Abstract
Agonists of the Gi protein-coupled A3 adenosine receptor (A3AR) have shown important pain-relieving properties in preclinical settings of several pain models. Active as a monotherapy against chronic pain, A3AR agonists can also be used in combination with classic opioid analgesics. Their safe pharmacological profile, as shown by clinical trials for other pathologies, i.e., rheumatoid arthritis, psoriasis and fatty liver diseases, confers a realistic translational potential, thus encouraging research studies on the molecular mechanisms underpinning their antinociceptive actions. A number of pathways, involving central and peripheral mechanisms, have been proposed. Recent evidence showed that the prototypical A3AR agonist Cl-IB-MECA and the new, highly selective, A3AR agonist MRS5980 inhibit neuronal (N-type) voltage-dependent Ca2+ currents in dorsal root ganglia, a known pain-related mechanism. Other proposed pathways involve reduced cytokine production, immune cell-mediated responses, as well as reduced microglia and astrocyte activation in the spinal cord. The aim of this review is to summarize up-to-date information on A3AR in the context of pain, including cellular and molecular mechanisms underlying this effect. Based on their safety profile shown in clinical trials for other pathologies, A3AR agonists are proposed as novel, promising non-narcotic agents for pain control.
Keywords: A3 adenosine receptor, neuropathic pain, visceral pain, dorsal root ganglion neurons, Ca2+ currents, T cells, interleukin-10, adenosine
1. Introduction
Chronic pain is a highly debilitating condition, disturbing all aspects of our daily experience in social and career-related contexts. The pharmacological tools currently available are sometimes inadequate, or, as in the case of opioids, limited by serious adverse effects [1]. Thus, efforts are being made to pursue research into innovative, non-opioid, pain-relieving compounds.
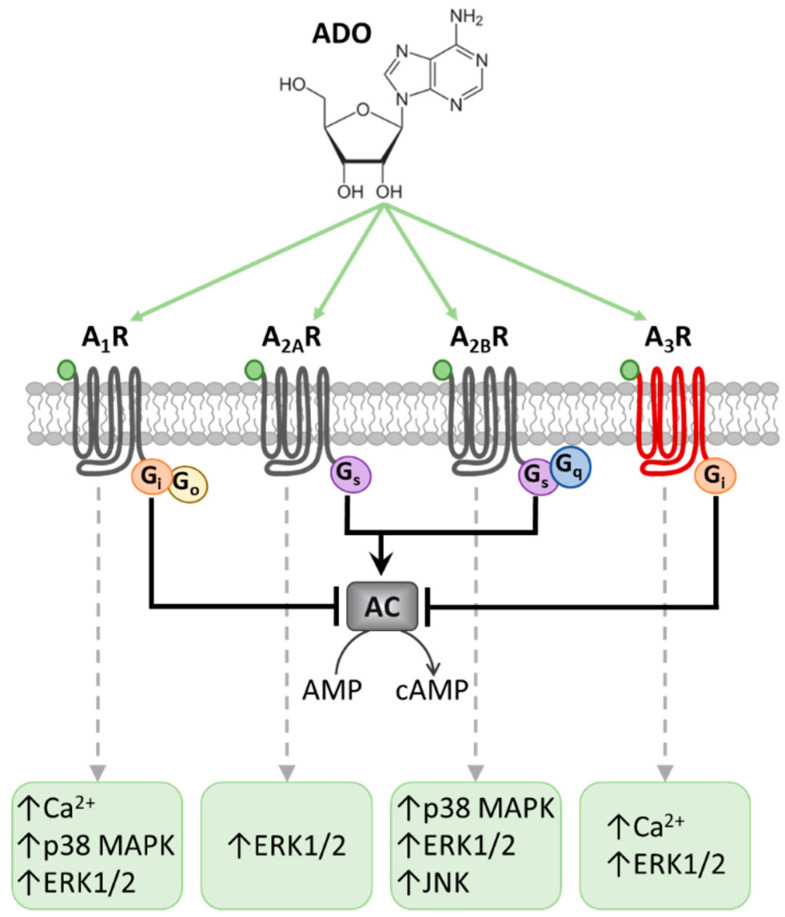
Many experimental reports have identified adenosine receptors (ARs) as potential targets for acute or chronic pain management. Adenosine is a ubiquitous endogenous neuromodulator whose actions are mediated by four G protein-coupled receptors (GPCR), namely A1, A2A, A2B and A3 receptors (A1Rs, A2ARs, A2BRs and A3Rs). A1Rs and A3Rs are coupled to Gi members of the G protein family, while A2ARs and A2BRs are Gs-coupled receptors [2]. The consequent modulation of cyclic adenosine monophosphate (cAMP) levels activates or inhibits a number of signaling pathways, depending on the specific type of cell involved (Figure 1). In some cases, A2BR might also couple to Gq proteins, as well as A1Rs to the Go protein. All ARs are coupled to mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK and Jc-Jun-NH2 terminal Kinase (JNK) [3].
Figure 1.
Adenosine receptors and the main transduction pathways involved in their activation. Schematic representation of G protein-coupled A1, A2A, A2B and A3 receptor (A1R, A2AR, A2BR and A3R) subtypes activated by extracellular adenosine (ADO), and the main intracellular pathways involved. A2ARs and A2BRs are coupled to the Gs protein, which leads to adenylyl cyclase (AC) activation and cyclic AMP (cAMP) increase. On the other hand, A1R and A3R are coupled to the Gi protein that inhibits AC and reduces cAMP. In some districts, A2BRs are also coupled with Gq proteins and A1R with Go, which stimulate Ca2+ release from intracellular stores. All adenosine receptors are coupled to mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK and Jc-Jun-NH2 terminal Kinase (JNK).
A variety of functions are regulated by purines: cardiovascular, respiratory, inflammatory and immune events [4,5,6], as well as neuronal maturation [7,8] and hypoxic damage [9,10]. Of note, adenosine receptors are expressed within the pain-related areas in the peripheral or central nervous system (CNS). It is known that ARs are widely distributed on neurons and glia; hence, considerable interest is focused on the development of selective AR ligands able to control neurological alterations in nervous system diseases [11,12,13,14]. Importantly, local levels of adenosine are significantly enhanced during events of tissue inflammation, stress or trauma, as well as during hypoxia-ischemia [15,16]. Adenosine is a potent anti-inflammatory autacoid that inhibits a number of inflammatory mechanisms, including phagocytosis, the generation of toxic oxygen metabolites, cytokine release and cellular adhesion [17]. Most of these anti-inflammatory effects were first ascribed to the activation of “A2Rs” [18], before further investigation attributed the anti-inflammatory effect of adenosine to A2ARs [19], which are widely expressed on peripheral blood and immune cells [20].
