Significance
Genomes contain signatures of past gene exchange between species. However, genomic data are not available for many organisms. For these, morphology may substitute for genes, as exemplified by Darwin’s finches on the Galápagos island of Floreana. In 1835, Darwin and companions collected seven specimens of a uniquely large form of Geospiza magnirostris that became extinct in the next few decades. A surviving population of Geospiza fortis shows evidence of hybridization in a pronounced skew in the distribution of beak size in the direction of the absent G. magnirostris. The genetic and morphological residuum of an extinct species in an extant one has implications for its future evolution, as well as for conservation programs of reintroduction in extinction-depleted communities.
Keywords: Darwin’s finches, introgression, beak size, extinction, reintroduction
Abstract
Many species of plants, animals, and microorganisms exchange genes well after the point of evolutionary divergence at which taxonomists recognize them as species. Genomes contain signatures of past gene exchange and, in some cases, they reveal a legacy of lineages that no longer exist. But genomic data are not available for many organisms, and particularly problematic for reconstructing and interpreting evolutionary history are communities that have been depleted by extinctions. For these, morphology may substitute for genes, as exemplified by the history of Darwin’s finches on the Galápagos islands of Floreana and San Cristóbal. Darwin and companions collected seven specimens of a uniquely large form of Geospiza magnirostris in 1835. The populations became extinct in the next few decades, partly due to destruction of Opuntia cactus by introduced goats, whereas Geospiza fortis has persisted to the present. We used measurements of large samples of G. fortis collected for museums in the period 1891 to 1906 to test for unusually large variances and skewed distributions of beak and body size resulting from introgression. We found strong evidence of hybridization on Floreana but not on San Cristóbal. The skew is in the direction of the absent G. magnirostris. We estimate introgression influenced 6% of the frequency distribution that was eroded by selection after G. magnirostris became extinct on these islands. The genetic residuum of an extinct species in an extant one has implications for its future evolution, as well as for a conservation program of reintroductions in extinction-depleted communities.
The classic, cladistic, view of the origin of biological diversity is a series of bifurcating processes with the products, species, remaining distinct as a result of genetic, behavioral, ecological, or geographical barriers to gene exchange (1, 2). Modern genomic data have necessitated a replacement of this view with a concept of networks in which lineages diverge but nonetheless exchange genes, especially in the early stages of diversification (3–10). Genomic data not only contain signatures of past gene exchange (9, 11–13)—the ghosts of introgression past—in some cases they reveal a legacy of lineages that no longer exist, for example in the phylogenies of bonobos (14, 15), dogs (16), cats (17), birds (18) and Homo sapiens (13). Persistence of introgressed genes for long periods of time implies either a slow rate of loss through random processes or selective retention (9, 19–25). The more genomic analyses are carried out, and the more refined they become, the more such “missing” history will be revealed, including our own (26). Studies of organisms for which fossils do not exist will profit the most.
Can hybridization in the past be detected in the absence of genomic data? The question is important to ask because genomic data are not available for many organisms, such as rare, endangered, and protected species and those living in remote regions. Moreover, gene exchange may be difficult to detect between weakly differentiated species (27). Morphology may provide an answer. The signs of introgressive hybridization can be identified in the frequency distributions of continuously varying heritable traits (28–30). Hybrids are expected to be approximately intermediate between the parental species in body size, appendage size, and the size of trophic traits (but see ref. 31), and by back-crossing to one of the parental species they contribute to the proximal half of the frequency distribution. The result is a broader distribution of trait values of the recipient population that is skewed toward the donor population. If back-crossing is bidirectional, the frequency distributions of the two species should be skewed toward each other.
Island populations of birds are good candidates to search for morphological evidence of past introgressive hybridization. Populations are often small and fluctuate in size, sex ratios are occasionally unbalanced, conspecific mates are then relatively scarce, and hybridization with related species may ensue (32–40). Darwin’s finches in the Galápagos archipelago are particularly suitable because there are many populations that vary in size. Indeed, introgressive hybridization is known from long-term field studies of individually marked birds (38, 41–47), and inferred from genetic data in other studies (48–50). In an earlier study we suggested that introgressive hybridization might occur when the population of one of two or more related species declines toward extinction, leaving a signature in the morphology of the surviving species (51). Geospiza fortis and Geospiza magnirostris on the southern islands of Floreana and San Cristóbal are an exemplary pair of species for testing this proposal. In 1835, Darwin, Fitzroy, and assistants collected seven specimens of a uniquely large form of G. magnirostris, five of them from Floreana, one from San Cristóbal, and the other probably from San Cristóbal (52). The populations became extinct, unobserved, in the next few decades, whereas G. fortis has persisted on both islands to the present. In clarifying the island of origin of each of the large specimens, Sulloway (52) assigned two other and much smaller specimens to San Cristóbal: one definitely and the other possibly to that island (S1 Appendix, section 1). Sulloway suggested one might be a hybrid between G. fortis and G. magnirostris, but did not comment on the other. We have since learned from a study of fossils that the large form of G. magnirostris was abundant on Floreana prior to human settlement in 1832 (53). San Cristóbal has a similar history of human settlement, introduction of goats, and habitat destruction (54, 55), but there are no reports of fossils.
Thousands of specimens of Darwin’s finches were collected for museums after Darwin’s visit, more than 95% of them in the period 1888 to 1906 and most of the remainder afterward. All specimens of the ground finch genus Geospiza were measured and compared in a previous study (56). Included among them were almost 500 specimens of G. fortis from Floreana and close to 200 from San Cristóbal. Here we use the measurements to test for effects of introgression on the skewness of beak size and body size frequency distributions. To place the results in context, we compare these two populations with conspecific populations on other islands (Table 1). In one group, comprising populations on Marchena, Pinta, and Rábida, the two species currently coexist, frequency distributions are well separated, and hybridization is neither known nor suspected. Remaining populations of G. fortis are combined in another group of potential hybridizers. On Santa Cruz, blurring of the morphological distinction between the species is indicative of hybridization. Interbreeding has been observed once (44, 57), and large members of the population are genetically intermediate between small members and G. magnirostris (45). Lack of distinctiveness is also present in the frequency distributions on Santiago and Isabela. Pinzón and Seymour have suffered considerable habitat damage from introduced goats (54, 55), leading to the probable extinction of G. magnirostris. In view of uncertainty over the status of G. magnirostris, we have added G. fortis from these islands to the group of G. fortis populations known or suspected to have hybridized.
