Significance
There are no biomarkers for schizophrenia (SCZ), a disorder of dysfunctional neural networks. We demonstrate that a master regulator of cytoskeleton (“CRMP2”) and, hence, neural circuitry, may form the basis for such a biomarker because its activity is uniquely imbalanced in SCZ patients. We show that SCZ patients are characterized by an excess of active CRMP2 not only in their brains (where it is correlated with dendritic abnormalities) but also in their peripheral blood lymphocytes. The abundance of active CRMP2 and insufficiency of opposing inactive p-CRMP2 likely disrupts neuronal function. Because peripheral blood CRMP2 appears to reflect intracerebral processes, it could form the basis of a rapid, minimally invasive, sensitive, and specific clinical diagnostic aid for SCZ in young patients.
Keywords: collapsin response mediator protein-2 (CRMP2), dendritic morphology, cytoskeleton, biomarker, blood test
Abstract
There are no validated biomarkers for schizophrenia (SCZ), a disorder linked to neural network dysfunction. We demonstrate that collapsin response mediator protein-2 (CRMP2), a master regulator of cytoskeleton and, hence, neural circuitry, may form the basis for a biomarker because its activity is uniquely imbalanced in SCZ patients. CRMP2’s activity depends upon its phosphorylation state. While an equilibrium between inactive (phosphorylated) and active (nonphosphorylated) CRMP2 is present in unaffected individuals, we show that SCZ patients are characterized by excess active CRMP2. We examined CRMP2 levels first in postmortem brains (correlated with neuronal morphometrics) and then, because CRMP2 is expressed in lymphocytes as well, in the peripheral blood of SCZ patients versus age-matched unaffected controls. In the brains and, more starkly, in the lymphocytes of SCZ patients <40 y old, we observed that nonphosphorylated CRMP2 was higher than in controls, while phosphorylated CRMP2 remained unchanged from control. In the brain, these changes were associated with dendritic structural abnormalities. The abundance of active CRMP2 with insufficient opposing inactive p-CRMP2 yielded a unique lowering of the p-CRMP2:CRMP2 ratio in SCZ patients, implying a disruption in the normal equilibrium between active and inactive CRMP2. These clinical data suggest that measuring CRMP2 and p-CRMP2 in peripheral blood might reflect intracerebral processes and suggest a rapid, minimally invasive, sensitive, and specific adjunctive diagnostic aid for early SCZ: increased CRMP2 or a decreased p-CRMP2:CRMP2 ratio may help cinch the diagnosis in a newly presenting young patient suspected of SCZ (versus such mimics as mania in bipolar disorder, where the ratio is high).
Although the molecular pathogenesis of schizophrenia (SCZ) is poorly understood, altered structure and function of neural networks in the brain have been implicated (1–5). In developing brains, neural circuitry is formed through neurogenesis, differentiation, axon guidance, dendritogenesis, synaptogenesis, and activity-dependent and microglial refinement of immature synapses (6). Because synapses are the sites of neurotransmitter signal transduction, dendritic spine dysfunction may play an important etiological role in SCZ. Indeed, genetic linkage studies have suggested that aberrations in genes responsible for synapse formation and maturation may be SCZ risk factors (4, 6–8). The lack of reliable, pathogenetically related, clinically accessible biomarkers hinders not only understanding underlying etiological mechanisms, but also the diagnosis of SCZ. Enhancing the speed, sensitivity, and specificity of diagnosing early-stage SCZ would facilitate that systematic examination of genes across larger more homogenous patient populations, improve clinical management, and accelerate the development of new therapeutic options.
Collapsin response mediator protein 2 (CRMP2), also known as Dihydropyrimidinase-like 2 (DPYSL2), is a master regulator of axon guidance, dendritic branching, and spine formation: hence, a neural network modulator. It was first identified as an intracellular molecule that mediates the signaling of Semaphorin3A, a repulsive axon guidance molecule (9), but has since been recognized as playing a much larger role in neural development and maintenance of homeostasis in the adult nervous system. The CRMP family of proteins are now known to consist of five homologous cytosolic proteins, CRMP1 through CRMP5 (10, 11). CRMP2 actively binds cytoskeletal elements in its nonphosphorylated active state; phosphorylation of CRMP2—which is a two-step process—inactivates it and induces it to release cytoskeletal elements. Cdk5 first phosphorylates CRMP2 at Ser522, priming it for glycogen synthase kinase 3β (GSK3β) to phosphorylate it at Thr514 and Ser518 (12, 13). We now know that “toggling” between inactive (phosphorylated) and active (nonphosphorylated) CRMP2 is an ongoing physiologic adaptive mechanism for preventing abnormal neuronal sprouting. Overall, a balance exists between active and inactive CRMP2. We previously described how this balance is pivotal for proper dendritic branching and spine organization in vivo (14). Dendritic spines are the point of contact for interneuronal synaptic communication. Nonphosphorylated CRMP2 is expressed throughout the neuron, including the dendritic spines; phosphorylated CRMP2 is not expressed in the spines, suggesting that when CRMP2 becomes inactivated, it leaves or is excluded from the spines. There appears to be a continuous dynamic in which CRMP2 enters and fills the spines when it is activated/dephosphorylated and is absent from or leaves the spines when it becomes inactivated/phosphorylated. Agents known to decrease inactivated CRMP2 (e.g., lithium) also increase dendritic spine volume and density, an action abrogated by the elimination of CRMP2. Constitutive Crmp2 knockout mice are characterized by defects in dendritic morphology, including diminished spine density and dendritic length, in regions where CRMP2 is expressed (e.g., the cerebral cortex, hippocampus, cerebellum, striatum).