Moving in more detail to pain transmission, the first proof of adenosine’s involvement in anti-nociception dates from the 1970s, when the systemic and spinal (intrathecal: i.th.) administration of selective agonists proved effective in pain control. These studies emphasized the role of adenosine A1Rs in producing anti-nociception, with some effects ascribed to the A2AR subtype [21,22]. Adenosine involvement in peripheral nociception was further confirmed, e.g., the exogenous administration of A1R agonists locally to the hind paw of a rat produces anti-nociception in a pressure hyperalgesia model [23], whereas the local administration of A2R agonists enhances pain responses [24], an action due to adenosine A2AR activation, as confirmed by using the selective agonist CGS21680 [25].
The anti-nociceptive action of A1R agonists has been attributed to adenylate cyclase (AC) inhibition and to the decreased production of cAMP in sensory nerve terminals [26,27]. While not visualized directly on sensory terminals, A1Rs are present on the cell body of dorsal root ganglion (DRG) neurons [28], and on primary afferent neuronal terminals [29]. In the last decade, investigations into the adenosine-mediated effects in a variety of models for acute or chronic pain were undertaken [30], and the robust protective role of A1R emerged [31].
Conversely, the effects of A2AR activation on the promotion of cutaneous inflammatory pain have been proposed to result from the stimulation of AC, leading to increased cAMP levels in the sensory nerve terminal [26,27], thus producing opposite effects to those elicited by the anti-hyperalgesic A1R subtype. However, the relation between A2ARs and pain has been controversial, with evidence implying either pro-nociceptive or anti-nociceptive activity depending on the receptors’ localization and animal models of pain [30]. Indeed, a relevant A2AR-mediated anti-nociceptive effect has been described in a recent study demonstrating that central neuropathic pain evoked by dorsal root avulsion could be reversed by a single intrathecal injection of A2AR agonists [32]. The beneficial effects of A2AR agonists were associated with reduced reactive gliosis in the CNS. However, A2AR antagonists reduced chemotherapy-induced neuropathic pain when administered orally [33]. The discrepancies between the reported effects of A2ARs in pain control could be due to the possible opposing roles that this AR subtype exerts in the periphery (anti-inflammatory effect) versus the CNS (pro-excitatory effect) (for reviews, see [21,22,23,24,25,26,27,28,29,30,31,32,33,34]).
By using an animal model of acute peripheral pain, e.g., subcutaneous glutamate injection into the hind paw of mice, Macedo and co-workers recently confirmed the anti-nociceptive effect of peripheral A1R activation by demonstrating that intraplantar (i.pl.) N6-cyclohexyl-adenosine (CHA, A1R agonist) administration reduced glutamate-evoked nociception. On the other hand, the A2AR agonist CGS21680 increased—whereas the A2AR antagonist ZM241385 decreased—pain behavior in the same experimental model [35]. Of note, neither the A2R agonist, N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)-ethyl]adenosine (DPMA), nor the A3R agonist, 2-hexyn-1-yl-N6-methyladenosine (HEMADO), had any effect on glutamate-induced pain. The authors conclude that peripheral A1Rs alleviate, while peripheral A2AR exacerbate, acute nociception, whereas A2BRs and A3Rs do not participate in this particular pain mechanism [35]. Of note, this in vivo experimental model, even if useful to investigate acute pain mechanisms, does not cover the issue of pain chronicization.
Regarding the less studied and less characterized AR subtype in chronic models of pain, A2BR, contrasting evidence exists in the literature. On the one hand, A2BR activation promotes pain states by increasing the release of interleukin-6 (IL-6) [36,37,38], a pro-inflammatory cytokine also known to cause nociceptor hyperexcitability [36]. On the other hand, the selective A2BR agonism, similarly to A2AR activation, alleviates the mechanical allodynia induced by different rat models of long-lasting neuropathic pain states, i.e., by long-established chronic constriction injury (CCI; monitored up to 6 weeks after surgery), spinal nerve ligation, or sciatic inflammatory neuropathy [39]. In these experimental paradigms, both A2AR and A2BR receptor subtypes shared a common intracellular pathway to produce pain relief, consisting in the attenuation of tumor necrosis factor-α (TNFα) production in microglia and astrocytes by protein kinase A (PKA) and/or protein kinase C (PKC) activation [39]. Of note, in the same paper, the authors also confirmed the anti-allodynic effect of selective A1R agonism. Interestingly, they found that A2AR- or A2BR-mediated pain control lasted for a significantly longer time (i.e., up to 6 weeks) than the A1R-mediated anti-allodynic effect. This concept could be crucial to gaining insight into the different pain-relieving mechanisms mediated by distinct AR subtypes.
Unfortunately, the clinical translation of A1R or A2AR agonists for pain relief was hindered by important side effects caused by A1Rs being expressed in conducting tissues or A2ARs in vascular smooth muscle, e.g., bradycardia and vasodilation, respectively [40]. Furthermore, data on A2AR’s role in pain are elusive and often contradictory [41], thus failing to concretize into a clinical approach.
In contrast, the activation of the A3R in humans by potent, selective, and orally bioavailable A3R agonists, e.g., IB-MECA (1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purine-9-yl]-N-methyl-β-D-ribofuranuronamide) and its chlorinated counterpart Cl-IB-MECA (2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide), is not associated with cardiac or hemodynamic effects [42], thus pointing to these compounds as potential therapeutics for the treatment of a number of central or peripheral diseases. It is important to note that these A3R-selective nucleosides are already in phase 2 and/or 3 clinical trials for autoimmune inflammatory diseases, liver cancer, and non-alcoholic steatohepatitis (see NCT00556894 at https://www.clinicaltrials.gov/ct2/show/NCT00556894?id=NCT00556894&draw=2&rank=1&load=cart; or NCT02927314 at https://www.clinicaltrials.gov/ct2/show/NCT02927314?term=NCT02927314&draw=2&rank=1) [43]. Hence, further attention was focused on this AR subtype for the investigation of innovative pain-relieving strategies, as detailed below.
2. A3R and Pain
The A3R is Gi-coupled, similarly to the A1R, and its activation decreases intracellular cAMP levels, an effect classically related to pain control [44]. However, in the late 1990s, when the first A3R-mediated effects on pain were described, it was found that the local application of the A3R agonist produced an intrinsic, formalin-like, nociceptive response, and potentiated the algesic effect of low formalin concentrations [44,45]. The pro-nociceptive role of peripheral A3R activation was later ascribed to the stimulation of this receptor subtype on mast cells [46], where it is highly expressed and represents one of the most efficacious stimuli to achieve degranulation and histamine release [47]. However, A3R activation does not induce degranulation and histamine release in human mast cells [48].