Table 1.
Occurrence of Geospiza fortis and G. magnirostris on Galápagos islands, and known or suspected hybridization
| Island | G. fortis | G. magnirostris | Hybridization |
| Daphne | Present | Present | No |
| Darwin | Present | ||
| Floreana | Present | Extinct | In the past? |
| Genovesa | Present | ||
| Isabela | Present | Present | Suspected |
| Marchena | Present | Present | No |
| Pinta | Present | Present | No |
| Pinzón | Present | Extinct? | In the past? |
| Rábida | Present | Present | No |
| San Cristóbal | Present | Extinct | In the past? |
| Santa Cruz | Present | Present | Yes |
| Santiago | Present | Present | Suspected |
| Seymour | Present | Extinct | In the past? |
| Wolf | Present |
We present morphological evidence of an imprint of hybridization in the Floreana population of G. fortis, and show that the imprint faded with time following the disappearance of G. magnirostris. We discuss why hybridization occurred and the fate of hybrids, consider alternative sources of genetic input that might be discriminated with genomic testing, and conclude by discussing two implications of these findings for evolution and conservation. For the purpose of this paper, we use the term mega-magnirostris to distinguish populations on Floreana and San Cristóbal from all other populations of G. magnirostris. The average beak depth of the mega-magnirostris sample (21.9 mm) is ∼20% larger than the largest average (17.8 mm) beak depth of the G. magnirostris samples (Genovesa Island).
Results
Skewness.
The beak size distribution of G. fortis on Floreana is positively skewed, as expected from the hybridization hypothesis, whereas the distribution on San Cristóbal is not. (Fig. 1 and Table 2). Parallel results are found with an analysis of body size (S1 Appendix, section 2). Frequency distributions of G. fortis are positively skewed on three of four islands where hybridization is known or suspected: Santa Cruz, Santiago, and Pinzón, but not Isabela (Table 2). In contrast to these results, there is no skew in G. fortis populations on three islands where hybridization is not known or suspected, and variances are also much lower (S1 Appendix, section 3). Fig. 2 illustrates the contrast in frequency distributions on Pinta, where hybridization is neither known nor suspected, Santiago, where hybridization is suspected, and Santa Cruz where it is known.
Fig. 1.
Frequency distributions of beak size of G. fortis on the two islands previously inhabited by G. magnirostris.
Table 2.
Variance and skew in beak size frequency distributions
| Island | Species | Sample | Skew | SE | Variance |
| Floreana | G. fortis | 489 | 1.01**** | 0.111 | 2.41 |
| Pinzón | G. fortis | 74 | 1.00*** | 0.285 | 2.67 |
| Santiago | G. fortis | 135 | 0.98**** | 0.211 | 0.21 |
| Seymour | G. fortis | 62 | 0.62 | 0.311 | 2.31 |
| Santa Cruz | G. fortis | 259 | 0.57** | 0.152 | 1.55 |
| Pinta | G. fortis | 77 | 0.32 | 0.279 | 0.07 |
| San Cristóbal | G. fortis | 192 | 0.06 | 0.177 | 2.39 |
| Isabela | G. fortis | 367 | 0.00 | 0.128 | 1.69 |
| Daphne | G. fortis | 43 | −0.02 | 0.374 | 1.91 |
| Marchena | G. fortis | 60 | −0.32 | 0.316 | 0.08 |
| Rábida | G. fortis | 18 | −0.64 | 0.577 | 0.14 |
| Isabela | G. magnirostris | 29 | 1.04 | 0.455 | 2.19 |
| Santa Cruz | G. magnirostris | 65 | 0.04 | 0.304 | 1.55 |
| Rábida | G. magnirostris | 62 | −0.02 | 0.311 | 0.43 |
| Pinta | G. magnirostris | 87 | −0.15 | 0.263 | 0.16 |
| Darwin | G. magnirostris | 25 | −0.47 | 0.490 | 2.58 |
| Marchena | G. magnirostris | 60 | −0.55 | 0.316 | 0.40 |
| Genovesa | G. magnirostris | 64 | −0.60 | 0.306 | 2.25 |
| Wolf | G. magnirostris | 22 | −0.72 | 0.522 | 1.59 |
| Santiago | G. magnirostris | 87 | −1.26**** | 0.263 | 0.33 |
SE refers to the SE of the Skew (g1) estimate, and statistical significance is indicated by **P < 0.005, ***P < 0.001, and ****P < 0.0001. P > 0.1 for all others.
Fig. 2.
Disproportionately large beak size of G. magnirostris (green) compared with G. fortis (blue) on three islands where hybridization is known (Santa Cruz), suspected (Santiago), and not known or suspected (Pinta) (Table 1).
The opposite (negative) skew is manifested by G. magnirostris on Santiago (Table 2). Unlike positive skewness, a negative skew could be the result of incomplete growth of some individuals in brown immature plumage. Therefore, we repeated the analysis with males in black plumage because the plumage indicates they were fully grown (n = 23). The negative skew (g1 = −1.93, P < 0.005) validates the previous result. The Santiago pair of distributions (Fig. 3) provide an example of the theoretical expectation of opposite skewness. It is noteworthy that none of the populations of either species is significantly skewed in the opposite direction to the predicted direction (Table 2).
Fig. 3.
Reciprocally skewed beak size distributions of G. fortis and G. magnirostris on Santiago Island.
Allometry.
