Abstract
To investigate the importance of light on healing and acclimatization, in the present study, grafted watermelon seedlings were exposed to darkness (D) or light, provided by blue (B), red (R), a mixture of R (68%) and B (RB), or white (W; 35% B, 49% intermediate spectra, 16% R) LEDs for 12 days. Survival ratio, root and shoot growth, soluble carbohydrate content, photosynthetic pigments content, and photosynthetic performance were evaluated. Seedling survival was not only strongly limited in D but the survived seedlings had an inferior shoot and root development, reduced chlorophyll content, and attenuated photosynthetic efficiency. RB-exposed seedlings had a less-developed root system. R-exposed seedlings showed leaf epinasty, and had the smallest leaf area, reduced chlorophyll content, and suppressed photosynthetic apparatus performance. The R-exposed seedlings contained the highest amount of soluble carbohydrate and together with D-exposed seedlings the lowest amount of chlorophyll in their scions. B-exposed seedlings showed the highest chlorophyll content and improved overall PSII photosynthetic functioning. W-exposed seedling had the largest leaf area, and closely resembled the photosynthetic properties of RB-exposed seedlings. We assume that, during healing of grafted seedlings monochromatic R light should be avoided. Instead, W and monochromatic B light may be willingly adopted due to their promoting effect on shoot, pigments content, and photosynthetic efficiency.
Keywords: chlorophyll fluorescence imaging, healing and acclimatization, light quality, O–J–I–P-transient, vegetable grafting
1. Introduction
In vegetable crops, grafting has been widely employed to improve fruit quality, as well as to achieve better yields under biotic (e.g., soil-borne diseases) and abiotic (e.g., drought, salinity, non-optimum temperatures) stresses [1]. It is a widely applied agricultural practice on species of the Cucurbitaceae and Solanaceae families [2,3], and in particular in tomato and watermelon, with the latter showing the lowest survival rates [4].
For gaining the advantages of grafting, high-quality seedlings ought to be produced [5]. This necessitates a successful connection between rootstock and scion, a process that takes place during the healing and acclimatization period [6]. During the first days of this period (healing), the newly-grafted seedlings have no vascular connections, and the scion part often encounters desiccation. Optimizing environmental factors during healing and acclimatization stages is thus vital for seedling production [3]. Healing and acclimatization are generally carried out under shaded plastic-covered tunnels, often referred to as conventional tunnel systems. In these systems, relative air humidity is maintained at high levels to prevent excessive water loss and the resultant scion wilting. Letting aside that direct exposure to natural light is avoided (by shading), light conditions are generally poorly controlled since they are prone to diurnal and seasonal changes [7]. Therefore, among environmental factors, the role of light quality on mediating seedling growth and vigor is currently the most neglected one [8,9]. This topic has been currently addressed by a handful of studies, where different light quality regimes were reported for driving optimal growth during healing and acclimatization [5,6,10]. For instance, monochromatic red (R) light led to optimum grafted tomato seedling quality [5], whereas a spectrum including both blue (B) and R spectral regions was required in pepper [6]. Given the noted interspecific variation in response to light spectrum, seedling quality ought to be individually evaluated in the species of interest before commercial implementation.
Generally, high-quality seedlings are regarded as the ones showing uniformity in greenness, a criterion that is often employed throughout the production-distribution chain (e.g., vegetable nurseries, growers). Instead, yellowing symptoms of scion cotyledon are a clear sign of improper healing and acclimatization. Therefore, assessing the greening in different parts of the grafted seedlings is an index of their vigor [2].
Carbohydrates are the main source of energy in plants, and their abundance is a prerequisite for cell division activity and region differentiation. In this respect, the carbohydrate status is expected to play a major role in the healing of grafted seedlings. This status is related to both photosynthetic activity, and to the available amount of carbohydrates in the scion. Although carbohydrate status has been earlier associated to light quality, the respective effects on seedling growth and vigor have received limited attention [11,12].
De novo carbohydrate synthesis through photosynthesis is vital for the grafting success [13]. During healing and acclimatization, environmental conditions may exert a considerable impact on the reactions involved in the photosynthetic process [14]. For instance, the photosynthesis of grafted seedlings may be interrupted by mechanical stress, water deficit in scion, or these two combined. On top of this, seedlings are very sensitive to light stress at this stage. Chlorophyll fluorescence is often utilized for non-invasive evaluation of electron transport system efficiency. Moreover, polyphasic chlorophyll fluorescence induction curves can be employed to investigate the fate of absorbed light energy and knowledge about the structure and function of the photosynthetic apparatus [15,16].
The present investigation was done to gain insight in the effect of light quality during healing and acclimatization of grafted watermelon seedlings on survival, morphology, carbohydrate status, and photosynthetic pigment content in different leaf types. In addition, chlorophyll fluorescence parameters were quantified as well as total biomass to assess the impact of light spectrum on photosynthetic performance. All these parameters are directly associated to the seedling quality. Thus, selecting the most appropriate light quality for healing and acclimatization would pave the way for the expansion of vertical-pattern systems, which rely exclusively on artificial light sources [17,18].
2. Results
2.1. Survival, Growth and Morphology of Grafted Seedlings
Following grafting and 2 days of light deprivation, seedlings were exposed to either D or different light quality regimes for 12 days prior to sampling. Survival ratio was 26% following exposure to D, whereas all seedlings survived under light irrespective of spectral quality (Table 1). Shoot dry weight, root dry weight, and leaf area per plant were highly suppressed under D, as compared to seedlings exposed to light. Shoot dry weight was not significantly different among seedlings exposed to different light spectra, while root dry weight was significantly lower in RB-exposed seedlings. The treatments with enhanced proportion of blue (i.e., B and W) resulted in taller scions. Among light treatments, leaf area per plant was the lowest in R-exposed seedlings, mainly owing to smaller individual leaf area rather than a lower number of leaves (Table 1).
Table 1.
Growth and morphology of grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Means within a column followed by the same letters are not significantly different at p ≤ 0.05 according to Duncan’s multiple range test.
| Light Regime | Survival Ratio (%) | Rootstock Length (cm) | Scion Length (cm) | Scion Stem Diameter (cm) | Leaf Number (plant−1) | Leaf Area (cm2 leaf−1) | Leaf Area (cm2 plant−1) | Plant Dry Weight (g) | Shoot Dry Weight (g) | Specific Leaf Area (SLA; cm2 g−1) | Leaf Mass Ratio (LMR; g g−1) | Root Length (cm) | Root Dry Weight (g) | Shoot to Root Ratio (g/g) | Dickson’s Quality Index (DQI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | 26.3 b | 7.51 | 2.45 b | 0.31 | 2.3 c | 5.01 d | 26.4 d | 0.174 b | 0.165 b | 247 c | 0.38 a,b | 4.14 b | 0.009 c | 19.17 a | 0.006 b |
| B | 100 a | 5.87 | 4.15 a | 0.33 | 5.0 b | 8.24 b | 65.9 b | 0.290 a | 0.265 a | 417 b | 0.35 c | 6.38 a | 0.025 a | 10.53 b | 0.015 a |
| W | 100 a | 6.05 | 4.10 a | 0.38 | 5.0 b | 10.11 a | 80.8 a | 0.251 a | 0.230 a | 540 a | 0.37 b | 6.33 a | 0.021 a | 11.02 b | 0.013 a |
| RB | 100 a | 6.27 | 2.42 b | 0.40 | 6.0 a | 8.15 b | 73.4 a,b | 0.282 a | 0.270 a | 411 b | 0.39 a | 4.24 b | 0.012 b | 23.17 a | 0.013 a |
| R | 100 a | 6.38 | 2.25 b | 0.38 | 5.3 b | 6.28 c | 52.2 c | 0.265 a | 0.256 a | 376 b | 0.34 c | 6.72 a | 0.024 a | 10.16 b | 0.016 a |
| p | <0.0001 | 0.0963 | 0.0075 | 0.23 | <0.0001 | <0.0001 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | 0.0065 | <0.0001 | <0.0001 | 0.0009 |
W-exposed seedlings had the highest SLA, while the lowest one was noted under D. B-, RB- and R-exposed seedlings showed intermediate SLA values. D- and RB-exposed seedlings had the highest LMR, while the lowest one was noted in B- and R-exposed seedlings. Shoot-to-root ratio of D- and RB-exposed seedlings was significantly lower as compared to seedlings exposed to the other light regimes. By using the DQI, as a quality measure, the seedlings exposed to D were of inferior quality, as compared to the ones exposed to light irrespective of quality (Table 1).
Leaf morphology was also affected by light spectra (Figure 1). Downward curling of leaf margins was only observed in R-exposed seedlings, whereas flattened leaves were apparent in B-exposed seedlings (Figure 1II).
Figure 1.

Images of grafted watermelon seedlings following 12 days of exposure to the respective light environment (from left to right: darkness, blue, white, red and blue, red; see spectrum in Supplementary Figure S3). Images include cultivation (I); where blue as well as red are magnified in (II), shoot (III), root (IV), and leaf (V) levels.
2.2. Leaf Soluble Carbohydrates
The lowest concentration of soluble carbohydrates was noted in seedlings under D (Figure 2). Among light treatments, the treatments with enhanced proportion of blue (i.e., B and W) had the lowest soluble carbohydrate concentration, while the highest concentration was noted in R-exposed seedling (Figure 2).