More recently, extensive evidence has highlighted the opposite, i.e., anti-hyperalgesic, effect of A3R activation in different rodent models of neuropathic pain. Works performed by the team of Salvemini demonstrated that a single, intraperitoneal (i.p.) injection (at day 7 after injury) of prototypical (IB-MECA, Cl-IB-MECA) or newly synthesized (MRS1898) A3R agonists proved effective in relieving neuropathic pain caused by CCI or chemotherapy treatment in rodents [49]. These effects were sensitive to the prototypical A3R antagonist MRS1523, but not to the A1R and A2AR blockers DPCPX and SCH-442416, respectively, nor to the opioid antagonist naloxone [49]. Of note, A3R-mediated pain control was achieved after a single dose of IB-MECA administered at the peak of CCI-induced allodynia (on day 7 after nerve ligation: D7), and was not different from that produced by consecutive daily injections of the compound (from D8 to D15). Thus, it was shown that efficient A3R-mediated pain relief is achieved after a single acute administration of the agonist, and that the A3R does not become tolerant to agonist activation [49]. It is worth noting that A3R agonist treatment did not affect the physiological pain threshold in the contralateral paw, indicating a specific anti-hyperalgesic effect in conditions of altered sensitivity that causes the selective alleviation of persistent neuropathic pain states, but without analgesic effects [49,50]. A3R agonist-mediated pain relief was absent in A3R knock-out (KO) mice [50].
The potent pain-relieving effect of A3Rs was further confirmed in mice and rats using a variety of pain models [50,51,52,53,54] and different A3R ligands, including innovative, highly selective A3R agonists synthesized by Jacobson and coworkers (MRS5841: [55]; MRS7220: [56]; MRS7154: [57]; for a review see [58]), among which are the “first in class” compounds MRS5980 [59] and MRS5698 [60,61] and the water-soluble prodrug MRS7476 [62]. Interestingly, the efficacy of A3R stimulation was recently demonstrated against colitis-induced visceral pain [63].
Of note, extracellular adenosine overload during injury or stress conditions can be further increased by inhibiting the enzyme responsible for its extracellular degradation, adenosine kinase (AK) [64]. So, in addition to treatment with AR agonists, the local administration of an AK inhibitor can also modify behaviors produced by an inflammatory stimulus. Accordingly, systemic administration of the AK inhibitor ABT-702, at the peak of CCI-induced pain (D7), significantly increased intraspinal adenosine levels and reversed mechanical allodynia in CCI mice. This effect was partially attenuated by pre- or post-treatment with the selective A3R antagonist MRS1523 [50]. Again, no effects of the tested compounds were observed on the mechanical pain threshold in non-injured animals [50]. Similar results were obtained, in the same work, via paclitaxel or oxaliplatin treatment in animals developing peripheral neuropathy, i.e., chemotherapy-induced neuropathic pain (CINP) [50]. This mechanism of action was further confirmed by later work demonstrating that the upregulation of spinal AK exacerbates oxaliplatin-induced allodynia [61] by astrocyte-dependent signaling in the spinal cord, an effect prevented by i.th. injections of the A3R antagonist MRS1523 [61].
Taken together, these data indicate that A3R-mediated pain control is efficiently achieved in mice and rats [51], and is evident after either systemic (i.p.: [49,50,51,52,53]), oral [49,50,51,52,53,54,55,56,57,58,59,60], subcutaneous [50] or spinal (intrathecal, i.t.: [49,50,51]) administration of A3R agonists. All effects were prevented by the spinal administration of the selective A3R antagonist MRS1523 [50,51], thus pointing to the predominantly central mode of action of this receptor subtype. However, the exact mechanism(s) of A3R-mediated anti-hyperalgesia are still not fully understood and, as stated below, peripheral routes of action have also been described.
3. Mechanisms of A3R-Mediated Pain Control
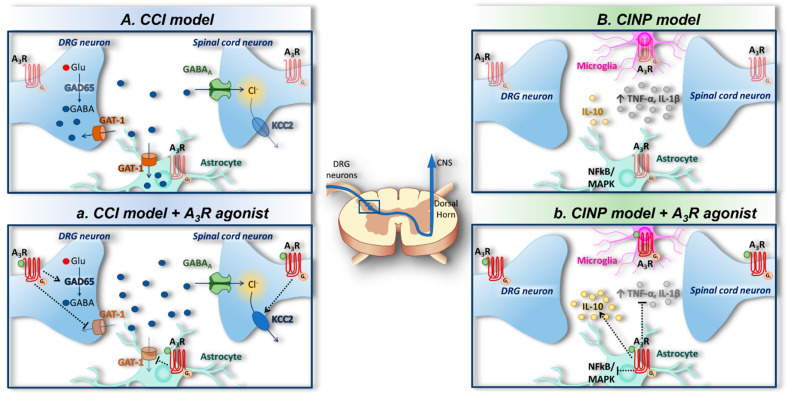
Various molecular mechanisms have been proposed to mediate A3R-induced pain relief. Janes et al. (2014) demonstrated that A3R agonists block the development of chemotherapy-induced neuropathic pain (CINP) by exerting beneficial effects associated with the modulation of spinal neuro-glial communication and neuroinflammatory processes. IB-MECA administered i.p. 15 min prior to each paclitaxel dose attenuated astrocytic activation in the spinal cord by inhibiting NADPH oxidase, and by enhancing redox-sensitive nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) and MAPK kinases such as ERK1/2 and p38. This results in a reduced level of the neuroexcitatory/proinflammatory cytokines TNF-α and interleukin-1β (IL-1β), whereas an increase in the neuroprotective/anti-inflammatory interleukin-10 (IL-10) was observed, as well as restored synaptic glutamate homeostasis at the dorsal horns [52] (Figure 2A). These data suggest that the inhibition of an astrocyte-associated neuroinflammatory response in the spinal cord contributes to the protective actions of A3R, as later data demonstrate that microglial activation was not observed during the development of oxaliplatin-induced mechano-hypersensitivity in rats [53]. Of note, the authors add important information to this scenario by demonstrating that the A3R-mediated inhibition of redox state alterations in the spinal cord during chemotherapy treatment results in a second A3R-mediated protective mechanism, e.g., the maintenance of glutamate homeostasis at the synapse, because IB-MECA prevented post-transcriptional alterations in glutamate transporter-1 and glutamine synthetase [52].
Figure 2.
Adenosine A3 receptors and spinal mechanisms of pain control. (A) Chemotherapy-induced neuropathic pain (CINP) is characterized by high neuroexcitatory/proinflammatory cytokine (TNF-α and IL-1β) release and the activation of NFκB and MAPK in the spinal dorsal horn. (a) Glial A3Rs inhibit NFκB and MAPK activation, thus leading to a decreased release of pro-inflammatory TNF-α and IL-1β and an increased release of anti-inflammatory interleukin-10 (IL-10) [52]. (B) Chronic constriction injury (CCI), an animal model of neuropathic pain, induces deregulation of γ-aminobutyric acid (GABA) signaling via a reduction in GABA synthesis by glutamic acid decarboxylase 65-kDa (GAD65), and enhanced GABA reuptake via the GABA transporter 1- (GAT-1) and the loss of Cl− gradient by K+-Cl− co-transporter 2 (KCC2) inhibition, leading to an overall reduction in GABA at the synapse. (b) A3 receptor (A3R) activation prevents CCI-induced GAT-1 enhancement and GAD65 inhibition as well as KCC2 loss of function [51], augmenting extracellular GABA levels.
