Abstract
There is still limited data available from real-world experience studies on the pangenotypic regimens in patients with genotype (GT) 3 hepatitis C virus (HCV) infection and liver cirrhosis. The current study aimed to evaluate the efficacy and safety of pangenotypic regimens in this difficult-to-treat population. A total of 236 patients with mean age 52.3 ± 11.3 years and male predominance (72%) selected from EpiTer-2 database were included in the analysis; 72% of them were treatment-naïve. The majority of patients (55%) received the combination of sofosbuvir/velpatasvir (SOF/VEL), 71 without and 58 with ribavirin (RBV), whereas the remaining 107 individuals were assigned to glecaprevir/pibrentasvir (GLE/PIB). The effectiveness of the treatment following GLE/PIB and SOF/VEL regimens (96% and 93%) was higher compared to SOF/VEL + RBV option (79%). The univariate analysis demonstrated the significantly lower sustained virologic response in males, in patients with baseline HCV RNA ≥ 1,000,000 IU/mL, and among those who failed previous DAA-based therapy. The multivariate logistic regression analysis recognized only the male gender and presence of ascites at baseline as the independent factors of non-response to treatment. It should be emphasized that despite the availability of pangenotypic, strong therapeutic options, GT3 infected patients with cirrhosis still remain difficult-to-treat, especially those with hepatic impairment and DAA-experienced.
Keywords: hepatitis C, genotype 3, liver cirrhosis, pangenotypic
1. Introduction
Chronic infection with the hepatitis C virus (HCV) seems to be one of the significant health problems worldwide. Approximately 71 million people are affected globally, of whom 400,000 died annually due to the consequences of the disease [1]. The most severe complications of chronic hepatitis C (CHC) with a risk of death are liver cirrhosis and hepatocellular carcinoma (HCC). The development of liver fibrosis leading to cirrhosis occurs in nearly 20% of patients, and, on average, two decades of HCV infection are needed for this [2]. However, the rate of progression of fibrosis varies between different patients and depends on both viral and host predictors [2]. Male gender, the age of infection over 40 years, coinfection with hepatitis B virus (HBV) and human immunodeficiency virus (HIV), obesity, alcohol abuse are listed among variables related to the infected person, whereas the most important viral predictor for the accelerated fibrosis is genotype (GT) 3 HCV, which is second in frequency worldwide accounting for 25–30% all HCV cases [3,4,5,6]. In the era of interferon (IFN) based therapy, patients with liver cirrhosis had limited access to antiviral treatment due to safety issues and low effectiveness [7]. The implementation of the IFN-free DAA regimens has removed those safety-related limitations, but sofosbuvir (SOF) plus ribavirin (RBV), the only option available initially for GT3 patients, had still relatively low efficacy as compared to the cure rate achieved with DAA therapies in other GTs-infected individuals and treatment with daclatasvir (DCV) plus SOF was not available worldwide [8,9,10]. Therefore, at the beginning of the IFN-free era, cirrhotics infected with GT3 were assumed to be the most difficult-to-treat patients with CHC. The latest development in the antiviral treatment of this subpopulation was the registration of pangenotypic regimens. According to the recent guidelines, two options are recommended in patients with liver cirrhosis in the course of GT3 infection, the combination of protease inhibitor glecaprevir (GLE) with the inhibitor of non-structural protein 5A (NS5A) pibrentasvir (PIB), and SOF, polymerase inhibitor with velpatasvir (VEL), acting by inhibition of NS5A HCV [11,12,13,14]. However, available data in this population are based on limited studies, which usually included a small number of patients. The current study aimed to evaluate the efficacy of pangenotypic regimens in patients with liver cirrhosis infected with GT3 in the real-world experience.
2. Materials and Methods
The analyzed population consisted of CHC patients with liver cirrhosis infected with GT3 HCV selected from EpiTer-2 database. This sizeable national project supported by the Polish Association of Epidemiologists and Infectiologists includes 13,554 individuals treated with DAA regimens in 22 Polish hepatology centers between 1 July 2015 and 31 December 2020. Clinical data, including the severity of liver disease, the presence of the extrahepatic manifestations, coexisting medical conditions, concomitant medications, coinfections, the history of previous antiviral treatment and currently used regimen, and laboratory parameters were collected at baseline.
The severity of liver disease was assessed based on the non-invasive fibrosis evaluation either by transient elastography (TE) or shear-wave elastography (SWE), and cirrhosis was diagnosed according to recommendations of the European Association for the Study of the Liver (EASL) if liver stiffness ≥13 kilopascals corresponding to a METAVIR score of F4 [11]. In addition, cirrhotic patients were assessed for the oesophageal varices, past or present hepatic decompensation, history of liver transplantation, and scored in Child-Pugh (CP) scale and Model of End Stage Liver Disease (MELD).
HCV RNA was measured at baseline, at the end of treatment (EOT), and 12 weeks after therapy completion. The efficacy endpoint was sustained virologic response (SVR) defined as undetectable HCV RNA post-treatment week 12. The intent-to-treat (ITT) population included all patients who initiated the treatment, whereas per-protocol (PP) analysis was performed after excluding lost follow-up patients considered to be a non-virologic failure. Safety data in terms of adverse events (AE) and deaths were collected during the treatment course and in the 12-weeks follow-up period. Data were collected retrospectively and submitted by an online questionnaire administered by Tiba sp. z o.o.
Statistical Analysis
Results were expressed as mean (SD) or number (percentage). A P value less than 0.05 was considered significant. The significance of differences was calculated by the χ2 or Fisher exact tests for nominal variables and by the Mann–Whitney test and the Kruskal-Wallis analysis of variance for continuous variables. Univariable comparisons were calculated using the GraphPad Prism 5.1 software (GraphPad Software, Inc., La Jolla, CA, USA). The general logistic regression model was performed with SVR as the dependent variable. Among independent variables tested for the best model were age, sex, response to previous therapy, anamnesis of hepatic decompensation, baseline ascites, serum bilirubin, albumin, platelets, and HCV RNA. Logistic regression models were calculated by use of Statistica 13.0 (TIBCO Software Inc., Palo Alto, CA, USA).
3. Results
A total of 236 patients with liver cirrhosis infected with GT3 with mean age 52.3 ± 11.3 years and male predominance (72%) treated with pangenotypic regimens were included in the analysis. One hundred and seven (45%) were assigned to GLE/PIB, whereas the remaining 129 patients received SOF/VEL including 58 on the regimen with RBV. The choice of the therapeutic option was made by treating physicians in line with guidelines of the Polish Group of Experts for HCV and the recommendations of the National Health Fund, taking into consideration patients’ characteristics and drug labels.
No significant differences in demographic variables, as well as rates of comorbidities and concomitant medications, were observed between patients treated with two pangenotypic regimens (Table 1).
Table 1.
