Abstract
Objective
Patients with sickle cell disease (SCD) face inconsistent effective analgesic management, leading to high inpatient healthcare utilization and significant financial burden for healthcare institutions. Current evidence does not provide guidance for inpatient management of acute pain in adults with sickle cell disease. We conducted a retrospective analysis of a longitudinal cohort quality improvement project to characterize the role of individualized care plans on improving patient care and reducing financial burden in high healthcare-utilizing patients with SCD-related pain.
Methods
Individualized care plans were developed for patients with hospital admissions resulting from pain associated with sickle cell disease. A 2-year prospective longitudinal cohort quality improvement project was performed and retrospectively analyzed. Primary outcome measure was duration of hospitalization. Secondary outcome measures included: pain intensity; 7, 30, and 90-day readmission rates; cost per day; total admissions; total cost per year; analgesic regimen at index admission; and discharge disposition.
Results
Duration of hospitalization, the primary outcome, significantly decreased by 1.23 days with no worsening of pain intensity scores. Seven-day readmission decreased by 34%. Use of intravenous hydromorphone significantly decreased by 25%. The potential cost saving was $1,398,827 as a result of this quality initiative.
Conclusions
Implementation of individualized care plans reduced both admission rate and financial burden of high utilizing patients. Importantly, pain outcomes were not diminished. Results suggest that individualized care plans are a promising strategy for managing acute pain crisis in adult sickle cell patients from both care-focused and utilization outcomes.
Keywords: Sickle Cell, Pain, Utilization, LOS, Admission
Introduction
Sickle cell disease (SCD), an inheritable genetic disorder, is associated with high rates of acute care, hospitalization, and financial resource utilization [1–4]. Painful vaso-occlusive crisis (VOC) is the most common manifestation of SCD requiring acute intervention [5]. The unpredictable nature and complexity of acute VOC pain makes inpatient pain care challenging. In addition to acute VOC events, approximately 30% of adults develop chronic SCD-related pain that may worsen with age, potentially due to neuropathic components [6]. Managing acute on chronic SCD pain does not have a gold standard treatment paradigm and is complicated in medical environments without integrated and multidisciplinary care teams [3, 4, 7–9]. Misconceptions and stigma regarding SCD-related pain among clinicians and physicians leads to inconsistent and undertreated pain. This is compounded by barriers to care including the lack of an ambulatory transition plan of care or lack of follow-up after discharge. Ineffective pain management increases the likelihood of readmission within days to weeks of discharge [10–12]. Lack of consistency in care leads to loss of the patient’s confidence in pain treatments and worsened pain control. Ultimately, this results in longer duration of care or in departures against medical advice (AMA) and/or increased rates of rapid readmission [2, 8]. This clinical care challenge can compound the financial burden of inpatient stays related to SCD [4] and negatively affect the care given to SCD patients who already suffer from stigma [13].
SCD related hospitalizations resulted in over $800 million dollars in expenditures for healthcare systems in 2016 (2). With approximately 100,000 patients diagnosed with SCD [14] and high hospital admission rates associated with SCD [4], both resource allocation and pain management care need optimization. Similar to other medical conditions with a small portion of high-utilization patients needing the majority of healthcare associated with the condition [15, 16], prior studies have indicated that a subset of SCD patients contribute to the majority of acute care hospital utilization [17]. Previously, the high utilization patient (HUP) subset has been defined as patients with greater than or equal to 4 hospital admissions per year [17]. The HUP in the sickle cell population are typically young adults (18 to 30 years old) with acute exacerbations and chronic pain, are prescribed opioids for daily use and have a high prevalence of psychological comorbidities [2, 18, 19]. These factors may unconsciously bias the pain care provided and add to stigma in the healthcare environment [13].
Lack of consistent and mechanistic-based pain treatment protocols contribute to patients repeatedly seeking inpatient care [8]. Following established and accepted care plans can reduce the impact of bias and stigma by lessening the individual clinician’s care plan variability. In pediatric populations [9], the use of individualized care plans (ICP) have demonstrated to reduced admission rates and hospital length of stay (LOS) for pediatric SCD patients as inpatients [20–24]. ICPs can protocolize both the pharmacological care and the treatment team delivering the medical care. ICPs may reduce inconsistency between encounters for pain care and improve the patient’s confidence in the clinician [22]. However, the effectiveness of ICPs within the adult inpatient population has not previously been evaluated.
Opioid analgesics have been the pain management mainstay of SCD-related inpatient pain treatment [9]. Although the use of opioids for the treatment of acute and severe pain related to SCD is guideline supported; the timing and approach to the transition from intravenous to oral opioids of equivalent efficacy is not [9]. SCD patients, such as the HUP patient, who have high frequency and persistent pain fit the definition for chronic pain [25, 26]. The concept of acute on chronic SCD-related pain is gaining acceptance. A specific paradigm shift is that the neurobiological mechanism of the condition is likely neuropathic and different from acute SCD pain [25–27]. Neuropathic pain is less effectively treated with opioids as monotherapy due to patients developing tolerance and hyperalgesia [28]. Non-opioid analgesics have support in the treatment of SCD-related chronic pain [9] and have been shown to mitigate the side effects of chronic opioid therapy when managing acute on chronic pain [29–31]. However, the effectiveness of non-opioid analgesics has not focused on prescribing these medications within the context of standardized ICPs.
