Abstract
The pathological aggregation of the presynaptic protein α-synuclein (α-syn) and propagation through synaptically coupled neuroanatomical tracts is increasingly thought to underlie the pathophysiological progression of Parkinson’s disease (PD) and related synucleinopathies. Although the precise molecular mechanisms responsible for the spreading of pathological α-syn accumulation in the CNS are not fully understood, growing evidence suggests that de novo α-syn misfolding and/or neuronal internalization of aggregated α-syn facilitates conformational templating of endogenous α-syn monomers in a mechanism reminiscent of prions. A refined understanding of the biochemical and cellular factors mediating the pathological neuron-to-neuron propagation of misfolded α-syn will potentially elucidate the etiology of PD and unravel novel targets for therapeutic intervention. Here, we discuss recent developments on the hypothesis regarding trans-synaptic propagation of α-syn pathology in the context of neuronal vulnerability and highlight the potential utility of novel experimental models of synucleinopathies.
Keywords: Parkinson’s disease, alpha-synuclein, prion-like, neurodegeneration
1. Introduction
Parkinson’s disease (PD) is a major neurodegenerative disease causing progressive motor disability in individuals over 55–60 years of age and affects both genders with a slight male preponderance [1,2,3]. Clinical PD is defined by the cardinal signs of TRAP (resting Tremor, Rigidity, Akinesia/bradykinesia and Postural/gait instability) [4], which respond to L-dopa therapy (especially tremor and rigidity) [1,2]. The etiology of PD-like motor disability, termed parkinsonism, is predominantly of idiopathic nature, but can also be observed in other neurological conditions (e.g., post-encephalitis, repetitive traumatic brain injury, progressive supranuclear palsy) [5]. Moreover, the clinical presentation of idiopathic PD can also be heterogeneous and is further sub-classified into additional variants (e.g., tremor dominant, akinetic-rigid, early disease onset or mixed) with distinct responses to the existing therapeutic modalities [6,7,8]. Lastly, long-standing PD invariably results in cognitive decline and dementia in as many as 30–40% of the cases, especially in patients with the late-onset disease [1,2]. However, it is becoming increasingly evident that PD has a long (15–20 years) prodromal phase during which the affected individuals experience non-motor symptoms, particularly anosmia, autonomic dysfunction, constipation and REM sleep behavior disorder (RBD) [9,10]. In the backdrop of clinical scenarios, the neuropathological diagnosis of PD requires two features: (i) depigmentation/demelanization of the substantia nigra-pars compacta (SNpc) due to the pathological loss of dopaminergic neurons and (ii) the presence of Lewy related α-synuclein (α-syn; gene symbol SNCA/PARK1) pathology (LRP), e.g., Lewy bodies (LBs) and Lewy neurites (LNs), across several brain regions, primarily in the brainstem nuclei [1,11,12]. A detailed overview of PD neuropathology is beyond the scope of this review and can be accessed elsewhere [5,11]. However, it suffices to mention that aggregation and deposition of α-syn in the CNS, either due to genetic predisposition and/or the presence of factors in the local microenvironment that are conducive to α-syn aggregation (i.e., impaired redox homeostasis, ionic imbalance and neuroinflammation [13,14,15]), contribute to the proteopathic stress with detrimental consequences for the neuronal function and/or survival [16,17]. Hence, currently prevailing consensus maintains that PD is the result of chronical loss of dopaminergic neurons in SNpc, which culminates with dysregulated modulatory innervations into the striatum and resultant dysfunction of the nigro-striatal-cortical circuitry in the basal ganglia [1,2,11]. However, post-mortem studies in pathologically diagnosed PD show that a variable degree of LRP is also found in several extra-nigral locations (nuclei) in the brainstem, including the dorsal motor nucleus of vagus-dmX and intermediate reticular zone in the caudal brainstem, and more rostrally in the gigantocellular reticular nucleus (GRN), locus coeruleus (LC) and subcoeruleus complex, raphe nuclei, the tractus solitarius, SNpc, the pedunculopontine nucleus-PPN and the ventral tegmental area [18,19]. In late-stage PD, localized LRP has also been detected in distinct nuclei of the basal forebrain, thalamus, hypothalamus, the olfactory and basolateral portions of the amygdala, the anterior olfactory nucleus, CA2 of the hippocampus, as well as the insular, cingulate and prefrontal cortices [11,20]. These observations suggest that, although not unique to PD, the distribution of LRP in PD is not random and exhibits a predilection for distinct neuronal populations and their connectivity [11,18,21,22]. Accordingly, recent opinions have sought to reconcile the symptomatology of PD with the known aspects of α-syn LRP, especially the pattern(s) of LRP initiation and propagation in the nervous system and the consequent dysfunction in affected neuronal populations [9,20,23].
2. α-Syn Aggregation and Cytotoxicity
α-Syn is a 140-amino-acid cytoplasmic protein that is mostly found within presynaptic nerve terminals and is involved in the assembly of the SNARE complexes [24,25]. α-Syn contains an amphipathic lysine-rich N-terminal region, which plays a crucial role in lipid-binding [26], and an acidic carboxyl-terminal region, which is enriched with acid residues and that has been implicated in the protein’s chaperone-like activity [27]. The central domain of α-syn is known as the non-amyloid-component (NAC) (61–95) and contains a highly hydrophobic motif, essential for α-syn aggregation [28]. Burgeoning genetic and neuropathological evidence suggests that the abnormal aggregation of monomeric α-syn into intracellular insoluble protein inclusions in the brain (e.g., LBs and LNs) plays a key role in the development of several adult-onset neurodegenerative diseases, including PD, Multiple System Atrophy (MSA) and Dementia with Lewy Bodies (DLB), all collectively known as synucleinopathies. LBs are spherical cytoplasmic inclusions, which are 5–25 µm in diameter, and mainly composed of aggregated α-syn, with a dense eosinophilic core surrounded by aggregated α-syn brighter halo [29]. Several point mutations (A30P, E46K, H50Q, G51D or A53T) within the SNCA, as well as duplication and triplication of SNCA, are closely associated with rare early-onset familial PD and DLB cases, demonstrating an unequivocal connection between α-syn and neurological disease [30,31,32,33]. Nevertheless, with the exception of the aforementioned rare familial forms, the great majority of PD cases are sporadic, suggesting that PD etiology is most likely multifactorial, involving a complex interplay between aging, genetic susceptibility and environmental factors [1]. Therefore, understanding the earliest physiological to pathological events underlying α-syn misfolding and abnormal aggregation is of utmost importance, since they provide opportunities for therapeutical intervention.
Despite recent advances, the nature of precise native conformation of α-syn under physiological conditions remains elusive. However, it is widely accepted that α-syn primarily occurs as an intrinsically disordered monomer in the cytosol [34], with few tertiary interactions between the C-terminus and the central hydrophobic NAC region and the N-terminus of the protein [35,36]. A wide variety of conditions have been found to induce α-syn misfolding and aggregation in vitro, including acidic pH [37,38], increased temperature [37], molecular crowding [39], divalent and trivalent metal ions such as aluminum, copper(II), iron(III), cobalt(III) and manganese(II) [40], organic solvents [41], lipids with high solubility in aqueous solution and short hydrocarbon chains [42], heparin and other glycosaminoglycans [43], polycations [44], pesticides [45] and α-syn binding proteins [46,47,48]. In addition, α-syn can undergo extensive post-–translational modifications (PTMs) that are known to modulate its neurotoxicity and its propensity to aggregate, including phosphorylation [49,50,51,52], ubiquitination [53,54], nitration [55,56], sumoylation [57,58], truncation [59,60] and N–terminal acetylation [61,62]. Among the PTMs, approximately 90% of the α-syn aggregates present in LBs are phosphorylated on the serine residue-129 (p-S129), hence S129 hyperphosphorylation of α-syn has been widely regarded as a pathological hallmark of PD and related synucleinopathies [63]. Whether the disease-associated α-syn phosphorylation stimulates or hampers α-syn aggregation, and its neurotoxicity in a pathophysiological context, remain debatable [64,65]. Resembling other aggregation-prone proteins, α-syn self-assembly exhibits a sigmoidal profile in biochemical assays measuring protein aggregation [66,67,68,69,70,71]. The formation of α-syn fibrils typically follows a nucleation-dependent mechanism, consisting of an initial lag phase, followed by a growth phase of elongation and a plateau phase of fibril maturation [72,73]. The oligomeric aggregates can be structurally categorized according to their size and shape, and functionally classified as on-pathway and off-pathway, depending on whether they evolve to form mature amyloid fibrils or, alternatively, result in amorphous, non-fibrillar assemblies [74,75]. Moreover, whether low molecular weight α-syn oligomers, rather than mature fibrils, are the most toxic entities underlying α-syn toxicity remains uncertain. In this regard, a robust dopaminergic loss in the substantia nigra in transgenic animals expressing α-syn variants that form ring/pore-like oligomers has been reported (i.e., E57K and E35K), whereas the α-syn variants that rapidly form fibrils were found to be comparatively less toxic in these experiments [76]. Of note, this study was based on an ectopic lentivirus expressing system, thus, partly limited by the fact that α-syn is overexpressed locally. It is plausible that the fibril forming variants can be actively recruited by LBs into aggresome-like structures, and prevent their abnormal interactions with other cytoplasmic proteins and deleterious effects on the function of cellular organelles [77,78]. A supporting role of oligomeric α-syn neurotoxicity is also suggested by immunotherapy using antibodies targeting oligomeric α-syn, which rescued motor dysfunction in a PD transgenic mouse model [79]. In line with these observations, increased levels of soluble α-syn oligomers have been detected in brain and cerebrospinal fluid (CSF) of patients with LB pathology compared to healthy age-matched controls [80,81,82,83].
Compounding evidence in animal models and cell cultures, including neuronal cultures, implicate a pathogenic role of pathological α-syn aggregation in triggering detrimental effects on the synaptic function, purportedly via calcium dyshomeostasis, mitochondrial impairment, endoplasmic reticulum (ER) stress, defective autophagy, neuroinflammation and oxidative stress [15,17,84]. It has also been suggested that α-syn aggregation in the presynaptic terminals and sequestration into the inclusions affects the assembly of SNARE complexes, thus decreasing the efficiency of dopamine release [85]. Moreover, several synaptic proteins and neurotransmitter receptors (e.g., NMDA glutamate receptors) have been identified as putative interaction partners of α-syn, and these aspects have been reviewed elsewhere [86,87].
3. α-Syn Cell-to-Cell Propagation
Prions are infectious agents in which the conformationally altered protein PrPSc recruits and corrupts its normal counterpart PrPC generating self-propagating misfolded species, which can spread from cell-to-cell [88]. In recent years, it has been demonstrated that several amyloid-forming proteins possibly share an analogous prion-like spreading mechanism, including α-syn [89,90], β-amyloid [91,92], tau [93,94] and Huntingtin [95,96]. Accordingly to the Braak model, PD neuropathological staging follows a highly stereotypical and spatiotemporal progression for the Lewy pathology, suggesting propagation of misfolded α-syn through vulnerable neuroanatomically connected pathways (elaborated below under Section 4) [18]. Initial evidence supporting a prion-like propagation of α-syn came from the observation of α-syn aggregation in grafted fetal mesencephalic progenitor neurons several years after transplantation, and implied host-to-graft Lewy pathology transmission [97,98]. Since then, accumulating evidence has shown that α-syn seeds formed from recombinant proteins or aggregate-containing lysates from diseased brains can propagate following the prion-like paradigm in neuronal cells, in organotypic slice cultures and in rodent models of PD [48,89,90,99,100,101,102,103,104,105,106].
Similar to PrPSc, α-syn can self-assemble into β-sheet-rich amyloid fibrils giving rise to epidemiologically and histopathologically distinct neurodegenerative diseases. In PD and DLB, widespread α-syn aggregation is observed not only in α-syn-expressing neuronal populations (such as LBs and LNs) but also in neighbouring astroglial cells [107,108]. In comparison, the neuropathology of MSA is principally characterized by α-syn aggregates in the oligodendroglia as glial cytoplasmic inclusions (GCIs) and in instances of neuronal nuclear inclusions in discrete brain regions (e.g., base of pons) [12]. In addition, α-syn pathology is also observed in Alzheimer’s disease (AD) [109,110], and in about 20% of neurologically normal elderly individuals as the incidental LB disease (iLBD) [111].
