Abstract
Objective: Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disorder where the disease activity itself and the medications used for its treatment, may have adverse effects on ovarian function. This study aimed to assess the ovarian reserve (OR) in SLE patients.
Materials and methods: The anti-müllerian hormone (AMH) and the antral follicle count (AFC), two markers to evaluate the OR was assessed in 64 SLE patients and compared to normal individuals. Additionally, we assessed whether the disease per se or the pharmacological treatments affect the OR.
Results: Patients with SLE displayed alterations in the OR regardless of the presence of alterations of the menstrual cycle. The AFC and AMH were significantly lower in SLE patients with and without menstrual alterations when compared to control individuals (p<0.0001). However, the AFC and AMH levels were significantly correlated (p=0.006) in the SLE patients with menstrual alterations. Except for hydroxychloroquine that was statistically higher in SLE patients with menstrual alterations (p=0.04), the cumulative dose for cyclophosphamide, corticosteroid, and methotrexate was similar in SLE patients regardless of the occurrence of menstrual alterations.
Conclusion: The monitoring of AMH and AFC in SLE patients should be used to detect the rapid and irreversible decline of the OR to provide a possibility of pregnancy to the SLE patients.
Key Words: Ovarian Reserve, Anti-Mullerian Hormone, Ovarian Follicle, Systemic Lupus Erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic and multisystemic autoimmune disorder. It has a variable course and prognosis and the symptoms may range from mild to severe (1, 2). SLE has a 9:1 female to male incidence ratio (3), affecting mostly females during childbearing age (4,5). Still, fertility is considered to be normal (3,4,6,7). However, fecundity naturally declines with women’s age (8,9). Specifically, ovarian reserve (OR), a term used to refer to the quantity and quality of the remaining oocytes in the ovaries (10) decreases as the women age.
Disease activity and the medications used for treatment can influence the ovarian function of SLE patients. The use of cytotoxic medications, like cyclophosphamide (CYC), may impact the ovarian function of these patients, leading to menstrual irregularities (11–15), infertility (16), and primary ovarian insufficiency (14,17–19). The menstrual disorders caused by CYC treatment are linked to the dose and patient’s age (11,12,20). Additionally, the use of non-steroidal anti-inflammatory drugs (NSAIDs) and high-dose corticosteroids, might contribute to infertility and menstrual abnormalities of SLE patients, respectively (17). Overall, medication-mediated immunosuppression may prone patients to a high risk of infections (1).
The prognosis of pregnancy for SLE patients is favorable, especially when the disease has been quiescent (21), however, predictors of maternal and obstetrical complications are active SLE, positive antiphospholipid antibodies, antihypertensive treatments, and low platelet count (1,21,22). The risks of those pregnancies include flares of disease, preeclampsia, fetal loss, and preterm birth (4,17). Alternatively to natural pregnancies, assisted reproduction techniques (ART) offer the same safety and efficacy as in the general population and have been successfully performed in SLE patients (23,24).
Autoimmune mechanisms have been associated with the reduction of the OR causing premature ovarian failure (POF) (25,26) as shown by the detection of anti-ovarian antibodies whose presence was found to be related to early ovarian aging. However, infertility in SLE patients has been mainly attributed to CYC therapy. For instance; a study from Thailand reported that 12 % of SLE patients (n=11) developed premature ovarian failure. In a retrospective study from Lucknow India, 17% of SLE patients (n=6) had premature menopause (27). A study that included 71 SLE patients, reported ovarian failure and premature menopause in 15% (n=11) and 11% (n=9) of the patients, respectively (28). Infertility was reported in 16% (n=33) of the SLE patients from Helsinki, Finland (29). Sustained amenorrhea was reported in 80% of patients that presented amenorrhea due to CYC (16). Also, Appenzeller et al. reported sustained amenorrhea in SLE patients (13). Also, a prospective study that included 110 SLE patients from Kolkata, India, reported gonadal insufficiency and premature ovarian failure in 33% (n=22) and 2.7% (n=2) patients, respectively (12).
Therefore, timely and appropriate evaluation of subclinical ovarian damage in SLE patients has important clinical implications. In this regard, the OR can be evaluated directly by ultrasound imaging of the ovaries in which the follicles are counted for the antral follicle count (AFC) or indirectly by the measurement of the circulating levels of the day-3 follicle-stimulating hormone (FSH) or the anti-Müllerian hormone (AMH) (30). Since the AMH is produced by growing follicles it represents the remaining primordial follicles and can accurately estimate the OR (30,31).