Another mechanism was identified by Ford and co-workers, who highlighted the fact that the A3R-mediated anti-hyperalgesic effect was completely lost following the intraspinal injection of the γ-aminobutyric acid A (GABAA) receptor antagonist bicuculline [51], indicating recruitment of the GABAergic system in the effect. It is known that tonic GABA inhibition in the dorsal horn is inhibited in neuropathic pain states, thus leading to a net neuronal hyperexcitability [65]. Indeed, the authors [51] demonstrated that the deregulation of GABA signaling in CCI rats and mice was due to a reduced GABA synthesis by glutamic acid decarboxylase 65-kDa (GAD65), enhanced extracellular GABA reuptake via the GABA transporter 1 (GAT-1), and by the loss of the K+-Cl− co-transporter 2- (KCC2)-maintained Cl− gradient [51], collectively leading to reduced extracellular GABA levels. Interestingly, the systemic or spinal administration of A3R agonists (IB-MECA and MRS5698) prevented GAT-1 and GAD65 inactivation, and promoted Cl− gradient maintenance by preserving KCC2 functionality, thus counteracting extracellular GABA decrease and preventing mechanical allodynia (Figure 2B). These effects were blocked by spinal pretreatment with the A3R antagonist MRS1523, indicating a central mode of action of the A3R in this experimental model [51].
A further spinal mechanism was proposed, e.g., mechanical allodynia induced in rats or mice by CCI or CINP, or cancer-induced bone pain [66]. These were reversed by the A3R agonist MRS5698 by reducing the dynamic range of neuron excitability, causing the supraspinal inhibition of nociception through the activation of serotonergic and noradrenergic bulb spinal circuits [50]. Indeed, the authors demonstrated that subcutaneous MRS5698 administered to nerve-injured rats during maximal mechano-allodynia significantly reduced the evocation of wide dynamic-range neuron responses to non-noxious and noxious mechanical, thermal, and electrical stimulation, with peak effects at 1 h post-dosing. Critically, A3R agonism did not produce tolerance nor alter nociceptive thresholds in non-neuropathy animals, therefore causing the selective alleviation of persistent neuropathic pain states [50].
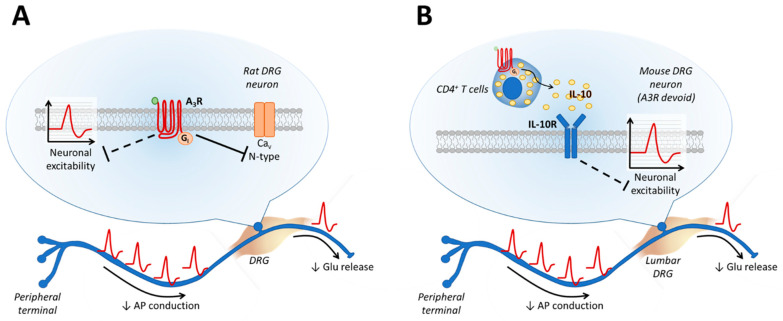
Beyond above mentioned effects on the CNS, further evidence from Coppi et al. (2019) shows that peripheral mechanisms could be involved in A3R-mediated anti-hyperalgesia, because selective agonists of the receptor (Cl-IB-MECA or MRS5980) applied to isolated DRG neurons decreased neuronal firing and inhibited pro-nociceptive Ca2+ currents sensitive to the N-type channel blocker PD732121, an analogue of ω-conotoxin [67] (Figure 3A). It is noteworthy that the selective activation of A1Rs by CPA led to a much smaller Ca2+ current (ICa) inhibition than Cl-IB-MECA, and the effect of the endogenous agonist adenosine was blocked to a higher extent by the A3R-selective antagonist VUF5574 than by the A1R-selective blocker DPCPX. Adenosine-mediated ICa inhibition was completely abolished by a combination of both agonists, demonstrating that A1R and A3R, but not the A2R subtypes, concurrently inhibit N-type ICa (Cav2.2) in rat DRG neurons, but that A3R-mediated effects predominate [67]. These results are in agreement with data from the 1980s showing that adenosine inhibits voltage-dependent Ca2+ channels (VDCCs) in isolated mouse DRG neurons [28], an effect that was only partially ascribed to A1R activation [68]. Hence, by using the DRG in vitro model, it was demonstrated that A3R activation inhibits Ca2+ entry into the neurons and action potential firing, suggesting an inhibition of synaptic transmission at the dorsal horn. However, direct evidence for decreased glutamate release into the spinal cord is still lacking. These results are also in line with previous evidence demonstrating that the aberrant expression and/or activity of N-type VDCCs is associated with neuropathic pain [69], and ziconotide, a derivative of ω-CTX, was FDA approved in 2000 (Prialt) for the intrathecal treatment of severe and refractory chronic pain [70,71,72]. However, severe adverse effects (e.g., hallucinations or other psychiatric symptoms) are associated with a direct Ca2+ channel block, likely due to their wide expression in the CNS.
Figure 3.
Adenosine A3 receptors and peripheral mechanisms for pain control. (A) A3 receptors (A3Rs) expressed on rat DRG neurons decrease action potential (AP) firing by blocking neuronal excitability. The same receptor subtype also inhibits N-type voltage-gated calcium channels (CaV), thus reducing glutamate (Glu) release at the synapse [67]. (B) In a mouse model of CCI, A3Rs expressed on CD4+ T cells, but not on mouse DRG neurons in this rodent species, promote interleukin-10 (IL-10) release, which, by activating IL-10 receptors (IL-10R) on DRG neurons, reduces neuronal excitability [73].
A similar mechanism of action, e.g., Cav2.2 inhibition, has been proposed by Lucarini et al. for A3R-mediated visceral pain relief in a rat model of experimental colitis reproduced by dinitrobenzenesulfonic acid (DNBS) treatment [63]. In this case, as in the CCI model, a single injection of Cl-IB-MECA, or the more selective A3R agonist MRS5980, at the peak of visceral hypersensitivity (day 14 after DNBS injection) was found to be effective in relieving visceral allodynia measured by quantifying the number of abdominal muscle contractions upon intestine distention [63]. Interestingly, the N-type Ca2+ channel blocker PD723212 mimicked A3R agonist-mediated pain control, thus confirming previous evidence implicating this ion channel in DRG electrical activity [67] (Figure 3a).
