Abstract
Filariae are vector-borne nematodes responsible for an enormous burden of disease. Human lymphatic filariasis, caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori, and onchocerciasis (caused by Onchocerca volvulus) are neglected parasitic diseases of major public health significance in tropical regions. To date, therapeutic efforts to eliminate human filariasis have been hampered by the lack of a drug with sufficient macrofilaricidal and/or long-term sterilizing effects that is suitable for use in mass drug administration (MDA) programs, particularly in areas co-endemic with Loa loa, the causative agent of loiasis.
Emodepside, a semi-synthetic cyclooctadepsipeptide, has been shown to have broad-spectrum efficacy against gastrointestinal nematodes in a variety of mammalian hosts, and has been approved as an active ingredient in dewormers for cats and dogs. This paper evaluates, compares (where appropriate) and summarizes the in vitro effects of emodepside against a range of filarial nematodes at various developmental stages.
Emodepside inhibited the motility of all tested stages of filariae frequently used as surrogate species for preclinical investigations (Acanthocheilonema viteae, Brugia pahangi, Litomosoides sigmodontis, Onchocerca gutturosa, and Onchocerca lienalis), human-pathogenic filariae (B. malayi) and filariae of veterinary importance (Dirofilaria immitis) in a concentration-dependent manner. While motility of all filariae was inhibited, both stage- and species-specific differences were observed. However, whether these differences were detected because of stage- and/or species-specific factors or as a consequence of variations in protocol parameters among the participating laboratories (such as purification of the parasites, read-out units, composition of media, incubation conditions, duration of incubation etc.) remains unclear.
This study, however, clearly shows that emodepside demonstrates broad-spectrum in vitro activity against filarial nematode species across different genera and can therefore be validated as a promising candidate for the treatment of human filariases, including onchocerciasis and lymphatic filariasis.
Keywords: Emodepside, Anthelmintics, Lymphatic filariasis, Onchocerciasis, River blindness, Filariae
Graphical abstract
Highlights
-
•
Emodepside causes concentration dependent paralysis on filariae.
-
•
Findings confirm the broad-spectrum nematicidal profile of emodepside.
-
•
Data reveal emodepside as a promising drug candidate for human filariasis.
1. Introduction
Worldwide, more than 1 billion people are at risk of acquiring one or more filarial diseases, the vast majority of which reside in areas of greatest poverty in tropical and subtropical regions (WHO, 2017a; WHO, 2017b). Approximately 198 million people across 36 countries are at risk of the filarial disease onchocerciasis (also known as river blindness), caused by Onchocerca volvulus (WHO, 2017a). According to estimates by the African Program for Onchocerciasis Control (APOC), 99 % of those at risk of O. volvulus infection live in sub-Saharan African countries (Kuesel, 2016). Furthermore, around 856 million people, spread across 52 countries, are threatened by lymphatic filariasis caused by Wuchereria bancrofti, Brugia malayi, or Brugia timori (WHO, 2017b). Filariae are transmitted by blood-feeding insect vectors that transmit the infective third-stage larvae (L3). Within the definitive host, L3 migrate to the species-specific site, e.g. the subcutaneous tissue for O. volvulus and lymphatic vessels for filariae causing lymphatic filariasis, molt into adult worms, mate and release the filarial progeny, the microfilariae. For transmission, the microfilariae are passaged via a specific blood-feeding insect vector, developing into the L3, the infective stage for the definitive host.
The Global Burden of Disease Study (GBD) is a landmark initiative that systematically quantifies prevalence, mortality and morbidity for hundreds of diseases considered to be of global health importance (GBD, 2017). Data modeling from the 2017 GBD suggested that (a potentially underestimated) 20.9 million people were infected with onchocerciasis (GBD, 2017; WHO, 2017a). The most severe complication attributed to onchocerciasis is vision loss, which is observed in approximately 1.15 million people (GBD, 2017). However, onchocerciasis is a systemic disease that is also associated with musculoskeletal pain, reduced body mass index, and decreased work productivity (Basanez et al., 2006). Moreover, the immunological response to the death of the O. volvulus microfilariae is also associated with severe itching, disfiguring skin lesions and depigmentation, which together comprise the vast majority of symptoms observed in infected people (Kuesel, 2016).
Both lymphatic filariasis and onchocerciasis are considered to be potentially eradicable (Townson et al., 2007). However, despite the tremendous burden of these diseases, treatment options remain insufficient (Mackenzie, 2000; Bockarie and Deb, 2010; Osei-Atweneboana et al., 2011; Stolk et al., 2018). For decades, the control of onchocerciasis exclusively relied on the administration of a single macrocyclic lactone (ML), ivermectin, administered through mass drug administration (MDA) programs (some 2 billion treatments were donated by Merck & Co over the past 30 years) (Campbell, 2016). Finally, in June 2018, a New Drug Application was approved by the U.S. Food and Drug Administration for the use of another ML, moxidectin, for treatment of onchocerciasis in patients aged 12 years and older (FDA, 2018). A single ML dose clears the skin-dwelling O. volvulus microfilariae and temporarily interrupts fertility of the adult female to some degree, however adulticidal effects are minimal (Walker et al., 2017). Consequently, MDA programs need to be repeated for multiple years in order to encompass the reproductive lifespan of the long-lived adult O. volvulus (estimated at 9–11 years), with high population coverage, to greatly reduce or interrupt transmission in a given endemic area (WHO, 1993). More recently, it was shown that four or more ivermectin treatments have partial adulticidal effects and permanently sterilize adult females (Walker et al., 2017).
Nonetheless, a number of barriers to the elimination of onchocerciasis remain, including drawbacks that come from the reliance on ivermectin. For example, the emergence of suboptimal responses to ivermectin has been observed in some O. volvulus-infected human populations in Ghana and Cameroon (Osei-Atweneboana et al., 2007; Doyle et al., 2017). In addition, ivermectin is contraindicated in people heavily co-infected with another filarial nematode, Loa loa, due to the risk of life-threatening adverse events such as encephalitis (Gardon et al., 1997; Akue, 2011; Vinkeles Melchers et al., 2020), and thus cannot be simply distributed in areas co-endemic for L. loa. Therefore, although recent studies suggest triple therapy with diethylcarbamazine (DEC) (which also causes L. loa associated encephalitis), ivermectin, and albendazole may exert some macrofilaricidal activity in lymphatic filariasis, this combination is also contraindicated in areas co-endemic for loiasis (Thomsen et al., 2016).
Other barriers to elimination include the logistical and financial challenges to increasing MDA program frequency from annual to biannual, which would facilitate sustained interruption of microfilariae production, thereby interrupting transmission (Hotez et al., 2015). Additionally, with lower prevalence of filarial diseases, the cost-effectiveness of community-directed MDA treatments will decrease, but short-term treatments with a macrofilaricidal or long-term sterilizing drug could reduce the program time frames required to reach elimination of onchocerciasis (Dunn et al., 2015).
An orally active anthelmintic with an ivermectin-independent mode of action that kills or at least permanently sterilizes adult worms – preferably safe in patients co-infected with L. loa (Gardon et al., 1997; Vinkeles Melchers et al., 2020) – would therefore add significant value towards the ambitious goal of eliminating onchocerciasis. The cyclooctadepsipeptide emodepside exhibits striking anthelmintic efficacy against gastrointestinal nematodes in a wide range of hosts (Krücken et al., 2012). Due to its broad spectrum of anthelmintic activity, favorable mammalian safety profile and unique mode of action through the calcium-activated and voltage-dependent potassium channel SLO-1 (Kulke et al., 2014), emodepside is considered to be one of the most promising anthelmintic drug candidates for potential human use (Geary et al., 2010; Keiser and Utzinger, 2010; Olliaro et al., 2011; Kuesel, 2016). In 2014, the Drugs for Neglected Diseases initiative (DNDi) and Bayer AG agreed to jointly develop emodepside as an adulticidal treatment for onchocerciasis. Clinical development has started with first-in-human studies to determine the safety, tolerability and pharmacokinetics of emodepside in healthy male volunteers having been recently completed (Kuesel, 2016).
