Decades of preclinical studies indicate that dopaminergic neurons in the ventral tegmental area (VTA) of the midbrain modulate animal behavior and cognition (1). The role of dopaminergic VTA neurons in wakefulness, and hence consciousness, emerged more recently from VTA stimulation experiments utilizing pharmacologic (2, 3), electrophysiologic (4), optogenetic (5, 6), and chemogenetic (7) methods, as well as behavioral experiments in dopamine knockout mice (8). Yet, confirmation of VTA modulation of human consciousness has until now been elusive—inferred from pharmacologic studies of dopaminergic therapies (9) and positron emission studies of dopamine receptor dynamics (10), but unconfirmed due to a lack of suitable techniques for measuring VTA function in humans. This absence of a translational link between animal and human VTA function has hindered the construction of a comprehensive subcortical–cortical connectivity model of human consciousness and impeded the development of new therapies to promote recovery of consciousness in patients with severe brain injuries (11).
In PNAS, Spindler et al. report groundbreaking results from complementary observational and interventional studies that shed light on VTA modulation of human consciousness, bridging the gap between animal and human VTA research (12). In a series of resting-state functional MRI experiments performed in healthy volunteers undergoing propofol-induced sedation (n = 24), patients with chronic disorders of consciousness (DoC) caused by severe brain injuries (n = 22), and patients with traumatic brain injury treated with methylphenidate (n = 12), Spindler et al. (12) provide convergent evidence that the subcortical VTA modulates human consciousness via connectivity with the cortical default mode network (DMN). Although VTA neurons connect with multiple regions of the cerebral cortex via monosynaptic and polysynaptic pathways that traverse the mesocortical, mesolimbic, and nigrostriatal bundles (13), the experiments here focus on VTA connectivity with the precuneus/posterior cingulate cortex (PCu/PCC), a well-established “hub node” of the DMN. Electrophysiologic and neuroimaging studies have revealed the DMN’s role in consciousness (14, 15) and the PCu/PCC’s role as a DMN hub (16, 17), but the role of VTA–PCu/PCC connectivity in modulating human consciousness has not been previously demonstrated.
By analyzing VTA–PCu/PCC connectivity in anesthetized and brain-injured human subjects, Spindler et al. (12) generate parallel observations about the mechanisms linking pharmacological and pathological states of unconsciousness. Specifically, they demonstrate that VTA–PCu/PCC functional connectivity is similarly disrupted in states of unconsciousness produced by propofol sedation and severe brain injury. Moreover, in subjects undergoing propofol sedation, VTA functional disconnections showed anatomic specificity for the PCu/PCC, and the magnitude of these disconnections correlated with the dose of sedation. VTA–PCu/PCC connectivity was also associated with recall of stimuli presented during sedation.
Importantly, in patients with chronic DoC, VTA–PCu/PCC disconnections were associated with disruption of PCu/PCC connectivity with other DMN nodes, suggesting that VTA–PCu/PCC connectivity modulates the broader DMN connectome. Spindler et al. (12) further show that methylphenidate, a dopamine reuptake inhibitor, promotes VTA–PCu/PCC connectivity in a separate cohort of patients with traumatic brain injury. Collectively, these findings provide a putative mechanism—reintegration of VTA–PCu/PCC connections and downstream reactivation of the DMN—by which dopaminergic drugs promote recovery of consciousness.
The potential translational impact of this groundbreaking work is perhaps best viewed through the lens of therapy development for patients with DoC, a population for which few proven therapies exist (18). To date, only amantadine has shown benefit in a phase-3 randomized controlled trial, specifically for patients in the subacute stage of recovery from traumatic DoC (4 to 16 wk postinjury). Multiple additional pharmacologic, electrophysiologic, and ultrasonic therapies have been tested (18), but evidence of efficacy is limited. Of the many barriers to therapy development, the paucity of predictive biomarkers to identify patients who are likely to respond and the lack of pharmacodynamic biomarkers to measure subclinical therapeutic effects in early-stage trials are of paramount importance (11). The present results suggest that VTA–PCu/PCC connectivity has potential utility as both a predictive and pharmacodynamic biomarker—the former because it may identify likely responders to VTA neuromodulation, and the latter because it may reveal whether a dopaminergic therapy has engaged its target VTA network. The use of dopaminergic neuroimaging biomarkers in clinical trials is rapidly accelerating (10, 11, 19), and the findings from Spindler et al. (12) provide a strong foundation for future efforts to develop VTA-based predictive and pharmacodynamic biomarkers. These results thus have potential to improve the design of clinical trials and expand the therapeutic landscape for patients with DoC.
