Abstract
Introduction
There is an urgent need for effective therapies against bacterial infections, especially those caused by antibiotic-resistant Gram-negative pathogens.
Hypothesis
Synergistic combinations of existing antimicrobials show promise due to their enhanced efficacies and reduced dosages which can mitigate adverse effects, and therefore can be used as potential antibacterial therapy.
Aim
In this study, we sought to characterize the in vitro interaction of 5-nitrofurans, vancomycin and sodium deoxycholate (NVD) against pathogenic bacteria.
Methodology
The synergy of the NVD combination was investigated in terms of growth inhibition and bacterial killing using checkerboard and time-kill assays, respectively.
Results
Using a three-dimensional checkerboard assay, we showed that 5-nitrofurans, sodium deoxycholate and vancomycin interact synergistically in the growth inhibition of 15 out of 20 Gram-negative strains tested, including clinically significant pathogens such as carbapenemase-producing Escherichia coli , Klebsiella pneumoniae and Acinetobacter baumannii , and interact indifferently against the Gram-positive strains tested. The time-kill assay further confirmed that the triple combination was bactericidal in a synergistic manner.
Conclusion
This study demonstrates the synergistic effect of 5-nitrofurans, sodium deoxycholate and vancomycin against Gram-negative pathogens and highlights the potential of the combination as a treatment for Gram-negative and Gram-positive infections.
Keywords: antibiotic resistance, enterobacteria, nitrofurans, sodium deoxycholate, synergy, vancomycin
Introduction
Antimicrobial resistance that renders existing antimicrobial therapies ineffective poses a significant threat to public health with an enormous social and economic burden globally. The issue is particularly more severe with regard to Gram-negative bacterial pathogens than their Gram-positive counterparts because the former group is inherently resistant to many antimicrobial agents due to a highly impermeable outer membrane and a range of powerful efflux pumps [1]. This is reflected in the World Health Organization (WHO)’s list of priority pathogens, against which research and development of new antibiotics is urgently needed [2]. The majority of these pathogens are Gram-negative bacteria, including three that are deemed critical: carbapenem-resistant Acinetobacter baumannii , Pseudomonas aeruginosa and Enterobacteriaceae . As multidrug resistance continues to emerge and spread globally, and with the current clinical development pipeline insufficient to keep pace [3], this problem needs to be rectified through alternative strategies. In this study, we propose the revival of ‘old’ drugs by employing them in novel synergistic antibacterial combinations.
One such example of ‘old’ drugs are 5-nitrofurans, a broad-spectrum class of antibacterials. Although they have had a controversial past due to their mutagenicity and carcinogenicity [4, 5], they remain effective, have low prevalence of resistance [6–10] and are still clinically used today. The 5-nitrofuran class of antibacterials includes commercially available furazolidone (FZ), nitrofurantoin (NIT) and nitrofurazone (NFZ). FZ is used to treat diarrhoea and as a component of combination therapy against Helicobacter pylori , NIT to treat urinary tract infections, and NFZ as a topical treatment for burns and wounds [11].
The glycopeptide vancomycin (Van) is another ‘old’ antibiotic that is clinically used to treat Gram-positive infections. However, due to its toxicity [12–14] and complications with its intravenous administration [15], it is not a first-choice antibiotic. Over the past few years, new glycopeptides with lipophilic substituents, termed lipoglycopeptides, have been developed and introduced to the market. They include telavancin, dalbavancin and oritavancin [16]. Having lipophilic side-chains and other modifications give these drugs better antimicrobial properties and safety profiles compared to Van [17]. The glycopeptide antibiotics exert their antibacterial activity by inhibiting peptidoglycan synthesis [18]. Large molecule size (≥1449 Da) prevents the passage of glycopeptides across the Gram-negative outer membrane, blocking their access to the target and rendering them ineffective against this group of organisms at sub-toxic concentrations.
Sodium deoxycholate (DOC) is a secondary bile salt, playing important roles in lipid digestion, intestinal homeostasis and antimicrobial protection [19, 20]. Gram-negative bacteria have evolved resistance to DOC through mechanisms such as preventing the accumulation of DOC inside the cells by expulsion mediated by multidrug efflux pumps [21–23], and activation of stress responses including DNA repair mechanisms [24, 25].
Having identified pairwise synergies between nitrofurans and DOC [26], and between nitrofurans and Van in a high-througput screen of 180 000 small molecules that included known antibiotics (J. Spagnuolo, F. Glickman and J. Rakonjac, 2012, unpublished data) and in light of a published nitrofurantoin–Van synergy against Escherichia coli [27] we explored whether combining these three antibacterials will further decrease the inhibitory concentration of individual components. We now demonstrate the in vitro three-way synergy of 5-nitrofurans, Van and DOC (NVD) in growth inhibition and killing of a range of clinical Gram-negative pathogenic isolates, including multidrug-resistant and carbepenemase-producing enterobacteria for which new therapies are urgently needed [2]. To explore the possibility of also using the combination in treating WHO priority Gram-positive pathogens, the interaction was also evaluated in Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus .
Methods
Bacterial strains and growth conditions
All bacterial strains used in this study are listed in Table 1. The bacterial cell cultures were grown in trypticase soy agar at 37 °C. For Staphylococcus, Streptococcus and Pasteurella strains, the growth medium was further supplemented with 5 % sheep blood. Cation-adjusted Mueller Hinton II broth (Becton-Dickinson) was used in checkerboard and time-kill assays. Because 5-nitrofuran stocks were made in DMSO, final DMSO concentrations for all treatments and controls in the following assays were maintained at 1 %.
Table 1.