To evaluate the filaricidal activity spectrum of emodepside as part of the preclinical package, this study investigates emodepside in vitro susceptibility of a range of filariae, including Acanthocheilonema viteae, B. malayi, B. pahangi, Dirofilaria immitis, Litomosoides sigmodontis, O. gutturosa, and O. lienalis. These species are commonly used as model organisms for human filariasis (Townson et al., 2005; Morris et al., 2013; Risch et al., 2021). Different stages including microfilariae, third-stage (L3) and fourth-stage (L4) larvae, as well as adult male and female worms, were exposed to varying concentrations of emodepside in order to measure drug effects on helminth motility, using established and adapted protocols (Tagboto and Townson, 1996; Townson et al., 2007; Storey et al., 2014; Maclean et al., 2017).
2. Material and methods
2.1. Ethics statement
All animal housing conditions and the procedures used in this work were in accordance with the Animal Care and Use Committees of each institution and the respective governmental authorities. More detail is provided in the following sub-sections.
2.2. Emodepside in vitro assays
The effect of emodepside on motility was tested on a variety of different filarial nematode species and developmental stages. Parasite maintenance and experimental setup for each species/stage is provided in the following sub-sections as well as summarized in Supplementary Table 1. A range of at least three concentrations of emodepside was used in the various in vitro assays described below.
Table 1.
Overview of the emodepside in vitro assay results. A variety of developmental stages of seven filarial nematode species were assayed for their emodepside susceptibility in vitro. Pharmacological values for emodepside-induced motility inhibition are shown.
| Species | Stage | IC50 (95 % CI) | IC90 | MIC100 | Time point |
|---|---|---|---|---|---|
| Acanthocheilonema viteae | MF | 0.01 μM (0.004–0.017) | 0.21 μM (0.05–1.351) | >10 μM | 72 h |
| Brugia malayi | MF | 0.064 μM (0.045–0.090) | 0.3 μM (xxx-0.654) | >8.93 μM | 72 h |
| Brugia pahangi | MF | 0.025 μM (xxx-0.035) | 0.06 μM (xxx-0.119) | >8.93 μM | 72 h |
| ♂ | 0.14 μM (0.088–0.235) | 3.3 μM (1.238–10.35) | >12.5 μM | 24 h | |
| ♀ | 0.24 μM (0.170–0.356) | 2.2 μM (0.885–6.061) | >12.5 μM | 24 h | |
| Dirofilaria immitis | MF | 0.01 μM (0.010–0.011) | 0.046 μM (0.042–0.049) | 0.36 μM | 72 h |
| L3 | 0.006 μM (0.005–0.008) | 0.076 μM (0.047–0.126) | 0.36 μM | 72 h | |
| L4 | 0.009 μM (0.008–0.011) | 0.042 μM (0.031–0,060) | 0.07 μM | 72 h | |
| ♂ | <0.003 μM | <0.003 μM | ≤0.003 μM | 72 h | |
| ♀ | <0.003 μM | <0.003 μM | ≤0.003 μM | 72 h | |
| Litomosoides sigmodontis | MF | 0.009 μM (0.006–0.011) | 0.1 μM (0.059–0.213) | 10 μM | 96 h |
| L3 | 0.35 μM (0.287–0.437) | >10.00 μM | >10 μM | 72 h | |
| ♂ | <0.01 μM | <0.01 μM | ≤0.01 μM | 96 h | |
| ♀ | <0.01 μM | <0.01 μM | ≤0.01 μM | 96 h | |
| Onchocerca lienalis | MF | 0.02 μM (0.014–0.035) | 0.76 μM (0.302–2.243) | 3.13 μM | 120 h |
| Onchocerca gutturosa | ♂ | 0.001 μM (0.0009–0.0015) | 0.005 μM (0.003–0.008) | 0.05 μM | 120 h |
MF: microfilariae; L3: third-stage larvae; L4: fourth-stage larvae; ♂: adult males; ♀: adult females.
IC50 and IC90 represent the concentrations at which 50 % and 90 % of the drug effect can be observed, respectively; MIC100 describes the lowest drug concentration at which 100 % motility inhibition is induced.
xxx: no value was reported using calculation of the confidence interval in GraphPad Prism.
Acanthocheilonema viteae microfilariae in vitro assays.
All experiments on A. viteae were performed in the laboratory of the Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Bonn, Germany in accordance with the European Union animal welfare guidelines, and all protocols were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz, Cologne, Germany (AZ 84–02.04.2012.A140).
To obtain A. viteae microfilariae, blood was collected via cardiac puncture from euthanized infected gerbils (Meriones unguiculatus) and transferred into ethylenediaminetetraacetic acid (EDTA)-coated tubes. A total of 20 μL phytohemagglutinin A (1 mg/mL) was added per 100 μL of blood, and 100 μL aliquots of agglutinating blood were immediately transferred to tilted petri dishes and covered with 500 μl supplemented Minimum Essential Medium (MEM) containing 10 % heat-inactivated fetal calf serum (FCS), 1 % glutamine (2 mM), 1 % penicillin (10,000 units/mL) and streptomycin (10 mg/mL) (all from PAA Pasching, Austria). Microfilariae could migrate from the blood clot to the medium for 1.5 h before the supernatant was collected and replaced with fresh medium for an additional 1.5 h. After two repetitions of supernatant collection, supernatants were pooled and centrifuged (400 g, 5 min), followed by three washing steps with supplemented MEM. The obtained microfilariae were then re-suspended in supplemented MEM to a final concentration of 80 microfilariae per mL. A 125 μL microfilariae suspension (~10 microfilariae) was added to each well of a 96-well microtiter plate and incubated at 37 °C in an atmosphere of 5 % CO2. An emodepside dilution series in pure dimethylsulfoxide (DMSO) was prepared from a 10 mM stock solution to obtain final concentrations of 10 μM, 1 μM, 0.1 μM, and 0.01 μM and compared with the negative control (0.5 % DMSO only). For each well, motility of microfilariae was evaluated under the microscope after 2 h, 24 h, 48 h, 72 h, and 96 h of exposure using a 4-point scoring system where 3 represented microfilariae with normal motility (vigorous, fidgeting movements), 2 microfilariae with impaired motility (slow movements), 1 microfilariae with minimal motility (single movements observable), and 0 microfilariae exhibiting no movement within the 20 s observation period per well (adapted from Townson et al., 2007). A single experiment with three technical replicates was performed.
2.2.1. Brugia spp. in vitro assays
2.2.1.1. Brugia malayi and Brugia pahangi microfilariae in vitro assays
All experiments on Brugia spp. microfilariae were performed in the laboratory of Dr. Adrian Wolstenholme at the University of Georgia, Athens, GA, USA. B. malayi and B. pahangi microfilariae were obtained from the NIH/NIAID Filariasis Research Reagent Resource Center (FR3) (College of Veterinary Medicine, University of Georgia, Athens, GA, USA) and adjusted to a final concentration of 1.5 microfilariae/μL in supplemented RPMI 1640 medium containing 10 % heat-inactivated FCS, 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 40 μg/mL gentamicin. Microfilariae were cultured in 384 microtiter plates (NUNC black with optically clear bottom Thermo Fisher Scientific, Rochester NY, USA) with approximately 150 microfilariae/well at 37 °C, 5 % CO2. An emodepside dilution series in pure DMSO was prepared from a 10 mM stock solution to obtain final concentrations of 8.93 μM, 1.79 μM, 0.36 μM, 0.0715 μM, 0.0143 μM, and 0.0029 μM emodepside. The final concentration of DMSO was 1 % in all wells; medium containing 1 % DMSO only was used as the negative control. Motility of microfilariae was evaluated after 24 h, 48 h, and 72 h using the WormAssay system (Marcellino et al., 2012), a pixel based motility recording algorithm in a version modified for microfilariae (Storey et al., 2014). To this end, each well was scanned for approximately 30 s at 40X magnification. Three experiments with three replicates were performed.