Baseline characteristics of GT3 HCV infected patients with liver cirrhosis treated with pangenotypic regimens.
| Parameter | GLE/PIB n = 107 |
SOF/VEL n = 71 |
SOV/VEL + RBV n = 58 |
p |
|---|---|---|---|---|
| Gender, females/males, n (%) | 30 (28)/77 (72) | 23 (32.4)/48 (67.6) | 13 (22.4)/45 (77.6) | 0.45 |
| Age [years] mean (SD) | 51.8 (10.6) | 53.2 (12.5) | 53.0 (11.3) | 0.96 |
| BMI mean (SD) | 27.8 (4.7) | 27.5 (4.8) | 29.0 (5.6) | 0.31 |
| Comorbidities, n (%) | 75 (70.1) | 50 (70.4) | 40 (69) | 0.98 |
| Concomitant medications, n (%) | 70 (65.4) | 47 (66.2) | 45 (77.6) | 0.24 |
| ALT IU/L, mean (SD) | 141 (116) | 132 (92) | 106 (70) | 0.17 |
| Bilirubin mg/dL, mean (SD) | 1.0 (0.6) | 0.8 (0.4) | 1.3 (0.8) | 0.003 |
| Albumin g/dL, mean (SD) | 3.9 (0.5) | 3.9 (0.5) | 3.7 (0.5) | 0.02 |
| Creatinine mg/dL, mean (SD) | 0.9 (0.6) | 0.8 (0.2) | 0.8 (0.2) | 0.74 |
| Hemoglobin g/dL, mean (SD) | 14.4 (1.8) | 14.5 (1.5) | 13.9 (1.7) | 0.27 |
| Platelets, ×1000/µL, mean (SD) | 139 (82) | 128 (54) | 95 (53) | <0.001 |
| HCV RNA × 106 IU/mL, mean (SD) | 2.17 (4.31) | 1.45 (1.79) | 1.49 (2.29) | 0.62 |
HCV, hepatitis C virus; GLE, glecaprevir; PIB, pibrentasvir; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; SD, standard deviation; BMI, body mass index; ALT, alanine transaminase; HCV RNA, ribonucleic acid of hepatitis C virus.
Significantly higher bilirubin concentration, lower albumin level, and platelet count were found among patients treated with SOF/VEL + RBV. In addition, in this subpopulation, a significantly higher percentage of those with past and present hepatic decompensation were observed, and a higher rate of individuals in category B of the Child-Pugh scale (Table 2).
Table 2.
Characteristics of the liver disease in GT3 HCV infected patients with liver cirrhosis treated with pangenotypic regimens.
| Parameter | GLE/PIB n = 107 |
SOF/VEL n = 71 |
SOF/VEL + RBV n = 58 |
p |
|---|---|---|---|---|
| History of hepatic decompensation, n (%) | ||||
| Number of patients | 2 (1.8) | 3 (4.2) | 9 (15.5) | 0.001 |
| Ascites | 1 (0.9) | 3 (4.2) | 9 (15.5) | <0.001 |
| Encephalopathy | 1 (0.9) | 1 (1.4) | 1 (1.7) | 0.91 |
| Documented esophageal varices, n (%) | 22 (20.6) | 11 (15.5) | 12 (20.7) | 0.66 |
| Hepatic decompensation at baseline, n (%) | ||||
| Moderate ascites—responded to diuretics | 0 | 1 (1.4) | 6 (10.3) | <0.001 |
| Tense ascites—not responded to diuretics | 0 | 0 | 0 | na |
| Encephalopathy | 0 | 0 | 0 | na |
| HCC history, n (%) | 4 (3.7) | 2 (2.8) | 1 (1.7) | 0.76 |
| OLTx history, n (%) | 0 | 0 | 0 | na |
| Child-Pugh, n (%) | ||||
| A | 102 (95.3) | 70 (98.6) | 53 (91.4) | 0.15 |
| B | 5 (4.7) | 1 (1.4) | 5 (8.6) | 0.15 |
| C | 0 | 0 | 0 | na |
| MELD, n (%) | ||||
| <15 | 100 (93.6) | 67 (94.4) | 58 (100) | 0.15 |
| 15–18 | 3 (2.8) | 1 (1.4) | 0 | na |
| 19–20 | 2 (1.8) | 1 (1.4) | 0 | na |
| >20 | 1 (0.9) | 0 | 0 | na |
| No data | 1 (0.9) | 2 (2.8) | 0 | na |
| HBV coinfection (HBsAg+), n (%) | 2 (1.8) | 3 (4.2) | 0 | 0.24 |
| HIV coinfection, n (%) | 7 (6.5) | 9 12.7) | 3 (5.1) | 0.22 |
HCV, hepatitis C virus; GLE, glecaprevir; PIB, pibrentasvir; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; hepatocellular carcinoma; OLTx, orthotopic liver transplantation; MELD, Model End-Stage Liver Disease; HBV, hepatitis B virus; HBsAg+, hepatitis B surface antigen; HIV, human immunodeficiency virus.
The significantly lower percentage of patients treated with SOF/VEL+RBV were treatment-naïve as compared to SOF/VEL and GLE/PIB regimens, 55.2%, 77.5%, and 77.6%, respectively. The relapse rate was the highest among those assigned to SOF/VEL + RBV option, and SOF + RBV was the most frequently used previous regimen in all subpopulations. A total of 30 patients were nonresponders to previous DAA-containing therapy without IFN, and eight of them were treated in the past with NS5A inhibitors. Six of those who previously failed NS5A-containing regimens were treated with SOF/VEL + RBV; the remaining two patients received GLE/PIB in re-therapy.
The majority of patients on the GLE/PIB option received a 12-weeks regimen (60.7%); more than half (55%) of those assigned to SOF/VEL therapy were treated for 12 weeks without RBV (Table 3).
Table 3.
Previous and current treatment characteristics of GT3 HCV infected patients with liver cirrhosis treated with pangenotypic regimens.
| Parameter | GLE/PIB n = 107 |
SOF/VEL n = 71 |
SOF/VEL + RBV n = 58 |
p |
|---|---|---|---|---|
| History of previous therapy, n (%) | ||||
| Treatment-naïve | 83 (77.6) | 55 (77.5) | 32 (55.2) | 0.004 |
| Nonresponder | 3 (2.8) | 3 (4.2) | 4 (6.9) | 0.46 |
| Relapser | 16 (14.9) | 12 (16.9) | 20 (34.5) | 0.008 |
| Discontinuation due to safety reasons | 0 | 0 | 1 (1.7) | na |
| Unknown type of response | 5 (4.7) | 1 (1.4) | 1 (1.7) | 0.37 |
| Previous regimen in patients with treatment failure, n (%) | n = 24 | n = 16 | n = 26 | |
| PegIFNα + RBV | 5 (20.8) | 6 (37.5) | 4 (15.4) | 0.24 |
| SOF + PegIFNα + RBV | 4 (16.7) | 3 (19) | 4 (15.4) | 0.96 |
| SOF + RBV | 12 (50) | 7 (43.8) | 11 (42.3) | 0.85 |
| SOF/VEL ± RBV | 2 (8.3) | 0 | 0 | na |
| SOF/LDV | 0 | 0 | 1 (3.8) | na |
| GLE/PIB | 0 | 0 | 4 (15.4) | na |
| Other | 0 | 0 | 2 (7.7) * | na |
| No data | 1 (4.2) | 0 | 0 | na |
| Current treatment regimens, n (%) | ||||
| GLE/PIB, 8 weeks | 20 (18.7) | na | na | |
| GLE/PIB, 12 weeks | 65 (60.7) | na | na | |
| GLE/PIB, 16 weeks | 22 (20.6) | na | na | |
| SOF/VEL, 12 weeks | na | 71 (100) | na | |
| SOF/VEL + RBV, 12 weeks | na | na | 48 (82.7) | na |
| SOF/VEL + RBV, 24 weeks | na | na | 10 (16.3) |
HCV, hepatitis C virus; GLE, glecaprevir; PIB, pibrentasvir; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; PegIFNα, pegylated interferon alpha; LDV, ledipasvir. * IFNα + RBV, Uprifosbuvir + Grazoprevir + Elbasvir/Ruzasvir.
