Abstract
Human papillomavirus oral papilloma is often sexually transmitted, but non‐sexual modes of transmission should be considered, including autoinoculation from skin lesions. A patient‐centered multimodality approach should be utilized in the pediatric population.
Keywords: adolescent medicine, HPV, oral papilloma, pediatrics
Human papillomavirus oral papilloma is often sexually transmitted, but non‐sexual modes of transmission should be considered, including autoinoculation from skin lesions. A patient‐centered multimodality approach should be utilized in the pediatric population.
1. INTRODUCTION
Squamous papilloma is the most common benign oral epithelial growth in children, accounting for 8% of oral soft tissue tumors among the pediatric population. 1 It is characterized by slow‐growing “cauliflower”‐like exophytic projections with a predilection for the tongue and palate within the oral cavity. 2 Squamous papilloma is most commonly associated with infection by human papillomavirus (HPV), types 6 and 11. While HPV is considered the most common sexually transmitted infection, it may be transmitted non‐sexually through various routes. 3 Management of oral HPV‐positive papillomas is warranted to decrease transmissibility and remove cosmetically and functionally impairing lesions. Surgical excision has been described in the literature as a curative treatment 4 ; however, recurrence may occur due to incomplete removal of infected epithelium or autoinoculation from other infection sites. 3 Additional treatment methods include CO2 laser ablation, electrosurgery, and cryotherapy, 4 although there is little research on the efficacy of these. We present a case of recurrent, diffuse HPV‐positive oral squamous papillomas of unknown etiology in an adolescent female treated with surgical excision and CO2 laser ablation.
2. CASE REPORT
A healthy 13‐year‐old female presented to Penn State Otolaryngology—Head and Neck outpatient clinic with a 9‐month history of intraoral papillomas first noticed at the dentist. She denied pain, dysphagia, or contact bleeding. Past medical history was only significant for multiple palmar verrucae 2 years prior that were treated by dermatology with Candida