2.2.1.2. Brugia pahangi adult worms in vitro assay
All experiments on B. pahangi adult worms were performed in the laboratory of Simon Townson (Griffin Institute, formerly Northwick Park Institute for Medical Research, London, UK). Adult male and female B. pahangi (90 days old) were purchased from TRS Inc. Athens, GA, USA. Worms were maintained individually in single wells of a 24-well plate containing 2 mL of supplemented MEM (10 % heat-inactivated newborn calf serum [NCS], 200 units/mL penicillin, 200 μg/mL streptomycin, 0.5 μg/mL amphotericin) and a monkey kidney-cell (LLCMK2; ECACC, UK) feeder layer at 36.5 °C, under an atmosphere of 5 % CO2 in air (Townson et al., 2007). Using serial dilutions, each emodepside concentration (12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM, 3.0 nM, 0.75 nM and 0.188 nM) was tested against two worms of each sex in total. At the highest concentration of 12.5 μM, the final DMSO solvent concentration was 0.25 % and 0.25 % for the comparison untreated control groups (six worms each for both sexes). The effect of drug exposure on worm motility was evaluated at 2 h, 24 h, 48 h, 72 h, 96 h and 120 h post exposure, using an Olympus inverted microscope (Olympus, Hamburg, Germany). Motility scores were assessed on a scale of 0 (immotile) to 10 (maximum) (Townson et al., 2007).
2.2.2. Dirofilaria immitis in vitro assays
Experiments on D. immitis microfilariae, L3 and L4 were performed in the laboratories of Bayer Animal Health GmbH (Monheim, Germany) in accordance with the local Animal Care and Use Committee and governmental authorities (LANUV #200/A176 and #200/A154). The Missouri D. immitis isolate used for assays with microfilariae, L3 and L4 stages, was originally isolated from an infected dog from Missouri (USA). From 2005 onwards, the isolate was maintained and passaged in beagle dogs at the University of Georgia (Athens, GA, USA) (Evans et al., 2017). From 2012 onward, the isolate was also maintained at the laboratories of Bayer Animal Health GmbH in Monheim, Germany. For the experiments with microfilariae, blood was sampled from beagle dogs (Marshall BioResources, North Rose, NY, USA) with patent infections, and microfilariae were purified according to the protocol described by the FR3 (FR3, 2009). L3s were obtained by feeding microfilaremic canine blood to Aedes aegypti mosquitoes (black-eyed Liverpool strain). Fourteen days after feeding, L3s were isolated from infected A. aegypti according to the protocol by Evans and colleagues (Evans et al., 2017). L4s were obtained by incubating freshly isolated L3s in a 96-well microtiter plate (one L3 per cavity) containing supplemented RPMI 1640 medium for 72 h. Only the worms that exhibited a fully separated cuticula and showed normal motility were utilized for in vitro assays.
Experiments on adult D. immitis were carried out in the laboratory of Dr. Adrian Wolstenholme at the University of Georgia Athens, GA, USA using nematodes of the Georgia-2 isolate (GA-2) supplied by TRS Labs Inc. Athens, GA, USA. The GA-2 isolate originated from a blood collection from a dog from Vidalia, GA, USA, in 2013. Following an initial passage through the mosquito vector (A. aegypti; black-eyed Liverpool strain), it was maintained in laboratory beagle dogs at TRS Labs Inc. (Berrafato et al., 2019).
2.2.2.1. Dirofilaria immitis microfilariae in vitro assay
Approximately 250 freshly purified microfilariae were cultured in single wells of a 96-well microtiter plate containing supplemented RPMI 1640 medium as described in section 2.2.2.1. Emodepside was added in the following concentrations: 8.93 μM, 1.79 μM, 360 nM, 71.5 nM, 14.3 nM, 2.9 nM, 0.57 nM, and 0.11 nM. Microfilariae exposed to medium substituted with 1 % DMSO were used as negative controls. Microfilariae motility was evaluated 72 h after drug exposure using an image-based approach – DiroImager, developed by Bayer Technology Services. This device is a fully automated high-throughput platform, allowing high-resolution optical imaging of an entire 96-well microtiter plate. The DiroImager integrates a high-resolution video camera (Prosilica GT6600; Allied Vision) with a telecentric lens (S5LPJ3005; Sill Optics) that prevents perspective distortion of the recorded images, ensuring high accuracy of measured values across all samples.
In brief, a series of 20 high-resolution images were recorded (one per second). In a first step, image processing filters were used that discriminate larger objects to avoid the detection of crystallized or undissolved particles. In the actual calculation, pixel-wise differences between sequential images were calculated to determine worm movement between single images of a series; emodepside activity was determined as the reduction of motility in comparison to the solvent control. Based on the evaluation of a wide concentration range, concentration–response curves as well as IC50 values were calculated. Ten experiments with three replicates were performed.
2.2.2.2. Dirofilaria immitis L3 in vitro assay
Freshly isolated L3s were cultured in wells of a 96-well microtiter plate with approximately 10 L3 per well. All wells contained supplemented RPMI 1640 medium and emodepside at one of the following concentrations: 8.93 μM, 1.79 μM, 360 nM, 71.5 nM, 14.3 nM, 2.9 nM, 0.57 nM, and 0.11 nM L3s exposed to DMSO only (1 %) were used as negative controls. All emodepside concentrations were tested in triplicate and drug effects were evaluated after 72 h of incubation. Motility was scored on a 4-point scale, where 0 represented complete paralysis and 3 represented full motility as observed before any drug was added. Emodepside activity was determined as the reduction of motility in comparison to the negative control. Data were only considered as valid if the worms in the negative control group remained as motile as observed at the beginning of the experiment. Based on the evaluation of a wide concentration range, concentration–response curves as well as IC50 values were calculated. Ten experiments with three replicates were performed.
2.2.2.3. Dirofilaria immitis L4 in vitro assay
Freshly isolated L3s were cultured individually in wells of a 96-well microtiter plate. After 72 h of incubation at 37 °C and 5 % CO2, each well was screened for the presence of motile larvae and a fully separated cuticula, indicating a completed moulting process from L3 to L4 stage. In vitro molted and motile L4 were transferred and cultured individually in wells of a 96-well microtiter plate with approximately 10 L4 per well. All wells contained supplemented RPMI 1640 medium and emodepside at one of the following concentrations: 8.93 μM, 1.79 μM, 360 nM, 71.5 nM, 14.3 nM, 2.9 nM, 0.57 nM, and 0.11 nM L4s exposed to 1 % DMSO were used as negative controls. All emodepside concentrations were tested in triplicate and drug effects were evaluated after 72 h of incubation. Motility was scored on a 4-point scale, where 0 represented complete paralysis and 3 represented full motility as observed before any drug was added. Emodepside activity was determined as the reduction of motility in comparison to the negative control. Data were only considered as valid if the worms in the negative control group remained as motile as observed at the beginning of the experiment. Based on the evaluation of a wide concentration range, concentration–response curves as well as IC50 values were calculated. Three experiments were conducted in triplicate.
2.2.2.4. Dirofilaria immitis adult worm in vitro assay
Individual adult worms, either male or female, were cultured in 75 cm2 tissue culture flasks in supplemented RPMI 1640 medium. Emodepside was added at the following concentrations: 8.93 μM, 1.79 μM, 360 nM, 71.5 nM, 14.3 nM, 2.9 nM, 0.57 nM, and 0.11 nM. Worms exposed to DMSO only (final concentration 1 %) were used as negative controls. Adult worm motility was scored on a 4-point scale at 72 h post treatment, where 0 represented complete paralysis and 3 represented full motility as observed before any drug was added. Two experiments were carried out in triplicate (three males and three females).