Figure 2.
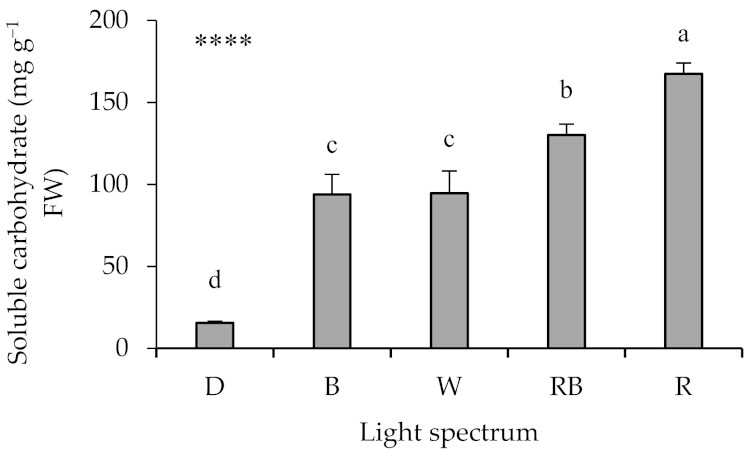
Total leaf carbohydrate content in grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.0001 probability level is indicated by ****.
2.3. Leaf Photosynthetic Pigments
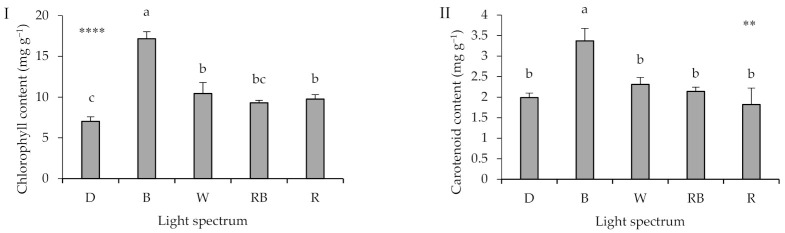
Optimum greenness in different parts of grafted seedlings is indicative of proper healing, and is therefore, vital for their marketability. Under this background, pigments concentration was evaluated in rootstock cotyledon, scion cotyledon and scion leaf (Figure 3). The effect of light regime during healing and acclimatization on pigment concentration varied among the tissue under study. In rootstock cotyledon, B-exposed seedlings had increased chlorophyll and carotenoid contents (Figure 3I,II). In both scion cotyledon and leaf, R- and D-exposed seedlings had the lowest chlorophyll content (Figure 3III,V). In scion cotyledons and leaves, carotenoid content was not affected by light environment (Figure 3IV). In both scion cotyledons and leaves, D stimulated carotenoid content, as compared to the light treatments (Figure 3VI).
Figure 3.

Chlorophyll and carotenoid contents of rootstock cotyledon (I, II, respectively), scion cotyledon (III, IV, respectively), and scion leaf (V, VI, respectively) in grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.05, 0.01, 0.001 and 0.0001 probability levels are indicated by *, **, *** and ****, respectively.
2.4. Chlorophyll Fluorescence Imaging
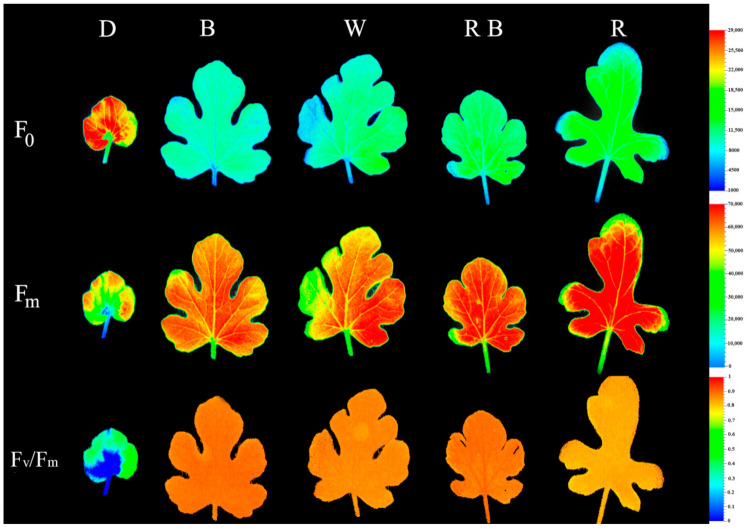
The effect of light environment during healing and acclimatization on overall photosynthetic functionality was assessed by evaluating the spatial pattern of fluorescence emission through pseudo-color images of F0, Fm, and Fv/Fm (Figure 4; equations in Table 2). Exposure to D led to the highest F0 and the lowest Fv/Fm, as compared to the other treatments. Among light treatments, R-exposed seedlings had the highest F0, Fm and the lowest Fv/Fm. B-exposed seedlings achieved the highest Fv/Fm value.
Figure 4.
Pseudo-color images of F0, Fm, and Fv/Fm (equations in Table 2) exhibited by leaves sampled from grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Results from all nine plants were similar, representative images are shown.
Table 2.
Abbreviations, definitions, and formulas of the O–J–I–P parameters assessed in the current study.
| Basic Parameters | ||
|---|---|---|
| F0 | Minimum fluorescence, when all PSII reaction centers (RCs) are open (O-step of OJIP transient) | F50µs |
| FJ | Fluorescence intensity at the J-step (2 ms) of OJIP | F2 ms |
| FI | Fluorescence intensity at the I-step (30 ms) of OJIP | F30ms |
| Fluorescence Parameters | ||
| Fm | Maximum fluorescence, when all PSII RCs are closed (P-step of OJIP transient) | F1s = Fp |
| Fv | Variable fluorescence of the dark-adapted leaf | Fm − F0 |
| VJ | Relative variable fluorescence at time 2 ms (J-step) after start of actinic light pulse | (FJ − F0)/(Fm − F0) |
| VI | Relative variable fluorescence at time 30 ms (I-step) after start of actinic light pulse | (F30ms − F0)/(Fm − F0) |
| Fv/Fm | Maximal quantum yield of PSII photochemistry | 1 − (F0/Fm) = (Fm − F0)/Fm = φP0 = TR0/ABS |
| Quantum Yields and Efficiencies/Probabilities | ||
| φE0 | The quantum yield of electron transport | [1 − (F0/Fm)](1 − VJ) = ET0/ABS |
| φD0 | Quantum yield of energy dissipation | F0/Fm |
| φPAV | Average quantum yield for primary photochemistry | φP0 (1 − VJ) = φP0 (SM/tFM) |
| Specific Energy Fluxes (Per QA Reducing PSII RC) | ||
| ABS/RC | The specific energy fluxes per RC for energy absorption | M0 (1/VJ)(1/φP0) |
| TR0/RC | Trapped energy flux (leading to QA reduction) per RC | M0 (1/VJ) |
| ET0/RC | Electron transport flux (further than QA−) per RC | M0 (1/VJ)(1 − VJ) |
| DI0/RC | Dissipated energy flux | (ABS/RC) − (TR0 /RC) |
| Performance Indexes (Products of Terms Expressing Partial Potentials at Steps of Energy Bifurcations) | ||
| PIABS | Performance index for the photochemical activity | [(γRC/1 − γRC) (φP0 /1 − φP0) (ψE0 /1 − ψE0)] |
2.5. Polyphasic Chlorophyll Fluorescence Transient (OJIP) Evaluation
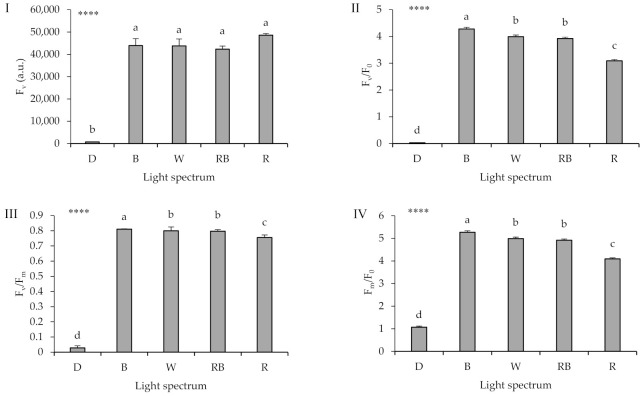
Transient chlorophyll fluorescence analysis was recorded in leaves expanded under different light regimes. Minor changes were detected in the OJIP steps of B-, W-, and RB-exposed seedlings, whereas striking changes were observed under either D or R regimes (Supplementary Figure S1). Under D, fluorescence intensity of I and P steps was the lowest as compared to the other treatments, while fluorescence intensity of the O step was the highest. Under R light, all steps of the OJIP graph (F0, FJ, FI, and Fm) were increased as compared to the other light quality regimes.
Seedling exposure to D led to a dramatic decrease in the values of Fv, Fv/F0, Fv/Fm, and Fm/F0 (Figure 5; equations and explanations in Table 2). Among light treatments, B led to the highest Fv/F0, Fv/Fm, and Fm/F0 values, while R light led to the lowest values in these parameters.
Figure 5.