antigen injections, squaric acid 2%, and cryotherapy. She also endorsed a history of frequent nail‐biting, and based on our records, had not received the HPV vaccination series prior to presentation. On physical examination, there were multiple clusters of papillomas on the bilateral buccal mucosa with one large isolated papilloma on the right buccal mucosa measuring approximately 2 cm × 3 cm (Figure 1). There were also multiple papillomas in the intraoral mucosa of the lower lip and the right and left commissures. Biopsies of the lesions were positive for HPV types 6 and 11, and negative for types 16, 18, 31, and 33. The concern for possible sexual abuse was raised due to the HPV positivity; however, the patient adamantly denied any previous sexual activity and further investigation by child services did not identify evidence of sexual abuse. Given the diffuse nature of the lesions, the patient wished to proceed with operative intervention. The large right buccal papilloma was excised and repaired primarily due to its size. To minimize scarring along the lower lip, CO2 laser ablation of the smaller papillomas was performed. Histopathology of one of the excised lesions is demonstrated in Figure 2.
FIGURE 1.
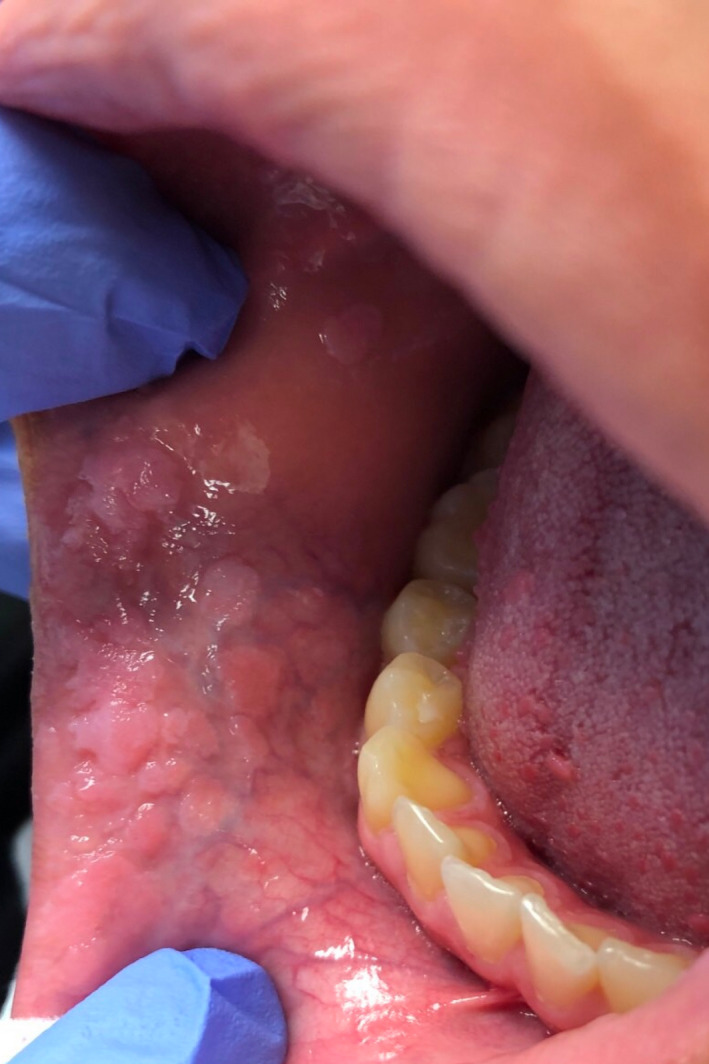
Demonstrates the patient's physical examination on initial evaluation at our facility and reveals a cluster of papillomas of the intraoral right commissure
FIGURE 2.
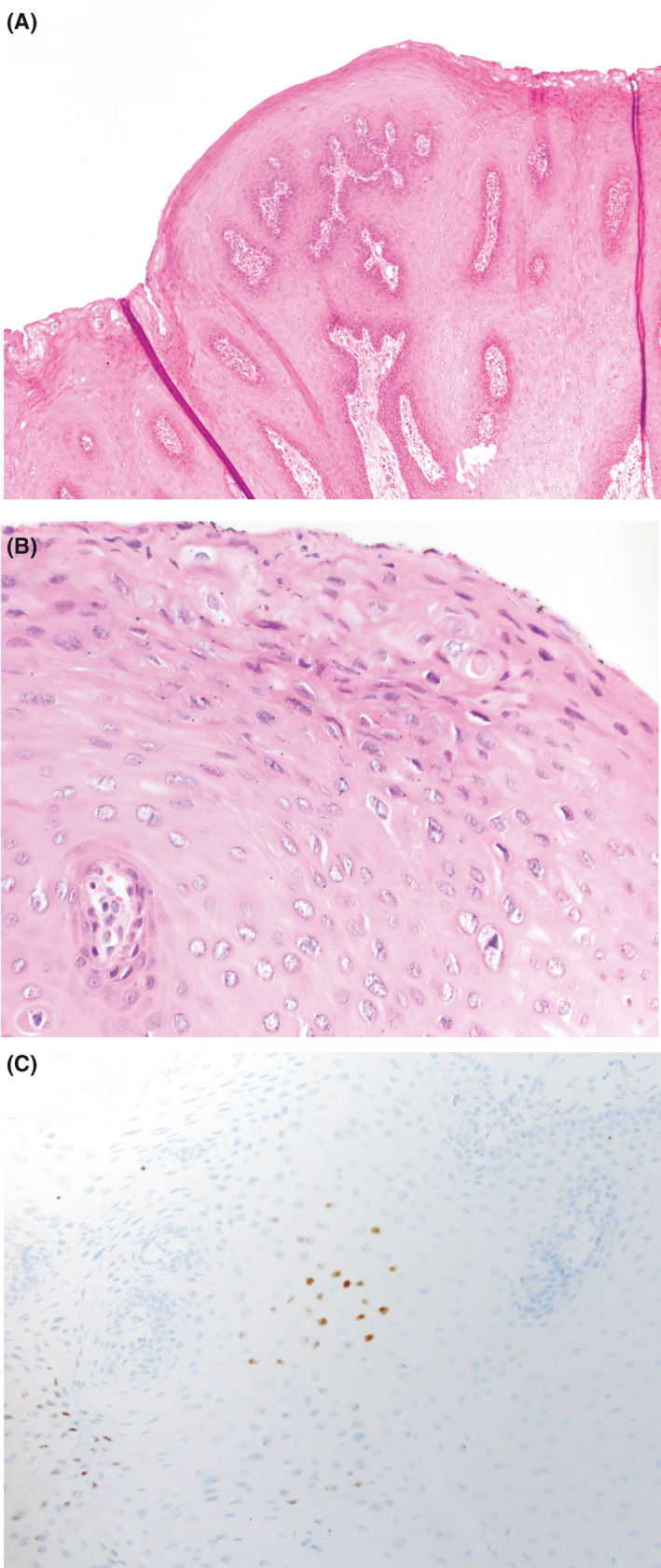
Sections demonstrate a squamous lesion with papillary architecture (A, H&E, 100× magnification). There was focal koilocytic atypia in the lesion (B, H&E, 400× magnification). RNA in situ hybridization showed lesional cells were positive for low‐risk HPV in a nuclear localization (C, in situ hybridization, 400× magnification), as seen in HPV‐mediated proliferations
Six months later on routine follow‐up, the patient noticed recurrence of the smaller papillomas. Physical examination revealed recurrent lesions on the right lower lip, left buccal mucosa adjacent to the oral commissure, and right superior sublabial mucosa. At that time, no treatment was pursued because the papillomas were not bothersome and the patient denied any pain or