Inpatient pain consultation services are becoming an established component of managing hospitalized pain patients [32–34]. The newest rebranding of this concept is the transitional pain service [35, 36]. Given the complexity of acute pain, specialized consultation has a critically important role in improving the quality of pain control patients receive [32]. Despite the increased cost associated with specialized care, prior studies have shown that specialized inpatient pain management was cost-effective for patients who had undergone major elective surgery and was valued by patients [37]. Within the context of managing pain related to SCD, an inpatient pain consultation can be an option within an ICP. Including the option of consulting such a specialized service is important for adoption of use of ICPs for SCD.
To our knowledge, ICPs have not been developed, implemented, and studied in adult hospitalized patients with pain related to SCD. Our interdisciplinary team of clinicians with expertise in the management of SCD-related pain developed multidisciplinary ICPs for SCD patients who were admitted frequently to the hospital as a prospective quality improvement (QI) project. The goal for these tailored plans was to improve inpatient pain care leading to decreased duration of SCD-related hospitalizations. The ICPs involved implementing and prescribing novel combinations of therapies that were specific to a patient’s pain needs. This work reflects the evaluation of this clinical program through a retrospective analysis of this novel initiative.
Methods
Quality Improvement Initiative
From January 1, 2018, to December 31, 2019, we performed a prospective quality improvement initiative within a large integrated academic learning health system to address pain care of hospitalized patients with SCD. A multidisciplinary team consisting of advanced practice clinicians and physicians from Pain Medicine, Hematology, Hospital Medicine, and Emergency Medicine came together for a series of planning meetings as the SCD Working Group (WG). The WG reviewed quality data that revealed extended hospital stays were related to patient reported inadequate pain care. The WG was provided with health system financial data showing hospitalization duration that was significantly higher than national averages for the adult SCD patients admitted for pain. The diagnosis related group (DRG) 812 (Red Blood Cell Disorders without Major Complication or Comorbidity) was used and filtered by ICD-10 diagnosis code D57 (Sickle-Cell Disorders), and age filter (>18 years old). The other primary DRG applicable to SCD patients, DRG811 (Red Blood Cell Disorders with Major Complication or Comorbidity Data) was not included to reduce overestimation of hospital stay due to non-pain related conditions increasing the LOS. Therefore, at the initial meeting, the WG decided to address the longer than average hospital stays through a QI project to improve pain care (Figure 1). A barrier analysis was conducted using a modified-Delphi strategy to refine and finalize the most significant barriers [38]. As shown in Table 1, variability in treatment approaches among primary medical teams, lack of pharmaceutical diversity, and lack of pain specialty consultation availability were identified as significant barriers that could be addressed in this initiative.
Figure 1.
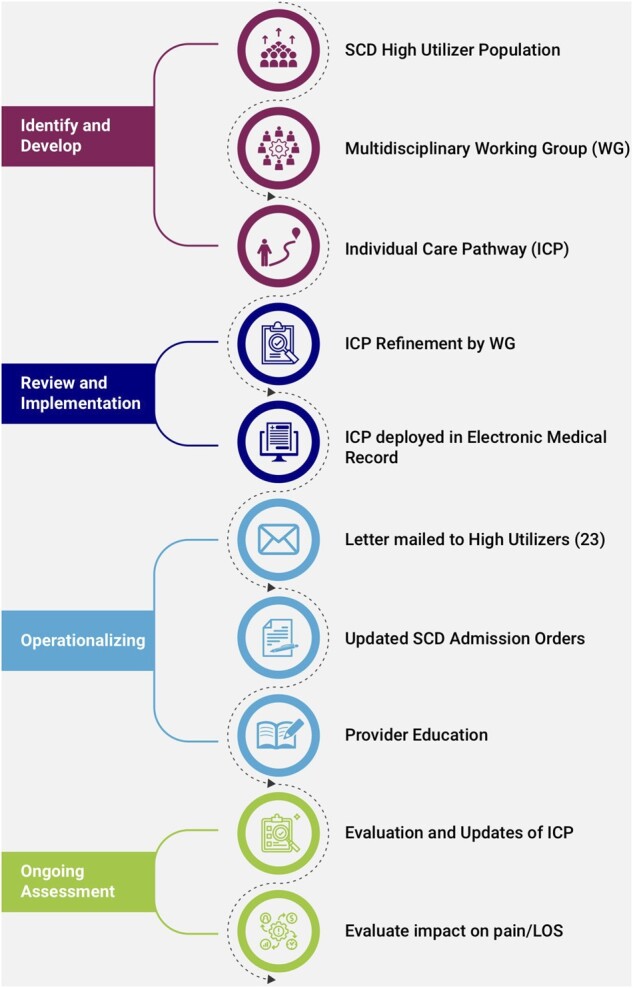
Quality improvement (QI) process for the sickle cell pain high utilization program.
