Abstract
The aim of the present research was to describe the consequences of hyperlipidemia (HL) on the pharmacokinetics of glibenclamide (Gb) in poloxamer 407-induced hyperlipidemic rats. Rats were given intraperitoneal dose of poloxamer 407 to cause hyperlipidemia. A single oral dose of Gb (10 mg/Kg) was given to normal and HL rats. The Cmax and tmax after oral dose of Gb in normal rats were 340.10 µg/ml and 3.67 h, respectively. Whereas, the Cmax and tmax after oral dose of Gb in HL rats were noted as 773.39 µg/ml and 2.50 h respectively. The AUC value of Gb was found considerably higher in the HL rats. While the plasma clearance (CL) after oral dose of Gb was 2.53 ml/h and 1.39 ml/h in normal and HL rats respectively. The improved plasma concentration of Gb following oral dosing in rats with HL seems to be due to a direct influence on hepatic clearance or metabolizing enzymes. In conclusion, the Gb pharmacokinetics was considerably affected by the HL in rats. Such findings play an important role for predicting the alterations in the pharmacokinetics of drugs including GB, in cases having hyperlipidemia.
Keywords: Diabetes, Glyburide, Hyperlipidemia, Lipoproteins, Rats
1. Introduction
Hyperlipidemia (HL) is a clinical condition that is defined by rise in plasma lipoproteins. This situation presents a clinical concern, because chronic rises in low-density lipoproteins are a key factor contributing to an increased risk of coronary heart disease, atherosclerosis and hypertension. Previous reports have reported that HL could contributes certain changes in the toxicodynamic, pharmacodynamic and pharmacokinetic profiles of lipoprotein-bound drugs (Hamdy and Brocks, 2009, Hamdy and Brocks, 2016, Khalil et al., 2016, Lee et al., 2019). HL might be associated with increased plasma binding and a lower unbound plasma fraction, with a decrease in total drug volume of distribution (Vd) and likely reduce the clearance (CL) of the drug. In general, decreased in clearance of drug leads to increase the concentration of drug in systemic circulation to toxic level and that apparently contribute to increased potential for adverse drug reactions. Conversely, increased in clearance of drug contributes to lesser side effect of drug. However, in cases where drug clearance is greater, higher weight-based doses and more frequent dosing intervals are needed. Hence, when it comes to medication dosing, knowing the importance of drug clearance is vital. Drug dosages and their frequency of administration must also be adjusted for cases with HL in attempt to avoid undesirable drug effects (Malangu, 2018).
HL has also been associated with a decreased level of expression and some activity of cytochrome P450 (CYP) isoforms that are primarily engaged in drug metabolism (Patel and Brocks, 2009). Antecedently, HL has been shown to modify the pharmacokinetic profile of several candidate in rats (Khalil et al., 2017, Khalil et al., 2016, Lee et al., 2012, Lee et al., 2019 Bin Jardan and Brocks, 2016).
Glibenclamide (Gb, Fig. 1) is an oral 2nd generation sulfonylurea, with a potent and sustained hypoglycemic impact, often prescribed to manage type 2 diabetes mellitus (Ju et al., 2020, Zou et al., 2020). Gb is also widely considered to treat gestational diabetes mellitus (Affres et al., 2020, Bouchghoul et al., 2020, Shuster et al., 2020), Gb has been observed to accumulate in pancreatic beta cells after chronic use (Hellman et al., 1984). These variables can lead to the extended action of the drug and cause hypoglycemia in certain circumstances. Gb is extensively metabolized in the liver through the CYP system (Naritomi et al., 2004, Zharikova et al., 2009, Zhou et al., 2010). Several studies have shown that the main CYP enzyme concerned in the in vitro metabolism of Gb is CYP3A4 (Zhou et al., 2010). Although, other CYPs, for instance, CYP2C8, CYP2C9 and CYP2C19 could also play a role in Gb in vitro metabolism (Zharikova et al., 2009, Zhou et al., 2010). The pharmacokinetics of Gb and its excretion in hyperlipidemia condition is unclear. Gb is sparingly soluble in water and categorized in the list of BCS class II drugs (Ahad et al., 2015) and having log p of 4.7 (Spiller, 2014). Therefore, Gb is a possible candidate for lipoprotein binding and therefore might show potential changes in its pharmacokinetics. Consequently, the objective of this research was to examine the potential effects of raised lipoprotein levels on the Gb pharmacokinetics in the poloxamer induced -HL rats.