The detailed molecular pathways underlying α-syn exocytosis, the potential interaction of α-syn with extracellular components and the conformational properties of released α-syn in terms of aggregation state remain largely elusive. Misfolded α-syn seeds are secreted from donor cells to the interstitial compartment as naked protein or in vesicles (e.g., exosomes) [112,113,114,115,116,117]. Although the initial focus of this mechanistic research was primarily in neurons, compounding evidence has shown that microglial exosomes may significantly contribute to the progression of α-syn pathology, and potentially serve as a therapeutic target for PD [118,119]. Once in the extracellular space, α-syn seeds have been shown to be taken up by neighbouring cells in culture via several routes, including direct penetration of the plasma membrane, fluid-phase or receptor-mediated endocytosis (e.g, via lymphocyte-activation gene 3 in cultures of primary neurons) or fusion of plasma-exosomal membranes [112,113,114,120,121]. In addition to the release and internalisation mechanisms, tunnelling nanotubes that directly connect two adjacent cells have also been reported to play a role in cell-to-cell transfer of pathological α-syn assemblies [122,123]. Once inside the recipient cell, α-syn seeds purportedly undergo multiple PTMs (e.g., truncation, phosphorylation, ubiquitination), that facilitate interactions with endogenous α-syn monomers and other cytosolic proteins, and further promote α-syn aggregation and propagation [112,113,114]. Therefore, potential therapeutic approaches to modulate these processes include antibodies that specifically target the α-syn seeds or the cellular release and uptake machinery.
Lastly, the involvement of α-syn aggregates in several diseases that exhibit dissimilar phenotypic traits, together with the fact that synthetic α-syn monomers can form polymorphs with distinct conformations and biological activities [124,125,126,127], has led to the recent hypothesis that multiple α-syn strains may underlie the clinical heterogeneity observed in synucleinopathies and other neurodegenerative diseases [48,128,129,130]. Indeed, α-syn inclusions isolated from MSA brains have unique ultrastructural features that differ from those of individuals with DLB [131,132]. Interestingly, it has been shown that oligodendrocytes, but not neurons, phenoconvert LB-like α-syn fibrils into a GCI-like strain, highlighting the fact that the oligodendroglial intracellular milieu determines how MSA-associated α-syn strains are generated [130]. Supporting these findings, it has been found that sub-stoichiometric concentrations of oligodendroglial protein p25α redirects α-syn aggregation into a unique α-syn/p25α strain with a different structure and enhanced in vivo neurodegenerative properties [48]. Taken together, these observations highlight the importance of both misfolded seeds and intracellular milieu in the formation of α-syn strains.
4. α-Syn Propagation in the Clinical Pathology of PD: Models and Hypotheses
Although a single unifying hypothesis is still lacking, the prevailing viewpoints on the pathogenic role of α-syn aggregation in PD mainly posit the following set of arguments: (i) pathological α-syn aggregation is triggered in extra-nigral location(s), purportedly in contact with the external environment, and subsequently propagates in the nervous system following neural connectivity [18,133]; and (ii) the profound neurodegeneration of neuronal populations (primarily SNpc) in PD reflects selective vulnerability to pathogenic processes, including α-syn induced proteopathic stress [20,134]. Despite some differences, largely due to the basis of the primary evidence, advances in investigative methodology such as brain imaging and refinements in the experimental models will possibly reveal some degree of overlap in these viewpoints. In the following sections, we will summarize the salient features of these hypotheses and provide some perspectives on the significance of extra-nigral α-syn LRP for PD.
4.1. ‘Dual-Hit’ Hypothesis
According to this hypothesis, pathological α-syn aggregation is initiated in peripheral (i.e., extra-cerebral) locations such as olfactory epithelium and/or gut mucosa in response to the exposure to environmental factors, presumably a neurotropic viral pathogen or a toxin [135]. Based on post-mortem neuropathological assessment of LRP in PD and iLBD, Braak and colleagues proposed that the α-syn LRP in PD develops in defined spatiotemporal patterns (stages) and is possibly multifocal in origin. Briefly, in the earliest phase (stage 1), LRP is detected in the dmX/IX and within few projections of the medullary intermediate reticular zone, peripheral autonomic ganglia and spinal cord (and anterior olfactory nucleus). Subsequently, there is a caudo-rostral propagation into the pontine tegmentum (stage 2; GRN, LC and raphe magnus), midbrain (stage 3; SNpc), basal forebrain and olfactory areas (stage 4) and eventually neocortical regions (stages 5–6) [18,133]. Given the observations that symptomatic PD usually indicates the loss of 30–70% of SNpc dopaminergic neurons in the ventrolateral tier of SNpc (and their striatal terminals) [136,137,138], i.e., stage 3 of Braak scheme, this neuropathological scheme in its simplest form arguably serves to categorize PD as presymptomatic (Braak stages 1–2), early symptomatic (Braak stages 3–4) and late symptomatic (Braak stages 5–6) phases [20]. It is noteworthy that not all PD cases, between 17% and 47%, show clinical correlation with the distribution of LRP following this scheme [8,20,139]. For instance, in some studies there was a remarkable lack of LRP in dmX despite significant involvement of higher brainstem or cortical regions in up to 8% of the examined specimen [140]. Nevertheless, these data show that pathological α-syn accumulation in the form of LRP preferentially affects distinct neuronal populations/nuclei and that neuronal susceptibility to LRP accumulation is possibly modulated by genetic factors [20,141]. Furthermore, non-physiological α-syn deposition has also been reported in peripheral sites, presumably in the areas of innervations of peripheral autonomic nerves [134,142].
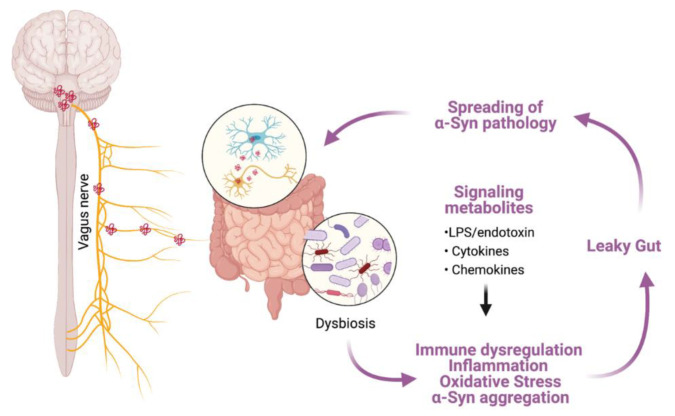
In this mechanistic model of caudo-rostral α-syn neuroinvasion, the enteric nervous system (ENS) of the gastrointestinal tract (GIT) and the associated autonomic ganglia have been proposed as the putative sites of origin for α-syn aggregation in the periphery [133]. α-Syn aggregation induced dysfunction in the ENS has, in turn, been implicated in several GIT related non-motor symptoms in PD (i.e., constipation) [143]. The neuropathological findings that support the role of ‘gut-brain’ axis in PD have recently been discussed in several review opinions [144,145,146] and some key aspects of this model are presented below. Within the ENS, α-syn immunopositivity has been reported in the intramuscular myenteric and submucosal Meissner’s plexuses in the gastric, duodenal and colonic biopsies during the prodromal stages of PD (i.e., in the absence of motor disability) [18,147,148], as well as in patients with idiopathic rREM sleep behaviour disorder (iRBD), which is present in as much as one-third of PD patients and considered a strong indicator of prodromal PD [149]. In population-based studies, truncal vagotomy (i.e., division of the anterior and posterior trunks proximal to the gastro-esophageal junction; used mainly in the treatment of complicated peptic ulcer disease) has been shown to reduce the risk of developing PD by 40–50% after 5 years post-procedure [144,150,151]. Furthermore, positron emission tomography (PET) studies using [11C]-donepzil, a surrogate marker for assessing cholinergic parasympathetic gut innervation, show reduced signal in the colon and small intestine during early-stage PD [152] and in the cohorts of subjects with iRBD [153]. These observations reinforce the idea that, in a subset of PD cases, initial α-syn pathology may originate within the ENS and subsequently involve autonomic ganglia peripherally and dmX centrally. In support of this viewpoint, exogenous inoculation of PD brain lysates or preformed fibrillar (PFF) α-syn into the GIT of wild type rats was followed by the emergence of α-syn accumulation in ENS and subsequently in the dmX as early as 2–3 days post-inoculation [154]. Similarly, PFF α-syn inoculation in the gastric or intestinal wall of C57BL/6 mice resulted in substantial α-syn phosphorylation (p-S129) in the dmX, which was prevented by vagotomy prior to the PFF α-syn inoculation [146,155]. Although, the spreading of α-syn inclusions beyond the dmX has not been consistently observed in these experiments, their findings provide support for the notion that α-syn aggregation in ENS can spread centrally into the CNS, and mimics a pattern observed following ectopic induction of α-syn expression in the vagus nerve of rodents using recombinant adeno-associated viruses (rAAV). With regards to the factors that promote de novo α-syn in the ENS, several mechanisms have been implicated including the altered composition of gut microbiome in PD [156], chronic helicobacter pylori infection [157], disruption of intestinal epithelial barrier due to bacterial and environmental toxins leading to a ‘leaky gut’ [158,159] and potentially a pro-inflammatory milieu due to dysregulated immune response in the GIT [160,161] (Figure 1).
Figure 1.
A schematic representation of hypothesized α-syn aggregation and spreading from the ENS towards the CNS via vagus nerve. Environmental factors, including changes in the gut microbiota (dysbiosis), are hypothesized to initiate pathological processes within the enteric nerve cell plexus, provoking mucosal inflammation and oxidative stress and thereby inducing abnormal aggregation of α-syn. Increased permeability of the intestinal barrier (‘leaky gut’) will ultimately provide a route of transmission for the ENS-formed α-syn seeds into the brain. Structures are not drawn to scale. The illustration was created in biorender.com (accessed on 3 August 2021).
However, it is worth mentioning that none of the neuropathological studies published to date have identified isolated α-syn accumulation that is localized only to the gut component of ENS and, in the absence of intracerebral LRP, that eventually progressed into clinical PD [145]. Even if such cases of localized α-syn pathology in ENS do exist, their detection is a highly challenging task technically as the human GIT is 8–10 m long and rigorous analyses would necessitate a large number of tissue sections. Furthermore, some studies have also shown instances of α-syn immunodetection in the intestinal biopsy specimen from neurologically normal individuals [162,163]. As in the case of intracerebral LRP in iLBD [164], it remains unsettled if these individuals eventually will progress to develop a Lewy body disorder or represent a population with an intact ENS function (as revealed by the expression of ENS neurotransmitter molecules [163] that potentially compensates in response to local α-syn aggregation.
Based on these findings, a significant development in the field has been to investigate if α-syn misfolding and propagation in the nervous system occurs in a ‘prion-like’ fashion, i.e., templating of endogenous physiological α-syn into misfolded conformers formed in situ or received from a neuroanatomically connected location [23,165]. Due to the absence of validated biomarkers that can be used in longitudinal studies and the absence of clinical assessment that could reveal PD progression from non/early-symptomatic to symptomatic stage, unequivocally demonstrating a causative role of LRP propagation with the disease stage is certainly a daunting task [8,20]. Pivotal evidence in PD brain was provided by the histological assessment of heterologous fetal transplants in striatum, which revealed that some of the neurons developed LRP after approximately a decade [97,98]. The implication that these observations indicate bona fide host-to-graft propagation has been contested [134]. For instance, even at late stage PD, LRP in medulla remains largely confined within specific cell populations and does not invade neighboring neurons, e.g., LRP is usually found in dmX, intermediate reticular zone and raphe magnus, while the neighboring nuclei are spared [166]. However, these findings emphasize the role of pre-existing LRP in altering local microenvironment conducive to de novo LRP formation and/or progression. In this regard, several studies in cellular and animal models support such ‘prionoid’ behavior of α-syn, i.e., trans-synaptic propagation and templating, although fully recapitulating the spectrum of PD associated LRP combined with preferential loss of SNpc dopaminergic neurons has been challenging [23,167,168,169]. Despite some limitations, these animal models have been instrumental in elucidating that in vivo inoculation with exogenous fibrillar α-syn, LRP containing human brain extracts or the overexpression of α-syn via viral mediated somatic gene transfer induces the aggregation of endogenous α-syn in recipient neurons (and glial cells) [23,90,101,170,171,172,173]. Such de novo induced aggregated α-syn inclusions, in most instances, also contain markers of PD associated LRP, including α-syn phosphorylation at serine residue 129 (p-S129) along with co-detection of ubiquitin and/or the general inclusion marker p62 (sequestosome) protein [23,169]. Importantly, in animal studies several groups have shown that intracerebral or peripherally induced α-syn aggregation (including direct nerve injections) spreads into connected neuronatomical tracts and the aggregation is also not random [101,102,174,175]. Nevertheless, widespread, but circumscribed, LRP-like α-syn deposition in the CNS seems to be more pronouncedly observed in models using fibrillar α-syn and less so using oligomeric α-syn or virally induced α-syn overexpression models [20,23,169,171]. Overall, existing evidence suggests that, in these experimental models, the initial phase of (arguably) trans-synaptic pathological α-syn spreading occurs in a retrograde fashion, although the identification of factors mediating and/or promoting α-syn cell-to-cell transfer remains an evolving field [23].
