Abstract
Objectives
The main objectives are: to evaluate the feasibility and effectiveness of a community-pharmacy based medication therapy management programme; to explore patients’ experiences and views towards medication therapy management service delivered by community pharmacists.
Research design and methods
A mixed-methods research design consisting of a pilot randomized controlled trial embedded with qualitative study will be used in this study. The study consists of two phases: a 6-month follow-up pilot randomized control trial (quantitative approach) to assess the feasibility and effectiveness of a community pharmacy-based medication therapy management programme. The primary outcome is HbA1C and secondary outcomes include: clinical and health services utilization and process measures, medication adherence, diabetes distress as well as satisfaction with care. Phase two consists of an embedded qualitative study using semi-structured interviews to explore patients’ experiences and views with the medication therapy management programme. Study data collection will be collected between April 2021 and December 2021.
Ethics consideration
The study has been approved by institutional review boards from Princess Nourah bent Abdulrahman University (Approval # 20–0240), King Fahad Medical City (Approval # 20-388E) and Birmingham University (Approval # ERN_20-0768).
Keywords: Community pharmacy, Pharmacist, Medication review, Medication therapy management
1. Introduction
1.1. Introduction
Over the past couple of decades, pharmacists worldwide have assumed a range of direct patient responsibilities such as medication prescribing, performing point of care diagnostic testing, emergency medication refills, administering vaccination and medication review programmes, with most of these services provided in the community setting. (Amien et al., 2013, Mossialos et al., 2015, Al-Saleh et al., 2017). Despite such evolution in the role of pharmacists, the community pharmacy practice in Saudi Arabia, albeit sporadic and limited new initiatives of patient-centred care services, are still at a very nascent stage and in need of substantial upgrades. (Al-Hassan, 2009a). In addition, increase in life expectancy of the population and growing burden of non-communicable chronic diseases and their risk factors are among the significant challenges facing the Saudi’s healthcare system and healthcare professionals across all specialities (World Health Organization, 2013).
Under the Vision 2030 (Saudi Vision 2030, 2016), the Saudi healthcare system is undergoing a significant transition to adapt to the epidemiological and demographic changes that impact its citizens' health and wellness. The Ministry of Health (MOH) launched several initiatives within the new healthcare models, including persistent support for cross-cutting interventions to enhance primary care services, continuity of care services, providing value-based care and promoting integrated care at the appropriate settings (Health sector transformation strategy, 2017). It is also expected that pharmaceutical care services will switch from inpatient to ambulatory and community setting (Alomi, 2017). Recently, the MOH published the updated executive regulations (Ministry of health, 2019) that allowed the provision of providing pharmaceutical care services such as vaccination, vital signs assessment, health and medical devices education. Furthermore, non-urgent care services such as medication therapy management (MTM), minor illnesses care, pharmaceutical consultations and compounding are among the new interventions a community pharmacist can provide under the updated regulations (Ministry of health 2019).
Extending community pharmacists’ roles could result in many patient-related benefits, including improvement in the quality of care, optimisation of drug therapy (Smith et al., 2011), decrease in general practitioner workload and potential reduction in long-term healthcare costs (Dunlop and Shaw, 2002, Giberson et al., 2011). In addition, it will ease the pressure on governmental hospitals and healthcare centres (Smith et al., 2011), which is one of the key objectives of the healthcare reformation in Saudi Arabia. Multiple studies from various countries have evaluated the impact of community pharmacy-based medication review. Results from these studies suggest that pharmacists have a significant role in advancing patient health outcomes through different medication review models (Bernsten et al., 2001, Mott et al., 2003, Planas et al., 2012, Stewart et al., 2014, Jodar-Sanchez et al., 2015, Tsuyuki et al., 2016, Cheema et al., 2018, Verdoorn et al., 2019, Schulz et al., 2019). Likewise, community pharmacists in KSA are easily accessible with a broad range of services (Al-Arifi, 2012). Pharmacists are authorized to provide over-the-counter medication consultations, procuring prescription medications (Alfadl et al., 2018, Al-Saleh et al., 2017) through e-services such as Wasfaty “My Prescription” (Wasfaty, 2020), vaccination and specialized education such as diabetes education (Al Nahdi, 2018), however, the depth and impact of such services are still at the initial stage. For the vast majority of community pharmacists, their role in direct patient care is still limited (Rasheed et al., 2019).
1.2. Medication therapy management programme
MTM is a distinct service or group of services that optimize therapeutic outcomes for individual patients who are beneficiaries of part D of Medicare health coverage enacted in 2003 in the United States (De Oliveira et al., 2010). Core elements of an MTM service model encompasses the provision of medication therapy review (MTR), personal medication record (PMR), medication-related action plan (MAP), intervention and/or referral and documentation and follow-up (American Pharmacists Association and the National Association of Chain Drug Stores Foundation, 2008). The sequence and delivery of the core elements may be modified to meet an individual patient’s needs. Recognition of the pharmacist as one of the providers of MTM under the Medicare Modernization Act of 2003 (effective January 2006) represents a valuable opportunity for community pharmacists to enhance patient care and address the nationally recognized need to identify and resolve medication therapy problems (American Pharmacists Association and the National Association of Chain Drug Stores Foundation, 2008).