Table 1.
Identified barriers to care for high utilization patients with sickle cell disease
| General Barriers | Specific Examples/Areas |
|---|---|
| Variability in treatment among clinicians and staff |
Emergency medicine Hospital medicine Hematology service Inpatient nursing |
| Lack of pharmaceutical diversity offered |
Pharmacy—medication availability Clinical Providers—education on multimodal analgesic options |
| Lack of pain specialist consultation availability/use | Less than 5% of SCD patients received pain consultation |
High utilization patients (HUP) were defined as those patients with four or more hospital admissions [39] in the 2018 calendar year related to their acute exacerbations of their chronic SCD pain. Patient chart review was conducted by an interdisciplinary team of inpatient pain specialists (J.R.C., C.L.A., R.W.H.) and ICPs were developed to address each of the three identified barriers. Each ICP incorporated past inpatient treatments that resulted in the greatest reduction in pain intensity scores and shortest hospital duration. Two pain management pathways were then developed around the primary opioid analgesic: one for full opioid agonists and one for partial opioid agonists (Table 2a and b; and Figure 2). Medication doses were tailored at the level of the individual. Both pathways included a multimodal approach including non-opioid medications (Table 2c) and non-pharmacologic treatments (e.g., heat, distraction, meditation, and physical therapy) that were not part of previous treatment regimens. The option of inpatient pain management consult was added to both pathways. The full multidisciplinary WG then reviewed each ICP. A modified-Delphi strategy was used to refine and finalize the ICPs for each patient [38]. Within the EMR, the ICPs were placed in an easily accessible location in patients charts viewable by all clinicians accessing the record. This intervention included a detailed letter sent to all SCD patients who received an ICP regarding the pathway. Education about the ICPs was provided to the patients via written communication and the health system using our clinical communication platforms prior to releasing the ICPs.
Table 2a.
Inpatient pain management pathway dosing: Full agonists
| Drug | Route |
|---|---|
| Fentanyl | PCA |
| Morphine | PCA |
| Morphine | IVP |
| Tramadol | PO |
| Hydrocodone-acetaminophen | PO |
| Oxycodone-acetaminophen | PO |
| Oxycodone | PO |
| Morphine sulfate | PO |
| Hydromorphone | PO |
Figure 2.
Sickle cell pain standard order set used with the individualized care plans developed for our high utilization program.
Table 2c.
Inpatient pain management pathway dosing: Non-opioids
| Drug | Route | Dose | Frequency |
|---|---|---|---|
| Ketamine | PO | 20–70 mg | 4× daily |
| Ketamine | IV | 0.1–1.0 mg/kg/hr (Max 70 mg/hr) | Continuous × 48–72 hr |
Table 2b.
Inpatient pain management pathway dosing: Partial agonists
| Drug | Route | Dose | Frequency |
|---|---|---|---|
| Buprenorphine | IVP | 0.15–0.3 mg | Q6 hr PRN |
| Buprenorphine (Subutex) | SL | 2–8 mg | Q6–8 hr |
| Buprenorphine-naloxone (Suboxone) | SL | 2–0.5 mg, 8–2 mg | Q6–8 hr |
Retrospective Study
The retrospective analysis of this QI project met internal review board standards that did not require informed consent due to the retrospective nature of the study. Review of records was approved by the Wake Forest University Health Sciences Institutional Review Board (IRB00063471).
Study Measures and Outcome
Outcomes from pre- and post-individual care pathways were compared in the SCD patients included in the QI project. The primary outcome measure was the duration of the hospital admission. This value was determined by taking the difference between the discharge time and the admission time rounded to the nearest hour and converted into days. Secondary outcome measures included change in pain score between admission and discharge, total number of admissions in each calendar year, readmission after an index admission at 7, 30, and 90 days, discharge disposition including AMA departures, total hospitalization cost, analgesic usage/regimen and use of the inpatient pain consult. LOS was also included for cost calculation and calculations were determined with standard hospital accounting principles by counting admission as day 1 and sequentially each “midnight” accumulated during a hospital stay [21]. In addition, we also sought to determine the impact of our program on neighboring health systems and hospitals. To ensure all admissions were captured, our analysis evaluated admission data to outside hospitals linked to our electronic medical record (EMR) system. All costs were determined using data from our health system for SCD-related to the target DRG 812 (Red Blood Cell Disorders without Major Complication or Comorbidity) of these HUP patients. Total direct cost was used and assumed to be fixed for the purposes of this analysis. Hospital charges were not used for any financial analysis. Index admissions were defined as the first hospital admission to begin and end in the same calendar year [2].