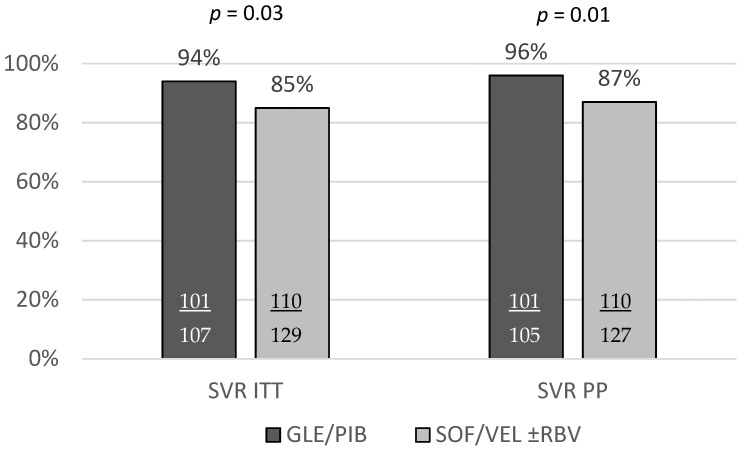
A total of 211 patients achieved an SVR corresponding to 89.4% in the ITT analysis, and after exclusion of four patients lost to follow-up, 91% in the PP analysis. The SVR rate was significantly higher among patients treated with GLE/PIB compared to those receiving SOF/VEL ± RBV both in ITT and PP analyses, 94.4% vs. 85.3% (p = 0.03), and 96.2% vs. 86.6%, (p = 0.01), respectively (Figure 1).
Figure 1.
The comparison of the SVR rates between GT3 HCV infected patients with liver cirrhosis treated with GLE/PIB and SOF/VEL ± RBV regimens.
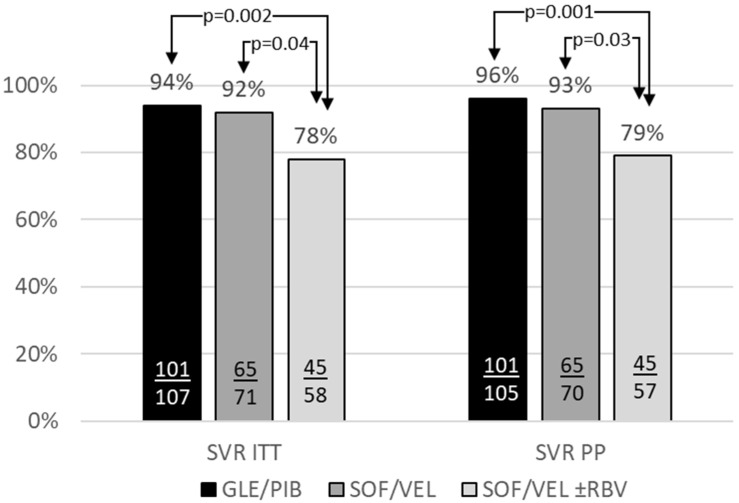
The detailed comparison of an SVR rates revealed no significant difference between GLE/PIB and SOF/VEL regimens, whereas cirrhotics on SOV/VEL + RBV option had significantly lower SVR as compared to both remaining options, 77.6% vs. 91.5% (p = 0.04), vs. 94.4% (p = 0.002), and 78.9% vs. 92.9% (p = 0.003), vs. 96.2% (p = 0.001), in ITT and PP analysis, respectively (Figure 2).
Figure 2.
The effectiveness of the GLE/PIB, SOF/VEL, and SOF/VEL + RBV options in GT3 infected patients with liver cirrhosis.
A total of twenty-three virologic failures were documented, 6 on GLE/PIB and 17 on SOF/VEL ± RBV regimen (Table 4 and Table 5).
Table 4.
Characteristics of 6 virologic failures to GLE/PIB regimen.
| Patient | Age | CP | Regimen | History of Previous Therapy | Baseline HCV RNA IU/mL | Treatment Course | EOT | Comment (Possible Reason for Non-Response) |
|---|---|---|---|---|---|---|---|---|
| Female 1 | 56 | A | GLE/PIB 12 | treatment-naive | 2,518,022 | according to plan | TD | |
| Male 1 | 48 | A | GLE/PIB 8 | treatment-naive | 942,000 | according to plan | TND | |
| Male 2 | 51 | A | GLE/PIB 8 | treatment-naive | 1,621,033 | according to plan | TD | |
| Male 3 | 52 | A | GLE/PIB 8 | treatment-naive | 1,483,266 | according to plan | TND | |
| Male 4 | 30 | A | GLE/PIB 12 | treatment-naive | 1,580,000 | according to plan | TND | |
| Male 5 | 54 | A | GLE/PIB 16 | relapse (SOF + RBV) | 4,030,000 | according to plan | TND | DAA failure |
GLE, glecaprevir; PIB, pibrentasvir; CP, Child-Pugh scale; HCV RNA, ribonucleic acid of hepatitis C virus; EOT, end of treatment; TD, target detected; TND, target not detected; SOF, sofosbuvir; RBV, ribavirin; DAA, direct-acting antivirals.
Table 5.