1.3. Study rational
Several studies (Al-Hassan, 2009b, Alanazi et al., 2016, Al-Tannir et al., 2016, Nahid, 2016, Gillani et al., 2017, Alhaddad, 2019, Rasheed et al., 2019) explored pharmacist’s preparedness and attitudes towards direct patient care activities, but to our knowledge there is no available evidence of establishing a MTM service at the community level in Saudi Arabia. Therefore, there is a need to demonstrate the benefits of a community pharmacist-led medication review model through high-quality study design. This paper presents protocol of a pilot randomized controlled trial which will evaluate feasibility and effectiveness of community-pharmacy based MTM service in Riyadh, Saudi Arabic. We will adapt the MTM core elements to build a culturally relevant service considering the unique healthcare structure in KSA and patients’ values and perspectives. The results of this study will illustrate the feasibility of implementing such services and affirming the role of the pharmacist as an integral member of primary care services to maximize the quality of provided care.
1.4. Aim and objectives
This study has two main aims:
-
•
To determine the effectiveness of a community-pharmacist-led MTM programme in Saudi Arabia
-
•
To explore patients' views around their experiences with community pharmacist-led MTM programme.
To achieve these aims, three main objectives will guide this project:
-
•
To determine the impact of a pharmacist-led MTM service on patient clinical and health services utilization outcomes.
-
•
To assess a patient’s medication adherence, diabetes distress and patient satisfaction of MTM service.
-
•
To describe the feasibility of MTM service through process measures such as number and type of referrals, type and frequency of services provided, number of visits/follow up per patient and pharmacist consultation time.
2. Methods and analysis
2.1. Overall study design
A mixed-methods methodology with embedded study design will be used to evaluate the effectiveness of community-pharmacist-led MTM programme. The embedded design will consist of two phases; a pilot randomized controlled trial (RCT) and a qualitative study. The data from both quantitative and qualitative components will be collected sequentially and independently. The main question (effectiveness of the programme) requires a quantitative approach and therefore it is the principal method while the qualitative method will be used to explore patients’ experience and views with the service and have a supportive role, helping to understand and explain why and how the intervention works and how the service can be improved. Because of the concept is still new, we favored the use of mixed-methods to allow the generation of multiple data sets and perspectives, thus providing a broader understanding than using either a qualitative or quantitative approach alone. The use mixed-methods design also allowed to answer different research questions within a single study. (Hadi et al., 2013a, Hadi et al., 2013b, Hadi et al., 2014, Hadi and Closs, 2016)
2.2. Setting
The study will be carried out at the Health Kingdom Community Pharmacy (HKCP) in Riyadh City, KSA. HKCP is one of a medium-sized-chain pharmacy group. The pharmacy is linked to a private Polyclinic Medical Center located east of Riyadh City that serves a medium to upper middle-income population. The center comprises of 19 clinics with a broad range of specialties, laboratory facility and clientele across the spectrum of all age groups. The electronic medical record system used in the center is Ministry of Communications and Information Technology (MCIT) has been designed by Hospital Informations Management System (HIMS).
2.3. Phase one: The quantitative study
2.3.1. Study design
An open-label, two-arm, parallel-group, pilot RCT with a follow up period of 6-months to examine potential impact of the intervention. The two arms include an active arm where patients will receive MTM service and a control arm where patients will receive usual care. Since patients from both groups will attend the same pharmacy, treatment contamination is highly plausible. A cluster-randomized design could help resolve this issue, but this is not applicable due to the complex intervention and the lack of similar setup. We will try to minimize the potential contamination risk by having two different pharmacists providing care, the patients in the intervention group will receive the MTM service from the MTM-trained pharmacist and patients in the usual care will receive usual care from the store-based community pharmacist.
2.3.2. Eligibility criteria
Patients will be selected according to the following eligibility criteria:
2.3.2.1. Inclusion criteria
-
•
Age 18 years and older
-
•
Patients with uncontrolled diabetes
-
•
Male or female
-
•
Prescribed at least three chronic medications.
-
•
Provided informed consent.
-
•
The patients should have a continuous active status with the clinic (defined as having a record of at least one visit in the six months before screening)
2.3.2.2. Exclusion criteria
-
•
Patients diagnosed with mental illness or dementia, and those with significant cognitive impairment (score < 24 on the Mini-Mental State Examination)
-
•
Patients with unstable acute complications/illness or with advanced illness as judged by the enrolling physician
-
•
Patients with gestational diabetes
-
•
Patients who cannot consent to take part in the study
2.3.3. Patients recruitment
All eligible patients attending the health care center within the study period will be informed about the study by their physicians. Posters describing the study will be posted inside clinics, and in the lobby of the medical center. Information about the study objectives and details will be explained to willing patients. Recruitment will be conducted until the required number of patients are enrolled.
