Abstract
Ten strains, BG-AF3-AT, pH52_RY, WF-MT5-AT, BG-MG3-A, Lr3000T, RRLNB_1_1, STM3_1T, STM2_1, WF-MO7-1T and WF-MA3-C, were isolated from intestinal or faecal samples of rodents, pheasant and primate. 16S rRNA gene analysis identified them as Limosilactobacillus reuteri . However, average nucleotide identity and digital DNA–DNA hybridization values based on whole genomes were below 95 and 70 %, respectively, and thus below the threshold levels for bacterial species delineation. Based on genomic, chemotaxonomic and morphological analyses, we propose five novel species with the names Limosilactobacillus balticus sp. nov. (type strain BG-AF3-AT=DSM 110574T=LMG 31633T), Limosilactobacillus agrestis sp. nov. (type strain WF-MT5-AT=DSM 110569T=LMG 31629T), Limosilactobacillus albertensis sp. nov. (type strain Lr3000T=DSM 110573T=LMG 31632T), Limosilactobacillus rudii sp. nov. (type strain STM3_1T=DSM 110572T=LMG 31631T) and Limosilactobacillus fastidiosus sp. nov. (type strain WF-MO7-1T=DSM 110576T=LMG 31630T). Core genome phylogeny and experimental evidence of host adaptation of strains of L. reuteri further provide a strong rationale to consider a number of distinct lineages within this species as subspecies. Here we propose six subspecies of L. reuteri : L. reuteri subsp. kinnaridis subsp. nov. (type strain AP3T=DSM 110703T=LMG 31724T), L. reuteri subsp. porcinus subsp. nov. (type strain 3c6T=DSM 110571T=LMG 31635T), L. reuteri subsp. murium subsp. nov. (type strain lpuph1T=DSM 110570T=LMG 31634T), L. reuteri subsp. reuteri subsp. nov. (type strain F 275T=DSM 20016T=ATCC 23272T), L. reuteri subsp. suis subsp. nov. (type strain 1063T=ATCC 53608T=LMG 31752T) and L. reuteri subsp. rodentium subsp. nov. (type strain 100-23T=DSM 17509T=CIP 109821T).
Keywords: evolution, host adaptation, Limosilactobacillus reuteri subspecies, novel Limosilactobacillus species, phylogenetic lineages
Introduction
The vertebrate gastrointestinal tract harbours diverse microbial communities that are referred to as the gut microbiota. Composed of microbes that often function as symbionts, these communities contribute substantially to the health and performance of their vertebrate hosts through the provision of nutrients and vitamins, cues for the development of the immune system and protection from bacterial and viral pathogens [1–3]. Limosilactobacillus reuteri (formerly Lactobacillus reuteri ) [4], classified in the genus Limosilactobacillus of the family Lactobacillaceae , is a member of the intestinal microbiota of humans and other vertebrates and has been used as a model organism to study the adaptation of intestinal micro-organisms to their hosts [5–8]. The family Lactobacillaceae currently comprises more than 300 species [4], including species that are adapted to vertebrate and invertebrate hosts, free-living lactobacilli and nomadic organisms which oscillate between host-associated and environmental habitats [9]. The genus Limosilactobacillus (previously designated as the L. reuteri group) contains 17 species, including L. alvi , L. antri , L. caviae , L. coleohominis , L. equigenerosi , L. fermentum , L. frumenti , L. gastricus , L. gorillae , L. ingluviei , L. mucosae , L. oris , L. panis , L. pontis , L. reuteri , L. secaliphilus and L. vaginalis [4, 9, 10], and is considered adapted to vertebrates with very few exceptions ( L. fermentum and L. secaliphilus ) [9].
The species L. reuteri is extremely well studied due to its wide use as probiotics. Isolates have been obtained mainly from humans and several animal species including mice, rats, pigs, ruminants and birds, as well as fermented food [7, 8]. This species is divided into six phylogenetic clusters that show a clear association with particular vertebrate species [5–7, 11], and the genome content of these lineages is reflective of the niche characteristics in different vertebrate species [6]. These findings point to host specialization, which has been experimentally demonstrated in mice and chicken [5–7, 11]. Molecular factors that confer host specialization and allow stable persistence of L. reuteri in the intestinal tract of animals have been identified and relate to acid resistance, syntrophic interactions with Bifidobacterium , facilitation in biofilms with other lactobacilli, adhesion to non-secretory epithelia and biofilm formation, and sucrose-dependent biofilm formation [11–16]. Species in Limosilactobacillus including L. reuteri are also stable elements in food fermentation, particularly cereal fermentation [17, 18].
Isolation and ecology
To expand our understanding of the evolution and host adaptation of L. reuteri , we sampled wild and zoo animals with the goal of isolating a wider array of L. reuteri strains. Ten strains, BG-AF3-AT, pH52_RY, WF-MT5-AT, BG-MG3-A, Lr3000T, RRLNB_1_1, STM3_1T, STM2_1, WF-MO7-1T and WF-MA3-C were selected and analysed in the current study. Five of them were obtained from wild rodents during a previous study [13]: strains BG-AF3-AT, WF-MT5-AT, BG-MG3-A, WF-MO7-1T and WF-MA3-C were isolated from the jejunum of yellow-necked mouse (Apodemus flavicollis), field vole (Microtus agrestis), bank vole (Myodes glareolus), root vole (Microtus oeconomus) and common vole (Microtus arvalis), respectively, in the Vilnius area (Lithuania) (Table 1). Strain pH52_RY was isolated from the intestine of a pheasant (Phasianus colchicus) in Sweden (Swedish University of Agricultural Sciences, Uppsala, Sweden), Lr3000T from the stomach of a hamster in the USA by BioGaia (Stockholm, Sweden), RRLNB_1_1 from the faecal sample of a red ruffed lemur (Varecia rubra) raised at San Francisco Zoo (CA, USA), and STM3_1T and STM2_1 from faeces of a striped mouse (Rhabdomys pumilio) raised at the Henry Doorly Zoo and Aquarium (Omaha, NE, USA) (Table 1). Strains were either isolated on MRS (De Man, Rogosa, Sharpe) or modified MRS (mMRS) medium, and cultivated under anaerobic conditions at 37 °C. mMRS refers to MRS supplied with 10 g l−1 maltose and 5 g l−1 fructose. Colonies were then purified, sub-cultured and preserved as glycerol stocks at −80 °C.
Table 1.
Genomic characteristics, host origins and quality of genome assemblies of ten strains classified as novel Limosilactobacillus species
|
|
L. balticus sp. nov. |
L. agrestis sp. nov. |
L. albertensis sp. nov. |
L. rudii sp. nov. |
L. fastidiosus sp. nov. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
BG-AF3-AT |
pH52_RY |
WF-MT5-AT |
BG-MG3-A |
Lr3000T |
RRLNB_1_1 |
STM3_1T |
STM2_1 |
WF-MO7-1T |
WF-MA3-C |
|
|
Host origin |
Yellow-necked mouse (Apodemus flavicollis) |
Pheasant (Phasianus colchicus) |
Field vole (Microtus agrestis) |
Bank vole (Myodes glareolus) |
Hamster |
Red ruffed lemur (Varecia rubra) |
Striped mouse (Rhabdomys pumilio) |
Striped mouse (Rhabdomys pumilio) |
Root vole (Microtus oeconomus) |
Common vole (Microtus arvalis) |
|
GenBank ID |
GCA_014145615.1 |
GCA_014145605.1 |
GCA_014145585.1 |
GCA_014145545.1 |
GCA_014145555.1 |
GCA_014145525.1 |
GCA_014145455.1 |
GCA_014145435.1 |
GCA_014145505.1 |
GCA_014145425.1 |
|
IMG Genome ID |
2860350408 |
2860354513 |
2860352526 |
2860356824 |
2860358791 |
2860361239 |
2860373262 |
2860375653 |
2860378031 |
2860379800 |
|
Genome size (bp) |
2 088 966 |
2 169 741 |
1 899 103 |
1 863 625 |
2 452 361 |
2 354 505 |
2 316 339 |
2 307 466 |
1 720 134 |
1 728 435 |
|
Coverage (×) |
874 |
940 |
937 |
896 |
768 |
1027 |
1194 |
889 |
1121 |
1107 |
|
G+C content (mol%) |
38.3 |
38.2 |
38.0 |
37.9 |
38.8 |
38.5 |
38.5 |
38.5 |
39.1 |
39.1 |
|
No. of contigs |
76 |
94 |
73 |
67 |
78 |
72 |
63 |
61 |
44 |
64 |
|
N50 (bp) |
136 912 |
105 538 |
73 671 |
115 417 |
116 418 |
121 174 |
131 342 |
118 051 |
112 343 |
99 702 |
|
Total genes |
2117 |
2310 |
1986 |
1966 |
2447 |
2383 |
2390 |
2377 |
1768 |
1820 |
|
No. of CDS* |
1992 |
2183 |
1881 |
1845 |
2317 |
2259 |
2247 |
2233 |
1668 |
1718 |
*CDS, coding sequence.
A previous study classified several of these strains as L. reuteri on the basis of partial sequences of 16S rRNA genes that were >98.5 % identical to L. reuteri [13]. However, analysis of whole-genome sequences not only revealed that these strains fell into phylogenetic lineages that were distinct from the lineages described for L. reuteri [5, 7], but also showed that the average nucleotide identity (ANI) and digital DNA–DNA hybridization (dDDH) values with L. reuteri type strain DSM 20016T ranged from 73.4 to 93.6% and from 20.8 to 54.9 %, respectively, which are below the ANI (95 %) and dDDH (70 %) thresholds that are currently accepted for a bacterial species [19–23]. We therefore concluded that these ten strains could not be assigned to any validly published species in the genus Limosilactobacillus . Therefore, in the present study, we propose that these strains represent five novel Limosilactobacillus species based on whole-genome sequencing, 16S rRNA gene sequence analysis, chemotaxonomic analysis and morphological analysis.
Phylogeny based on the 16S rRNA gene
Genomic DNA of each of the ten strains was extracted from overnight cultures using the Wizard Genomic DNA Purification Kit (Promega) according to the manufacturer’s protocol for Gram-positive bacteria. DNA quality and quantity were estimated using a NanoDrop Spectrophotometer ND-1000 (Thermo Fisher Scientific). Libraries for whole-genome sequencing were constructed using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs) and sequenced on the Illumina HiSeqX platform to produce paired-end reads with the length of 150 bp at the McGill University and Génome Québec Innovation Centre (Montréal, QC, Canada). Quality control (QC) of each sequencing dataset was conducted using Trimmomatic (version 0.36) [24] to trim adapters and cut low-quality bases (quality scores <20). Post-QC reads with read length no less than 100 bp were de novo assembled using ABySS (version 2.0) [10, 25] and contigs assigned to the PhiX sequence (NCBI accession: NC_001422.1) were removed. Only contigs with more than 200 bp were kept for downstream analysis. The completeness and the contamination of each genome assembly were estimated using CheckM [26]; the completeness and the contamination of genomes were more than 98 % and less than 2 %, respectively, demonstrating that strains or DNA from these strains were not contaminated during incubation or DNA isolation.
Genome sequences for type strains of L. reuteri (DSM 20016T; GenBank: GCA_000016825.1), L. antri (DSM 16041T; GenBank: GCA_000160835.1), L. coleohominis (DSM 14060T; GenBank: GCA_001435055.1), L. equigenerosi (DSM 18793T; GenBank: GCA_001435245.1), L. fermentum (DSM 20052T; GenBank: GCA_000159215.1), L. frumenti (DSM 13145T; GenBank: GCA_001436045.1), L. gastricus (DSM 16045T; GenBank: GCA_001434365.1), L. gorillae (DSM 28356T; GenBank: GCA_001293735.1), L. ingluviei (DSM 15946T; GenBank: GCA_001435775.1), L. mucosae (DSM 13345T; GenBank: GCA_001436025.1), L. oris (DSM 4864T; GenBank: GCA_001434465.1), L. panis (DSM 6035T; GenBank: GCA_001435935.1), L. pontis (DSM 8475T; GenBank: GCA_001435345.1), L. secaliphilus (DSM 17896T; GenBank: GCA_001437055.1) and L. vaginalis (DSM 5837T; GenBank: GCA_001435915.1) were retrieved from the GenBank database. An additional 32 genome sequences of published L. reuteri strains were obtained from the Joint Genome Institute (JGI) genome portal or the GenBank database (Table S1, available in the online version of this article).