Chlorophyll a fluorescence of the OJIP-test including Fv (I), Fv/F0 (II), Fv/Fm (III) and Fm/F0 (IV); equations and explanations in Table 2) from leaves sampled from grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.0001 probability level is indicated by ****.
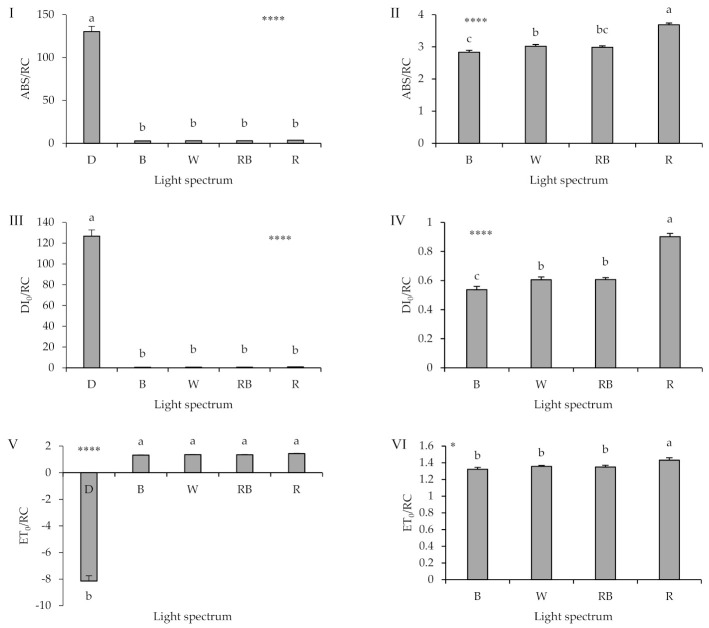
A striking difference in the parameters related to specific energy fluxes per reaction center (RC) (DI0/RC, ABS/RC, and ET0/RC; equations and explanations in Table 2) was noted between seedlings exposed to either D or light irrespective of quality (Figure 6). DI0/RC and ABS/RC increased by up to two orders under D. ET0/RC was −8.13 under D, while the respective values in light treatments were between 1.32 and 1.43.
Figure 6.
Specific energy fluxes per reaction center (RC) for energy absorption (ABS/RC; I, II), dissipated energy flux (DI0/RC; III, IV), electron transport flux (ET0/RC; V, VI); equations and explanations in Table 2) from the fluorescence transient exhibited by leaves sampled from grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. The Panels II, IV, and VI (on the right) represent the same data as the Panels I, III, and V (on the left), besides the darkness treatment which was excluded. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.05, and 0.0001 probability levels is indicated by * and ****, respectively.
By applying statistics to all treatments (i.e., including D), no significant differences in DI0/RC, ABS/RC, and ET0/RC values were noted among seedlings exposed to B, W, RB, and R light (Figure 6I,III,V). Given the considerable difference in the values noted under D, these values were removed, and the statistical analysis was repeated (i.e., excluding D). This new analysis indicated that seedling exposure to R light led to increased DI0/RC, ABS/RC, and ET0/RC values, as compared to the other light treatments (Figure 6II,IV,VI). In addition, it was noted that seedling exposure to B light resulted in a decreased DI0/RC and ABS/RC, though the latter was not significantly different when compared to RB light.
TR0/RC was the highest in seedlings healed and acclimatized under D, followed by those exposed to R (Figure 7). No significant difference was noted in TR0/RC value between seedlings exposed to B, W, or RB light.
Figure 7.
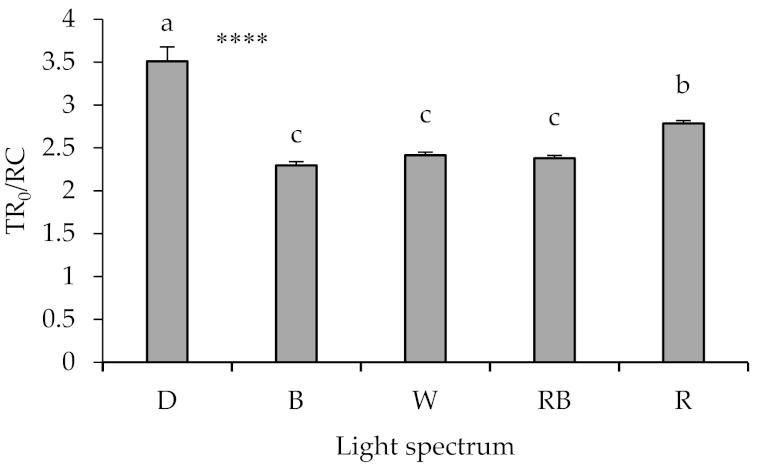
Specific energy fluxes per reaction center (RC) for trapped energy flux (TR0/RC; equations and explanations in Table 1), from the fluorescence transient exhibited by leaves sampled from grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.0001 probability level is indicated by ****.
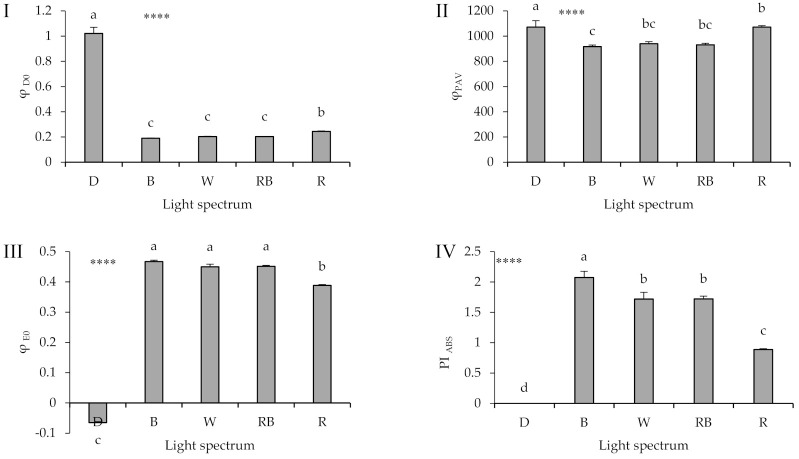
A striking difference between healing and acclimatization under either D or light was also noted in φD0 and φE0 values (Figure 8I,III; equations and explanations in Table 2). A difference between exposure to D or light treatments was also evident in φPAV parameter (equation in Table 2), though the magnitude of the effect was considerably less (Figure 8II). Among the light treatments, R-exposed seedlings showed increased φD0 value, in combination with decreased φE0 value.
Figure 8.
Quantum yield of energy dissipation (φD0; I), quantum yield for primary photochemistry (φPAV; II), quantum yield of electron transport (φE0; III), performance index in light absorption basis (PIABS; IV); equations and explanations in Table 2) from the fluorescence transient exhibited by leaves sampled from grafted watermelon seedlings exposed for 12 days to either darkness (D) or different light quality regimes [blue (B), white (W), red and blue (RB), as well as red (R); see spectrum in Supplementary Figure S3]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. Nine plants per treatment were assessed. Different letters indicate that values are significantly different at p < 0.01 according to Duncan’s multiple range tests. Bars represent SEM. Significance at the 0.0001 probability level is indicated by ****.
The PIABS (equation and explanation in Table 2) was 0 in the seedlings that were exposed to D (Figure 8IV). Among the light treatments, B-exposed seedlings showed the largest PIABS value, while R-exposed plants showed the lowest one.
To facilitate the comparison of light quality regimes (i.e., B, W, RB, and R, thus excluding D), the parameters (values relative to W treatment) of the OJIP test were plotted in a spider plot (Supplementary Figure S2(II)). Light spectra caused substantial changes in the fate of the absorbed light by PSII. Seedling exposure to light containing B (i.e., B, W, and RB treatments) led to an enhanced electron transport flow, whereas monochromatic R caused an adverse effect on the photosynthetic functionality (Supplementary Figure S2(II)). Seedlings exposed to RB were comparable to the ones under the W regime, since the parameters related to fluorescence transient induction showed similar trends.
Figure 9 shows the energy pipeline model of watermelon seedlings exposed to different lighting environments, regrouping fluctuations of the specific energy parameters (i.e.; ABS/RC, TR0/RC, DI0/RC and ET0/RC) and the average antenna size per one active reaction center. The pale thylakoids are illustrated as heat sinks that could not absorb the light energy in the same way as active RCs do. These heat sinks are not able to store and transfer the excitation energy as redox energy in the electron transport chain; thereby, dissipating their total excitation energy as heat which is called heat-sink centers or silent centers. The greenness of the leaves demonstrate their total chlorophyll content. It is shown that the D-exposed seedlings (Figure 9I) had much less chlorophyll content accompanied by much lower electron transport, while, other seedlings especially those exposed to B-contained spectra contained more chlorophyll that was accompanied by high electron transport.
Figure 9.