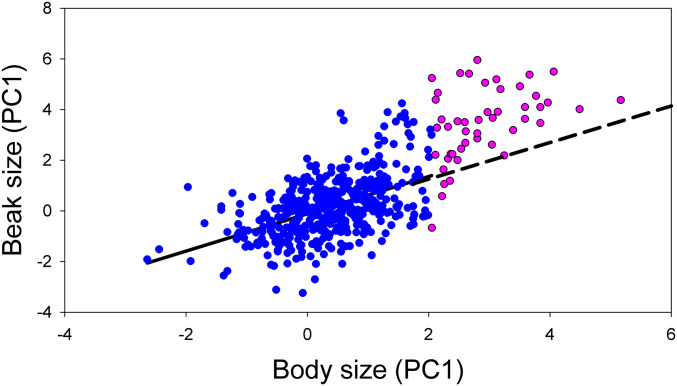
Positive skew in the G. fortis beak distributions could be the result of disruptive selection and assortative mating, with or without hybridization (58). Bimodal frequency distributions have been documented in a population of G. fortis on the south side of Santa Cruz Island (58), and models provide evidence of ongoing disruptive selection (57, 59). Bimodality is not evident in the distribution of measurements of museum specimens (30), although there is perhaps a hint of a second peak in the frequency distribution on Floreana (Fig. 1). Regardless, unlike a hypothesis of disruptive selection, the hybridization hypothesis makes a specific prediction about the positive tail of the distribution based on different allometric relationships between beak and body size in G. fortis and G. magnirostris: beak size is disproportionately large in G. magnirostris (Fig. 2). Thus, G. fortis individuals in the extreme portion of the frequency distribution closest to G. magnirostris are predicted to have a higher regression slope than other segments of the distribution on Floreana. The expected pattern is observed (Table 3), and is illustrated in Fig. 4, where an extrapolation of the regression line is a null hypothesis expectation for beak sizes of birds in the group of largest body size. Of 48 points in this region, 43 are above the line, which is significantly different from the expected 24 (two-tailed binomial test, z = 5.30, P < 0.0001). Similar patterns of covariation are observed in populations of G. fortis known or suspected to hybridize on Santa Cruz and Santiago (Table 3).
Table 3.
Correlations between beak and body size of G. fortis in segments of frequency distributions of body size ordered from the smallest (group 1) to the largest (group 2)
| Island | Group | n | b | SE | r | P |
| Floreana | 1 | 49 | 0.30 | 0.247 | 0.01 | 0.23 |
| Floreana | 2 | 49 | 2.27 | 1.190 | 0.05 | 0.06 |
| Floreana | 3 | 49 | 0.92 | 1.972 | 0 | 0.64 |
| Floreana | 4 | 49 | 0.73 | 2.152 | 0 | 0.74 |
| Floreana | 5 | 49 | 0.10 | 1.537 | 0 | 0.95 |
| Floreana | 6 | 49 | −0.74 | 2.520 | 0 | 0.77 |
| Floreana | 7 | 49 | 1.37 | 1.613 | 0 | 0.40 |
| Floreana | 8 | 49 | 1.48 | 1.905 | 0 | 0.44 |
| Floreana | 9 | 49 | −0.03 | 1.007 | 0 | 0.98 |
| Floreana | 10 | 47 | 0.87 | 0.270 | 0.17 | 0.002 |
| Santiago | 1 | 27 | 0.08 | 0.241 | 0 | 0.74 |
| Santiago | 2 | 27 | 0.02 | 0.798 | 0 | 0.98 |
| Santiago | 3 | 27 | 1.10 | 0.820 | 0.18 | 0.19 |
| Santiago | 4 | 27 | 0.06 | 0.722 | 0 | 0.93 |
| Santiago | 5 | 27 | 0.96 | 0.202 | 0.67 | < 0.0001 |
| Santa Cruz | 1 | 43 | −0.04 | 0.192 | 0 | 0.85 |
| Santa Cruz | 2 | 43 | 0.70 | 0.643 | 0.06 | 0.29 |
| Santa Cruz | 3 | 43 | 0.05 | 1.100 | 0 | 0.96 |
| Santa Cruz | 4 | 43 | 1.29 | 0.771 | 0.20 | 0.10 |
| Santa Cruz | 5 | 43 | 1.76 | 0.581 | 0.30 | 0.004 |
| Santa Cruz | 6 | 44 | 0.41 | 0.179 | 0.40 | 0.029 |
The slope (b) of least square regressions of beak size (Y) on body size (X) are given with one SE (SE). P is the significance of the correlation coefficients (r).
Fig. 4.
Beak size variation in relation to body size in G. fortis on Floreana Island. The largest one-sixth (n = 48) of the total sample are indicated in purple. A least-squares regression line is fitted to the points to the remainder in blue (Table 3).
To estimate the extent of the putative effect of introgression in the Floreana G. fortis sample, we ordered the individuals by body size and used a sliding-window technique with groups of 50. Starting with the largest group, at each step we deleted the largest five individuals and added the largest five individuals from the remainder, tested the slopes of regression for significance, and repeated the procedure until statistical significance was lost. In the zone of transition from significance to nonsignificance, we repeated the procedure with deletions and additions of single individuals. Significance was lost after deleting the 30 largest individuals, approximately 6% of the total sample.
After Extinction.
The hybridization hypothesis makes two predictions concerning the beak size frequency distribution of G. fortis in the years after extinction of mega-magnirostris and cessation of genetic input: reversion to a normal distribution and reduction in mean size. Alternatively, a skewed distribution might be maintained ecologically by disruptive selection. The data allow us to discriminate these alternatives because most specimens were collected in two time periods, 1891 to 1906 and 1924 to 1941 (S1 Appendix, section 4). The strong skew in the first period (g1 = 1.06 ± 0.122 SEM, n = 400) is not present in the second period (g1 = 0.64 ± 0.455, n = 29). The expected reversion was realized (Table 4).
Table 4.