Bacterial strains used in this study
|
Strain |
Genotype/description |
Source |
|---|---|---|
|
E. coli ATCC 25922 |
Antibiotic susceptibility reference/quality control strain |
ATCC 25922; NZRM 916* |
|
E. coli K12 strain K1508 |
MC4100 [F− araD− Δlac U169 relA− thiA rpsL (StrR)] ΔlamB106 |
[41] |
|
E. coli ERL034336 |
O157, human isolate |
Dr Ann Midwinter, School of Veterinary Sciences, Massey University, Palmerston North, New Zealand (NZ) |
|
E. coli UPEC P191 |
Isolate from a feline urinary tract infection |
New Zealand Veterinary Pathology (NZVP) diagnostic labs, Palmerston North, NZ |
|
E. coli NZRM 4402 |
Plasmid-mediated AmpC β-lactamase-producing (CMY-2) |
NZRM 4402 |
|
E. coli NZRM 4364 |
Carbapenemase (IMP-4) producing. Human isolate from hospital outbreak (Melbourne, Australia) |
NZRM 4364 |
|
E. coli NZRM 4457 |
NDM-1 metallo-β-lactamase-producing |
ARL09/232 [42]; NZRM 4457 |
|
E. coli NZRM 4524 |
Verotoxin-producing E. coli (VTEC) Serotype 045: H Rough |
NZRM 4524 |
|
Citrobacter gillenii PMR001 |
Isolate from a municipal sewage processing (water purification) plant, Palmerston North, NZ |
[26] |
|
Klebsiella pneumoniae PMR001 |
Isolate from a municipal sewage processing (water purification) plant, Palmerston North, NZ |
[26] |
|
Klebsiella pneumoniae NZRM 4387 |
Beta-lactamase-producing. SHV-11, TEM-1, CTX-M-15 |
NZRM 4387 |
|
Klebsiella pneumoniae NZRM 4412 |
Klebsiella pneumoniae carbapenemase-producing |
ATCC BAA-170; NZRM 4412 |
|
Klebsiella pneumoniae NZRM 4498 |
OXA-181 carbapenemase-producing |
NZRM 4498 |
|
Type strain, S. enterica subsp. enterica , serovar Typhimurium |
ATCC 43971 |
|
|
Salmonella enterica NZRM 4533 |
Human isolate (NZ) |
NZRM 4533 |
|
Shigella dysenteriae 1015 |
Type strain |
ATCC 13313; NZRM 1015 |
|
Acinetobacter lwoffi NZRM 1218 |
Knee wound isolate (NZ) |
NZRM 1218 |
|
Acinetobacter baumannii NZRM 3697 |
Human isolate from outbreak (Christchurch, NZ) |
NZRM 3697 |
|
Acinetobacter baumannii NZRM 4408 |
OXA-27 carbapenemase-producing |
NCTC 13304; NZRM 4408 |
|
Pasteurella dagmatis NZRM 959 |
Isolate from a feline oral cavity |
NZRM 959 |
|
Staphylococcus aureus NZRM 3478 |
Methicillin-resistant. human wound isolate (Auckland, NZ) |
NZRM 3478 |
|
Staphylococcus aureus NZRM 4315 |
Human wound isolate. Erythromycin (ermA) resistant |
ATCC BAA-977; NZRM 4315 |
|
Staphylococcus aureus NZRM 4548 |
WR/AK1 strain. Panton-Valentine-leukocidin (PVL)-positive methicillin-resistant. Human isolate (NZ) |
NZRM 4548 |
|
Staphylococcus aureus NZRM 4549 |
AK3 methicillin-resistant. Human isolate (NZ) |
NZRM 4549 |
|
Streptococcus pneumoniae NZRM 2764 |
Susceptibility testing control |
CDC 78-008107; NZRM 2764 |
|
Streptococcus pyogenes NZRM 4366 |
Exotoxin positive control |
NZRM 4366 |
*NZRM, The New Zealand Reference Culture Collection: Medical Section.
Susceptibility testing
The broth microdilution assay for antimicrobial susceptibility was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [28] in a 384-well plate format. Each well contained 5×105 c.f.u. ml−1 at exponential growth phase, 1 % DMSO, and the antimicrobial at specific concentrations prepared by two-fold serial dilutions, in a total volume of 50 µl. Each treatment was performed in triplicate. The plate was incubated at 37 °C for 18 h for enterobacteria and Staphylococcus and 24 h for Acinetobacter and Streptococcus as recommended by the CLSI guidelines [28]. The optical density at 600 nm was measured to assess growth inhibition [29]. The MIC is defined as the concentration that inhibits at least 90 % of growth at the endpoint.
Checkerboard assay
The synergy between the test antimicrobials was examined using a growth inhibition three-dimensional (3D) checkerboard microdilution assay in 384-well plates according to the CLSI guidelines [28]. Two-fold dilutions with a concentration range of 2× MIC to 0.06× MIC of the antimicrobials and exponential-phase bacteria were prepared in BBL cation-adjusted Mueller Hinton II broth (Becton-Dickinson). Each well contained 5×105 c.f.u. ml−1, 1 % DMSO and the antimicrobials in a total volume of 50 µl. Each plate was incubated at 37 °C for the required time according to CLSI guidelines, and the optical density at 600 nm was measured to quantify growth [29]. Each combination of concentrations was performed in triplicate and the mean growth inhibition was used to determine MIC values which correspond to concentrations of combinations that result in at least 90 % of growth inhibition [29]. For most of the strains tested, FZ was used as a representative of the 5-nitrofurans in the checkerboard assays.