Characteristics of 17 virologic failures to SOF/VEL ± RBV regimen.
| Patient | Age | CP | Regimen | History of Previous Therapy | Baseline HCV RNA IU/mL | Treatment Course | EOT | Comment (Possible Reason for Non-Response) |
|---|---|---|---|---|---|---|---|---|
| Female 1 | 44 | A | SOF/VEL + RBV 12 | treatment-naive | 3,560,000 | RBV dose reduction | TD | |
| Male 1 | 50 | A | SOF/VEL 12 | relapse (SOF + RBV) | 2,190,000 | according to plan | TND | DAA failure |
| Male 2 | 54 | A | SOF/VEL 12 | relapse (SOF + RBV) | 5279 | according to plan | TND | DAA failure |
| Male 3 | 49 | A | SOF/VEL 12 | treatment-naive | 1,014,206 | according to plan | TD | |
| Male 4 | 38 | A | SOF/VEL 12 | treatment-naive | 4,910,000 | according to plan | TD | |
| Male 5 | 50 | A | SOF/VEL 12 | treatment-naive | 70,000 | according to plan | TD | |
| Male 6 | 58 | A | SOF/VEL + RBV 12 | treatment-naive | 1,620,000 | according to plan | TND | |
| Male 7 | 54 | A | SOF/VEL + RBV 12 | relapse (SOF + RBV) | 667,000 | according to plan | TND | DAA failure |
| Male 8 | 53 | A | SOF/VEL + RBV 12 | relapse (PR + SOF) | 261,902 | according to plan | TND | DAA failure |
| Male 9 | 29 | A | SOF/VEL + RBV 12 | relapse (PR + SOF) | 534,255 | according to plan | TND | DAA failure |
| Male 10 | 50 | A | SOF/VEL + RBV 12 | relapse (Uprifosbuvir + Grazoprevir + Elbasvir or Ruzasvir) | 2,230,000 | according to plan | TND | DAA failure |
| Male 11 | 58 | A | SOF/VEL + RBV 12 | relapse (GLE/PIB) | 1,270,000 | according to plan | TND | DAA failure |
| Male 12 | 51 | A | SOF/VEL + RBV 12 | treatment-naive | 1,790,000 | according to plan | TND | |
| Male 13 | 70 | A | SOF/VEL + RBV 12 | treatment-naive | 2,420,000 | according to plan | TND | |
| Male 14 | 52 | A | SOF/VEL + RBV 12 | treatment-naive | 1,220,000 | according to plan | TD | |
| Male 15 | 73 | A | SOF/VEL + RBV 12 | treatment-naive | 4,270,000 | discontinued | TD | Treatment discontinuation |
| Male 16 | 56 | A | SOF/VEL + RBV 24 | relapse (GLE/PIB) | 1,080,000 | according to plan | TND | DAA failure |
SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; CP, Child-Pugh scale; HCV RNA, ribonucleic acid of hepatitis C virus; EOT, end of treatment; TD, target detected; TND, target not detected; DAA, direct-acting antivirals; PR, PegIFNα + RBV; GLE, glecaprevir; PIB, pibrentasvir.
All of them were scored as category A on the CP scale; one experienced RBV dose reduction, and another one discontinued therapy by his own decision. Twenty-one of them were males, and nine were nonresponders to previous DAA-containing therapy, of whom two were treated in the past with pegylated IFN alpha (pegIFNα) + RBV + SOF, 4 received SOF + RBV, two another with GLE/PIB and one patient as a participant of the clinical trial did not respond to uprifosbuvir + grazoprevir + elbasvir/ruzasvir.
A significantly higher rate of males (91.3% vs. 69.4%, p = 0.03) was documented in GT3-infected nonresponders to pangenotypic regimens than those who achieved an SVR (Table 6).
Table 6.
Virologic nonresponders vs. responders to pangenotypic regimens.
| Parameter | Virologic Nonresponders n = 23 |
Responders n = 209 |
p |
|---|---|---|---|
| Gender, females/males, n (%) | 2 (8.7)/21 (91.3) | 64 (30.6)/145 (69.4) | 0.03 |
| Age [years] mean (SD) | 51.3 (10) | 52.8 (11.5) | 0.67 |
| BMI mean (SD) | 28.8 (4.6) | 28.0 (5.1) | 0.44 |
| Any comorbidity, n (%) | 16 (69.6) | 147 (70.3) | 1.00 |
| Concomitant medications, n (%) | 18 (78.3) | 143 (68.4) | 0.47 |
| HBV coinfection (HBsAg+), n (%) | 0 | 5 (2.4) | 1.00 |
| HIV coinfection, n(%) | 2 (8.7) | 16 (7.7) | 0.69 |
| Liver stiffness kPa, mean (SD) | 28 (13.3) | 28.8 (17.5) | 0.71 |
| History of hepatic decompensation, n (%) | 3 (13) | 11 (5.3) | 0.15 |
| HCC history, n (%) | 1 (4.3) | 6 (2.9) | 0.52 |
| Hepatic decompensation at baseline, n (%) | 2 (8.7) | 5 (2.4) | 0.14 |
| Child-Pugh B, n (%) | 0 | 10 (4.8) | 0.60 |
| Treatment-experienced, n (%) | 9 (39.1) | 54 (25.8) | 0.22 |
| IFN-free DAA-experienced, n (%) | 7 (30.4) | 29 (13.9) | 0.06 |
| ALT IU/L, mean (SD) | 143 (85) | 129 (102) | 0.24 |
| Bilirubin mg/dL, mean (SD) | 1.15 (0.38) | 1.0 (0.64) | 0.01 |
| Albumin g/dL, mean (SD) | 3.87 (0.55) | 3.86 (0.49) | 0.99 |
| Creatinine mg/dL, mean (SD) | 0.85 (0.14) | 0.85 (0.45) | 0.14 |
| Hemoglobin g/dL, mean (SD) | 14 (1.9) | 14.3 (1.7) | 0.51 |
| Platelets, ×1000/µL, mean (SD) | 100 (54) | 128 (71) | 0.04 |
| HCV RNA ×106 IU/mL, mean (SD) | 1.79 (1.33) | 1.8 (3.45) | 0.03 |
BMI, body mass index; SD, standard deviation; HBV, hepatitis B virus; HBsAg+, hepatitis B surface antigen; HIV, human immunodeficiency virus; HCC, hepatocellular carcinoma; IFN, interferon; DAA, direct-acting antivirals; ALT, alanine transaminase; HCV RNA, ribonucleic acid of hepatitis C virus.
The univariate analysis demonstrated the significantly lower SVR in males, in patients with baseline HCV RNA ≥ 1,000,000 IU/mL compared to <1,000,000 IU/mL, and among those who failed previous DAA-based therapy (Table 7).
Table 7.
Treatment effectiveness in subpopulations.
| Females, n = 66 | Males, n = 170 | p | |
| SVR ITT | 97% (64/66) | 85.3% (145/170) | 0.01 |
| SVR PP | 97% (64/66) | 87.3% (145/166) | 0.03 |
| HCV RNA < 1,000,000, n = 131 | HCV RNA ≥ 1,000,000, n = 105 | ||
| SVR ITT | 93.1% (122/131) | 82.9% (87/105) | 0.02 |
| SVR PP | 95.3% (122/128) | 83.7% (87/104) | 0.004 |
| Treatment-experienced, n = 66 | Treatment-naive, n = 170 | ||
| SVR ITT | 81.8% (54/66) | 91.2% (155/170) | 0.07 |
| SVR PP | 85.7% (54/63) | 91.7% (155/169) | 0.22 |
| DAA-experienced, n = 49 | Treatment-naive, n = 170 | ||
| SVR ITT | 77.5% (38/49) | 91.2% (155/170) | 0.02 |
| SVR PP | 80.9% (38/47) | 91.7% (155/169) | 0.06 |
| BMI < 30, n = 161 | BMI ≥ 30, n = 64 | ||
| SVR ITT | 88.2% (142/161) | 92.2% (59/64) | 0.48 |
| SVR PP | 89.9% (142/158) | 92.2% (59/64) | 0.80 |
SVR, sustained virologic response; ITT, intent to treat; PP, per protocol; HCV RNA, ribonucleic acid of hepatitis C virus; IFN, interferon; DAA, direct-acting antivirals; SOF, sofosbuvir; BMI, body mass index; The bold represent the same level as gender.