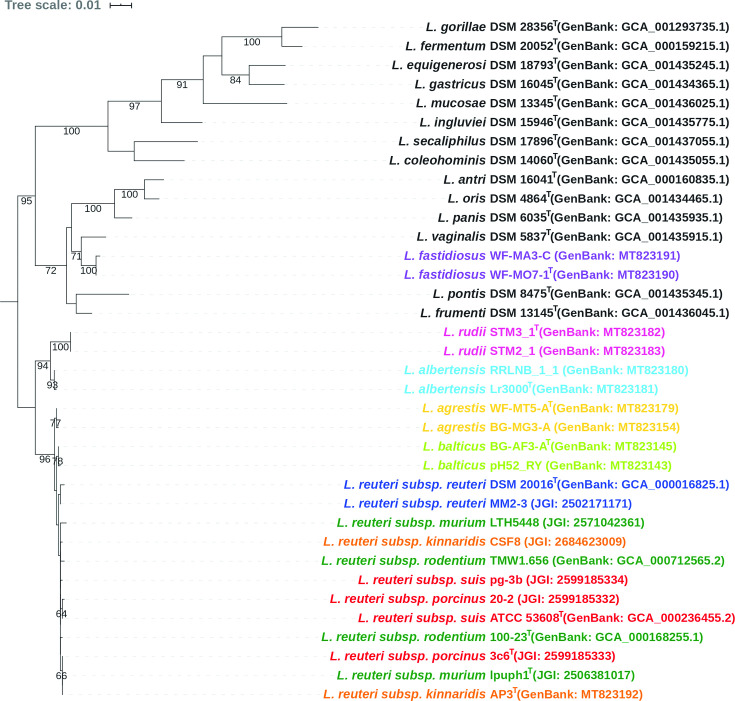
Full length (1566–1570 bp) 16S rRNA gene sequences of these strains were extracted from their draft genomes through mapping contigs to the 16S rRNA gene of L. reuteri DSM 20016T. The 16S rRNA gene similarities among these strains (Table 2) were calculated using the blast algorithm [27]. To infer the phylogeny of these ten strains and other related species in Limosilactobacillus , their 16S rRNA gene sequences were aligned using muscle [28] and the maximum-likelihood (ML) phylogenetic tree was inferred based on the generalized time-reversible model and the Gamma distribution (GTR+G) with 1000 bootstrap replicates through RAxML [29] (Fig. 1). Sequence similarity of strains BG-AF3-AT, pH52_RY, WF-MT5-AT, BG-MG3-A, Lr3000T, RRLNB_1_1, STM3_1T and STM2_1 was ≥98 % when compared to L. reuteri DSM 20016T (Table 2); these strains were closely related to L. reuteri according to the 16S-based phylogenetic tree (Fig. 1), which led to the initial classification of these strains to L. reuteri [13]. Strains WF-MO7-1T and WF-MA3-C were most closely related to L. vaginalis (Fig. 1) and the identities of their 16S rRNA genes to L. vaginali s DSM 5837T were 98.5 and 98.4 %, respectively (Table 2).
Table 2.
16S rRNA gene similarity (%) between five novel Limosilactobacillus species, six L. reuteri subspecies and other closely related species in the genus Limosilactobacillus
|
sp. nov. |
sp. nov. |
sp. nov. |
sp. nov. |
sp. nov. |
subsp. kinnaridis subsp. nov. |
subsp. porcinus subsp. nov. |
subsp. murium subsp. nov. |
subsp. reuteri subsp. nov. |
subsp. suis subsp. nov. |
subsp. rodentium subsp. nov. |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Strain |
BG- AF3- AT |
pH52_ RY |
WF- MT5- AT |
BG- MG3- A |
Lr 3000T |
RRLNB _1_1 |
STM3 _1T |
STM2_1 |
WF-MO7 -1T |
WF- MA3- C |
AP3T |
CSF8 |
3c6T |
20–2 |
lpuph1T |
LTH 5448 |
DSM 20016T |
MM2 -3 |
ATCC 53608T |
pg- 3b |
100- 23T |
TMW 1.656 |
|
sp. nov. |
||||||||||||||||||||||
|
BG-AF3-AT |
– |
|||||||||||||||||||||
|
pH52_RY |
99.9 |
– |
||||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MT5-AT |
99.8 |
99.8 |
– |
|||||||||||||||||||
|
BG-MG3-A |
99.8 |
99.7 |
99.9 |
– |
||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
Lr3000T |
98.7 |
98.7 |
98.7 |
98.6 |
– |
|||||||||||||||||
|
RRLNB_1_1 |
98.8 |
98.7 |
98.7 |
98.7 |
99.9 |
– |
||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
STM3_1T |
98.2 |
98.2 |
98.3 |
98.2 |
99.2 |
99.2 |
– |
|||||||||||||||
|
STM2_1 |
98.2 |
98.2 |
98.3 |
98.2 |
99.2 |
99.2 |
100.0 |
– |
||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MO7-1T |
97.1 |
97.1 |
97.2 |
97.1 |
97.3 |
97.3 |
97.3 |
97.3 |
– |
|||||||||||||
|
WF- MA3-C |
97.0 |
96.9 |
97.1 |
97.0 |
97.2 |
97.1 |
97.2 |
97.2 |
99.9 |
– |
||||||||||||
|
subsp. kinnaridis subsp. nov. |
||||||||||||||||||||||
|
AP3T |
99.8 |
99.7 |
99.7 |
99.6 |
98.6 |
98.7 |
98.2 |
98.2 |
97.3 |
97.1 |
– |
|||||||||||
|
CSF8 |
99.8 |
99.7 |
99.7 |
99.6 |
98.5 |
98.5 |
98.2 |
98.2 |
97.2 |
97.1 |
99.9 |
– |
||||||||||
|
L. reuteri subsp. porcinus subsp. nov. |
||||||||||||||||||||||
|
3c6T |
99.7 |
99.6 |
99.6 |
99.6 |
98.5 |
98.6 |
98.2 |
98.2 |
97.2 |
97.1 |
99.9 |
99.8 |
– |
|||||||||
|
20–2 |
99.6 |
99.6 |
99.6 |
99.5 |
98.3 |
98.4 |
98.0 |
98.0 |
97.1 |
97.0 |
99.8 |
99.8 |
99.7 |
– |
||||||||
|
subsp. murium subsp. nov. |
||||||||||||||||||||||
|
lpuph1T |
99.8 |
99.7 |
99.7 |
99.6 |
98.6 |
98.7 |
98.2 |
98.2 |
97.3 |
97.1 |
100.0 |
99.9 |
99.9 |
99.8 |
– |
|||||||
|
LTH5448 |
99.6 |
99.6 |
99.6 |
99.6 |
98.3 |
98.4 |
98.0 |
98.0 |
97.0 |
96.9 |
99.8 |
99.8 |
99.7 |
99.6 |
99.8 |
– |
||||||
|
subsp. reuteri subsp. nov. |
||||||||||||||||||||||
|
DSM 20016T |
99.7 |
99.6 |
99.6 |
99.7 |
98.4 |
98.5 |
98.0 |
98.0 |
96.9 |
96.8 |
99.7 |
99.7 |
99.6 |
99.6 |
99.7 |
99.7 |
– |
|||||
|
MM2-3 |
99.8 |
99.8 |
99.8 |
99.8 |
98.5 |
98.6 |
98.2 |
98.2 |
97.1 |
96.9 |
99.8 |
99.8 |
99.8 |
99.7 |
99.8 |
99.8 |
99.9 |
– |
||||
|
subsp. suis subsp. nov. |
||||||||||||||||||||||
|
ATCC 53608T |
99.8 |
99.7 |
99.7 |
99.6 |
98.5 |
98.5 |
98.1 |
98.1 |
97.3 |
97.1 |
99.9 |
99.9 |
99.8 |
99.9 |
99.9 |
99.8 |
99.7 |
99.8 |
– |
|||
|
pg-3b |
99.8 |
99.7 |
99.7 |
99.6 |
98.6 |
98.7 |
98.2 |
98.2 |
97.3 |
97.1 |
99.9 |
99.9 |
99.8 |
99.8 |
99.9 |
99.8 |
99.7 |
99.8 |
99.9 |
– |
||
|
subsp. rodentium subsp. nov. |
||||||||||||||||||||||
|
100-23T |
99.8 |
99.7 |
99.7 |
99.6 |
98.5 |
98.6 |
98.2 |
98.2 |
97.3 |
97.1 |
99.9 |
99.9 |
99.8 |
99.8 |
99.9 |
99.8 |
99.7 |
99.8 |
99.9 |
99.9 |
– |
|
|
TMW1.656 |
99.7 |
99.6 |
99.6 |
99.6 |
98.5 |
98.5 |
98.2 |
98.2 |
97.3 |
97.1 |
99.8 |
99.8 |
99.8 |
99.7 |
99.8 |
99.7 |
99.6 |
99.8 |
99.8 |
99.8 |
99.8 |
– |
|
DSM 4864T |
96.1 |
96.1 |
96.2 |
96.2 |
96.3 |
96.3 |
96.7 |
96.7 |
97.3 |
97.2 |
96.2 |
96.2 |
96.1 |
96.0 |
96.2 |
95.9 |
96.1 |
96.1 |
96.1 |
96.2 |
96.2 |
96.2 |
|
DSM 16041T |
95.7 |
95.7 |
95.7 |
95.7 |
96.0 |
95.9 |
96.3 |
96.3 |
97.2 |
97.1 |
95.8 |
95.7 |
95.7 |
95.6 |
95.8 |
95.5 |
95.7 |
95.7 |
95.7 |
95.8 |
95.8 |
95.8 |
|
DSM 6035T |
96.1 |
96.0 |
96.2 |
96.1 |
96.2 |
96.2 |
96.4 |
96.4 |
97.5 |
97.3 |
96.1 |
96.1 |
96.1 |
95.9 |
96.1 |
95.9 |
96.0 |
96.1 |
96.0 |
96.1 |
96.1 |
96.0 |
|
DSM 8475T |
97.3 |
97.2 |
97.3 |
97.2 |
97.3 |
97.2 |
96.9 |
96.9 |
97.6 |
97.5 |
97.3 |
97.2 |
97.2 |
97.0 |
97.3 |
97.0 |
97.1 |
97.2 |
97.1 |
97.3 |
97.3 |
97.1 |
|
DSM 5837T |
96.7 |
96.6 |
96.8 |
96.7 |
96.9 |
96.9 |
96.8 |
96.8 |
98.5 |
98.4 |
96.7 |
96.6 |
96.6 |
96.4 |
96.7 |
96.4 |
96.6 |
96.6 |
96.6 |
96.7 |
96.7 |
96.7 |
|
DSM 13145T |
97.3 |
97.2 |
97.2 |
97.1 |
97.4 |
97.4 |
97.1 |
97.1 |
98.4 |
98.3 |
97.1 |
97.1 |
97.1 |
96.9 |
97.1 |
96.9 |
97.0 |
97.1 |
97.0 |
97.1 |
97.1 |
97.0 |
|
DSM 14060T |
95.0 |
94.9 |
95.2 |
95.2 |
94.9 |
94.9 |
95.2 |
95.2 |
95.6 |
95.5 |
95.0 |
95.0 |
95.0 |
94.9 |
95.0 |
94.9 |
95.0 |
95.1 |
95.0 |
95.0 |
95.0 |
94.9 |
Fig. 1.