Morphological contrasts between samples of G. fortis on Floreana in early and late periods after extinction of G. magnirostris (mega-magnirostris)
| Period | Trait | Sample | Mean | SEM | Skew |
| Early | PC1 body | 400 | 0.58 | 0.054 | 0.73**** |
| Late | PC1 body | 28 | 0.38 | 0.169 | −0.13 |
| Early | PC1 beak | 400 | 0.48 | 0.078 | 1.06**** |
| Late | PC1 beak | 29 | −0.23 | 0.273 | 0.64 |
| Early | Beak length | 400 | 11.33 | 0.044 | 0.85**** |
| Late | Beak length | 29 | 11.10 | 0.139 | 0.12 |
| Early | Beak depth | 400 | 11.12 | 0.050 | 0.84**** |
| Late | Beak depth | 29 | 10.20 | 0.219 | 0.17 |
| Early | Beak width | 400 | 9.60 | 0.042 | 1.05**** |
| Late | Beak width | 29 | 9.49 | 0.146 | 0.76 |
Statistical significance at ****P < 0.0001. P > 0.1 for all others.
We tested the second prediction with sex-corrected means and found, as expected, the second sample was significantly smaller in average beak size than the first sample (F1, 428 = 5.39, P = 0.021). Time-dependent shrinkage of museum specimens is a potentially biasing factor, but it should work in the opposite direction to the one observed because the second sample should have a larger mean. To investigate the contributions of each beak dimension to the difference in beak size, we performed univariate analyses, expecting approximately equal contributions. Unexpectedly, we discovered a surprisingly large difference in beak depth (F1, 428 = 24.07, P < 0.0001) (Fig. 5) in contrast to no difference in beak length (F1, 428 = 1.89, P = 0.17) or beak width (F1, 428 = 0.47, P = 0.49) (Table 4). We consider alternative explanations in the Discussion.
Fig. 5.
Reduction in average beak size in G. fortis on Floreana Island from 1891 to 1906 (red symbol) to 1924 to 1941 (blue symbol). The least-squares regression line is fitted to mean depth and width of 10 populations of G. fortis after excluding the 1924 to 1941 sample. = 0.22 ± 0.78 + 1.12 ± 0.08, and the adjusted R2 value is 0.94.
Discussion
Morphological Ghosts.
The study supports the hypothesis of introgressive hybridization of G. fortis and G. magnirostris on Floreana island. Hybridization is suspected to have occurred between Darwin’s visit in 1835 and the start of a 16-y burst of collecting activity for museums in 1891. During this period, mega-magnirostris became extinct on Floreana and San Cristóbal. The evidence of past introgression on Floreana is a skewed frequency distribution of beak measurements of G. fortis toward G. magnirostris. A possible alternative explanation for the skewness is persistent directional selection against large body size and beak size before and during the 16-y period. We consider this unlikely, and it is beyond our ability to test it. Instead, hybridization is supported by similarly skewed distributions in samples of museum specimens of other populations of G. fortis that are known or suspected to hybridize with G. magnirostris. Furthermore, skewed distributions are manifested in contemporary populations of interbreeding Geospiza propinqua and G. magnirostris on Genovesa (41) and G. fortis with Geospiza fuliginosa and Geospiza scandens on Daphne Major Island (41–43). To validate the hypothesis of introgression, genomic analysis should be performed on DNA extracted from the museum specimens (60, 61). This will require large samples because species differ in allele frequencies (11, 42, 49, 50). Also desirable is a combined genomic and morphological analysis of contemporary G. fortis on Floreana to test if there are residual indications of past introgression in the present population, despite erosion of a conspicuous influence on morphology ∼100 y ago.
The evidence for introgression on San Cristóbal island is much weaker and ambiguous, and is discussed separately in S1 Appendix, section 1. The variance is large, as on Floreana (S1 Appendix, section 3), but the expected skew in the frequency distribution is absent. Furthermore, only one specimen of mega-magnirostris is known with certainty to come from San Cristóbal.
Extinction, Diets, and Competition.
Persistence of populations in a changing environment may depend on the presence of adequate genetic variation for adaptation (62–65). The likelihood of persistence of hybridizing species is governed by the balance of beneficial genetic effects of hybridization (66, 67) and the adverse effects and speed of environmental change (68, 69). Extinction of mega-magnirostris on Floreana occurred in less than 60 y, or 12 generations, taking an average generation time to be 5 y (70). This may be too short for a population to adapt to a profoundly changing environment, despite possible beneficial effects of introgressive hybridization with G. fortis. On the other hand, instead of saving a population from extinction, hybridization can have the opposite effect of contributing to its demise through genetic swamping by introgressed alleles (39, 46, 71–75). This may have happened; however, it does not explain why mega-magnirostris became rare after a long period of abundance (53). There is general agreement that extinction was caused by humans (52, 53, 76), and an important contributor was habitat destruction by introduced goats. Goats were present at the initiation of human settlement of Floreana in 1832, and known to be present on San Cristóbal in 1847 (55). Goats had a particularly devastating early effect on Opuntia cactus in arid zones where cactus was a primary source of water (54). We do not know the time interval over which mega-magnirostris declined to extinction. It could have been short in view of the rapid expansion of goat populations (54).
For mega-magnirostris, cactus was a source of seeds. This is presumed. Although there are no published observations of the diet on the southern island populations, elsewhere in the archipelago G. magnirostris and other ground finch species are known to feed on Opuntia seeds (41, 77). The seeds of Opuntia megasperma on Floreana and satellite islands of Champion and Gardner are larger than those of all other Opuntia species elsewhere in the archipelago (78). The beaks of the extinct mega-magnirostris on Floreana were also larger than all others in the archipelago, except for those on San Cristóbal (52). The exceptionally large beaks were capable of cracking the large and hard seeds on Floreana. This is shown by extrapolation of a least-squares line of best fit to the maximum seed size and hardness function in relation to beak size for contemporary ground finch species (Fig. 6). The correspondence of beak size and seed characteristics suggests a history of directional coevolution on Floreana (51).