The fractional inhibitory concentration index (FICI) was calculated using the equation [30]:
where MIC5-nitrofuran(com), MICDOC(com) and MICVan(com) is the MIC of 5-nitrofuran, DOC or oxgall bile salts, and Van or lipoglycopeptides (dalbavancin or oritavancin) when used in combination, and MIC5-nitrofuran(alone), MICDOC(alone) and MICVan(alone) is the MIC when used alone. Using the lowest FICI, the interactions were interpreted as synergistic if FICI≤0.5, indifferent if 0.5<FICI≤4.0 and antagonistic if FICI>4.0 [31].
Time-kill assay
Exponentially growing bacterial cultures were prepared at 5×105 c.f.u. ml−1 and treated with individual antimicrobial agents (FZ, DOC, Van) or combinations thereof at specified concentrations in a final volume of 10 ml. The cultures treated with 1 % DMSO were included as vehicle controls. Each treatment was performed in triplicate. The bacterial cultures were incubated at 37 °C with shaking at 200 r.p.m. At time points 0, 2, 4, 6, 8, and 24 h, 500 µl was sampled from each culture and centrifuged at 10 000 g for 1 min. The pellet was then resuspended in 100 µl of the maximum recovery diluent (0.1 % peptone, 0.85 % NaCl), and 10-fold serial dilutions were drop-plated (10 µl) on 2×YT agar. Plates were incubated at 37 °C overnight before counting colonies. The limit of detection is 60 c.f.u. ml−1 or 1.78 log10 c.f.u. ml−1. The synergy of the combination was defined as a ≥2log10 decrease in the cell count (c.f.u. ml−1) compared to the most potent single drug at 24 h.
Results
Synergy of 5-nitrofurans, Van and DOC against Gram-negative bacteria
Growth-inhibition checkerboard assays were performed to evaluate the synergy between 5-nitrofurans, Van and DOC against a range of Gram-negative bacteria. The MICs for these antibacterials were determined according to the CLSI guidelines and are summarized in Table 2. As a proof of concept that the 5-nitrofuran drug, as an antibacterial class, is synergistic with Van and DOC, we evaluated the antibacterial effect of combinations of these two agents with either of the three 5-nitrofurans (FZ, NIT and NFZ) in a checkerboard assay against the reference strain E. coli ATCC 25922. We found that all the three 5-nitrofurans were synergistic with DOC and Van, with FICIs ranging from 0.11 to 0.15 (Fig. S1).
Table 2.
MICs against Gram-negative pathogens
|
|
MIC (μg ml−1) |
||||
|---|---|---|---|---|---|
|
Strain |
FZ |
DOC |
Van |
NIT |
NFZ |
|
E. coli ATCC 25922 |
1.25 |
80 000 |
500 |
16 |
8 |
|
E. coli K1508 |
1.25 |
>80 000 |
250 |
16 |
8 |
|
E. coli ERL034336 |
1.25 |
80 000 |
250 |
16 |
8 |
|
E. coli UPEC P191 |
1.25 |
80 000 |
250 |
16 |
8 |
|
E. coli NZRM 4364 |
0.25 |
>80 000 |
125 |
nt |
nt |
|
E. coli NZRM 4402 |
0.625 |
40 000 |
500 |
nt |
nt |
|
E. coli NZRM 4457 |
>128 |
80 000 |
250 |
128 |
64 |
|
E. coli NZRM 4524 |
1.25 |
80 000 |
500 |
nt |
nt |
|
C. gillenii PMR001 |
5 |
80 000 |
500 |
16 |
16 |
|
K. pneumoniae PMR001 |
1.25 |
80 000 |
1000 |
64 |
32 |
|
K. pneumoniae NZRM 4387 |
2.5 |
80 000 |
2000 |
nt |
nt |
|
K. pneumoniae NZRM 4412 |
32 |
80 000 |
2000 |
>128 |
128 |
|
K. pneumoniae NZRM 4498 |
16 |
>80 000 |
2000 |
>128 |
64 |
|
Salmonella enterica sv. Typhimurium LT2 |
2.5 |
40 000 |
500 |
16 |
8 |
|
Salmonella enterica NZRM 4533 |
2 |
80 000 |
1000 |
32 |
8 |
|
Shigella dysenteriae NZRM 1015 |
4 |
>80 000 |
250 |
8 |
8 |
|
A. lwoffi NZRM 1218 |
16 |
40 000 |
62.5 |
nt |
nt |
|
A. baumannii NZRM 3697 |
32 |
80 000 |
125 |
nt |
nt |
|
A. baumannii NZRM 4408 |
>128 |
80 000 |
250 |
>128 |
32 |
|
P. dagmatis NZRM 959 |
2 |
1250 |
31.25 |
4 |
4 |
FZ, furazolidone; DOC, sodium deoxycholate, Van, vancomycin; NIT, nitrofurantoin; NFZ, nitrofurazone; nt, not tested.
Among the 5-nitrofurans included in this study, FZ was the most potent against the strains tested (Table 2). Therefore, we chose FZ as a representative of the 5-nitrofurans in the expanded strain profiling by checkerboard and time-kill assays. The FICIs for the two-drug and three-drug combinations of FZ, Van and DOC are listed in Table 3. For some of the strains, where the MIC could not be determined, the FICI was calculated using the highest tested concentration. In this case, the actual FICI would be lower than the calculated value, and for some strains, it may not be possible to classify an interaction when the calculated FICI is bordering between synergistic and indifferent, such as in E. coli NZRM 4457. With the 20 Gram-negative bacterial strains tested, the FZ, Van and DOC combination was synergistic against 15 strains, indifferent against four strains (S. Typhimurium LT2, K. pneumoniae PMR001, K. pneumoniae NZRM 4412 and P. dagmatis NZRM 959), and unable to be classified as synergistic or indifferent against one strain ( E. coli NZRM 4457). Of importance is the synergy of the combination against some of the WHO critical priority pathogens, namely carbapenemase-producing E. coli NZRM 4364 and Acinetobacter baumannii NZRM 4408, and extended-spectrum β-lactamase-producing K. pneumoniae NZRM 4387 (Table 3).