A maximum-likelihood phylogenetic tree reconstructed using 16S rRNA gene sequences. GenBank or JGI accession numbers of these genomes are provided in parentheses. The tree was inferred based on the GTR+G model with 1000 bootstrap replicates and only bootstrap values above 60 % are shown. Strains of five novel Limosilactobacillus species are labelled by different colours; labels of six L. reuteri subspecies are colour representing vertebrate host origin: green for rodents, red for pigs, blue for humans and orange for poultry. The tree was drawn with iTOL [54].
Genomic analyses
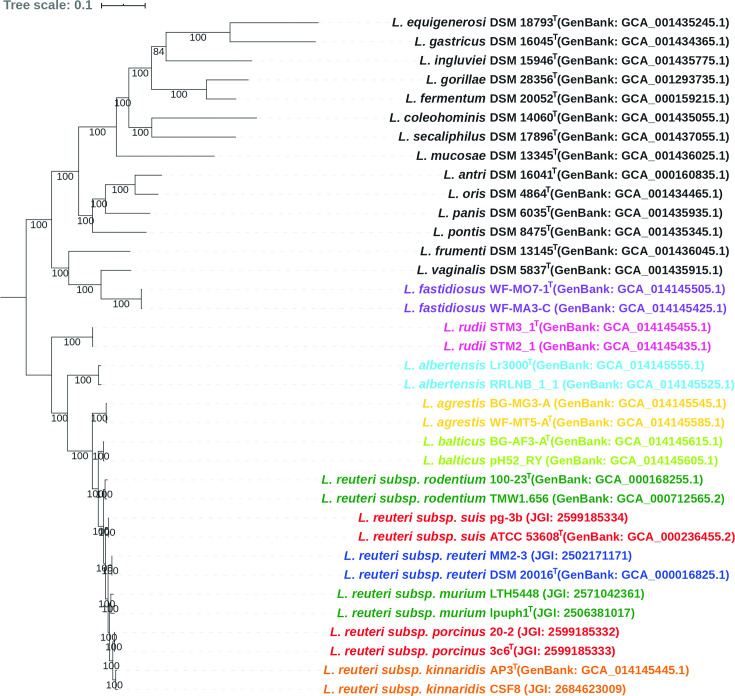
Draft genomes of the ten Limosilactobacillus strains were annotated using the Integrated Microbial Genomes (IMG) system from the Joint Genome Institute [30] and genomic characteristics of these strains are summarized in Table 1. The G+C content of the draft genomes ranged from 37.9 to 39.1 mol% and the genome size ranged from 1.72 to 2.45 Mbp (Table 1). A phylogenetic tree of these strains was reconstructed based on the alignments of the genes shared in all genomes (n=100). Briefly, draft genomes of the ten Limosilactobacillus strains, published L. reuteri strains and other type strains of the genus Limosilactobacillus were re-annotated using Prokka with default settings [31]. The Roary pipeline was applied to identify core genes based on these re-annotated assemblies [32] and concatenated core gene alignments were used as the input for RAxML to reconstruct the maximum-likelihood (ML) tree using the GTR+G model with 1000 bootstrap replicates [29]. The core-gene-based phylogenetic tree confirmed that strains BG-AF3-AT, pH52_RY, WF-MT5-AT, BG-MG3-A, Lr3000T, RRLNB_1_1, STM3_1T and STM2_1 were closely related to L. reuteri lineages but formed distinct phylogenetic clades, while the strains WF-MO7-1T and WF-MA3-C clustered with L. vaginalis (Fig. 2).
Fig. 2.
A maximum-likelihood phylogenetic tree reconstructed using core genes (n=100) identified from whole-genome sequences, showing the evolutionary relationships among five novel Limosilactobacillus species, six L. reuteri subspecies and other recognized species in the genus Limosilactobacillus . GenBank or JGI accession numbers of these genomes are provided in parentheses. The tree was inferred based on the GTR+G model with 1000 bootstrap replicates and only bootstrap values above 60 % are shown. Strains of five novel Limosilactobacillus species are labelled by different colours; labels of six L. reuteri subspecies are colour representing vertebrate host origin: green for rodents, red for pigs, blue for humans and orange for poultry. The tree was drawn with iTOL [54].
To determine which of the distinct phylogenetic clades should be considered as separate species, pairwise ANI values were calculated based on entire genomes using JSpeciesWS and JSpecies with the blast algorithm [21, 33] and pairwise dDDH values were estimated using the Genome-To-Genome Distance Calculator (GGDC) [22]. Pairwise ANI and dDDH values of the ten strains of Limosilactobacillus with other species of Limosilactobacillus and two representative strains from each of six L. reuteri lineages are shown in Tables 3 and 4, respectively. The ANI and dDDH values of strains from different phylogenetic clades to type strains of known Limosilactobacillus species were lower than 93.6 and 54.9 %, respectively, and therefore lower than the threshold for assignment to established species (95 % of ANI and 70 % of dDDH) [19–23]. In contrast, strains within the same phylogenetic clade had ANI values between 97.0 and 100 %, and dDDH values between 77.0 and 100 % (Tables 3 and 4). Strains WF-MO7-1T and WF-MA3-C were most closely related to the type strain of L. vaginalis (DSM 5837T) (Fig. 2), but their ANI and dDDH values with L. vaginalis DSM 5837T were below 82 and 26 %, respectively (Tables 3 and 4). Strains Lr3000T and RRLNB_1_1, as well as STM3_1T and STM2_1 were most closely related to L. reuteri , but the ANI and dDDH values of these strains with L. reuteri DSM 20016T were below 82 and 25 %, respectively (Tables 3 and 4). The ANI and dDDH values of strains BG-AF3-AT and pH52_RY as well as WF-MT5-AT and BG-MG3-A with L. reuteri DSM20016T were much higher (90.7–93.6 % of ANI and 43.2–54.9 % of dDDH) but still below 94 and 55 %, respectively. These analyses demonstrated that the ten strains represent five novel species.
Table 3.
Average nucleotide identity values (%) between five novel Limosilactobacillus species, six L. reuteri subspecies and other closely related species in the genus Limosilactobacillus
|
L. balticus sp. nov. |
L. agrestis sp. nov. |
L. albertensis sp. nov. |
L. rudii sp. nov. |
L. fastidiosus sp. nov. |
subsp. kinnaridis subsp. nov. |
subsp. porcinus subsp. nov. |
subsp. murium subsp. nov. |
subsp. reuteri subsp. nov. |
subsp. suis subsp. nov. |
subsp. rodentium subsp. nov. |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Strain |
BG-AF3-AT |
pH52_RY |
WF-MT5-AT |
BG-MG3-A |
Lr3000T |
RRLNB_1_1 |
STM3_1T |
STM2_1 |
WF-MO7- 1T |
WF-MA3-C |
AP3T |
CSF8 |
3c6T |
20–2 |
lpuph1T |
LTH5448 |
DSM 20016T |
MM2-3 |
ATCC 53608T |
pg-3b |
100-23T |
TMW1.656 |
|
sp. nov. |
||||||||||||||||||||||
|
BG-AF3-AT |
– |
|||||||||||||||||||||
|
pH52_RY |
98.3 |
– |
||||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MT5-AT |
91.0 |
91.2 |
– |
|||||||||||||||||||
|
BG-MG3-A |
91.1 |
91.4 |
98.8 |
– |
||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
Lr3000T |
81.9 |
82.1 |
81.3 |
81.8 |
– |
|||||||||||||||||
|
RRLNB_1_1 |
81.6 |
81.9 |
81.4 |
81.7 |
97.0 |
– |
||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
STM3_1T |
79.5 |
79.2 |
79.1 |
79.2 |
79.8 |
79.7 |
– |
|||||||||||||||
|
STM2_1 |
79.5 |
79.2 |
79.1 |
79.2 |
79.7 |
79.7 |
100.0 |
– |
||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MO7-1T |
73.4 |
73.4 |
73.0 |
73.2 |
73.0 |
73.0 |
73.4 |
73.4 |
– |
|||||||||||||
|
WF-MA3-C |
73.5 |
73.5 |
73.2 |
73.2 |
73.3 |
73.2 |
73.6 |
73.5 |
99.7 |
– |
||||||||||||
|
subsp. kinnaridis subsp. nov. |
||||||||||||||||||||||
|
AP3T |
93.2 |
93.3 |
90.6 |
90.7 |
82.2 |
82.4 |
79.4 |
79.4 |
73.5 |
73.5 |
– |
|||||||||||
|
CSF8 |
93.2 |
93.2 |
90.5 |
90.7 |
82.1 |
81.9 |
79.2 |
79.2 |
73.3 |
73.3 |
98.2 |
– |
||||||||||
|
subsp. porcinus subsp. nov. |
||||||||||||||||||||||
|
3c6T |
92.8 |
93.0 |
90.4 |
90.6 |
81.5 |
81.5 |
79.0 |
79.0 |
73.2 |
73.2 |
96.3 |
96.6 |
– |
|||||||||
|
20–2 |
92.7 |
93.0 |
90.3 |
90.6 |
81.8 |
81.6 |
79.0 |
79.0 |
73.0 |
73.4 |
96.3 |
96.6 |
99.1 |
– |
||||||||
|
subsp. murium subsp. nov. |
||||||||||||||||||||||
|
lpuph1T |
93.0 |
93.1 |
90.5 |
90.7 |
81.5 |
81.5 |
79.1 |
79.1 |
73.3 |
73.3 |
95.9 |
95.8 |
96.4 |
96.3 |
– |
|||||||
|
LTH5448 |
93.0 |
93.1 |
90.5 |
90.7 |
81.5 |
81.5 |
79.0 |
79.0 |
73.3 |
73.2 |
95.5 |
95.7 |
95.9 |
95.8 |
97.0 |
– |
||||||
|
subsp. reuteri subsp. nov. |
||||||||||||||||||||||
|
DSM 20016T |
93.6 |
93.6 |
90.7 |
90.8 |
81.7 |
81.9 |
79.4 |
79.4 |
73.5 |
73.4 |
95.4 |
95.8 |
96.1 |
96.0 |
96.5 |
96.5 |
– |
|||||
|
MM2-3 |
93.5 |
93.5 |
90.7 |
90.7 |
81.7 |
81.7 |
79.2 |
79.2 |
73.4 |
73.4 |
95.3 |
95.8 |
96.0 |
96.0 |
96.4 |
96.3 |
100.0 |
– |
||||
|
L. reuteri subsp. suis subsp. nov. |
||||||||||||||||||||||
|
ATCC53608T |
94.1 |
94.2 |
90.9 |
91.0 |
82.2 |
82.4 |
79.4 |
79.4 |
73.6 |
73.6 |
95.4 |
95.2 |
94.7 |
94.7 |
95.6 |
95.6 |
95.7 |
95.7 |
– |
|||
|
pg-3b |
93.9 |
94.0 |
90.6 |
90.8 |
81.5 |
81.7 |
79.2 |
79.2 |
73.1 |
73.2 |
95.2 |
95.3 |
95.1 |
95.1 |
95.5 |
95.7 |
96.1 |
96.1 |
99.0 |
– |
||
|
subsp. rodentium subsp. nov. |
||||||||||||||||||||||
|
100-23T |
93.8 |
94.0 |
90.8 |
90.9 |
81.7 |
81.8 |
79.1 |
79.2 |
73.3 |
73.5 |
94.8 |
94.7 |
94.8 |
94.8 |
95.6 |
95.8 |
96.2 |
96.1 |
96.3 |
96.2 |
– |
|
|
TMW1.656 |
94.2 |
94.3 |
90.6 |
90.7 |
81.4 |
81.5 |
78.8 |
78.9 |
72.8 |
73.0 |
94.6 |
94.7 |
94.9 |
94.9 |
95.5 |
95.6 |
95.9 |
95.9 |
96.2 |
95.9 |
97.3 |
– |
|
DSM 4864T |
71.6 |
71.1 |
70.9 |
71.1 |
71.4 |
71.0 |
71.5 |
71.4 |
71.3 |
71.5 |
71.6 |
71.3 |
71.0 |
70.9 |
71.1 |
71.0 |
71.5 |
71.0 |
72.1 |
70.9 |
71.5 |
70.8 |
|
DSM 16041T |
70.7 |
70.6 |
70.5 |
70.8 |
71.1 |
70.6 |
71.0 |
70.8 |
71.0 |
71.1 |
71.3 |
70.8 |
70.7 |
70.7 |
70.7 |
70.7 |
71.1 |
70.6 |
71.5 |
70.5 |
71.0 |
70.3 |
|
DSM 6035T |
71.7 |
71.7 |
71.5 |
71.7 |
72.0 |
71.6 |
71.8 |
71.7 |
71.8 |
72.1 |
72.3 |
71.9 |
71.5 |
71.4 |
71.7 |
71.5 |
72.0 |
71.7 |
72.2 |
71.5 |
72.2 |
71.4 |
|
DSM 8475T |
70.8 |
70.5 |
70.4 |
70.7 |
70.8 |
70.6 |
70.6 |
70.7 |
71.0 |
71.1 |
71.4 |
70.8 |
70.5 |
70.9 |
70.7 |
70.6 |
71.1 |
70.7 |
71.3 |
70.6 |
71.0 |
70.4 |
|
DSM 5837T |
73.4 |
73.3 |
73.1 |
73.2 |
73.0 |
73.3 |
73.5 |
73.4 |
81.5 |
81.7 |
74.4 |
73.5 |
73.1 |
73.1 |
73.2 |
73.4 |
73.6 |
73.0 |
73.6 |
73.0 |
73.9 |
72.9 |
|
DSM 13145T |
72.4 |
72.4 |
72.4 |
72.6 |
72.0 |
72.4 |
72.5 |
72.5 |
74.0 |
74.0 |
73.0 |
72.6 |
72.4 |
72.3 |
72.5 |
72.5 |
73.1 |
72.3 |
73.1 |
72.1 |
73.2 |
71.9 |
|
DSM 14060T |
71.1 |
71.1 |
70.6 |
71.1 |
71.2 |
71.1 |
71.6 |
71.4 |
71.2 |
71.1 |
72.0 |
71.0 |
70.9 |
70.8 |
71.0 |
70.7 |
71.3 |
70.8 |
71.6 |
70.9 |
71.6 |
70.3 |
Table 4.