The processes in which we’ve learned CRMP proteins play critical roles are also now recognized as pivotal to the network abnormalities central to neuropsychiatric disorders (10, 11, 13–19). Phenotypic analysis of crmp1 and crmp2 gene-deficient mice (crmp−/− and crmp2−/−, respectively) revealed that both of these gene-deficient animals show behavioral abnormalities that model neuropsychiatric symptoms (15–17). We recently implicated aberrant CRMP2 posttranslational regulation as central to the pathogenesis of lithium-responsive (LiR) bipolar disorder (BPD) (14, 20). Specifically, the “set-point” for the ratio of phosphorylated (inactive)-to-nonphosphorylated (active) CRMP2 was abnormally high in LiR BPD human brains and patient-specific human induced pluripotent stem cell (hiPSC)-derived neurons; lithium, a CRMP2 pathway modulator, normalized that set-point by reducing the levels of phosphorylated CRMP2 (pCRMP2) and, concomitantly, dendritic structural pathology and neuronal hyperexcitability. Hence, we viewed BPD as a disorder not of a gene per se, but rather of the posttranslational regulation of a developmentally critical molecule. The specificity of the elevated pCRMP2:CRMP2 ratio for LiR BPD—an elevation not seen in unaffected controls, in other psychiatric and neurological disorders, or even in lithium-nonresponsive (LiNR) BPD patients—suggested that it might serve as a biomarker for that disorder.
A series of recent genome-wide association studies have, intriguingly, shown BPD to cluster with cognitive disorders, such as SCZ, more so than with mood disorders (21–23). Given CRMP2’s role as a master regulator of cytoskeletal and hence neural network modulation, we investigated whether its activity state might serve as a potential biomarker for SCZ as well, a condition also suspected of being characterized by abnormal synaptogenesis, neuritogenesis, and neural network activity. We, indeed, found CRMP2 anomalies in the postmortem brains of SCZ patients: the levels of active CRMP2 were abnormally high and correlated with relevant dendritic morphometric aberrations. Because CRMP2 is expressed also in lymphocytes, imbalances in the phosphorylation state of this intracerebral neural network modulator were reflected in the peripheral blood of SCZ patients, as confirmed by a blinded, prospective clinical study, making this finding pertinent to the search for a mechanistically based “bedside” diagnostic marker.
Results
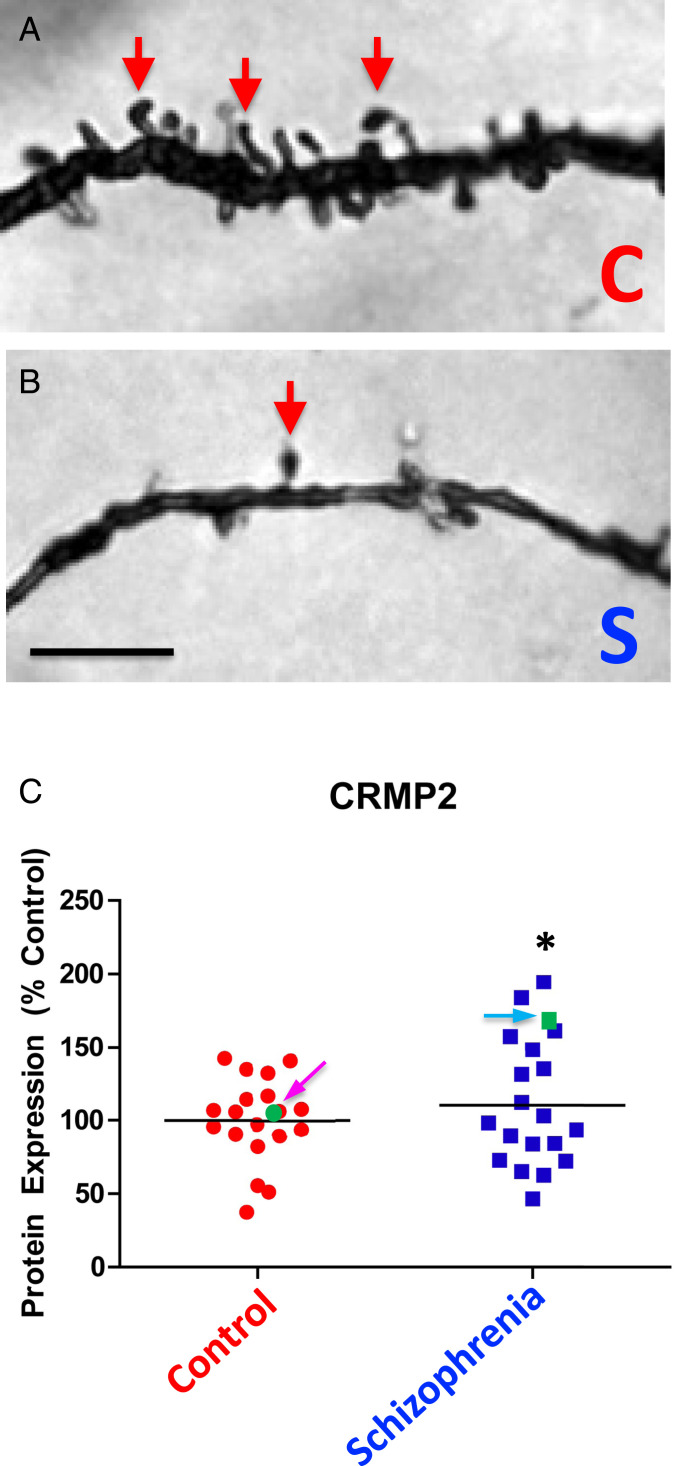
To get a first approximation of whether there might be any differences in CRMP2 expression in the brains of patients with SCZ—known to have dendritic abnormalities (24–26)—compared with the brains of unaffected patients without such abnormalities, we blindly examined frozen, postmortem, human brain tissue obtained from the Harvard Brain Tissue Resource Center. Because several lines of evidence have implicated the dorsolateral prefrontal cortex (DLPFC) in SCZ pathophysiology (24), we focused on that region. As previously reported by us and others, the presence of cortical dendritic arbor pathology was confirmed (Fig. 1 A and B). Indeed, both shortened basilar dendrites and basilar dendritic spine loss were detected on pyramidal neurons (with somata in the deep layer III of the DLPFC) from SCZ patients (25, 26).
Fig. 1.