2.2.3. Litomosoides sigmodontis in vitro assays
All experiments on L. sigmodontis were performed at the Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Bonn, Germany in accordance with the European Union animal welfare guidelines and all protocols were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz, Cologne, Germany (AZ 84–02.04.2015.A507; 84–02.04.2012.A140; 81–02.05.40.18.057). To obtain microfilariae, peripheral blood was collected from L. sigmodontis-infected cotton rats (Sigmodon hispidus), which were bred at the Institute and originally obtained from Envigo, in EDTA-coated tubes. Microfilariae were purified from blood by Percoll gradient centrifugation consisting of a bottom layer of 30 % iso-osmotic Percoll in 0.25 M sucrose and a top layer of 25 % iso-osmotic Percoll in 0.25 M sucrose solution (Chandrashekar et al., 1984). The blood was diluted 1:2 in 37 °C phosphate-buffered saline and gently pipetted onto the upper Percoll layer. Subsequently, the gradient was centrifuged at 400×g at room temperature for 30 min. Following centrifugation, microfilariae appeared as a white ring in the 25–30 % Percoll gradient and were collected and washed three times with 37 °C supplemented MEM (as described in section 2.2.1) at 450×g and room temperature for 5 min. After the final washing step, microfilariae were re-suspended in 1 mL supplemented MEM and counted using a Neubauer chamber.
Gerbils (Janvier Labs, Saint-Berthevin, France) were exposed to Ornithonyssus bacoti mites containing infective L. sigmodontis L3. Necropsy was performed 5 days after infection and infective L3 were obtained by pleural lavage with supplemented MEM.
L. sigmodontis adult worms were flushed from the pleural cavity of a patently infected donor animal (gerbil or cotton rat) and individual, intact adult worms were washed with supplemented MEM and transferred into individual wells of a 12-well plate, containing a confluent layer of monkey kidney cells (LLC-MK2, ECACC, UK).
2.2.3.1. Litomosoides sigmodontis microfilariae in vitro assay
Freshly isolated microfilariae were adjusted to a concentration of 80 microfilariae/mL; 125 μL of this suspension (~10 microfilariae) was then added to each well of a 96-well microtiter plate containing confluent LLC-MK2 cells in supplemented MEM at 37 °C, 5 % CO2.
To assess emodepside's effects on microfilariae motility, medium was removed from the culture plates and 125 μL of supplemented MEM containing emodepside at concentrations of 10 μM, 1 μM, 0.1 μM, and 0.01 μM or 0.5 % DMSO as the negative control was added and incubated at 37 °C, 5 % CO2. Motility of microfilariae was assessed under the microscope after 96 h of exposure. Media containing emodepside at the indicated concentrations, as well as the negative control media, were changed after 48 h. A 4-point scoring system was used for evaluating microfilariae motility, in which 3 represented microfilariae with normal motility (vigorous, fidgeting movements), 2 microfilariae with impaired motility (slow movements), 1 worms with minimal motility (single movements observable), and 0 microfilariae exhibiting no movement within the 20 s observation period per well. Two independent experiments were performed in triplicate.
2.2.3.2. Litomosoides sigmodontis L3 in vitro assay
Using a 96-well plate, approximately 13 L3s in a volume of 198 μL supplemented MEM were seeded per well. Emodepside was tested at four concentrations (10 μM, 1 μM, 0.1 μM, and 0.01 μM) with two replicates per condition. L3s exposed to 0.5% DMSO served as negative control. Motility was evaluated after 72 h of incubation at 37 °C and 5 % CO2 using a 3-point scoring system, in which 0 % activity represented larvae showing no motility impairment, 80 % activity for L3s with motility impairment and 100 % activity for dead or completely immobile larvae. This scoring system was due to the fact that weaker effects on L3 motility were difficult to quantify, so only drastic effects resulting in either 80 or 100 % motility reduction were recorded. Each L3 was individually scored and mean activity was determined per well. Ten independent experiments were performed in duplicate.
2.2.3.3. Litomosoides sigmodontis adult worms in vitro assay
Adult worms (3–4 per experiment) were individually co-cultured with LLC-MK2 cells at 37 °C and 5 % CO2, analogue to the above described microfilariae in vitro culture in wells of a 12-well plate. The effect of emodepside was tested at final concentrations of 10 μM, 1 μM and 0.1 μM, and treatment with 0.5 % DMSO only served as negative control. Media containing emodepside at the indicated concentrations, as well as the negative control media, were changed after 48 h. Emodepside's activity on female and male adult worm motility was evaluated after 96 h using a 5-point scoring system (Lentz et al., 2013) in which 0 represented absence of movement, 1 described worms that were entirely stretched with single shivering movements, 2 were worms that were mostly stretched with non-continuous wave-like movements and no change in their general position, 3 was for worms that had longer immobile periods but changed their position, 4 described worms with slower movements that were not continuous, and 5 was for worms with continuous and vigorous movements. All experiments (two experiments for adult female worms, one experiment for adult male worms) were performed with at least three technical replicates.
2.2.4. Onchocerca spp. in vitro assays
2.2.4.1. Onchocerca lienalis microfilariae in vitro assay
All experiments on O. lienalis microfilariae were performed in the laboratory of Simon Townson (Griffin Institute, formerly Northwick Park Institute for Medical Research, London, UK). A single large batch of microfilariae was obtained from the peri-umbilical skin area of freshly euthanized, naturally infected cattle from an abattoir in the UK following the procedure described by Tagboto and Townson (1991). The extracted microfilariae were cryopreserved using a two-step incubation technique with ethanediol as a cryoprotectant (Ham et al., 1981), stored in liquid nitrogen, and thawed when required for immediate use. Upon thawing, five worms were transferred into each well of a 96-well plate containing 200 μL of MEM supplemented with 10 % heat-inactivated NCS, 200 units/mL penicillin, 200 μg/mL streptomycin, 0.5 μg/mL amphotericin and an LLCMK2 (ECACC, UK) cell feeder layer at 36.5 °C under an atmosphere of 5 % CO2 in air (Townson et al., 2007). The activity of emodepside on microfilariae was assessed using two wells (10 worms) for the following drug concentrations (serial dilutions): 12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM, and 3.0 nM. At the highest concentration of 12.5 μM, the final DMSO solvent concentration was 0.25 % and 0.25 % for the comparison untreated control groups (10 worms). Using an Olympus inverted microscope (Olympus, Hamburg, Germany), the motility of the microfilariae was scored on a scale of 0 (immotile) to 3 (continuous, rapid sinuous movement) after 120 h of drug exposure.
2.2.4.2. Onchocerca gutturosa adult worm in vitro assay
All experiments on O. gutturosa adult worms were performed under the supervision of Simon Townson (Griffin Institute, formerly Northwick Park Institute for Medical Research, London, UK) at The West Africa Livestock Innovation Center (WALIC), The Gambia. The nuchal ligaments of naturally infected cattle were purchased from the local abattoir from which the adult O. gutturosa males were dissected free from bovine tissues. Using serial dilutions, each emodepside concentration (12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM, 3.0 nM, 0.75 nM, and 0.188 nM) was tested against four worms per drug group, with each worm maintained in individual wells of a 24-well plate containing 2 mL of MEM supplemented with 10 % heat-inactivated NCS, 200 units/mL penicillin, 200 μg/mL streptomycin, 0.5 μg/mL amphotericin and an LLCMK2 (ECACC, UK) cell feeder layer at 36.5 °C under an atmosphere of 5 % CO2 in air (Townson et al., 2007). At the highest concentration of 12.5 μM, the final DMSO solvent concentration was 0.25 % and 0.25 % for the comparison untreated control groups (six worms). Using an Olympus inverted microscope (Olympus, Hamburg, Germany), motility scores were assessed on a 10-point scale of 0 (immotile) to 10 (maximum) after 120 h of drug exposure.