Table 3.
FICI values for the two-drug and three-drug combinations of furazolidone, vancomycin and sodium deoxycholate
|
|
FICI |
|||
|---|---|---|---|---|
|
Strain |
FZ+DOC |
FZ+Van |
DOC+Van |
FZ+Van+DOC |
|
E. coli ATCC 25922 |
0.16 |
0.75 |
0.52 |
0.11 |
|
E. coli K1508 |
<0.50 |
0.75 |
<1.02 |
<0.13 |
|
E. coli ERL034336 |
0.27 |
0.50 |
1.01 |
0.30 |
|
E. coli UPEC P191 |
0.19 |
0.75 |
0.28 |
0.17 |
|
E. coli NZRM 4364 |
<0.31* |
0.9 |
<0.52* |
<0.35* |
|
E. coli NZRM 4402 |
0.19 |
0.74 |
0.23 |
0.19 |
|
E. coli NZRM 4457 |
<0.75* |
<1* |
1 |
<0.54* |
|
E. coli NZRM 4524 |
0.19 |
0.35 |
0.53 |
0.17 |
|
C. gillenii PMR001 |
0.17 |
0.5 |
1 |
0.27 |
|
K. pneumoniae PMR001 |
1 |
0.53 |
1 |
0.51 |
|
K. pneumoniae NZRM 4387 |
0.31 |
0.63 |
1 |
0.28 |
|
K.pneumoniae NZRM 4412 |
1 |
1 |
0.53 |
0.54 |
|
K. pneumoniae NZRM 4498 |
<0.52* |
0.38 |
<1* |
<0.26* |
|
Salmonella enterica sv. Typhimurium LT2 |
0.31 |
0.56 |
1 |
0.53 |
|
Salmonella enterica NZRM4533 |
0.38 |
0.5 |
1 |
0.27 |
|
Shigella dysenteriae NZRM 1015 |
<0.25* |
0.5 |
<0.52* |
<0.28* |
|
A. lwoffi NZRM 1218 |
0.25 |
0.82 |
0.46 |
0.29 |
|
A. baumannii NZRM 3697 |
0.28 |
0.75 |
1 |
0.32 |
|
A. baumannii NZRM 4408 |
<0.09* |
0.56 |
1 |
<0.13* |
|
P. dagmatis NZRM 959 |
0.75 |
0.75 |
0.63 |
0.66 |
FZ, furazolidone; DOC, sodium deoxycholate; Van, vancomycin.
*MIC is higher than the highest tested concentration which was used to calculate the FICI, and therefore the actual FICI is lower than the calculated value; values in bold indicate synergy (FICI≤0.5).
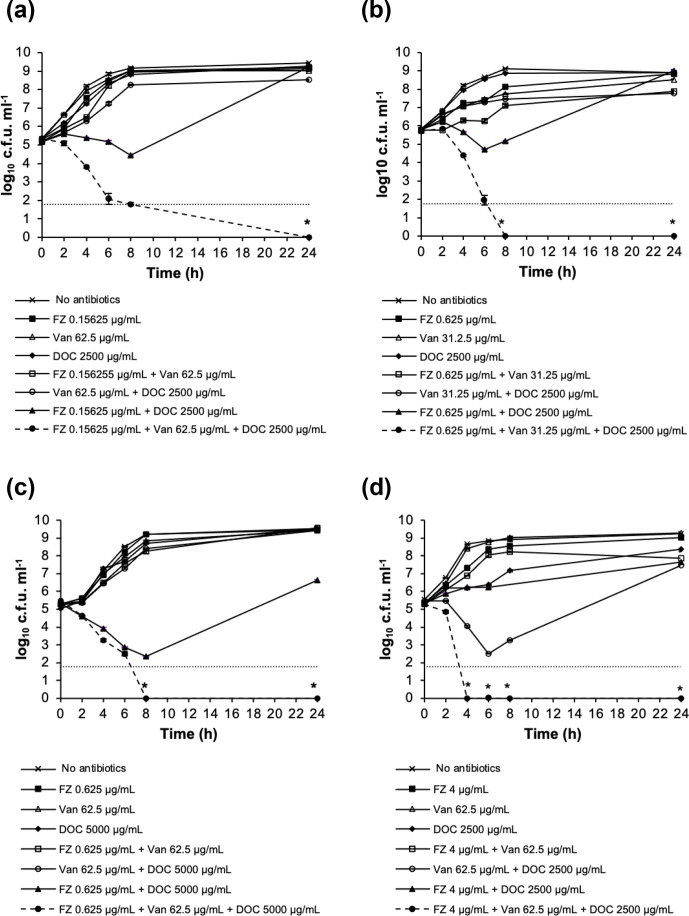
To further investigate the interaction between FZ, Van and DOC in terms of bacterial killing, we performed time-kill assays on some representative pathogens for which the triple combination showed growth inhibition synergy in the checkerboard assay. The strains were exposed to subinhibitory concentrations of FZ, Van and DOC or two-drug and three-drug combinations of these concentrations over a time course of 24 h. The time-kill analysis for E. coli ATCC 25922, Shigella dysenteriae NZRM 1015, Citrobacter gillenii PMR001 and Acinetobacter baumannii NZRM 3697 is shown in Fig. 1. The combination of subinhibitory concentrations of FZ, Van and DOC resulted in >2log10 reduction in viable cell count after 24 h of exposure in comparison with the most active single drug, demonstrating the synergy in the bacterial killing of the three-drug combination in these strains. In these four examples, the triple combination led to the extinction of the challenged bacterial population at the end of the assay (i.e. below the limit of detection), which was not achieved by single drugs or double combinations at those same concentrations.