4.2. Selective Neuronal Vulnerability/Threshold Hypothesis
The basic tenet of this viewpoint is that certain neuronal populations, due to their inherent cellular and network properties, are more vulnerable to α-syn induced proteopathic stress and susceptible to changes in the local microenvironment (e.g., neuroinflammation, metabolic deficits) [20,134,176]. As suggested earlier, the pathogenic roles of misfolded α-syn on mitochondrial function, autophagic flux and altered ion balance (e.g., calcium) are very well studied in cell-based paradigms and have been supported by findings in animal models of synucleinopathies [84,177]. Current opinion suggests that the neurons typically affected by pathological α-syn accumulation are CNS projection neurons with thin, long unmyelinated or poorly myelinated axons, and with comparatively higher axonal terminals (i.e., hyperbranching axons) [138,178]. In this regard, it has been estimated that a single dopaminergic neuron makes up to a quarter million striatal synapses in the rat brain, while in human brain the number of vesicle release sites can be 10-fold higher [20,179]. Several studies show that the neurodegenerative changes initially comprise the loss of terminals, with subsequent swelling of neuronal soma and some degree of neuronophagia by microglial cells [180,181,182]. In addition, the extensive hyperbranching places extra burden on metabolic regulation, (e.g., to meet the demand for axonal transport) and compromises scavenging capacity to mitigate oxidative stress (highlighted in Figure 2) [183,184,185].
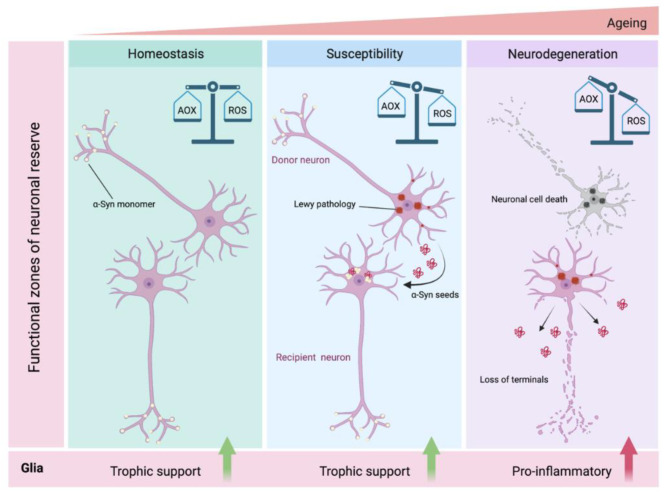
Figure 2.
Schematic depiction of hypothesized α-syn neuron-to-neuron transmission and intracellular redox imbalance resulting in neurodegeneration. Under normal homeostatic conditions, neuronal α-syn exists in soluble non-aggregated conformations and the anti-oxidant (AOX) scavenging mechanisms are at equilibrium with intracellular reactive oxygen species (ROS) generation. Misfolded α-syn perturbs cellular redox balance in favor of excessive ROS, which is further aggravated by additional susceptibility/risk factors (e.g., genetic risk factors and ageing) that promote pathological α-syn aggregation and proteopathic stress [1,2]. Subsequently, cell-to-cell transmission of α-syn seeds from the affected neurons (depicted as donor neuron) via the neuroanatomical projections onto additional neuronal populations results in transmission of α-syn pathology into the recipient neurons. In the receiving neuron, the newly internalized seeds recruit endogenous soluble α-syn and further template a vicious cycle of α-syn aggregation and neurotoxicity. In established (i.e., long-term) α-syn neuronal pathology, there is profound dysregulation of AOX/ROS balance which is associated with loss of synaptic terminals and neuronal demise. The neuroglial cells modulate these processes by providing trophic support (e.g., glia derived neurotrophic factor- GDNF) which serves to maintain pro-survival local microenvironment [185,186,187,188,189]. However, relentless disease progression and ensuing neurodegeneration are strong triggers for neuroinflammatory response. Structures are not drawn to scale. The illustration was created in biorender.com (accessed on 3 August 2021).
Supporting this notion of impaired redox homeostasis, several immunohistochemical studies have shown aberrant localization of redox regulating molecules in association with LRP in PD SN, including nuclear factor erythroid 2-related factor 2 (NRF2/Nrf2) [186,187], NRF2 inhibitor Kelch-like ECH-associated protein 1 (Keap1) [188], anti-oxidant heme oxygenase (HO-1) [189] and anti-xenobiotic NAD(P)H quinone dehydrogenase 1 (NQO1) [190]. Moreover, the neuronal populations containing LRP in PD belong to diverse neurotransmitter systems (dopamine, serotonin, noradrenaline and acetylcholine) [23], yet not all neurons containing pathological α-syn inclusions show relentless neurodegeneration (e.g., tuberomamillary nucleus in hypothalamus [191]) as observed in the SNpc.
Moreover, in SNpc, the number of LRP/total α-syn immunopositive neurons does not correlate with disease severity and is stable over time, with ~3.6% of the neurons affected on average [192,193]. In contrast, dopamine transporter (DAT) density is reported to be inversely correlated to the total α-syn in SN than LRP burden, arguably favoring defective axonal transport [194,195]. Conversely, some brain regions exhibit variable loss of neurons (e.g., supraoptic nucleus) in the relative absence of LRP [191]. Apart from these structural/cytoarchitectural features discussed above, certain functional properties have also been posited as factors that predispose neurons to α-syn induced neurotoxicity (i.e., the threshold hypothesis) [134], which do not necessarily correlate with LRP burden in PD. Electrophysiological measurements show that the SNpc dopaminergic neurons possess slow, tonic and autonomic pacemaking activity characterized by broad spikes [196,197,198]. These neurons also exhibit a sustained opening of calcium cav1 channels, with large intracellular Ca2+ oscillations and low intrinsic Ca2+ buffering [196,199,200,201]. The neighboring dopaminergic neurons in VTA, which are less susceptible to neurodegeneration, also exhibit autonomic pacemaking and broad spikes, but possess comparatively smaller cav1 currents and robust Ca2+ buffering predominantly mediated by calbindin [202,203]. One drawback of the slow Ca2+ oscillations in SNpc dopaminergic neurons is the Ca2+ entry into the mitochondria, which is needed to sustain ATP production with potential for creating redox imbalance [204,205,206].
A second aspect is the functional reserve/resilience of neuronal populations and network compensation under extrenuous demands [134]. In this regard, a common feature of subcortical motor networks is their extensive connectivity and considerable redundancy in the control of motor function [19]. Although we do not have a complete map of human connectome, extrapolation of the known connectivity in the rodent nervous system reveals extensive projections among the brainstem (SNpc, LC and reticular nuclei, including GRN and PAG), striatum, pallidum, thalamus and cortical nuclei, which often are reciprocal [207]. Hence, the following can be argued: (i) Networks with high network threshold (i.e., redundancy) are relatively less sensitive to major dysfunction, while networks with low threshold exhibit impaired compensatory response [134]. This implies that comparatively lower loss of neurons would lead to dysfunction in autonomic systems (i.e., due to the LRP affection in autonomic ganglia and dmX/IX) and result in prodromal PD [9,10,142] compared to the estimated 30–50% neuronal loss observed in SNpc prior to the emergence of clinical motor disability [1,2,20]; and (ii) the network dysfunction in PD does not necessarily correlate with the trans-synaptic LRP propagation [8,20,134]. For instance, not all projection/reciprocal innervation regions of LC, which are considered the ‘hotspot’ of LRP in early PD, develop robust LRP lesions, e.g., cerebellum and central nucleus of amygdale are relatively spared [20,208].
Lastly, animal studies suggest that neuroinflammatory response of glial cells in response to fibrillar α-syn and/or formation of α-syn inclusion pathology in glial cells [107,108] may also render the local microenvironment conducive to α-syn propagation and neurotoxicity [23,101,169,175]. Human post-mortem studies in synucleinopathies show variable degrees of diffuse α-syn accumulation in the astroglial cells, which is morphologically distinct from the compact neuronal LRP and lacks histological markers of LB pathology (e.g., ubiquitin/p62 immunopositivity) [108]. Cultured astroglia readily internalize extracellular α-syn in vitro, either added exogenously to the culture medium or within the conditioned media from α-syn expressing neuronal cells and in neuron-glia co-culture experiments [108,209]. A substantial number of animal studies also support the view of possible neuron-to-astroglia transmission of aggregated α-syn. For instance, transgenic mice overexpressing human α-syn (wild type or mutant A53T; under the PDGFβ or mouse Prnp promoters, respectively) in neurons show glial accumulation of α-syn aggregates, which appears at stages when neuronal α-syn inclusion pathology is clearly established [209] or occurs significantly later, implicating neuron-to-astroglia transmission [101,175]. Moreover, inducible expression of the aggregation prone human mutant A53T α-syn in astrocytes (under the astroglial GFAP promoter; GFAP-tTA/tetO-α-syn) results in the loss of dopaminergic neurons and neuroinflammation, although it was not clear if glia-to-neuron transmission of aggregated α-syn occurred since no histological data on neuronal α-syn pathology were reported [210].
It is worthwhile to mention that neuronal loss in SNpc is associated with extracellular release of neuromelanin, which is phagocytosed by glial cells and there is evidence for melanin-induced microglial activation in early PD and in the rat SN [180,181,211]. Although this implies that the neuroinflammatory processes are contributors to the disease, some recent reports indicate that activated microglia may also be a source of neurotrophic factors and play a neuroprotective role (Figure 2) [180,212].
5. Future Prospects and Opportunities
Apart from the rare forms of familial PD, idiopathic PD runs a protracted course over 15–20 years. With an average number of ~3.6% SNpc neurons affected by LRP, and an estimated lifespan of ~6.2 months in neurons bearing pathological α-syn inclusions before demise, the observed loss of neurons in SNpc at the time of clinical presentation seems to support a relentless course driven by α-syn aggregation in the CNS [192,213]. As more evidence becomes available, i.e., brain imaging, biomarkers in biological fluids or digital tools in clinical practice, it is likely that the two viewpoints discussed above (i.e., Dual-hit hypothesis and Neuronal vulnerability hypothesis) may not be as irreconcilable and could potentially guide a refined understanding of the etiology and symptomatology of PD, especially with respect to the prodromal non-motor symptoms [10]. In this regard, the roles of co-existing neuropathologies and the involvement of white matter in PD are often overlooked [214]. For instance, deposition of tau has been observed in association with LRP, particularly in neurons of LC, basal forebrain and amygdala, and recent studies indicate that tauopathy in PD preferentially affects the nigrostrial neurons than compared with global tauopathy of the Alzheimer type [215,216,217]. Moreover, some clinical features of PD (i.e., rigidity and gait apraxia) in the absence of resting tremor are also seen in rare movement disorders such as progressive supranuclear palsy and corticobasal degeneration [5]. In these disorders, tauopathy affects the basal ganglia and brainstem nuclei, including the SN [5,218]. The akinetic-rigid PD, which is diagnosed in ~50% of patients, preferentially affects the elderly (in contrast to the tremor dominant, which has a younger age of onset), and is frequently associated with cognitive declinesimilar to the age related tauopathies [5,7]. These observations suggest that LRP and tauopathy could engage common pathogenic processes and research in the two fields has the potential to be mutually informative.