An additional peripheral pathway of A3R-medited anti-hyperalgesia has recently been identified by Durante and co-workers. Surprisingly, it was found that transgenic Rag-KO mice, lacking T and B cells, are insensitive to the anti-allodynic effects of A3R agonist MRS5980, whereas the adoptive transfer of CD4+ T cells from wild-type (wt) mice infiltrated the inflamed DRG and restored A3R agonist-mediated anti-allodynia [73]. Of note, the adoptive transfer of CD4+ T cells from A3R-KO or IL-10-KO mice did not restore the A3R-mediated anti-hyperalgesic effect, demonstrating, for the first time, that A3R activation on CD4+ T cells elicits IL-10 release, which, in turn, is responsible for the anti-hyperalgesic effect of MRS5980 [73]. Further downstream mechanisms of the A3R-mediated anti-allodynic effect were elucidated in the same work by use of an innovative in vitro model consisting of co-cultures of DRG neurons and T cells isolated from the same animal, either naïve or CCI mice. By this paradigm, CD4+ or CD8+ T cell infiltration into the ganglion was reproduced in vitro via a circumscribed experimental model, wherein any eventual effects of the cytokine(s) released by the T cells on DRG neurons upon A3R stimulation could be evaluated in isolation. Notably, the application of the A3R agonist MRS5980 to DRG-CD4+ T cell co-cultures significantly reduced the action potential (AP) firing evoked by a depolarizing current ramp and increased the current threshold for AP initiation. The effect was not observed in DRG-CD8+ T cell co-cultures, or in DRG neurons cultured alone, demonstrating the selective engagement of A3Rs expressed on CD4+ T cells [73]. Importantly, the effect of MRS5980 on DRG neuronal firing was also abolished by pre-treatment with an anti-IL-10 selective antibody, thus unequivocally pointing to this anti-inflammatory cytokine as the main effector produced by CD4+ T cells upon A3R activation, inhibiting neuronal excitability (Figure 3b). The results were not different when either DRG or CD4+ T cells were isolated from naïve or CCI animals [73]. Interestingly, the involvement of IL-10 in A3R-mediated pain control was already evident in a previous work, where attenuating IL-10 signaling in oxaliplatin-treated rats was achieved with an intrathecal neutralizing IL-10 antibody, or in IL-10-/- mice [61].
Interestingly, a recent paper outlined that the pain-relief properties of some tricyclic antidepressants, such as amitriptyline, one of the most widely prescribed antidepressants for neuropathic pain management [74], might be at least partly ascribed to AR activation, since it is blocked by caffeine pre-treatment [75,76]. In particular, it was demonstrated that A3R antagonists prevent amitriptyline-induced anti-hyperalgesia in CCI rats by preventing the decrease in pro-inflammatory cytokines produced by spinal nerve ligation and by the phosphorylation of ERK1/2 and cAMP response element-binding protein (CREB) [77]. This suggests that A3R activation might facilitate the balance between pro-inflammatory and anti-inflammatory pathways in favor of the latter.
Interestingly, a certain degree of sexual dimorphism in A3R-mediated pain control has recently been outlined. Indeed, differences in the effects of A3R agonists in male and female rodents with paclitaxel-, oxaliplatin- or bortezomib-induced peripheral neuropathy have been reported. The study shows that the A3R agonist MRS5698 failed to prevent bortezomib-induced neuropathic pain in female, but not male, rodents [78]. Of note, morphine and duloxetine, both clinical analgesics, were equally effective in both sexes, demonstrating that, on one hand, different chemotherapeutic treatments engage distinct intracellular pathways to produce mechanical allodynia, and, on the other, the lack of response to bortezomib-induced hyperalgesia in females is specific to A3R ligands. In the same work, the authors suggested that the lack of effect of MRS5980 in female rats was likely due to the absence of A3R overexpression in the spinal cord after bortezomib treatment, in contrast to male rats, in which A3R is overexpressed [78].
Finally, A3R agonists could be attractive co-therapeutics with opioids. Indeed, the prevention of morphine-induced AK overexpression in the dorsal horn of the spinal cord provided a >90% attenuation of antinociceptive tolerance in CCI mice, an effect mimicked by the selective A3R agonists IB-MECA and MRS5698 [79]. Interestingly, and in line with the above results, the beneficial effects of A3R stimulation in mechanical allodynia were associated with the inhibition of the NOD-like receptor pyrin domain-containing 3 inflammasome in the spinal cord, leading to decreased IL-1β production and increased IL-10 levels [79]. Furthermore, A3R activation following treatment with levels of A3R agonist (i.th.) that had no effect on nociceptive threshold in morphine-naïve rats potentiated/restored antinociception (tail flick assay) in morphine-tolerant rats [80].
4. Conclusions
The modulation of A3Rs induces potent anti-hypersensitive effects in diverse preclinical models of chronic pain. Nevertheless, the mechanism by which this AR subtype exerts anti-hyperalgesic and anti-allodynic effects is still to be clarified, and before now, both peripheral (CD4+ T cell-mediated production of anti-inflammatory IL-10 or DRG neuronal inhibition) and central (spinal astrocyte reactivity inhibition, increased GABA release in the spinal cord, inhibition of spinal and supraspinal activation of serotonergic and noradrenergic circuits) effects have been described, with some gender-specific effects outlined in particular cases (e.g., bortezomib-induced neuropathic pain). Of note, the efficacy of A3R ligands for the pharmacological control of chronic pain states is particularly relevant because it is devoid of tolerance effects (in animal models) or significant side effects (in animals and humans). However, the clinical relevance of A3R-mediated pain control still needs to be demonstrated, and, even if results from clinical trials for other pathologies encourage the use of these ligands for their safe profile, future work will help us elucidate the feasibility of A3R-based treatments in humans.
Acknowledgments
We thank J. Eissenberg (Saint Louis University) for editing the manuscript.