In brains of patients with SCZ compared with those of unaffected age-matched controls, CRMP2 levels are elevated and dendritic spine densities and basilar dendrite lengths are reduced. (A and B) Representative brightfield photomicrographs of the basilar dendrites of Golgi-stained pyramidal neurons in the DLPFC from a representative control subject (A) compared with a representative patient with SCZ (B). Red arrows point to dendritic spines, which are markedly reduced in the SCZ patient. (Scale bar, 5 µm.) (C) Graph depicting the relative protein expression of CRMP2 in control and SCZ patients (whose representative neuropathology is illustrated in B). The SCZ values were normalized to control values (percent control) collected in parallel from the same gel. CRMP2 was significantly increased by 10% in SCZ patients relative to controls (*P = 0.05) and CRMP2 protein expression was inversely correlated with basilar dendrite length (r = –0.37, P = 0.04). However, as indicated in SI Appendix, Table S1, this cohort of brain donors tended to be older (a mean age of ∼60 y old). Focusing on the few patients <40 y-old (among the SCZ patients, indicated by the blue arrow and green square, and among the unaffected controls, indicated by the pink arrow and green dot) suggested that we should explore additional CRMP2 parameters—such as diminished p-CRMP2:CRMP2 ratios—as we did in SI Appendix, Fig. S1, and found to be distinctively low in SCZ patients. It also prompted us to focus on young patients (defined as <40 y old) in our second prospective clinical study (Fig. 2) where, in this more highly powered cohort 2, the differences were significantly greater. Please see the Introduction for a summary of the relationship between CRMP2 and dendritogenesis.
We next determined whether differences in CRMP2 expression might be associated with this dendritic arbor pathology in SCZ. Using quantitative Western blotting, the relative protein expressions of CRMP2 and p-CRMP2, and the resultant pCRMP2:CRMP2 ratio were assessed in the above-mentioned archived DLPFC gray matter specimens from patients with SCZ (n = 19) and from unaffected control subjects (n = 19) (note: there were no demographic or pharmacological differences between the two cohorts) (SI Appendix, Table S1). Protein expression and ratios were then correlated with dendritic parameters (e.g., basilar dendrite spine density, dendrite length, and the number of spines per dendrite) of pyramidal neurons in the deep layer III of the DLPFC. Relative to unaffected controls, CRMP2 was significantly increased by 10% (P = 0.05) (Fig. 1C). In addition, CRMP2 protein expression was inversely correlated with basilar dendrite length (r = –0.37, P = 0.04) (Fig. 1B). [Note: we and others have previously demonstrated a causal relationship between normal levels of active CRMP2 and normal dendritogenesis (14–19, 27).]
Although CRMP2 levels were significantly higher in brains from patients with SCZ compared with brains from control subjects, phosphorylated CRMP2 (p-CRMP2) protein expression (SI Appendix, Fig. S1A) and, hence, the ratio of p-CRMP2:CRMP2 protein expression (SI Appendix, Fig. S1B), was not statistically different in brains from SCZ patients compared with brains from control subjects (*P > 0.05), when the data were analyzed in the aggregate. However, a few findings caught our attention. First, the finding of an increase in CRMP2 in SCZ contrasted starkly with our previously reported data (14) from the DPLFCs of patients with LiR BPD where CRMP2 was not significantly different from age-matched unaffected patients but, rather, it was p-CRMP2 that was increased. Hence, the p-CRMP2:CRMP2 ratio was significantly elevated in LiR BPD patients compared with unaffected controls (as well as compared with patients with other psychiatric and neurological diseases, including LiNR BPD) because the numerator (p-CRMP2) was abnormally high while the denominator (CRMP2) remained unchanged. In other words, there was a striking difference between the brains of patients with SCZ versus BPD from the same brain bank, processed similarly, by the same investigators.