The OR of SLE patients has been previously assessed. Ulung et al. reported a lower AFC and ovarian volume along with higher FSH and LH levels in SLE patients (n=20) when compared to healthy controls (32). De Araujo reported, lower AFC and AMH levels in SLE patients when compared to controls (33). Similarly, the AMH levels were found to be lower in SLE-patients in contrast to controls (34–36). Malheiro et al. in 2014, also reported low AMH values however FSH levels were not different from controls (37). Recently, AMH levels (< 1.0 ng/mL) representative of a diminished OR, were reported from a group of African-American women with SLE (38). In contrast, Li et al. showed the opposite (39). While others have not found a difference in serum AMH levels of SLE patients and the general population (40,41).
Usually, the low AMH levels in SLE patients have been dichotomized for the presence of menstrual alterations, finding that the patients with abnormal menstruation had lower AMH levels than those of SLE patients without menstrual alterations (35). However, this has not been observed consistently (37,42).
This study aimed to assess the AFC and AMH serum levels in a cohort of Mexican SLE patients and compare them to those of healthy controls and evaluate whether the pharmacological treatments used to treat this disease affect the OR.
Materials and methods
Subjects and samples: This study was approved by the Bioethics Committee of the Hospital Universitario “Dr. José Eleuterio González” (#GI13-009). This study complied with the principles of the Declaration of Helsinki. Patients were provided with written information about the study before giving consent.
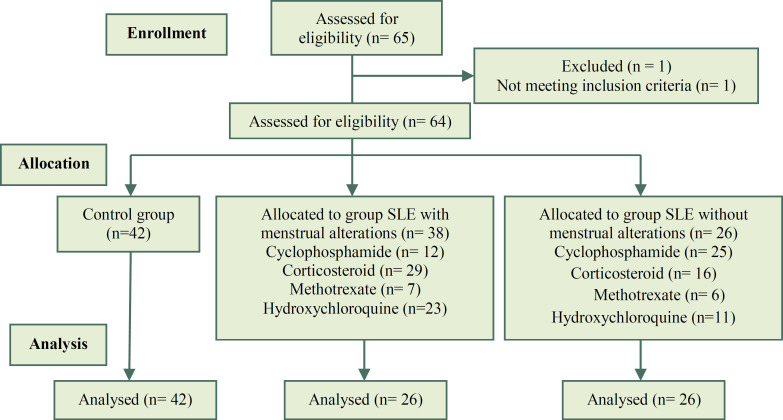
A prospective and comparative study was performed. In which, 64 patients who attended the Rheumatology Service of the Hospital Universitario “Dr. Jose Eleuterio Gonzalez”, and that were diagnosed with SLE according to the American College of Rheumatology (ACR) criteria were included. The SLE patients included in this study were 18-39 years old. The SLE patients were divided into two groups: 1) SLE with alterations in the menstrual cycle (n=38); 2) SLE without alterations in the menstrual cycle (n=26) (Figure 1). Amenorrhea was considered when there was an absence of at least three menstrual periods in a row.
Patients with a clinical history of endometriosis, Polycystic Ovarian Syndrome (PCOS), hysterectomy, oophorectomy, or any other kind of ovarian surgery, pregnant, with primary amenorrhea, or treated for cancer (either with radiotherapy or chemotherapy) were excluded from the study. A group of 42 healthy women that were part of the oocyte donation program of the University Center of Human Reproduction of the same hospital was included as a control.
Transvaginal ultrasonography and antral follicle count: On the third day of the menstrual cycle, transvaginal ultrasonography was performed using a Toshiba Xario 100 (Toshiba Medical Systems Corporation, Nasu, Japan) with a 7.5-MHz vaginal transducer. Briefly, with an empty bladder and the patient in a lithotomy position, the transducer was advanced into the vagina angling laterally until the ovary was seen. The length and Antero-Posterior (AP) measurements were obtained in the longitudinal plane, while in the transverse plane the antral follicles measuring 2–9 mm in diameter were counted. The total number of follicles in the ovaries were added for the total antral follicle count (AFC).
