Constraints on CO2 emissions are confronting society with multiple massive challenges: creating new sources of clean energy beyond solar and wind; electrifying our transportation systems and using lighter weight materials; decarbonizing the industrial sector; and dealing with the economic fallout associated with shrinking the fossil hydrocarbon industry, which accounts for about 7% of the world economy
Widespread use of carbon nanotubes could make alternative energy and transportation systems more efficient by providing lighter-weight materials. It could also mean using less oil and gas for fuel and more for both hydrogen and carbon production—resulting in greener fuels and a variety of desirable materials that effectively serve as carbon sinks. Nanotube image credit: Shutterstock/Gl0ck; Field image credit: Shutterstock/Evgeny Karandaev.
At first blush, the solutions to these dilemmas appear to be at odds with one another. Coming at this challenge with perspectives from academia and industry, we believe there may be a way forward that positively influences all of these aims: Stop using oil and gas as fuels and instead use them as sources of both hydrogen for fuel and carbon for useful, pervasive materials.
As early as the 1880s, solid carbon was industrially generated from natural gas and other hydrocarbons for use in products (1). One problem: The grades of carbon generated in the past (e.g., carbon black) lack structural integrity and have limited applications. But carbon nanomaterials, a type of solid carbon first described about 30 years ago (2, 3), can be synthesized by splitting hydrocarbons and could displace steel, aluminum, and cement (i.e., massive markets that account for more than 50% of the industrial sector CO2 emissions). All of this could be possible while also providing light-weight solutions for structural and electrical components in transportation systems and generating CO2-free hydrogen. It’s a potentially win–win scenario. But charting a way forward will require carefully mapping and navigating the interconnections between energy and material systems.
Material–Energy Nexus
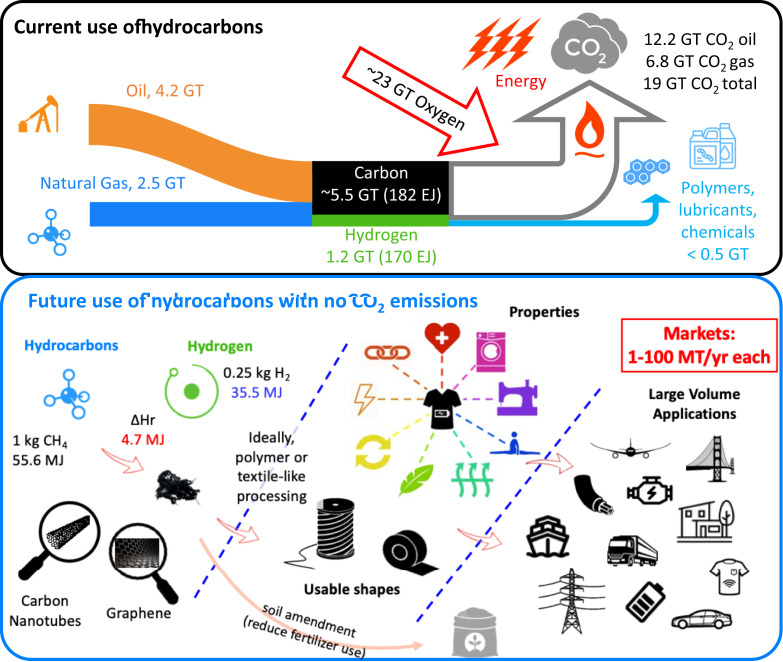
Every year we extract more than 10 gigatonnes (Gt) of carbon and 1.3 Gt of hydrogen as oil, natural gas, and coal (Table 1). Almost all these resources are burned to generate energy, causing more than 30 Gt of CO2 to enter the atmosphere, which is unsustainable in light of climate change. The only significant exception is polymers, which fix 0.35 Gt per year of hydrocarbon resources into valuable solid materials (Fig. 1). At the same time, every year the world devotes more than 12% of its energy production to primary metals; most of this energy goes into mining, refining, and processing metal ores into usable metals (Table 2). Unlike hydrocarbons, which are mined at high concentration and in reduced form, metals are mined at low concentration [typically about 50% for iron ore, 15% for aluminum ore, and below 1% for copper ore (4)]. They’re in an oxidized form and must be reduced using carbon, which generates 3.7 Gt of CO2 emissions—more than half of the emissions of the industrial sector—in addition to the well-known environmental impacts of mining metal ores. Concrete (via the production of cement from calcium carbonate) is the other primary offender.
Table 1.