2.3. Statistics
All statistical analyses were performed with the GraphPad Prism software version 8.4.3 (GraphPad Software, San Diego, CA, USA). To determine IC50, IC90 and MIC100 values of emodepside for each nematode species, concentration–response curves were calculated from each phenotype assay at a given time point using the following equation:
| Y = 100*(X^HillSlope)/(EC50^HillSlope + (X^HillSlope)) |
Prior to calculation, all treatment groups were normalized to the corresponding solvent control. IC50 and IC90 represent the concentrations at which 50 % and 90 % of the drug effect can be observed, respectively. MIC100 describes the lowest drug concentration at which 100 % motility inhibition is induced. Plotted data points of concentration–response curves represent mean values and statistical error is indicated as standard error of the mean (SEM). In addition, the time-course of emodepside motility inhibition for A. viteae microfilariae was plotted using mean motility values of each time point, including SEM.
3. Results
In the present study, the susceptibility of filarial nematodes to emodepside was evaluated in different in vitro motility assays using a variety of filarial species and developmental stages. Emodepside inhibited the motility of all tested species and development stages in a concentration-dependent manner (Table 1).
3.1. In vitro effects of emodepside on Acanthocheilonema viteae microfilariae
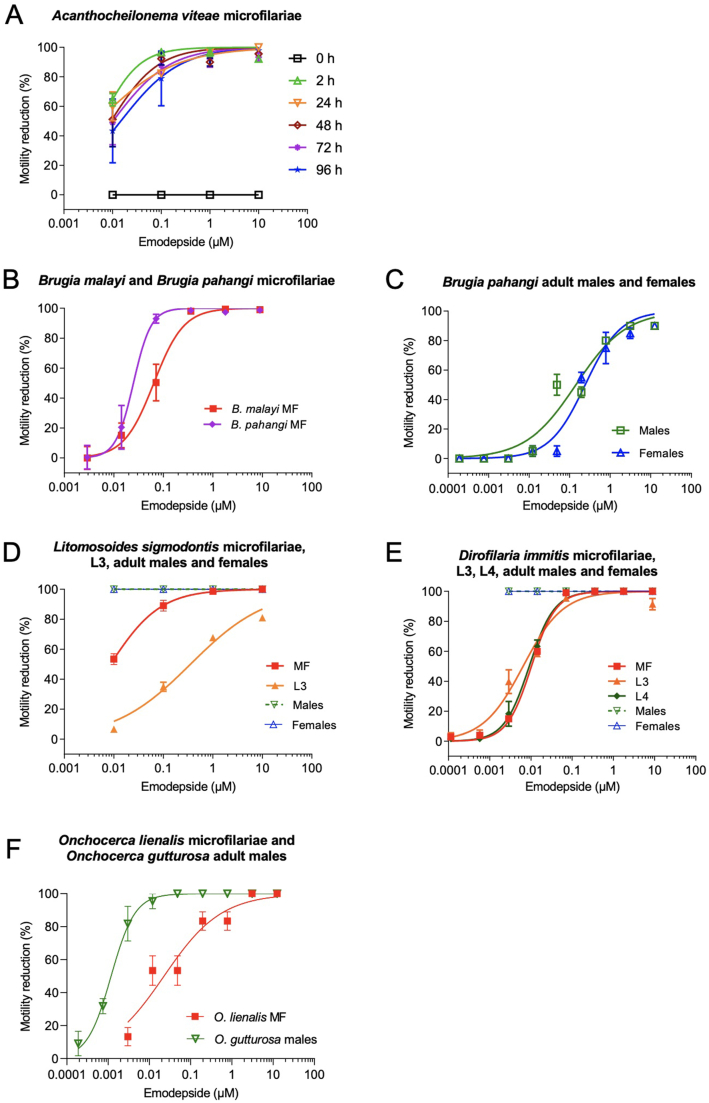
To investigate the anthelmintic effect of emodepside on A. viteae, microfilariae were incubated with different drug concentrations over a period of up to 96 h in vitro. During incubation, emodepside inhibited A. viteae microfilariae motility in a dose-dependent manner, with an IC50 value of 0.01 μM after 72 h (Fig. 1A). Over the time course of the experiment, microfilariae motility was already considerably impaired after 2 h of exposure to 0.01 μM emodepside. Motility impairment increased with higher emodepside concentrations (0.1 μM, 1 μM, and 10 μM) and led to complete paralysis of the majority of microfilariae throughout the observation period from 2 h to 96 h for emodepside concentrations of 1 and 10 μM (Fig. 1A). Microfilariae exposed to lower concentrations of 0.01 μM and 0.1 μM emodepside recovered some of their motility during the experiment (Fig. 1A), which was reflected by the IC50 values over time (2 h: 0.0062 μM; 24 h: 0.0051 μM; 48 h: 0.0098 μM; 72 h: 0.0096 μM; 96 h: 0.0145 μM).
Fig. 1.
Dose dependent inhibition of filarial motility by emodepside.
Emodepside was able to inhibit the motility of all tested filarial species and filarial life-cycle stages in vitro. (A) Calculated dose-response curve for the motility inhibition of A. viteae microfilariae after 2, 24, 48, 72 and 96 h of treatment at emodepside concentrations of 10 μM, 1 μM, 0.1 μM, 0.01 μM. Dose response curves of motility inhibition for (B) B. malayi and B. pahangi microfilariae (MF) after 72 h and (C) adult B. pahangi males and females after 24 h of treatment at emodepside concentrations of 12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM, 3.0 nM, 0.75 nM and 0.188 nM. (D) Dose-response curve for all tested developmental stages of D. immitis (MF: microfilariae; L3: third stage larvae; L4: fourth stage larvae; adult males; adult females) using different emodepside concentrations (8.93 μM, 1.79 μM, 360 nM, 71.5 nM, 14.3 nM, 2.9 nM, 0.57 nM, and 0.11 nM) after 72 h of drug exposure. (E) Dose-response curve for all tested developmental stages of L. sigmodontis (MF: microfilariae; L3: third stage larvae; adult males; adult females) using different emodepside concentrations (10 μM, 1 μM, 0.1 μM, 0.01 μM) after 72 (L3) or 96 h (MF, adult worm) of drug exposure. (E) Dose-response curve for different emodepside concentrations for O. gutturosa (12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM, 3.0 nM, 0.75 nM, and 0.188 nM) and for O. lienalis (12.5 μM, 3.13 μM, 0.78 μM, 0.195 μM, 0.048 μM, 0.012 μM and 3.0 nM) for the duration of both assays after 120 h.
3.2. In vitro effects of emodepside on Brugia spp. microfilariae and adult worms
Emodepside inhibited Brugia spp. microfilariae motility in a concentration-dependent manner over a time period of up to 72 h in vitro (Fig. 1B). For both B. malayi and B. pahangi, microfilariae were completely paralyzed at the tested concentrations of 360 nM and 71.5 nM, respectively. Thus, B. malayi microfilariae (IC50 = 0.064 μM; 95 % CI 0.045–0.09) were less sensitive to emodepside, as shown by non-overlapping 95 % CI, than B. pahangi microfilariae (IC50 = 0.025 μM; 95 % CI xxx-0.035) in an assay that was performed at the same institute under identical conditions (Fig. 1B). Emodepside-induced motility inhibition was also observed for B. pahangi adult worms, with IC50 values of 0.14 μM for males and 0.24 μM for females after 24 h of treatment (Fig. 1C).