Measurement of the anti-Mullerian hormone: Blood samples were obtained from the subjects after an overnight fast. Briefly, the blood was drawn into plain serum tubes and centrifuged within one hour, the serum was separated and stored at -20°C until further analysis.
Anti-Müllerian hormone (AMH) serum levels were assessed in a reference laboratory by using the ACTIVE Müllerian Inhibiting Substance/Anti-Müllerian (MIS/AMH) enzyme-linked immunosorbent assay (Beckmann Coulter). The AMH assay demonstrated stable intra- and inter-assay coefficients of variation of 5% and a functional sensitivity of 0.35 ng/ml.
Statistical analysis: Data are expressed as mean ± standard deviation (SD). Normality was assessed with the Kolmogorov-Smirnov test and statistical comparisons performed by Welch t-test, U Mann-Whitney, or Kruskal-Wallis with Dunn’s multiple comparison test. Additionally, the correlation between AMH and AFC was performed through the Spearman correlation test. Calculations were performed with GraphPad Prism version 8.4.2 for Windows (GraphPad Software, San Diego, CA, USA). A p-value <0.05 was considered statistically significant.
Figure 1.
CNSORT Flow chart of the study
Results
A total of 106 individuals were enrolled in this study. The SLE group consisted of 64 patients with SLE; within the SLE group, 38 patients had menstrual alterations while the remaining 26 did not. Also, 42 normal healthy women were included as a control group.
Regarding the obstetrical history, there was no difference (p=0.93) in the number of pregnancies of SLE patients (1.14±1.43) and the controls (1.02±1.17). Additionally, no difference (p=0.14) in the number of abortions was found between the SLE (0.22±0.52) and control (0.10±0.38) groups.
The clinical features of the patients with SLE are shown in Table 1. There was not a significant difference (p=0.55) in the age of SLE patients with and without menstrual alterations (29.08 ±6.11 vs 28.08 ±7.00), respectively. As expected, the date of last menstruation was statistically different between the groups (2.92 months vs 0; p<0.005).
Table 1.
Comparisons of the clinical features of SLE patients with and without menstrual alterations and control
|
Menstrual alterations (n=38) vs
no menstrual alterations (n=26) |
Menstrual alterations (n=38) vs
control (n=42) |
No menstrual alterations (n=26)
vs control (n=42) |
||||
|---|---|---|---|---|---|---|
| Difference SD | p-value | Difference SD | p-value | Difference ± SD | p-value | |
| Age (years) | 1±1.645 | 0.556 | 0.63±1.46 | 0.669 | -0.37±1.725 | 0.828 |
| Pregnancy | 0.44±0.366 | 0.193 | -0.03±0.41 | 0.934 | -0.47± 0.462** | 0.269 |
| AMH (ng/dl) | 0.04±0.084 | 0.404 | -1.17±0.12 | <0.0001* | -1.21±0.14** | <0.0001* |
| AFC | 0.18±0.452 | 0.684 | -3.79±0.54 | <0.0001* | -3.97± 0.783 ** | <0.0001* |
AMH: anti mullerian hormone, AFC: antral follicular count, SD: standard deviation
Age was compared with Welch’s T-test.
The number of pregnancies, AMH, and AFC comparisons were made with U de Mann-Whitney
We also compared the patients with SLE and menstrual alterations with the control group and we did not find a significant difference between their ages (0.669).
However, the AMH and AFC were significantly lower in the SLE patients with menstrual alterations when compared to the control group (p<0.0001).
Next, the age of SLE patients without menstrual alterations was compared with the age of the control group, finding those were not statistically different (p=0.828). The time when the last menstruation occurred was similar. The SLE patients that did not have menstrual alterations also had significantly lower AMH and AFC levels than those found in the control group (p<0.0001) (Table 1).
A statistically significant positive correlation of the AMH and the AFC was observed in patients with SLE and menstrual alterations (p<0.006), however, the AMH and AFC were not correlated in the other groups (Table 2).
Table 2.