The next point that prompted scrutiny was the ostensible lack of a statistically significant decrease in the p-CRMP2:CRMP2 ratio between SCZ brains and age-matched control brains despite the fact that CRMP2, the denominator, was increased. This finding suggested the following two possibilities: 1) the brain’s machinery for phosphorylating (and hence, inactivating) the increased CRMP2 substrate seen in SCZ could keep pace with the greater burden, or 2) p-CRMP2 was also rising for reasons independent of SCZ. Apropos to the latter possibility, the mean age of this cohort was relatively old (59.5 ± 12.7 y of age) (SI Appendix, Table S1); it is known that p-CRMP2 often rises in association with aging-related conditions (12). For example, hyperphosphorylated forms of CRMP2 accumulate in Alzheimer’s disease brains (12, 28–30).
Furthermore, the nature of psychiatric brain banking and the stochastic, often serendipitous, manner in which specimens are voluntarily donated as anatomical gifts makes most such SCZ collections skew to older brains. [Our BPD study (14) was comprised of younger patients because that disorder’s demographics skew younger, with many sufferers dying prematurely from suicide or substance abuse, less so seen in SCZ.] Notwithstanding the limitations in SCZ brain banking, there was an observation within this retrospective unselected cohort that attracted our scrutiny and informed our next larger, statistically powered prospective clinical study. If we focused on the few “young” patients (defined in psychiatry as <40 y old) in cohort 1 (Fig. 1C and SI Appendix, Fig. S1)—one with SCZ (Fig. 1C, blue arrow and green square) and one unaffected (Fig. 1C, pink arrow and green dot)—we saw that, while CRMP2 (the denominator) was significantly elevated in the young SCZ patient (as with all of the 19 SCZ patients), p-CRMP2 (the numerator) was indistinguishable from the young unaffected subject, making the p-CRMP2:CRMP2 ratio much lower than normal (0.8 compared with 1.3) in the young SCZ patient. In other words, this observation suggested (begging further confirmation) that, when aging as a confounder was eliminated, p-CRMP2 may not be elevated and the p-CRMP2:CRMP2 ratio may, hence, actually be low in young patients with SCZ.
While data from single patients are, of course, unpersuasive, with this latter observation in mind—prompting us to entertain the hypothesis that the p-CRMP2:CRMP2 ratio in SCZ is diminished (in contrast to that ratio in BPD, which is elevated)—we designed our next clinical study so that cohorts could be stratified by age to avoid the results being potentially confounded by conditions or morbidities unrelated to SCZ. Such a study, we recognized, would not only need to be larger, prospective, blinded, and case-controlled, but also—in order to include a sufficient number of young SCZ patients and their unaffected age-matched controls—employ assays obtainable from living subjects. Such a study would provide the statistical power unachievable in most brain bank studies.
Knowing that CRMP2 is also expressed in lymphocytes, we wondered whether the elevated CRMP2 levels detected in the brains of patients with SCZ could be reflected in the peripheral blood mononuclear cells (PBMCs) of such patients. Interestingly, as detailed below, the differences in CRMP2 between patients with SCZ and unaffected age-matched controls, as detected through assaying this more accessible peripheral blood-derived cell type, was actually more striking than in the brain; in addition, this approach in this cohort revealed the p-CRMP2:CRMP2 ratio to be the informative metric we had suspected it would be.
We first established a detection system for CRMP2 and p-CRMP2 in PBMC samples from living patients (SI Appendix, Figs. S2 and S3). Because our first set of studies (Fig. 1 and SI Appendix, Fig. S2) were performed on archived brains from anatomical gift donors from whom blood samples, while alive, were not available, a new cohort of living patients was recruited prospectively. Prospective recruitment enabled us to address our concern that the postmortem brain cohort was comprised mostly of older patients. In order to examine patients that likely had “pure” SCZ, and also to eliminate the confounding effects of aging-related diseases as well as poly-pharmacy (including for nonpsychiatric conditions), we focused our evaluation on patients with SCZ <40 y of age, a time before diseases-of-aging and unrelated comorbidities could intervene. In other words, we had much better control of this prospectively enrolled patient cohort than we did for the archived postmortem brains, although the latter were critical for suggesting the phenomenon of an abnormally elevated CRMP2 level and for confirming its neuronal morphometric and neural network consequences. Demographic information for this group of SCZ patients and for their unaffected age-matched controls is summarized in SI Appendix, Table S2 (total n = 58). There were no significant differences between the two groups in that regard. As might be expected, the years of education and the demands of the careers pursued were lower for SCZ patients than for the controls.