3.3. In vitro effects of emodepside on Dirofilaria immitis microfilariae, L3, L4, and adult worms
To investigate the effect of emodepside on the canine heartworm D. immitis, developmental stages of the parasite were incubated with different drug concentrations over a period of 72 h in vitro. Emodepside was found to inhibit the motility of the investigated D. immitis stages in a dose-dependent manner (Fig. 1D). Remarkably, adult worms showed a higher susceptibility to emodepside than larval stages as indicated by non-overlapping 95 % CI (Table 1), using the same assay (including scoring system and media), although at different institutes. While male and female worms were already completely paralyzed at the lowest assay concentration of ~3 nM, complete inhibition of L4 motility was only achieved after exposure to an emodepside concentration of 72 nM and complete inhibition of microfilariae and L3 motility was achieved at a concentration of 360 nM. Overall, IC50 values for all larval stages were close together, with concentrations of 0.01 μM (microfilariae), 0.006 μM (L3), and 0.009 μM (L4) of emodepside.
3.4. In vitro effects of emodepside on Litomosoides sigmodontis microfilariae, L3, and adult worms
Emodepside inhibited L. sigmodontis microfilariae motility in a dose-dependent manner over a period of up to 96 h in vitro. Over the time course of the experiment, inhibition of microfilariae motility was already observed 2 h post emodepside exposure; at a concentration of 0.01 μM emodepside, microfilariae motility was impaired after 2 h (score 1–2) and this effect was maintained throughout the observation period of 96 h (data not shown). Microfilariae had a motility reduction of 89 % at an emodepside concentration of 0.1 μM, with 99 % and complete inhibition of motility obtained with emodepside concentrations of 1 μM and 10 μM, respectively, starting at 2 h of culture and continuing until the end of the observation period (96 h). The calculated IC50 value was 0.009 μM after 96 h of treatment.
To assess the effect of emodepside on L. sigmodontis L3 motility, L3s were evaluated after 72 h in vitro culture in the presence of varying concentrations of the drug. In contrast to microfilariae, none of the emodepside concentrations tested were able to inhibit L3 motility completely. Emodepside 10 μM had an efficacy of 80.9 % in inhibiting L3 motility, while lower concentrations resulted in reduced efficacy from 67.6 % (1 μM), 35.5 % (0.1 μM), and 6.5 % (0.01 μM) (Fig. 1E). These results demonstrate that emodepside inhibits L3 motility in a dose-dependent manner with an IC50 of 0.35 μM.
The highest emodepside sensitivity was observed for adult worms, where the drug completely inhibited L. sigmodontis female and male motility at the lowest assay concentration tested (0.01 μM). Two hours after in vitro emodepside exposure at 0.01 μM, L. sigmodontis female adult worms were generally immotile with non-continuous movements; higher concentrations of emodepside (1 μM and 10 μM) completely inhibited female adult worm motility at this time point. From 24 h to the end of the observation period at 96 h, no male or female adult worm motility was observed for all emodepside concentrations tested.
3.5. In vitro effects of emodepside on Onchocerca spp
To investigate the effect of emodepside on the bovine filarial nematode O. lienalis, microfilariae were incubated with different drug concentrations over a period of 120 h in vitro. Emodepside inhibited O. lienalis microfilariae motility in a dose-dependent manner (Fig. 1F). At the highest emodepside concentration of 12.5 μM, microfilariae were completely paralyzed and partial but marked effects could be seen down to a concentration of 12 nM (IC50 = 0.02 μM).
Compared with O. lienalis microfilariae, the effect of emodepside on adult males of O. gutturosa was more pronounced. When emodepside was incubated for 120 h with O. gutturosa males in vitro, the drug inhibited motility completely from a concentration of 48 nM, with an IC50 value of 0.001 μM.
4. Discussion
Filarial nematodes have a tremendous impact on global health, with over 1 billion people at risk of infection from the parasitic diseases onchocerciasis or lymphatic filariasis (WHO, 2017a; WHO, 2017b). Since existing registered drugs do not kill the adult worms and have the potential to induce resistance, elimination programs will greatly benefit from innovative treatments that provide long-term sterilization and ideally kill the adult worms (Mackenzie, 2000; Bockarie and Deb, 2010; Osei-Atweneboana et al., 2011; Stolk et al., 2018). Emodepside belongs to the cyclooctadepsipeptide class of anthelmintics, and has been shown to exhibit anthelmintic activity against a broad range of parasitic nematodes of medical and veterinary importance, including filariae, roundworms, hookworms and strongylids (Krücken et al., 2012), and can successfully eliminate nematodes resistant to other anthelmintic classes (von Samson-Himmelstjerna et al., 2005; Jimenez Castro et al., 2020). In the present study, we tested the in vitro efficacy of emodepside against a broad range of related filarial parasite species and development stages using motility assays.
Overall, emodepside inhibited the motility of all tested filarial nematode species and stages in a dose-dependent manner. Species-specific and developmental stage differences in the response to emodepside were observed, although an overall susceptibility ranking is not advised because of the different experimental designs used to culture the various species and stages. Those various in vitro assays were originally established for different reasons, e.g. to allow screening of compound libraries, resistance-monitoring, evaluation of plant extracts or understanding of PK/PD relationships. Furthermore, culture conditions were optimized for the different lifecycle stages and filarial species used. Therefore, assays varied in multiple parameters, including composition of the media, usage of feeder cells, incubation time, drug preparation, solvents used, concentrations tested, and the readout (e.g. microscopy vs. imager-based assays). In addition, protocols for isolation/purification of certain filarial life-cycle stages from intermediate or definitive hosts vary in complexity and might even affect their susceptibility to test compounds under in vitro conditions. Therefore, a direct comparison of the respective results is not advised from our perspective for most conditions. Keeping those restrictions in mind, we provided 95 % CI for the IC50 and IC90 results for comparison (Table 1).
Microfilariae of the rodent filarial nematode A. viteae showed a moderate response to emodepside with a complete inhibition of motility starting at a concentration of 1 μM in vitro. These results are in line with previous findings of Zahner and colleagues that indicated an adequate efficacy of emodepside in the multimammate mouse (Mastomys coucha) A. viteae in vivo model with an almost complete clearance of the microfilaremia (Zahner et al., 2001a, 2001b).
An interesting observation from these in vitro experiments on A. viteae microfilariae was that at lower concentrations of emodepside, i.e. 0.01 μM and 0.1 μM, the motility of the microfilariae was initially inhibited, but the worms showed a partial recovery from the drug effects as the experiment proceeded, which was reflected by the IC50 over time. Similar experiments investigating the effect of emodepside on nematode motility have described worm death as the point at which there is complete paralysis (Karpstein et al., 2019). It is clear from the findings with A. viteae that partial inhibition of motility is not a lethal effect. Thus, at least the lowest concentrations of emodepside at which complete and maintained inhibition of motility is achieved should be considered as minimal systemic exposure in the host.
Microfilariae of other related filarial species (B. malayi and B. pahangi, D. immitis, L. sigmodontis, and O. lienalis) also showed a moderate response to emodepside. In all cases, motility was completely inhibited at concentrations of 0.36 μM–10 μM. This suggests that microfilariae are generally susceptible to emodepside, as has been indicated in vivo in previous studies of emodepside efficacy in filariae-infected rats, and gastrointestinal nematodes of sheep and cattle (Zahner et al., 2001a; Zahner et al., 2001b; von Samson-Himmelstjerna et al., 2005).
Given the above-mentioned limitations of this study, several observations on the susceptibility to emodepside were made and based on non-overlapping 95 % CI, as shown in Table 1. Using the same protocol at the same institute, microfilariae of B. malayi were less susceptible to emodepside than those of B. pahangi, despite their high genomic similarity (Lau et al., 2015). Within the same laboratory, but differences in the readout and the culture media, testing of L3 and L4 stages of D. immitis showed comparable sensitivity to the microfilariae experiments. In contrast, within the same laboratory but differences in culture media, the L3 stage of L. sigmodontis was less sensitive than microfilariae as none of the tested emodepside concentrations were able to inhibit L3 L. sigmodontis completely. L. sigmodontis is used as a rodent model for human filarial infections (Hübner et al., 2009; Risch et al., 2021), and previous in vivo studies of emodepside in a rodent model have already indicated that emodepside is not effective at killing larval and pre-adult stages of L. sigmodontis or B. malayi (Zahner et al., 2001a). Only microfilariae of B. malayi were tested in this study, and therefore the in vitro susceptibility of the L3 stage of B. malayi remains unknown.