Yearly production of carbon, hydrogen, and energy via oil, gas, and coal (from IEA World Energy Outlook)
| Production | Specific combustion energy | Total combustion energy | Carbon content | Hydrogen content | CO2 emissions (combustion) | |||
| Energy source | Mt per year | MJ per kg | EJ per year | Mt per year | EJ per year | Mt per year | EJ per year | Mt per year |
| Oil | 4,200 | 44 | 185 | 3,600 | 119 | 600 | 85 | 12,200 |
| Gas | 2,500 | 55 | 138 | 1,900 | 63 | 600 | 85 | 6,800 |
| Coal | 7,500 | 20 | 150 | 4,800 | 158 | 100 | 14 | 16,300 |
| Total | 473 | 10,300 | 340 | 1,300 | 184 | 35,300 | ||
For natural gas, most of the energy is contained in the hydrogen. The energies of combustion of carbon and hydrogen are ∼33 megajoules (MJ) per kilogram and ∼142 MJ per kilogram, respectively. Most fossil coal is lignite, which has low carbon content (∼60%) and 3 to 5% hydrogen. The total combustion energy of oil, gas, and coal (column 4) is not the same as the combustion energy of their carbon (column 6) and hydrogen (column 8) components; the difference is the standard energy of formation of their constituent hydrocarbons (e.g., 4.7 MJ per kg for methane). 1 ExaJoule = 1018 J.
Fig. 1.
In principle, and with sufficient investments, natural gas and hydrocarbons can become feedstock to produce hydrogen and carbon nanotube materials that can be used at a large scale to decarbonize industrial emissions, provide clean energy, and reduce the weight and CO2 footprint of vehicles and electrical systems.
Table 2.
Properties, embodied energies and CO2 footprints, and yearly production of main industrial materials (adapted from Ref. 4 and the United States Geological Survey)
| Density | Cost | Embodied energy | CO2 footprint | Yearly production | Yearly Energy consumption | Yearly CO2 emissions | |||
| Material | Kilograms per cubic meter | US dollars per kilogram | MJ per kilogram | GJ per cubic meter | Kilograms CO2 per kilogram | t CO2 per cubic meter | Mt per year | EJ per year | Mt per year |
| Steel | 7,800 | 0.8 | 30 | 6.2 | 1.8 | 14 | 1,600 | 48 | 2,900 |
| Aluminum | 2,700 | 2 | 210 | 567 | 12 | 24 | 60 | 12.6 | 720 |
| Copper | 9,000 | 6 | 60 | 54 | 3.7 | 33 | 20 | 1.2 | 74 |
| C-Fibers | 1,800 | 30-200 | 370 | 666 | 25 | 45 | 0.1 | 0.037 | 2.5 |
| Concrete | 2,500 | 0.05 | 1.2 | 3 | 0.12 | 0.3 | 16,000 | 19.2 | 1,900 |
Within the same class of materials (e.g., steel), there are subclasses with major markets and significantly higher economic value and energy and CO2 footprints. For example, stainless steel is produced at 50 Mt per year and sold at 3 to 8 US dollars per kg; its energy and CO2 intensity are about three times higher than low carbon steel. CO2 footprints are given on a by-weight and by-volume basis, because materials are traded on a mass basis but used on the basis of function, which tracks more closely with volumetric properties. Because of this accounting, heavier materials appear to be cheaper and less environmentally impactful. Most of the concrete emissions are attributable to cement production; however, cement alone is not used as a structural material.
Why, then, don’t we make more effective use of the carbon contained in oil and gas in making materials? Surprisingly, researchers, industry, policymakers, and the general public are not focusing on this “materials–energy” nexus. The concept of creating materials out of hydrocarbons was not appealing when hydrocarbons were considered scarce and climate change concerns were minimal; industry used fossil fuels to generate energy and reduce metal oxide ores. However, it is now commonly accepted that hydrocarbons can be extracted in vast excess of the ecosystem’s ability to absorb their conversion into CO2 to generate energy (in the absence of CO2 capture and sequestration).