As for the prospects in PD research in the near future, we expect significant developments in three areas: (i) Novel therapeutic modalities, especially stem cells and viral gene therapy; (ii) biomarker discovery, including the use of digital technologies; and (iii) refinements in the disease models, ideally towards prodromal PD-like phenotypes. The status of the development of therapeutic modalities and novel candidates for therapy has been reviewed elsewhere [219,220,221,222], hence, we will focus on the latter two aspects. The first is the advancement in the biomarker discovery and technologies that can allow the monitoring of the disease extent (e.g., neuronal loss) and possibly subtle neuronal dysfunction.
Measurements of total or modified forms of α-syn (e.g., p-S129) in biological fluids have been the focus of investigation over the last decade with conflicting results in the levels detected by immunodetection ELISA methods or correlation to the disease severity. Recent studies indicate that the detection of oligomeric and p-S129-α-syn in the cerebrospinal fluid may be promising biomarker candidates in PD, with the ratio of oligomeric/total α-syn greatly improving the sensitivity and specificity of these assays [223,224]. Significant technical improvements have also been made for the detection of protein aggregates in biological fluids that rely on the templated seeding mechanism (e.g., amyloidogenic seeds converting the native protein into misfolded conformers), such as real-time quaking-induced conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA) [223]. However, to date, these methods are still restricted to research purposes. By comparison, a number of functional neuroimaging approaches, such as the measurement of presynaptic dopamine or dopamine transporter (DAT) and metabolism of L-dopa, has shown to differentiate PD from controls with impressive specificity and sensitivity [225,226]. An easily applicable and low-cost neuroimaging approach using transcranial sonography also distinguishes the pathological affection of SN, i.e., abnormal extension of SN echogenicity, in the majority of patients [227]. Similarly, myocardial scintigraphy (using 123I-metaiodobenzylguanidine) has also been successfully used to detect cardiac sympathetic denervation in early PD and in the differential diagnosis [228]. Another exciting development is in the area of digital/telemetric technologies, which can serve as an aid in detecting early neurological dysfunction, guide in patient monitoring and response to therapy [229,230]. Although, more scientific data and rigorous analyses on their utility are still missing, these can be useful clinical tools in the detection of sleep disorders, gastrointestinal problems (i.e., dysphagia, salivation, constipation) and tremor.
Lastly, there is a dire need for refinements in experimental models that can be used to study motor deficits largely due to the extranigral α-syn pathology and non-motor symptoms, such as postural instability and pain [9,10]. Several animal models, based on transgenic α-syn overexpression or viral mediated α-syn somatic gene transfer, recapitulate aspects of PD-like α-syn pathology, such as loss of dopamine and motor phenotypes due to basal ganglia dysfunction [23,167,169]. Among the transgenic models, the mice expressing human wild type α-syn under the Thy1 promoter have been consistently reported to exhibit non-motor phenotypes that are relevant to PD, such as cognitive impairment, olfactory dysfunction, constipation and changes in the circadian rhythm [231,232]. Given the nigro-centric neuropathological context of PD, it is understandable that the animal models have largely focused on the nigrostriatal dysfunction. However, it would be interesting to study sensorimotor phenotypes by selective α-syn lesions in extra-nigral locations, such as LC, GRN, PAG and the hypothalamus. In this regard, two recent studies show that viral mediated induction of mutant A53T α-syn in LC [233] or transgenic overexpression of human wild-type α-syn under control of the noradrenergic-specific dopamine β-hydroxylase promoter [234] results in the development of PD-like α-syn pathology, neuroinflammation and behavioral deficits in the latter [234].
The GRN is part of the brainstem reticular nuclei, which, according to Braak staging, is also affected very early in the disease [18,133]. The neuronal populations in GRN are extensively connected to several brain regions including LC and cerebellar nuclei and via their descending projections to spinal motor systems (i.e., premotor and motor neurons) serve as the ‘gain-setting mechanism’ in the control of movement and posture [19]. In a PFF model of synucleinopathy (M83 transgenic line expressing the human mutant A53T α-syn) [235] several laboratories, including our own, have shown that, after the initial appearance of α-syn pathology (p-S129) in the spinal cord following intramuscular PFF inoculation, periventricular regions of brainstem (i.e., medullary reticular nuclei, LC, pontine GRN and midbrain PAG) are affected long before the emergence of movement disability [170,175,236]. This ‘prodromal’ phase coincides with the emergence of a sensorimotor deficit exhibited as mild degree of hindlimb clasping [102,236], which is a behavior observed in rodents with lesions in basal ganglia and cerebellum [237]. Moreover, the (intramuscularly) PFF inoculated M83 mice exhibit a hunched posture at the end-stage, although it is not clear if this phenotype is due to neuronal dysfunction in higher the brain region or if it results from the significant loss of spinal motor neurons [101]. It is worthwhile to indicate that the PFF based models have several limitations to qualify as bona fide PD models, including the lack of both the dopaminergic cell loss and significant α-syn pathology in SN [23,169,236]. Nevertheless, several groups have demonstrated that these models exhibit α-syn pathology beyond the site of PFF inoculation (i.e., intracerebral or peripheral) and putative ‘trans-synaptic’ spreading [23].
The neuronal populations in PAG are also heterogeneous and have several projections that link forebrain structures to the reticular nuclei of the brainstem [238]. This phylogenetically ancient region has been implicated in autonomic regulation (possibly via hypothalamus), circadian rhythm and pain modulation (via descending projections to the raphe magnus and spinal nociceptor neurons) [238,239]. In the PFF inoculated (via the intramuscular route) M83 transgenic mice, abundant α-syn pathology (p- S129 α-syn) in the PAG (and spinal cord) was associated with mechanical allodynia and impaired pain response [102]. However, we are still in the preliminary stages in terms of inferring whether the impaired pain perception was due to central neuronal/nociceptive dysfunction or the loss of normal nerve function as measured by nerve conduction velocity and myelin damage in the nerve dorsal roots [102]. Hence, quasi-selective induction of α-syn aggregation in central pain processing centers, i.e., PAG, may unravel relevant mechanisms for pain, which is reported in a considerable number of PD patients [10].
6. Conclusions
Since the early discovery of α-syn as a major component of LB pathology in PD, and genetic linkage between mutations in the SNCA with rare forms of familial PD [30,240,241,242], significant progress has been made to make a compelling case for a pathogenic role of α-syn aggregation in PD and related diseases [12,17,240]. However, there are several aspects that represent the missing links between α-syn aggregation in the CNS and the onset and/or progression of clinical PD symptomatology. This is illustrated by the fact that despite a growing consensus on the putative downstream mechanisms of α-syn neurotoxicity following its misfolding and/or aggregation [15,17], the identity of causative factor(s) that promote the initial pathological conversion of α-syn into neurotoxic species in PD is largely unsettled. Apart from the case of rare familial forms due to specific genetic mutations in the SNCA locus (i.e., increased protein expression due the gene dosage effect and tendency to form oligomers as a result of certain point mutations), the nature of mechanism(s) that promote α-syn aggregation in other forms of PD (genetic or idiopathic) remains elusive, and the mechanisms are likely to be of multi-factorial origin [1]. For instance, a sizeable proportion (8–14%) of autopsy proven PD cases reveal mutations in the gene encoding glucocerebrosidase (GBA) associated with perturbed lysosomal function [243] and potentially favor α-syn aggregation as a result of ensuing lipid accumulation and defective autophagy [15,243]. A pathogenic relevance of defective autophagy promoting α-syn aggregation in PD is also supported by the studies in cellular and animal models overexpressing PD-associated mutations in the leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) [244], vacuolar protein sorting-associated protein 35 (VPS35) [245] and showing putative interactions of parkin with mutant glucocerebrosidase [246]. Thus, it could be argued that rectifying the pathological decrease in autophagic flux may hold promise in mitigating the neurotoxic effects of aggregated α-syn in at least a subset of PD cases. Mechanistic studies in different PD populations using refined approaches, e.g., advanced genetic studies in patient derived induced pluripotent stem cells [247], will potentially reveal whether the defects in autophagic flux of lipids and/or proteins are a generalized feature in PD or if additional contributing mechanisms underlie the etiology of pathological α-syn accumulation in different PD populations.
Another relevant consideration is the establishment of a framework for the identification of patient specific factors, such as co-existing neuropathology (e.g., tau [218]) and co-morbidities that predispose to age related neurological dysfunction (e.g., impaired glycemic control) that may inform on the clinical phenotypes in relation to the distribution and/or progression of α-syn pathology [248,249]. For instance, while tau pathology is a pronounced post-mortem feature in the cases of familial PD due to LRRK2 mutations, not all PD cohorts with pathogenic LRRK2 mutations exhibit α-syn LRP [250,251]. It is worth considering that the paucity of LRP (inclusions containing fibrillar α-syn, which is ubiquinated [114]) in these subsets of PD cases does not rule out the existence of oligomeric α-syn in the brain, since α-syn oligomers have been reported in the cerebrospinal fluid of individuals who carry LRRK2 mutations, either with a PD diagnosis [252] or in neurologically normal volunteers [253]. However, research reagents (e.g., conformational antibodies) that can detect oligomeric α-syn have not been systematically studied to the extent that they are generally accepted in their own accord as the tissue biomarkers of pathological α-syn accumulation. Moreover, the specificity of these reagents to unambiguously recognize ‘oligomeric’ α-syn conformations without binding to fibrillar α-syn has also been contested [254].
In conclusion, the wealth of information about PD symptomatology, extensive characterization of the neuropathological findings and refinements in animal models hold promise for meaningful discoveries that may yield potential biomarkers of disease as well as guide the development of disease modifying therapies.
Author Contributions
Conceptualization, A.J. and N.F.; writing and original draft preparation, A.J., N.P.G. and N.F.; review and editing, A.J., N.P.G., C.B.V., P.H.J. and N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding to AJ in the form of a Marie Skłodowska Curie Fellowship from European Union’s Horizon 2020 Research and Innovation Programme (MSCA-IF-2017, grant #786433) and the Lundbeckfonden, Denmark (grant #R250-2017-1131), Lundbeck Foundation grants R223-2015-4222 for PHJ, R248-2016-2518 for Danish Research Institute of Translational Neuroscience-DANDRITE, Nordic-EMBL Partnership for Molecular Medicine, Aarhus University, Denmark, Postdoctoral Fellowship R171-2014-591 to N.F.
Institutional Review Board Statement
Not applicable (The article does not contain any experimental data involving human or animal subjects).
Informed Consent Statement
Not applicable (The article does not contain any experimental data involving human subjects).