Digital DNA–DNA hybridization values (%) between five novel Limosilactobacillus species, six L. reuteri subspecies and other closely related species in the genus Limosilactobacillus
|
Strain |
L. balticus sp. nov. |
L. agrestis sp. nov. |
L. albertensis sp. nov. |
L. rudii sp. nov. |
L. fastidiosus sp. nov. |
subsp. kinnaridis subsp. nov. |
subsp. porcinus subsp. nov. |
subsp. murium subsp. nov. |
subsp. reuteri subsp. nov. |
subsp. suis subsp. nov. |
subsp. rodentium subsp. nov. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BG-AF3-AT |
pH52_RY |
WF-MT5-AT |
BG-MG3-A |
Lr3000T |
RRLNB_1_1 |
STM3_1T |
STM2_1 |
WF-MO7-1T |
WF-MA3-C |
AP3T |
CSF8 |
3c6T |
20–2 |
lpuph1T |
LTH5448 |
DSM 20016T |
MM2-3 |
ATCC 53608T |
pg-3b |
100-23T |
TMW1.656 |
|
|
sp. nov. |
||||||||||||||||||||||
|
BG-AF3-AT |
– |
|||||||||||||||||||||
|
pH52_RY |
89.0 |
– |
||||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MT5-AT |
45.0 |
45.2 |
– |
|||||||||||||||||||
|
BG-MG3-A |
45.1 |
45.2 |
91.2 |
– |
||||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
Lr3000T |
25.4 |
25.5 |
24.5 |
25.2 |
– |
|||||||||||||||||
|
RRLNB_1_1 |
25.1 |
25.1 |
24.6 |
25.0 |
77.0 |
– |
||||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
STM3_1T |
22.5 |
22.1 |
22.3 |
22.3 |
22.8 |
22.6 |
– |
|||||||||||||||
|
STM2_1 |
22.5 |
22.1 |
22.3 |
22.3 |
22.8 |
22.6 |
100.0 |
– |
||||||||||||||
|
sp. nov. |
||||||||||||||||||||||
|
WF-MO7-1T |
20.5 |
20.4 |
20.6 |
20.8 |
20.5 |
20.2 |
20.8 |
20.8 |
– |
|||||||||||||
|
WF-MA3-C |
20.5 |
20.3 |
20.8 |
20.9 |
20.8 |
20.5 |
20.9 |
20.9 |
98.6 |
– |
||||||||||||
|
subsp. kinnaridis subsp. nov. |
||||||||||||||||||||||
|
AP3T |
52.8 |
53.0 |
42.4 |
42.4 |
25.5 |
25.5 |
22.4 |
22.4 |
20.5 |
20.5 |
– |
|||||||||||
|
CSF8 |
53.6 |
53.1 |
42.5 |
42.8 |
25.3 |
25.0 |
22.4 |
22.4 |
20.4 |
20.4 |
89.7 |
– |
||||||||||
|
L. reuteri subsp. porcinus subsp. nov. |
||||||||||||||||||||||
|
3c6T |
52.2 |
52.3 |
42.9 |
43.0 |
24.7 |
24.7 |
22.3 |
22.3 |
20.6 |
20.6 |
72.0 |
75.5 |
– |
|||||||||
|
20–2 |
51.7 |
51.9 |
42.6 |
43.1 |
25.1 |
24.7 |
22.3 |
22.3 |
20.4 |
21.1 |
71.3 |
74.8 |
95.0 |
– |
||||||||
|
subsp. murium subsp. nov. |
||||||||||||||||||||||
|
lpuph1T |
52.5 |
52.5 |
43.2 |
43.1 |
24.7 |
24.6 |
22.5 |
22.5 |
20.4 |
20.5 |
68.2 |
68.1 |
71.2 |
70.7 |
– |
|||||||
|
LTH5448 |
52.7 |
52.8 |
42.8 |
42.7 |
24.6 |
24.6 |
22.3 |
22.3 |
20.3 |
20.1 |
67.0 |
67.2 |
69.5 |
69.0 |
75.6 |
– |
||||||
|
subsp. reuteri subsp. nov. |
||||||||||||||||||||||
|
DSM 20016T |
54.9 |
54.7 |
43.2 |
43.2 |
24.9 |
24.9 |
22.5 |
22.5 |
20.8 |
20.8 |
65.1 |
68.4 |
70.0 |
70.1 |
71.5 |
72.0 |
– |
|||||
|
MM2-3 |
54.6 |
54.4 |
43.0 |
42.9 |
24.6 |
24.7 |
22.1 |
22.1 |
20.3 |
20.3 |
64.6 |
68.2 |
69.5 |
69.8 |
70.9 |
71.4 |
99.9 |
– |
||||
|
subsp. suis subsp. nov. |
||||||||||||||||||||||
|
ATCC53608T |
57.4 |
57.8 |
43.7 |
43.8 |
25.8 |
25.9 |
22.6 |
22.6 |
21.2 |
21.2 |
65.1 |
64.2 |
62.4 |
62.1 |
65.5 |
65.7 |
68.2 |
68.1 |
– |
|||
|
pg-3b |
57.0 |
57.5 |
43.5 |
43.6 |
24.9 |
24.7 |
22.4 |
22.4 |
20.1 |
20.1 |
63.8 |
64.0 |
64.3 |
64.1 |
65.3 |
67.0 |
70.4 |
70.7 |
94.9 |
– |
||
|
subsp. rodentium subsp. nov. |
||||||||||||||||||||||
|
100-23T |
57.1 |
57.7 |
43.6 |
43.7 |
25.3 |
25.1 |
22.7 |
22.7 |
21.0 |
21.0 |
62.0 |
61.5 |
63.0 |
62.8 |
65.6 |
66.3 |
69.0 |
68.6 |
70.7 |
70.9 |
– |
|
|
TMW1.656 |
58.6 |
58.7 |
42.9 |
42.9 |
24.6 |
24.5 |
22.0 |
22.0 |
19.4 |
19.4 |
61.3 |
61.1 |
63.3 |
63.2 |
65.6 |
66.0 |
67.8 |
67.9 |
70.7 |
69.8 |
80.1 |
– |
|
DSM 4864T |
22.2 |
20.6 |
20.0 |
20.3 |
20.0 |
20.5 |
20.3 |
20.3 |
21.8 |
21.8 |
20.8 |
20.1 |
19.9 |
19.8 |
20.2 |
19.9 |
21.3 |
20.2 |
22.0 |
20.1 |
21.7 |
19.7 |
|
DSM 16041T |
20.2 |
19.8 |
18.9 |
19.1 |
21.1 |
20.5 |
19.7 |
19.7 |
21.9 |
21.8 |
21.1 |
20.1 |
19.7 |
19.9 |
20.4 |
20.3 |
20.8 |
19.8 |
21.1 |
19.9 |
21.3 |
19.7 |
|
DSM 6035T |
19.2 |
19.1 |
19.4 |
19.5 |
19.8 |
19.5 |
20.0 |
20.0 |
21.8 |
21.8 |
19.5 |
19.2 |
19.1 |
18.9 |
19.1 |
19 |
19.7 |
18.8 |
20.1 |
19.3 |
20.2 |
18.9 |
|
L. pontis DSM 8475T |
18.7 |
18.6 |
18.5 |
18.6 |
18.7 |
18.6 |
19.5 |
19.5 |
20.5 |
20.7 |
19.6 |
18.9 |
18.9 |
19.1 |
18.4 |
18.4 |
19.6 |
18.8 |
19.9 |
18.8 |
19.4 |
18.2 |
|
DSM 5837T |
20.6 |
20.5 |
20.9 |
20.9 |
20.9 |
20.7 |
20.7 |
20.7 |
25.0 |
25.3 |
21.6 |
20.8 |
21.0 |
20.8 |
20.7 |
21.1 |
21.7 |
20.6 |
21.2 |
20.1 |
21.9 |
20.6 |
|
DSM 13145T |
20.8 |
20.9 |
20.1 |
20.2 |
20.1 |
19.9 |
20.6 |
20.6 |
20.0 |
20.2 |
20.8 |
20.5 |
19.9 |
19.8 |
20.4 |
20.6 |
21.1 |
19.8 |
21.8 |
20.6 |
23.0 |
19.7 |
|
DSM 14060T |
26.2 |
25.7 |
24.2 |
23.3 |
26.0 |
25.0 |
27.1 |
27.2 |
24.9 |
25.5 |
23.3 |
23.6 |
23.9 |
23.9 |
23.8 |
24.4 |
28.0 |
24.4 |
27.5 |
23.4 |
26.7 |
22.2 |
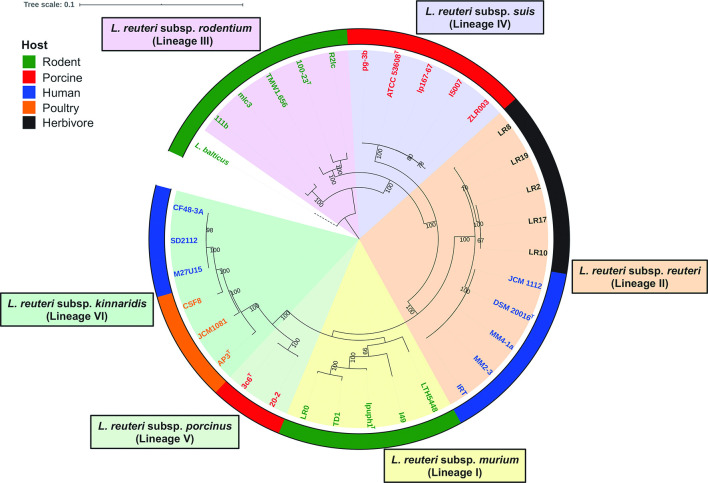
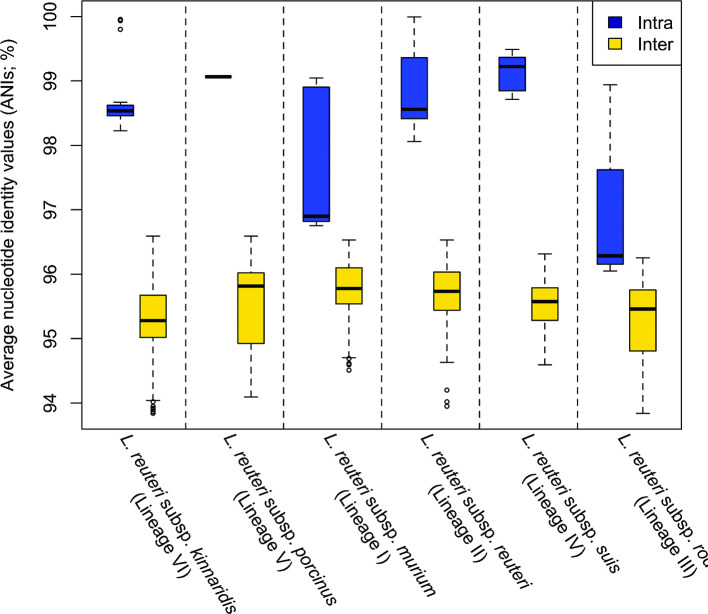
Representative strains of L. reuteri that represent the six host-adapted phylogenetic lineages were not sufficiently resolved by the analysis based on 16S rRNA gene sequences (Fig. 1) but formed distinct phylogenetic clades using the core-gene-based analysis (Fig. 2), which is consistent with previous findings [5, 7]. However, ANI and dDDH values of strains belonging to different L. reuteri lineages were from 94.6 to 96.6 % and from 61.1 to 75.5 %, respectively, indicating genetic dissimilarity (Tables 3 and 4). To gain further insight into the genetic dissimilarities and evolutionary relationships among strains belonging to different L. reuteri lineages, a core-gene-based phylogenetic tree was reconstructed for 33 L . reuteri genomes available in public databases (Table S1) with the BG-AF3-AT strain as an outlier. In accordance with previous analyses that were based on multi-locus sequence analysis (MLSA), amplified-fragment length polymorphism (AFLP) or core genome phylogeny [5, 7, 34], strains of L. reuteri clustered into six cohesive host-adapted lineages (Fig. 3). Pairwise ANI values of L. reuteri strains within the same lineages (98.5±1.0 %; mean±SD) were higher than those of strains belonging to different lineages (95.5±0.6 %), with little overlap (Fig. 4). These analyses provide phylogenetic and genomic support for the assignation of subspecies status to the six L. reuteri lineages.