Fig. 1.

Chemical structure of Gb.
2. Materials and methods
2.1. Materials
“Glibenclamide was purchased from Alfa Aesar (Ward Hill, MA)”. Poloxamer-407 was purchased from “Anatrace Products, LLC Maumee, Ohio, USA”. HPLC grade methanol and acetonitrile were procured from “Panreac Quimica (Barcelona, Espana)”. Formic acid was acquired from “Loba Chemie Pvt. Ltd. (Mumbai, India)”. “Purified water was arranged using Milli-QR Gradient A10R (“Millipore, Molsheim Cedex, France”).
2.2. Animals and induction of HL
Experimental protocols were duly approved by the “King Saud University Research Ethics Committee with ethical reference number KSU-SE-21-10”. Wistar rats (250 ± 20 g) were used in this study. All rats were housed in temperature-controlled rooms with 12 h of light per day. Rats were distributed into two groups and named as NL (control group, n = 3) and HL (n = 4) groups. HL was induced by single intraperitoneal injection of 1 g/kg of Poloxamer-407. Control and the HL groups were dosed with the drug; HL group were treated after 36 h from the P407 injection (Chaudhary and Brocks, 2013, Khalil et al., 2017, Khalil et al., 2016).
2.3. Drug administration and sampling
For the pharmacokinetic study, the both groups of rats received Gb 10 mg/kg via oral feeding needle. Blood samples were taken in heparinized tubes from NL and HL groups at the time interval of 0, 0.5, 1, 2, 3, 4, 12, 24 h after the Gb oral dose. The plasma was separated by centrifugation of the blood at 13,000 rpm for 10 min and transferred to clean eppendorf tubes. The separated plasma samples were analyzed using a UPLC-MS/MS method. Briefly, plasma protein precipitation method was used to prepare the samples. As an internal standard, glimepiride was included. An Acquity UPLC®BEH C18 column was used for the analysis. A mixture of acetonitrile (0.1% formic acid) and water (0.1% formic acid) was used as mobile phase which was pumped at a flow rate of 150 μl/min in binary gradient mode. Positive electrospray ionization was used to operate the triple- quadrupole mass spectrometer. In MRM mode, sodium adducts [M+Na]+ of Gb and internal standard were observed. The sample 10 μl was injected and the autosampler temperature was maintained at 20 ± 3 °C. Total sample run time was 2.0 min (Alam et al., 2018). The non-compartmental pharmacokinetic parameters were estimated using PK Solver software (version 1.0). The calculated parameters were as follows: Cmax, maximum plasma concentration; Tmax, time to reach maximum plasma concentration; AUC0-t, area under the plasma concentration–time curve; t1/2, elimination half-life. The CL, total clearance was calculated as dose/AUC.
2.4. Statistical analysis
“Differences in means were analyzed by unpaired t-test; using GraphPad InStat® 3.06 (GraphPad Software, Inc, CA, USA). p < 0.05 were considered significant”.
3. Results and discussion
A single dose of poloxamer-407 caused HL in rats; this model is an acceptable choice to evaluate the effect of HL in the pharmacokinetics of drugs, since it is non-inflammatory and not linked with diabetes or cardiovascular disruption (Gabr et al., 2017).