Statistical Analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) [40, 41]. Analysis of inpatient measures of care was performed comparing the difference in primary and secondary outcome measures for the index admissions of 2018 and 2019. All ICPs were in place and education complete by January 1, 2019. All HUP admissions in 2019 used ICPs. Univariate statistical analysis was performed on the outcome variables. Continuous data were analyzed with two independent samples t-test and ANOVA. Categorical data were analyzed with Pearson’s χ2 test. Discrete data were analyzed with a two-sample t-test. Categorical data were recorded as count data and reported as percentages. Continuous data were presented with means, standard deviations, and 95% confidence intervals. A P values of < .05 was used as the statistically significant threshold.
Results
Demographic information for the SCD HUP with ICPs is presented in Table 3. The average age of the cohort was 27 ± 6 (18 to 38) years at the beginning of the QI initiative. The majority of the cohort identified as male. All of the cohort self-identified as Black and/or African American. Nineteen patients in the cohort were Hb-SS, three patients with Hb-Sc, and one patient with Hb-β0 thalassemia. No patients in this cohort were Hb-β+.
Table 3.
Demographic information for all patients included in the quality improvement study
| 2018–2019 (N = 23) | |
|---|---|
| Total admissions | 319 |
| Age (yr) | 27.0 ± 5.8 |
| Sex (%) | |
| Male | 69.6 |
| Female | 31.8 |
| Race/Ethnicity (%, (N)) | |
| Black or African American | 100 (23) |
| Type of sickle cell (%, (N)) | |
| Hb-SS | 82.6 (19) |
| Hb-Sc | 13.0 (3) |
| Hb-β0 | 4.35 (1) |
| Hb-β+ | (0) |
Table 5b.
Display length of stay and number of admissions for the 7, 30, and 90 day readmission rates for 2019 from index admission
| 2019 | Number of Readmissions | Total Hospital Days | Mean |
|---|---|---|---|
| 7 | 1 | 3 | N/A |
| 30 | 14 | 80 | 5.71 |
| 90 | 29 | 143 | 4.93 |
The introduction of ICPs was associated with a reduction in average SCD pain related hospitalization treatment time from 5.23 to 3.96 days and 34% reduction in readmissions within 7 days of the index admission (Table 4). The 30-day readmission rate decreased by 23.7% with the introduction of ICPs but was not found to be statistically significant. The 90-day readmission rate did not differ pre- or post- the QI intervention. The mean length of stay of hospitalization during each re-admission period was also reduced (Table 5a and b). Patient-reported pain intensity upon discharge did not change after ICP introduction. However, discharge pain intensity was lower than admission pain intensity. The use of the inpatient pain consultation team increased by 17% with the introduction of the ICPs.
Table 4.
Treatment time, readmission rates and pain intensity score of HUP admissions
| Measurements | 2018 | 2019 | Difference | P-value |
|---|---|---|---|---|
| Treatment time (days) | 5.23 ± 4.5 | 3.96 ± 2.9 | 1.23 | .020 |
| Readmission | ||||
| 7 Day readmission (%) | 39.1 | 4.54 | 34.6 | .005 |
| 30 Day readmission (%) | 78.3 | 54.5 | 23.7 | .12 |
| 90 Day readmission (%) | 87.0 | 77.2 | 9.68 | .46 |
| Pain | ||||
| Score admission | 8.55 ± 1.26 | 7.86 ± 2.78 | 0.69 | .28 |
| Score discharge | 6.18 ± 2.36 | 6.27 ± 2.58 | −0.09 | .85 |
| Pain consult (%) | 25.7 (62) | 43.2 (35) | 17.5 | .0017 |
Table 5a.
Display length of stay and number of admissions for 7, 30, and 90 day readmissions 2018 from index admission
| 2018 | Number of Readmissions | Total Hospital Days | Mean |
|---|---|---|---|
| 7 | 9 | 55 | 6.11 |
| 30 | 23 | 156 | 6.78 |
| 90 | 50 | 268 | 5.36 |
The total direct cost of treating an average uncomplicated (DRG 812) SCD patient admitted to our health system was determined to be $1248 per day. LOS decreased (6.21 days (2018) vs 4.97 days (2019)) which resulted in a potential decrease in treatment cost per admission ($7,755 (2018) vs. $6,207 (2019)). The total number of admissions of the HUP cohort decreased by 165 resulting in 1121 fewer days spent hospitalized following the introduction of ICPs. Admissions to outside hospitals increased by 79 admissions during this same period. Patient departures AMA significantly decreased from 7.9% to 1.9% in 2019 (P = .046). Potential hospital cost savings would be over $1.3 million from 2018 to 2019 in this patient population (Table 6).
Table 6.