Fig. 3.
A maximum-likelihood phylogenetic tree reconstructed using core genes (n=100) identified from whole-genome sequences, showing the evolutionary relationships among six L. reuteri subspecies. The tree was reconstructed using 33 L . reuteri genomes available in public databases (n=6 for L. reuteri subsp. kinnaridis , n=2 for L. reuteri subsp. porcinus , n=5 for L. reuteri subsp. murium , n=10 for L. reuteri subsp. reuteri , n=5 for L. reuteri subsp. suis and n=5 for L. reuteri subsp. rodentium ) and L. balticus BG-AF3-AT was used as an outgroup. Further information on the involved genome sequences is listed in Table S1. The tree was inferred based on the GTR+G model with 1000 bootstrap replicates and only bootstrap values above 60 % are shown. The tree was drawn with iTOL [54].
Fig. 4.
Pairwise average nucleotide identity values (ANI; %) of genome sequences belonging to the same or different L. reuteri subspecies. ANI values within the same subspecies and between different subspecies were calculated for 33 L. reuteri genomes available in public databases (n=6 for L. reuteri subsp. kinnaridis , n=2 for L. reuteri subsp. porcinus , n=5 for L. reuteri subsp. murium , n=10 for L. reuteri subsp. reuteri , n=5 for L. reuteri subsp. suis and n=5 for L. reuteri subsp. rodentium ). Further information on the involved genome sequences is listed in Table S1.
Physiology and chemotaxonomy
Carbohydrate utilization of the five novel Limosilactobacillus species and the six L. reuteri subspecies was determined with the API 50 CH system (bioMérieux) following the manufacturer’s instructions; results are shown in Table 5. Aspects of the fermentation phenotypes of the strains are in agreement with presence/absence patterns of the phosphofructokinase gene, the mannitol dehydrogenase gene (mdh) and the gene (adhE) encoding a two-domain enzyme combining acetyl coenzyme A (acetyl-CoA) and alcohol dehydrogenase domains in the annotated draft genomes [10]. The phosphofructokinase gene was absent, while the mdh and adhE genes were present in all of these ten strains, which matches the gene content of heterofermentative lactobacilli but differs from the gene content of homofermentative lactobacilli [4]. This in silico prediction was verified for all five proposed type strains (BG-AF3-AT, WF-MT5-AT, Lr3000T, STM3_1T and WF-MO7-1T) by observation of gas formation from glucose in MRS broth with Durham tubes. The lactate isomers fermented from glucose of these five putative type strains were determined using QuantiQuik d-Lactic Acid Quick Test Strips and QuantiQuik l-Lactic Acid Quick Test Strips (BioAssay Systems): both d-lactate and l-lactate were produced by these five strains from the glucose fermentation. To evaluate the optimal temperature conditions for growth, the five putative type strains were incubated anaerobically in MRS broth (in the 96-well microplate; each well was overlaid with paraffin to keep the oxygen out) at 15, 30, 37 and 45 °C, and OD600 was measured hourly using SpectraMax M3 Multi-Mode Microplate Readers (Molecular Devices) for 24 h (Fig. S1). None of these five strains grew at 15 °C. Optimum growth of BG-AF3-AT and Lr3000T occurred at 45 °C, while 37 °C was the optimum temperature for the growth of WF-MT5-AT and STM3_1T (Fig. S1). WF-MO7-1T did not grow in the 96-well microplate (Fig. S1), but its growth occurred at 30, 37 and 45 °C (optimum) in Falcon 15 ml tubes (data not shown). To investigate the pH range for growth, the pH of MRS broth was adjusted to 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0. Growth of BG-AF3-AT, WF-MT5-AT, Lr3000T and STM3_1T occurred at pH 4.0–8.0, and growth of WF-MO7-1T occurred at pH 4.5–7.5. The Gram-staining pattern of each strain was checked using Bactident Aminopeptidase strips (Merck) and the potassium hydroxide test (KOH test) [35]. The ten Limosilactobacillus strains were aminopeptidase- and KOH-negative, suggesting that they are Gram-positive bacteria. The catalase activity was examined using the standard methods as described previously [36] and further confirmed through checking the absence of catalase and NADPH peroxidase genes in the annotated draft genomes [37], which showed that these ten Limosilactobacillus strains are catalase-negative.
Table 5.
Carbohydrate utilization phenotypes of five novel Limosilactobacillus species, six L. reuteri subspecies and L. vaginalis
Carbohydrates with negative or not determined results for all strains: glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate and potassium 5-ketogluconate. Grey shading, acid production from sugar indicated; white, no acid production from sugar indicated or not determined.
|
Characteristic |
L. Balticus sp. nov. |
sp. nov. |
L. Albertensis sp. nov. |
L. Rudii sp. nov. |
L. Fastidiosus sp. nov. |
L. Reuteri subsp. kinnaridis subsp. nov. |
L. Reuteri subsp. porcinus subsp. nov. |
L. Reuteri subsp. murium subsp. nov.* |
L. Reuteri subsp. reuteri subsp. nov.† |
L. Reuteri subsp.suis subsp. nov. |
L. Reuteri subsp. rodentium subsp. nov.* |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BG-AF3-AT |
pH52 _RY |
WF-MT5-AT |
BG-MG3-A |
Lr 3000T |
RRLNB _1_1 |
STM3_1T |
STM2_1 |
WF-MO7-1T |
WF-MA3-C |
AP3T |
CSF8 |
3 c6T |
20–2 |
lpuph1T |
LTH 5448 |
DSM 20016T |
MM2-3 |
ATCC 53608T |
lp167-67 |
100–23T |
TMW 1.656 |
DSM 5837T |
|
|
l-Arabinose |
|||||||||||||||||||||||
|
d-Ribose |
|||||||||||||||||||||||
|
d-Xylose |
|||||||||||||||||||||||
|
d-Galactose |
|||||||||||||||||||||||
|
d-Glucose |
|||||||||||||||||||||||
|
d-Fructose |
|||||||||||||||||||||||
|
d-Mannose |
|||||||||||||||||||||||
|
Methyl- α-d- glucopyranoside |
|||||||||||||||||||||||
|
Aesculin/ ferric citrate |
|||||||||||||||||||||||
|
Maltose |
|||||||||||||||||||||||
|
Lactose |
|||||||||||||||||||||||
|
Melibiose |
|||||||||||||||||||||||
|
Sucrose |
|||||||||||||||||||||||
|
Raffinose |
|||||||||||||||||||||||
|
Turanose |
|||||||||||||||||||||||
|
Potassium gluconate |
|||||||||||||||||||||||
*API 50 CH test data of L. reuteri subsp. murium (lpuph1T and LTH5448) and L. reuteri subsp. rodentium (100-23T and TMW1.656) were obtained from [51].
†API 50 CH test data of L. reuteri DSM 20016T and L. vaginalis DSM 5837T were retrieved from the Bacterial Diversity Metadatabase (BacDive; https://bacdive.dsmz.de/).
Peptidoglycan structure of five putative type strains (BG-AF3-AT, WF-MT5-AT, Lr3000T, STM3_1T and WF-MO7-1T) was analysed as described previously [38]. The cell morphology of cells grown on MRS agar plates for 24–48 h under anaerobic conditions was observed with a Scope-A1 microscope (Carl Zeiss) (Fig. S2). Results are presented in the species description section. Cellular fatty acid profiles were generated using the Sherlock MIS (midi) system by the Identification Service of the DSMZ, for which TSBA40 was used for the initial analysis and TSBA6 was used for calculation. Fatty acid profiles of these ten strains showed variations: C16 : 0 was the major fatty acid in BG-AF3-AT, pH52_RY, WF-MT5-AT, BG-MG3-A, STM3_1T, STM2_1, WF-MO7-1T and WF-MA3-C, while summed feature 7 (combination of C19 : 1 ω6c and/or C19 : 0 cyclo ω10c) was more abundant than other fatty acids in Lr3000T and RRLNB_1_1 (from the same putative novel species) (Table 6). Cluster analysis based on fatty acid compositions suggested that two strains representing each putative novel species had generally more similar fatty acid profiles to each other, compared to strains from other putative novel species, with the only exceptions of BG-AF3-AT and pH52_RY (Table 6, Fig. S3). This further confirms the delineation of five novel species.
Table 6.