From a neuroscientific perspective, the present results raise intriguing new questions about the neural correlates of consciousness. There is ongoing debate about whether the neural correlates of consciousness emerge from the anterior (i.e., prefrontal, anterior cingulate, and orbitofrontal) or posterior (i.e., parietal, occipital, and lateral temporal) regions of the cerebral cortex (20). Evidence from lesion, stimulation, and electrophysiologic recording studies indicates that the PCu/PCC is located within a “posterior hot zone” that contributes to the contents of consciousness (20). The present study did not test specifically for VTA connectivity with anterior DMN nodes such as the medial prefrontal cortex, nor was the study designed to distinguish between the level and the contents of consciousness. Nevertheless, the findings highlight the importance of the posterior cortex in human consciousness, raising the possibility that the PCu/PCC is not only a hub node for the DMN but also a hub for subcortical–cortical integration of arousal and awareness in human consciousness (Fig. 1). As such, these VTA–PCu/PCC connectivity findings add to a growing body of evidence that network-based models of human consciousness may provide clinically actionable therapeutic targets (11).
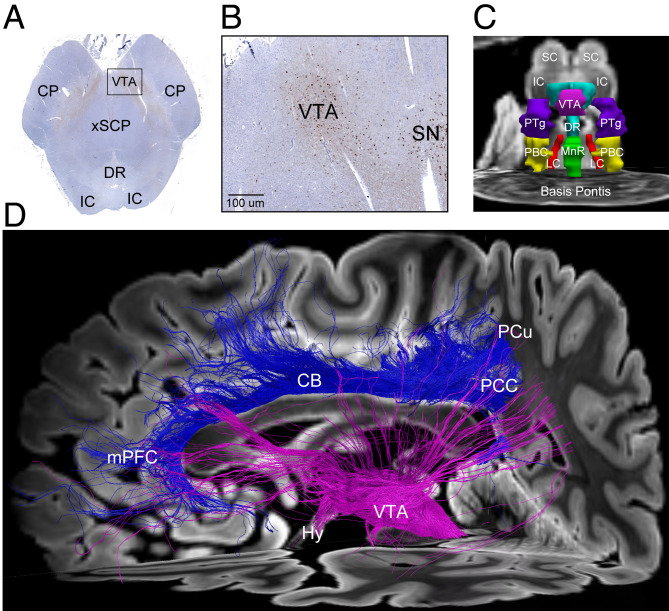
Fig. 1.
Integration of subcortical and cortical connections in human consciousness. (A) Tyrosine hydroxylase immunostaining of dopaminergic neurons (brown), counterstained with hematoxylin (blue), in a caudal midbrain tissue section. The human brain specimen was donated by a 53-year-old woman who died of nonneurological causes and whose brainstem histology data provided the basis for the Harvard Ascending Arousal Atlas (22) used by Spindler et al. (12). (B) Zoomed-in view of the region indicated by the black rectangle in A. Dopaminergic VTA neurons are visualized posterior to the cerebral peduncle (CP), medial to the substantia nigra (SN) and anterior to the decussation of the superior cerebellar peduncles (xSCP). (C) Anterior view of three-dimensional brainstem arousal nuclei, manually traced on an ex vivo MRI dataset from the same brain specimen using histological guidance. (D) A diffusion tractography analysis from an ex vivo MRI scan of a second brain specimen donated by a 60-year-old woman who died of nonneurological causes, as previously described (24). Tracts emanating from the VTA (pink) project diffusely to the cerebral cortex, including the PCu/PCC. Tracts connecting the PCu/PCC node of the DMN to the medial prefrontal cortex (mPFC) node via the cingulum bundle (CB) are shown in blue. Collectively, these tractography findings provide a structural correlate for the functional connectivity findings reported by Spindler et al. (12), whereby VTA–PCu/PCC connectivity appears to modulate the DMN. Abbreviations: DR, dorsal raphe; Hy, hypothalamus; IC, inferior colliculus; LC, locus coeruleus; MnR, median raphe; PBC, parabrachial complex; PTg, pedunculotegmental nucleus; SC, superior colliculus. Written informed consent for brain donation was provided by surrogate decision makers, in accordance with an Institutional Review Board–approved protocol. A higher-quality version of the figure is available at Figshare (https://figshare.com/articles/figure/PNAS_Commentary_Figure_20210719_pdf/15015297).
