Abstract
This cohort study examines antibody responses in infection-naive and previously infected individuals after 1 and 2 doses of the BNT162b2 vaccine.
Introduction
The Pfizer/BioNTech (BNT162b2) SARS-CoV-2 mRNA vaccine demonstrated 95% efficacy after 2 doses during clinical trials.1 There are reports that individuals with previous SARS-CoV-2 infection elicit stronger antibody responses after 1 dose compared with individuals without prior infection.2,3,4 Consequently, individuals with a documented prior COVID-19 infection may be sufficiently protected from reinfection after a single mRNA vaccine dose, which could free up availability of millions of additional doses. We evaluated the SARS-CoV-2 spike immunoglobin (Ig) G antibody levels after 1 and 2 BNT162b2 doses in previously infected individuals compared with those without previous infection.
Methods
This study was approved by the Rush University institutional review board, and all participants provided written informed consent. Participants for this cohort study (n = 59) were recruited at Rush University Medical Center and assigned to 2 groups based on evidence of previous SARS-CoV-2 infection. Evidence of previous SARS-CoV-2 infection included previous positive polymerase chain reaction (PCR) test and/or positive SARS-CoV-2 antibody result at baseline. Self-reported demographic data including age, sex, and race/ethnicity were collected for statistical analysis. The self-reported categories for race/ethnicity were Black, White, Asian, and Hispanic. Group 1 (n = 30) had no evidence of previous SARS-CoV-2 infection and Group 2 (n = 29) did. SARS-CoV-2 spike IgG antibody levels from plasma were measured at baseline and after receiving 1 and 2 BNT162b2 doses. Samples were run on an Abbott ARCHITECT i2000SR and tested with CE marked SARS-CoV-2 Quant IgG II (List 6S60), which quantitatively measures IgG antibodies against the SARS-CoV-2 spike Receptor Binding Domain (spike-RBD). Results were reported in arbitrary units (AU)/mL (≥50.0 AU/mL is positive). Graphing and statistical analysis was conducted with Prism version 8.0.2 (GraphPad Software) using unpaired 2-tailed t tests from April to May 2021. Statistical significance was defined as P < .05.
This manuscript was written following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. Additional information about the study design and participant demographics can be found in the eMethods and eTable in the Supplement.
Results
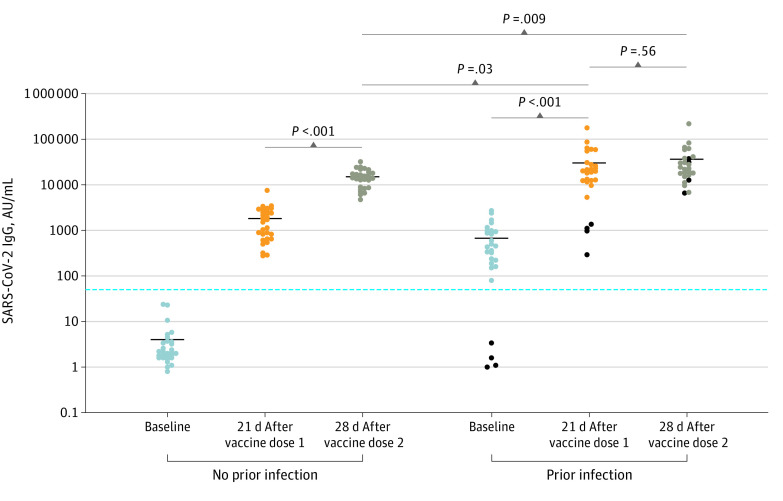
Among 59 participants, 29 (49%) were White individuals and 43 (73%) were female individuals. The mean (SD) age was 42 (12) years. Infection-naive participants (n = 30) had mean SARS-CoV-2 spike-RBD IgG levels at baseline of 4.03 AU/mL (95% CI, 1.92-6.13 AU/mL) and increasing to 1822 AU/mL (95% CI, 1266-2377 AU/mL) after 1 vaccine dose and to 15 005 AU/mL (95% CI, 12 533-17 476 AU/mL) after 2 vaccine doses (Figure). Previously infected individual’s (n = 29) mean IgG levels increased from 621.3 AU/mL (95% CI, 388.90-853.70 AU/mL) at baseline to 30 173 AU/mL (95% CI, 15 571-44 775 AU/mL) after 1 dose and 36 600 AU/mL (95% CI, 19 563-53 637 AU/mL) after 2 doses (Figure). Mean (standard error of the mean [SEM]) differences between second and first doses were 13 183 (1218) AU/mL (P < .001) in infection-naive participants. Mean (SEM) IgG differences were 6427 (10 876) AU/mL (P = .56) between second and first doses in previously infected individuals. Four previously infected participants reported a previous positive PCR but did not develop antibodies. Vaccine responses in these 4 participants resembled infection-naive individuals. Mean (SEM) antibody differences after 2 vaccine doses in infection-naive individuals compared with 1 and 2 vaccine doses in previously infected individuals were 15 168 (6963) AU/mL (P = .03) and 21 595 (7927) AU/mL (P = .009), respectively.
Figure. SARS-CoV-2 Immunoglobin (Ig) G Serological Response in Infection-Naive and Previously Infected Individuals After 1 or 2 Doses of BNT162b2.
Quantitative SARS-CoV-2 IgG levels at baseline (blue dots), 21 days after vaccine dose 1 (orange dots), and 28 days after vaccine dose 2 (brown dots) in infection-naive or previously infected individuals. Horizontal black bars represent mean quantitative IgG levels (AU/mL) within the indicated groups. Black dots represent 4 individuals with a previous positive SARS-CoV-2 polymerase chain reaction but did not have detectable SARS-CoV-2 IgG at baseline. Horizontal blue line denotes the assay limit of detection cutoff. Statistical analysis was performed using unpaired 2-tailed t test. AU indicates arbitrary unit.
Discussion
We observed higher SARS-CoV-2 antibody levels in previously infected individuals after 1 dose of BNT162b2 compared with infection-naive individuals after 2 doses. Importantly, in previously infected individuals with positive SARS-CoV-2 spike IgG levels, the second dose did not significantly increase IgG levels compared with the first dose, suggesting that 1 dose may be acceptable in this group. However, it is important to note that a positive PCR diagnosis alone was not enough to discount the need for a second vaccine dose. In 4 participants who reported a positive PCR, but did not develop S-protein antibodies, the response to the first vaccine dose was more similar to that of the infection-naive group. Furthermore, these results highlight that even in previously infected individuals, baseline serological testing should be performed prior to deciding whether to forego a second vaccine dose.
This study highlights the potential for recommending a single dose for previously infected individuals and may be useful for discussions surrounding vaccination strategy. Limitations of this study include small sample size, diversity of participants (sex, race, nationality), lack of neutralization studies, and lack of T-cell response studies.
eMethods. Materials and Methods
eTable. Participant Demographics
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057-1058. doi: 10.1016/S0140-6736(21)00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178-1181. doi: 10.1016/S0140-6736(21)00502-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley T, Grundberg E, Selvarangan R, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(20):1959-1961. doi: 10.1056/NEJMc2102051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Materials and Methods
eTable. Participant Demographics