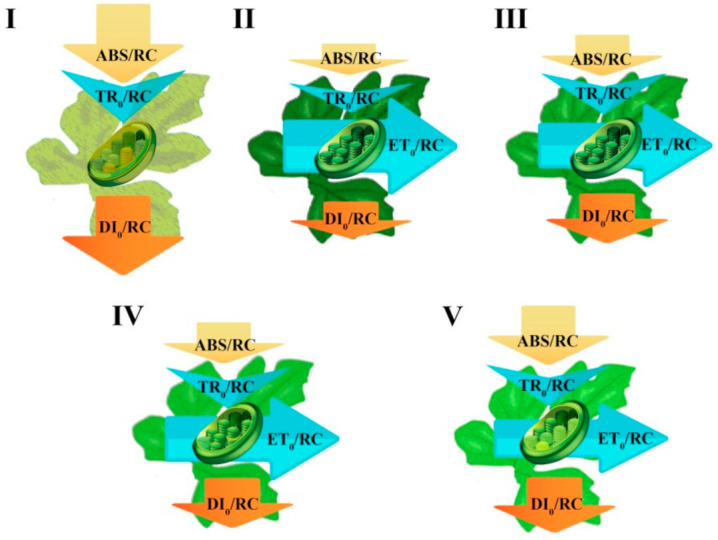
Energy pipeline models of grafted watermelon seedlings exposed for 12 days to either darkness (D; I) or different light quality regimes [blue (B; II), white (W; III), red and blue (RB; IV), as well as red (R; V); see spectrum and parameters’ equations in Supplementary Figure S3 and Table 2, respectively]. Photosynthetic photon flux density was 20 ± 1 µmol m−2 s−1 during the first 2 days, and 50 ± 1 µmol m−2 s−1 during the following days. The relative magnitude of each activity or fluctuation is shown by the size of the corresponding arrow. The intensity of green color of the leaves and chloroplasts illustrates their overall health functionality.
3. Discussion
The effect of light quality during healing and acclimatization was evaluated by examining a wide range of quality attributes in grafted watermelon seedlings.
3.1. Light during Healing and Acclimatization Improves Survival Ratio of the Grafted Seedlings
The role of presence or absence of light and its quality during healing (~0–7 days) and acclimatization (~8–14 days) on determining growth and vigor of grafted seedlings was investigated. Grafted seedling survival ratio was reduced to 26% under D, whereas all seedlings survived in the presence of light irrespective of its quality (Table 1). Therefore, light is essential for seedling survival during healing and acclimatization. Enhanced mortality ratio under D has also been earlier documented in tomato grafted seedling [5,19]. In other taxa, light during healing has been shown to stimulate callus induction [20], vascular connections’ formation [21] and scion-to-rootstock vascular system functioning (thus restoring water flow) [22]. The D-induced impairment of callus formation generally results in leaf shedding, and decreasing grafted seedlings’ survival [5]. The seedlings that survived after the 12-day period in D also showed inferior shoot development (indicated by both shoot dry weight and leaf number) and considerably weaker root system (indicated by both root length and dry weight), as compared to the seedlings exposed to light (Table 1). Since carbon assimilation is absent under light deprivation, plants survive by consuming the carbohydrates present in the scion [23]. Prolonged exposure to D leads to a rapid exhaustion of transitory starch reserves [24], and plants sustain metabolism by consumption of lipids and amino acids [25,26,27]. Prolonged light deprivation also disrupts developmental, transcriptional and hormonal changes, which are vital for growth [28]. Overall, the reduced (above and below ground) growth of the D-exposed seedlings in combination with their inferior quality set them as not marketable.
3.2. Morphology and Growth of the Grafted Seedlings Depends on the Light Spectrum during Healing and Acclimatization
Shoot dry weight and the DQI were not significantly affected by light quality during healing and acclimatization (Table 1). By examining other traits, light regime-induced differences in grafted seedling quality were clearly evident. Leaf area is an important indicator of seedling quality, and thus of marketability. Among light treatments, W-exposed seedlings had the largest leaf area as compared to seedlings exposed to the remaining light regimes, besides RB light. By contrast, R-exposed seedlings had the lowest leaf area. The difference in leaf area between W- and R-exposed seedlings was attributed to the area of individual leaves rather than their number. The increased SLA (thus thinner leaves) of W-exposed seedlings contributed to the enhanced area of individual leaves [5]. Although the reduced leaf area of R-exposed seedlings did not compromise shoot and root dry weight at this stage, it may slow down field establishment. Therefore, light quality during healing and acclimatization imposed considerable variation in seedling quality as reflected by leaf area.
LMR was affected by both presence of light and its quality; increased under D and RB and decreased under B and R, while W-exposed seedlings showed an intermediate value. The high value of LMR under D can be attributed to both decreased shoot (mostly scion) and root dry weights, while in RB-exposed seedlings the increase in LMR is mainly attributed to just decrease in root dry weight. Since differences of shoot dry weight among light treatments were not significant, the lower LMR of B- and R-exposed seedlings is the result of their increased root dry weight.
A high proportion of B in the growth spectrum (i.e., B and W) led to increased scion length as compared to the other light treatments, while scion stem diameter remained unaffected. These findings are in accordance with previous studies showing that B light generally promotes stem elongation as compared to monochromatic R light in the seedling stage [29,30,31], though in subsequent growth stages the opposite trend is often apparent [32]. In the seedling stage, the B-induced enhancement of stem elongation has been associated with increased accumulation of bioactive gibberellins, as compared to monochromatic R light [33]. The increase in seedling length without a reduction in stem diameter under the light regimes with a high proportion of B (i.e., B and W) is considered as a positive factor, enhancing seedling quality.
Root length and dry weight were significantly lower in RB-exposed seedlings, as compared to the other light quality treatments. The reduced root dry weight of these seedlings also led to increased shoot-to-root ratio. A well-developed root system and an optimum shoot-to-root ratio have been earlier suggested to be critical for successful establishment in the field [7]. Therefore, the reduced root development of RB-exposed seedlings may impose maintaining them for longer periods in the nurseries (thus increased associated costs), until roots spread over and seedlings are ready for transferring to the field. In this regard, the RB growth spectrum appears to be less advantageous for seedling healing and acclimatization, as compared to the remaining light treatments.
Regimes with increased proportion of B in the light spectrum stimulate stomatal conductance, resulting in increased gas exchange [34]. Although increased transpiration during the first days of healing (~0–4 days) is generally regarded as a negative factor and has been related to scion wilting [14], it was here counteracted by both darkness (0–2 days) and using elevated relative air humidity conditions (≥95% RH) [35,36]. Following healing, relative air humidity was gradually decreased, and the increased stomatal conductance noted under enhanced B conditions is expected to be a positive factor contributing to growth during the remaining healing and acclimatization period, through facilitating carbon dioxide intake.
Although the importance of light quality during healing and acclimatization has been earlier highlighted, different regimes have been proposed to be optimal. For instance, grafted tomato seedling quality was improved through monochromatic R light during healing and acclimatization [5]. Instead, in grafted pepper seedlings, growth was promoted by application of both B and R LEDs [6]. A combination of R and B lights was also suggested to be optimum in terms of growth in grafted watermelon seedlings [3], though different (rootstock and scion) cultivars were used as compared to our investigation. Our results and those of earlier studies [3,5,6], clearly suggest that the effects of the light quality on the grafted seedling growth appear to be not only species- but even cultivar- dependent.
Monochromatic R light during healing and acclimatization induced downward curling of leaf margins (the so-called leaf epinasty; Figure 1II), whereas monochromatic B light led to flattened leaves. Earlier studies attributed this variation in lamina curvature to differences in the epidermal cell extension among the two leaf sides, which are driven by the imbalance of metatopoline and auxin distribution across them [37,38,39]. This imbalance can be prevented by including a very low level (0.1 µmol m−2 s−1) of B in the spectrum [40,41], which promotes epidermal cell elongation on the abaxial leaf side [42]. The monochromatic R light-induced downward curling of leaf margins has been related to the reduced light interception [43]. On top of this, monochromatic R light during healing and acclimatization compromises the visual quality of the produced seedlings, by inducing a peculiar leaf form, and in this way is expected to decrease their marketability.
3.3. Light Spectra during Healing and Acclimatization Affects Pigment Content in Different Parts of the Seedlings
Chlorophyll concentration in different parts of the grafted seedlings is an important criterion of quality [2]. The light regime effect on photosynthetic pigment content, varied depending on the leaf type and location. D decreased rootstock cotyledon chlorophyll content, and increased both scion cotyledon and leaf carotenoid content. Earlier studies indicate that light deprivation initially stimulates chloroplast ultrastructure alterations (e.g., thylakoids become swollen), and eventually leads to chlorophyll degradation. The monochromatic B light effect was only apparent in rootstock cotyledon (higher chlorophyll and carotenoid content), whereas the effect of monochromatic R light was noted in both scion cotyledon and leaf (decreased chlorophyll content). The adverse effect of monochromatic R light on chlorophyll content is possibly related to the increased carbohydrate accumulation, since it has been associated with chloroplast deformation and depletion of chlorophyll [44,45]. Additionally, chlorophyll biosynthesis is negatively affected by B light deficiency in several taxa including spinach [46], rose [47], cucumber [29], lettuce [48] and basil [49]. Therefore, seedlings healed and acclimatized under either D or monochromatic R light are expected to have low marketability owing to reduced greening. By contrast, the grafted seedlings under monochromatic B light were found to be superior as compared to the ones exposed to either RB or W regime.