The study by Spindler et al. (12) thus helps to set the agenda for the next decade of research into new treatments for patients with DoC (21). VTA–DMN connectivity appears to be a prime target for a new generation of consciousness-promoting therapies. Whether VTA–DMN neuromodulation can be optimized via bottom-up (i.e., subcortical), top-down (i.e., cortical), or multidirectional (i.e., concurrent subcortical and cortical) stimulation remains to be determined. Elucidating the optimal timing, dosing, and duration of therapy, as well as the patient characteristics that predict a response to VTA–DMN neuromodulation, will require a sustained, international commitment to clinical trials for patients with DoC. It is also important to acknowledge that pharmacologic therapy development should not proceed in a purely dopamine-centric manner. The extensive interconnectivity of subcortical nodes within the ascending arousal network (22) and the network’s resilience to injury (23) suggest that there may be multiple structural scaffolds upon which functional recovery can be built. Indeed, preliminary evidence suggests that there is a role for serotonergic, noradrenergic, glutamatergic, cholinergic, orexinergic, and even GABA-ergic therapies to promote consciousness (18), depending on which components of an individual patient’s connectome are preserved or disrupted (11). Nevertheless, the findings by Spindler et al. (12) provide a strong mechanistic rationale for future investigation into dopaminergic neuromodulation, an enterprise with great potential to advance understanding about the connectivity of human consciousness and improve the lives of patients with DoC.
Acknowledgments
B.L.E.’s research is supported by the NIH Director’s Office (DP2HD101400), NIH National Institute of Neurological Disorders and Stroke (R21NS109627, RF1NS115268), James S. McDonnell Foundation, and Tiny Blue Dot Foundation.
Footnotes
The author declares no competing interest.
See companion article, “Dopaminergic brainstem disconnection is common to pharmacological and pathological consciousness perturbation,” 10.1073/pnas.2026289118.
Data Availability
A higher-quality version of the figure is available at Figshare (https://figshare.com/articles/figure/PNAS_Commentary_Figure_20210719_pdf/15015297).
References
- 1.Morales M., Margolis E. B., Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Solt K., et al., Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 115, 791–803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenny J. D., Taylor N. E., Brown E. N., Solt K., Dextroamphetamine (but not atomoxetine) induces reanimation from general anesthesia: Implications for the roles of dopamine and norepinephrine in active emergence. PLoS One 10, e0131914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solt K., et al., Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 121, 311–319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor N. E., et al., Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. U.S.A. 113, 12826–12831 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eban-Rothschild A., Rothschild G., Giardino W. J., Jones J. R., de Lecea L., VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 19, 1356–1366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oishi Y., et al., Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct. Funct. 222, 2907–2915 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Palmiter R. D., Dopamine signaling as a neural correlate of consciousness. Neuroscience 198, 213–220 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Giacino J. T., et al., Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 366, 819–826 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Fridman E. A., Osborne J. R., Mozley P. D., Victor J. D., Schiff N. D., Presynaptic dopamine deficit in minimally conscious state patients following traumatic brain injury. Brain 142, 1887–1893 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlow B. L., et al., Personalized connectome mapping to guide targeted therapy and promote recovery of consciousness in the intensive care unit. Neurocrit. Care 33, 364–375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spindler L. R. B., et al., Dopaminergic brainstem disconnection is common to pharmacological and pathological consciousness perturbation. Proc. Natl. Acad. Sci. U.S.A. 118, e2026289118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yetnikoff L., Lavezzi H. N., Reichard R. A., Zahm D. S., An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience 282, 23–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhaudenhuyse A., et al., Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133, 161–171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox K. C. R., Foster B. L., Kucyi A., Daitch A. L., Parvizi J., Intracranial electrophysiology of the human default network. Trends Cogn. Sci. 22, 307–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner R. L., DiNicola L. M., The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 17.van den Heuvel M. P., Sporns O., Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Edlow B. L., Claassen J., Schiff N. D., Greer D. M., Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 17, 135–156 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins P. O., et al., Stratifying drug treatment of cognitive impairments after traumatic brain injury using neuroimaging. Brain 142, 2367–2379 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Boly M., et al., Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J. Neurosci. 37, 9603–9613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provencio J. J., et al., The Curing Coma Campaign: Framing initial scientific challenges—Proceedings of the first Curing Coma Campaign Scientific Advisory Council meeting. Neurocrit. Care 33, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlow B. L., et al., Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 71, 531–546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianciardi M., Izzy S., Rosen B., Wald L. L., Edlow B. L., Location of subcortical microbleeds and recovery of consciousness after severe traumatic brain injury. Neurology 97, e113–e123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan A. L., et al., Tractography-pathology correlations in traumatic brain injury: A TRACK-TBI study. J. Neurotrauma 38, 1620–1631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A higher-quality version of the figure is available at Figshare (https://figshare.com/articles/figure/PNAS_Commentary_Figure_20210719_pdf/15015297).