Total sickle cell disease-related pain admissions, cost and discharge disposition in 2018 and 2019
| 2018 | 2019 | Difference | P-value | |
|---|---|---|---|---|
| No. of admissions | 242 | 77 | 165 | |
| Length of stay | 6.21 | 4.97 | 1.24 | .022 |
| Total days in hospital | 1504 | 383 | 1121 | |
| Total potential hospital cost ($) | 1 876 811 | 477 984 | 1 398 827 | |
| Against medical advice (%) | 7.88 | 1.94 | 5.94 | .046 |
Inpatient analgesic options for cohort patients are shown in Table 7. Use of intravenous hydromorphone significantly decreased. Additionally, use of oral ketamine significantly increased (0% (2018) vs 22.73% (2019), P = .015). Otherwise, analgesic regimen remained consistent across the 2-year period with no other statistically significant changes in analgesic medications. No change in outpatient or inpatient regimen of disease modifying treatments (transfusion, hydroxyurea), and no use of crizanlizumab, and/or voxelotor was noted.
Table 7.
Comparing inpatient medication management for patients with vaso-occlusive crisis in 2018 and 2019
| Inpatient Pain Medication | 2018 | 2019 | Difference(absolute) | P-value |
|---|---|---|---|---|
| Hydromorphone IV (%) | 95.7 | 72.7 | 23.0 | .034 |
| Hydromorphone PO (%) | 26.1 | 40.9 | 14.8 | .052 |
| Morphine IV (%) | 4.35 | 4.55 | 0.2 | .974 |
| Morphine PO (%) | 13.0 | 9.09 | 3.91 | .673 |
| Oxycodone PO (%) | 52.2 | 54.55 | 2.30 | .873 |
| Methadone (%) | 21.7 | 13.6 | 8.10 | .477 |
| Fentanyl IV (%) | 0 | 4.55 | 4.55 | .301 |
| Buprenorphine PO (%) | 0 | 4.55 | 4.55 | .301 |
| Ketamine IV (%) | 17.4 | 9.09 | 8.31 | .413 |
| Ketamine PO (%) | 0 | 22.73 | 22.7 | .015 |
Discussion
Moving beyond stigma and inconsistent pain care for patients with SCD is important from both a patient-centric and hospital utilization perspective. Despite the adult SCD population requiring frequent acute care in the inpatient setting and previously demonstrated success in pediatric SCD patients, prior studies have not focused on developing ICPs in adults. The transition from pediatric to adult care also poses serious health risks and complications [42, 43] with high utilization patients (HUP) at increased risk for mental health comorbidities and healthcare stigma [44]. Relative to the pediatric population, the adult SCD population more often experience acute on chronic SCD pain and is especially vulnerable to bias [6]. Care for adult SCD patients continues to remain inconsistent and unsatisfactory to patients [8]. In addition to benchmarking the hospitalization LOS, our work focused on measuring the quality of pain care, measured through impact on pain outcomes for patients.
Our work found that the implementation of ICPs to manage acute on chronic pain episodes was effective at reducing hospital LOS, reducing hospital readmissions at 7 days, and keeping pain scores at admission and discharge consistent despite significantly shorter treatment time while reducing the number of patients that left AMA. The decrease in hospital admissions and LOS resulted in substantial improvement in financial resource utilization. Reduction in admission rates, consistent pain scores during admissions, and a decreased financial cost demonstrate the efficacy of the ICPs for managing acute on chronic painful episodes within the high utilizer population.
Within the pediatric population, multidisciplinary care for acute pain related to vaso-occlusive crisis (VOC) reduced hospital LOS by approximately 1 day [21]. Similarly, our QI initiative found a reduction in LOS from 6 days to 5 days. When comparing pediatric SCD care to adult SCD care, it is important to take into account the difference in pediatric acute pain and adult acute pain due to recent literature indicating that adults with SCD experience acute on chronic pain [45]. Moreover, these pediatric results were not specific to the high utilization population and could not be attributed to ICPs.
LOS and readmission rates are used as the standard reference metrics for measuring improved care outcomes for acute painful crisis within the SCD population. As a result, there is minimal literature on the effect of ICPs on pain intensity scores or pain experience and none on the effect of ICPs in the hospitalized setting. ICPs have been found to reduce pain in the ambulatory setting [46]. However, this is an entirely different care setting and the analgesic management in this study was exclusively with opioid medications. Recent literature indicates that acute on chronic pain is exacerbated by opioids use [28]. Our ICPs implemented multimodal analgesic treatment, which included non-opioid analgesics as an option as they have been shown to reduce opioid use in SCD [47].