Abbreviations
| A1Rs, A2ARs, A2BRs or A3Rs | A1, A2A, A2B or A3 receptors |
| AC | adenylyl cyclase |
| AK | adenosine kinase |
| AR | adensoine receptor |
| cAMP | cyclic adenosine monophosphate |
| CINP | chemotherapy-induced neuropathic pain |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| DNBS | dinitrobenzenesulfonic acid |
| DRG | dorsal root ganglion |
| ERK1/2 | extracellular signal-regulated kinase ½ |
| GABA | γ-aminobutyric acid |
| GAD65 | Glutamic Acid Decarboxylase 65-kDa |
| GAT-1 | GABA transporter 1 |
| GPCR | G protein-coupled receptors |
| ICa | Ca2+ current |
| IL | interleukin: |
| i.p. | intraperitoneal |
| i.pl | intraplantar |
| i.t. | intrathecal |
| JNK | Jc-Jun-NH2 terminal Kinase JUN N-terminal kinase |
| KCC2 | K+-Cl− co-transporter 2 |
| KO | knock out |
| MAPK | mitogen-activated protein kinase |
| NFκB | nuclear factor k-light-chain-enhancer of activated B cells |
| PKA | protein kinase A |
| PKC | protein kinase C |
| TNFα | tumor necrosis factor-α |
| VDCC | voltage-dependent Ca2+ channel |
Author Contributions
Conceptualization, E.C., L.D.C.M. and D.S.; writing—original draft preparation, E.C. and F.C.; review and editing, A.M.P., L.D.C.M., E.L., C.G. and E.C.; supervision F.P., A.M.P., D.S. and K.A.J.; funding acquisition, A.M.P., D.S. and K.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was supported by the University of Florence (Fondi Ateneo, AMP); Fondazione Italiana Sclerosi Multipla (FISM): 2019/R-Single/036 (AMP and EC); NIH/NCI R01 CA169519 (DS); NIDDK Intramural Research Program (ZIADK31117; KJ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Gilron I., Haanpaa M., Hansson P., Jensen T.S., et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Muller C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacol. Rev. 2011;63 doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonioli L., Blandizzi C., Pacher P., Hasko G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019;71:345–382. doi: 10.1124/pr.117.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guieu R., Deharo J.C., Maille B., Crotti L., Torresani E., Brignole M., Parati G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020;9:1366. doi: 10.3390/jcm9051366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G., Krugel U., Abbracchio M.P., Illes P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Coppi E., Pedata F., Gibb A.J. P2Y1 receptor modulation of Ca2+-activated K+ currents in medium-sized neurons from neonatal rat striatal slices. J. Neurophysiol. 2012;107:1009–1021. doi: 10.1152/jn.00816.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maraula G., Traini C., Mello T., Coppi E., Galli A., Pedata F., Pugliese A.M. Effects of oxygen and glucose deprivation on synaptic transmission in rat dentate gyrus: Role of A2A adenosine receptors. Neuropharmacology. 2013;67:511–520. doi: 10.1016/j.neuropharm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Fusco I., Ugolini F., Lana D., Coppi E., Dettori I., Gaviano L., Nosi D., Cherchi F., Pedata F., Giovannini M.G., et al. The Selective Antagonism of Adenosine A2B Receptors Reduces the Synaptic Failure and Neuronal Death Induced by Oxygen and Glucose Deprivation in Rat CA1 Hippocampus in Vitro. Front. Pharmacol. 2018;9:399. doi: 10.3389/fphar.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fusco I., Cherchi F., Catarzi D., Colotta V., Varano F., Pedata F., Pugliese A.M., Coppi E. Functional characterization of a novel adenosine A2B receptor agonist on short-term plasticity and synaptic inhibition during oxygen and glucose deprivation in the rat CA1 hippocampus. Brain Res. Bull. 2019;151:174–180. doi: 10.1016/j.brainresbull.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppi E., Dettori I., Cherchi F., Bulli I., Venturini M., Pedata F., Pugliese A.M. New Insight into the Role of Adenosine in Demyelination, Stroke and Neuropathic Pain. Front. Pharmacol. 2021;11:2403. doi: 10.3389/fphar.2020.625662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherchi F., Pugliese A.M., Coppi E. Oligodendrocyte precursor cell maturation: Role of adenosine receptors. Neural Regen. Res. 2021;16:1686–1692. doi: 10.4103/1673-5374.306058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzzi M., Coppi E., Pugliese A.M., Chiarugi A. Anticonvulsant effect of AMP by direct activation of adenosine A1 receptor. Exp. Neurol. 2013;250:189–193. doi: 10.1016/j.expneurol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Coppi E., Dettori I., Cherchi F., Bulli I., Venturini M., Lana D., Giovannini M.G., Pedata F., Pugliese A.M. A2B Adenosine Receptors: When Outsiders May Become an Attractive Target to Treat Brain Ischemia or Demyelination. Int. J. Mol. Sci. 2020;21:9697. doi: 10.3390/ijms21249697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedata F., Dettori I., Coppi E., Melani A., Fusco I., Corradetti R., Pugliese A.M. Purinergic signalling in brain ischemia. Neuropharmacology. 2016;104:105–130. doi: 10.1016/j.neuropharm.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Cronstein B.N. Adenosine, an endogenous antiinflammatory agent. J. Appl. Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Cronstein B.N. A novel-approach to the development of antiinflammatory agents—Adenosine release at inflamed sites. J. Investig. Med. 1995;43:50–57. [PubMed] [Google Scholar]
- 19.Cronstein B.N., Sitkovsky M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017;13:41–51. doi: 10.1038/nrrheum.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasko G., Antonioli L., Cronstein B.N. Adenosine metabolism, immunity and joint health. Biochem. Pharmacol. 2018;151:307–313. doi: 10.1016/j.bcp.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338 doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Yoon M.H., Bae H.B., Choi J.I. Antinociception of intrathecal adenosine receptor subtype agonists in rat formalin test. Anesth. Analg. 2005;101:1417–1421. doi: 10.1213/01.ANE.0000180994.10087.6F. [DOI] [PubMed] [Google Scholar]
- 23.Taiwo Y.O., Levine J.D. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–762. doi: 10.1016/0306-4522(90)90068-F. [DOI] [PubMed] [Google Scholar]
- 24.Karlsten R., Gordh T., Post C. Local antinociceptive and hyperalgesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacol. Toxicol. 1992;70 Pt 1:434–438. doi: 10.1111/j.1600-0773.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 25.Doak G.J., Sawynok J. Complex role of peripheral adenosine in the genesis of the response to subcutaneous formalin in the rat. Eur. J. Pharmacol. 1995;281:311–318. doi: 10.1016/0014-2999(95)00257-L. [DOI] [PubMed] [Google Scholar]
- 26.Taiwo Y.O., Levine J.D. further confirmation of the role of adenyl-cyclase and of camp-dependent protein-kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-M. [DOI] [PubMed] [Google Scholar]