In different animal models, poloxamer-407 can stimulate hyperlipidemia, which greatly enhances the triglycerides and cholesterol in plasma (Johnston and Palmer, 1993, Kim et al., 2019, Palmer et al., 1998, Wout et al., 1992, Yeom et al., 2018). Antecedently, the pharmacokinetics of several medications, including amiodarone, docetaxel, nifedipine, and cyclosporine A, have been shown to substantially changed in hyperlipidemia. A marked increase in plasma amiodarone concentrations and reductions in clearance, were documented in hyperlipidemic rats (Shayeganpour et al., 2005). In another study, the unbound fraction and intrinsic liver metabolism of docetaxel were considerably lowered; this could be owing to the lower expression of CYP3A (Lee et al., 2011). In another study, the clearance of nifedipine in hyperlipidemic rats was slightly lower owing to the decline in unbound fraction in plasma (Eliot et al., 1999). On the other hand, the CL of cyclosporine A was not altered in hyperlipidemic rats, but the unbound plasma fraction, and Vd, were substantially reduced (Brocks et al., 2006).
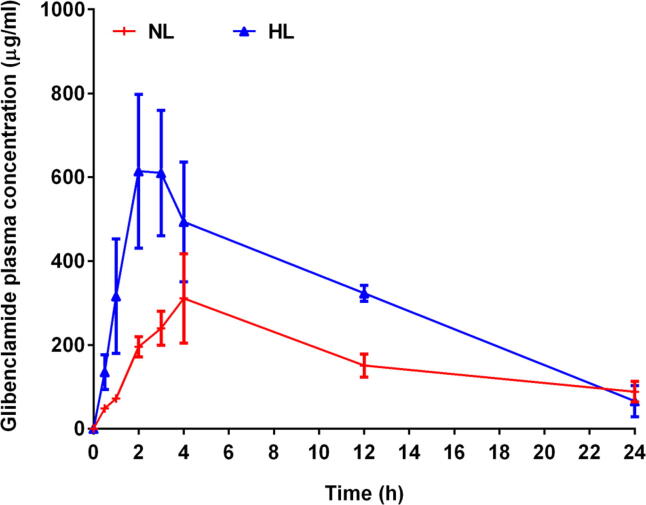
Based on above literature, the pharmacokinetics of Gb, which has a lipophilic property and is metabolized by CYP3A, could also be modified in the hyperlipidemic state. The Gb plasma concentration–time profiles in normolipidemic (NL) and HL rats after oral treatment are illustrated in Fig. 2, and the estimated pharmacokinetic parameters are displayed by bar graphs in Fig. 3.
Fig. 2.
Gb plasma concentration verses time profile in NL and HL rats.
Fig. 3.
Pharmacokinetic parameters of Gb such as (A) Cmax, (B) Tmax, (C) AUC0-t and (D) t1/2, and (E) CL, in NL and HL rats.
In this study, a single dose of poloxamer-407 successfully caused HL in rats. It was observed that the blood lipid levels of treated rats (data not shown) were considerably higher than those of the control group, suggesting that the hyperlipidemia rat model has been successfully established. In present study, the Cmax of Gb in NL group was found to be 340.10 µg/ml, whereas the Cmax of Gb in HL group was found to be 773.39 µg/ml. There is an about 127% increase in Cmax of Gb was observed in HL group. On the other hand, the Tmax of Gb in HL rats was decreased by 32%, the Tmax of Gb in NL rats was found to be 3.67 h, whereas the Tmax of Gb was little shifted to earlier time 2.50 h in HL rats. The AUC0-t of Gb in NL rats was found to be 3956.21 µg.h/ml, interestingly the AUC0-t of Gb in HL rats was increased by 87% and reached to 7380.38 µg.h/ml. On other side, the CL of Gb was deceased by 45% (Fig. 3). The t1/2 of Gb in NL rats was calculated as 19.51 h while the t1/2 in HL rats was noted as 7.59 h, although the difference was not significant. The results of present study are in agreement with the previous findings that reported lesser t1/2 of investigated drug in HL rats (Katnapally et al., 2009, Khalil et al., 2017, Khalil et al., 2016). The CL of Gb in NL rats was recorded as 2.53 ml/h while the CL in HL rats was noted as 1.39 ml/h. Clearly, the findings of present study demonstrated better pharmacokinetics parameters such as Cmax, and AUC0-t of Gb in poloxamer 407-induced hyperlipidemic rats as compared to control group rats. While the Tmax and CL of Gb was decreased in hyperlipidemic rats as compared to control group rats. It was observed here that the bioavailability of Gb was found to increase by 1.88 fold in rats with HL. Based on the previous reports, it concluded that reduced hepatic clearance of Gb alone could not cause an increase in AUC0-t value in HL rats after oral administration. It was reported that in HL rats, lowered intestinal metabolism associated by reduced hepatic metabolism of Gb following oral administration could be the reason of the higher AUC0-t value (Lee et al., 2012). It was reported that the overall CYP content was markedly lower in the liver microsomal protein of HL rats than in control rats (Shayeganpour et al., 2008). Investigators presented noteworthy reductions in the protein expressions of CYP2C11, CYP3A1, and CYP3A2 in HL rat hepatic microsomes as compared to control rats. The GB is extensively metabolized by hepatic enzymes, hence this could be postulated that such low clearance of Gb in the HL rats could be due to the down regulation of hepatic CYP3A1/2; when the 73% homology among the human CYP3A4 and rat CYP3A1 protein was taken into consideration (Gay et al., 2010, Guengerich et al., 2016, Manikandan and Nagini, 2018).