The multivariate logistic regression analysis recognized the male gender and presence of ascites at baseline as the independent factors of non-response to pangenotypic treatment (Table 8).
Table 8.
Baseline factors associated with SVR based on the logistic regression model.
| Estimate of β | SE | t-Stat | p Value | |
|---|---|---|---|---|
| (Intercept) | 550.76 | <0.001 | ||
| Gender (male) | −0.16 | 0.07 | −2.47 | 0.01 |
| Baseline ascites (no) | 0.17 | 0.07 | 2.43 | 0.03 |
| Previous decompensation (no) | 0.04 | 0.07 | 0.59 | 0.55 |
| Response to previous therapy (non-response) | 0.04 | 0.09 | 0.51 | 0.61 |
| Response to previous therapy (naive) | 0.11 | 0.09 | 1.22 | 0.22 |
| Bilirubin | 0.03 | 0.07 | 0.34 | 0.73 |
| Platelets | 0.05 | 0.07 | 0.71 | 0.48 |
| HCV RNA | 0.02 | 0.06 | 0.34 | 0.73 |
HCV RNA, ribonucleic acid of hepatitis C virus.
The majority of patients completed the treatment course according to schedule, 98.2% in GLE/PIB and 93% in SOF/VEL ± RBV, 6.2% of patients receiving RBV experienced dose modification, three patients discontinued treatment, two due to adverse events (AE), and one by his own decision. A similar proportion of patients in both subpopulations reported at least one AE, with the most common pruritus/skin changes in the course of GLE/PIB treatment and weakness/fatigue during SOF/VEL ± RBV therapy (Table 9).
Table 9.
Safety of GLE/PIB and SOF/VEL ± RBV in GT3 infected patients with liver cirrhosis.
| Parameter | GLE/PIB n = 107 |
SOF/VEL ± RBV n = 129 |
p |
|---|---|---|---|
| Treatment course, n (%) | |||
| according to schedule | 105 (98.2) | 120 (93) | 0.12 |
| modified RBV dosage | Na | 8 (6.2) | Na |
| therapy discontinuation | 2 (1.8) | 1 (0.8) * | 0.59 |
| Patients with at least one AE | 24 (22.4) | 28 (21.7) | 1.00 |
| Most common AEs | |||
| weakness/fatigue | 7 (6.5) | 12 (9.3) | 0.48 |
| gastrointestinal symptoms | 4 (3.7) | 7 (5.4) | 0.76 |
| pruritus/skin changes | 8 (7.5) | 2 (1.6) | 0.05 |
| anemia | 0 | 9 (7) | 0.004 |
| Death | 0 | 0 | na |
| Other serious adverse events | 0 | 3 (2.3) ** | 0.25 |
| AEs leading to treatment discontinuation | 2 (1.8) *** | 0 | 0.20 |
| AEs of particular interest | |||
| ascites | 2 (1.8) | 2 (1.6) | 1.00 |
| hepatic encephalopathy | 0 | 1 (0.8) | 1.00 |
| gastrointestinal bleeding | 0 | 2 (1.6) | 0.50 |
* patient’s decision; ** hepatic decompensation, HCC, pneumonia; *** worsening of depression, exacerbation of heart failure; GLE, glecaprevir; PIB, pibrentasvir; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse event.
Three serious AE in patients treated with SOF/VEL + RBV, but not related to this regimen, were documented. In addition, seven AEs of particular interest related to the deterioration of the liver function were reported, including ascites in 4 patients, gastrointestinal bleeding in 2 individuals, and hepatic encephalopathy in one person.
4. Discussion
After more than four years elapsed since the registration of the highly potent pangenotypic regimens, the published data from real-world experience (RWE) studies on the use of these medications in GT3 infected patients with liver cirrhosis are still limited, and most of them included a small number of patients.
The single tablet SOF/VEL combination was the first available highly effective option registered for patients with CHC regardless of the HCV genotype, the history of previous therapy, and liver fibrosis. For those with GT3 infection and liver cirrhosis, a 12-week treatment duration was approved based on the results of clinical trials demonstrating cure rates of 91–93%, which is comparable to 93% reported in our analysis [15,16,17]. The better efficacy of 97.5% was achieved in RWE analysis performed by Mangia et al. among 205 Italian GT3 infected patients with liver cirrhosis despite the higher percentage of CP B patients compared to our cohort [18]. However, it should be noted that no DAA-experienced patients were included in the study in contrast to our analysis. The population treated with SOF/VEL in 16 clinical practice cohorts worldwide comprising also DAA-experienced individuals except NS5A-containing regimens achieved an SVR of 93% (332/356) [19]. On the other hand, the cure rate following the SOF/VEL option reported among the RWE cohort of American Veterans, including previously untreated and those who received both IFN- and DAA-based regimens, was 86.5%, lower compared to our result [20].
Even lower efficacy of 79% was achieved in the current analysis in patients treated with SOF/VEL and RBV. It should be noted that the addition of RBV is an option to consider in compensated cirrhotics infected with GT3, whereas it is recommended in the case of decompensated individuals for whom the SOF/VEL is the only registered DAA pangenotypic regimen [21]. The differences in baseline characteristics of patients with a significantly higher number of those with more severe liver disease and the higher rate of treatment-experienced ones among individuals receiving therapy with RBV seem to be the difference of great importance that affects the effectiveness of the treatment with SOF/VEL regimen. Our findings on lower SVR with the SOF/VEL + RBV regimen contradict the results of clinical trials with 96% cure rates, but both studies included only IFN-based treatment-experienced individuals [16,17].