- 27.Khasar S.G., Wang J.F., Taiwo Y.O., Heller P.H., Green P.G., Levine J.D. Mu-opioid agonist enhancement of prostaglandin-induced hyperalgesia in the rat—A G-protein beta-gamma subunit-mediated effect. Neuroscience. 1995;67:189–195. doi: 10.1016/0306-4522(94)00632-F. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald R.L., Skerritt J.H., Werz M.A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurons in cell-culture. J. Physiol. 1986;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santicioli P., Delbianco E., Maggi C.A. Adenosine-a1-receptors mediate the presynaptic inhibition of calcitonin gene-related peptide release by adenosine in the rat spinal-cord. Eur. J. Pharmacol. 1993;231:139–142. doi: 10.1016/0014-2999(93)90695-E. [DOI] [PubMed] [Google Scholar]
- 30.Vincenzi F., Pasquini S., Borea P.A., Varani K. Targeting Adenosine Receptors: A Potential Pharmacological Avenue for Acute and Chronic Pain. Int. J. Mol. Sci. 2020;21:8710. doi: 10.3390/ijms21228710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zylka M.J. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwilasz A.J., Ellis A., Wieseler J., Loram L., Favret J., McFadden A., Springer K., Falci S., Rieger J., Maier S.F., et al. Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav. Immun. 2018;69:470–479. doi: 10.1016/j.bbi.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Falsini M., Catarzi D., Varano F., Ceni C., Dal Ben D., Marucci G., Buccioni M., Volpini R., Di Cesare Mannelli L., Lucarini E., et al. Antioxidant-Conjugated 1,2,4-Triazolo 4,3-a pyrazin-3-one Derivatives: Highly Potent and Selective Human A(2A) Adenosine Receptor Antagonists Possessing Protective Efficacy in Neuropathic Pain. J. Med. Chem. 2019;62:8511–8531. doi: 10.1021/acs.jmedchem.9b00778. [DOI] [PubMed] [Google Scholar]
- 34.Magni G., Riccio D., Ceruti S. Tackling Chronic Pain and Inflammation through the Purinergic System. Curr. Med. Chem. 2018;25:3830–3865. doi: 10.2174/0929867324666170710110630. [DOI] [PubMed] [Google Scholar]
- 35.Macedo S.J., Nascimento F.P., Luiz-Cerutti M., Santos A.R.S. The role of peripheral adenosine receptors in glutamate-induced pain nociceptive behavior. Purinergic Signal. 2021;17:303–312. doi: 10.1007/s11302-021-09781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu X., Adebiyi M.G., Luo J.L., Sun K.Q., Le T.T.T., Zhang Y.J., Wu H.Y., Zhao S.S., Karmouty-Quintana H., Liu H., et al. Sustained Elevated Adenosine via ADORA2B Promotes Chronic Pain through Neuro-immune Interaction. Cell Rep. 2016;16:106–119. doi: 10.1016/j.celrep.2016.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotanska M., Szafarz M.L., Mika K., Dziubina A., Bednarski M., Muller C.E., Sapa J., Kiec-Kononowicz K. PSB 603—A known selective adenosine A2B receptor antagonist—Has anti-inflammatory activity in mice. Biomed. Pharmacother. 2021;135:111164. doi: 10.1016/j.biopha.2020.111164. [DOI] [PubMed] [Google Scholar]
- 38.Wei W., Du C.S., Lv J., Zhao G.X., Li Z.X., Wu Z.Y., Hasko G., Xie X. Blocking A2B Adenosine Receptor Alleviates Pathogenesis of Experimental Autoimmune Encephalomyelitis via Inhibition of IL-6 Production and Th17 Differentiation. J. Immunol. 2013;190:138–146. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loram L.C., Taylor F.R., Strand K.A., Harrison J.A., RzasaLynn R., Sholar P., Rieger J., Maier S.F., Watkins L.R. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav. Immun. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deb P.K., Deka S., Borah P., Abed S.N., Klotz K.N. Medicinal Chemistry and Therapeutic Potential of Agonists, Antagonists and Allosteric Modulators of A1 Adenosine Receptor: Current Status and Perspectives. Curr. Pharm. Des. 2019;25:2697–2715. doi: 10.2174/1381612825666190716100509. [DOI] [PubMed] [Google Scholar]
- 41.Luongo L., Salvemini D. Targeting metabotropic adenosine receptors for neuropathic pain: Focus on A2A. Brain Behav. Immun. 2018;69:60–61. doi: 10.1016/j.bbi.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Silverman M.H., Strand V., Markovits D., Nahir M., Reitblat T., Molad Y., Rosner I., Rozenbaum M., Mader R., Adawi M., et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: Data from a phase II clinical trial. J. Rheumatol. 2008;35:41–48. [PubMed] [Google Scholar]
- 43.Jacobson K.A., Tosh D.K., Jain S., Gao Z.G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019;13:124. doi: 10.3389/fncel.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawynok J., Reid A. Role of G-proteins and adenylate-cyclase in antinociception produced by intrathecal purines. Eur. J. Pharmacol. 1988;156:25–34. doi: 10.1016/0014-2999(88)90143-4. [DOI] [PubMed] [Google Scholar]
- 45.Sawynok J. Adenosine receptor activation and nociception. Eur. J. Pharmacol. 1998;347 doi: 10.1016/S0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 46.Sawynok J., Zarrindast M.R., Reid A.R., Doak G.J. Adenosine A3 receptor activation produces nociceptive behaviour and edema by release of histamine and 5-hydroxytryptamine. Eur. J. Pharmacol. 1997;333 doi: 10.1016/S0014-2999(97)01110-2. [DOI] [PubMed] [Google Scholar]
- 47.Ramkumar V., Stiles G.L., Beaven M.A., Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast-cells. J. Biol. Chem. 1993;268:16887–16890. doi: 10.1016/S0021-9258(19)85277-8. [DOI] [PubMed] [Google Scholar]
- 48.Leung C.T., Li A., Banerjee J., Gao Z.G., Kambayashi T., Jacobson K.A., Civan M.M. The role of activated adenosine receptors in degranulation of human LAD2 mast cells. Purinergic Signal. 2014;10:465–475. doi: 10.1007/s11302-014-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z.M., Janes K., Chen C., Doyle T., Bryant L., Tosh D.K., Jacobson K.A., Salvemini D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;26:1855–1865. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little J.W., Ford A., Symons-Liguori A.M., Chen Z.M., Janes K., Doyle T., Xie J., Luongo L., Tosh D.K., Maione S., et al. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain. 2015;138:28–35. doi: 10.1093/brain/awu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford A., Castonguay A., Cottet M., Little J.W., Chen Z., Symons-Liguori A.M., Doyle T., Egan T.M., Vanderah T.W., De Koninck Y., et al. Engagement of the GABA to KCC2 Signaling Pathway Contributes to the Analgesic Effects of A3AR Agonists in Neuropathic Pain. J. Neurosci. 2015;35:6057–6067. doi: 10.1523/JNEUROSCI.4495-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janes K., Esposito E., Doyle T., Cuzzocrea S., Tosh D.K., Jacobson K.A., Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155:2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janes K., Wahlman C., Little J.W., Doyle T., Tosh D.K., Jacobson K.A., Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav. Immun. 2015;44:91–99. doi: 10.1016/j.bbi.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tosh D.K., Paoletta S., Ford A., Janes K., Salvemini D., Jacobson K.A. Abstracts of Papers of the American Chemical Society. Volume 247. Amer Chemical Soc; Washington, DC, USA: 2014. Discovery of next generation A3 adenosine receptor selective agonists for treatment of chronic neuropathic pain; p. 53. 1155 16TH ST, NW. [Google Scholar]
