognosis for treatment response or survival. Based on our current preliminary exploratory study, we cannot make any definitive statements nor draw any conclusions regarding the prognostic value of our results using only 1 cell line. However, we hope that these preliminary results provide insight to direct future studies expanding to multiple cell lines, in vivo modeling, and tumor sample testing that may provide better prognostic data. Moreover, knowing which checkpoint molecules are present within the tumor plays a valuable role in drug choice for treatment. Presence of certain molecules in the DoxR compared to WT cell lines may imply that certain treatments, such as PD-1 and PD-L1 pathway inhibition, are more effective once chemoresistance or metastasis is established. Aside from the PD-1 and PD-L1 pathway, which currently has 6 commercially available inhibitory FDA-approved drugs, the CD86 and CTLA pathway also has an FDA-approved drug, ipilimumab, commercially available [33]. CD86 is a needed costimulatory molecule when bound to CTLA-4 which inhibits the early activation of naive and memory T cells [35]. Clinical trials for adult cancer treatment are also ongoing with anti–LAG-3 (NCT03005782) and anti–TIM-3 (NCT02817633) drugs. A better understanding of the osteosarcoma checkpoint molecule landscape may better elucidate pathways leading to drug resistance and metastasis, serve as prognostic biomarkers, or provide insight when selecting targeted drug treatment.
Finally, we evaluated the cell culture media for soluble checkpoint protein molecules. Cancer cells can shed various molecules via exosomes or via proteolytic cleavage of the membrane-bound form and induce immunosuppression and cancer survival [36,37]. The former has recently been shown in osteosarcoma with identification of tumor-derived exosomal PD-L1 [24]. However, in other cancers such as esophageal adenocarcinoma, soluble PD-L1 has been shown to be generated by the latter [37]. Regardless, levels of these soluble markers may vary based on the health of the patient and advancement or chemoresistance of the disease [36,37]. Therefore, identifying levels of these soluble markers may provide value by detecting osteosarcoma early, aiding in prognosis, or directing treatment depending on the checkpoint molecule biomarker present. In this study, we identified HVEM, CD40, and PD-L2 to be present in both the WT and DoxR cell lines without significant difference between the two. We did not detect soluble PD-L1, although this may be due to overly dilute media making detection of small levels difficult. These results are promising in that we confirmed that osteosarcoma does have soluble checkpoint molecules which warrant further investigation. Unfortunately, in this exploratory study which uses only one cell line, we cannot draw any conclusions regarding their prognostic value as more data ideally using patient serum samples are needed.
The main limitation of this study is that it used only 1 cell line. This was primarily due to the time necessary to create a resistant cell line and the fact that this study was exploratory in nature to direct future research. To partially account for this, all experiments were done in triplicate using cell lysates or media from different cell passages and maintained by different researchers within the laboratory. We also were not able to draw any conclusions regarding the prognostic value of the biomarkers we identified. However, because this study was exploratory in nature, its main goal was to assist with directing future checkpoint molecule research. We plan to expand this study to multiple cell clines and create an in vivo model. Moreover, we also plan to test our own institutional biorepository to further validate our results.
In conclusion, this is one of the pioneer studies to demonstrate that drug-resistant osteosarcoma cells overexpress PD-L1 in vitro. Select checkpoint molecules are expressed in osteosarcoma cells, and the immune-checkpoint protein landscape in osteosarcoma likely changes as it becomes more chemoresistant or metastatic. Therefore, this study may provide insight into directing future in vitro and in vivo studies as they relate to future drug treatment with checkpoint inhibitors, pathways of drug resistance, and metastasis. Additionally, this study introduces the possible value of checkpoint molecules as tumor biomarkers.
Acknowledgments
Acknowledgments
We would like to thank the Beckley Foundation and Alex Manderino Foundation for their generous support. We would also like to thank the Rush Biomarker Development Core for processing the immunobead assays.
Conflict of Interest
The following authors have no financial disclosures: NJS, FC, IT, SS, JB, and MBM.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding Source
The Beckley Foundation, the Alex Mandarino Foundation, and Rush University Medical Center Department of Surgery.
Footnotes
Declarations of Interest: None.
Meeting: Presented as a Podium Presentation at the Academic Surgical Congress, 2021.