Data Availability Statement
All the data cited in the article can be accessed in original research studies provided in the bibliography under the references.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 3.Schapira A.H.V. Parkinson’s Disease. Oxford University Press; Oxford, NY, USA: 2010. p. 116. [Google Scholar]
- 4.Frank C., Pari G., Rossiter J.P. Approach to diagnosis of Parkinson disease. Can. Fam. Physician Med. Fam. Can. 2006;52:862–868. [PMC free article] [PubMed] [Google Scholar]
- 5.Gray F.O., Duyckaerts C., De Girolami U., Escourolle R., Gray F.O. Escourolle & Poirier’s Manual of Basic Neuropathology. 5th ed. Oxford University Press; Oxford, NY, USA: 2014. p. xiii.406p [Google Scholar]
- 6.Rajput A.H., Voll A., Rajput M.L., Robinson C.A. Course in Parkinson disease subtypes: A 39-year clinicopathologic study. Neurology. 2009;73:206–212. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- 7.Selikhova M., Williams D.R., Kempster P.A., Holton J.L., Revesz T., Lees A.J. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger K.A. Is Braak staging valid for all types of Parkinson’s disease? J. Neural Transm. 2018;126:423–431. doi: 10.1007/s00702-018-1898-9. [DOI] [PubMed] [Google Scholar]
- 9.Dickson D.W., Fujishiro H., Orr C., DelleDonne A., Josephs K.A., Frigerio R., Burnett M., Parisi J.E., Klos K.J., Ahlskog J.E. Neuropathology of non-motor features of Parkinson disease. Park. Relat. Disord. 2009;15:S1–S5. doi: 10.1016/S1353-8020(09)70769-2. [DOI] [PubMed] [Google Scholar]
- 10.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18:509. doi: 10.1038/nrn.2017.91. [DOI] [PubMed] [Google Scholar]
- 11.Dickson D.W. Neuropathology of Parkinson disease. Park. Relat. Disord. 2018;46:S30–S33. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCann H., Stevens C., Cartwright H., Halliday G. α-Synucleinopathy phenotypes. Park. Relat. Disord. 2014;20:S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 13.Scudamore O., Ciossek T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J. Neuropathol. Exp. Neurol. 2018;77:443–453. doi: 10.1093/jnen/nly024. [DOI] [PubMed] [Google Scholar]
- 14.Beal M.F. Mitochondria, Oxidative Damage, and Inflammation in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2006;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong Y.C., Krainc Y.C.W.D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017;23:1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz-Schaeffer W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lashuel H.A., Overk C.R., Oueslati A., Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2012;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braak H., Del Tredici K., Rüb U., de Vos R.A., Steur E.N.J., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Braak H., Rüb U., Sandmann-Keil D., Gai W.P., De Vos R.A.I., Steur E.N.H.J., Arai K., Braak E. Parkinson’s disease: Affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol. 2000;99:489–495. doi: 10.1007/s004010051150. [DOI] [PubMed] [Google Scholar]
- 20.Surmeier D.J., Obeso J.A., Halliday G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017;18:101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach T.G., Adler C.H., Lue L., Sue L.I., Bachalakuri J., Henry-Watson J., Sasse J., Boyer S., Shirohi S., Brooks R., et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dijkstra A.A., Voorn P., Berendse H.W., Groenewegen H.J., Rozemuller A.J., van de Berg W., Bank N.B. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 2014;29:1244–1251. doi: 10.1002/mds.25952. [DOI] [PubMed] [Google Scholar]
- 23.Uchihara T., Giasson B.I. Propagation of alpha-synuclein pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2015;131:49–73. doi: 10.1007/s00401-015-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M.R., Südhof T.C. α-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassen L.B., Reimer L., Ferreira N., Betzer C., Jensen P.H. Protein Partners of α-Synuclein in Health and Disease. Brain Pathol. 2016;26:389–397. doi: 10.1111/bpa.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmer T.S., Bax A., Cole N.B., Nussbaum R.L. Structure and Dynamics of Micelle-bound Human α-Synuclein. J. Biol. Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 27.Souza J.M., Giasson B.I., Lee V.M.-Y., Ischiropoulos H. Chaperone-like activity of synucleins. FEBS Lett. 2000;474:116–119. doi: 10.1016/S0014-5793(00)01563-5. [DOI] [PubMed] [Google Scholar]
- 28.Giasson B.I., Murray I., Trojanowski J.Q., Lee V.M.-Y. A Hydrophobic Stretch of 12 Amino Acid Residues in the Middle of α-Synuclein Is Essential for Filament Assembly. J. Biol. Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 29.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 31.Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M.B., Kösel S., Przuntek H., Epplen J.T., Schols L., Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 32.Zarranz J.J., Alegre J., Gomez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., et al. The new mutation, E46K, of α-synuclein causes parkinson and Lewy body dementia. Ann. Neurol. 2003;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 33.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., et al. α-Synuclein Locus Triplication Causes Parkinson’s Disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 34.Burré J., Vivona S., Diao J., Sharma M., Brunger A., Südhof T.C. Properties of native brain α-synuclein. Nat. Cell Biol. 2013;498:E4–E6. doi: 10.1038/nature12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedmon M.M., Lindorff-Larsen K., Christodoulou J., Vendruscolo M., Dobson C.M. Mapping Long-Range Interactions in α-Synuclein using Spin-Label NMR and Ensemble Molecular Dynamics Simulations. J. Am. Chem. Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 36.Bertoncini C.W., Jung Y.-S., Fernandez C.O., Hoyer W., Griesinger C., Jovin T.M., Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured -synuclein. Proc. Natl. Acad. Sci. USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uversky V.N., Li J., Fink A.L. Evidence for a Partially Folded Intermediate in α-Synuclein Fibril Formation. J. Biol. Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 38.Wu K.-P., Weinstock D.S., Narayanan C., Levy R.M., Baum J. Structural Reorganization of α-Synuclein at Low pH Observed by NMR and REMD Simulations. J. Mol. Biol. 2009;391:784–796. doi: 10.1016/j.jmb.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shtilerman M.D., Ding A.T.T., Lansbury J.P.T. Molecular Crowding Accelerates Fibrillization of α-Synuclein: Could an Increase in the Cytoplasmic Protein Concentration Induce Parkinson’s Disease? Biochemistry. 2002;41:3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- 40.Uversky V.N., Li J., Fink A.L. Metal-triggered Structural Transformations, Aggregation, and Fibrillation of Human α-Synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 41.Munishkina L.A., Phelan C., Uversky V.N., Fink A.L. Conformational Behavior and Aggregation of α-Synuclein in Organic Solvents: Modeling the Effects of Membranes. Biochemistry. 2003;42:2720–2730. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 42.Galvagnion C., Brown J., Ouberai M.M., Flagmeier P., Vendruscolo M., Buell A.K., Sparr E., Dobson C.M. Chemical properties of lipids strongly affect the kinetics of the membrane-induced aggregation of α-synuclein. Proc. Natl. Acad. Sci. USA. 2016;113:7065–7070. doi: 10.1073/pnas.1601899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohlberg J.A., Li J., Uversky A.V.N., Fink A.L. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from α-Synuclein in Vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 44.Goers J., Uversky V.N., Fink A.L. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uversky V.N., Li J., Fink A.L. Pesticides directly accelerate the rate of α-synuclein fibril formation: A possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/S0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 46.Engelender S., Kaminsky Z., Guo X., Sharp A.H., Amaravi R.K., Kleiderlein J.J., Margolis R.L., Troncoso J.C., Lanahan A.A., Worley P.F., et al. Synphilin-1 associates with α-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 47.Lindersson E., Lundvig D., Petersen C., Madsen P.S., Nyengaard J.R., Højrup P., Moos T., Otzen D., Gai W.-P., Blumbergs P.C., et al. p25α Stimulates α-Synuclein Aggregation and Is Co-localized with Aggregated α-Synuclein in α-Synucleinopathies. J. Biol. Chem. 2005;280:5703–5715. doi: 10.1074/jbc.M410409200. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira N., Gram H., Sorrentino Z.A., Gregersen E., Schmidt S.I., Reimer L., Betzer C., Perez-Gozalbo C., Beltoja M., Nagaraj M., et al. Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol. 2021;142:87–115. doi: 10.1007/s00401-021-02316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson J.P., Walker D.E., Goldstein J.M., de Laat R., Banducci K., Caccavello R.J., Barbour R., Huang J., Kling K., Lee M., et al. Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J. Biol. Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 50.Paleologou K.E., Oueslati A., Shakked G., Rospigliosi C.C., Kim H.-Y., Lamberto G.R., Fernandez C.O., Schmid A., Chegini F., Gai W.P., et al. Phosphorylation at S87 Is Enhanced in Synucleinopathies, Inhibits α-Synuclein Oligomerization, and Influences Synuclein-Membrane Interactions. J. Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura T., Yamashita H., Takahashi T., Nakamura S. Activated Fyn Phosphorylates α-Synuclein at Tyrosine Residue 125. Biochem. Biophys. Res. Commun. 2001;280:1085–1092. doi: 10.1006/bbrc.2000.4253. [DOI] [PubMed] [Google Scholar]
- 52.Kofoed R.H., Zheng J., Ferreira N., Lykke-Andersen S., Salvi M., Betzer C., Reimer L., Jensen T.H., Fog K., Jensen P.H. Polo-like kinase 2 modulates α-synuclein protein levels by regulating its mRNA production. Neurobiol. Dis. 2017;106:49–62. doi: 10.1016/j.nbd.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa M., Fujiwara H., Nonaka T., Wakabayashi K., Takahashi H., Lee V.M.-Y., Trojanowski J.Q., Mann D., Iwatsubo T. Phosphorylated α-Synuclein Is Ubiquitinated in α-Synucleinopathy Lesions. J. Biol. Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 54.Tofaris G., Razzaq A., Ghetti B., Lilley K.S., Spillantini M.G. Ubiquitination of α-Synuclein in Lewy Bodies Is a Pathological Event Not Associated with Impairment of Proteasome Function. J. Biol. Chem. 2003;278:44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- 55.Giasson B.I., Duda J.E., Murray I.V.J., Chen Q., Souza J.M., Hurtig H.I., Ischiropoulos H., Trojanowski J.Q., Lee V.M.-Y. Oxidative Damage Linked to Neurodegeneration by Selective alpha -Synuclein Nitration in Synucleinopathy Lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 56.Burai R., Ait-Bouziad N., Chiki A., Lashuel H.A. Elucidating the Role of Site-Specific Nitration of α-Synuclein in the Pathogenesis of Parkinson’s Disease via Protein Semisynthesis and Mutagenesis. J. Am. Chem. Soc. 2015;137:5041–5052. doi: 10.1021/ja5131726. [DOI] [PubMed] [Google Scholar]
- 57.Rott R., Szargel R., Shani V., Hamza H., Savyon M., Elghani F.A., Bandopadhyay R., Engelender S. SUMOylation and ubiquitination reciprocally regulate α-synuclein degradation and pathological aggregation. Proc. Natl. Acad. Sci. USA. 2017;114:13176–13181. doi: 10.1073/pnas.1704351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krumova P., Meulmeester E., Garrido M., Tirard M., Hsiao H.-H., Bossis G., Urlaub H., Zweckstetter M., Kügler S., Melchior F., et al. Sumoylation inhibits α-synuclein aggregation and toxicity. J. Cell Biol. 2011;194:49–60. doi: 10.1083/jcb.201010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crowther R., Jakes R., Spillantini M.G., Goedert M. Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett. 1998;436:309–312. doi: 10.1016/S0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 60.Choi D.-H., Kim Y.-J., Kim Y.-G., Joh T.H., Beal M.F., Kim Y.-S. Role of Matrix Metalloproteinase 3-mediated α-Synuclein Cleavage in Dopaminergic Cell Death. J. Biol. Chem. 2011;286:14168–14177. doi: 10.1074/jbc.M111.222430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang L., Janowska M.K., Moriarty G.M., Baum J. Mechanistic Insight into the Relationship between N-Terminal Acetylation of α-Synuclein and Fibril Formation Rates by NMR and Fluorescence. PLoS ONE. 2013;8:e75018. doi: 10.1371/journal.pone.0075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dikiy I., Eliezer D. N-terminal Acetylation Stabilizes N-terminal Helicity in Lipid- and Micelle-bound α-Synuclein and Increases Its Affinity for Physiological Membranes. J. Biol. Chem. 2014;289:3652–3665. doi: 10.1074/jbc.M113.512459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M.S., Shen J., Takio K., Iwatsubo T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 64.Oueslati A., Fournier M., Lashuel H.A. Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog. Brain Res. 2010;183:115–145. doi: 10.1016/s0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 65.Oueslati A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? J. Park. Dis. 2016;6:39–51. doi: 10.3233/JPD-160779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgold S., Filser S., Dorostkar M.M., Schmidt B., Herms J. In vivo imaging reveals sigmoidal growth kinetic of beta-amyloid plaques. Acta. Neuropathol. Com. 2014;2:30. doi: 10.1186/2051-5960-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramachandran G., Udgaonkar J.B. Understanding the Kinetic Roles of the Inducer Heparin and of Rod-like Protofibrils during Amyloid Fibril Formation by Tau Protein. J. Biol. Chem. 2011;286:38948–38959. doi: 10.1074/jbc.M111.271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saraiva M.J., Magalhaes J., Ferreira N., Almeida M. Transthyretin Deposition in Familial Amyloidotic Polyneuropathy. Curr. Med. Chem. 2012;19:2304–2311. doi: 10.2174/092986712800269236. [DOI] [PubMed] [Google Scholar]