- 55.Paoletta S., Tosh D.K., Finley A., Gizewski E.T., Moss S.M., Gao Z.G., Auchampach J.A., Salvemini D., Jacobson K.A. Rational Design of Sulfonated A3 Adenosine Receptor-Selective Nucleosides as Pharmacological Tools to Study Chronic Neuropathic Pain. J. Med. Chem. 2013;56:5949–5963. doi: 10.1021/jm4007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tosh D.K., Ciancetta A., Warnick E., O’Connor R., Chen Z.M., Gizewski E., Crane S., Gao Z.G., Auchampach J.A., Salvemini D., et al. Purine (N)-Methanocarba Nucleoside Derivatives Lacking an Exocyclic Amine as Selective A3 Adenosine Receptor Agonists. J. Med. Chem. 2016;59:3249–3263. doi: 10.1021/acs.jmedchem.5b01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosh D.K., Crane S., Chen Z.M., Paoletta S., Gao Z.G., Gizewski E., Auchampach J.A., Salvemini D., Jacobson K.A. Rigidified A3 Adenosine Receptor Agonists: 1-Deazaadenine Modification Maintains High in Vivo Efficacy. ACS Med. Chem. Lett. 2015;6:804–808. doi: 10.1021/acsmedchemlett.5b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobson K.A., Giancotti L.A., Lauro F., Mufti F., Salvemini D. Treatment of chronic neuropathic pain: Purine receptor modulation. Pain. 2020;161:1425–1441. doi: 10.1097/j.pain.0000000000001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tosh D.K., Finley A., Paoletta S., Moss S.M., Gao Z.G., Gizewski E.T., Auchampach J.A., Salvemini D., Jacobson K.A. In Vivo Phenotypic Screening for Treating Chronic Neuropathic Pain: Modification of C2-Arylethynyl Group of Conformationally Constrained A3 Adenosine Receptor Agonists. J. Med. Chem. 2014;57:9901–9914. doi: 10.1021/jm501021n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tosh D.K., Padia J., Salvemini D., Jacobson K.A. Efficient, large-scale synthesis and preclinical studies of MRS5698, a highly selective A3 adenosine receptor agonist that protects against chronic neuropathic pain. Purinergic Signal. 2015;11:371–387. doi: 10.1007/s11302-015-9459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahlman C., Doyle T.M., Little J.W., Luongo L., Janes K., Chen Z., Esposito E., Tosh D.K., Cuzzocrea S., Jacobson K.A., et al. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain. 2018;159:1025–1034. doi: 10.1097/j.pain.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suresh R.R., Jain S., Chen Z.M., Tosh D.K., Ma Y.L., Podszun M.C., Rotman Y., Salvemini D., Jacobson K.A. Design and in vivo activity of A3 adenosine receptor agonist prodrugs. Purinergic Signal. 2020;16:367–377. doi: 10.1007/s11302-020-09715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucarini E., Coppi E., Micheli L., Parisio C., Vona A., Cherchi F., Pugliese A.M., Pedata F., Failli P., Palomino S., et al. Acute visceral pain relief mediated by A3AR agonists in rats: Involvement of N-type voltage-gated calcium channels. Pain. 2020;161:2179–2190. doi: 10.1097/j.pain.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cronstein B.N., Naime D., Firestein G. The antiinflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthritis Rheum. 1995;38:1040–1045. doi: 10.1002/art.1780380804. [DOI] [PubMed] [Google Scholar]
- 65.Zeilhofer H.U., Wildner H., Yevenes G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lozano-Ondoua A.N., Hanlon K.E., Symons-Liguori A.M., Largent-Milnes T.M., Havelin J.J., Ferland H.L., Chandramouli A., Owusu-Ankomah M., Nikolich-Zugich T., Bloom A.P., et al. Disease Modification of Breast Cancer-Induced Bone Remodeling by Cannabinoid 2 Receptor Agonists. J. Bone Miner. Res. 2013;28:92–107. doi: 10.1002/jbmr.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coppi E., Cherchi F., Fusco I., Failli P., Vona A., Dettori I., Gaviano L., Lucarini E., Jacobson K.A., Tosh D.K., et al. Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain. 2019;160:1103–1118. doi: 10.1097/j.pain.0000000000001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gross R.A., Macdonald R.L., Ryanjastrow T. 2-Chloroadenosine reduces the n-calcium current of cultured mouse sensory neurons in a pertussis toxin-sensitive manner. J. Physiol. 1989;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hannon H.E., Atchison W.D. Omega-Conotoxins as Experimental Tools and Therapeutics in Pain Management. Mar. Drugs. 2013;11:680–699. doi: 10.3390/md11030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain K.K. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expert Opin. Investig. Drugs. 2000;9:2403–2410. doi: 10.1517/13543784.9.10.2403. [DOI] [PubMed] [Google Scholar]
- 71.McDowell G.C., Pope J.E. Intrathecal Ziconotide: Dosing and Administration Strategies in Patients with Refractory Chronic Pain. Neuromodulation. 2016;19:522–532. doi: 10.1111/ner.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brookes M.E., Eldabe S., Batterham A. Ziconotide Monotherapy: A Systematic Review of Randomised Controlled Trials. Curr. Neuropharmacol. 2017;15:217–231. doi: 10.2174/1570159X14666160210142056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durante M., Squillace S., Lauro F., Giancotti L.A., Coppi E., Cherchi F., Di Cesare Mannelli L., Ghelardini C., Kolar G., Wahlman C., et al. Adenosine A3 agonists reverse neuropathic pain via T cell-mediated production of IL-10. J. Clin. Investig. 2021;131:e139299. doi: 10.1172/JCI139299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McQuay H.J., Tramer M., Nye B.A., Carroll D., Wiffen P.J., Moore R.A. Systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217–227. doi: 10.1016/S0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 75.Esser M.J., Sawynok J. Caffeine blockade of the thermal antihyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur. J. Pharmacol. 2000;399:131–139. doi: 10.1016/S0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 76.Ulugol A., Karadag H.C., Tamer M., Firat Z., Aslantas A., Dokmeci I. Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci. Lett. 2002;328:129–132. doi: 10.1016/S0304-3940(02)00491-3. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y., Kwon S.Y., Jung H.S., Park Y.J., Kim Y.S., In J.H., Choi J.W., Kim J.A., Joo J.D. Amitriptyline inhibits the MAPK/ERK and CREB pathways and proinflammatory cytokines through A3AR activation in rat neuropathic pain models. Korean J. Anesthesiol. 2019;72:60–67. doi: 10.4097/kja.d.18.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stockstill K., Wahlman C., Braden K., Chen Z.M., Yosten G.L., Tosh D.K., Jacobson K.A., Doyle T.M., Samson W.K., Salvemini D. Sexually dimorphic therapeutic response in bortezomib-induced neuropathic pain reveals altered pain physiology in female rodents. Pain. 2020;161:177–184. doi: 10.1097/j.pain.0000000000001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doyle T.M., Largent-Milnes T.M., Chen Z.M., Staikopoulos V., Esposito E., Dalgarno R., Fan C., Tosh D.K., Cuzzocrea S., Jacobson K.A., et al. Chronic Morphine-Induced Changes in Signaling at the A3 Adenosine Receptor Contribute to Morphine-Induced Hyperalgesia, Tolerance, and Withdrawal. J. Pharmacol. Exp. Ther. 2020;374:331–341. doi: 10.1124/jpet.120.000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leduc-Pessah H., Xu C., Fan C.Y., Dalgarno R., Kohro Y., Sparanese S., Burke N.N., Jacobson K.A., Altier C., Salvemini D., et al. Spinal A3 adenosine receptor activation acutely restores morphine antinociception in opioid tolerant male rats. J. Neurosci. Res. 2021 doi: 10.1002/jnr.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.