Most remarkably, adult worms of all tested species except for B. pahangi exhibited the strongest susceptibility to emodepside compared with all other development stages (see Table 1), which is a promising finding with respect to the weak adulticidal effects observed for currently used MDA compounds (Walker et al., 2017). If this finding is also confirmed in vivo, emodepside concentrations may be used for treatment of filariasis that have macrofilaricidal efficacy without resulting in rapid microfilariae killing, thus avoiding severe adverse events. In this case, treatment in areas co-endemic for loiasis may be also safe.
Although in vitro activity cannot be easily translated to the in vivo situation, given that formulations, drug uptake, distribution and metabolism, accessibility of the parasite to the drug, PK/PD relationship are altered in vivo and dependent on the host species, we are certain that our study provides new insights into the potential of emodepside as a novel anthelmintic compound for humans with broad-spectrum efficacy against nematodes.
5. Conclusion
In summary, emodepside exhibits good in vitro efficacy against the various filarial nematode species and developmental stages included in our study. The present data strengthens the potential of emodepside as a promising anthelmintic drug with broad-spectrum activity against microfilarial, third larval, fourth larval, and adult stages of a variety of filarial genera and species. Based on these findings, emodepside is validated as a promising candidate for treatment of human filarial infections.
Declaration of competing interest
Achim Harder, Steffen Hahnel and Daniel Kulke were employees of Bayer Animal Health, which has since been taken over by Elanco Animal Health. Martin Glenschek-Sieberth was employee of Bayer AG. Bayer Animal Health a division of Bayer AG developed and sold veterinary pharmaceuticals including dewormers. Except for the authors, Bayer Animal Health was not involved in the preparation of the manuscript. The decision to publish the data was jointly taken. Simon Townson, Suzanne Gokool, Senyo Tagboto, Mary J. Maclean, Guilherme G. Verocai, Adrian J. Wolstenholme, Stefan J. Frohberger and Achim Hörauf declare no competing interests.
Role of the funding source
This work was supported by the World Health Organization and Drugs for Neglected Diseases initiative. The funding for studies with L. sigmodontis and A. viteae and for experiments at the University of Georgia was obtained from Bayer Animal Health, which has since been taken over by Elanco Animal Health. Except for the co-authors Achim Harder, Steffen Hahnel and Daniel Kulke, Bayer Animal Health was neither involved in the study design nor in collection, analysis and interpretation of the data. However, Bayer Animal Health approved the decision to publish the work.
Acknowledgements
The authors would like to thank Martina Fendler, Marianne Koschel, Alexandra Ehrens and Christian Lentz for their help with the L. sigmodontis and A. viteae in vitro experiments. They would also like to thank Barbara Reaves for her assistance with the adult D. immitis in vitro experiments and to acknowledge the NIH/NIAID Filariasis Research Reagent Resource Center (FR3) (www.filariasiscenter.org) for providing some parasite materials and advice in parasite maintenance (Brugia spp., Dirofilaria immitis). The authors acknowledge Highfield Communication, Oxford, United Kingdom, sponsored by Bayer AG, for editorial support.
Contributor Information
Marc P. Hübner, Email: huebner@uni-bonn.de.
Simon Townson, Email: s.townson@imperial.ac.uk.
Suzanne Gokool, Email: s.gokool@ucl.ac.uk.
Senyo Tagboto, Email: senyo2@hotmail.com.
Mary J. Maclean, Email: mary.maclean@nih.gov.
Guilherme G. Verocai, Email: gverocai@cvm.tamu.edu.
Adrian J. Wolstenholme, Email: adrianw@uga.edu.
Stefan J. Frohberger, Email: stefan.frohberger@gmx.de.
Achim Hoerauf, Email: hoerauf@uni-bonn.de.
Sabine Specht, Email: sspecht@dndi.org.
Ivan Scandale, Email: iscandale@dndi.org.
Achim Harder, Email: achim_harder@hotmail.de.
Martin Glenschek-Sieberth, Email: mglenscheksieberth@t-online.de.
Steffen R. Hahnel, Email: steffen.hahnel@elancoah.com.
Daniel Kulke, Email: dkulke@iastate.edu, dkulke@iastate.edu.
References
- Akue J.P. Encephalitis due to Loa loa. In: Tkachev S., editor. Non-flavivirus Encephalitis. InTech; London: 2011. [Google Scholar]
- Basáñez M.G., Pion S.D., Churcher T.S., Breitling L.P., Little M.P., Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrafato T., Coates R., Reaves B.J., Kulke D., Wolstenholme A.J. Macrocyclic lactone anthelmintic-induced leukocyte binding to Dirofilaria immitis microfilariae: influence of the drug resistance status of the parasite. Int. J. Parasitol. Drugs Drug Resist. 2019;10:45–50. doi: 10.1016/j.ijpddr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockarie M.J., Deb R.M. Elimination of lymphatic filariasis: do we have the drugs to complete the job? Curr. Opin. Infect. Dis. 2010;23:617–620. doi: 10.1097/QCO.0b013e32833fdee5. [DOI] [PubMed] [Google Scholar]
- Campbell W.C. Vol. 55. Angew. Chem. Int; 2016. Ivermectin: a reflection on simplicity (nobel lecture) pp. 10184–10189. (Engl). [DOI] [PubMed] [Google Scholar]
- Chandrashekar R., Rao U.R., Rajasekariah G.R., Subrahmanyam D. Separation of viable microfilariae free of blood cells on Percoll gradients. J. Helminthol. 1984;58:69–70. doi: 10.1017/s0022149x00028078. [DOI] [PubMed] [Google Scholar]
- Doyle S.R., Bourguinat C., Nana-Djeunga H.C., Kengne-Ouaf J.A., Pion S.D.S., Bopda J., Kamgno J., Wanji S., Che H., Kuesel A.C., Walker M., Basáñez M., Boakye D.A., Osei-Atweneboana M.Y., Boussinesq M., Prichard R.K., Grant W.N. Genome-wide analysis of ivermectin response by Onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005816. e0005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C., Callahan K., Katabarwa M., Richards F., Hopkins D., Withers P.C., Jr., Buyon L.E., McFarland D. The contributions of onchocerciasis control and elimination programs toward the achievement of the millennium development goals. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003703. e0003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Blagburn B.L., Wolstenholme A.J., Kaplan R.M. Evaluation of the larval migration inhibition assay for detecting macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2017;246:76–81. doi: 10.1016/j.vetpar.2017.09.003. [DOI] [PubMed] [Google Scholar]
- FDA NDA 210867: NDA APPROVAL. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/210867Orig1s000Ltr.pdf Available at:
- Gardon J., Gardon-Wendel N., Demanga N., Kamgno J., Chippaux J.P., Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T.G., Woo K., McCarthy J.S., Mackenzie C.D., Horton J., Prichard R.K., de Silva N.R., Olliaro P.L., Lazdins-Helds J.K., Engels D.A., Bundy D.A. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int. J. Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Ham P.J., Townson S., James E.R., Bianco A.E. An improved technique for the cryopreservation of Onchocerca microfilariae. Parasitology. 1981;83:139–146. doi: 10.1017/s0031182000050113. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Bottazzi M.E., Zhan B., Makepeace B.L., Klei T.R., Abraham D., Taylor D.W., Lustigman S. The onchocerciasis vaccine for Africa – TOVA – initiative. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003422. e0003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner M.P., Torrero M.N., McCall J.W., Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp. Parasitol. 2009;123:95–98. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez Castro P.D., Mansour A., Charles S., Hostetler J., Settje T., Kulke D., Kaplan R.M. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. Int. J. Parasitol. Drugs Drug Resist. 2020;13:22–27. doi: 10.1016/j.ijpddr.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpstein T., Pasche V., Häberli C., Scandale I., Neodo A., Keiser J. Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections. Parasites Vectors. 2019;12:226. doi: 10.1186/s13071-019-3476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv. Parasitology. 2010;73:197–230. doi: 10.1016/S0065-308X(10)73008-6. [DOI] [PubMed] [Google Scholar]