3.4. Photosynthetic Functionality Is Affected by the Presence of Light and Its Spectrum during Healing and Acclimatization
During healing and acclimatization, functional photosynthesis is essential for the overall quality of grafted seedlings, determining their marketability. The functionality of photosynthetic apparatus was assessed by both Fv/Fm imaging and OJIP test. Both methods pointed out a strong negative effect of D on photosynthetic performance. The decrease in PSII activity, owing to D exposure, has been correlated to chlorophyll degradation and ultrastructure alteration [50]. Therefore, D-exposed seedlings not only have lower chlorophyll content but also the remaining chlorophyll could facilitate photosynthesis in a less efficient manner.
Healing and acclimatization under D also led to an increase in the parameters related to the energy dissipation in PSII [51], such as DI0/RC and ΦD0 (Figure 6III and Supplementary Figure S2(I)). DI0/RC is related to partial deactivation of PSII reaction centers, and is generally increased when these centers cannot transfer energy upstream of PSII owing to thylakoid membrane damage. A high rate of DI0/RC as compared to the corresponding ET0/RC value is most probably associated with the reduced Fv/Fm value and a higher chance of photoinhibition occurrence. This is because a reduction in Fv/Fm value generally occurs when the PSII function and structure are damaged by stress, causing most of the light energy absorbed to be dissipated from the PSII reaction center. Following 14 days exposure to D, the light energy absorbed by one active reaction center (ABS/RC) was dramatically higher. This striking increase may be the result of either PSII response centers’ inactivation or receptors’ size increase. Additionally, the energy used to reduce QA by the RC unit of PSII (TR0/RC) was the highest in grafted seedlings under D. The effect of D on overall PSII photosynthetic activity is also clearly manifested by examining PIABS (Figure 8IV), which is the most sensitive OJIP-test parameter. A decrease in PIABS value is generally associated with a lower capacity for development of the trans-thylakoid proton gradient. The decrease in PIABS also indicates that the system structure, potential activity of PSII, damage/repair ratio of protein D1 of PSII may be damaged or unable to completely advance under certain light conditions. Short (24 h) exposure to D already caused a drastic (64%) Fv/Fm decrease in Asterochloris erici [52]. Prolonged light deprivation has been earlier associated with attenuated activity of PSI, PSII, cytb6f and ATP synthase in Arabidopsis thaliana [53]. The high F0 intensity of dark-developed chloroplasts is attributed to an enhanced fraction of closed PSII core, which disturbs PSII activity and electron transport [54,55]. Among light treatments, monochromatic R light significantly down-regulated the biophysical parameters related to the PSII efficiency such as Fv/F0, Fm/F0, and Fv/Fm, which has been associated to either damage or long-term down-regulation of PSII owing to stress [38]. This negative effect of monochromatic R light has also been noted in other taxa (the so-called red light syndrome; [38,39,56], though species not undergoing any adverse effect have also been referred [57]. Although the processes underlying the attenuated PSII efficiency in R-exposed plants have not been clarified, impaired PSII core proteins’ synthesis, D1 protein damage and PSII reaction centers’ inactivation have been implicated as contributing factors [38,49].
Seedling growth under monochromatic R light led to an effect on the parameters related to the energy dissipation in PSII such as DI0/RC and ΦD0, which was similar to D in terms of direction, but at smaller magnitude. Similar results have been reported for three pot plants exposed to monochromatic R light [58]. The increased ABS/RC under monochromatic R light has been related to the reduction in the number of active PSII reactive centers, which might serve as a defense mechanism to alleviate the light stress pressure on the photosynthetic system [59]. The adverse effect of monochromatic R light on overall PSII photosynthetic activity is also evident by examining the PIABS parameter.
The highest soluble carbohydrate content was noted in R-exposed seedlings. An intermediate one was noted under RB light, while the lowest was noted in the regimes with a high proportion of B (i.e., B and W). The same trend has been earlier noted in lettuce leaves [11]. The monochromatic R light effect on photosynthetic product accumulation has been validated in several taxa. Since both auxin biosynthesis and transport are affected by carbohydrate content, the latter may underlie the noted leaf epinasty under R light [60]. Although carbohydrate synthesis is reduced owing to disturbed photosynthetic apparatus, the increased carbohydrate content in R-exposed seedlings is possibly the result of reduced translocation out of the leaves [61]. This increased carbohydrate accumulation has been associated with both down-regulation of photosynthesis [62] and feedback inhibition on sucrose synthesis and sugar phosphates’ cytosol accumulation [63]. Since B light generally up-regulates the genes encoding key enzymes in the Calvin-Benson cycle, feedback control is ruled out in the inhibition of photosynthesis under monochromatic B light [63,64].
3.5. High Proportion of B (i.e., B, W, RB) in the Overall Spectrum Up-Regulates Electron Transport, Leading to Improved Photosynthetic Functionality
Several parameters of both employed methods (Fv/Fm imaging and OJIP test) pointed out a superiority of seedlings that were exposed to B light. Among light treatments, B light led to the highest values in Fv/F0, Fv/Fm, as well as Fm/F0, and the lowest value in DI0/RC. This enhancement following exposure to monochromatic B light is also reflected by the PIABS parameter. In these parameters (Fv/F0, Fv/Fm, Fm/F0, DI0/RC, and PIABS), no significant difference was noted between seedlings exposed to W or RB light. Therefore, B-exposed seedlings have not only elevated chlorophyll content (Figure 3I), but also chloroplasts are able to perform photosynthesis in a more efficient context, as compared to the other light quality regimes.
Treatments containing high proportion of B (i.e., B, W, RB) had a decreased energy absorption per active RC but overall increased electron transport, due to more active RCs (Figure 9). Dissipation occurs as heat, fluorescence and energy transfer to other systems. It is also influenced by the ratios of active/inactive RCs. ABS/RC in D-exposed seedlings increased mainly due to the impairment of the RCs. Among light-exposed seedlings, the same increase (but with lower magnitude) happened for R-exposed seedlings. D-exposed seedlings also revealed an increased ratio of total dissipation to the amount of active RCs (DI0/RC) mainly due to the high dissipation of the inactive RCs.
Although the LED efficiency is determined by several factors, a photon at shorter wavelengths (i.e., B) fundamentally carries more energy, as compared to one at longer wavelengths. This increased energy per photon in the B region impedes the B LEDs’ efficiency. Another downside of applying monochromatic B light in practice is the difficulty of detecting both nutrient deficiency and disease symptoms. That said, W light appears to be the best alternative. Although most of the assessed traits were similar between exposure to W or RB light, W light better stimulated scion length and root growth.
4. Materials and Methods
4.1. Plant Material and Exposure Conditions
A commercial watermelon cultivar [Citrullus lanatus (Thunb.) Matsum. and Nakai. cv. Crimson] and a commercial rootstock cultivar [Cucurbita maxima Duchesne ex Poir. × Cucurbita moschata (Duchesne ex Lam.) cv. Marvel] were employed. This rootstock was selected as it is frequently used in commercial practice in Iran due to its compatibility with melon, and its tolerance to low temperature and soilborne pathogens. Graded seeds of scion and rootstock were soaked in distilled water at room temperature (25 °C), a day prior to sowing. To produce seedlings with approximately the same size and diameter of hypocotyls, the scion seeds were sown 7 days earlier than the rootstock seeds. This was done in 50-cell trays filled with a commercial growing mixture containing peatmoss, perlite and cocopeat in a ratio of 7:2:1 (v/v/v). The trays were placed in a glass-covered greenhouse (Karaj, 35°51′21’’ N), where day/night temperature was controlled to 26/18 °C, and photoperiod to 12 h.
When the scion and rootstock cotyledons had fully opened, one-cotyledon grafting was conducted. In rootstock, one cotyledon along with the visible growing point were removed (45° angled cut) by using a semi-automatic grafting robot (model type GR 300/3; Atlantic Man s.r.l., Castelnovo di Sotto, Italy). Thereafter, the scion (bearing two cotyledons) was placed on the rootstock hypocotyl by using a plastic clip. In this step, the rootstock roots were removed to prevent water accumulation in the graft union, owing to enhanced (rootstock) root pressure.
Grafted seedlings were then transferred to a specialized healing room equipped with an automatically-controlled air conditioning system. These were initially exposed to darkness (D) for 2 days to prevent leaf dehydration. Then, the seedlings were placed in five healing and acclimation cabinets (LED-equipped vertical systems with three floors; Supplementary Figure S3), each under different light regime, including B (peak at 460 nm), white [W; 35% blue (400–500 nm), 49% intermediate (500–600 nm), and 16% red (600–700 nm)], R (68%) and B (RB), as well as R (peak at 660 nm; Supplementary Figure S3). Light was provided by LED modules (Iraneon Co, Birjand, South Khorasan, Iran), and spectra were monitored using a Sekonic light meter (Sekonic C-7000, Tokyo, Japan). In all four healing and acclimation cabinets with illumination, light intensity was adjusted to 20 ± 1 µmol m−2 s−1 photosynthetic photon flux density (PPFD) at the top of the plant canopy for 2 days, and then to 50 ± 2 µmol m−2 s−1 PPFD for the next 10 days. All plants were exposed to the same controlled conditions, i.e., 16 h photoperiod, and 25/20 °C day/ night air temperature. Relative air humidity was gradually decreased, being 95–98% (Days 0–2), 85–90% (Days 3–4), 80–85% (Days 5–7), and 60–70% (Days 8–14). Growth-media moisture was maintained at or near maximum water holding capacity by regular watering.