Fig. 6.
The maximum seed size-hardness index [√(DH) from ref. 91] is strongly correlated with average beak depth across nine finch populations (r = 0.88, P = 0.0012) excluding G. magnirostris (mega-magnirostris) on Floreana Island (large green circle). A least-squares line of best fit is shown for the nine populations (slope = 1.13 ± 0.21 SE), and extrapolated to the point for G. magnirostris on Floreana. The point for the next largest, the more typical G. magnirostris on Genovesa, is indicated by a split green and black symbol. The remaining populations are Geospiza conirostris (gray circle), G. propinqua (green square) G. fortis (blue inverted triangle and diamond), G. scandens (red inverted triangle and diamond), G. fuliginosa (black inverted triangle), and Geospiza acutirostris (purple square). Two populations of G. fortis and G. scandens, from Santa Cruz (inverted triangles) and Daphne Major (diamonds) are included, each with contrasting mean beak sizes.
Extinction, whether or not introgression contributed to it, would have removed competitive constraints on G. fortis, not for Opuntia seeds but for other, jointly exploited, components of their diets. This is one possible factor responsible for the persistence of morphological effects of introgression clearly beyond the point at which G. magnirostris became extinct. It would have slowed the reversion to a normal distribution under stabilizing selection in the absence of gene flow. A second possible factor is assortative mating, which would have slowed the decay of linkage disequilibrium and the integration of introgressed genes into the genomes of G. fortis. Evidence of disruptive selection and assortative mating in contemporary populations of G. fortis on Santa Cruz Island make these suggestions plausible (44, 45, 57, 59). Assortative mating in relation to beak morphology has been demonstrated in the population of G. fortis on Daphne Major Island (79). Song is also involved in mate choice and species discrimination, so there is an intriguing possibility of a song legacy in the descendants of G. fortis that should be investigated (S1 Appendix, section 5).
Source of Introgressed Genes.
G. magnirostris is the only species of ground finch larger than G. fortis; however, it is not obvious that the declining resident population on Floreana (mega-magnirostris) was the sole source of introgressed genes. The reason for doubt is the fact that premating isolation of the ground finches is based in part on beak size differences (80), and the difference in mean beak sizes of G. magnirostris and G. fortis on Floreana is exceptionally large. For example, beaks of the extinct G. magnirostris were approximately twice as deep as the Floreana G. fortis (81). This is larger than the pronounced difference on two islands where hybridization does not occur, namely Pinta (71%; from Fig. 2) and Daphne Major (82%; from ref. 42).
Immigrants are a potential alternative source of introgressed genes. While this hypothesis is more complex than a hypothesis of residents as the source, it is supported by three facts. First, contemporary G. magnirostris do fly to other islands. For example, a new population was founded on Daphne Major in 1982 by two female and three male immigrants (82). Second, two specimens of G. magnirostris typical of populations on Santa Cruz and elsewhere are in the 1835 collection of specimens from San Cristóbal (S1 Appendix, section 1). These, together with a single specimen collected by Robert Bowman on Floreana in 1957, show that the southern islands are occasionally visited by G. magnirostris from other islands. Establishment of G. magnirostris immigrants might have been facilitated by the presence of a few resident mega-magnirostris if they sang the song typical of the species (50) (S1 Appendix, section 5). Third, the average beak depth of G. magnirostris from Santa Cruz is only 45% larger than Floreana G. fortis (Fig. 2).
We conclude that immigrant G. magnirostris may have bred with one or both resident species and contributed to the G. fortis genetic variance. If genomic studies confirm the presence of G. magnirostris genes in specimens of G. fortis on Floreana, they may also be able to identify the population from which they came. They should also consider two other possible sources, Geospiza difficilis, which became extinct after 1852 (52), and G. fuliginosa (next section).
Morphological Ghosts Exorcised or Replaced?
We discovered a surprisingly large reduction in average beak size from samples of G. fortis collected roughly 30 y apart in the period after the disappearance of mega-magnirostris. Reduction was expected in the absence of gene flow, but was not expected to be restricted to the beak depth dimension, nor was the magnitude expected. Without observations or measurements of living birds from that time, it is not possible to do more than speculate on the causes. Unlike the situation with G. fortis in response to mega-magnirostris, we have no a priori hypothesis and no basis for invoking a bias in the collecting of samples. Selection alone is not likely to be a viable post hoc explanation for a major trait-specific change in fewer than 10 generations, in view of the strong positive genetic correlations between beak depth and the other two beak dimensions in the related G. fortis population on Daphne Island (28). A possible explanation is hybridization with the smaller G. fuliginosa. Beak depth and width measurements are approximately equal in this species (56). The average beak depth in G. fortis is 14% larger than beak width, and this relationship holds strongly across all populations in Fig. 6. Average beak depth in relation to beak width of the last sample of G. fortis collected on Floreana in 1924 to 1941 is a conspicuous outlier in Fig. 6, and at 7% it is midway between 0% (G. fuliginosa) and 14%. However, there is no negative skew in the distribution of the late G. fortis sample (g1 = 0.36 ± 0.45) that would indicate introgressive hybridization. Genetic data from specimens in museums may help to resolve the question of whether the morphological ghost of introgression from mega-magnirostris in the early sample was almost completely exorcised by selection, replaced by a different ghost of hybridization with G. fuliginosa, or both (S1 Appendix, section 4).
Implications.
Assuming the skewed distributions reflect morphological expression of introgressed genes, the study joins a growing number of examples of extant species carrying genes of extinct species (8, 15–18). Introgression implies a potential for further evolution through increased genetic variation (19–22, 28, 73, 83, 84), possibly along lines of different allometry (28), as indicated by the unusual morphology of the positive tail of the G. fortis frequency distribution of beak size on Floreana. While the unusual morphology disappeared, some of the contributing alleles may have remained.