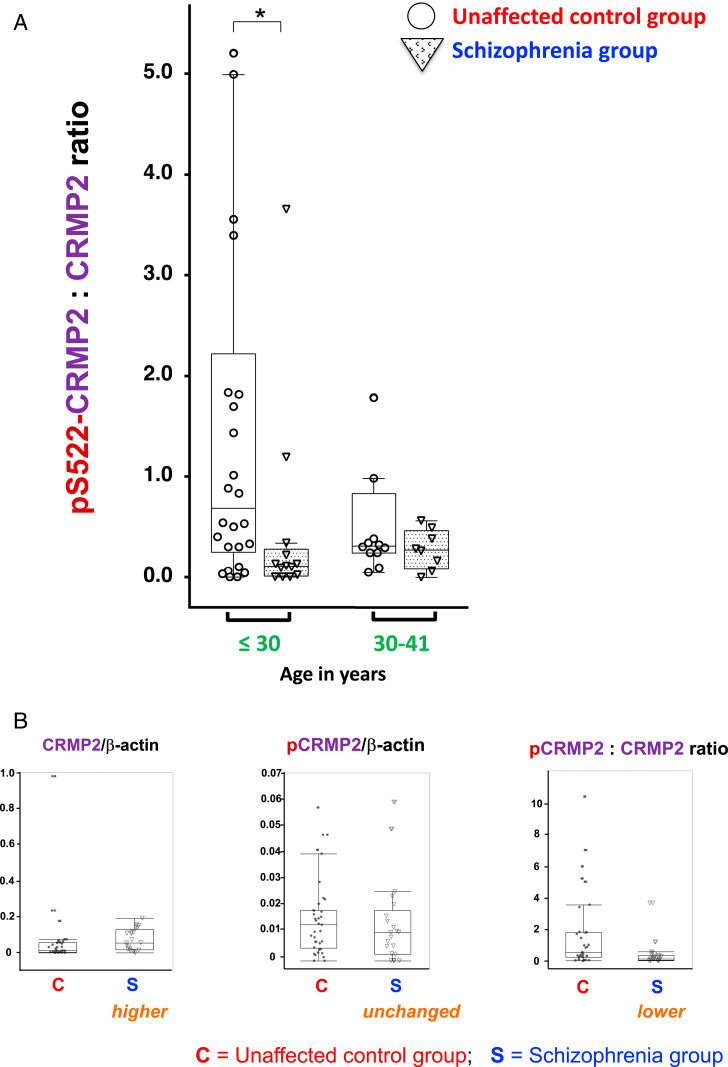
Significant differences did exist between SCZ patients (n = 21) and unaffected controls (n = 37) with regard not only to the levels of CRMP2 but also the resultant p-CRMP2:CRMP2 ratios (Fig. 2). As in brains, CRMP2 levels were significantly higher in the PBMCs of SCZ patients, most strikingly so in SCZ patients <30 y old (*P < 0.01). On the other hand, phosphorylation of CRMP2 did not increase concomitantly with an increase in the CRMP2 substrate, and the ratio of p-CRMP2:CRMP2 was, therefore, lower in the SCZ group (compared with the unaffected age-matched control group) because the denominator (CRMP2) was larger based on its increased abundance (Fig. 2). With an excess of active (nonphosphorylated) CRMP2—in contrast to the equilibrium between active and inactive (phosphorylated) CRMP2 that normally exists—neural network function, particularly at dendritic spines, might be expected to be aberrant (14). Notably, the lower p-CRMP2:CRMP2 ratio in SCZ contrasted dramatically with the higher ratio previously reported by us in patients with LiR BPD (14, 20), enhancing the potential utility of this diagnostic aid.
Fig. 2.
The p-CRMP2-to-CRMP2 ratio in lymphocytes from patients with SCZ was significantly lower than in lymphocytes from unaffected age-matched control subjects. CRMP2 phosphorylated at Serine 522 (p-CRMP2) was examined via quantitative Western blots using a specific well-authenticated antibody (SI Appendix, Fig. S1). (A) Differences were most striking between cohorts that were <30 y old (*P < 0.01). (B) Examination (in the aggregate) of the absolute values of CRMP2 (Left) and p-CRMP2 (Center) (using arbitrary values normalized to β-actin as an internal standard) shows that the p-CRMP2:CRMP2 ratio in human PBMC fractions from patients with SCZ (S) compared with unaffected controls (C) (Right) was significantly lower (P = 0.0051) because the level of CRMP2 (the denominator) was greater (P = 0.0146) while pCRMP2 remained unchanged (P = 0.4373).
In short, clinically, in brain and—for diagnostic purposes—in blood, excessive amounts of active (nonphosphorylated) CRMP2 distinguishes SCZ patients (with fair sensitivity and specificity) from unaffected age-matched subjects, and likely from psychiatric disorders that might mimic SCZ in a newly presenting young patient, for example, the manic phase of BPD.