Reduction in readmission is a successful care outcome for care of SCD patients. Nationally the 7-day readmission rate for acute painful crisis related to SCD is approximately 7.6% [4]. Demonstrating an improvement from the standard of care, our QI intervention was able to reduce 7-day post-intervention from 39.1% to 4.54%. Previous studies have shown 30-day readmission was approximately between 30% and 40% for general SCD population admitted for pain associated to SCD [2,4]. Although not statistically significant, the 30-day readmission rate decreased by 24%. This reduction is positive and consistent with general pediatric SCD literature [21]. It should be noted that the population in this initiative are high utilizers of healthcare services and have expected higher rates of readmission, therefore the reduction observed after the intervention is clinically important. Departures AMA have a significant impact on hospital readmission [4,48]. Patients leaving AMA may be indicative of dissatisfaction with care [48]. There is no population-specific data for AMA in the HUP population of SCD patients; however, hospitalized SCD patients account for 2.86% AMA [4]. Post-intervention, our analysis indicated that patients discharged AMA significantly decrease to a rate of 2.0%. This is below the national average for all SCD patients and may indicate an overall improvement in their satisfaction with care despite the decrease in treatment duration.
In addition to improved patient care outcomes, there was significant reduction in financial burden for the hospital system related to pain-related SCD hospitalization. Reduction in cost of care was a direct result of decrease in LOS and admission rates post-intervention. Although the observed decrease in hospital admissions was influenced by admissions to outside hospitals, only one patient left the cohort post-intervention, while the rest of these patients continued to return to our institution. The increase in admissions to external hospitals is outweighed by the substantial decrease in admissions seen within our healthcare system. The admissions to outside hospitals may reflect patient care preference and/or patient household relocation. Patients included in this QI program continued to receive care within our healthcare system following receipt of the ICP letters. No disease modifying treatments were altered during the initiative and no newly introduced pharmacological treatments were included, therefore the results are unlikely related to non-ICP improvements in care. Although previous interventions in pediatric SCD found a more modest reduction in cost that was attributed to a decrease in LOS [21]. Our cost reduction was driven by decrease in both admissions and hospital LOS despite a reduced elopement rate quantified by a lower AMA incidence.
Decreased use of IV opioids, while maintaining stable pain scores indicates that non-opioid, multimodal analgesia contributed to the management of acute on chronic SCD pain. The findings align with the evolving paradigm of sickle cell pain as partially neuropathic, which could then be exacerbated by chronic opioid treatments [28, 49]. The ICP analgesic regimen operationalized current guidelines that recommend the use of ketamine for acute pain related to SCD [30, 31, 47] and multimodal analgesia.
Our inpatient pain consultation service experienced the expected increase in utilization during this quality improvement project. However, we did not observe >50% utilization of inpatient pain consultation in these patients. We believe this reflects the usability of the ICPs by general practitioners and non-pain trained clinicians. We did observe an important trend of decreased consultation with stable LOS and pain scores after six months of the QI implementation. We believe that the involvement of a multidisciplinary care team with physician champion(s) on the admitting service likely increased the acceptance and use of ICPs without the need for specialty consultation. The rate of consultation did not fall to zero because a small population of patients had ketamine as a part of their ICP. In our health system, the inpatient pain service manages the use of ketamine for analgesia in the hospital. Recognizing that not all hospitals have inpatient pain services, a future area of study would be implementation at other hospital sites without a specialized consult service.
ICPs provided consistent and predictable care personalized to an individual patient’s needs that lead to decreased treatment duration and decrease financial cost without negatively affecting the patient’s pain care. In developing the ICPs, our multidisciplinary SCD team was able to identify specific barriers to care for individuals. This included creating order sets that were easily accessible within the EMR and making inpatient pain consultation possible, altering the pharmacological regimen and education of healthcare clinicians.
Limitations
Although ICPs appear to be clinically beneficial, further evidence is needed due to the limits of a single academic institution study over the course of 1 year and the potential for clinician bias during an unblinded QI project. When compared to blinded, controlled studies, previous research demonstrates observational QI studies do not overstate the results of treatment changes [50, 51]. However, we do understand the limitations of an unblinded QI protocol, and encourage results be interpreted cautiously. Patients within the cohort were admitted to outside hospital systems more frequently in 2019 than in 2018. However, in totality, the difference in admissions at outside hospitals does not account for the majority of the decrease in admissions at the index hospital. However, it is crucial to continue to follow this and future initiatives will follow-up with patients who sought care at difference institutions. Use of “index admission” methodology may not fully represent the patient’s healthcare utilization or pain intensity throughout the entire year. Although this is a possibility, the index admission was similar to subsequent admissions for that year. Continued QI analysis will be needed to continue to prove the efficacy and the generalizability of these findings over a longer timeframe. The cost savings reported in this initiative would only be realized if the 2018 data would have occurred in 2019. We recognize that this cannot be assumed or known, however based on our analysis of SCD HUP data in the years preceding this QI initiative we believe this to be an accurate assumption. We did not involve patients or patient representatives in the QI planning, this limits the knowledge of satisfaction with care and is therefore reliant on indirect measures (decrease readmission, reduction of AMA, and a reduction in discharge pain scores despite shorter treatment time) of pain care success. It will be important to include a robust set of patient-reported outcomes including pain interference, satisfaction with care and potentially qualitative structured interviews in future studies. An analysis of changes in emergency department visits that did not result in admission will also need to be conducted.