Nine plants were sampled (three per vertical system floor) per light regime. To minimize border effects, these were surrounded by border plants (periphery of the tray) that were not sampled. After 14 days in the healing and acclimation cabinets, measurements were performed. Sampled leaves were young, fully-expanded, and grown under direct light. In all cases, the time between sampling and the start of the evaluation did not exceed 15 min.
4.2. Survival Ratio, Plant Biomass and Leaf Morphology
The effect of light regime on seedling survival, growth and morphology was assessed. The survived seedlings relative to the ones that were initially placed (the so-called survival ratio) was calculated. Evaluations also included rootstock length (from the root-to-shoot junction to the graft union), scion length (from the graft union to the apical meristem), scion diameter (1 cm above the graft union), number of leaves, leaf area, leaf and aboveground dry masses. For measuring dry weight, samples were placed in a forced-air drying oven for 72 h at 80 °C [65]. For leaf area assessment, leaves were scanned (HP Scanjet G4010, Palo Alto, CA, USA) and then evaluated by using the Digimizer software (version 4.1.1.0, MedCalc Software, Ostend, Belgium). Before scanning, curled leaves (noted under monochromatic R light) were flattened out on a white paper. In this way, the leaf surface curvature-induced reduction in projected area was minimized. Thereafter, the specific leaf area (SLA; leaf area/leaf dry mass), and leaf mass ratio (LMR; leaf dry mass/plant dry mass) were calculated [66]. Following removal of the substrate from the roots via gentle washing, root length and dry mass were also measured.
was also calculated. This index is commonly employed for the evaluation of tree seedlings’ quality, and it has also been recently introduced for assessing the quality of grafted vegetable seedlings [2].
Prior to biomass evaluation, plant images were also obtained by using a digital camera (Canon EOS M2; Canon Inc., Tokyo, Japan). The captured images allowed the evaluation of plant morphology. The plants were manually moved to the image capture station. The imaging station included top and side lighting units (fluorescent tubes; Pars Shahab Lamp Co., Tehran, Iran). A side view perspective (perpendicular to the shoot axis) was employed [67]. The camera-to-plant distance was maintained at 1 m. One image (RGB) was obtained per plant (including shoot, one representative leaf, and root), and nine plants (three per vertical system floor) were imaged per treatment.
4.3. Determination of Soluble Carbohydrates
Leaf samples were ground in liquid nitrogen, and 0.2 g tissue was sampled and blended with 7 mL of 70% ethanol (w/v) for 5 min on ice and centrifuged (6700× g) for 10 min at 4 °C. After adding 200 mL of the supernatant to 1 mL of an anthrone solution (0.5 g anthrone, 250 mL 95% H2SO4, and 12.5 mL distilled water), the absorbance was spectrophotometrically (UV-1800, Shimadzu, Japan) recorded at 625 nm. Nine scion leaves (three per vertical system floor) were assessed per treatment. Replicate leaves were collected from separate plants.
4.4. Photosynthetic Pigments
The light regime effect on photosynthetic pigment (chlorophyll, carotenoids) content was assessed in rootstock cotyledon, scion cotyledon, and scion leaf. Leaf samples were processed immediately after collection. Following fine chopping, portions weighing 0.5 g were homogenized with the addition of 10 mL of 80% acetone. This primary acetone extract was then filtered, and the filtered extract was diluted by adding 2 mL of 80% acetone per mL of extract. Since chlorophyll is light sensitive, extraction took place in a dark room [68]. The obtained extract was subjected to reading on a spectrophotometer (Mapada UV-1800; Shanghai. Mapada Instruments Co., Ltd., Shanghai, China). Total chlorophyll and carotenoid contents were calculated [69]. Nine leaves (three per vertical system floor) were assessed per treatment. Replicate leaves were collected from separate plants.
4.5. Chlorophyll Fluorescence Imaging
As a sensitive indicator of plant photosynthetic performance, dark-adapted values of the maximum quantum yield of PSII (Fv/Fm; equation in Table 2) were recorded in leaves detached from plants of each light regime. Measurements were conducted on leaves by using a FluorCam FC 1000-H (Photon Systems Instruments, Drásov, Czech Republic). By turning LEDs off, the leaves were dark adapted (≥20 min) prior to evaluation. Then, Fv/Fm was evaluated by applying a saturated PPFD of 3900 µmol m−2 s−1 [17,70,71]. Nine scion leaves (three per vertical system floor) were assessed per treatment. Replicate leaves were collected from separate plants.
4.6. Polyphasic Chlorophyll Fluorescence Transient (OJIP) Evaluation
A polyphasic chlorophyll fluorescence induction curve (O–J–I–P-transient) was obtained in leaves attached to plants of each light regime. By employing the OJIP test, the shape changes of the OJIP transient are quantitatively translated to a set of parameters (equations and explanations in Table 2), which relate to the in vivo adaptive behavior of the photosynthetic apparatus (especially PSII) to the growth environment [70,72]. Measurements were conducted on attached leaves by using a PAR-fluorPen FP 100-MAX (Photon Systems Instruments, Drásov, Czech Republic) following dark adaptation (≥20 min). The employed light intensity (3900 μmol m−2 s−1 PPFD) was sufficient to generate maximal fluorescence for all light quality treatments.
Following dark adaptation, leaves exhibit a polyphasic chlorophyll fluorescence rise during the first second of illumination. The fluorescence transient, plotted on a logarithmic time scale, typically includes the following phases: O to J, J to I, and I to P. F0 represents the so-called “open” (O) state of the O–J–I–P-transient [73] measured at 50 µs. For non-stressed plants, the F0 value drastically differs, and thus a simple range cannot be reported. Yet, higher F0 values indicate exposure to more intense stress. F0 primarily originates from the light-harvesting antenna pigments [74,75]. Fj and FI originate from the inflections at 2 and 30 ms, respectively [76]. Fm, on the other hand, comes from the reduction–oxidation state of the primary quinone electron acceptor of PSII (QA). The highest Fv/Fm value is around 0.84 for horticultural plants, while values higher than 0.8 are often detected in healthy plants. φE0 is a metric of the quantum yield of the electron transport from QA- to plastoquinone (PQ), and drops under stress conditions [77]. Overall, decreasing values of Fv/F0, Fv/Fm, Fm/F0, φE0, and PIABS indicate stress in planta [75,76]. Nine scion leaves (three per vertical system floor) were assessed per treatment. Replicate leaves were collected using separate plants.
4.7. Energy Pipeline Model
To study the mechanisms of photosynthesis in each treatment, the so-called energy pipeline model has been developed and illustrated (Figure 9). Energy pipeline is a dynamic model in which the value of each energy flux, here, modified by the different imposed lighting conditions, which is expressed by the appropriately adjusted size of the corresponding arrow. In general, two types of models can be presented, one of which refers to the RC in the membrane and thus deals with the specific energy fluxes per RC and the other refers to the excited cross-section (CS) of a leaf, thereby dealing with the phenomenological energy fluxes per CS. In the present study, we developed the former one (i.e., per RC; membrane model). The membrane energy pipeline model includes also a demonstration of the average “antenna size”, which follows the value of the ABS/RC. This value expresses the total absorption of PSII antenna chlorophylls divided by the number of active (in the sense of QA reducing) reaction centers.
4.8. Statistical Analysis
The experiments were arranged in a completely randomized design. Data were analyzed using SAS software (v. 9.4, SAS Institute Inc., Cary, NC, USA). Mean separations were calculated using Duncan’s multiple range test at p ≤ 0.05.
5. Conclusions
The effect of light and its quality during healing and acclimatization of grafted watermelon seedling was assessed. Seedling survival was strongly decreased by D condition. The seedlings that survived showed inferior shoot development, weaker root system, reduced (rootstock cotyledon) chlorophyll content, and reduced photosynthetic apparatus functionality (assessed by both Fv/Fm imaging and OJIP test). Seedling survival was 100% under all light regimes irrespective of their quality. Shoot dry weight and the DQI were not illustrative in quantifying variation in grafted seedling quality among light quality treatments. Although W and RB light-produced seedlings were similar in the majority of the assessed traits, W light better promoted seedling scion length and root growth. RB-exposed seedlings had a less-developed root system, requiring more time to produce marketable plants. R-exposed seedlings had the smallest leaf area, and underwent leaf epinasty, which not only compromises visual quality, but also reduces light interception. Seedlings that were exposed to monochromatic R light showed reduced (scion cotyledon and leaf) chlorophyll content, limiting their subsequent marketability. The photosynthetic apparatus functionality was also suppressed in R-exposed seedlings, as compared to the remaining light treatments. B-exposed seedlings had advantageous shoot morphology (elongated scion, flattened leaves), the highest (rootstock cotyledon) chlorophyll content, and could facilitate photosynthesis in a more efficient manner.
Acknowledgments
We thank the laboratory staff for their contributions, continued diligence, and dedication to their craft. We also thank Saeid Eilkhani for his help during the healing and acclimatization period. The valuable comments of the editor and two anonymous reviewers are also greatly acknowledged.