The SVR rate of 95.5% (192/201) was reported for SOF/VEL + RBV option in analysis from multinational RWE presented by Fagiuoli et al., but the range was between 88% and 100% [19]. Mangia et al. documented a 90.5% cure rate with SOF/VEL + RBV regimen in the RWE population, but only ten GT3 infected patients with liver cirrhosis were included [22]. The efficacy of 88% was demonstrated in a real-life population consisted of 34 patients, including 31 treatment-experienced with both IFN- and DAA-based except NS5A-containing regimens [23]. The much more numerous RWE cohort comprising 267 cirrhotic American Veterans treated with SOF/VEL + RBV analyzed by Belperio et al., including NS5A-experienced individuals, responded in 84.5% [20]. Since the failure of prior antiviral therapy, especially DAA containing antiviral therapy, is well recognized as a negative predictor of SVR, the low efficacy documented in our analysis may be influenced by a high percentage of nonresponders in the SOF/VEL + RBV arm, 26/58 (45%), with of whom 21 were treated with DAA [24]. Nine of them received a longer therapy duration 24 weeks, seven responded to treatment, and one was lost to follow-up, giving an 87.5% SVR rate in PP analysis. According to the label, the longer treatment course of SOF/VEL + RBV may be considered in patients who have failed therapy with an NS5A-containing regimen based on analysis from phase 2 and 3 clinical trials. However, there are no clinical data to support this recommendation [21,25]. Therefore further studies are needed to clarify the need for ribavirin in the treatment of decompensated genotype 3 infected cirrhotics who failed previous DAA-based therapy. In the current analysis, six of eight NS5A-experienced patients were treated with SOF/VEL + RBV; three of them failed to achieve an SVR, two with 12-week and another with a 24-week regimen. The remaining two NS5A-experienced patients underwent successful treatment with a 16-week GLE/PIB regimen; however, the numbers are too small to draw conclusions.
The dual therapy of GLE/PIB was approved for GT3 infected patients with compensated liver cirrhosis, and initially, a 12-week option was recommended for treatment-naïve and a 16-week regimen for treatment-experienced individuals based on the results from the clinical trials [26]. With the update of the label made upon the findings from the EXPEDITION-8 trial treatment-naïve, GT3 infected cirrhotics received the possibility to shorten the therapy length to 8 weeks without losing efficacy [27]. In our analysis, the majority of treatment-naïve patients were assigned to a 12-week regimen with an efficacy rate of 97%, while treatment-experienced individuals responded in 95% to 16-week therapy, which is comparable to 98% and 96% SVR rates documented in SURVEYOR-II part 3 study [28]. The data pooled from five phase 2 and 3 clinical trials, including a total of 120 patients with compensated liver cirrhosis, documented a 97% efficacy rate in treatment-naïve following 12-week therapy and 94% as a result of 16-week regimen in treatment-experienced patients [29]. A higher cure rate of 100% was reported in 12 cirrhotic patients from the German Hepatitis-C Registry receiving GLE/PIB, and among Italian cirrhotics treated for 12 or 16 weeks depending on the history of previous treatment, but no precise information on the number of patients, in this case, was added [30,31]. A lower SVR of 83% was demonstrated in 6 treatment-naïve cirrhotic GT3 infected individuals by Toyoda et al. [32]. Very limited RWE data based on small numbers of patients are available for treatment-naïve GT3 infected patients with liver cirrhosis treated with GLE/PIB for 8 weeks. The first published paper from the USA reported a 100% response rate in a group of 4 patients [33]. The same effectiveness was documented by Lampertico et al. following the 8-week GLE/PIB treatment duration in 19 cirrhotic patients with GT3 infection from seven small RWE studies included in the summary analysis [34,35]. A much lower SVR of 72% in PP analysis was demonstrated in nine GT3 infected cirrhotics in our previous study from the EpiTer-2 database, but it was due to a small subset of patients [36]. In the current study, 16 patients treated for 8 weeks achieved SVR, which gives an unsatisfactory rate of 84% in PP analysis, lower than demonstrated for a 12-week regimen with statistical significance for ITT analysis (80% vs. 95.4%, p = 0.05), however, it should be noted that a number of patients on 8-week regimen was still low. Further investigations in a large population of GT3 infected cirrhotics are needed to assess the real-world efficacy of an 8-week GLE/PIB regimen. According to label glecaprevir as a protease inhibitor included in the glecaprevir/pibrentasvir regimen is not recommended in moderate hepatic impairment (Child-Pugh B), and is contraindicated in Child-Pugh C patients only. Our study did not include patients with Child-Pugh C and only 4.7% of those treated with glecaprevir/pibrentasvir were classified as Child-Pugh B. The decision to use a protease inhibitor (glecaprevir) in a patient with Child-Pugh B was made by the treating physician.
According to the best of our knowledge, only two available studies made a direct comparison between different pangenotypic regimens in GT3 infected patients, including those with compensated liver cirrhosis. One of them is the analysis performed among 76 Spanish patients with GT3 infection, of whom 12 were diagnosed as cirrhotics, nine were treated with SOF/VEL, including three receiving RBV additionally, and three were assigned to GLE/PIB. The reported efficacy rates were 89% for SOF/VEL ± RBV (8/9) regimen and 67% (2/3) for GLE/PIB option [37]. The second available RWE study comparing SOF/VEL ± RBV, GLE/PIB, and SOF/DCV regimens in GT3 infected patients was made by Soria et al. in a multicentre cohort of Italian patients [38]. Ninety-nine of 2082 individuals included in the study had liver cirrhosis, and despite the difference in SVR rates with 100% in 21 patients treated with GLE/PIB and 93.6% among 78 those receiving SOF/VEL ± RBV regimen, no statistical significance was demonstrated. The comparative analysis concerning demographic, clinical, and laboratory variables between two cirrhotic subpopulations was not provided since the primary comparison was performed among GT3 patients regardless of the liver fibrosis.
No specific safety issues were observed during the treatment course, and we confirmed comparable tolerability across regimens with only a higher rate of RBV-related anemia in SOF/VEL ± RBV. Our findings are in line with the results of clinical trials and RWE studies [15,16,17,18].
The several limitations of the current study related to its real-world nature and retrospective observational design could be identified. Firstly, some clinical data might have been under-reported, including mild adverse events, the prevalence of comorbidities, and concomitant medications usage. No drug monitoring during the therapy hampers the assessment of compliance and its impact on the treatment efficacy. Electronic data capture might result in possible data entry errors. No resistance-associated substitutions (RAS) in previously DAA-nonresponders were tested at baseline. The choice of a therapeutic regimen in all patients was based on the treating physician’s decision regarding recommendations and regulations. However, according to the most recent EASL guidelines, if resistance testing is available and performed, only DAA-experienced patients with the NS5A Y93H RAS at baseline should be treated with SOF/VEL plus RBV, whereas those without should receive SOF/VEL alone, so we assumed that this factor did not affect efficacy reported in our analysis, no NS5A-experienced patient was treated with SOL/VEL [11,39]. Noteworthy, the other regimen prescribed in GT3 infected patients with the presence of Y93H RAS is the combination of SOF/VEL and protease inhibitor voxilaprevir is not recommended in decompensated cirrhotics; moreover, it was not available in Poland within a reimbursed therapeutic program in the analyzed period. Furthermore, finally, since the possibility for a shorter 8-weeks treatment course with GLE/PIB in treatment-naïve GT3 infected patients with liver cirrhosis has emerged very recently, the subset of this population in our analysis is relatively small. However, the study’s major strength is collecting data from the real-world, heterogeneous population representative of routine practice. Moreover, in this study, we included a high number of patients with a low rate of those lost to follow-up (<2%).