Fig. 1.
Time-kill analysis of furazolidone (FZ), vancomycin (Van) and sodium deoxycholate (DOC) combinations in killing (a) E. coli ATCC 25922, (b) Shigella dysenteriae NZRM 1015, (c) C. gillenii PMR001 and (d) A. baumannii NZRM 3697. The data are presented as mean±sem of three independent measurements. The lower limit of detection was 60 c.f.u. ml−1; an asterisk (*) indicates a data point that is below the limit of detection.
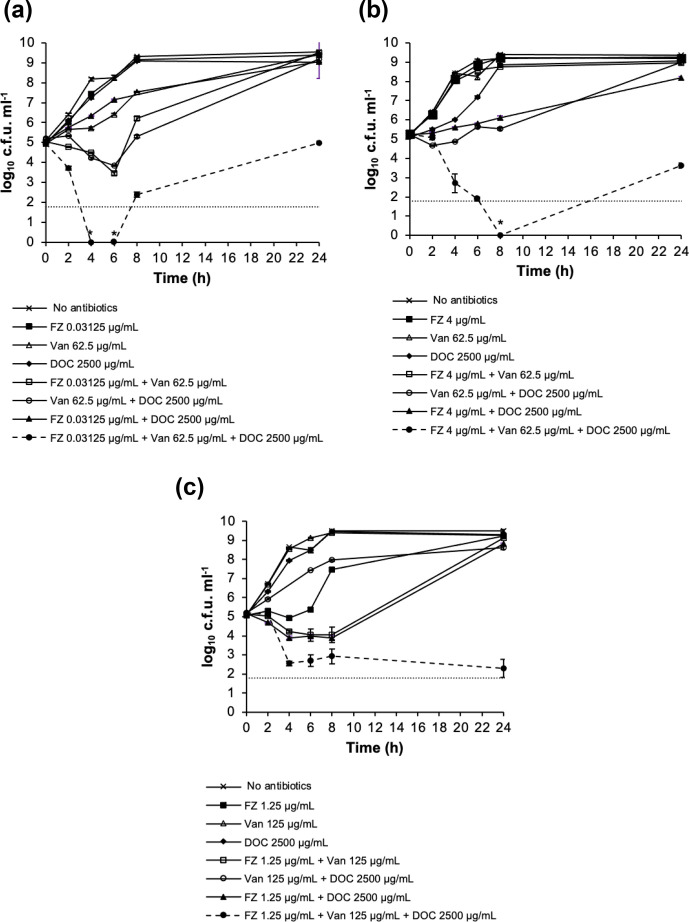
We also performed the time-kill assay for three multidrug-resistant pathogens, E. coli NZRM 4364, A. baumannii NZRM 4408 and K. pneumoniae NZRM 4387. Interestingly, bactericidal synergy was still retained against these strains, although the effect was less profound than against the drug-sensitive strains (Fig. 2).
Fig. 2.
Time-kill analysis of furazolidone (FZ), vancomycin (Van) and sodium deoxycholate (DOC) combinations in killing carbapenemase-producing (a) E. coli NZRM 4364 and (b) A. baumannii NZRM 4408, and (c) multidrug-resistant extended-spectrum β-lactamase-producing K. pneumoniae NZRM 4387. The data are presented as mean±sem of three independent measurements. The lower limit of detection was 60 c.f.u. ml−1; an asterisk (*) indicates a data point that is below the limit of detection.
Indifferent interaction of FZ, DOC and Van against Gram-positive bacteria
Van is not the first choice for the treatment of Gram-positive infections due to its adverse effects [32]. We questioned whether the triple combination would be synergistic against Gram-positive bacteria, achieving eradication with a lower dose of Van, and thus mitigating its adverse effects. To do so, we examined the interaction between FZ, Van and DOC against some important Gram-positive pathogens such as Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes using a checkerboard assay. In the six Gram-positive strains tested, all two-drug and three-drug combinations of FZ, Van and DOC were classified as indifferent, with FICIs ranging from 0.53 to 1.05 (Table 4). The inhibitory concentrations of at least two out of these three antibacterials were lower against Gram-positive bacteria than concentrations within the Gram-negative-inhibitory triple combination, and hence the combination would be effective against these pathogens despite an indifferent interaction.
Table 4.
MICs and FICIs for the combinations of FZ, Van and DOC against Gram-positive pathogens
|
|
MIC* (μg ml−1) |
|||
|---|---|---|---|---|
|
Strain |
FZ |
DOC |
Van |
|
|
Staphylococcus aureus NZRM 3478 |
4 |
312.50 |
2 |
|
|
Staphylococcus aureus NZRM 4315 |
2 |
625 |
1 |
|
|
Staphylococcus aureus NZRM 4548 |
2 |
625 |
1 |
|
|
Staphylococcus aureus NZRM 4549 |
2 |
625 |
1 |
|
|
Streptococcus pyogenes NZRM 4366 |
16 |
156.25 |
0.78 |
|
|
Streptococcus pneumoniae NZRM 2764 |
2 |
625 |
0.78 |
|
|
|
FICI |
|||
|
|
FZ+DOC |
FZ+Van |
DOC+Van |
FZ+Van+DOC |
|
Staphylococcus aureus NZRM 3478 |
1 |
1 |
1 |
0.81 |
|
Staphylococcus aureus NZRM 4315 |
1 |
1.03 |
1 |
0.88 |
|
Staphylococcus aureus NZRM 4548 |
1 |
1.03 |
1.02 |
1.05 |
|
Staphylococcus aureus NZRM 4549 |
1 |
1.03 |
1 |
1.03 |
|
Streptococcus pyogenes NZRM 4366 |
0.63 |
0.63 |
0.53 |
0.56 |
|
Streptococcus pneumonia NZRM 2764 |
0.53 |
0.75 |
1 |
0.58 |
*MIC of antibacterials when used as monotherapy.