- 69.Sant’Anna R., Gallego P., Robinson L.Z., Pereira-Henriques A., Ferreira N., Pinheiro F., Esperante S., Pallares I., Huertas O., Almeida M., et al. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat. Commun. 2016;7:10787. doi: 10.1038/ncomms10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira N., Saraiva M.J., Almeida M. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585:2424–2430. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira N., Saraiva M.J., Almeida M.R. Uncovering the Neuroprotective Mechanisms of Curcumin on Transthyretin Amyloidosis. Int. J. Mol. Sci. 2019;20:1287. doi: 10.3390/ijms20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wood S.J., Wypych J., Steavenson S., Louis J.-C., Citron M., Biere A.L. α-Synuclein Fibrillogenesis Is Nucleation-dependent—Implications for the pathogenesis of Parkinson’s disease. J. Biol. Chem. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 73.Li X., Dong C., Hoffmann M., Garen C.R., Cortez L.M., Petersen N.O., Woodside M.T. Early stages of aggregation of engineered α-synuclein monomers and oligomers in solution. Sci. Rep. 2019;9:1734. doi: 10.1038/s41598-018-37584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alam P., Bousset L., Melki R., Otzen D.E. α-synuclein oligomers and fibrils: A spectrum of species, a spectrum of toxicities. J. Neurochem. 2019;150:522–534. doi: 10.1111/jnc.14808. [DOI] [PubMed] [Google Scholar]
- 75.Lorenzen N., Otzen D.E. Oligomers of α-synuclein: Picking the culprit in the line-up. Essays Biochem. 2014;56:137–148. doi: 10.1042/bse0560137. [DOI] [PubMed] [Google Scholar]
- 76.Winner B., Jappelli R., Maji S.K., Desplats P., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahul-Mellier A.-L., Burtscher J., Maharjan N., Weerens L., Croisier M., Kuttler F., Leleu M., Knott G.W., Lashuel H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA. 2020;117:4971–4982. doi: 10.1073/pnas.1913904117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olanow C.W., Perl D.P., DeMartino G.N., McNaught K.S.P. Lewy-body formation is an aggresome-related process: A hypothesis. Lancet Neurol. 2004;3:496–503. doi: 10.1016/S1474-4422(04)00827-0. [DOI] [PubMed] [Google Scholar]
- 79.Lindström V., Fagerqvist T., Nordström E., Eriksson F., Lord A., Tucker S., Andersson J., Johannesson M., Schell H., Kahle P.J., et al. Immunotherapy targeting α-synuclein protofibrils reduced pathology in (Thy-1)-h[A30P] α-synuclein mice. Neurobiol. Dis. 2014;69:134–143. doi: 10.1016/j.nbd.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Paleologou K.E., Kragh C.L., Mann D.M.A., Salem S.A., Al-Shami R., Allsop D., Hassan A.H., Jensen P.H., El-Agnaf O.M.A. Detection of elevated levels of soluble α-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2008;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- 81.Sharon R., Bar-Joseph I., Frosch M.P., Walsh D.M., Hamilton J., Selkoe D.J. The Formation of Highly Soluble Oligomers of α-Synuclein Is Regulated by Fatty Acids and Enhanced in Parkinson’s Disease. Neuron. 2003;37:583–595. doi: 10.1016/S0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 82.Groveman B.R., Orrù C.D., Hughson A.G., Raymond L.D., Zanusso G., Ghetti B., Campbell K., Safar J., Galasko D., Caughey B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018;6:1–10. doi: 10.1186/s40478-018-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansson O., Hall S., Öhrfelt A., Zetterberg H., Blennow K., Minthon L., Nägga K., Londos E., Varghese S., Majbour N., et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Res. Ther. 2014;6:25. doi: 10.1186/alzrt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bose A., Beal M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016;139:216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 85.Reitboeck P.G., Anichtchik O., Bellucci A., Iovino M., Ballini C., Fineberg E., Ghetti B., Della Corte L., Spano P., Tofaris G., et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghiglieri V., Calabrese V., Calabresi P. Alpha-Synuclein: From Early Synaptic Dysfunction to Neurodegeneration. Front. Neurol. 2018;9:295. doi: 10.3389/fneur.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bridi J., Hirth F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018;12:80. doi: 10.3389/fnins.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan K.M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R.J., Cohen F.E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luk K., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M.-Y. Pathological α-synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luk K., Kehm V.M., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A.L., et al. Exogenous Induction of Cerebral -Amyloidogenesis Is Governed by Agent and Host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 92.Riquelme A.I.R., Lau H.H.C., Stuart E., Goczi A.N., Wang Z., Schmitt-Ulms G., Watts J.C. Prion-like propagation of β-amyloid aggregates in the absence of APP overexpression. Acta Neuropathol. Commun. 2018;6:1–16. doi: 10.1186/s40478-018-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo J.L., Lee V.M.-Y. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J. Biol. Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren P.-H., Lauckner J.E., Kachirskaia I., Heuser J.E., Melki R., Kopito R.R. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearce M.P., Kopito R.R. Prion-Like Characteristics of Polyglutamine-Containing Proteins. Cold Spring Harb. Perspect. Med. 2017;8:a024257. doi: 10.1101/cshperspect.a024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., Olanow C.W. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 98.Li J.-Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Björklund A., et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 99.Luk K., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., Lee V.M.-Y. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prusiner S.B., Woerman A.L., Mordes D.A., Watts J., Rampersaud R., Berry D.B., Patel S., Oehler A., Lowe J.K., Kravitz S.N., et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. USA. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sacino A.N., Brooks M., Thomas M.A., McKinney A.B., Lee S., Regenhardt R., McGarvey N.H., Ayers J., Notterpek L., Borchelt D.R., et al. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferreira N., Gonçalves N.P., Jan A., Jensen N.M., van der Laan A., Mohseni S., Vægter C.B., Jensen P.H. Trans-synaptic spreading of alphaα-synuclein pathology through sensory afferents leads to sensory nerve degeneration and neuropathic pain. Acta Neuropathol. Commun. 2021;9:1–17. doi: 10.1186/s40478-021-01131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berge N.V.D., Ferreira N., Gram H., Mikkelsen T.W., Alstrup A.K.O., Casadei N., Tsung-Pin P., Riess O., Nyengaard J.R., Tamgüney G., et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019;138:535–550. doi: 10.1007/s00401-019-02040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Volpicelli-Daley L.A., Luk K., Patel T., Tanik S.A., Riddle D.M., Stieber A., Meaney D., Trojanowski J.Q., Lee V.M.-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elfarrash S., Jensen N.M., Ferreira N., Betzer C., Thevathasan J.V., Diekmann R., Adel M., Omar N.M., Boraie M.Z., Gad S., et al. Organotypic slice culture model demonstrates inter-neuronal spreading of alpha-synuclein aggregates. Acta Neuropathol. Commun. 2019;7:1–16. doi: 10.1186/s40478-019-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berge N.V.D., Ferreira N., Mikkelsen T.W., Alstrup A.K.O., Tamgüney G., Karlsson P., Terkelsen A.J., Nyengaard J.R., Jensen P.H., Borghammer P. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain. 2021 doi: 10.1093/brain/awab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song Y.J.C., Halliday G.M., Holton J.L., Lashley T., O’Sullivan S.S., McCann H., Lees A.J., Ozawa T., Williams D.R., Lockhart P., et al. Degeneration in Different Parkinsonian Syndromes Relates to Astrocyte Type and Astrocyte Protein Expression. J. Neuropathol. Exp. Neurol. 2009;68:1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- 108.Sorrentino Z.A., Giasson B.I., Chakrabarty P. α-Synuclein and astrocytes: Tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol. 2019;138:1–21. doi: 10.1007/s00401-019-01977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamilton R.L. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mikolaenko I., Pletnikova O., Kawas C.H., O’Brien R., Resnick S.M., Crain B., Troncoso J.C. Alpha-Synuclein Lesions in Normal Aging, Parkinson Disease, and Alzheimer Disease: Evidence from the Baltimore Longitudinal Study of Aging (BLSA) J. Neuropathol. Exp. Neurol. 2005;64:156–162. doi: 10.1093/jnen/64.2.156. [DOI] [PubMed] [Google Scholar]
- 111.Markesbery W.R., Jicha G.A., Liu H., Schmitt F.A. Lewy Body Pathology in Normal Elderly Subjects. J. Neuropathol. Exp. Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uemura N., Uemura M., Luk K., Lee V.M.-Y., Trojanowski J.Q. Cell-to-Cell Transmission of Tau and α-Synuclein. Trends Mol. Med. 2020;26:936–952. doi: 10.1016/j.molmed.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng C., Trojanowski J.Q., Lee V.M.-Y. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020;16:199–212. doi: 10.1038/s41582-020-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fares M.B., Jagannath S., Lashuel H.A. Reverse engineering Lewy bodies: How far have we come and how far can we go? Nat. Rev. Neurosci. 2021;22:256. doi: 10.1038/s41583-021-00447-7. [DOI] [PubMed] [Google Scholar]
- 115.Wu X., Zheng T., Zhang B. Exosomes in Parkinson’s Disease. Neurosci. Bull. 2016;33:331–338. doi: 10.1007/s12264-016-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi M., Liu C.-Q., Cook T.J., Bullock K.M., Zhao Y., Ginghina C., Li Y., Aro P., Dator R., He C., et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stuendl A., Kunadt M., Kruse N., Bartels C., Moebius W., Danzer K.M., Mollenhauer B., Schneider A. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2015;139:481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo M., Wang J., Zhao Y., Feng Y., Han S., Dong Q., Cui M., Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain. 2020;143:1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xia Y., Zhang G., Han C., Ma K., Guo X., Wan F., Kou L., Yin S., Liu L., Huang J., et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019;10:174. doi: 10.1038/s41419-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang S., Liu Y.-Q., Jia C., Lim Y.-J., Feng G., Xu E., Long H., Kimura Y., Tao Y., Zhao C., et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2011196118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mao X., Ou M.T., Karuppagounder S., Kam T.-I., Yin X., Xiong Y., Ge P., Umanah G.E., Brahmachari S., Shin J.-H., et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353 doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tardivel M., Bégard S., Bousset L., Dujardin S., Coens A., Melki R., Buée L., Colin M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016;4:1–14. doi: 10.1186/s40478-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abounit S., Bousset L., Loria F., Zhu S., de Chaumont F., Pieri L., Olivo-Marin J., Melki R., Zurzolo C. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 2016;35:2120–2138. doi: 10.15252/embj.201593411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bousset L., Pieri L., Ruiz-Arlandis G., Gath J., Jensen P.H., Habenstein B., Madiona K., Olieric V., Böckmann A., Meier B., et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gath J., Bousset L., Habenstein B., Melki R., Böckmann A., Meier B.H. Unlike Twins: An NMR Comparison of Two α-Synuclein Polymorphs Featuring Different Toxicity. PLoS ONE. 2014;9:e90659. doi: 10.1371/journal.pone.0090659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gath J., Bousset L., Habenstein B., Melki R., Meier B.H., Böckmann A. Yet another polymorph of α-synuclein: Solid-state sequential assignments. Biomol. NMR Assign. 2013;8:395–404. doi: 10.1007/s12104-013-9526-y. [DOI] [PubMed] [Google Scholar]
- 127.Gath J., Habenstein B., Bousset L., Melki R., Meier B.H., Böckmann A. Solid-state NMR sequential assignments of α-synuclein. Biomol. NMR Assign. 2011;6:51–55. doi: 10.1007/s12104-011-9324-3. [DOI] [PubMed] [Google Scholar]