Cellular fatty acid profiles of the ten strains classified as novel Limosilactobacillus species
|
Fatty acid (%) |
L. balticus sp. nov |
L. agrestis sp. nov. |
L. albertensis sp. nov. |
L. rudii sp. nov. |
L. fastidiosus sp. nov. |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BG-AF3-AT |
pH52_RY |
WF-MT5-AT |
BG-MG3-A |
Lr3000T |
RRLNB_1_1 |
STM3_1T |
STM2_1 |
WF-MO7-1T |
WF-MA3-C |
DSM 20016T |
DSM 5837T |
|
|
C14 : 0 |
2.6 |
3.6 |
1.8 |
5.3 |
0.5 |
0.6 |
1.8 |
2.0 |
7.6 |
7.2 |
2.3 |
7.4 |
|
iso-C15 : 0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.2 |
0.0 |
0.0 |
|
iso-C16 : 0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.7 |
0.0 |
0.0 |
|
C16 : 0 |
42.3 |
32.4 |
48.3 |
50.4 |
16.5 |
16.9 |
42.2 |
46.0 |
33.4 |
30.2 |
37.4 |
23.7 |
|
C17 : 0 cyclo |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.5 |
0.0 |
0.0 |
0.0 |
|
C16 : 0 3-OH |
0.0 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.5 |
0.6 |
0.0 |
0.0 |
|
C18 : 1 ω9c |
6.4 |
21.7 |
5.7 |
7.3 |
31.4 |
30.1 |
6.5 |
6.6 |
17.2 |
21.2 |
6.7 |
33.3 |
|
C18 : 0 |
3.0 |
2.5 |
5.0 |
2.5 |
3.3 |
3.3 |
5.3 |
5.7 |
1.7 |
2.2 |
7.1 |
0.0 |
|
C18 : 1 ω7c 11-methyl |
0.0 |
0.0 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
C17 : 0 3-OH |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.3 |
0.0 |
0.0 |
0.0 |
0.0 |
|
iso-C19 : 0 |
1.0 |
1.3 |
0.4 |
0.0 |
2.3 |
2.5 |
1.3 |
1.2 |
0.0 |
1.0 |
0.0 |
0.0 |
|
C19 : 0 cyclo ω8c |
18.9 |
8.4 |
20.4 |
13.6 |
0.0 |
0.0 |
14.2 |
15.7 |
0.0 |
0.0 |
7.0 |
0.0 |
|
Summed feature 3† |
1.5 |
1.7 |
1.5 |
1.9 |
1.2 |
1.3 |
1.3 |
1.5 |
2.7 |
2.0 |
3.0 |
4.4 |
|
Summed feature 5† |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
17.1 |
0.0 |
|
Summed feature 7† |
14.5 |
19.3 |
13.8 |
15.1 |
36.8 |
38.4 |
20.6 |
21.2 |
31.4 |
30.1 |
5.4 |
15.7 |
|
Summed feature 8† |
9.9 |
8.7 |
2.6 |
4.0 |
8.1 |
7.0 |
7.0 |
0.0 |
5.2 |
4.6 |
13.9 |
15.5 |
*Cellular fatty acid data of L. reuteri DSM 20016T and L. vaginalis DSM 5837T were retrieved from the Bacterial Diversity Metadatabase (BacDive; https://bacdive.dsmz.de/).
†Summed feature 3 comprises C16 : 1 ω7c and/or C16 : 1 ω6c; summed feature 5 comprises C18 : 2 ω6,9c and/or C18 : 0 anteiso; summed feature 7 comprises C19 : 1 ω6c and/or C19 : 0 cyclo ω10c; summed feature 8 is an unknown combination of C18 : 1 ω7c and/or C18 : 1 ω6c.
Proposal of novel species and subspecies within L. reuteri
According to aforementioned polyphasic analyses and the chemotaxonomic and phenotypic characterization, we propose that these ten strains represent five novel species of the genus Limosilactobacillus . We propose the names Limosilactobacillus balticus sp. nov. (type strain BG-AF3-AT=DSM 110574T=LMG 31633T), Limosilactobacillus agrestis sp. nov. (type strain WF-MT5-AT=DSM 110569T=LMG 31629T), Limosilactobacillus albertensis sp. nov. (type strain Lr3000T=DSM 110573T=LMG 31632T), Limosilactobacillus rudii sp. nov. (type strain STM3_1T=DSM 110572T=LMG 31631T) and Limosilactobacillus fastidiosus sp. nov. (type strain WF-MO7-1T=DSM 110576T=LMG 31630T) for these novel species.
Based on the cohesive phylogenetic lineages that showed little overlap in ANI values and the experimental proof of differences in host adaptation between the phylogenetic lineages [5, 7, 11] and characteristic physiological differences related to the utilization of glycerol [6, 8, 16, 39, 40], decarboxylation of histidine [34], synthesis of folate [34] and the expression of mucus-binding large surface proteins [8, 41, 42], we also propose that the six phylogenetic lineages within L. reuteri represent six subspecies. The following names are proposed: L. reuteri subsp. kinnaridis subsp. nov. (type strain AP3T=DSM 110703T=LMG 31724T), L. reuteri subsp. porcinus subsp. nov. (type strain 3c6T=DSM 110571T=LMG 31635T), L. reuteri subsp. murium subsp. nov. (type strain lpuph1T=DSM 110570T=LMG 31634T), L. reuteri subsp. reuteri subsp. nov. (type strain DSM 20016T=ATCC 23272T=F 275T [original designation]), L. reuteri subsp. suis subsp. nov. (type strain ATCC 53608T=LMG 31752T=1063T [original designation]) and L. reuteri subsp. rodentium subsp. nov. (type strain 100-23T=DSM 17509T=CIP 109821T).
Description of Limosilactobacillus balticus sp. nov.
Limosilactobacillus balticus (bal.ti′cus. L. adj. balticus pertaining to the Baltic region where the type strain was isolated).
Cells are Gram-positive, non-motile, non-spore-forming, catalase-negative and heterofermentative. Cells are rod-shaped, measuring 0.9–3.0×0.6–1.0 µm. Colonies of the type strain BG-AF3-AT on MRS agar plate incubated at the anaerobic condition at 37 °C for 2 days are whitish, opaque, raised, circular and entire, with a diameter of 1.2–3.2 mm; no colony appears on MRS agar plate incubated at the aerobic condition at 37 °C for 2 days. d-Lactate, l-lactate and gas are produced from glucose fermentation by the type strain BG-AF3-AT. Growth occurs at 30, 37 and 45 °C (optimum), but not at 15 °C. Growth occurs at pH 4.0–8.0 in MRS broth. For BG-AF3-AT, the most abundant fatty acid is C16 : 0, followed by C19 : 0 cyclo ω8c. Acid is produced from l-arabinose, d-ribose, d-xylose, d-galactose, d-glucose, aesculin, maltose, lactose, melibiose, sucrose and raffinose; acid is not produced from d-fructose, d-mannose, methyl α-d-glucopyranoside, potassium gluconate, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. The cell-wall peptidoglycan of BG-AF3-AT contains the amino acids alanine (Ala), glutamic acid (Glu), lysine (Lys), and aspartic acid (Asp), with the molar ratio 1.3 (Ala):0.8 (Asp):1.0 (Glu):1.0 (Lys), suggesting the cell-wall peptidoglycan type A4α l-Lys–d-Asp by [38]. The DNA G+C content of BG-AF3-AT is 38.3 mol%. The type strain is BG-AF3-AT (=DSM 110574T=LMG 31633T), which was isolated from the jejunum of yellow-necked mouse (Apodemus flavicollis) caught in the Vilnius area in Lithuania [13].
Description of Limosilactobacillus agrestis sp. nov.
Limosilactobacillus agrestis (a.gres′tis. L. masc. adj. agrestis, wild, referring to the isolation of the type strain from wild rodents).
Cells are Gram-positive, non-motile, non-spore-forming, catalase-negative and heterofermentative. Cells are rod-shaped, measuring 0.9–2.7×0.6–0.8 µm. Colonies of the type strain WF-MT5-AT on MRS agar plate incubated at the anaerobic condition at 37 °C for 2 days are yellowish, translucent, flat, circular shape but irregular edge, with a diameter of 2.2–5.2 mm; no colony appears on MRS agar plate incubated at the aerobic condition at 37 °C for 2 days. d-Lactate, l-lactate and gas are produced from glucose fermentation by the type strain WF-MT5-AT. Cell growth occurs at 37 °C, slow growth is observed at 30 and 45 °C and no growth occurs at 15 °C. Growth occurs at pH 4.0–8.0 in MRS broth. For WF-MT5-AT, the major fatty acids are C16 : 0, followed by C19 : 0 cyclo ω8c. Acid is produced from l-arabinose and aesculin; the fermentation of d-ribose, d-galactose, d-glucose, maltose, melibiose, sucrose and raffinose is strain-specific; acid is not produced from d-xylose, d-fructose, d-mannose, methyl α-d-glucopyranoside, lactose, potassium gluconate, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. The cell-wall peptidoglycan of WF-MT5-AT contains the amino acids alanine (Ala), glutamic acid (Glu), lysine (Lys), aspartic acid (Asp), serine (Ser) and threonine (Thr), with the molar ratio 1.1 (Ala):0.8 (Asp):1.0 (Glu):0.7 (Lys):0.1 (Thr):0.1 (Ser), suggesting the cell-wall peptidoglycan type A4α l-Lys–d-Asp by [38]. The DNA G+C content of WF-MT5-AT is 38.0 mol%. The type strain is WF-MT5-AT (=DSM 110569T=LMG 31629T), which was isolated from the jejunum of field vole (Microtus agrestis) caught in the Vilnius area in Lithuania [13].
Description of Limosilactobacillus albertensis sp. nov.
Limosilactobacillus albertensis (al.ber.ten′sis. N.L. masc. adj. albertensis, pertaining to Alberta, a province of Canada where the isolates were characterized and identified).
Cells are Gram-positive, non-motile, non-spore forming, catalase-negative and heterofermentative. Cells are rod-shaped, measuring 0.8–2.4×0.6–1.2 µm. Colonies of the type strain Lr3000T on MRS agar plate incubated at the anaerobic condition at 37 °C for 2 days are whitish, opaque, raised, circular and entire, with a diameter of 0.8–1.5 mm; colonies on MRS agar plate at the aerobic condition show similar morphological characteristics as colonies on MRS agar incubated anaerobically, but with a smaller diameter of 0.5–1 mm. d-Lactate, l-lactate and gas are produced from glucose fermentation by the type strain Lr3000T. Cell growth occurs at 30, 37 and 45 °C (optimum), but not at 15 °C. Growth occurs at pH 4.0–8.0 in MRS broth. The major fatty acids of Lr3000T are summed feature 7 (combination of C19 : 1 ω6c and/or C19 : 0 cyclo ω10c) and C18 : 1 ω9c, followed by C16 : 0. Acid is produced from l-arabinose, d-ribose, d-xylose, d-galactose, d-glucose, methyl α-d-glucopyranoside, aesculin, maltose, lactose, melibiose, sucrose and raffinose; the fermentation of potassium gluconate is strain-specific; acid is not produced from d-fructose, d-mannose, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. The cell-wall peptidoglycan of Lr3000T contains the amino acids alanine (Ala), glutamic acid (Glu), lysine (Lys) and aspartic acid (Asp), with the molar ratio 1.1 (Ala):0.8 (Asp):1.0 (Glu):1.0 (Lys), suggesting the cell-wall peptidoglycan type A4α l-Lys–d-Asp [38]. The DNA G+C content of Lr3000T is 38.8 mol%. The type strain, Lr3000T (=DSM 110573T=LMG 31632T), was isolated from the stomach of a hamster in the USA.
Description of Limosilactobacillus rudii sp. nov.
Limosilactobacillus rudii [ru′di.i.i. N.L. gen. n. rudii of Rudi (Vogel), in recognition of the German scientist Rudi F. Vogel, in recognition of his significant contributions to the taxonomy of lactic acid bacteria as well as the technology and microbial ecology of fermented foods].