Interactions of bile salts mixture with FZ and Van against E. coli
We further investigated whether a natural bile combination present in the human gut is able to aid treatment of enterobacteria-caused gastrointestinal illness in combination with FZ and Van. Ox gall powder from Sigma-Aldrich (Cat. No. B3883) was reported to have bile salt ratios closest to human bile [33] and was therefore used in this study. The content of DOC in the ox gall powder, according to Hu et al. [33], is 0.09 % (w/w) or 2.09 mmol kg−1.
The MIC of the ox gall powder for the E. coli strain ATCC 25922 was 200 mg ml−1, a much higher value than that of DOC (80 mg ml−1). A checkerboard assay was performed to investigate interactions between FZ, Van and ox gall bile salts. In the two-way combinations of the ox gall bile salts with FZ or Van, the FICIs were both 1.02, indicating an indifferent interaction in the double combinations. The three-way combination of ox-gall bile salts, Van and FZ led to a modest decrease in the FICI (0.63), yet this value corresponds to an indifferent interaction.
Interactions of lipoglycopeptides with FZ and DOC against E. coli
Dalbavancin and oritavancin are two modified versions of Van that have been reported to possess higher efficacy against Gram-positive bacteria and a better safety profile than Van [17]. Given that they share the same mechanism of action with Van, we hypothesized that dalbavancin and oritavancin were also synergistic with FZ and DOC in inhibiting Gram-negative pathogens. The highest achievable concentration of dalbavancin and oritavancin, due to limited solubility, is 200 μg ml−1. This concentration was too low to inhibit the growth of E. coli ATCC 25922. The combination of 200 µg ml−1 dalbavancin or oritavancin with FZ (0.04 µg ml−1) and DOC (625 µg ml−1) resulted in an FICI of <1.04, indicating indifferent interactions.
Discussion
Novel effective therapies are urgently needed against Gram-negative bacteria, in particular carbapenemase-producing Acinetobacter baumannii and enterobacteria. The latter two groups top the list of WHO priority pathogens for which development of novel therapies is urgent. With the conventional approach of small-molecule antibiotic development unable to keep pace with the continuing emergence and spread of multidrug resistance, alternative strategies are necessary to expand the therapeutic space. Synergistic combinations of currently available antibiotics show promise due to their enhanced activity with the advantage of improved clearance of pathogens, slowed resistance development and decreased toxicity [34].
Van and DOC, on their own, are not effective against Gram-negative bacteria, as evidenced by their high MICs when applied individually. Due to the relative impermeability of the outer membrane, large antibiotics such as Van are not able to reach their targets in Gram-negative bacteria [35, 36]. These bacteria have also evolved to be highly resistant to bile salts, including DOC [37–39]. As shown in this study, a Gram-negative-active agent, 5-nitrofuran, undermines tolerance of these bacteria to Van and DOC, thereby allowing the use of these antimicrobials to be expanded beyond Gram-positive bacteria.
Because of the synergistic interaction, effective doses of the individual drugs in the combinations have been significantly reduced. This reduction is especially significant for drugs that have adverse effects, such as Van and 5-nitrofurans. Decline in the use of these drugs after their introduction to the market is due to their toxicity. Adverse reactions of Van, including nephrotoxicity and ototoxicity [12, 14], are partly the reasons why this antibiotic is considered a last resort treatment for Gram-positive infections. Similarly, 5-nitrofuran use has been controversial due to its mutagenicity [11]. Taking advantage of the NVD synergy and using these antibiotics in a combination at lowered concentrations will allow the revival of these ‘old’ drugs due to the reduction or elimination of adverse effects.
In addition to testing the combination in Gram-negative pathogens, we also investigated the drug interaction in Gram-positive bacteria that cause skin and soft tissue infections such as Staphylococcus aureus and Streptococcus pyogenes . Although the three molecules are not synergistic against Gram-positive pathogens, the individual MICs of DOC and Van are very low in comparison with those for Gram-negative bacteria. The combination therapy effective against Gram-negative bacteria will therefore contain at least two molecules at concentrations above the Gram-positive MIC and will therefore inhibit these latter organisms. The NVD combination therapy could therefore be considered an alternative therapeutic option for the Gram-positive WHO priority pathogens such as methicillin-resistant Staphylococcus aureus . Besides these Gram-positive bacteria, some Gram-negative pathogens, such as enterobacteria, can also cause skin and soft tissue infections, albeit at lower rates compared to Gram-positive pathogens [40]. This study therefore provides evidence for the potential of the NVD combination as a broad-spectrum treatment for Gram-negative and Gram-positive skin infections.