Hydrocarbons can be directly split into their elements: hydrogen and solid carbon in the zero-oxidation state; for methane, such a transformation occurs spontaneously at moderate temperature (above 700 °C) with no CO2 emissions, via the pyrolysis reaction CH4 → C + 2H2. This slightly endothermal reaction consumes 4.7 gigajoules (GJ) per tonne of pyrolyzed methane; however, it produces 250 kilograms of hydrogen, with an energy content of 35.5 GJ (63% of the total energy content of methane). Hence, in the thermodynamic limit (i.e., an ideal process), one tonne of solid carbon can be produced while cogenerating 41.1 GJ of net clean energy as hydrogen. Hydrogen is extremely valuable because it can be converted into electricity at high efficiency (60% to 80%) using fuel cells and, as the simplest and most effective transportation fuel, it could displace liquid hydrocarbons. In fact, hydrogen can be derived from direct splitting of methane using eight times less renewable energy than “green” hydrogen obtained via water electrolysis. Still, for this notion to be feasible on the massive scale of energy systems (billion of tonnes per year of products), a productive use must be found for the solid carbon byproduct—lest we create a massive solid waste problem.
The idea of direct conversion of methane into hydrogen and solid carbon gets revisited every two decades or so (5, 6). The alternatives for solid carbon were limited until now: Carbon exists in its elemental (zero-oxidation) state as diamond and graphitic carbon; a large-scale example of elemental carbon is carbon black, which can be produced via pyrolysis. Carbon black use dates to pre-industrial times and has been produced from natural gas for well over a century (1); yet, the global carbon black market is below ∼15 million tonnes (Mt) per year (7) because of its limited applications, chiefly the result of its lack of structural integrity. Hence, carbon black is unlikely to provide a major outlet for natural gas or to make any contribution to the material–energy nexus.
So why could large-scale methane splitting be fruitful now? The emergence of nanoscale carbon is the real major change (Fig. 1). Because of this material’s potential use in cars, heavy vehicles, and aircrafts, mass-scale production of carbon nanomaterials from methane could cogenerate significant amounts of hydrogen (tens to hundreds of Mt per year), providing additional economic value as well as clean energy. Moreover, compared with aluminum and carbon fibers (Table 2), such lightweight carbon nanomaterials could provide a low-CO2 pathway to reducing the weight of electrified transportation fleets. Table 1
Enter the Nanotube
Carbon nanotubes (CNTs), a subclass of Fullerenes discovered in the early 1990s (3, 8, 9), share many features with polymers because of their thin diameter and elongated shape and can form self-supporting macroscopic materials (10).
Intriguingly, routes for converting methane into CNTs were published as early as 1997 (11, 12), and natural gas and light hydrocarbons are currently the preferred feedstock of several CNT producers. However, over the past two decades, producers developed CNT materials, and later graphene (13), within a framework of specialty chemicals (essentially, high-value replacement for carbon black) and with little to no guidance from the application market in terms of which subclasses of CNTs can make useful macro-materials. The field has now evolved to the point that high-quality CNTs are available in multi-tonnes per year (14), and they have been converted into fibers, sheets, and other macroscopic materials that could displace steel, aluminum, and copper in a multitude of applications (15–21). Yet they have exotic materials prices ($2,000 to $100,000 per kilogram), making their introduction impossible beyond a very limited range of high-end applications (chiefly in aerospace, electronics, and medicine).
However, production costs have dropped by three orders of magnitude in the past twenty years and are within striking distance of becoming competitive—by comparison, solar panels took four decades to attain comparable cost reduction and two more to become cost-competitive for civilian use. CNT-derived materials prices in the range of $20 to $30 per kilogram could enable penetration in most of the copper, aluminum, and stainless steel markets; at $10 per kilogram, CNT materials could be used in vehicles to replace steel components (more than 100 Mt per year). Below $3 per kilogram, displacement of construction materials appears feasible in large infrastructures (e.g., bridges) and even office buildings and houses. Yet, process knowledge needed for scale-up is incomplete and broken up into various organizations and countries, which does not augur well for continued rapid cost reduction.
So, can CNTs replace metals on a large scale (100+ Mt per year)? The rapid rise of plastics over the course of a few short decades shows that such a revolution is possible. That explosive growth was propelled in part by the need to use the light byproducts of oil refining, which were being flared by refineries. CNTs could provide a comparable solution to hydrocarbon combustion.
Historical parallels with the development of steel, aluminum, and polyolefins, combined with thermodynamic limit analysis, indicate that CNTs’ energy production cost should be lower than polyethylene. In essence, it may be possible to achieve superior production efficiencies with CNTs as compared with steel or polyethylene because the raw material required for their production is in abundant supply and hydrogen is increasingly in demand. The challenge is shifting CNTs’ perspective away from high-performance materials and additives and towards large-scale use, while concurrently developing the appropriate synthesis and process technologies.