bleeding. However, 7 months later, the patient returned to clinic for re‐evaluation due to continued growth of the papillomas. On examination, there were now two small papillomas on the upper mucosal lip, each 1‐2 mm in size. Additional papillomas were noted in the right retromolar trigone, right superior gingivobuccal sulcus, as well as the right and left buccal mucosa at the oral commissures (Figure 3A,B). The patient again denied any sexual activity. The plan for management was for surgical intervention with CO2 laser ablation, but due to the COVID‐19 pandemic, the patient was unfortunately lost to follow‐up.
FIGURE 3.
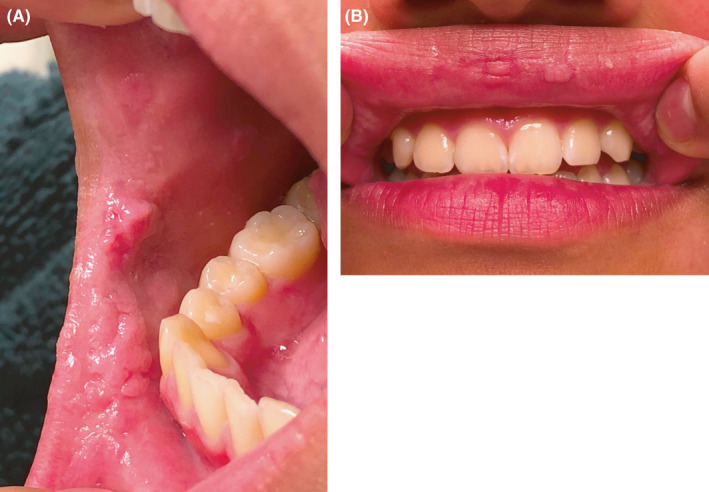
(A) Demonstrates the patient's physical examination on evaluation about 1 year after surgical intervention; reveals recurrence of papillomas of the intraoral right commissure. (B) Demonstrates the patient's physical examination on evaluation about 1 year after surgical intervention; reveals appearance of new papillomas of the red upper lip
3. DISCUSSION
We report a case of a young, sexually naive female presenting with multiple recurrent HPV‐positive oral papillomas despite operative intervention. There is sparse literature examining the natural history of HPV infection among sexually unexposed adolescents. While oral HPV is thought to be transmitted via sexual contact of genitalia to oral mucosa, transmission of HPV infection from warts on hands to the anogenital area is reported, suggesting alternate modes of transmission. 5 There is also evidence of transmission from fomites found on towels and other objects, although this is not well‐established. 3 With a history of nail‐biting and no prior history of sexual behavior or claims of sexual abuse, we hypothesize that autoinoculation may have occurred via hand‐to‐mouth transmission from our patient's cutaneous verrucae of her fingers. Due to the slow‐growing nature of papillomas, the time between inoculation and gross appearance may have led these oral lesions to go undetected for months to years. Since the patient's cutaneous verrucae were previously treated by dermatologic intervention, we were unable to identify a proven relationship between these lesions and the oral papillomas.