Bile salts are present along the gastrointestinal tract. To assess the potential contribution of endogenous bile salts to NVD therapy of gastrointestinal infections, we tested the combination of FZ, Van and a human-gut-like in vitro bile mixture (ox gall) against E. coli and have found that this combination interacts indifferently. These findings suggest that components other than DOC (e.g. other bile salts) of ox gall powder have antibacterial properties, which act only additively with FZ and/or Van, and that the DOC content of ox gall powder is not sufficient to result in synergy. An important consideration, however, is that bile salts undergo transformations such as deconjugation and dehydroxylation along the gastrointestinal tract after they are released from the gall bladder [20]. Therefore, ox gall powder may not be a suitable human bile model due to under-representation of DOC in comparison with the human intestine, where bile salt transformations lead to a higher DOC concentration. Notwithstanding this caveat, it can be concluded from the ox gall experiment that for treating gastrointestinal infections, DOC would need to be supplemented together with FZ and Van to achieve the synergistic effect of the triple therapy.
Because the lipoglycopeptide antibiotics dalbavancin and oritavancin have better safety profiles than Van, we sought to expand their use beyond Gram-positive bacteria by employing them in the triple combination instead of Van. Therefore, we tested the combination of these lipoglycopeptides with FZ and DOC, and found that at the highest soluble concentration in liquid media, dalbavancin and oritavancin are not inhibitory to E. coli . The FICI values of these lipoglycopeptides could not be calculated because insolubility precluded determination of their MIC for E. coli . Using maximal soluble concentrations as proxies, checkerboard analyses showed at least indifferent interaction with FZ and DOC; a synergistic interaction, however, cannot be excluded.
The variation among strains in MIC and type of interaction between 5-nitrofurans, Van and DOC was observed in this study. No obvious pattern in terms of individual drug MICs can be seen that would dictate whether synergy would be observed or not. In addition, the mechanism of the NVD combination synergy is unknown. These reasons make it difficult to predict whether the NVD combination will be synergistic in a specific bacterial strain. Elucidation of the triple synergy mechanism will help understand why the combination is not synergistic in some of the Gram-negative strains.
A limitation of our study is that time-kill assays were only performed to confirm synergistic interaction identified by the checkerboard assay. It is known that FICIs from checkerboard tests can differ depending on the various interpretation criteria. This could mean that in some strains where the combination was interpreted as indifferent, it could actually be synergistic, or vice versa. In this regard, for strains showing borderline interactions between synergistic and indifferent interactions by checkerboard growth inhibition assays, further time-kill analyses are expected to define more precisely the NVD interaction with respect to their bactericidal effect. Finally, this was an in vitro study, and the ability of in vitro combination assays to predict clinical synergy is unknown. In vivo studies are therefore needed to confirm the clinical relevance of our findings.
In summary, we present here a synergistic combination of 5-nitrofurans, Van and DOC (NVD) against Gram-negative pathogens. This synergistic interaction allows the use of antimicrobials, such as Van and DOC, that would otherwise be ineffective against Gram-negative bacteria.
Funding information
C.O. was supported by a Massey University PhD Scholarship. We are indebted to Bryce and Ann Carmine for their generous donation that made this work possible. Funding from the School of Fundamental Sciences and a Massey University-MBIE PSAF grant is gratefully acknowledged.
Acknowledgements
We thank Dr Anne Midwinter, School of Veterinary Sciences, Massey University, for providing an E. coli human O157 isolate and Ian Bruce from the New Zealand Veterinary Pathology Ltd for an isolate of a canine E. coli uropathogenic strain (P191). We are grateful to Kristin Dyet (ESR Kenepuru, Porirua, New Zealand) for selecting and providing NZMS strains for profiling.
Author contributions
Conceptualization, C.O., V.L. and J.R. Methodology, C.O. Investigation, C.O. and C.D. Original draft preparation, C.O. Review and editing, V.L. and J.R.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; 3D, three-dimensional; DOC, sodium deoxycholate; FICI, fractional inhibitory concentration index; FZ, furazolidone; NFZ, nitrofurazone; NIT, nitrofurantoin; NVD, 5-nitrofurans, vancomycin and sodium deoxycholate; Van, vancomycin; WHO, World Health Organization.
References
- 1.Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 3.World Health Organization Antibacterial Agents in Clinical Development: an Analysis of the Antibacterial Clinical Development Pipeline. Vol. 2019. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 4.McCalla DR, Voutsinos D. On the mutagenicity of nitrofurans. Mutat Res-Fund Mol M. 1974;26:3–16. doi: 10.1016/S0027-5107(74)80065-5. [DOI] [PubMed] [Google Scholar]
- 5.Obaseiki-Ebor EE, Akerele JO. Nitrofuran mutagenicity: induction of frameshift mutations. Mutat Res Lett. 1986;175:149–152. doi: 10.1016/0165-7992(86)90114-4. [DOI] [PubMed] [Google Scholar]
- 6.Ny S, Edquist P, Dumpis U, Gröndahl-Yli-Hannuksela K, Hermes J, et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J Glob Antimicrob Re. 2019;17:25–34. doi: 10.1016/j.jgar.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Raja NS. Oral treatment options for patients with urinary tract infections caused by extended spectrum βeta-lactamase (ESBL) producing Enterobacteriaceae. J Infect Public Heal. 2019;12:843–846. doi: 10.1016/j.jiph.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Kwon DH, Lee M, Kim JJ, Kim JG, El-Zaatari FAK, et al. Furazolidone- and nitrofurantoin-resistant Helicobacter pylori: Prevalence and role of genes involved in metronidazole resistance. Antimicrob Agents Chemother. 2001;45:306–308. doi: 10.1128/AAC.45.1.306-308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YI, Jeong S-H, Chung J-W, Park DK, Kim KO, et al. Rifabutin and furazolidone could be the candidates of the rescue regimen for antibiotic-resistant H. pylori in Korea. Can J Infect Dis Med. 2019;2019:1–7. doi: 10.1155/2019/9351801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamani M, Rahbar A, Shokri-Shirvani J. Resistance of Helicobacter pylori to furazolidone and levofloxacin: A viewpoint. WJG. 2017;23:6920–6922. doi: 10.3748/wjg.v23.i37.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vass M, Hruska K, Fránek M. Nitrofuran antibiotics: a review on the application, prohibition and residual analysis. Vet Med (Praha) 2008;53:469–500. doi: 10.17221/1979-VETMED. [DOI] [Google Scholar]