4. Conclusion
In summary, the consequences of HL in rats were examined using Poloxamer 407 model. This method has been reported to provide a substantial impact on the pharmacokinetics of several drugs. In this study, following oral administration, the AUC value of Gb was substantially greater in the HL rats, this could be due to the decreased hepatic and intestinal metabolism of Gb. These observations have important role for predicting the alterations in the pharmacokinetics of drugs including GB, in cases having hyperlipidemia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this research project (RAED).
Footnotes
Peer review under responsibility of King Saud University.
References
- Affres H., Senat M.V., Letourneau A., Deruelle P., Coustols-Valat M., Bouchghoul H., Bouyer J. Glyburide therapy for gestational diabetes: Glycaemic control, maternal hypoglycaemia, and treatment failure. Diab. Metab. 2020:101210. doi: 10.1016/j.diabet.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Ahad A., Al-Saleh A.A., Akhtar N., Al-Mohizea A.M., Al-Jenoobi F.I. Transdermal delivery of antidiabetic drugs: formulation and delivery strategies. Drug Discov. Today. 2015;20:1217–1227. doi: 10.1016/j.drudis.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Alam M.A., Al-Jenoobi F.I., Al-Mohizea A.M. Rapid, Validated UPLC-MS/MS Method for Determination of Glibenclamide in Rat Plasma. Int. J. Anal. Chem. 2018;2018:2569027. doi: 10.1155/2018/2569027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin Jardan Y.A., Brocks D.R. The pharmacokinetics of dronedarone in normolipidemic and hyperlipidemic rats. Biopharm. Drug Dispos. 2016;37:345–351. doi: 10.1002/bdd.2016. [DOI] [PubMed] [Google Scholar]
- Bouchghoul H., Bouyer J., Senat M.V., Mandelbrot L., Letourneau A., Bourcigaux N., Becquemont L., Verstuyft C. Hypoglycemia and glycemic control with glyburide in women with gestational diabetes and genetic variants of cytochrome P450 2C9 and/or OATP1B3. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.2142. [DOI] [PubMed] [Google Scholar]
- Brocks D.R., Ala S., Aliabadi H.M. The effect of increased lipoprotein levels on the pharmacokinetics of cyclosporine A in the laboratory rat. Biopharm. Drug Dispos. 2006;27:7–16. doi: 10.1002/bdd.476. [DOI] [PubMed] [Google Scholar]
- Chaudhary H.R., Brocks D.R. The single dose poloxamer 407 model of hyperlipidemia; systemic effects on lipids assessed using pharmacokinetic methods, and its effects on adipokines. J. Pharm. Pharm. Sci. 2013;16:65–73. doi: 10.18433/j37g7m. [DOI] [PubMed] [Google Scholar]
- Eliot L.A., Foster R.T., Jamali F. Effects of hyperlipidemia on the pharmacokinetics of nifedipine in the rat. Pharm. Res. 1999;16:309–313. doi: 10.1023/a:1018896912889. [DOI] [PubMed] [Google Scholar]
- Gabr R.Q., El-Sherbeni A.A., Ben-Eltriki M., El-Kadi A.O., Brocks D.R. Pharmacokinetics of metformin in the rat: assessment of the effect of hyperlipidemia and evidence for its metabolism to guanylurea. Can. J. Physiol. Pharmacol. 2017;95:530–538. doi: 10.1139/cjpp-2016-0329. [DOI] [PubMed] [Google Scholar]