Cells are Gram-positive, non-motile, non-spore-forming, catalase-negative and heterofermentative. Cells are rod-shaped, measuring 1.1–2.7×0.7–1.2 µm. Colonies of the type strain STM3_1T on MRS agar plate incubated under the anaerobic condition at 37 °C for 2 days are whitish, opaque, raised, circular and entire, with a diameter of 1.0–2.2 mm; colonies on MRS agar plate at the aerobic condition show similar morphological characteristics as colonies on MRS agar incubated anaerobically, but with a smaller diameter of 0.4–0.8 mm. d-Lactate, l-lactate and gas are produced from glucose fermentation by the type strain STM3_1T. Cell growth occurs at 30 and 37 °C (optimum), but not at 15 or 45 °C. Growth occurs at pH 4.0–8.0 in MRS broth. The major fatty acid of STM3_1T is C16 : 0, followed by summed feature 7 (combination of C19 : 1 ω6c and/or C19 : 0 cyclo ω10c) and C19 : 0 cyclo ω8c. Acid is produced from l-arabinose, d-ribose, d-xylose, d-galactose, d-glucose, aesculin, maltose, lactose, melibiose, sucrose and raffinose; acid is not produced from d-fructose, d-mannose, methyl α-d-glucopyranoside, potassium gluconate, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. The cell-wall peptidoglycan of STM3_1T contains the amino acids alanine (Ala), glutamic acid (Glu), lysine (Lys), aspartic acid (Asp), serine (Ser) and threonine (Thr), with the molar ratio 1.1 (Ala):1.0 (Asp):1.0 (Glu):0.8 (Lys):0.1 (Thr), suggesting the cell-wall peptidoglycan type A4α l-Lys–d-Asp by [38]. The DNA G+C content of STM3_1T is 38.5 mol%. The type strain, STM3_1T (=DSM 110572T=LMG 31631T), was isolated from the faecal sample of striped mouse (Rhabdomys pumilio) raised at Henry Doorly Zoo and Aquarium (Omaha, NE, USA).
Description of Limosilactobacillus fastidiosus sp. nov.
Limosilactobacillus fastidiosus (fas.ti.di.o′sus. L. masc. adj. fastidious, fastidious, referring to the fastidious growth requirements of the type strain).
Cells are Gram-positive, non-motile, non-spore-forming, catalase-negative and heterofermentative. Cells are rod-shaped, measuring 0.9–3.0×0.6–0.9 µm. Colonies of the type strain WF-MO7-1T on MRS agar plate incubated at the anaerobic condition at 37 °C for 2 days are whitish, opaque, raised, circular and entire, with a diameter of 1.2–2.2 mm; no colony appears on MRS agar plate incubated at the aerobic condition at 37 °C for 2 days. d-Lactate, l-lactate and gas are produced from glucose fermentation by the type strain WF-MO7-1T. No growth occurs in 96-well microplate at 15, 30, 37 or 45 °C; in Falcon 15 ml tubes, cell growth occurs at 30, 37 and 45 °C (optimum) but not at 15 °C. Growth occurs at pH 4.5–7.5 in MRS broth. The most abundant fatty acids of WF-MO7-1T are C16 : 0, summed feature 7 (combination of C19 : 1 ω6c and/or C19 : 0 cyclo ω10c) and C18 : 1 ω9c. Acid is produced from l-arabinose and aesculin; acid production from d-galactose, d-glucose, d-fructose, maltose, lactose, melibiose and raffinose is strain-specific; acid is not produced from d-ribose, d-xylose, d-mannose, methyl α-d-glucopyranoside, sucrose, potassium gluconate, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. The cell-wall peptidoglycan of WF-MO7-1T contains the amino acids alanine (Ala), glutamic acid (Glu), aspartic acid (Asp) and ornithine (Orn), with the molar ratio 1.5 (Ala):0.9 (Asp):1.0 (Glu):1.1 (Orn), suggesting the cell-wall peptidoglycan type A4α l-Orn–d-Asp by [38]. The DNA G+C content of WF-MO7-1T is 39.1 mol%. The type strain is WF-MO7-1T (=DSM 110576T=LMG 31630T), which was isolated from the jejunum of root vole (Microtus oeconomus) caught in the Vilnius area in Lithuania [13].
Description of Limosilactobacillus reuteri subsp. reuteri subsp. nov.
Limosilactobacillus reuteri subsp. reuteri (reu′te.ri. N.L. gen. n. reuteri, of Reuter; named for G. Reuter, a German bacteriologist after whom the species L. reuteri was named).
L. reuteri strains clustered in lineage II (Fig. 3) belong to L. reuteri subsp. reuteri and they were isolated from humans and herbivores [7, 43]. Strains of this subspecies have ANI values of 98.1–100.0 % with each other and ANI values of 94.0–96.5 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from l-arabinose, d-ribose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose and raffinose; acid production from potassium gluconate is strain-specific; acid is not produced from d-xylose, d-fructose, d-mannose, methyl α-d-glucopyranoside, aesculin, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and previous studies [5, 43], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7], suggest that these strains are genetically homogeneous [8]. Strains of this subspecies possess the pdu-cbi-cob-hem cluster (pdu cluster) [6, 8], which equips them with the ability to utilize 1,2-propanediol and glycerol as electron acceptors [16, 39, 40] and to produce the antimicrobial compound reuterin [8]. They also produce histamine from histidine that has been linked to their anti-inflammatory phenotype [34]. Strains belonging to this subspecies have been considered as immunosuppressive because they could suppress the proinflammatory cytokines tumour necrosis factor (TNF), monocyte chemoattractant protein (MCP)-1, interleukin (IL)-1β and IL-12, as well as suppress intestinal inflammation [34]. The type strain, DSM 20016T (=ATCC 23272T=F 275T [original designation]), was isolated from the gastrointestinal tract of an adult human [6, 44, 45], with a DNA G+C content of 38.9 mol%.
Description of Limosilactobacillus reuteri subsp. kinnaridis subsp. nov.
Limosilactobacillus reuteri subsp. kinnaridis (kin.na′ri.dis. N.L. gen.n. kinnaridis of Kinnaris, referring to kinnaris, half-bird, half-woman creatures of South-East Asian mythology and reflecting occurrence of strains of this subspecies in birds and in humans. The name also reflects the use of this subspecies in probiotics, as according to south-east Asian mythology, Kinnaris are believed to come from the Himalayas and watch over the well-being of humans in times of trouble or danger).
L. reuteri strains clustered in lineage VI (Fig. 3) belong to L. reuteri subsp. kinnaridis and they were isolated from poultry and humans [5, 7]. Strains of this subspecies have ANI values of 98.2–100.0 % with each other and ANI values of 93.8–96.6 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from d-ribose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose, raffinose and potassium gluconate; acid production from l-arabinose, methyl α-d-glucopyranoside and turanose is strain-specific; acid is not produced from d-xylose, d-fructose, d-mannose, aesculin, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and a previous study [5], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7] indicate that strains clustered in this lineage are adapted to poultry and also occur in humans. Experimental test has revealed that strains of L. reuteri subsp. kinnaridis displayed elevated fitness in chickens but not in humans [5], suggesting that this subspecies is autochthonous of chicken and share an evolutionary history with poultry. Strains of this subspecies possess the pdu-cbi-cob-hem cluster (pdu cluster) [6, 8], which equips them with the ability to utilize 1,2-propanediol and glycerol as electron acceptors [16, 39, 40] and to produce the broad-spectrum antimicrobial compound reuterin [8, 34]. These strains are immunostimulatory; specifically, they stimulate the production of IL-7, IL-12 and IL-13, but suppress the production of IL-5 [34]. In addition, strains belonging to this subspecies synthesize folate de novo [34]. The type strain, AP3T (=DSM 110703T=LMG 31724T), was isolated from the gastrointestinal tract of an Argus Pheasant, with a DNA G+C content of 38.6 mol%.
Description of Limosilactobacillus reuteri subsp. porcinus subsp. nov.
Limosilactobacillus reuteri subsp. porcinus (por.ci′nus. L. masc. adj. porcinus of swine, referring to the host origin of most strains of this subspecies being swine).
L. reuteri strains clustered in lineage V (Fig. 3) belong to L. reuteri subsp. porcinus and they were isolated from pigs [5, 7]. Strains (3c6T and 20-2) of this subspecies have an ANI value of 99.1 % with each other and ANI values of 93.8–96.6 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from d-ribose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose, raffinose and potassium gluconate; acid production from methyl-α-d-glucopyranoside is strain-specific; acid is not produced from l-arabinose, d-xylose, d-fructose, d-mannose, aesculin, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and previous studies [5, 43, 46], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7] indicate that strains clustered in this lineage are pig-specific. Both 3c6T and 20-2 possess the pdu-cbi-cob-hem cluster (pdu cluster) [5, 46], which equips them with the ability to utilize 1,2-propanediol and glycerol as electron acceptors [16, 39, 40] and to produce the antimicrobial compound reuterin [8]. The type strain, 3c6T (=DSM 110571T=LMG 31635T), was isolated from porcine gastrointestinal tract [7, 46], with a DNA G+C content of 38.6 mol%.
Description of Limosilactobacillus reuteri subsp. murium subsp. nov.
Limosilactobacillus reuteri subsp. murium (mu′ri.um. L. plur. gen. n. murium of mice, referring to the adaptation of strains of the subspecies to rodents including mice).
L. reuteri strains clustered in lineage I (Fig. 3) belong to L. reuteri subsp. murium and they were isolated from rodents [5–7]. Strains of this subspecies have ANI values of 96.8–99.1 % with each other and ANI values of 94.5–96.5 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from l-arabinose, d-ribose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose and raffinose; acid production from potassium gluconate is strain-specific; acid is not produced from d-xylose, d-fructose, d-mannose, methyl α-d-glucopyranoside, aesculin, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and a previous studies [5], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7] indicate that strains clustered in this lineage are rodent-specific. Strains of L. reuteri subsp. murium displayed elevated fitness in mice through the colonization and biofilm formation on the forestomach epithelium [5, 7, 11], suggesting that their evolution with rodents was adaptive and led to host specificity. Large surface proteins (>750 aa) exist among strains belonging to this subspecies, which involve in epithelial adhesion and biofilm formation [6]. Strains of this subspecies produce the enzyme urease for acid resistance and rarely produce the antimicrobial compound reuterin [6, 8]. The type strain, lpuph1T (=DSM 110570T=LMG 31634T), was isolated from mouse gastrointestinal tract [6, 7], with a DNA G+C content of 38.4 mol%.
Description of Limosilactobacillus reuteri subsp. suis subsp. nov.
Limosilactobacillus reuteri subsp. suis (su′is. L. gen. n. suis, of swine, reflecting the host origin of most strains of this subspecies being the swine intestinal tract).
L. reuteri strains clustered in lineage IV (Fig. 3) belong to L. reuteri subsp. porcinus and were isolated from pig [5, 7]. Strains belonging to this subspecies have ANI values of 98.7–99.5 % with each other and ANI values of 94.6–96.3 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from l-arabinose, d-ribose, d-xylose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose and raffinose; acid is not produced from d-fructose, d-mannose, methyl α-d-glucopyranoside, aesculin, potassium gluconate, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and previous studies [5, 43, 46, 47], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7] indicate that strains clustered in this lineage are pig-specific. A mucus-binding protein (Mub) that could bind mucus and/or IgA [8, 41, 42] exists within this subspecies and it specifically supports the colonization of this subspecies to the porcine gastrointestinal tract. Strains within this subspecies have been applied as probiotics to improve porcine intestinal health, enhance production, prevent diarrhoea, release stress and immune modulation [48]. The type strain, ATCC 53608T (=LMG 31752T=1063T [original designation]), was isolated from porcine gastrointestinal tract [7, 49, 50], with a DNA G+C content of 39.0 mol%.