Introgression also has implications for programs of ecosystem restoration by introducing species that have become locally extinct (85–87). In the Galápagos, tortoises have been reintroduced from captivity to islands that have lost their native populations, and new ones are planned with individuals produced by selective breeding from others of mixed ancestry, including on Floreana (88, 89). The finch study raises two concerns where hybridization precedes extinction. First, to avoid a repetition of extinction, environmental disturbance that led to the original extinction needs to be reversed. Second, genetic swamping may undermine success, and to minimize the chances of that happening the individuals to be introduced need to be carefully chosen, and perhaps genetically reconstituted by a program of selective breeding. For Floreana, the tasks would be formidable, because reconstructing the former community requires rebuilding the plant community and especially the Opuntia cactus component, as well as selective breeding of G. magnirostris to produce a form that is distinctly larger than all others currently in existence.
Methods
Sampling Design.
We used our previously published measurements of museum specimens in millimeters (56). On three islands, Santa Cruz, Santiago, and Isabela, collectors and taxonomists were not consistent in assigning individuals to species. For these samples we ranked all individuals of the combined species from smallest to largest beak size, identified a region of intermediate sizes where assignments by collectors or taxonomists to G. fortis or G. magnirostris were intermingled in rankings, divided these intermediates into two equal groups, and assigned individuals with the largest beaks to G. magnirostris and those with the smallest beaks to G. fortis. Percent intermediates were 18.2 (Santa Cruz sample), 7.8 (Isabela), and 3.9 (Santiago). We then performed principal components analyses (PCA) on the total sample (one or both species) on each island. The first component from an analysis of wing and tarsus lengths provides a synthetic index of body size. The first component from an analysis of beak length, depth, and width provides an index of beak size (56). Percent variance accounted for by the first components in each analysis varied from 52% to 96%. On average, males are larger than females by up to 4%. To correct for these differences, we calculated the difference in sex-specific mean PC scores for males and females on each island and then added them to each of the female measurements prior to analysis. We ln-transformed data prior to testing differences in mean values to minimize differences in variance and reduce skewness. To test for a genetic influence of G. magnirostris (mega-magnirostris) on the frequency distribution of beak sizes of G. fortis on Floreana, we analyzed individual segments of the distribution in size classes, and since segment length is an arbitrary variable, we performed a methodological check for possible bias by varying segment length and found no bias (S1 Appendix, section 6).
Statistical Analyses.
Statistical tests were two-tailed and performed in JMP (SAS Institute). In assessing the significance of g1 values (skewness) by t tests, we lowered the criterion of significance from 0.05 to 0.01 in view of the approximate nature of the SE (90), the small sizes of most samples, and heterogeneity in the collections.
Supplementary Material
Acknowledgments
We thank Erik Enbody for comments on the manuscript. Our past research was supported by grants from the National Sciences and Engineering Research Council of Canada and the NSF.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107434118/-/DCSupplemental.
Data Availability
All study data are included in the article and supporting information.
References
- 1.Mayr E., Animal Species and Evolution (Harvard University Press, Harvard, MA, 1963). [Google Scholar]
- 2.Coyne J. A., Orr A. R., Speciation (Sinauer, Sunderland, MA, 2004). [Google Scholar]
- 3.Rokas A., Carroll S. B., Bushes in the tree of life. PLoS Biol. 4, e352 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards S. V., Potter S., Schmitt C. J., Bragg J. G., Moritz C., Reticulation, divergence, and the phylogeography-phylogenetics continuum. Proc. Natl. Acad. Sci. U.S.A. 113, 8025–8032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallet J., Besansky N., Hahn M. W., How reticulated are species? BioEssays 38, 140–149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen D., Yu Y., Hahn M. W., Nakhleh L., Reticulate evolutionary history and extensive introgression in mosquito species revealed by phylogenetic network analysis. Mol. Ecol. 25, 2361–2372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malinsky M., et al., Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow. Nat. Ecol. Evol. 2, 1940–1955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor S. A., Larson E. L., Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat. Ecol. Evol. 3, 170–177 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Edelman N. B., et al., Genomic architecture and introgression shape a butterfly radiation. Science 366, 594–599 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottenburghs J., van Hooft P., van Wieren S. E., Ydenburgh R. C., Prins H. T., Birds in a bush: Toward an avian phylogenetic network. The Auk: Ornithological Advances 133, 577–582 (2016). [Google Scholar]
- 11.Lamichhaney S., et al., Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 518, 371–375 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lavretsky P., Engilis A. Jr, Eadie J. M., Peters J. L., Genetic admixture supports an ancient hybrid origin of the endangered Hawaiian duck. J. Evol. Biol. 28, 1005–1015 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Sankararaman S., Mallick S., Patterson N., Reich D., The combined landscape of Denisovan and Neanderthal ancestry in present-day humans. Curr. Biol. 26, 1241–1247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Manuel M., et al., Chimpanzee genomic diversity reveals ancient admixture with bonobos. Science 354, 477–481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhlwilm M. S., Han S., Sousa V. C., Excoffier L., Marques-Bonet T., Ancient admixture from an extinct ape lineage into bonobos. Nat. Ecol. Evol. 3, 957–965 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishnan S., et al., Interspecific gene flow shaped the evolution of the genus Canis. Curr. Biol. 28, 3441–3449.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiró H. V., et al., Genome-wide signatures of complex introgression and adaptive evolution in the big cats. Sci. Adv. 3, e1700299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D., et al., ‘Ghost introgression’ as a cause of deep mitochondrial divergence in a bird species complex. Mol. Biol. Evol. 36, 2375–2386 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Arnold M. L., Bulger M. R., Burke J. M., Hempel A. L., Williams J. H., Natural hybridization: How long can you go and still be important? Ecology 80, 371–381 (1999). [Google Scholar]