Discussion
We provide clinical evidence for an adjunctive diagnostic biomarker for SCZ that derives from growing evidence in the literature that this condition’s symptomatology rests upon disordered neural network function, prominently mediated by synaptic connections upon dendritic spines, a process driven, in part, by cytoskeletal regulation. CRMP2 is a master cytoskeleton regulator, hence, a neural network modulator. Phosphorylation of CRMP2 alters its binding affinity to specific subsets of cytoskeleton and related proteins, such as tubulin, RhoA, and filamin-A (11, 25, 26, 31). These alterations of phosphorylation status affect regulation of cytoskeletal dynamics. “Toggling” between inactive (phosphorylated) and active (nonphosphorylated) CRMP2 is physiologic (14, 20). However, an abnormal balance between active and inactive CRMP2—too much of one or the other—could plausibly lead to aberrations in synaptogenesis, synapse maturation, and synaptic transmission (11, 28, 32).
The first clue that CRMP2 levels might be abnormal in SCZ came from examining postmortem human brain specimens where it was found that nonphosphorylated (active) CRMP2 protein expression was elevated and inversely correlated with basilar dendrite length in the DLPFC from patients with SCZ. This observation contrasted strikingly with—indeed, was the obverse of—what we had previously reported for patients with LiR BPD from the same brain bank with CRMP2 assayed in an identical fashion (14). In patients with LiR BPD, the set-point ratio of p-CRMP2:CRMP2 was abnormal in that the amount of inactive (phosphorylated) CRMP2 was excessively high while CRMP2 itself remained normal, yielding an elevated p-CRMP2:CRMP2 ratio. In specimens from young patients with SCZ, the converse condition exists: active (nonphosphorylated) CRMP2 is increased but p-CRMP2 remains largely unchanged. In other words, in SCZ, as in BPD, CRMP2 regulation is abnormal; however, in SCZ, not only is the p-CRMP2:CRMP2 ratio not elevated, that ratio may actually be decreased because the denominator has increased (greater than the numerator) (see discussion of blood results, below). The correlation between increased CRMP2 protein (and consequent disordered CRMP2 function) and dendrite abnormalities would certainly be consistent with—and perhaps even provide a mechanism for—the abnormal regulation of dendritic morphology and function that is recognized as prevalent in SCZ (25, 26). (A detailed electrophysiological study of dendrite function is beyond the scope of this clinical report but should be pursued in the future.)
These observations in brain suggested to us that, if elevated phosphorylated CRMP2 (and an elevated p-CRMP2:CRMP2 ratio) could be a BPD-specific biomarker [as we previously reported (14, 20)], then an elevated nonphosphorylated CRMP2 (and a diminished ratio) might serve as a biomarker for SCZ. We sought to support that speculation prospectively in not only a larger and better stratified patient population (particularly with regard to age), but also by going to a clinically accessible cell type in living patients, PBMCs.
As noted above, CRMP2 is also present in lymphocytes. Indeed, studies have shown similarities between receptor expression and transduction processes in cells from the nervous system and lymphocytes (29, 30, 33–36). Using our PBMC assay, we found that, compared with age-matched unaffected control patients, the blood levels of CRMP2 were significantly higher. And, because the phosphorylated form of CRMP2 remained largely unchanged from control, the net effect of increasing the denominator while maintaining a constant numerator was lowering the ratio of p-CRMP2:CRMP2 in the SCZ group compared with the control group. These differences were most striking in the youngest group of SCZ patients: in those SCZ patients <30 y old, the ratio of p-CRMP2:CRMP2 was significantly lower than in control patients.
At the outset, we were uncertain whether the biochemical abnormalities we had identified in the brain could also be detected in peripheral blood. In fact, whether alterations in blood can reflect brain states has long been a vexing question in psychiatry and neurology (33–36). That our assessment of CRMP2 levels in the brain was similar to that noted in the peripheral blood raises the possibility that, at least for cytoskeletal molecules, lymphocytes (which contain a good deal of cytoskeleton) may, indeed, adequately reflect cytoskeletal dynamics ongoing elsewhere in the body, including in the brain.
Indeed, the differences between unaffected control subjects and patients with SCZ was even more striking in blood than in the brain. Admittedly, the patients whose brains were examined were, on average, older than the patient’s whose blood was examined (compare SI Appendix, Tables S1 and S2). As previously explained, in the second, larger cohort in which PCMCs were examined, we wished to eliminate confounders from aging-related diseases (12, 22–24), comorbidities, or extraneous medications and limit our initial assessment of PBMCs to “young” (<40 y old) patients that likely had “purer” SCZ (conventionally regarded as being the case when younger patients are diagnosed with the disorder and drug abuse has been ruled out). In other words, having somewhat younger patients with purer SCZ might account for the slightly greater sensitivity of the biomarker for identifying SCZ in the living subjects whose blood was assayed compared with the cohort whose postmortem brains were assessed. In addition, there may be fewer long-term confounders of cytoskeletal biochemistry in blood (which rapidly turns over) than in the brain, making assessment of cytoskeletal regulators in PBMCs more straightforward.