While our patient adamantly denied sexual activity, our case did raise concern for sexual abuse. Investigation by child services did not identify evidence of sexual abuse. A review of possible non‐sexual modes of HPV transmission concludes that sexual abuse is very low among children with genital warts and suggests that HPV acquisition occurs via close contacts. 3 They emphasize that clinical examination should be carried out to look for similar lesions in caregivers that may contribute to horizontal transmission. However, there was no knowledge of HPV‐related condyloma among household contacts to suggest person‐to‐person transmission as the primary mode of this patient's infection. Understanding alternative modes of transmission can also facilitate avoidance of investigative procedures in children and sexually unexposed adolescents. 3
The literature indicates similar rates of recurrence for surgical excision and CO2 laser ablation of oral squamous papillomas (10% and 14%, respectively). 6 The advantages of greater preservation of healthy oral mucosa and improved hemostasis provide growing support for CO2 ablation in the treatment of smaller or diffuse lesions. However, scarring may occur and lasting clearance of papillomas can be difficult to achieve. 7 , 8 A previous study indicates that CO2 laser ablation of respiratory papillomas prevented clinical recurrence for 5 years among just 17% of patients. 7 Our case utilized a multimodality approach with both excision and CO2 laser ablation, but did demonstrate multiple recurrences, likely due to a more conservative ablation initially. The decision of which surgical modality to implement may depend on the size of the lesions and surgeons’ preferences. The goal of treatment is to remove all of the papillomas while maximizing preservation of as much healthy tissue as possible; however, this may come at the cost of lesion recurrence. In our case, the lesions presented at follow‐up may have represented the consequence of either incomplete resection or de novo lesions.
4. CONCLUSION
The etiology of HPV‐positive oral squamous papillomas in sexually naive patients remains unclear. While we hypothesize that our patient's lesions may have been a result of autoinoculation by HPV‐positive skin lesions, this has not been proven and the exact etiology remains unknown. Further investigation into alternate non‐sexual modes of transmission is warranted to elucidate this diagnostic dilemma.
CONFLICTS OF INTEREST
All authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
SB collected the data, performed the literature search, and prepared the manuscript. AK collected data, prepared and revised the manuscript, formatted figures, prepared and submitted the manuscript. JW prepared, formatted and described pathology specimens and figures. KC revised, prepared, and submitted the manuscript. All authors read and approved the final manuscript.
ETHICAL APPROVAL
The Human Subjects Protection Office determined that the research for this manuscript does not meet the definition of human subject research and IRB review and approval is not required. The manuscript was created in compliance with the ethical standards of our institution.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Published with written consent of the patient.
Benyo S, Keane A, Warrick J, Choi KY. HPV‐positive oral papillomas in an adolescent—A diagnostic dilemma. Clin Case Rep. 2021;9:e04546. 10.1002/ccr3.4546
Funding information
There was no financial support or funding received for the preparation of this manuscript.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Praveen Kumar B, Khaitan T, Ramaswamy P, Pattipati S, Sudhakar S, Geethika VR. Squamous papilloma. Int J Stomatol Occlusion Med. 2013;6:106‐109. [Google Scholar]
- 2. Babaji P, Chaurasia VR, Masamatti VS, Sharma AM, Singh V. Squamous papilloma of the hard palate. Indian J Dent. 2014;5(4):211‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabeena S, Bhat P, Kamath V, et al. Possible non‐sexual modes of transmission of human papilloma virus. J Obstet Gynaecol Res. 2017;43(3):429‐435. [DOI] [PubMed] [Google Scholar]
- 4. Pringle GA. The role of human papillomavirus in oral disease. Dent Clin North Am. 2014;58(2):385‐399. [DOI] [PubMed] [Google Scholar]
- 5. Moscicki A‐B, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(Suppl 5):F24‐F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toledano‐Serrabona J, Lopez‐Ramirez M, Sanchez‐Torres A, Espana‐Tost A, Gay‐Escoda C. Recurrence rate of oral squamous cell papilloma after excision with surgical scalpel or laser therapy: a retrospective cohort study. Med Oral Patol Oral Cir Bucal. 2019;24(4):e433‐e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dedo HH, Yu KC. CO(2) laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111(9):1639‐1644. [DOI] [PubMed] [Google Scholar]
- 8. Benazzo M, Canzi P, Mauramati S, et al. Transoral robot‐assisted surgery in supraglottic and oropharyngeal squamous cell carcinoma: laser versus monopolar electrocautery. J Clin Med. 2019;8(12):2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