Yet, the target process efficiencies are far from intimidating. A 1 gigawatt (GW) natural gas power plant uses 1.13 Mt per year of natural gas and emits 3.12 Mt per year of CO2; at 20% efficiency, that same natural gas could make enough carbon nanomaterials to displace 0.85 Mt of aluminum while generating 0.1 Mt of net hydrogen (or 0.28 Mt of total hydrogen if 0.85 GWs of renewable energy are used to run the process). This would save 10.2 Mt per year of CO2 emissions and 2.8 GWs of power needed to make aluminum. If the hydrogen is used locally to power the plant, this yields a net reduction of 14.3 Mt per year of CO2 and net savings of 1.8 GWs of power, while generating 0.1 Mt per year of hydrogen. If green energy is used to power the process, CO2 emissions are reduced by 16.1 Mt per year while producing 0.28 Mt per year of hydrogen with a net grid energy saving of 0.95 GWs. Notably, generating 0.28 Mt per year of green hydrogen (via 66% efficient water electrolysis) would require 1.93 GWs of renewable energy! CO2 and energy savings would be about 50% lower if the carbon is used to displace steel—although a larger amount of steel would be displaced because of steel’s higher density.
The potential contribution to lowering CO2 emissions is massive; transitioning the projected nine billion humans to developed-world living standards (10 tonnes of installed steel per person) will require the production of more than 70 Gt of virgin steel by the end of the century; the associated CO2 emissions (125 Gt) will increase atmospheric CO2 by more than 30 parts per million—they will take up 16% of our remaining CO2 emission budget if we are to keep global temperature rise to below 2 °C. Any reduction of this baseline by displacing steel with CNTs will proportionally reduce CO2 growth—presently, the only option for limiting metal-induced CO2 growth is to reduce the amount of metals we use (22).
Of course, there are real challenges. Entire value, manufacturing, and supply chains must be developed for CNTs, including conversion of CNTs into semi-finished macroscopic articles such as fibers, sheets, and three-dimensional materials and incorporation of such articles into products and applications. In parallel, materials grades need to be standardized, a new workforce must be developed, and health and environmental standards must be established for materials, processes, and product use and life cycles. And, taking a lesson from cheap readily available plastics and the huge waste problem they have presented, we should consider recycling and end-of-life procedures early on for CNTs.
Creative Solutions
Unfortunately, despite steady progress on CNT properties and manufacturing, the appetite for CNT development has slowed down (and perhaps stalled). In the absence of a deliberate effort, the transition from using hydrocarbons as fuels to using them as a source of solid materials may never materialize. This transition will not happen by replicating the pre-1980s materials development models, in which just a few companies conducted research and development and commercialization, as was the case for aramids (Kevlar) or carbon fibers. The size of the problem is too large for any one corporation to tackle, and the current size of the opportunity is too small for any established corporation to care.
Yet, it’s not clear whether any government or coalition of governments has the ability or the political will to take on this challenge. Most dauntingly, whereas energy and hydrogen are commodities, materials are not. To be successful, every material class required the redesign of end-products and fabrication technologies—consider, for example, the differences in architecture and construction between a Roman arch stone bridge and a steel suspension bridge. CNTs and other new carbon materials will need to follow the same path of diversification in manufacturing and use, while retaining efficiency and economy of scale in primary production.
Governments will always have an important role, but researchers and industry players should consider sparking rapid advances through open innovation and self-organization—for example, lifting patent protections in select cases to allow more researchers to make rapid contributions. Such approaches have been successful in other areas, such as software, but are largely untested in large-scale materials, manufacturing, and energy systems. Philanthropists could have the biggest impact of all groups, by providing long-term support and requiring sharing of knowledge.
This effort could be organized by a nonprofit institute, or a network of connected institutes that cut across international boundaries, enabling companies to collaborate with each other and with academic researchers across the fledgling material value chains. Governments and philanthropists could fund these efforts by coordinating existing mechanisms or unlocking new ones (as in the case of Advanced Manufacturing Institutes and Department of Energy Hubs in the United States or Flagships in Europe); as in solar energy, governments could become early customers and accelerate introduction (e.g., via incentives). Coordination of CNT production and conversion with application development will be key, because many applications cannot be explored with gram-scale (or even kilogram-scale) levels of materials, and their pursuit will depend on demonstration-scale synthesis coming online.