- Gay S.C., Roberts A.G., Halpert J.R. Structural features of cytochromes P450 and ligands that affect drug metabolism as revealed by X-ray crystallography and NMR. Future Med. Chem. 2010;2:1451–1468. doi: 10.4155/fmc.10.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F.P., Waterman M.R., Egli M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016;37:625–640. doi: 10.1016/j.tips.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy D.A., Brocks D.R. Experimental hyperlipidemia causes an increase in the electrocardiographic changes associated with amiodarone. J. Cardiovasc. Pharmacol. 2009;53:1–8. doi: 10.1097/FJC.0b013e31819359d1. [DOI] [PubMed] [Google Scholar]
- Hamdy D.A., Brocks D.R. Effect of hyperlipidemia on ketoconazole-midazolam drug-drug interaction in rat. J. Pharm. Sci. 2016;100:4986–4992. doi: 10.1002/jps.22675. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Taljedal I.B. Glibenclamide is exceptional among hypoglycaemic sulphonylureas in accumulating progressively in beta-cell-rich pancreatic islets. Acta Endocrinol. (Copenh) 1984;105:385–390. doi: 10.1530/acta.0.1050385. [DOI] [PubMed] [Google Scholar]
- Johnston T.P., Palmer W.K. Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem. Pharmacol. 1993;46:1037–1042. doi: 10.1016/0006-2952(93)90668-m. [DOI] [PubMed] [Google Scholar]
- Ju Y.J., Kim N., Gee M.S., Jeon S.H., Lee D., Do J., Ryu J.S., Lee J.K. Glibenclamide modulates microglial function and attenuates Abeta deposition in 5XFAD mice. Eur. J. Pharmacol. 2020;884 doi: 10.1016/j.ejphar.2020.173416. [DOI] [PubMed] [Google Scholar]
- Katnapally P.K., Janaki A., Anreddy R.N.R., Yellu N.R. Pharmacokinetics and pharmacodynamics of atorvastatin alone and in combination with lercanidipine in hyperlipidemic rats. J. Pharm. Res. 2009;2:66–70. [Google Scholar]
- Khalil H.A., ElKhatib M.A.W., Belal T.S., El-Yazbi A.F., Hamdy D.A. Hyperlipidemia Alters the Pharmacokinetics of Posaconazole and Vincristine Upon Co-Administration in Rats. Drugs R D. 2017;17:287–296. doi: 10.1007/s40268-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H.A., Elnaggar M.M., Belal T.S., El-Yazbi A.F., Hamdy D.A. The effect of hyperlipidemia on the pharmacokinetics, hepatic and pulmonary uptake of posaconazole in rat. Eur. J. Pharm. Sci. 2016;91:190–195. doi: 10.1016/j.ejps.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Kim M., Kim T.W., Kim C.J., Shin M.S., Hong M., Park H.S., Park S.S. Berberine Ameliorates Brain Inflammation in Poloxamer 407-Induced Hyperlipidemic Rats. Int Neurourol J. 2019;23:S102–S110. doi: 10.5213/inj.1938216.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Oh J.H., Lee Y.J. Effects of experimental hyperlipidaemia on the pharmacokinetics of docetaxel in rats. Xenobiotica. 2011;41:797–804. doi: 10.3109/00498254.2011.580019. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Oh J.H., Lee Y.J. Effects of experimental hyperlipidemia on the pharmacokinetics of tadalafil in rats. J. Pharm. Pharm. Sci. 2012;15:528–537. doi: 10.18433/j35p59. [DOI] [PubMed] [Google Scholar]
- Lee U., Kwon M.H., Kang H.E. Pharmacokinetic alterations in poloxamer 407-induced hyperlipidemic rats. Xenobiotica. 2019;49:611–625. doi: 10.1080/00498254.2018.1466212. [DOI] [PubMed] [Google Scholar]
- Malangu, N., 2018. Introductory chapter: Linkages between pharmacokinetics and adverse effects of drugs. Pharmacokinetics and adverse effects of drugs - Mechanisms and risks factors IntechOpen. http://dx.doi.10.5772/intechopen.76511.