Conclusion
ICPs significantly improved clinical outcomes and healthcare utilization for adult SCD patients. This low-cost intervention did not worsen pain care quality metrics and led to a significant reduction in readmission rates, reducing financial burden within the hospital system. These results support the value of ICPs developed by a multidisciplinary and interdisciplinary team to treat acute pain episodes associated with SCD.
Disclosures and conflicts of interest: Dr. Adams is supported by National Institutes of Health funding through the NIH K08 EB022631, U24 NS115708, and R33 DA046085. Dr. Hurley holds funding through NIH R33 DA046085 and U24 NS115708, and Avanos Medical for research unrelated to Sickle Cell Disease treatment.
Jena L. Welch-Coltrane and Anthony A. Wachnik are co-first authors; these authors contributed equally to the publication.
Erik C. Summers and Robert W. Hurley share senior author responsibilities.
References
- 1. Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. Jama 2010;303(13):1288–94. [DOI] [PubMed] [Google Scholar]
- 2. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000–2016: Statistical Brief #251. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US; ); 2006. [PubMed] [Google Scholar]
- 3. Elixhauser A, Steiner C. Readmissions to U.S. Hospitals by Diagnosis, 2010: Statistical Brief #153. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. [PubMed]
- 4. Kumar V, Chaudhary N, Achebe MM. Epidemiology and predictors of all-cause 30-day readmission in patients with sickle cell crisis. Sci Rep 2020;10(1):2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: A descriptive study, 1999-2007. Am J Prev Med 2010;38(4 Suppl):S536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148(2):94–101. [DOI] [PubMed] [Google Scholar]
- 7. Telfer P, Bahal N, Lo A, Challands J. Management of the acute painful crisis in sickle cell disease: A re-evaluation of the use of opioids in adult patients. Br J Haematol 2014;166(2):157–64. [DOI] [PubMed] [Google Scholar]
- 8. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open 2020;3(5):e206016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: Management of acute and chronic pain. Blood Adv 2020;4(12):2656–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aljuburi G, Laverty AA, Green SA, Phekoo KJ, Bell D, Majeed A. Socio-economic deprivation and risk of emergency readmission and inpatient mortality in people with sickle cell disease in England: Observational study. J Public Health (Oxf) 2013;35(4):510–7. [DOI] [PubMed] [Google Scholar]
- 11. Glassberg J, Simon J, Patel N, Jeong JM, McNamee JJ, Yu G. Derivation and preliminary validation of a risk score to predict 30-day ED revisits for sickle cell pain. Am J Emerg Med 2015;33(10):1396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brodsky MA, Rodeghier M, Sanger M, et al. Risk factors for 30-day readmission in adults with sickle cell disease. Am J Med 2017;130(5):601.e9–e15. [DOI] [PubMed] [Google Scholar]
- 13. Blake A, Asnani V, Leger RR, et al. Stigma and illness uncertainty: Adding to the burden of sickle cell disease. Hematology 2018;23(2):122–30. [DOI] [PubMed] [Google Scholar]
- 14. Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: National and state estimates. Am J Hematol 2010;85(1):77–8. [DOI] [PubMed] [Google Scholar]
- 15. Wiendels NJ, Knuistingh Neven A, Rosendaal FR, et al. Chronic frequent headache in the general population: Prevalence and associated factors. Cephalalgia 2006;26(12):1434–42. [DOI] [PubMed] [Google Scholar]
- 16. Jansen LA, Knauff EA, Tan SS, Löwenberg M. Estimated hospital health costs of chronic abdominal pain in the Netherlands. Neth J Med 2014;72(2):102–6. [PubMed] [Google Scholar]
- 17. Carroll CP, Haywood C Jr., Fagan P, Lanzkron S. The course and correlates of high hospital utilization in sickle cell disease: Evidence from a large, urban Medicaid managed care organization. Am J Hematol 2009;84(10):666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergman EJ, Diamond NJ. Sickle cell disease and the “difficult patient” conundrum. Am J Bioeth 2013;13(4):3–10. [DOI] [PubMed] [Google Scholar]
- 19. Brennan-Cook J, Bonnabeau E, Aponte R, Augustin C, Tanabe P. Barriers to care for persons with sickle cell disease: The case manager's opportunity to improve patient outcomes. Prof Case Manag 2018;23(4):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schefft MR, Swaffar C, Newlin J, Noda C, Sisler I. A novel approach to reducing admissions for children with sickle cell disease in pain crisis through individualization and standardization in the emergency department. Pediatr Blood Cancer 2018;65(10):e27274. [DOI] [PubMed] [Google Scholar]
- 21. Balsamo L, Shabanova V, Carbonella J, et al. Improving care for sickle cell pain crisis using a multidisciplinary approach. Pediatrics 2019;143(5):e20182218. [DOI] [PubMed] [Google Scholar]