- 20.Hedrick P. W., Adaptive introgression in animals: Examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 22, 4606–4618 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Enciso-Romero J., et al., Evolution of novel mimicry rings facilitated by adaptive introgression in tropical butterflies. Mol. Ecol. 26, 5160–5172 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Meier J. I., et al., Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8, 14363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irisarri I., et al., Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes. Nat. Commun. 9, 3159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez-Gonzalez A., Lexer C., Cronk Q. C. B., Adaptive introgression: A plant perspective. Biol. Lett. 14, 20170688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean L. L., et al., Admixture between ancient lineages, selection, and the formation of sympatric stickleback species-pairs. Mol. Biol. Evol. 36, 2481–2497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinker D. C., et al., Neanderthal introgression reintroduced functional ancestral alleles lost in Eurasian populations. Nat. Ecol. Evol. 4, 1332–1341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarlane S. E., Pemberton J. M., Detecting the true extent of introgression during anthropogenic hybridization. Trends Ecol. Evol. 34, 315–326 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Grant P. R., Grant B. R., Phenotypic and genetic effects of hybridization in Darwin’s finches. Evolution 48, 297–316 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Grant P. R., Grant B. R., Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Grant P. R., Grant B. R., “Sympatric speciation, immigration, and hybridization in island birds” in The Theory of Island Biogeography Revisited, Losos J. B., Ricklefs R. E., Eds. (Princeton University Press, Princeton, NJ, 2009), pp. 326–357. [Google Scholar]
- 31.Thompson K. A., Urquhart-Cronish M., Whitney K. D., Rieseberg L. H., Schluter D., Patterns, predictors, and consequences of dominance in hybrids. Am. Nat. 197, E72–E88 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Gill F. B., Hybridization in Norfolk Island white-eyes (Zosterops). Condor 72, 481–482 (1970). [Google Scholar]
- 33.Ryan P. G., Moloney C. L., Hudon J., Color variation and hybridization among Nesospiza buntings on Inaccessible Island, Tristan da Cunha. Auk 111, 314–373 (1992). [Google Scholar]
- 34.Grant P. R., Grant B. R., Hybridization, sexual imprinting, and mate choice. Am. Nat. 149, 1–28 (1997). [Google Scholar]
- 35.McCracken K. G., Wilson R. E., Gene flow and hybridization between numerically imbalanced populations of two duck species in the Falkland Islands. PLoS One 6, e23173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren B. H., et al., Hybridization and barriers to gene flow in an island bird radiation. Evolution 66, 1490–1505 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Sardell J. M., Uy J. A. C., Hybridization following recent secondary contact results in asymmetric genotypic and phenotypic introgression between island species of Myzomela honeyeaters. Evolution 70, 257–269 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Peters K. J., Myers S. A., Dudaniec R. Y., O’Connor J. A., Kleindorfer S., Females drive asymmetrical introgression from rare to common species in Darwin’s tree finches. J. Evol. Biol. 30, 1940–1952 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Wells C. P., et al., Persistence of an endangered native duck, feral mallards, and multiple hybrid swarms across the main Hawaiian Islands. Mol. Ecol. 28, 5203–5216 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Gabrielli M., Nabholz B., Leroy T., Milá B., Thébaud C., Within-island diversification in a passerine bird. Proc. Biol. Sci. 287, 20192999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant B. R., Grant P. R., Evolutionary Dynamics of a Natural Population. The Large Cactus Finch of the Galápagos (University of Chicago Press, Chicago, 1989). [Google Scholar]
- 42.Grant P. R., Grant B. R., 40 Years of Evolution. Darwin’s Finches on Daphne Major Island (Princeton University Press, Princeton, NJ, 2014). [Google Scholar]
- 43.Grant P. R., Grant B. R., Triad hybridization via a conduit species. Proc. Natl. Acad. Sci. U.S.A. 117, 7888–7896 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber S. K., De León L. F., Hendry A. P., Bermingham E., Podos J., Reproductive isolation of sympatric morphs in a population of Darwin’s finches. Proc. Biol. Sci. 274, 1709–1714 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de León L. F., Bermingham E., Podos J., Hendry A. P., Divergence with gene flow as facilitated by ecological differences: Within-island variation in Darwin’s finches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1041–1052 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleindorfer S., et al., Species collapse via hybridization in Darwin’s tree finches. Am. Nat. 183, 325–341 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Lamichhaney S., et al., Female-biased gene flow between two species of Darwin’s finches. Nat. Ecol. Evol. 4, 979–986 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Farrington H. L., Lawson L. P., Clark C. M., Petren K., The evolutionary history of Darwin’s finches: Speciation, gene flow, and introgression in a fragmented landscape. Evolution 68, 2932–2944 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Chaves J., et al., Genomic variation at the tips of the adaptive radiation of Darwin’s finch radiation. Mol. Ecol. 25, 5282–5295 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Lawson L. P., et al., Slow motion extinction: Inbreeding, introgression, and loss in the critically endangered mangrove finch (Camarhynchus heliobates). Conserv. Genet. 18, 159–170 (2017). [Google Scholar]
- 51.Grant B. R., Grant P. R., Niche shifts and competition in Darwin’s finches: Geospiza conirostris and congeners. Evolution 36, 637–657 (1982). [DOI] [PubMed] [Google Scholar]
- 52.Sulloway F. J., The Beagle collections of Darwin’s finches (Geospizinae). Bull. Brit. Mus. (Nat. Hist.) Zool. Ser. 43, 49–94 (1982). [Google Scholar]