Correlation between AMH and AFC in the groups studied
| AFC | |||
|---|---|---|---|
| SLE with menstrual alterations | AMH (ng/dl) | Rho | 0.45 |
| p-value | 0.006* | ||
| n | 38 | ||
| Pregnancy | Rho | -0.185 | |
| p-value | 0.463 | ||
| n | 26 | ||
| AMH (ng/dl) | Rho | -0.0173 | |
| p-value | 0.286 | ||
| n | 42 |
AMH: anti mullerian hormone, AFC: antral follicular count, SLE: systematic lopus erythematous
AMH and AFC were correlated with the Spearman correlation.
Regarding the treatment, the cumulative dose for cyclophosphamide, corticosteroid, and methotrexate was similar in both SLE groups. However, the cumulative dose for hydroxychloroquine was statistically higher in SLE patients with menstrual alterations (p<0.04) (Table 3).
Table 3.
Cumulative doses (in grams) for cyclophosphamide, corticosteroid, hydroxychloroquine, and methotrexate
| With menstrual alterations | n | Without menstrual alterations | n | |
|---|---|---|---|---|
| Cyclophosphamide | 2.51±4.46 | 12 | 3.47±6.25 | 25 |
| Corticosteroid | 13.65±17.69 | 29 | 13.65±16.66 | 16 |
| Methotrexate | 11.42±4.04 | 7 | 10.41±2.45 | 6 |
| Hydroxychloroquine | 265.21±93.46 | 23 | 254.54±68.75 | 11 |
Comparisons were made with U de Mann-Whitney test.
Discussion
In this report, we found that individuals with SLE have a decreased OR by the assessment of the AMH and AFC. Nowadays, both the AMH and the AFC are extensively used to estimate the OR, to predict the ovarian response to hormonal stimulation in assisted reproduction techniques, and to estimate menopause (31). As such, the OR is used to determine the capacity of the ovary to provide egg cells that are capable of fertilization resulting in a healthy and successful pregnancy. In assisted reproductive clinics, this parameter is considered critical when assessing the female reproductive potential (43).
Aging naturally decreases the ovarian reserve. Although sometimes there is no apparent cause, several factors besides age can cause diminished ovarian reserve (44). These include ovarian surgery, chemo and radiotherapy, pelvic infection, genetic abnormalities, and autoimmune disorders. In this work, we have investigated the OR in a pathology related to this last condition: patients with SLE.
Previous reports showed that serum AMH levels were lower in SLE patients (33,35,37). However, other studies reported that patients with SLE do not display significant alterations in the OR (40,41). Due to the existing controversy, we aimed to study the AMH and AFC values in a group of patients with SLE. Our study revealed that patients with SLE have statistically significant lower values of both, AMH and AFC regardless of the occurrence of menstrual alterations when compared to normal individuals. A previous report also showed lower AMH and CFA levels in patients with SLE but not differences when these patients were classified as having menstrual alterations or not (45).
In this study, all the SLE patients were under treatment with cyclophosphamide, corticosteroids, methotrexate, or hydroxychloroquine. However, except for hydroxychloroquine, we did not find a significant difference between the doses used in the groups with or without menstrual alterations. A possible relationship between those drugs and the ovarian alterations need to be further explored. Cyclophosphamide is the most frequent treatment used in the therapy of SLE and is associated with menstrual disorders (20,46). This drug also affects the ovary resulting in ovarian failure or low AMH levels (47,48). Regarding hydroxychloroquine, previous reports showed no effect on AMH after hydroxychloroquine treatment (49).
The OR of the patients in the interquartile range of 23.25 - 34.50 years was diminished but not to levels that seriously compromise the response to hormonal stimulation that is commonly used in assisted reproductive programs. At the time of this study, none of the patients with SLE were seeking to become pregnant.
A clear limitation of this study is the number of patients that were included in the analysis. On the other hand, the major strength is associated with the fact that we were able to recruit valuable information about the fertility status of individuals with SLE. We consider that both AMH and AFC must be periodically assessed since the ovarian reserve may decrease faster in SLE patients. If a diminished ovarian reserve is diagnosed soon enough in these patients, it is possible to vitrify eggs for future use or to try an assisted reproduction treatment. In conclusion, we report that patients with SLE had a decreased OR regardless of the occurrence of menstrual cycle alterations
Conclusion
The monitoring of AMH and AFC in SLE patients should be used to detect the rapid and irreversible decline of the OR to provide a possibility of pregnancy to the SLE patients.