The stark separation between the low p-CRMP2:CRMP2 ratio in young patients with SCZ compared with a normal ratio in age-matched unaffected young control subjects, shown in Fig. 2A, suggests that assaying such a readily and repeatedly accessible and informative cell type as the PBMC might serve as the basis for a minimally invasive adjunctive diagnostic test in which CRMP2 and p-CRMP2 can be rapidly measured in the PBMCs of a patient suspected of having SCZ (to be performed, of course, in conjunction with psychiatric and neurobehavioral testing and medication trials). Having such an adjunctive test might help clinicians distinguish SCZ from other conditions that might mimic it, including other behavioral problems, personality disorders, or systemic diseases. For example, these values might aid clinicians in distinguishing, relatively expeditiously, between an episode of psychosis due to SCZ versus a manic episode in a patient with BPD: a normal CRMP2 level or an elevated p-CRMP2:CRMP2 ratio would speak to the latter; an increased CRMP2 level or a decreased ratio might help cinch the diagnosis of SCZ in a newly presenting young patient.
In conclusion, clinically—both in the brains and (perhaps more starkly) in the peripheral blood monocytes of patients with SCZ—the level of a master cytoskeletal modulator and neural network determinant, CRMP2, is higher than in unaffected age-matched controls with significant sensitivity. In the brain, these changes are associated with abnormalities in dendritogenesis. In contrast to other psychiatric disorders, including those in which CRMP2 has been implicated [e.g., LiR BPD (14, 20)], the higher active CRMP2 level with a constant inactive p-CRMP2 level makes for a unique lowering of the p-CRMP2:CRMP2 ratio below that of unaffected age-matched control subjects. This disruption in the normal equilibrium between active and inactive CRMP2 might plausibly lead to neural network imbalances. Because these changes in the brain can be detected in the peripheral blood of young (presumably early-stage and possibly not-yet-diagnosed) patients, the possibility exists that assaying these ratios may serve as an adjunctive, rapid, minimally invasive, sensitive and specific diagnostic aid when attempting to determine the etiology of a patient’s abnormal behavior (in conjunction with examination and imaging). Obviously, future clinical studies must be larger and multicenter; must include patients with other psychiatric and neurological disorders beyond SCZ and BPD, from a spectrum of racial and socio-economic backgrounds, and from middle and old age; and must resolve the influence of psychopharmacological agents and other medications. Given that CRMP2 does appear to be involved in the pathophysiology of SCZ [some have regarded it as a risk gene (37, 38)], further study of its dynamic regulation of cytoskeleton and neural networks might lead to greater insight into the molecular mechanisms underlying the neuropathological changes seen in SCZ as well as into novel therapeutic interventions. Screening the blood of patients for this cytoskeletal regulator may help increase the number and homogeneity of enrollees in such future clinical trials.
Materials and Methods
For cohort 1, archived frozen postmortem human brain tissue containing the DLPFC (obtained from the Harvard Brain Tissue Resource Center) was processed to enable pyramidal cell reconstruction. CRMP2 protein levels from this same tissue were correlated with dendrite parameters. For cohort 2, blood samples and medical histories were obtained from randomly selected young (defined as ≤40 y old) inpatients and outpatients at Yokohama City University Hospital, Yokohama Medical Center, and Yokohama Maioka Hospital with patient consent and approval by the Ethical Review Board of each hospital. Samples and data from age- and sex-matched normal (as determined by examination from two blinded psychiatrists) volunteers were similarly obtained (as sanctioned). Middle-aged (40 to 60 y old) and elderly (>60 y old) patients were not included in cohort 2 for the reasons described under Results. Immunoblots of wild-type and CRMP2-knockout mouse brains were used to confirm the specificity of the anti-CRMP2 antibodies that were subsequently applied (blindly and randomly) to all patient peripheral blood lymphocytes. Please see SI Appendix for details on methodology, consent, and statistical analysis.
Approvals, Registrations, and Patient Consent.
Immunoblots of wild-type and CRMP2-knockout mouse brains were used to confirm the specificity of the anti-CRMP2 antibodies, as approved by the institutional Animal Care and Use Committee of the Yokohama City University Graduate School of Medicine. Frozen postmortem human brain tissue containing the DLPFC was obtained from the Harvard Brain Tissue Resource Center. Because these patients are not identifiable, no Institutional Review Board approval was required. Permission to use the specimens for research was granted by the donors at the time they made their anatomical gifts. The use of blood samples and medical histories from inpatients and outpatients, as well as from normal volunteers, was approved by the Ethical Review Board of Yokohama City University Hospital, Yokohama Medical Center, and Yokohama Maioka Hospital. Informed consent was obtained from all participants. No data are provided in this manuscript that would allow the identity of any study participant to be discovered.
Supplementary Material
Acknowledgments
This work was supported in part by the fund for the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT; Grant 42890001) and the Japanese Smoking Research Foundation (SRF) Grant for Biomedical Research (to Y.G.). Support also came from grants from the United States National Institutes of Mental Health (NIMH): 1K08MH087640-01A1 (to G.T.K.), 5R01MH51290-08 (to J.T.C.), RC2MH090011 (to E.Y.S.); NIH’s Library of Integrated Network-based Cellular Signatures Program (E.Y.S.); the Viterbi Foundation Neuroscience Initiative (E.Y.S.); a California Institute of Regenerative Medicine training grant (to B.T.D.T.); a University of California, San Diego NIMH T32 training grant in psychiatry (to B.T.D.T.); and a SENS Research Foundation grant (to C.D.P. and E.Y.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100032118/-/DCSupplemental.