References
- 1.Durfee R.A., Mohammed M., Luu H.H. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3:221–224. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marulanda G.A., Henderson E.R., Johnson D.A., Letson G.D., Cheong D. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13–20. doi: 10.1177/107327480801500103. [DOI] [PubMed] [Google Scholar]
- 3.Goorin A.M., Schwartzentruber D.J., Devidas M. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K., Okamoto M., Sasaki J. Clinical outcome of osteosarcoma and its correlation with programmed death-ligand 1 and T cell activation markers. Onco Targets Ther. 2019;12:2513–2518. doi: 10.2147/OTT.S198421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.A., Cheung N.K.V. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev. 2017;58:22–33. doi: 10.1016/j.ctrv.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Chan T, Kroemer G, Wolchock J, Lopez-Sota A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459):1-15. doi:10.1126/scitranslmed.aat7807 LK - http://resolver.ebscohost.com/openurl?sid=EMBASE&issn=19466242&id=doi:10.1126%2Fscitranslmed.aat7807&atitle=The+hallmarks+of+successful+anticancer+immunotherapy&stitle=Sci.+Transl.+Med.&title=Science+Translational+Medicine&volume=10&issue=459&spage=&epage=&aulast=Galluzzi&aufirst=Lorenzo&auinit=L.&aufull=Galluzzi+L.&coden=&isbn=&pages=-&date=2018&auinit1=L&auinitm= [DOI] [PubMed]
- 7.Tocheva A.S., Mor A. Checkpoint inhibitors: applications for autoimmunity. Curr Allergy Asthma Rep. 2017;17(10):1–9. doi: 10.1007/s11882-017-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley J.L. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozali EN, Hato S V., Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012. doi:10.1155/2012/656340 [DOI] [PMC free article] [PubMed]
- 10.Tierney J.F., Vogle A., Poirier J. Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: an exploratory study. Surg (United States). 2019;165(1):196–201. doi: 10.1016/j.surg.2018.04.086. [DOI] [PubMed] [Google Scholar]
- 11.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med. Published online 2013. doi:10.1056/nejmoa1305133 [DOI] [PMC free article] [PubMed]
- 12.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. Published online 2014. doi:10.1056/nejmoa1411087 [DOI] [PMC free article] [PubMed]
- 13.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. Published online 2015. doi: 10.1056/nejmoa1501824 [DOI] [PubMed]
- 14.De Sousa Linhares A., Battin C., Jutz S. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-47910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi N.A., Hellmann M.D., Brahmer J.R. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.9861. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J.A., Cheung N.K.V. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev. 2017;58:22–33. doi: 10.1016/j.ctrv.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi F., O’Day S., McDermott D. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchant M.S., Wright M., Baird K. Phase i clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-0491. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer K., Kushner B.H., Modak S. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010 doi: 10.1007/s11060-009-0038-7. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y., Cao J., Zhao C., Li X., Zhou C., Hirsch F.R. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018 doi: 10.2147/OTT.S170385. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabir T.F., Chauhan A., Anthony L., Hildebrandt G.C. Immune checkpoint inhibitors in pediatric solid tumors: status in 2018. Ochsner J. 2018;18(4):370–376. doi: 10.31486/toj.18.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto N, Park JR, Murphy E, et al. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer. Published online 2017. doi:10.1002/pbc.26613 [DOI] [PubMed]
- 23.Rebbaa A., Chou P.M., Mirkin B.L. Factors secreted by human neuroblastoma mediate doxorubicin resistance by activating STAT3 and inhibiting apoptosis. Mol Med. 2001;7(6):393–400. doi: 10.1007/bf03402185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Zhang H., Sun X. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnology. 2020;18(1):151. doi: 10.1186/s12951-020-00710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toda Y., Kohashi K., Yamada Y. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol. 2020;146(10):1815–1824. doi: 10.1007/s00432-020-03242-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Chou X., Zhuang M. LINC00657 activates PD-L1 to promote osteosarcoma metastasis via miR-106a. J Cell Biochem. 2020;121(10):4188–4195. doi: 10.1002/jcb.29574. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Guenther L.M., Aronhalt A., Cardillo L., Janeway K.A., Church A. PD-1 and PD-L1 expression in osteosarcoma: which specimen to evaluate? J Pediatr Hematol Oncol. 2020;42(8):482–487. doi: 10.1097/MPH.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Hu C., Wang J. Checkpoint blockade in combination with doxorubicin augments tumor cell apoptosis in osteosarcoma. J Immunother. Published online. 2019 doi: 10.1097/CJI.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 29.Xie L., Xu J., Sun X. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Cesne A., Marec-Berard P., Blay J.Y. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur J Cancer. 2019;119:151–157. doi: 10.1016/j.ejca.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Geoerger B., Kang H., Yalon-Oren M. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21(1):121–133. doi: 10.1016/S1470-2045(19)30671-0. [DOI] [PubMed] [Google Scholar]
- 32.Davis K., Fox E., Merchant M. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21AD(4):541–550. doi: 10.1016/S1470-2045(20)30023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020;12(3):738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lou Y., Diao L., Cuentas E.R.P. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22(14):3630–3642. doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., Ni L., Dong C. Immune checkpoint receptors in cancer: redundant by design? Curr Opin Immunol. 2017;45:37–42. doi: 10.1016/j.coi.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Chen G., Huang A.C., Zhang W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida J., Ishikawa T., Doi T. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med Oncol. 2019;36(7):60. doi: 10.1007/s12032-019-1285-x. [DOI] [PubMed] [Google Scholar]