Each of these participants will have their own incentives: Oil and gas companies will preserve the value of their hydrocarbon feedstock and could capture additional value by implementing at scale direct-splitting technologies and supplying clean hydrogen and the base CNT materials. Companies in the industrial sector and customer products could capture value by converting CNTs into usable shapes and forms and enhanced products; more importantly, they will decarbonize their own operations and their entire supply chains, responding to consumer demand and societal pressures. Academics will be able to tackle exciting, impactful fundamental problems. Philanthropists and governments will have a route to address climate change while also providing a path for economic growth. Importantly, all of these pathways will generate robust growth in manufacturing jobs, most of which will stay at the local level where oil and gas are already established.
In the worst-case scenario, we will develop a new class of carbon materials that will complement and extend the capabilities of polymers, carbon fibers, and carbon black. In the best-case scenario, we will transform our current, frustratingly inefficient materials production systems, which require consuming valuable carbon to make materials that contain little to none of it, while emitting such carbon as waste CO2. Either scenario will produce a better future for all of us.
Supplementary Material
Footnotes
Competing interest statement: M.P. has a financial interest in a startup that makes CNT fibers (but does not convert oil and gas into CNTs), was on sabbatical at Shell in 2018-19, has funding from Shell (within a DOE/ARPA-E grant), and now directs Rice University’s Carbon Hub, which receives corporate funding from Shell, Mitsubishi Corporation (Americas), and Prysmian.
Any opinions, findings, conclusions, or recommendations expressed in this work are those of the authors and have not been endorsed by the National Academy of Sciences.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2112089118/-/DCSupplemental.
References
- 1.Cabot T. D., A short history of Cabot corporation. Daedalus 125, 113–136 (1996). [Google Scholar]
- 2.Kroto H. W., Heath J. R., O’Brien S. C., Curl R. F., Smalley R. E., C60: Buckminsterfullerene. Nature 318, 162–163 (1985). [Google Scholar]
- 3.Iijima S., Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991). [Google Scholar]
- 4.Ashby M. F., Materials and the Environment (Butterworth-Heinemann, New York, ed. 2, 2013). [Google Scholar]
- 5.Muradov N. Z., How to produce hydrogen from fossil fuels without CO2 emission. Int. J. Hydrogen Energy 18, 211–215 (1993). [Google Scholar]
- 6.Fulcheri L., Schwob Y., From methane to hydrogen, carbon black and water. Int. J. Hydrogen Energy 20, 197–202 (1995). [Google Scholar]
- 7.Dagle R., et al., An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products (PNNL-26726 Report) (U.S. Department of Energy, Office of Scientific and Technical Information, Oak Ridge, TN, 2017). [Google Scholar]
- 8.Iijima S., Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993). [Google Scholar]
- 9.Bethune D. S., et al., Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 363, 605–607 (1993). [Google Scholar]
- 10.Green M. J., Behabtu N., Pasquali M., Adams W. W., Nanotubes as polymers. Polymer (Guildf.) 50, 4979–4997 (2009). [Google Scholar]
- 11.Peigney A., Laurent C., Dobigeon F., Rousset A., Carbon nanotubes grown in situ by a novel catalytic method. J. Mater. Res. 12, 613–615 (1997). [Google Scholar]
- 12.Kong J., Soh H. T., Cassell A. M., Quate C. F., Dai H., Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature 395, 878–881 (1998). [Google Scholar]
- 13.Novoselov K. S., et al., Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Advanced Materials Primer: Carbon Nanotubes (BloombergNEF, February 8, 2021).
- 15.Vigolo B., et al., Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 290, 1331–1334 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Li Y.-L., Kinloch I. A., Windle A. H., Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 304, 276–278 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Ericson L. M., et al., Macroscopic, neat, single-walled carbon nanotube fibers. Science 305, 1447–1450 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Lee S.-H., et al., Deep-injection floating-catalyst chemical vapor deposition to continuously synthesize carbon nanotubes with high aspect ratio and high crystallinity. Carbon 173, 901–909 (2021). [Google Scholar]
- 19.Stallard J. C., et al., The mechanical and electrical properties of direct-spun carbon nanotube mats. Extreme Mech. Lett. 21, 65–75 (2018). [Google Scholar]
- 20.Behabtu N., et al., Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 339, 182–186 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Taylor L. W., et al., Improved properties, increased production, and the path to broad adoption of carbon nanotube fibers. Carbon 171, 689–694 (2021). [Google Scholar]
- 22.Allwood J. M., Cullen J. M., Sustainable Materials: With Both Eyes Open (UIT Cambridge, ed. 2, 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.