Data Availability
All data are included in the article and SI Appendix.
References
- 1.Schizophrenia Working Group of the Psychiatric Genomics Consortium , Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins-de-Souza D., et al., The protein interactome of collapsin response mediator protein-2 (CRMP2/DPYSL2) reveals novel partner proteins in brain tissue. Proteomics Clin. Appl. 9, 817–831 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Holmans P. A., et al., Genomewide linkage scan of schizophrenia in a large multicenter pedigree sample using single nucleotide polymorphisms. Mol. Psychiatry 14, 786–795 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Föcking M., et al., Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol. Psychiatry 20, 424–432 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Purcell S. M.et al.; International Schizophrenia Consortium , Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penzes P., Buonanno A., Passafaro M., Sala C., Sweet R. A., Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 126, 165–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirov G., et al., De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 17, 142–153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripke S.et al.; Multicenter Genetic Studies of Schizophrenia Consortium; Psychosis Endophenotypes International Consortium; Wellcome Trust Case Control Consortium 2 , Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 45, 1150–1159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goshima Y., Nakamura F., Strittmatter P., Strittmatter S. M., Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Quach T. T., Honnorat J., Kolattukudy P. E., Khanna R., Duchemin A. M., CRMPs: Critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol. Psychiatry 20, 1037–1045 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Yamashita N., Goshima Y., Collapsin response mediator proteins regulate neuronal development and plasticity by switching their phosphorylation status. Mol. Neurobiol. 45, 234–246 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Uchida Y., et al., Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 10, 165–179 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Ip J. P., Fu A. K., Ip N. Y., CRMP2: Functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist 20, 589–598 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Tobe B. T. D., et al., Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 114, E4462–E4471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita N., et al., Mice lacking collapsin response mediator protein 1 manifest hyperactivity, impaired learning and memory, and impaired prepulse inhibition. Front. Behav. Neurosci. 7, 216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura H., et al., Comprehensive behavioral study and proteomic analyses of CRMP2-deficient mice. Genes Cells 21, 1059–1079 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., et al., Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat. Commun. 7, 11773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higurashi M., et al., Localized role of CRMP1 and CRMP2 in neurite outgrowth and growth cone steering. Dev. Neurobiol. 72, 1528–1540 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Makihara H., et al., CRMP1 and CRMP2 have synergistic but distinct roles in dendritic development. Genes Cells 21, 994–1005 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Zhao W. N., et al., Discovery of suppressors of CRMP2 phosphorylation reveals compounds that mimic the behavioral effects of lithium on amphetamine-induced hyperlocomotion. Transl. Psychiatry 10, 76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandal M. J.et al.; CommonMind Consortium; PsychENCODE Consortium; iPSYCH-BROAD Working Group , Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anttila V.et al.; Brainstorm Consortium , Analysis of shared heritability in common disorders of the brain. Science 360, eaap8757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruderfer D. M.et al.; Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium , Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173, 1705–1715.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis D. A., Curley A. A., Glausier J. R., Volk D. W., Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konopaske G. T., Lange N., Coyle J. T., Benes F. M., Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 71, 1323–1331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glantz L. A., Lewis D. A., Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 57, 65–73 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Arimura N., Menager C., Fukata Y., Kaibuchi K., Role of CRMP-2 in neuronal polarity. J. Neurobiol. 58, 34–47 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Cole A. R., et al., Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. J. Neurochem. 103, 1132–1144 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Good P. F., et al., A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. J. Neurochem. 91, 716–736 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H., Watanabe A., Ihara Y., Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer’s disease. J. Biol. Chem. 273, 9761–9768 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Nakamura F., et al., Amino- and carboxyl-terminal domains of Filamin-A interact with CRMP1 to mediate Sema3A signalling. Nat. Commun. 5, 5325 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Brittain J. M., et al., Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat. Med. 17, 822–829 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin D. P., Sharma R. P., Chromatin from peripheral blood mononuclear cells as biomarkers for epigenetic abnormalities in schizophrenia. Cardiovasc. Psychiatry Neurol. 2009, 409562 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladkevich A., Kauffman H. F., Korf J., Lymphocytes as a neural probe: Potential for studying psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 559–576 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Rollins B., Martin M. V., Morgan L., Vawter M. P., Analysis of whole genome biomarker expression in blood and brain. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153B, 919–936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaros J. A., et al., Effects of olanzapine on serum protein phosphorylation patterns in patients with schizophrenia. Proteomics Clin. Appl. 9, 907–916 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Bader V., et al., Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits. Hum. Mol. Genet. 21, 4406–4418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee H., et al., Changes in Dpysl2 expression are associated with prenatally stressed rat offspring and susceptibility to schizophrenia in humans. Int. J. Mol. Med. 35, 1574–1586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the article and SI Appendix.