- 22. Koch KL, Karafin MS, Simpson P, Field JJ. Intensive management of high-utilizing adults with sickle cell disease lowers admissions. Am J Hematol 2015;90(3):215–9. [DOI] [PubMed] [Google Scholar]
- 23. Krishnamurti L, Smith-Packard B, Gupta A, Campbell M, Gunawardena S, Saladino R. Impact of individualized pain plan on the emergency management of children with sickle cell disease. Pediatr Blood Cancer 2014;61(10):1747–53. [DOI] [PubMed] [Google Scholar]
- 24. Ender KL, Krajewski JA, Babineau J, et al. Use of a clinical pathway to improve the acute management of vaso-occlusive crisis pain in pediatric sickle cell disease. Pediatr Blood Cancer 2014;61(4):693–6. [DOI] [PubMed] [Google Scholar]
- 25. Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain 2017;18(5):490–8. [DOI] [PubMed] [Google Scholar]
- 26. Field JJ, Ballas SK, Campbell CM, et al. AAAPT diagnostic criteria for acute sickle cell disease pain. J Pain 2019;20(7):746–59. [DOI] [PubMed] [Google Scholar]
- 27. Karafin MS, Chen G, Wandersee NJ, et al. Chronic pain in adults with sickle cell disease is associated with alterations in functional connectivity of the brain. PLoS One 2019;14(5):e0216994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carroll CP, Lanzkron S, Haywood C Jr, et al. Chronic opioid therapy and central sensitization in sickle cell disease. Am J Prev Med 2016;51(1 Suppl 1):S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lubega FA, DeSilva MS, Munube D, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: A randomized controlled trial. Scand J Pain 2018;18(1):19–27. [DOI] [PubMed] [Google Scholar]
- 30. Tawfic QA, Faris AS, Kausalya R. The role of a low-dose ketamine-midazolam regimen in the management of severe painful crisis in patients with sickle cell disease. J Pain Symptom Manage 2014;47(2):334–40. [DOI] [PubMed] [Google Scholar]
- 31. Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018;43(5):456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carr DB, Goudas LC. Acute pain. Lancet 1999;353(9169):2051–8. [DOI] [PubMed] [Google Scholar]
- 33. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2004;100(6):1573–81. [DOI] [PubMed] [Google Scholar]
- 34. Sun E, Dexter F, Macario A. Can an acute pain service be cost-effective? Anesth Analg 2010;111(4):841–4. [DOI] [PubMed] [Google Scholar]
- 35. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: Development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015;8:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17(2):131–57. [DOI] [PubMed] [Google Scholar]
- 37. Lee A, Chan SK, Chen PP, Gin T, Lau AS, Chiu CH. The costs and benefits of extending the role of the acute pain service on clinical outcomes after major elective surgery. Anesth Analg 2010;111(4):1042–50. [DOI] [PubMed] [Google Scholar]
- 38. Fitch KB, Aguilar MD, Burnand B, et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, Calif: RAND; 2001. [Google Scholar]
- 39. Carroll CP, Haywood C Jr., Lanzkron S. Prediction of onset and course of high hospital utilization in sickle cell disease. J Hosp Med 2011;6(5):248–55. [DOI] [PubMed] [Google Scholar]
- 40. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115(17):3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cronin RM, Hankins JS, Byrd J, et al. Risk factors for hospitalizations and readmissions among individuals with sickle cell disease: Results of a U.S. survey study. Hematology 2019;24(1):189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jonassaint CR, Jones VL, Leong S, Frierson GM. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br J Haematol 2016;174(1):136–47. [DOI] [PubMed] [Google Scholar]
- 45. Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers 2018;4:18010. [DOI] [PubMed] [Google Scholar]
- 46. Molokie RE, Montminy C, Dionisio C, et al. Opioid doses and acute care utilization outcomes for adults with sickle cell disease: ED versus acute care unit. Am J Emerg Med 2018;36(1):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Puri L, Morgan KJ, Anghelescu DL. Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease. Curr Opin Support Palliat Care 2019;13(4):402–7. [DOI] [PubMed] [Google Scholar]
- 48. Tan SY, Feng JY, Joyce C, Fisher J, Mostaghimi A. association of hospital discharge against medical advice with readmission and in-hospital mortality. JAMA Netw Open 2020;3(6):e206009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gupta K, Jahagirdar O, Gupta K. Targeting pain at its source in sickle cell disease. Am J Physiol Regul Integr Comp Physiol 2018;315(1):R104–r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342(25):1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernald DH, Coombs L, DeAlleaume L, West D, Parnes B. An assessment of the Hawthorne Effect in practice-based research. J Am Board Fam Med 2012;25(1):83–6. [DOI] [PubMed] [Google Scholar]