Abbreviations
| B | blue |
| D | darkness |
| DQI | Dickson’s quality index |
| LMR | leaf mass ratio |
| O–J–I–P | polyphasic chlorophyll fluorescence induction curve |
| PPFD | photosynthetic photon flux density |
| PSII | Photosystem II |
| R | red |
| RB | red and blue |
| RC | reaction center |
| SLA | specific leaf area |
| W | white |
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/article/10.3390/ijms22158043/s1.
Author Contributions
The following authors conceived and designed the study—M.M.-N., R.S. and S.A.; designed and constructed the healing and acclimation cabinets, conducted the experiment, and analyzed data—M.M.-N.; supervised cabinet construction, plant material preparation, and grafting process—R.S.; authored the first draft—M.M.-N. and R.S.; providing critical amendments for reaching the current version—D.F. and S.A.; data interpretation, providing valuable insights, and supervising the research—S.A., G.T., E.J.W. and D.F.; writing—review and editing—K.Ż.-G. and H.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the financial support provided by the University of Tehran. The research was financed by the University of Warmia and Mazury in Olsztyn, Poland: Project No. 30.610.013.110, and financially supported by Minister of Science and Higher Education in the range of the program entitled ‘Regional Initiative of Excellence’ for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not public.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rouphael Y., Kyriacou M.C., Colla G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018;8:2255. doi: 10.3389/fpls.2017.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantis F., Koukounaras A., Siomos A., Menexes G., Dangitsis C., Kintzonidis D. Assessing quantitative criteria for characterization of quality categories for grafted watermelon seedlings. Horticulturae. 2019;5:16. doi: 10.3390/horticulturae5010016. [DOI] [Google Scholar]
- 3.Bantis F., Koukounaras A., Siomos A.S., Fotelli M.N., Kintzonidis D. Bichromatic red and blue LEDs during healing enhance the vegetative growth and quality of grafted watermelon seedlings. Sci. Hortic. 2020;261:109000. doi: 10.1016/j.scienta.2019.109000. [DOI] [Google Scholar]
- 4.Johnson S.J., Miles C.A. Effect of healing chamber design on the survival of grafted eggplant, tomato, and watermelon. Horttechnology. 2011;21:752–758. doi: 10.21273/HORTTECH.21.6.752. [DOI] [Google Scholar]
- 5.Vu N.T., Kim Y.S., Kang H.M., Kim I.S. Influence of short-term irradiation during pre- and post-grafting period on the graft-take ratio and quality of tomato seedlings. Hortic. Environ. Biotechnol. 2014;55:27–35. doi: 10.1007/s13580-014-0115-5. [DOI] [Google Scholar]
- 6.Jang Y., Mun B., Seo T., Lee J., Oh S., Chun C. Effects of light quality and intensity on the carbon dioxide exchange rate, growth, and morphogenesis of grafted pepper transplants during healing and acclimatization. Korean J. Hortic. Sci. Technol. 2013;31:14–23. doi: 10.7235/hort.2013.12127. [DOI] [Google Scholar]
- 7.Lee J.M., Colla G., Kubota C., Tsao S.J., Bie Z., Echevarria P.H., Morra L., Oda M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010;127:93–105. doi: 10.1016/j.scienta.2010.08.003. [DOI] [Google Scholar]
- 8.Melnyk C.W. Plant grafting: Insights into tissue regeneration. Regeneration. 2017;4:3–14. doi: 10.1002/reg2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melnyk C.W., Schuster C., Leyser O., Meyerowitz E.M. A developmental framework for graft formation and vascular reconnection in arabidopsis thaliana. Curr. Biol. 2015;25:1306–1318. doi: 10.1016/j.cub.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousef A.F., Ali M.M., Rizwan H.M., Ahmed M.A.A., Ali W.M., Kalaji H.M., Elsheery N., Wróbel J., Xu Y., Chen F. Effects of light spectrum on morphophysiological traits of grafted tomato seedlings. PLoS ONE. 2021;16:e0250210. doi: 10.1371/journal.pone.0250210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X.L., Wang L.C., Li T., Yang Q.C., Guo W.Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-43498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L.Y., Wang L.T., Ma J.H., Ma E.D., Li J.Y., Gong M. Effects of light quality on growth and development, photosynthetic characteristics and content of carbohydrates in tobacco (Nicotiana tabacum L.) plants. Photosynthetica. 2017;55:467–477. doi: 10.1007/s11099-016-0668-x. [DOI] [Google Scholar]
- 13.Dabirian S., Miles C.A. Increasing survival of splice-grafted watermelon seedlings using a sucrose application. HortScience. 2017;52:579–583. doi: 10.21273/HORTSCI11667-16. [DOI] [Google Scholar]
- 14.Dabirian S., Miles C.A. Antitranspirant application increases grafting success of watermelon. Horttechnology. 2017;27:494–501. doi: 10.21273/HORTTECH03739-17. [DOI] [Google Scholar]
- 15.Kalaji H.M., Jajoo A., Oukarroum A., Brestic M., Zivcak M., Samborska I.A., Cetner M.D., Łukasik I., Goltsev V., Ladle R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- 16.Zagorchev L., Atanasova A., Albanova I., Traianova A., Mladenov P., Kouzmanova M., Goltsev V., Kalaji H., Teofanova D. Functional Characterization of the Photosynthetic Machinery in Smicronix Galls on the Parasitic Plant Cuscuta campestris by JIP-Test. Cells. 2021;10:1399. doi: 10.3390/cells10061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Fanourakis D., Tsaniklidis G., Aliniaeifard S., Yang Q., Li T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2020;172:111376. doi: 10.1016/j.postharvbio.2020.111376. [DOI] [Google Scholar]
- 18.Zou J., Zhang Y., Zhang Y., Bian Z., Fanourakis D., Yang Q., Li T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019;257:108725. doi: 10.1016/j.scienta.2019.108725. [DOI] [Google Scholar]
- 19.Nobuoka T., Nishimoto T., Toi K. Wind and light promote graft-take and growth of grafted tomato seedlings. J. Jpn. Soc. Hortic. Sci. 2005;74:170–175. doi: 10.2503/jjshs.74.170. [DOI] [Google Scholar]
- 20.Huang L., Hong Y., Zhang X. Effect of light quality on calluses induction and differentiation of Capsicum annuum. J. Hunan Agric. Univ. 2009;35:615–617. [Google Scholar]
- 21.Afshari R.T., Angoshtari R., Kalantari S. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (’Brassica napus L.’) genotypes. Plant Omics J. 2011;4:60–67. [Google Scholar]
- 22.Lee K.M., Lim C.S., Muneer S., Jeong B.R. Functional vascular connections and light quality effects on tomato grafted unions. Sci. Hortic. 2016;201:306–317. doi: 10.1016/j.scienta.2016.02.013. [DOI] [Google Scholar]
- 23.Zavala J.A., Ravetta D.A. Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crop. Res. 2001;69:143–149. doi: 10.1016/S0378-4290(00)00136-2. [DOI] [Google Scholar]
- 24.Smith A.M., Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007;30:1126–1149. doi: 10.1111/j.1365-3040.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 25.Fan J., Yu L., Xu C. A central role for triacylglycerol in membrane lipid breakdown, fatty acid β -oxidation, and plant survival under extended darkness. Plant Physiol. 2017;174:1517–1530. doi: 10.1104/pp.17.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunz H.-H., Scharnewski M., Feussner K., Feussner I., Flügge U.-I., Fulda M., Gierth M. The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of arabidopsis during extended darkness. Plant Cell. 2009;21:2733–2749. doi: 10.1105/tpc.108.064857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usadel B., Bläsing O.E., Gibon Y., Poree F., Höhne M., Günter M., Trethewey R., Kamlage B., Poorter H., Stitt M. Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant Cell Environ. 2008;31:518–547. doi: 10.1111/j.1365-3040.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- 28.Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández R., Kubota C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016;121:66–74. doi: 10.1016/j.envexpbot.2015.04.001. [DOI] [Google Scholar]
- 30.Johnson R.E., Kong Y., Zheng Y. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2020;169:103898. doi: 10.1016/j.envexpbot.2019.103898. [DOI] [Google Scholar]
- 31.Kong Y., Schiestel K., Zheng Y. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019;157:58–68. doi: 10.1016/j.envexpbot.2018.09.024. [DOI] [Google Scholar]
- 32.Seif M., Aliniaeifard S., Arab M., Mehrjerdi M.Z., Shomali A., Fanourakis D., Li T., Woltering E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021;48:515–528. doi: 10.1071/FP20280. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda N., Ajima C., Yukawa T., Olsen J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016;121:102–111. doi: 10.1016/j.envexpbot.2015.06.014. [DOI] [Google Scholar]
- 34.Savvides A., Fanourakis D., van Ieperen W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012;63:1135–1143. doi: 10.1093/jxb/err348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanourakis D., Aliniaeifard S., Sellin A., Giday H., Körner O., Rezaei Nejad A., Delis C., Bouranis D., Koubouris G., Kambourakis E., et al. Stomatal behavior following mid- or long-term exposure to high relative air humidity: A review. Plant Physiol. Biochem. 2020;153:92–105. doi: 10.1016/j.plaphy.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Fanourakis D., Bouranis D., Tsaniklidis G., Rezaei Nejad A., Ottosen C.O., Woltering E.J. Genotypic and phenotypic differences in fresh weight partitioning of cut rose stems: Implications for water loss. Acta Physiol. Plant. 2020;42:48. doi: 10.1007/s11738-020-03044-w. [DOI] [Google Scholar]