5. Conclusions
In summary, we confirmed the overall high effectiveness and safety of pangenotypic regimens in the real-world setting of cirrhotics with chronic genotype 3 HCV infection. The highest effectiveness was achieved in those treated with the GLE/PIB regimen, but it was suboptimal if therapy was carried out for 8 weeks. The addition of ribavirin to the SOF/VEL regimen was associated with significantly decreased effectiveness. However, it was related to hepatic decompensation at baseline and failure of previous DAA-based therapy, which are currently indications for ribavirin coadministration. Further studies are needed to clarify the real need for ribavirin in such a difficult-to-treat population of patients treated with SOF/VEL.
Acknowledgments
The authors wish to thank Tadeusz Łapiński, who helped in data collection but who had sadly passed away before the manuscript submission.
Author Contributions
Conceptualization—D.Z.-M., R.F., methodology—D.Z.-M., R.F., formal analysis—D.Z.-M., J.J., investigation—D.Z.-M., validation—R.F., writing—original draft preparation—D.Z.-M., writing—review and editing—R.F., supervision—R.F., project administration—R.F., data collection—D.Z.-M., J.J., A.P.-K., E.J., D.D., M.P., W.H., W.M., B.L., J.J.-L., K.S., A.P., H.B., J.K., P.S., B.S.-S., J.C., Ł.S., M.T.-Z., K.T., M.S., B.D., R.K., J.B.-W., Ł.L., R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This observational study was conducted in a real-world setting with approved drugs. Patients were not exposed to any experimental interventions nor did the study intervene with the clinical management of the patient. The study only collected information from patient medical records. The analysis included routine examinations and tests performed in patients treated within the therapeutic program of the National Health Fund. The data were originally collected to assess treatment efficacy and safety in individual patients, not for scientific purposes. Hence, the treating physicians did not obtain approval from the ethics committee. According to local law (Pharmaceutical Law of 6 September 2001, art. 37al), non-interventional studies do not require ethics committee approval.
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study.
Data Availability Statement
Data supporting reported results can be provided upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Global Health Sector Strategy on Viral Hepatitis 2016–2021. World Health Organization; Geneva, Switzerland: 2016. [(accessed on 13 June 2021)]. Available online: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1. [Google Scholar]
- 2.Westbrook R.H., Dusheiko G. Natural history of hepatitis C. J. Hepatol. 2014;61(Suppl. 1):S58–S68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F., Kramer J.R., Ilyas J., Duan Z., El-Serag H.B. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkontchou G., Ziol M., Aout M., Lhabadie M., Baazia Y., Mahmoudi A., Roulot D., Ganne-Carrie N., Grando-Lemaire V., Trinchet J.C., et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J. Viral. Hepat. 2011;18:e516–e522. doi: 10.1111/j.1365-2893.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 5.Messina J.P., Humphreys I., Flaxman A., Brown A., Cooke G.S., Pybus O.G., Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flisiak R., Pogorzelska J., Berak H., Horban A., Orłowska I., Simon K., Tuchendler E., Madej G., Piekarska A., Jabłkowski M., et al. Prevalence of HCV genotypes in Poland—The EpiTer study. Clin. Exp. Hepatol. 2016;2:144–148. doi: 10.5114/ceh.2016.63871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flisiak R., Pogorzelska J., Berak H., Horban A., Orłowska I., Simon K., Tuchendler E., Madej G., Piekarska A., Jabłkowski M., et al. Efficacy of HCV treatment in Poland at the turn of the interferon era—The EpiTer study. Clin. Exp. Hepatol. 2016;2:138–143. doi: 10.5114/ceh.2016.63870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster G.R., Pianko S., Brown A., Forton D., Nahass R.G., George J., Barnes E., Brainard D.M., Massetto B., Lin M., et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149:1462–1470. doi: 10.1053/j.gastro.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Poordad F., Shiffman M.L., Ghesquiere W., Wong A., Huhn G.D., Wong F., Ramji A., Shafran S.D., McPhee F., Yang R., et al. Daclatasvir and sofosbuvir with ribavirin for 24 wk in chronic hepatitis C genotype-3-infected patients with cirrhosis: A Phase III study (ALLY-3C) Antivir. Ther. 2019;24:35–44. doi: 10.3851/IMP3278. [DOI] [PubMed] [Google Scholar]
- 10.Zarębska-Michaluk D., Flisiak R., Jaroszewicz J., Janczewska E., Czauż-Andrzejuk A., Berak H., Horban A., Staniaszek A., Gietka A., Tudrujek M., et al. Is Interferon-Based Treatment of Viral Hepatitis C Genotype 3 Infection Still of Value in the Era of Direct-Acting Antivirals? J. Interferon Cytokine Res. 2018;38:93–100. doi: 10.1089/jir.2017.0113. [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver Clinical Practice Guidelines Panel: Chair, EASL Governing Board representative, Panel members. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Ghany M.G., Morgan T.R., AASLD-IDSA Hepatitis C Guidance Panel Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halota W., Flisiak R., Juszczyk J., Małkowski P., Pawłowska M., Simon K., Tomasiewicz K. Recommendations of the Polish Group of Experts for HCV for the treatment of hepatitis C in 2020. Clin. Exp. Hepatol. 2020;6:163–169. doi: 10.5114/ceh.2020.98606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarębska-Michaluk D. Genotype 3-hepatitis C virus’ last line of defense. World J. Gastroenterol. 2021;27:1006–1021. doi: 10.3748/wjg.v27.i11.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster G.R., Afdhal N., Roberts S.K., Bräu N., Gane E.J., Pianko S., Lawitz E., Thompson A., Shiffman M.L., Cooper C., et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 16.Pianko S., Flamm S.L., Shiffman M.L., Kumar S., Strasser S.I., Dore G.J., McNally J., Brainard D.M., Han L., Doehle B., et al. Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial. Ann. Intern. Med. 2015;163:809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 17.Esteban R., Pineda J.A., Calleja J.L., Casado M., Rodríguez M., Turnes J., Morano Amado L.E., Morillas R.M., Forns X., Pascasio Acevedo J.M., et al. Efficacy of Sofosbuvir and Velpatasvir, With and Without Ribavirin, in Patients With Hepatitis C Virus Genotype 3 Infection and Cirrhosis. Gastroenterology. 2018;155:1120–1127. doi: 10.1053/j.gastro.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Mangia A., Cenderello G., Copetti M., Verucchi G., Piazzolla V., Lorusso C., Santoro R., Squillante M.M., Orlandini A., Minisini R., et al. SVR12 Higher than 97% in GT3 Cirrhotic Patients with Evidence of Portal Hypertension Treated with SOF/VEL without Ribavirin: A Nation-Wide Cohort Study. Cells. 2019;8:313. doi: 10.3390/cells8040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagiuoli S., Agarwal K., Mangia A., Shafran S.D., Wedemeyer H., Terrault N., Feld J.J., Turnes J., Buggish P., Ciancio A., et al. Effectiveness of Sofosbuvir/Velpatasvir for 12 weeks in HCV genotype 3 patients with compensated cirrhosis in clinical practice cohorts from around the world. Hepatology. 2018;68(Suppl. 1):360A–361A. [Google Scholar]
- 20.Belperio P.S., Shahoumian T.A., Loomis T.P., Mole L.A., Backus L.I. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J. Hepatol. 2019;70:15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Epclusa Summary of Product Characteristics. [(accessed on 13 June 2021)];2020 Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/epclusa#product-information-section.