- 128.Guo J.L., Covell D.J., Daniels J.P., Iba M., Stieber A., Zhang B., Riddle D.M., Kwong L.K., Xu Y., Trojanowski J.Q., et al. Distinct α-Synuclein Strains Differentially Promote Tau Inclusions in Neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Haute C.V.D., Melki R., Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nat. Cell Biol. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 130.Peng C., Gathagan R., Covell D.J., Medellin C., Stieber A., Robinson J.L., Zhang B., Pitkin R.M., Olufemi M.F., Luk K., et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nat. Cell Biol. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schweighauser M., Shi Y., Tarutani A., Kametani F., Murzin A.G., Ghetti B., Matsubara T., Tomita T., Ando T., Hasegawa K., et al. Structures of α-synuclein filaments from multiple system atrophy. Nat. Cell Biol. 2020;585:464–469. doi: 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Surguchov A. Analysis of Protein Conformational Strains—A Key for New Diagnostic Methods of Human Diseases. Int. J. Mol. Sci. 2020;21:2801. doi: 10.3390/ijms21082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Del Tredici K., Braak H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2015;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 134.Engelender S., Isacson O. The Threshold Theory for Parkinson’s Disease. Trends Neurosci. 2017;40:4–14. doi: 10.1016/j.tins.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 137.Halliday G., McRitchie D., Cartwright H., Pamphlett R., Hely M., Morris J. Midbrain neuropathology in idiopathic Parkinson’s disease and diffuse Lewy body disease. J. Clin. Neurosci. 1996;3:52–60. doi: 10.1016/S0967-5868(96)90083-1. [DOI] [PubMed] [Google Scholar]
- 138.Cheng H.-C., Ulane C.M., Burke R. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jellinger K.A. A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Kalaitzakis M.E., Graeber M.B., Gentleman S.M., Pearce R.K.B. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: A critical analysis of α-synuclein staging. Neuropathol. Appl. Neurobiol. 2008;34:284–295. doi: 10.1111/j.1365-2990.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 141.Doherty K.M., Silveira-Moriyama L., Parkkinen L., Healy D.G., Farrell M., Mencacci N.E., Ahmed Z., Brett F.M., Hardy J., Quinn N., et al. Parkin Disease: A clinicopathologic entity? JAMA Neurol. 2013;70:571–579. doi: 10.1001/jamaneurol.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beach T.G., Adler C.H., Sue L.I., Vedders L., Lue L., Iii C.L.W., Akiyama H., Caviness J.N., Shill H.A., Sabbagh M.N., et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krogh K., Ostergaard K., Sabroe S., Laurberg S. Clinical aspects of bowel symptoms in Parkinson?s disease. Acta Neurol. Scand. 2007;117:60–64. doi: 10.1111/j.1600-0404.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 144.Breen D.P., Halliday G.M., Lang A.E. Gut–brain axis and the spread of α-synuclein pathology: Vagal highway or dead end? Mov. Disord. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 145.Menozzi E., Macnaughtan J., Schapira A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Ann. Med. 2021;53:611–625. doi: 10.1080/07853890.2021.1890330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim S., Kwon S.-H., Kam T.-I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braak H., de Vos R.A., Bohl J., Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 148.Stokholm M.G., Danielsen E.H., Hamilton-Dutoit S., Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 149.Sprenger F.S., Stefanova N., Gelpi E., Seppi K., Navarro-Otano J., Offner F., Vilas D., Valldeoriola F., Pont-Sunyer C., Aldecoa I., et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85:1761–1768. doi: 10.1212/WNL.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu B., Fang F., Pedersen N., Tillander A., Ludvigsson J.F., Ekbom A., Svenningsson P., Chen H., Wirdefeldt K. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Svensson E., Horváth-Puhó E., Thomsen R., Djurhuus J.C., Pedersen L., Borghammer P., Sørensen H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 152.Fedorova T.D., Seidelin L.B., Knudsen K., Schacht A.C., Geday J., Pavese N., Brooks D., Borghammer P. Decreased intestinal acetylcholinesterase in early Parkinson disease: An (11)C-donepezil PET study. Neurology. 2017;88:775–781. doi: 10.1212/WNL.0000000000003633. [DOI] [PubMed] [Google Scholar]
- 153.Knudsen K., Fedorova T.D., Hansen A.K., Sommerauer M., Otto M., Svendsen K.B., Nahimi A., Stokholm M.G., Pavese N., Beier C.P., et al. In-vivo staging of pathology in REM sleep behaviour disorder: A multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. doi: 10.1016/S1474-4422(18)30162-5. [DOI] [PubMed] [Google Scholar]
- 154.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Bjorklund T., Wang Z.-Y., Roybon L., Melki R., Li J.-Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 155.Uemura N., Yagi H., Uemura M.T., Hatanaka Y., Yamakado H., Takahashi R. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 2018;13:1–11. doi: 10.1186/s13024-018-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boertien J.M., Pereira P.A., Aho V.T., Scheperjans F. Increasing Comparability and Utility of Gut Microbiome Studies in Parkinson’s Disease: A Systematic Review. J. Park. Dis. 2019;9:S297–S312. doi: 10.3233/JPD-191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pierantozzi M., Pietroiusti A., Brusa L., Galati S., Stefani A., Lunardi G., Fedele E., Sancesario G., Bernardi G., Bergamaschi A., et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- 158.Schwiertz A., Spiegel J., Dillmann U., Grundmann D., Bürmann J., Fassbender K., Schäfer K.-H., Unger M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 159.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased Intestinal Permeability Correlates with Sigmoid Mucosa alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. PLoS ONE. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peter I., Dubinsky M., Bressman S., Park A., Lu C., Chen N., Wang A. Anti–Tumor Necrosis Factor Therapy and Incidence of Parkinson Disease Among Patients with Inflammatory Bowel Disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brudek T. Inflammatory Bowel Diseases and Parkinson’s Disease. J. Park. Dis. 2019;9:S331–S344. doi: 10.3233/JPD-191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harapan B.N., Frydrychowicz C., Classen J., Wittekind C., Gradistanac T., Rumpf J.-J., Mueller W. No enhanced (p-) α-synuclein deposition in gastrointestinal tissue of Parkinson’s disease patients. Park. Relat. Disord. 2020;80:82–88. doi: 10.1016/j.parkreldis.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 163.Barrenschee M., Zorenkov D., Böttner M., Lange C., Cossais F., Scharf A.B., Deuschl G., Schneider S.A., Ellrichmann M., Fritscher-Ravens A., et al. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun. 2017;5:1. doi: 10.1186/s40478-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adler C.H., Connor D.J., Ms J.G.H., Sabbagh M.N., Caviness J.N., Shill H.A., Noble B., Beach T.G. Incidental Lewy body disease: Clinical comparison to a control cohort. Mov. Disord. 2010;25:642–646. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goedert M., Masuda-Suzukake M., Falcon B. Like prions: The propagation of aggregated tau and α-synuclein in neurodegeneration. Brain. 2016;140:266–278. doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- 166.Kingsbury A.E., Bandopadhyay R., Moriyama L.S., Ayling H., Kallis C., Sterlacci W., Maeir H., Poewe W., Lees A.J. Brain stem pathology in Parkinson’s disease: An evaluation of the Braak staging model. Mov. Disord. 2010;25:2508–2515. doi: 10.1002/mds.23305. [DOI] [PubMed] [Google Scholar]
- 167.Blesa J., Przedborski S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Duty S., Jenner P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koprich J.B., Kalia L.V., Brotchie J. Animal models of α-synucleinopathy for Parkinson disease drug development. Nat. Rev. Neurosci. 2017;18:515–529. doi: 10.1038/nrn.2017.75. [DOI] [PubMed] [Google Scholar]
- 170.Ayers J.I., Riffe C.J., Sorrentino Z.A., Diamond J., Fagerli E., Brooks M., Galaleldeen A., Hart P.J., Giasson B.I. Localized Induction of Wild-Type and Mutant Alpha-Synuclein Aggregation Reveals Propagation along Neuroanatomical Tracts. J. Virol. 2018;92 doi: 10.1128/JVI.00586-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van der Perren A., Haute C.V.D., Baekelandt V. Viral Vector-Based Models of Parkinson’s Disease. Curr. Top. Behav. Neurosci. 2014;22:271–301. doi: 10.1007/7854_2014_310. [DOI] [PubMed] [Google Scholar]
- 172.Watts J., Giles K., Oehler A., Middleton L., Dexter D.T., Gentleman S.M., DeArmond S.J., Prusiner S.B. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Recasens A., Dehay B., Bové J., Carballo-Carbajal I., Dovero S., Pérez-Villalba A., Fernagut P.-O., Blesa J., Parent A., Perier C., et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2013;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 174.Schaser A.J., Stackhouse T.L., Weston L.J., Kerstein P.C., Osterberg V.R., López C.S., Dickson D.W., Luk K.C., Meshul C.K., Woltjer R.L., et al. Trans-synaptic and retrograde axonal spread of Lewy pathology following pre-formed fibril injection in an in vivo A53T alpha-synuclein mouse model of synucleinopathy. Acta Neuropathol. Commun. 2020;8:1–23. doi: 10.1186/s40478-020-01026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sorrentino Z.A., Xia Y., Funk C., Riffe C.J., Rutherford N.J., Diaz C.C., Sacino A.N., Price N., Golde T.E., Giasson B.I., et al. Motor neuron loss and neuroinflammation in a model of α-synuclein-induced neurodegeneration. Neurobiol. Dis. 2018;120:98–106. doi: 10.1016/j.nbd.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giguère N., Nanni S.B., Trudeau L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018;9:455. doi: 10.3389/fneur.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schapira A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 178.Braak H., Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv. Anat. Embryol. Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 179.Matsuda W., Furuta T., Nakamura K., Hioki H., Fujiyama F., Arai R., Kaneko T. Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum. J. Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hirsch E.C., Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 181.Tansey M.G., Goldberg M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cabello C.R., Thune J.J., Pakkenberg H., Pakkenberg B. Ageing of substantia nigra in humans: Cell loss may be compensated by hypertrophy. Neuropathol. Appl. Neurobiol. 2002;28:283–291. doi: 10.1046/j.1365-2990.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 183.Chan C.S., Gertler T.S., Surmeier D.J. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov. Disord. 2010;25:S63–S70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- 184.Fasano M., Bergamasco B., Lopiano L. Is neuromelanin changed in Parkinson’s disease? Investigations by magnetic spectroscopies. J. Neural Transm. 2006;113:769–774. doi: 10.1007/s00702-005-0448-4. [DOI] [PubMed] [Google Scholar]
- 185.Bolam J.P., Pissadaki E.K. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord. 2012;27:1478–1483. doi: 10.1002/mds.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Delaidelli A., Richner M., Jiang L., van der Laan A., Christiansen I.B.J., Ferreira N., Nyengaard J.R., Vægter C.B., Jensen P.H., Mackenzie I.R., et al. α-Synuclein pathology in Parkinson disease activates homeostatic NRF2 anti-oxidant response. Acta Neuropathol. Commun. 2021;9:1–16. doi: 10.1186/s40478-021-01209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramsey C., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C., Jordan-Sciutto K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanji K., Maruyama A., Odagiri S., Mori F., Itoh K., Kakita A., Takahashi H., Wakabayashi K. Keap1 Is Localized in Neuronal and Glial Cytoplasmic Inclusions in Various Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2013;72:18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- 189.Schipperab H.M., Liberman A., Stopa E. Neural Heme Oxygenase-1 Expression in Idiopathic Parkinson’s Disease. Exp. Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- 190.Lastres-Becker I., García-Yagüe J., Scannevin R.H., Casarejos M.J., Kügler S., Rábano A., Cuadrado A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 2016;25:61–77. doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ansorge O., Daniel S.E., Pearce R.K. Neuronal loss and plasticity in the supraoptic nucleus in Parkinson’s disease. Neurology. 1997;49:610–613. doi: 10.1212/WNL.49.2.610. [DOI] [PubMed] [Google Scholar]
- 192.Greffard S., Verny M., Bonnet A.-M., Seilhean D., Hauw J.-J., Duyckaerts C. A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol. Aging. 2010;31:99–103. doi: 10.1016/j.neurobiolaging.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 193.Porritt M.J., Kingsbury A.E., Hughes A.J., Howells D.W. Striatal dopaminergic neurons are lost with Parkinson’s disease progression. Mov. Disord. 2006;21:2208–2211. doi: 10.1002/mds.21129. [DOI] [PubMed] [Google Scholar]
- 194.Burke R.E., Dauer W., Vonsattel J.P.G. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann. Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kovacs G.G., Milenkovic I.J., Preusser M., Budka H. Nigral burden of α-synuclein correlates with striatal dopamine deficit. Mov. Disord. 2008;23:1608–1612. doi: 10.1002/mds.22207. [DOI] [PubMed] [Google Scholar]