Acknowledgments
This study complied with the principles of the 1964 Helsinki declaration and its later amendments or comparable ethical standards and was approved institutionally by the Bioethics Committee of the University Hospital “Dr. José Eleuterio González” (#GI13-009).
Conflict of Interests
Authors have no conflict of interests.
Notes:
Citation: Morales-Martínez FA, Salas-Castro C, García-Garza MR, Valdés-Martínez O, García-Luna SM, Garza-Elizondo M, et al. Evaluation of the Ovarian Reserve in Women With Systemic Lupus Erythematosus. J Fam Reprod Health 2021; 15(1): 38-44.
References
- 1.Justiz Vaillant AA, Goyal A, Bansal P, Varacallo M. StatPearls [Internet]. Treasure. Island (FL): StatPearls Publishing; 2020. Systemic Lupus Erythematosus. [PubMed] [Google Scholar]
- 2.Ugarte-Gil MF, González LA, Alarcón GS. Lupus: the new epidemic. Lupus. 2019;28:1031–50. doi: 10.1177/0961203319860907. [DOI] [PubMed] [Google Scholar]
- 3.Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: Clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011;40:42–9. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer-Betz R, Specker C. Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract Res Clin Rheumatol. 2017;31:397–414. doi: 10.1016/j.berh.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Kartoz CR. Reproductive Health Concerns in Women with Systemic Lupus Erythematosus. MCN Am J Matern Child Nurs. 2015;40:220–6. doi: 10.1097/NMC.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 6.Chambers C, Michaud K. Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64:668–74. doi: 10.1002/acr.21593. [DOI] [PubMed] [Google Scholar]
- 7.Ekblom-Kullberg S, Kautiainen H, Alha P, Helve T, Leirisalo-Repo M, Julkunen H. Reproductive Health in Women With Systemic Lupus Erythematosus Compared to Population Controls. Scand J Rheumatol. 2009;38:375–80. doi: 10.1080/03009740902763099. [DOI] [PubMed] [Google Scholar]
- 8.Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, et al. Fertility and ageing. Hum Reprod Update. 2005;11:261–76. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 9.Crawford NM, Steiner AZ. Age-related infertility. Obstet Gynecol Clin North Am. 2015;42:15–25. doi: 10.1016/j.ogc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. 2016;9:63–9. doi: 10.4103/0974-1208.183514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Crespo MR, Gomez-Reino JJ, Merino R, Ciruelo E, Gomez-Reino FJ, Muley R, et al. Menstrual Disorders in Girls With Systemic Lupus Erythematosus Treated With Cyclophosphamide. Br J Rheumatol. 1995;34:737–41. doi: 10.1093/rheumatology/34.8.737. [DOI] [PubMed] [Google Scholar]
- 12.Mandal D, Sircar G, Pandey A, Mandal S, Banerjee D, Ghosh A, et al. Menstrual and gonadal function alterations in women with systemic lupus erythematosus. J Assoc Physicians India. 2015;63:38–42. [PubMed] [Google Scholar]
- 13.Appenzeller S, Blatyta PF, Costallat LT. Ovarian Failure in SLE Patients Using Pulse Cyclophosphamide: Comparison of Different Regimes. Rheumatol Int. 2008;28:567–71. doi: 10.1007/s00296-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 14.Ulug P, Oner G, Kasap B, Akbas EM, Ozcicek F. Evaluation of Ovarian Reserve Tests in Women with Systemic Lupus Erythematosus. Am J Reprod Immunol. 2014;72:85–8. doi: 10.1111/aji.12249. [DOI] [PubMed] [Google Scholar]
- 15.Hickman RA, Gordon C. Causes and management of infertility in systemic lupus erythematosus. Rheumatology (Oxford) 2011;50:1551–8. doi: 10.1093/rheumatology/ker105. [DOI] [PubMed] [Google Scholar]
- 16.Wetzels JF. Cyclophosphamide-induced gonadal toxicity: A treatment dilemma in patients with lupus nephritis? Neth J Med. 2004;62:347–52. [PubMed] [Google Scholar]