- 22.Mangia A., Losappio R., Cenderello G., Potenza D., Mazzola M., De Stefano G., Terreni N., Copetti M., Minerva N., Piazzola V., et al. Real life rates of sustained virological response (SVR) and predictors of relapse following DAA treatment in genotype 3 (GT3) patients with advanced fibrosis/cirrhosis. PLoS ONE. 2018;13:e0200568. doi: 10.1371/journal.pone.0200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mushtaq S., Akhter T.S., Khan A., Sohail A., Khan A., Manzoor S. Efficacy and Safety of Generic Sofosbuvir Plus Daclatasvir and Sofosbuvir/Velpatasvir in HCV Genotype 3-Infected Patients: Real-World Outcomes from Pakistan. Front. Pharmacol. 2020;11:550205. doi: 10.3389/fphar.2020.550205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llerena S., Cabezas J., Cuadrado A., Manuel Olmos J., González M., García F., Cobo C., Crespo J. Rescue Therapy for Genotype-3 DAA Non-responders, Almost all Done. Ann. Hepatol. 2019;18:236–239. doi: 10.5604/01.3001.0012.7931. [DOI] [PubMed] [Google Scholar]
- 25.Gane E.J., Shiffman M.L., Etzkorn K., Morelli G., Stedman C.A.M., Davis M.N., Hinestrosa F., Dvory-Sobol H., Huang K.C., Osinusi A., et al. Sofosbuvir-velpatasvir with ribavirin for 24 weeks in hepatitis C virus patients previously treated with a direct-acting antiviral regimen. Hepatology. 2017;66:1083–1089. doi: 10.1002/hep.29256. [DOI] [PubMed] [Google Scholar]
- 26.European Medicines Agency [(accessed on 13 June 2021)];EMA/332999/2020. Maviret: Procedural Steps Taken and Scientific Information after the Authorization. Available online: https://www.ema.europa.eu/en/documents/procedural-steps-after/maviret-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf.
- 27.Brown R.S., Jr., Buti M., Rodrigues L., Chulanov V., Chuang W.L., Aguilar H., Horváth G., Zuckerman E., Carrion B.R., Rodriguez-Perez F., et al. Glecaprevir/pibrentasvir for 8 wk in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J. Hepatol. 2020;72:441–449. doi: 10.1016/j.jhep.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Wyles D., Poordad F., Wang S., Alric L., Felizarta F., Kwo P.Y., Maliakkal B., Agarwal K., Hassanein T., Weilert F., et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology. 2018;67:514–523. doi: 10.1002/hep.29541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flamm S., Mutimer D., Asatryan A., Wang S., Rockstroh J., Horsmans Y., Kwo P.Y., Weiland O., Villa E., Heo J., et al. Glecaprevir/Pibrentasvir in patients with chronic HCV genotype 3 infection: An integrated phase 2/3 analysis. J. Viral. Hepat. 2019;26:337–349. doi: 10.1111/jvh.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg T., Naumann U., Stoehr A., Sick C., John C., Teuber G., Schiffelholz W., Mauss S., Lohmann K., König B., et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: Data from the German Hepatitis C-Registry. Aliment. Pharmacol. Ther. 2019;49:1052–1059. doi: 10.1111/apt.15222. [DOI] [PubMed] [Google Scholar]
- 31.D’Ambrosio R., Pasulo L., Puoti M., Vinci M., Schiavini M., Lazzaroni S., Soria A., Gatti F., Menzaghi B., Aghemo A., et al. NAVIGATORE-Lombardia Study Group. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J. Hepatol. 2019;70:379–387. doi: 10.1016/j.jhep.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Toyoda H., Atsukawa M., Watanabe T., Nakamuta M., Uojima H., Nozaki A., Takaguchi K., Fujioka S., Iio E., Shima T., et al. Real-world experience of 12-week direct-acting antiviral regimen of glecaprevir and pibrentasvir in patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 2020;35:855–861. doi: 10.1111/jgh.14874. [DOI] [PubMed] [Google Scholar]
- 33.Flamm S.L., Kort J., Marx S.E., Strezewski J., Dylla D.E., Bacon B., Curry M.P., Tsai N., Wick N. Effectiveness of 8-Week Glecaprevir/Pibrentasvir for Treatment-Naïve, Compensated Cirrhotic Patients with Chronic Hepatitis C Infection. Adv. Ther. 2020;37:2267–2274. doi: 10.1007/s12325-020-01301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampertico P., Mauss S., Persico M., Barclay S.T., Marx S., Lohmann K., Bondin M., Zhang Z., Marra F., Belperio P.S., et al. Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis. Adv. Ther. 2020;37:4033–4042. doi: 10.1007/s12325-020-01449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belperio P., Shahoumian T., Loomis T., Mole L., Backus L. Real-world effectiveness of glecaprevir/pibrentasvir in 1,941 patients with hepatitis C genotypes 1 through 4. Hepatology. 2018;68:417A–418A. [Google Scholar]
- 36.Zarębska-Michaluk D., Jaroszewicz J., Pabjan P., Łapiński T.W., Mazur W., Krygier R., Dybowska D., Halota W., Pawłowska M., Janczewska E., et al. Is an 8-week regimen of glecaprevir/pibrentasvir sufficient for all hepatitis C virus infected patients in the real-world experience? J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15337. [DOI] [PubMed] [Google Scholar]
- 37.Margusino-Framiñán L., Cid-Silva P., Rotea-Salvo S., Mena-de-Cea Á., Suárez-López F., Vázquez-Rodríguez P., Delgado-Blanco M., Sanclaudio-Luhia A.I., Martín-Herranz I., Castro-Iglesias Á. Effectiveness and safety of sofosbuvir/velpatasvir ± ribavirin vs. glecaprevir/pibrentasvir in genotype 3 hepatitis C virus infected patients. Eur. J. Hosp. Pharm. 2020;27:e41–e47. doi: 10.1136/ejhpharm-2019-002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soria A., Fava M., Bernasconi D.P., Lapadula G., Colella E., Valsecchi M.G., Migliorino G.M., D’Ambrosio R., Landonio S., Schiavini M., et al. Comparison of three therapeutic regimens for genotype-3 hepatitis C virus infection in a large real-life multicentre cohort. Liver Int. 2020;40:769–777. doi: 10.1111/liv.14386. [DOI] [PubMed] [Google Scholar]
- 39.Sarrazin C. Treatment failure with DAA therapy: Importance of resistance. J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results can be provided upon request from the corresponding author.