- Manikandan P., Nagini S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets. 2018;19:38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- Naritomi Y., Terashita S., Kagayama A. Identification and relative contributions of human cytochrome P450 isoforms involved in the metabolism of glibenclamide and lansoprazole: evaluation of an approach based on the in vitro substrate disappearance rate. Xenobiotica. 2004;34:415–427. doi: 10.1080/00498250410001685728. [DOI] [PubMed] [Google Scholar]
- Palmer W.K., Emeson E.E., Johnston T.P. Poloxamer 407-induced atherogenesis in the C57BL/6 mouse. Atherosclerosis. 1998;136:115–123. doi: 10.1016/s0021-9150(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Patel J.P., Brocks D.R. The effect of oral lipids and circulating lipoproteins on the metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2009;5:1385–1398. doi: 10.1517/17425250903176439. [DOI] [PubMed] [Google Scholar]
- Shayeganpour A., Jun A.S., Brocks D.R. Pharmacokinetics of Amiodarone in hyperlipidemic and simulated high fat-meal rat models. Biopharm. Drug Dispos. 2005;26:249–257. doi: 10.1002/bdd.457. [DOI] [PubMed] [Google Scholar]
- Shayeganpour A., Korashy H., Patel J.P., El-Kadi A.O., Brocks D.R. The impact of experimental hyperlipidemia on the distribution and metabolism of amiodarone in rat. Int. J. Pharm. 2008;361:78–86. doi: 10.1016/j.ijpharm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Shuster D.L., Shireman L.M., Ma X., Shen D.D., Flood Nichols S.K., Ahmed M.S., Clark S., Caritis S., Venkataramanan R., Haas D.M., Quinney S.K., Haneline L.S., Tita A.T., Manuck T.A., Thummel K.E., Brown L.M., Ren Z., Brown Z., Easterling T.R., Hebert M.F. Pharmacodynamics of Glyburide, Metformin, and Glyburide/Metformin Combination Therapy in the Treatment of Gestational Diabetes Mellitus. Clin. Pharmacol. Ther. 2020;107:1362–1372. doi: 10.1002/cpt.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller H.A. Hypoglycemics, Oral. Encycl. Toxicol. (Third Ed.) 2014;2:989–991. [Google Scholar]
- Wout Z.G., Pec E.A., Maggiore J.A., Williams R.H., Palicharla P., Johnston T.P. Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injection to rats. J. Parenter. Sci. Technol. 1992;46:192–200. [PubMed] [Google Scholar]
- Yeom M., Park J., Lee B., Lee H.S., Park H.J., Won R., Lee H., Hahm D.H. Electroacupuncture ameliorates poloxamer 407-induced hyperlipidemia through suppressing hepatic SREBP-2 expression in rats. Life Sci. 2018;203:20–26. doi: 10.1016/j.lfs.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Zharikova O.L., Fokina V.M., Nanovskaya T.N., Hill R.A., Mattison D.R., Hankins G.D., Ahmed M.S. Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem. Pharmacol. 2009;78:1483–1490. doi: 10.1016/j.bcp.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Naraharisetti S.B., Liu L., Wang H., Lin Y.S., Isoherranen N., Unadkat J.D., Hebert M.F., Mao Q. Contributions of human cytochrome P450 enzymes to glyburide metabolism. Biopharm. Drug Dispos. 2010;31:228–242. doi: 10.1002/bdd.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Zhou C., Xu H., Yu J., Ye P., Zhang H., Chen S., Zhao J., Le S., Cui J., Jiang L., Wu J., Xia J. Glibenclamide ameliorates transplant-induced arteriosclerosis and inhibits macrophage migration and MCP-1 expression. Life Sci. 2020;241 doi: 10.1016/j.lfs.2019.117141. [DOI] [PubMed] [Google Scholar]