- Krücken J., Harder A., Jeschke P., Holden-Dye L., O'Connor V., Welz C., von Samson-Himmelstjerna G. Anthelmintic cyclcooctadepsipeptides: complex in structure and mode of action. Trends Parasitol. 2012;28:385–394. doi: 10.1016/j.pt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Kuesel A.C. Research for new drugs for elimination of onchocerciasis in Africa. Int. J. Parasitol. Drugs Drug Resist. 2016;6:272–286. doi: 10.1016/j.ijpddr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke D., von Samson-Himmelstjerna G., Miltsch S.M., Wolstenholme A.J., Jex A.R., Gasser R.B., Ballesteros C., Geary T.G., Keiser J., Townson S., Harder A., Krücken J. Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside. PLoS Neglected Trop. Dis. 2014;8:e3401. doi: 10.1371/journal.pntd.0003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.L., Lee W.C., Xia J., Zhang G., Razali R., Anwar A., Fong M. Draft genome of Brugia pahangi: high similarity between B. pahangi and B. malayi. Parasites Vectors. 2015;8:451. doi: 10.1186/s13071-015-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz C.S., Halls V., Hannam J.S., Niebel B., Strubing U., Mayer G., Hoerauf A., Famulok M., Pfarr K.M. A selective inhibitor of heme biosynthesis in endosymbiotic bacteria elicits antifilarial activity in vitro. Chem. Biol. 2013;20:177–187. doi: 10.1016/j.chembiol.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Mackenzie C.D. Human onchocerciasis: the essential partnership between research and disease control efforts. Curr. Opin. Infect. Dis.13. 2000:457–464. doi: 10.1097/00001432-200010000-00005. [DOI] [PubMed] [Google Scholar]
- Maclean M.J., Savadelis M.D., Coates R., Dzimianski M.T., Jones C., Benbow C., Storey B.E., Kaplan R.M., Moorhead A.R., Wolstenholme A.J. Does evaluation of in vitro microfilarial motility reflect the resistance status of Dirofilaria immitis isolates to macrocyclic lactones? Parasites Vectors. 2017;10:480. doi: 10.1186/s13071-017-2436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino C., Gut J., Lim K.C., Singh R., McKerrow J., Sakanari J. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Neglected Trop. Dis. 2012;6:e1494. doi: 10.1371/journal.pntd.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.P., Evans H., Larsen S.E., Mitre E. A comprehensive, model-based review of vaccine and repeat infection trials for filariasis. Clin. Microbiol. Rev. 2013;26:381–421. doi: 10.1128/CMR.00002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P., Seiler J., Kuesel A., Horton J., Clark J.N., Don R., Keiser J. Potential drug development candidates for human soil-transmitted helminthiases. PLoS Neglected Trop. Dis. 2011;5:e1138. doi: 10.1371/journal.pntd.0001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Eng J.K., Boakye D.A., Gyapong J.O., Prichard R.K. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Neglected Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0000998. e998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch F., Ritter M., Hoerauf A., Hübner M.P. Human filariasis-contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitol. Res. 2021 doi: 10.1007/s00436-020-07026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk W.A., Prada J.M., Smith M.E., Kontoroupis P., de Vos A.S., Touloupou P., Irvine M.A., Brown P., Subramanian S., Kloek M., Michael E., Hollingsworth T.D., de Vlas S.J. Are alternative strategies required to accelerate the global elimination of lymphatic filariasis? Insights from mathematical models. Clin. Infect. Dis. 2018;66:S260–S266. doi: 10.1093/cid/ciy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B., Marcellino C., Miller M., Maclean M., Mostafa E., Howell S., Sakanari J., Wolstenholme A., Kaplan R. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator". Int. J. Parasitol. Drugs Drug. Resist. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagboto S.K., Townson S. Onchocerca lienalis: comparison of techniques for the cryopreservation of microfilariae within skin-snips or free of host tissues. J. Helminthol. 1991;65:301–309. doi: 10.1017/s0022149x00010907. [DOI] [PubMed] [Google Scholar]
- Tagboto S.K., Townson S. Onchocerca volvulus and O. lienalis: the microfilaricidal activity of moxidectin compared with that of ivermectin in vitro and in vivo. Ann. Trop. Med. Parasitol. 1996;90:497–505. doi: 10.1080/00034983.1996.11813075. [DOI] [PubMed] [Google Scholar]
- Thomsen E.K., Sanuku N., Baea M., Satofan S., Maki E., Lombore B., Schmidt M.S., Siba P.M., Weil G.J., Kazura J.W., Fleckenstein L.L., King C.L. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin. Infect. Dis. 2016;62:334–341. doi: 10.1093/cid/civ882. [DOI] [PubMed] [Google Scholar]
- Townson S., Freeman A., Harris A., Harder A. Activity of the cyclooctadepsipeptide emodepside against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Am. J. Trop. Med. Hyg. 2005;73:93. [Google Scholar]
- Townson S., Ramirez B., Fakorede F., Mouries M.A., Nwaka S. Challenges in drug discovery for novel antifilarials. Expet Opin. Drug Discov. 2007;2:S63–S73. doi: 10.1517/17460441.2.S1.S63. [DOI] [PubMed] [Google Scholar]
- Vinkeles Melchers N.V.S., Coffeng L.E., Boussinesq M., Pedrique B., Pion S.D.S., Tekle A.H., Zoure H.G.M., Wanji S., Remme J.H., Stolk W.A. Projected number of people with onchocerciasis-loiasis coinfection in Africa, 1995 to 2025. Clin. Infect. Dis. 2020;70:2281–2289. doi: 10.1093/cid/ciz647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G., Harder A., Sangster N.C., Coles G.C. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitolology. 2005;130:343–347. doi: 10.1017/s0031182004006523. [DOI] [PubMed] [Google Scholar]
- Walker M., Pion S.D.S., Fang H., Gardon J., Kamgno J., Basanez M.G., Boussinesq M. Macrofilaricidal efficacy of repeated doses of ivermectin for the treatment of river blindness. Clin. Infect. Dis. 2017;65:2026–2034. doi: 10.1093/cid/cix616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 1993. Onchocerciasis and its Control: Report of a WHO Expert Committee on Onchocerciasis Control. WHO Technical Report Series 852.https://apps.who.int/iris/bitstream/handle/10665/37346/WHO_TRS_852.pdf?sequence=1&isAllowed=y Available at: [PubMed] [Google Scholar]
- WHO Progress report on the elimination of human onchocerciasis, 2016-2017. Wkly. Epidemiol. Rec. 2017;92:681–694. [PubMed] [Google Scholar]
- WHO Summary of global update on preventive chemotherapy implementation in 2016: crossing the billion. Wkly. Epidemiol. Rec. 2017;92:589–593. [PubMed] [Google Scholar]
- Zahner H., Taubert A., Harder A., von Samson-Himmelstjerna G. Effects of Bay 44-4400, a new cyclodepsipeptide, on developing stages of filariae (Acanthocheilonema viteae, Brugia malayi, Litomosoides sigmodontis) in the rodent Mastomys coucha. Acta Trop. 2001;80:19–28. doi: 10.1016/s0001-706x(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Zahner H., Taubert A., Harder A., von Samson-Himmelstjerna G. Filaricidal efficacy of anthelmintically active cyclodepsipeptides. Int. J. Parasitol. 2001;31:1515–1522. doi: 10.1016/s0020-7519(01)00263-6. [DOI] [PubMed] [Google Scholar]