APPLIED BIOLOGICAL SCIENCES Correction for “Mutational fitness landscapes reveal genetic and structural improvement pathways for a vaccine-elicited HIV-1 broadly neutralizing antibody,” by Bharat Madan, Baoshan Zhang, Kai Xu, Cara W. Chao, Sijy O’Dell, Jacy R. Wolfe, Gwo-Yu Chuang, Ahmed S. Fahad, Hui Geng, Rui Kong, Mark K. Louder, Thuy Duong Nguyen, Reda Rawi, Arne Schön, Zizhang Sheng, Rajani Nimrania, Yiran Wang, Tongqing Zhou, Bob C. Lin, Nicole A. Doria-Rose, Lawrence Shapiro, Peter D. Kwong, and Brandon J. DeKosky, which published March 1, 2021; 10.1073/pnas.2011653118 (Proc. Natl. Acad. Sci. U.S.A. 118, e2011653118).
The authors note that the legend for Fig. 4 appeared incorrectly. The figure and its corrected legend appear below. The authors also note that Table S3 appeared incorrectly. The SI Appendix has been corrected online.
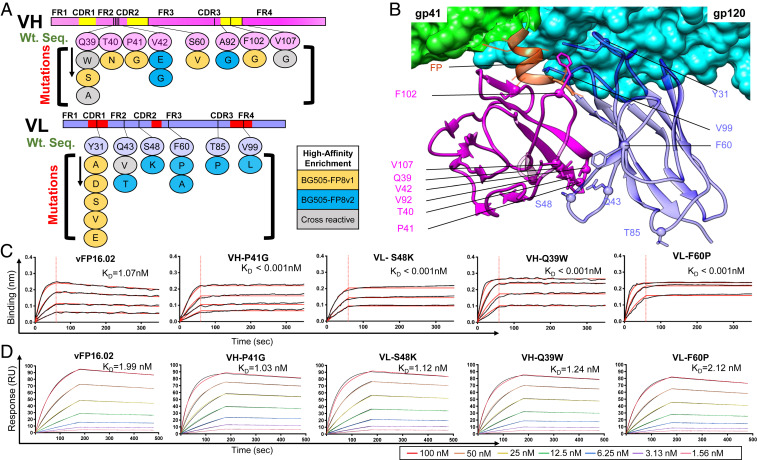
Fig. 4.
Selected single variants showed moderately enhanced affinity against the autologous BG505 trimer and orders of magnitude enhanced affinity against soluble FP. (A) Single mutational variants selected after FACS screening and bioinformatic analysis with locations highlighted within the antibody variable region. Twenty-three single-amino acid variants against BG505-FP8v1/v2 were selected (11 VH and 12 VL mutations). Some mutations were identified in library screening against both BG505-FP8 v1 and v2. (B) Structural mapping of enriched single mutations onto a cryo-electron microscopy structure of vFP16.02 in complex with a BG505.SOSIP trimer (PDB ID 6CDI) (16). (C) Bio-layer interferometry response curves for key variants binding against soluble FP showing over 1,000-fold enhanced binding affinity (SI Appendix, Fig. S5). (D) Surface plasmon resonance response curves for binding against BG505-FP8v1 trimer for key single mutation variants (SI Appendix, Fig. S6).