- 17.Bermas BL, Sammaritano LR. Fertility and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Fertil Res Pract. 2015;27:13. doi: 10.1186/s40738-015-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oktem O, Guzel Y, Aksoy S, Aydin E, Urman B. Ovarian function and reproductive outcomes of female patients with systemic lupus erythematosus and the strategies to preserve their fertility. Obstet Gynecol Surv. 2015;70:196–210. doi: 10.1097/OGX.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 19.Petri M. Pregnancy and Systemic Lupus Erythematosus. Best Pract Res Clin Obstet Gynaecol. 2020;64:24–30. doi: 10.1016/j.bpobgyn.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Boumpas DT, Austin HA, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for Sustained Amenorrhea in Patients With Systemic Lupus Erythematosus Receiving Intermittent Pulse Cyclophosphamide Therapy. Ann Intern Med. 1993;119:366–9. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Felten R, Sagez F, Gavand PE, Martin T, Korganow AS, Sordet C, et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med. 2019;6:e000303. doi: 10.1136/lupus-2018-000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76:476–85. doi: 10.1136/annrheumdis-2016-209770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orquevaux P, Masseau A, Le Guern V, Gayet V, Vauthier D, Guettrot-Imbert G, et al. In vitro fertilization in 37 women with systemic lupus erythematosus or antiphospholipid syndrome: A series of 97 procedures. J Rheumatol. 2017;44:613–8. doi: 10.3899/jrheum.160462. [DOI] [PubMed] [Google Scholar]
- 24.Reggia R, Andreoli L, Sebbar H, Canti V, Ceccarelli F, Favaro M, et al. An observational multicentre study on the efficacy and safety of assisted reproductive technologies in women with rheumatic diseases. Rheumatol Adv Pract. 2019;22:rkz005. doi: 10.1093/rap/rkz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoek A, Schoemaker J, Drexhage HA. Premature Ovarian Failure and Ovarian Autoimmunity. Endocr Rev. 1997;18:107–34. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 26.Goswami R, Marwaha RK, Goswami D, Gupta N, Ray D, Tomar N, et al. Prevalence of Thyroid Autoimmunity in Sporadic Idiopathic Hypoparathyroidism in Comparison to Type 1 Diabetes and Premature Ovarian Failure. J Clin Endocrinol Metab. 2006;91:4256–9. doi: 10.1210/jc.2006-1005. [DOI] [PubMed] [Google Scholar]
- 27.Singh G, Misra R, Aggarwal A. Ovarian insufficiency is major short-term toxicity in systemic lupus erythematosus patients treated with cyclophosphamide. J Assoc Physicians India. 2016;64:28–31. [PubMed] [Google Scholar]
- 28.Medeiros MM, Silveira VA, Menezes AP, Carvalho RC. Risk factors for ovarian failure in patients with systemic lupus erythematosus. Braz J Med Biol Res. 2001;34:1561–8. doi: 10.1590/s0100-879x2001001200008. [DOI] [PubMed] [Google Scholar]
- 29.Ekblom-Kullberg S, Kautiainen H, Alha P, Helve T, Leirisalo-Repo M, Julkunen H. Reproductive health in women with systemic lupus erythematosus compared to population controls. Scand J Rheumatol. 2009;38:375–80. doi: 10.1080/03009740902763099. [DOI] [PubMed] [Google Scholar]
- 30.Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217:129–40. doi: 10.1016/j.ajog.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Weenen C, Laven JS, von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 32.Ulug P, Oner G, Kasap B, Akbas EM, Ozcicek F. Evaluation of Ovarian Reserve Tests in Women with Systemic Lupus Erythematosus. Am J Reprod Immunol. 2014;72:85–8. doi: 10.1111/aji.12249. [DOI] [PubMed] [Google Scholar]
- 33.De Araujo DB, Yamakami LY, Aikawa NE, Bonfá E, Viana VS, Pasoto SG, et al. Ovarian reserve in adult patients with childhood-onset lupus: A possible deleterious effect of methotrexate? Scand J Rheumatol. 2014;43:503–11. doi: 10.3109/03009742.2014.908237. [DOI] [PubMed] [Google Scholar]