- 196.Guzman J.N., Sánchez-Padilla J., Chan C.S., Surmeier D.J. Robust Pacemaking in Substantia Nigra Dopaminergic Neurons. J. Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nedergaard S., Flatman J.A., Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J. Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- 198.Puopolo M., Raviola E., Bean B.P. Roles of Subthreshold Calcium Current and Sodium Current in Spontaneous Firing of Mouse Midbrain Dopamine Neurons. J. Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chan C.S., Guzman J.N., Ilijic E., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nat. Cell Biol. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 200.Mercuri N.B., Bond A., Calabresi P., Stratta F., Stefani A., Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br. J. Pharmacol. 1994;113:831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Foehring R.C., Zhang X.F., Lee J., Callaway J.C. Endogenous Calcium Buffering Capacity of Substantia Nigral Dopamine Neurons. J. Neurophysiol. 2009;102:2326–2333. doi: 10.1152/jn.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khaliq Z.M., Bean B.P. Pacemaking in Dopaminergic Ventral Tegmental Area Neurons: Depolarizing Drive from Background and Voltage-Dependent Sodium Conductances. J. Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Philippart F., Destreel G., Merino-Sepúlveda P., Henny P., Engel D., Seutin V. Differential Somatic Ca2+ Channel Profile in Midbrain Dopaminergic Neurons. J. Neurosci. 2016;36:7234–7245. doi: 10.1523/JNEUROSCI.0459-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Balaban R.S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Goldberg J., Guzman J.N., Estep C.M., Ilijic E., Kondapalli J., Sanchez-Padilla J., Surmeier D.J. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson’s disease. Nat. Neurosci. 2012;15:1414–1421. doi: 10.1038/nn.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guzman J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P.T., Surmeier D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nat. Cell Biol. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Watson C., Paxinos G., Puelles L. The Mouse Nervous System. 1st ed. Elsevier Academic Press; Amsterdam, The Netherlands: Boston, MA, USA: 2012. p. xvii.795p [Google Scholar]
- 208.Del Tredici K., Braak H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J. Neurol. Neurosurg. Psychiatry. 2012;84:774–783. doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- 209.Lee H.-J., Suk J.-E., Patrick C., Bae E.-J., Cho J.-H., Rho S., Hwang D., Masliah E., Lee S.-J. Direct Transfer of α-Synuclein from Neuron to Astroglia Causes Inflammatory Responses in Synucleinopathies*. J. Biol. Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gu X.-L., Long C.-X., Sun L., Xie C., Lin X., Cai H. Astrocytic expression of Parkinson’s disease-related A53T α-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Beach T.G., Sue L.I., Walker U.G., Lue L.F., Connor N.J., Caviness J.N., Sabbagh M.N., Adler C.H. Marked microglial reaction in normal aging human substantia nigra: Correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114:419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- 212.Knott C., Stern G., Kingsbury A., Welcher A., Wilkin G. Elevated glial brain-derived neurotrophic factor in Parkinson’s diseased nigra. Park. Relat. Disord. 2002;8:329–341. doi: 10.1016/S1353-8020(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 213.Riederer P., Gerlach M., Müller T., Reichmann H. Relating mode of action to clinical practice: Dopaminergic agents in Parkinson’s disease. Park. Relat. Disord. 2007;13:466–479. doi: 10.1016/j.parkreldis.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 214.Seidel K., Mahlke J., Siswanto S., Krueger R., Heinsen H., Auburger G., Bouzrou M., Grinberg L., Wicht H., Korf H., et al. The Brainstem Pathologies of Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2014;25:121–135. doi: 10.1111/bpa.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Muntane G., Dalfo E., Martinez A., Ferrer I. Phosphorylation of tau and α-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related α-synucleinopathies. Neuroscience. 2008;152:913–923. doi: 10.1016/j.neuroscience.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 216.Obi K., Akiyama H., Kondo H., Shimomura Y., Hasegawa M., Iwatsubo T., Mizuno Y., Mochizuki H. Relationship of phosphorylated α-synuclein and tau accumulation to A β deposition in the cerebral cortex of dementia with Lewy bodies. Exp. Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 217.Wills J., Jones J., Haggerty T., Duka V., Joyce J.N., Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Coakeley S., Strafella A.P. Imaging tau pathology in Parkinsonisms. NPJ Park. Dis. 2017;3:1–9. doi: 10.1038/s41531-017-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Axelsen T.M., Woldbye D.P. Gene Therapy for Parkinson’s Disease, An Update. J. Park. Dis. 2018;8:195–215. doi: 10.3233/JPD-181331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Coune P.G., Schneider B.L., Aebischer P. Parkinson’s Disease: Gene Therapies. Cold Spring Harb. Perspect. Med. 2012;2:a009431. doi: 10.1101/cshperspect.a009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 2020;21:103–115. doi: 10.1038/s41583-019-0257-7. [DOI] [PubMed] [Google Scholar]
- 222.McFarthing K., Buff S., Rafaloff G., Dominey T., Wyse R.K., Stott S.R.W. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J. Park. Dis. 2020;10:757–774. doi: 10.3233/JPD-202128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fayyad M., Salim S., Majbour N., Erskine D., Stoops E., Mollenhauer B., El-Agnaf O.M.A. Parkinson’s disease biomarkers based on α-synuclein. J. Neurochem. 2019;150:626–636. doi: 10.1111/jnc.14809. [DOI] [PubMed] [Google Scholar]
- 224.Atik A., Stewart T., Zhang J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016;26:410–418. doi: 10.1111/bpa.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mahlknecht P., Hotter A., Hussl A., Esterhammer R., Schocke M., Seppi K. Significance of MRI in Diagnosis and Differential Diagnosis of Parkinson’s Disease. Neurodegener. Dis. 2010;7:300–318. doi: 10.1159/000314495. [DOI] [PubMed] [Google Scholar]
- 226.Rizzo G., Martinelli P., Manners D., Scaglione C., Tonon C., Cortelli P., Malucelli E., Capellari S., Testa C., Parchi P., et al. Diffusion-weighted brain imaging study of patients with clinical diagnosis of corticobasal degeneration, progressive supranuclear palsy and Parkinson’s disease. Brain. 2008;131:2690–2700. doi: 10.1093/brain/awn195. [DOI] [PubMed] [Google Scholar]
- 227.Weise D., Lorenz R., Schliesser M., Schirbel A., Reiners K., Classen J. Substantia nigra echogenicity: A structural correlate of functional impairment of the dopaminergic striatal projection in Parkinson’s disease. Mov. Disord. 2009;24:1669–1675. doi: 10.1002/mds.22665. [DOI] [PubMed] [Google Scholar]
- 228.Rascol O., Schelosky L. 123 I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov. Disord. 2009;24:S732–S741. doi: 10.1002/mds.22499. [DOI] [PubMed] [Google Scholar]
- 229.Adams J.L., Lizarraga K.J., Waddell E.M., Myers T.L., Jensen-Roberts S., Modica J.S., Schneider R.B. Digital Technology in Movement Disorders: Updates, Applications, and Challenges. Curr. Neurol. Neurosci. Rep. 2021;21:1–11. doi: 10.1007/s11910-021-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Luis-Martínez R., Monje M.H.G., Antonini A., Sánchez-Ferro Á., Mestre T.A. Technology-Enabled Care: Integrating Multidisciplinary Care in Parkinson’s Disease Through Digital Technology. Front. Neurol. 2020;11:575975. doi: 10.3389/fneur.2020.575975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rockenstein E., Mallory M., Hashimoto M., Song D., Shults C.W., Lang I., Masliah E. Differential neuropathological alterations in transgenic mice expressing α-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 232.Chesselet M.-F., Richter F., Zhu C., Magen I., Watson M.B., Subramaniam S.R. A Progressive Mouse Model of Parkinson’s Disease: The Thy1-aSyn (“Line 61”) Mice. Neurotherapeutics. 2012;9:297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Henrich M., Geibl F.F., Lee B., Chiu W.-H., Koprich J.B., Brotchie J.M., Timmermann L., Decher N., Matschke L.A., Oertel W.H. A53T-α-synuclein overexpression in murine locus coeruleus induces Parkinson’s disease-like pathology in neurons and glia. Acta Neuropathol. Commun. 2018;6:39. doi: 10.1186/s40478-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Butkovich L.M., Houser M.C., Chalermpalanupap T., Porter-Stransky K.A., Iannitelli A.F., Boles J., Lloyd G.M., Coomes A.S., Eidson L.N., Rodrigues M.E.D.S., et al. Transgenic Mice Expressing Human α-Synuclein in Noradrenergic Neurons Develop Locus Ceruleus Pathology and Nonmotor Features of Parkinson’s Disease. J. Neurosci. 2020;40:7559–7576. doi: 10.1523/JNEUROSCI.1468-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M.-Y. Neuronal α-Synucleinopathy with Severe Movement Disorder in Mice Expressing A53T Human α-Synuclein. Neuron. 2002;34:521–533. doi: 10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 236.Ferreira N., Richner M., van der Laan A., Christiansen I.B.J., Vægter C.B., Nyengaard J.R., Halliday G.M., Weiss J., Giasson B.I., Mackenzie I.R., et al. Prodromal neuroinvasion of pathological α-synuclein in brainstem reticular nuclei and white matter lesions in a model of α-synucleinopathy. Brain Commun. 2021;3:fcab104. doi: 10.1093/braincomms/fcab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lalonde R., Strazielle C. Brain regions and genes affecting limb-clasping responses. Brain Res. Rev. 2011;67:252–259. doi: 10.1016/j.brainresrev.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 238.Linnman C., Moulton E., Barmettler G., Becerra L., Borsook D. Neuroimaging of the periaqueductal gray: State of the field. NeuroImage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wilson-Poe A., Pocius E., Herschbach M., Morgan M.M. The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol. Biochem. Behav. 2013;103:444–449. doi: 10.1016/j.pbb.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Goedert M., Jakes R., Spillantini M.G. The Synucleinopathies: Twenty Years On. J. Park. Dis. 2017;7:S51–S69. doi: 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Irizarry M.C., Growdon W., Gomez-Isla T., Newell K., George J.M., Clayton D.F., Hyman B.T. Nigral and Cortical Lewy Bodies and Dystrophic Nigral Neurites in Parkinsonʼs Disease and Cortical Lewy Body Disease Contain α-synuclein Immunoreactivity. J. Neuropathol. Exp. Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 242.Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R., Goedert M. α-synuclein in Lewy bodies. Nat. Cell Biol. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 243.Sidransky E., Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Volpicelli-Daley L.A., Abdelmotilib H., Liu Z., Stoyka L., Daher J.P.L., Milnerwood A.J., Unni V.K., Hirst W.D., Yue Z., Zhao H.T., et al. G2019S-LRRK2 Expression Augments α-synuclein Sequestration into Inclusions in Neurons. J. Neurosci. 2016;36:7415–7427. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tang F.-L., Erion J.R., Tian Y., Liu W., Yin D.-M., Ye J., Tang B., Mei L., Xiong W.-C. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for α-synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. J. Neurosci. 2015;35:10613–10628. doi: 10.1523/JNEUROSCI.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ron I., Rapaport D., Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: A possible link between Parkinson disease and Gaucher disease. Hum. Mol. Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 247.Doi D., Magotani H., Kikuchi T., Ikeda M., Hiramatsu S., Yoshida K., Amano N., Nomura M., Umekage M., Morizane A., et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-17165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brauer R., Wei L., Ma T., Athauda D., Girges C., Vijiaratnam N., Auld G., Whittlesea C., Wong I., Foltynie T. Diabetes medications and risk of Parkinson’s disease: A cohort study of patients with diabetes. Brain. 2020;143:3067–3076. doi: 10.1093/brain/awaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.De Pablo-Fernandez E., Goldacre R., Pakpoor J., Noyce A.J., Warner T.T. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology. 2018;91:e139–e142. doi: 10.1212/WNL.0000000000005771. [DOI] [PubMed] [Google Scholar]
- 250.Henderson M.X., Sengupta M., Trojanowski J.Q., Lee V.M.Y. Alzheimer’s disease tau is a prominent pathology in LRRK2 Parkinson’s disease. Acta Neuropathol. Commun. 2019;7:1–16. doi: 10.1186/s40478-019-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schneider S.A., Alcalay R.N. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature. Mov. Disord. 2017;32:1504–1523. doi: 10.1002/mds.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Garrido A., Fairfoul G., Tolosa E.S., Martí M.J., Green A., Barcelona L.S.G. α-synuclein RT-QuIC in cerebrospinal fluid of LRRK 2-linked Parkinson’s disease. Ann. Clin. Transl. Neurol. 2019;6:1024–1032. doi: 10.1002/acn3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Aasly J.O., Johansen K.K., Brã¸nstad G., War㸠B.J., Majbour N., Evarghese S., Ealzahmi F., Paleologou K.E., Amer D.A.M., Eal-Hayani A., et al. Elevated levels of cerebrospinal fluid α-synuclein oligomers in healthy asymptomatic LRRK2 mutation carriers. Front. Aging Neurosci. 2014;6:248. doi: 10.3389/fnagi.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kumar S.T., Jagannath S., Francois C., Vanderstichele H., Stoops E., Lashuel H.A. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct structures and morphology. Neurobiol. Dis. 2020;146:105086. doi: 10.1016/j.nbd.2020.105086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data cited in the article can be accessed in original research studies provided in the bibliography under the references.