- 12.Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102:459–469. doi: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinho DS, Huf G, Ferreira BLA, Castro H, Rodrigues CR, et al. The study of vancomycin use and its adverse reactions associated to patients of a Brazilian university hospital. BMC Res Notes. 2011;4:1. doi: 10.1186/1756-0500-4-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob Agents Chemother. 2009;53:483–486. doi: 10.1128/AAC.01088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roszell S, Jones C. Intravenous administration issues: a comparison of intravenous insertions and complications in vancomycin versus other antibiotics. J Infus Nurs. 2010;33:112–118. doi: 10.1097/NAN.0b013e3181cfcee4. [DOI] [PubMed] [Google Scholar]
- 16.Van Bambeke F. Lipoglycopeptide antibacterial agents in gram-positive infections: a comparative review. Drugs. 2015;75:2073–2095. doi: 10.1007/s40265-015-0505-8. [DOI] [PubMed] [Google Scholar]
- 17.Crotty MP, Krekel T, Burnham C-AD, Ritchie DJ. New gram-positive agents: the next generation of oxazolidinones and lipoglycopeptides. J Clin Microbiol. 2016;54:2225–2232. doi: 10.1128/JCM.03395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D, Debabov D, Hartsell TL, Cano RJ, Adams S, et al. Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb Perspect Med. 2016;6:a026989. doi: 10.1101/cshperspect.a026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Urdaneta V, Casadesús J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med. 2017;4 doi: 10.3389/fmed.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni . Infect Immun. 2003;71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli . J Bacteriol. 1997;179:2512–2518. doi: 10.1128/JB.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venter H, Mowla R, Ohene-Agyei T, Ma S. RND-type drug efflux pumps from gram-negative bacteria: molecular mechanism and inhibition. Front Microbiol. 2015;06 doi: 10.3389/fmicb.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prieto AI, Ramos-Morales F, Casadesús J. Repair of DNA damage induced by bile salts in Salmonella enterica . Genetics. 2006;174:575–584. doi: 10.1534/genetics.106.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández SB, Cota I, Ducret A, Aussel L, Casadesús J. Adaptation and preadaptation of Salmonella enterica to bile. PLoS Genet. 2012;8:e1002459. doi: 10.1371/journal.pgen.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le VVH, Olivera C, Spagnuolo J, Davies IG, Rakonjac J. In vitro synergy between sodium deoxycholate and furazolidone against enterobacteria. BMC Microbiol. 2020;20 doi: 10.1186/s12866-019-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou A, Kang TM, Yuan J, Beppler C, Nguyen C, et al. Synergistic interactions of vancomycin with different antibiotics against Escherichia coli: trimethoprim and nitrofurantoin display strong synergies with vancomycin against wild-type E. coli . Antimicrob Agents Chemother. 2015;59:276–281. doi: 10.1128/AAC.03502-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 10th ed. M07-A10 Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 29.Campbell J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr Protoc Chem Biol. 2011;3 doi: 10.1002/9780470559277.ch100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID) Eucast definitive document E.Def 1.2, may 2000: terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect. 2000;6:503–508. doi: 10.1046/j.1469-0691.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 31.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 32.Bruniera F, Ferreira F, Saviolli L, Bacci M, Feder D. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19:694–700. [PubMed] [Google Scholar]
- 33.PL H, Yuan YH, Yue TL, Guo CF. Bile acid patterns in commercially available oxgall powders used for the evaluation of the bile tolerance ability of potential probiotics. PLoS One. 2018;13:e0192964-e. doi: 10.1371/journal.pone.0192964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollenbach T. Antimicrobial interactions: mechanisms and implications for drug discovery and resistance evolution. Curr Opin Microbiol. 2015;27:1–9. doi: 10.1016/j.mib.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Hammes WP, Neuhaus FC. On the mechanism of action of vancomycin: inhibition of peptidoglycan synthesis in Gaffkya homari . Antimicrob Agents Chemother. 1974;6:722–728. doi: 10.1128/AAC.6.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieto M, Perkins HR. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971;123:789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/S1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 38.Raphael BH, Pereira S, Flom GA, Zhang Q, Ketley JM, et al. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J Bacteriol. 2005;187:3662–3670. doi: 10.1128/JB.187.11.3662-3670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul S, Alegre KO, Holdsworth SR, Rice M, Brown JA, et al. A single-component multidrug transporter of the major facilitator superfamily is part of a network that protects Escherichia coli from bile salt stress. Mol Microbiol. 2014;92:872–884. doi: 10.1111/mmi.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY antimicrobial surveillance program (1998–2004) Diagn Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Spagnuolo J, Opalka N, Wen WX, Gagic D, Chabaud E, et al. Identification of the gate regions in the primary structure of the secretin pIV. Mol Microbiol. 2010;76:133–150. doi: 10.1111/j.1365-2958.2010.07085.x. [DOI] [PubMed] [Google Scholar]
- 42.Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, et al. Identification and molecular characterisation of New Delhi metallo-β-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int J Antimicrob Agents. 2012;39:529–533. doi: 10.1016/j.ijantimicag.2012.02.017. [DOI] [PubMed] [Google Scholar]