- 37.Aliniaeifard S., Seif M., Arab M., Zare Mehrjerdi M., Li T., Lastochkina O. Growth and photosynthetic performance of Calendula officinalis under monochromatic red light. Int. J. Hortic. Sci. Technol. 2018;5:123–132. [Google Scholar]
- 38.Hogewoning S.W., Trouwborst G., Maljaars H., Poorter H., van Ieperen W., Harbinson J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010;61:3107–3117. doi: 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trouwborst G., Hogewoning S.W., van Kooten O., Harbinson J., van Ieperen W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016;121:75–82. doi: 10.1016/j.envexpbot.2015.05.002. [DOI] [Google Scholar]
- 40.Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 41.Haga K., Takano M., Neumann R., Iino M. The rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell. 2005;17:103–115. doi: 10.1105/tpc.104.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandalio L.M., Rodríguez-Serrano M., Romero-Puertas M.C. Leaf epinasty and auxin: A biochemical and molecular overview. Plant Sci. 2016;253:187–193. doi: 10.1016/j.plantsci.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Trouwborst G., Oosterkamp J., Hogewoning S.W., Harbinson J., van Ieperen W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant. 2010;138:289–300. doi: 10.1111/j.1399-3054.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 44.Krapp A., Quick W.P., Stitt M. Ribulose-1,5-bisphosphate carboxylase-oxygenase, other Calvin-cycle enzymes, and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta. 1991;186:58–69. doi: 10.1007/BF00201498. [DOI] [PubMed] [Google Scholar]
- 45.Nafziger E.D., Koller H.R. Influence of leaf starch concentration on CO2 assimilation in soybean. Plant Physiol. 1976;57:560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda R., Ohashi-Kaneko K., Fujiwara K., Kurata K. Effects of blue-light photon flux density on nitrogen and carbohydrate content and growth of spinach. Acta Hortic. 2008;801:1393–1398. doi: 10.17660/ActaHortic.2008.801.171. [DOI] [Google Scholar]
- 47.Terfa M.T., Solhaug K.A., Gislerød H.R., Olsen J.E., Torre S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa × hybrida but does not affect time to flower opening. Physiol. Plant. 2013;148:146–159. doi: 10.1111/j.1399-3054.2012.01698.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Lu W., Tong Y., Yang Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016;7:250. doi: 10.3389/fpls.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosseini A., Zare Mehrjerdi M., Aliniaeifard S., Seif M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants. 2019;25:741–752. doi: 10.1007/s12298-019-00647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan Q., Jiang W., Ding M., Lin Y., Huang D. Light affects the chloroplast ultrastructure and post-storage photosynthetic performance of watermelon (Citrullus lanatus) plug seedlings. PLoS ONE. 2014;9:e111165. doi: 10.1371/journal.pone.0111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falqueto A.R., da Silva Júnior R.A., Gomes M.T.G., Martins J.P.R., Silva D.M., Partelli F.L. Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Sci. Hortic. 2017;224:238–243. doi: 10.1016/j.scienta.2017.06.019. [DOI] [Google Scholar]
- 52.Gasulla F., Casano L., Guéra A. Chlororespiration induces non–photochemical quenching of chlorophyll fluorescence during darkness in lichen chlorobionts. Physiol. Plant. 2019;166:538–552. doi: 10.1111/ppl.12792. [DOI] [PubMed] [Google Scholar]
- 53.Podmaniczki A., Nagy V., Vidal-Meireles A., Tóth D., Patai R., Kovács L., Tóth S.Z. Ascorbate inactivates the oxygen-evolving complex in prolonged darkness. Physiol. Plant. 2021;171:232–245. doi: 10.1111/ppl.13278. [DOI] [PubMed] [Google Scholar]
- 54.Roosta H.R., Estaji A., Niknam F. Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce. Photosynthetica. 2018;56:606–615. doi: 10.1007/s11099-017-0696-1. [DOI] [Google Scholar]
- 55.Xue X., Wang Q., Qu Y., Wu H., Dong F., Cao H., Wang H.-L., Xiao J., Shen Y., Wan Y. Development of the photosynthetic apparatus of Cunninghamia lanceolata in light and darkness. New Phytol. 2017;213:300–313. doi: 10.1111/nph.14096. [DOI] [PubMed] [Google Scholar]
- 56.Ouzounis T., Rosenqvist E., Ottosen C. Spectral effects of artificial light on plant physiology and secondary metabolism: A Review. HortScience. 2015;50:1128–1135. doi: 10.21273/HORTSCI.50.8.1128. [DOI] [Google Scholar]
- 57.Yorio N.C., Goins G.D., Kagie H.R., Wheeler R.M., Sager J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience. 2001;36:380–383. doi: 10.21273/HORTSCI.36.2.380. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L., Van Labeke M.C. Chrysanthemum morphology, photosynthetic efficiency and antioxidant capacity are differentially modified by light quality. J. Plant Physiol. 2017;213:66–74. doi: 10.1016/j.jplph.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y., Wang T., Fang S., Zhou M., Qin J. Responses of morphology, gas exchange, photochemical activity of photosystem II, and antioxidant balance in Cyclocarya paliurus to light spectra. Front. Plant Sci. 2018;9:1704. doi: 10.3389/fpls.2018.01704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham M.D., Hwang H., Park S.W., Cui M., Lee H., Chun C. Leaf chlorosis, epinasty, carbohydrate contents and growth of tomato show different responses to the red/blue wavelength ratio under continuous light. Plant Physiol. Biochem. 2019;141:477–486. doi: 10.1016/j.plaphy.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Sæbø A., Krekling T., Appelgren M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell. Tissue Organ Cult. 1995;41:177–185. doi: 10.1007/BF00051588. [DOI] [Google Scholar]
- 62.Jeannette E., Reyss A., Grégory N., Gantet P., Prioul J.L. Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ. 2000;23:61–69. doi: 10.1046/j.1365-3040.2000.00519.x. [DOI] [Google Scholar]
- 63.Wang H., Gu M., Cui J., Shi K., Zhou Y., Yu J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009;96:30–37. doi: 10.1016/j.jphotobiol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Xin G., Liu C., Shi Q., Yang F., Wei M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020;20:318. doi: 10.1186/s12870-020-02523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassanvand F., Rezaei Nejad A., Fanourakis D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crop. Prod. 2019;134:19–25. doi: 10.1016/j.indcrop.2019.03.049. [DOI] [Google Scholar]
- 66.Żuk-Gołaszewska K., Upadhyaya M.K., Gołaszewski J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003;49:135–140. doi: 10.17221/4103-PSE. [DOI] [Google Scholar]
- 67.Fanourakis D., Briese C., Max J.F.J., Kleinen S., Putz A., Fiorani F., Ulbrich A., Schurr U. Rapid determination of leaf area and plant height by using light curtain arrays in four species with contrasting shoot architecture. Plant Methods. 2014;10:9. doi: 10.1186/1746-4811-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bergsträsser S., Fanourakis D., Schmittgen S., Cendrero-Mateo M.P., Jansen M., Scharr H., Rascher U. HyperART: Non-invasive quantification of leaf traits using hyperspectral absorption-reflectance-transmittance imaging. Plant Methods. 2015;11:1–17. doi: 10.1186/s13007-015-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- 70.Kalhor M.S., Aliniaeifard S., Seif M., Asayesh E.J., Bernard F., Hassani B., Li T. Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018;130:157–172. doi: 10.1016/j.plaphy.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Sørensen H.K., Fanourakis D., Tsaniklidis G., Bouranis D., Rezaei Nejad A., Ottosen C.O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020;267:109354. doi: 10.1016/j.scienta.2020.109354. [DOI] [Google Scholar]
- 72.Kalaji M.H., Goltsev V.N., Żuk-Gołaszewska K., Zivcak M., Brestic M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications. CRC Press LLC; Boca Raton, FL, USA: 2017. p. 222. [Google Scholar]
- 73.Küpper H., Benedikty Z., Morina F., Andresen E., Mishra A., Trtílek M. Analysis of OJIP chlorophyll fluorescence kinetics and QA reoxidation kinetics by direct fast imaging. Plant Physiol. 2019;179:369–381. doi: 10.1104/pp.18.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estaji A., Kalaji H.M., Karimi H.R., Roosta H.R., Moosavi-Nezhad S.M. How glycine betaine induces tolerance of cucumber plants to salinity stress. Photosynthetica. 2019;57:753–761. doi: 10.32615/ps.2019.053. [DOI] [Google Scholar]
- 75.Gorbe E., Calatayud A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012;138:24–35. doi: 10.1016/j.scienta.2012.02.002. [DOI] [Google Scholar]
- 76.Stirbet A., Lazár D., Kromdijk J. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica. 2018;56:86–104. doi: 10.1007/s11099-018-0770-3. [DOI] [Google Scholar]
- 77.Bayat L., Arab M., Aliniaeifard S., Seif M., Lastochkina O., Li T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants. 2018;10:ply052. doi: 10.1093/aobpla/ply052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not public.