Description of Limosilactobacillus reuteri subsp. rodentium subsp. nov.
Limosilactobacillus reuteri subsp. rodentium (ro.den′ti.um. L. pl. gen. n. rodentium of gnawing animals, reflecting adaptation of the subspecies to rodents).
L. reuteri strains clustered in lineage III (Fig. 3) belong to L. reuteri subsp. rodentium and were mainly isolated from rodents [5–7]. Strains of this subspecies have ANI values of 96.1–98.9 % with each other and ANI values of 93.8–96.3 % with other L. reuteri strains belonging to different subspecies (Fig. 4). Acid is produced from d-ribose, d-galactose, d-glucose, maltose, lactose, melibiose, sucrose, raffinose and potassium gluconate; acid production from l-arabinose and d-xylose is strain-specific; acid is not produced from d-fructose, d-mannose, methyl α-d-glucopyranoside, aesculin, glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, methyl β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-mannitol, d-sorbitol, methyl α-d-mannopyranoside, N-acetylglucosamine, amygdalin, arbutin, salicin, cellobiose, trehalose, inulin, melezitose, starch, glycogen, xylitol, gentiobiose, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, potassium 2-ketogluconate or potassium 5-ketogluconate. Phylogenetic analyses based on the core genes identified in this study (Fig. 3) and a previous study [5], AFLP and MLSA (using concatenated sequences of ddl, pkt, leuS, gyrB, dltA, rpoA and recA genes) [7] indicate that strains clustered in this lineage including the sourdough isolates are rodent-specific. Strains of L. reuteri subsp. rodentium displayed elevated fitness in mice through the colonization and biofilm formation on the forestomach epithelium [5, 7, 11], suggesting adaptive evolution with rodents that led to host specificity. Large surface proteins (>750 aa) exist among strains belonging to this subspecies, which involve in epithelial adhesion and biofilm formation [6]. A xylose operon is highly conserved for this subspecies, especially for strains originating from rodents [6], and thus most strains of this subspecies could metabolize xylose that is an important substrate for gut bacteria [51]. In addition, strains of this subspecies produce the enzyme urease for acid resistance and rarely produce the antimicrobial compound reuterin [6, 8]. Sourdough isolates of this subspecies (LTH2584, TMW1.106, TMW1.112 and TMW1.656) produce reutericyclin, a unique antimicrobial tetramic acid with activity against Gram-positive bacteria [52]. The type strain, 100-23T (=DSM 17509T=CIP 109821T), was isolated from the rat gastrointestinal tract [11, 53], with a DNA G+C content of 38.7 mol%.
Supplementary Data
Funding information
This work was supported by the Campus Alberta Innovates Program (CAIP), the Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Irish Government’s National Development Plan (Science Foundation Ireland) awarded to J.W. F.L. was financially supported by the Alberta Innovates Postgraduate Fellowship; M.G. is supported by the Canada Research Chairs Program.
Acknowledgements
The authors thank Drs. Yuan Yao Chen (University of Alberta, Canada) and Xiaoxi B. Lin (University of Alberta, Canada) for their advice and technical assistance. We are grateful to Drs. Susanne Verbarg and Peter Schumann at the DSMZ (Germany) for their assistance in chemotaxonomic and morphological analyses.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AFLP, amplified-fragment length polymorphism; ANI, average nucleotide identity; dDDH, digital DNA-DNA hybridization; GGDC, genome-to-genome distance calculator; MLSA, multi-locus sequence analysis.
Three supplementary figures and one supplementary table are available with the online version of this article.
References
- 1.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 5.Duar RM, Frese SA, Lin XB, Fernando SC, Burkey TE, et al. Experimental evaluation of host adaptation of Lactobacillus reuteri to different vertebrate species. Appl Environ Microbiol. 2017;83:e00132–17. doi: 10.1128/AEM.00132-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri . PLoS Genet. 2011;7:e1001314. doi: 10.1371/journal.pgen.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh PL, Benson AK, Peterson DA, Patil PB, Moriyama EN, et al. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. Isme J. 2010;4:377–387. doi: 10.1038/ismej.2009.123. [DOI] [PubMed] [Google Scholar]
- 8.Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4645–4652. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. 2017;41:S27–S48. doi: 10.1093/femsre/fux030. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J, Ruan L, Sun M, Ganzle M. A genomic view of Lactobacilli and Pediococci demonstrates that phylogeny matches ecology and physiology. Appl Environ Microbiol. 2015;81:7233–7243. doi: 10.1128/AEM.02116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frese SA, Mackenzie DA, Peterson DA, Schmaltz R, Fangman T, et al. Molecular characterization of host-specific biofilm formation in a vertebrate gut symbiont. PLoS Genet. 2013;9:e1004057. doi: 10.1371/journal.pgen.1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumbeck JA, Marsteller NL, Frese SA, Peterson DA, Ramer-Tait AE, et al. Characterization of the ecological role of genes mediating acid resistance in Lactobacillus reuteri during colonization of the gastrointestinal tract. Environ Microbiol. 2016;18:2172–2184. doi: 10.1111/1462-2920.13108. [DOI] [PubMed] [Google Scholar]
- 13.Lin XB, Wang T, Stothard P, Corander J, Wang J, et al. The evolution of ecological facilitation within mixed-species biofilms in the mouse gastrointestinal tract. Isme J. 2018;12:2770–2784. doi: 10.1038/s41396-018-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter J, Chagnaud P, Tannock GW, Loach DM, Dal Bello F, et al. A high-molecular-mass surface protein (Lsp) and methionine sulfoxide reductase B (MsrB) contribute to the ecological performance of Lactobacillus reuteri in the murine gut. Appl Environ Microbiol. 2005;71:979–986. doi: 10.1128/AEM.71.2.979-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter J, Loach DM, Alqumber M, Rockel C, Hermann C, et al. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract. Environ Microbiol. 2007;9:1750–1760. doi: 10.1111/j.1462-2920.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CC, Duar RM, Lin X, Perez-Munoz ME, Tollenaar S, et al. Ecological importance of cross-feeding of the intermediate metabolite 1,2-propanediol between bacterial gut symbionts. Appl Environ Microbiol. 2020;86:e00190–20. doi: 10.1128/AEM.00190-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganzle MG, Zheng J. Lifestyles of sourdough lactobacilli - Do they matter for microbial ecology and bread quality? Int J Food Microbiol. 2019;302:15–23. doi: 10.1016/j.ijfoodmicro.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, Zhao X, Lin XB, Ganzle M. Comparative genomics Lactobacillus reuteri from sourdough reveals adaptation of an intestinal symbiont to food fermentations. Sci Rep. 2015;5:18234. doi: 10.1038/srep18234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Oh H-S, Park S-C, Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 20.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 21.Richter M, Rossello-Mora R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 24.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 2009;19:1117–1123. doi: 10.1101/gr.089532.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen IA, Chu K, Palaniappan K, Pillay M, Ratner A, et al. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 2019;47:D666–D677. doi: 10.1093/nar/gky901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 32.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter M, Rossello-Mora R, Oliver Glockner F, Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, et al. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol. 2014;6:1772–1789. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregersen T. Rapid method for distinction of gram-negative from gram-positive bacteria. Eur J Appl Microbiol Biotechnol. 1978;5:123–127. doi: 10.1007/BF00498806. [DOI] [Google Scholar]
- 36.Gerhardt P, American Society for M . Manual of methods for general bacteriology. In: Gerhardt Philipp, Murray RGE., editors. Morphology. American Society for Microbiology; 1981. [Google Scholar]
- 37.McFrederick QS, Vuong HQ, Rothman JA. Lactobacillus micheneri sp. nov., Lactobacillus timberlakei sp. nov. and Lactobacillus quenuiae sp. nov., lactic acid bacteria isolated from wild bees and flowers. Int J Syst Evol Microbiol. 2018;68:1879–1884. doi: 10.1099/ijsem.0.002758. [DOI] [PubMed] [Google Scholar]
- 38.Schumann P. Peptidoglycan structure. In: Rainey F, Oren A, editors. Methods in Microbiology. Academic Press; 2011. pp. 101–129. [Google Scholar]
- 39.Lüthi-Peng Q, Dileme FB, Puhan Z. Effect of glucose on glycerol bioconversion by Lactobacillus reuteri . Appl Microbiol Biotechnol. 2002;59:289–296. doi: 10.1007/s00253-002-1002-z. [DOI] [PubMed] [Google Scholar]
- 40.Dishisha T, Pereyra LP, Pyo SH, Britton RA, Hatti-Kaul R. Flux analysis of the Lactobacillus reuteri propanediol-utilization pathway for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1,3-propanediol from glycerol. Microb Cell Fact. 2014;13:76. doi: 10.1186/1475-2859-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos S, Jonsson H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology. 2002;148:433–442. doi: 10.1099/00221287-148-2-433. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie DA, Tailford LE, Hemmings AM, Juge N. Crystal structure of a mucus-binding protein repeat reveals an unexpected functional immunoglobulin binding activity. J Biol Chem. 2009;284:32444–32453. doi: 10.1074/jbc.M109.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Zhao J, Song Y, Zhang J, Yu Z, et al. Comparative genomics of the herbivore gut symbiont Lactobacillus reuteri reveals genetic diversity and lifestyle adaptation. Front Microbiol. 2018;9:1151. doi: 10.3389/fmicb.2018.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morita H, Toh H, Fukuda S, Horikawa H, Oshima K, et al. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008;15:151–161. doi: 10.1093/dnares/dsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandler O, Stetter K-O, Köhl R, sp Lreuteri. Lactobacillus reuteri sp. nov., a new species of heterofermentative lactobacilli . Zentralbl Bakteriol Hyg Abt IOrig C. 1980;1:264–269. [Google Scholar]
- 46.Wegmann U, MacKenzie DA, Zheng J, Goesmann A, Roos S, et al. The pan-genome of Lactobacillus reuteri strains originating from the pig gastrointestinal tract. BMC Genomics. 2015;16:1023. doi: 10.1186/s12864-015-2216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JY, Han GG, Choi J, Jin GD, Kang SK, et al. Pan-genomic approaches in Lactobacillus reuteri as a porcine probiotic: investigation of host adaptation and antipathogenic activity. Microb Ecol. 2017;74:709–721. doi: 10.1007/s00248-017-0977-z. [DOI] [PubMed] [Google Scholar]
- 48.Hou C, Zeng X, Yang F, Liu H, Qiao S. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol. 2015;6:14. doi: 10.1186/s40104-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacKenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, et al. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri . Microbiology. 2010;156:3368–3378. doi: 10.1099/mic.0.043265-0. [DOI] [PubMed] [Google Scholar]
- 50.Axelsson L, Lindgren S. Characterization and DNA homology of Lactobacillus strains isolated from pig intestine. J Appl Bacteriol. 1987;62:433–440. doi: 10.1111/j.1365-2672.1987.tb02673.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X, Gänzle MG. Genetic and phenotypic analysis of carbohydrate metabolism and transport in Lactobacillus reuteri . Int J Food Microbiol. 2018;272:12–21. doi: 10.1016/j.ijfoodmicro.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Gänzle MG, Vogel RF. Contribution of reutericyclin production to the stable persistence of Lactobacillus reuteri in an industrial sourdough fermentation. Int J Food Microbiol. 2003;80:31–45. doi: 10.1016/S0168-1605(02)00146-0. [DOI] [PubMed] [Google Scholar]
- 53.Wesney E, Tannock GW. Association of rat, pig, and fowl biotypes of Lactobacilli with the stomach of gnotobiotic mice. Microb Ecol. 1979;5:35–42. doi: 10.1007/BF02010576. [DOI] [PubMed] [Google Scholar]
- 54.Letunic I, Bork P. Interactive Tree of Life (iTOL) V4: recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.