- 34.Lawrenz B, Henes J, Henes M, Neunhoeffer E, Schmalzing M, Fehm T, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: Evaluation by using anti-Muellerian hormone. Lupus. 2011;20:1193–7. doi: 10.1177/0961203311409272. [DOI] [PubMed] [Google Scholar]
- 35.Gao H, Ma J, Wang X, Lv T, Liu J, Ren Y, et al. Preliminary study on the changes of ovarian reserve, menstruation, and lymphocyte subpopulation in systemic lupus erythematosus (SLE) patients of childbearing age. Lupus. 2018;27:445–53. doi: 10.1177/0961203317726378. [DOI] [PubMed] [Google Scholar]
- 36.Luo W, Mao P, Zhang L, Chen X, Yang Z. Assessment of ovarian reserve by serum anti-Müllerian hormone in patients with systemic lupus erythematosus: A meta-analysis. Ann Palliat Med. 2020;9:207–15. doi: 10.21037/apm.2020.02.11. [DOI] [PubMed] [Google Scholar]
- 37.Malheiro OB, Rezende CP, Rocha AL, Del Puerto HL, Ferreira GA, Reis FM. Regular menstrual cycles do not rule out ovarian damage in adult women with systemic lupus erythematosus. Gynecol Endocrinol. 2014;30:701–4. doi: 10.3109/09513590.2014.922949. [DOI] [PubMed] [Google Scholar]
- 38.Angley M, Lim SS, Spencer JB, Howards PP. Infertility Among African American Women With Systemic Lupus Erythematosus Compared to Healthy Women: A Pilot Study. Arthritis Care Res (Hoboken) 2020;72:1275–81. doi: 10.1002/acr.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LI Yan-Mei, ZHAO Yi, CI Chun-zeng, LI Zhan. Anti-Müllerian hormone:its significance in female lupus patients and relatin with anti-ovarian antibody. Chinese J Rheumatol. 2010;14:305–7. [Google Scholar]
- 40.Di Mario C, Petricca L, Gigante MR, Barini A, Barini A, Varriano V, et al. Anti-Müllerian hormone serum levels in systemic lupus erythematosus patients: Influence of the disease severity and therapy on the ovarian reserve. Endocrine. 2019;63:369–75. doi: 10.1007/s12020-018-1783-1. [DOI] [PubMed] [Google Scholar]
- 41.Gasparin AA, Souza L, Siebert M, Xavier RM, Chakr RM, Palominos PE, et al. Assessment of anti-Müllerian Hormone Levels in Premenopausal Patients With Systemic Lupus Erythematosus. Lupus. 2016;25:227–32. doi: 10.1177/0961203315598246. [DOI] [PubMed] [Google Scholar]
- 42.Pasoto SG, Mendonça BB, BonfáE E. Menstrual disturbances in patients with systemic lupus erythematosus without alkylating therapy: Clinical, hormonal and therapeutic associations. Lupus. 2002;11:175–80. doi: 10.1191/0961203302lu163oa. [DOI] [PubMed] [Google Scholar]
- 43.Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet. 2020;37:89–100. doi: 10.1007/s10815-019-01622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amanvermez R, Tosun M. An update on ovarian aging and ovarian reserve tests. Int J Fertil Steril. 2015;9:411–5. doi: 10.22074/ijfs.2015.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghaleb RM, Fahmy KA. Anti-Müllerian hormone: A marker for ovarian function in systemic lupus erythematosus patients treated with cyclophosphamide. Joint Bone Spine. 2013;80:434–5. doi: 10.1016/j.jbspin.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Crespo MR, Gomez-Reino JJ, Merino R, Ciruelo E, Gomez-Reino FJ, Muley R, et al. Menstrual disorders in girls with systemic lupus erythematosus treated with cyclophosphamide. Br J Rheumatol. 1995;34:737–41. doi: 10.1093/rheumatology/34.8.737. [DOI] [PubMed] [Google Scholar]
- 47.Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365–72. doi: 10.1095/biolreprod52.2.365. [DOI] [PubMed] [Google Scholar]
- 48.Morel N, Bachelot A, Chakhtoura Z, Ghillani-Dalbin P, Amoura Z, Galicier L, et al. Study of anti-müllerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab. 2013;98:3785–92. doi: 10.1210/jc.2013-1235. [DOI] [PubMed] [Google Scholar]
- 49.Mok CC, Chan PT, To CH. Anti-müllerian hormone and ovarian reserve in systemic lupus erythematosus. Arthritis Rheum. 2013;65:206–10. doi: 10.1002/art.37719. [DOI] [PubMed] [Google Scholar]