- 53.Steadman D., Holocene Vertebrate Fossils from Isla Floreana, Galápagos (Smithsonian Contributions to Zoology No. 413, Smithsonian, 1986). [Google Scholar]
- 54.Stewart A., Some observations concerning the botanical conditions on the Galápagos Islands. Trans. Wisconsin Acad. Sci. 18, 272–340 (1915). [Google Scholar]
- 55.Hoeck H. N., “Introduced fauna” in Key Environments. Galapagos, Perry R., Ed. (Pergamon Press, New York, 1984), pp. 233–245. [Google Scholar]
- 56.Grant P. R., Abbott I., Schluter D., Curry R. L., Abbott L. K., Variation in the size and shape of Darwin’s finches. Biol. J. Linn. Soc. Lond. 25, 1–39 (1985). [Google Scholar]
- 57.Hendry A. P., Huber S. K., De León L. F., Herrel A., Podos J., Disruptive selection in a bimodal population of Darwin’s finches. Proc. Biol. Sci. 276, 753–759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hendry A. P., et al., Possible human impacts on adaptive radiation: Beak size bimodality in Darwin’s finches. Proc. Biol. Sci. 273, 1887–1894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beausoleil M.-O., et al., Temporally varying disruptive selection in the medium ground finch (Geospiza fortis). Proc. Biol. Sci. 286, 20192290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petren K., Grant P. R., Grant B. R., Clack A. A., Lescano N. V., Multilocus genotypes from Charles Darwin’s finches: Biodiversity lost since the voyage of the Beagle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1009–1018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrington H. L., Lawson L. P., Petren K., Predicting population extinctions in Darwin’s finches. Conserv. Genet. 20, 825–836 (2019). [Google Scholar]
- 62.Bürger R., Lynch M., Evolution and extinction in a changing environment—A quantitative genetic analysis. Evolution 49, 151–163 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Gomulkiewicz R., Holt R. D., When does evolution by natural selection prevent extinction? Evolution 49, 201–207 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Lande R., Shannon S., The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50, 434–437 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Bell G., Gonzalez A., Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Baskett M. L., Gomulkiewicz R., Introgressive hybridization as a mechanism for species rescue. Theor. Ecol. 4, 223–239 (2011). [Google Scholar]
- 67.Stelkens R. B., Brockhurst M. A., Hurst G. D. D., Greig D., Hybridization facilitates evolutionary rescue. Evol. Appl. 7, 1209–1217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlson S. M., Cunningham C. J., Westley P. A., Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521–530 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Lindsey H. A., Gallie J., Taylor S., Kerr B., Evolutionary rescue from extinction is contingent on a lower rate of environmental change. Nature 494, 463–467 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Grant P. R., Grant B. R., Demography and the genetically effective sizes of two populations of Darwin’s finches. Ecology 73, 766–784 (1992). [Google Scholar]
- 71.Levin D. A., Francisco-Ortega J. K., Jansen R. K., Hybridization and extinction of rare plant species. Conserv. Biol. 10, 10–16 (1996). [Google Scholar]
- 72.Rhymer J. M., Simberloff D., Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 27, 83–109 (1996). [Google Scholar]
- 73.Buerkle C. A., Wolf D. E., Rieseberg L. H., “The origin and extinction of species through hybridization” in Population Viability in Plants. Ecological Studies 165, Brigham C. A., Schwartz M. W., Eds. (Springer, New York, 2003), pp. 117–141. [Google Scholar]
- 74.Gómez J. M., González-Megías A., Lorite J., Abdelziz M., Perfectti F., The silent extinction: Climate change and the potential hybridization-mediated extinction of endemic high-mountain plants. Biodivers. Conserv. 24, 1843–1857 (2015). [Google Scholar]
- 75.Todesco M., et al., Hybridization and extinction. Evol. Appl. 9, 892–908 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lack D., The Galápagos finches (Geospizinae). A study in variation. Occas. Pap. Cal. Acad. Sci. 21, 1–159 (1945). [Google Scholar]
- 77.Grant B. R., Grant P. R., Exploitation of Opuntia cactus by birds on the Galápagos. Oecologia 49, 179–187 (1981). [DOI] [PubMed] [Google Scholar]
- 78.Wiggins I. L., Porter D. M., The Flora of the Galápagos Islands (Stanford University Press, Stanford, CA, 1971). [Google Scholar]
- 79.Grant P. R., Grant B. R., Role of sexual imprinting in assortative mating and premating isolation in Darwin’s finches. Proc. Natl. Acad. Sci. U.S.A. 115, E10879–E10887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant B. R., Grant P. R., Cultural inheritance of song and its role in the evolution of Darwin’s finches. Evolution 50, 2471–2487 (1996). [DOI] [PubMed] [Google Scholar]
- 81.Steadman D. W., The status of Geospiza magnirostris on Isla Floreana, Galápagos. Bull. Br. Ornithol. Club 194, 99–102 (1984). [Google Scholar]
- 82.Grant P. R., Grant B. R., The founding of a new population of Darwin’s finches. Evolution 49, 229–240 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Lewontin R. C., Birch L. C., Hybridization as a source of variation for adaptation to new environments. Evolution 20, 315–336 (1966). [DOI] [PubMed] [Google Scholar]
- 84.Seehausen O., Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Frankham R., Ballou J. D., Briscoe D. A., Introduction to Conservation Genetics (Cambridge University Press, Cambridge, 2002). [Google Scholar]
- 86.Brekke P., Bennett P. M., Santure A. W., Ewen J. G., High genetic diversity in the remnant island population of hihi and the genetic consequences of re-introduction. Mol. Ecol. 20, 29–45 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Grossen C., Keller L., Biebach I., Croll D., International Goat Genome Consortium , Introgression from domestic goat generated variation at the major histocompatibility complex of Alpine ibex. PLoS Genet. 10, e1004438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garrick R. C., et al., Lineage fusion in Galápagos giant tortoises. Mol. Ecol. 23, 5276–5290 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Quinzin M. C., et al., Genetically informed captive breeding of hybrids of an extinct species of Galapagos tortoise. Conserv. Biol. 33, 1404–1414 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Sokal R., Rolf F. J., Biometry (W. H. Freeman & Co., San Francisco, ed. 2, 1981). [Google Scholar]
- 91.Abbott I., Abbott L. K., Grant P. R., Comparative ecology of Galápagos ground finches (Geospiza Gould): Evaluation of the importance of floristic diversity and interspecific competition. Ecol. Monogr. 47, 151–184 (1977). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.