2.3.4. Randomization
Patients will be randomized in a 1:1 ratio to receive either MTM service or usual care. Randomization will be provided by one of the researchers not directly involved in patient care. An allocation sequence will be generated on a computer-generated list of random numbers to allocate patients to either group to avoid selection bias. Sequentially numbered tamper-proof opaque sealed envelopes will be used to conceal sequence allocation.
2.3.5. Blinding
Considering the nature of the intervention, patients and pharmacists cannot be blinded. However, the researchers who responsible for data collection and analysis will be blinded which will reduce bias and increase fidelity.
2.3.6. Description and delivery of the study intervention
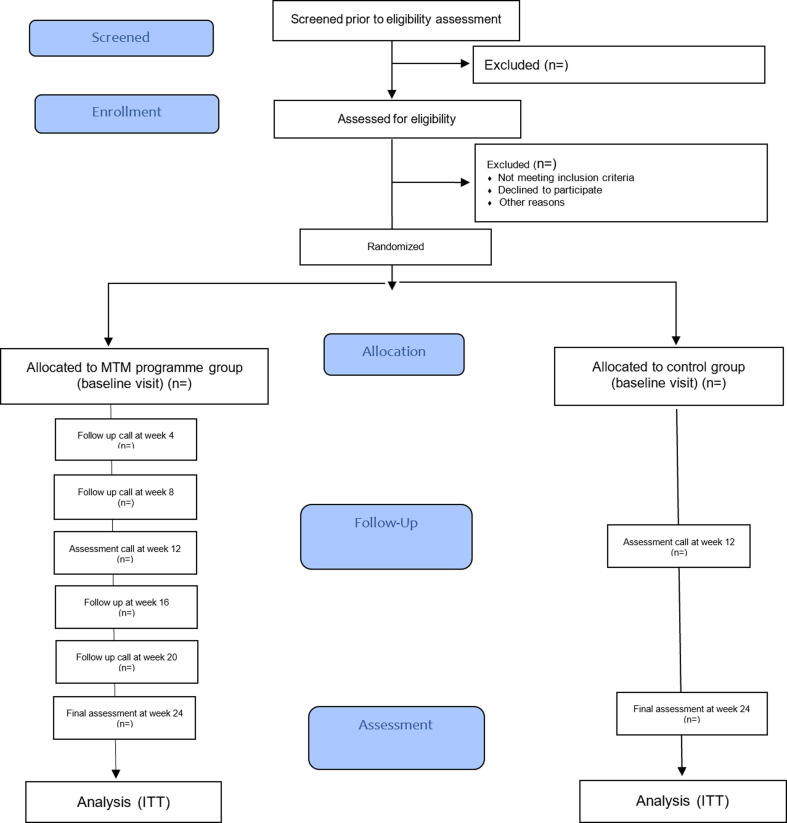
All patients will be followed for six months. During the study period the patients in both arms will have one face to face visit at baseline in addition to two follow up assessment points (at week 12 and 24). During the follow up the patients will be contacted by phone for any hospitalization or ED visits and an updated medication list. Medical record will be accessed to obtain vitals and laboratory data (glycosylated hemoglobin (HbA1c), lipid profile serum creatinine (Scr) and albumin to creatinine ratio). Patients will also be required to fill two questionnaires (medication adherence and diabetes distress) at baseline visits and at the end of the study. A satisfaction questionnaire will be administered at the end of study to all study population. Fig. 1 shows the study design in the CONSORT flow diagram (Fig. 1).
Fig. 1.
Consort flow diagram of the study design.
2.3.6.1. Usual care (control)
Patients randomized to the control arm will receive the usual care provided by the community pharmacist during the routine dispensing of medications. Two follow-up calls, one each at three months and six months will be arranged to obtain information as described above. Patients in this arm will continue to visit the pharmacy to fill prescriptions without further intervention and no restriction on contacting the pharmacist for advice, should they wish to.
2.3.6.2. Description of the intervention (MTM programme)
Patients randomized to the MTM arm will receive a total of 7 visits (at week 0, 4, 8, 12, 16, 20 and 24). Initial face to face visit with the MTM-pharmacist to complete a comprehensive medication review and to fill the baseline questionnaires. At the end of the session, the patient will receive a personalized medication record that will include all their medications by name, indication, direction and special instruction. The patient will also receive a medication-related action plan (MAP) as agreed upon with the MTM pharmacist to resolve identified drug related problems (DRP). Patients will also receive a monthly follow up by phone (10–20 mins duration) to provide support. Phone calls time and date will be agreed upon with the patient. Additional clinic visits/phone calls will be arranged based on patient’s need. During the follow up encounters, the pharmacist will assess patient’s understanding of their medications, review medications and home monitoring (blood glucose, blood pressure, etc), if applicable, identify and resolve any new DRPs and provide additional education if needed. Pharmacist will document patient visits, phone calls in the patient medical record (MCIT).
2.3.6.3. Plan of contact for those who do not attend
Patients who do not answer, will be contacted for up to 3 times. If the person does not respond, they will be considered lost to follow-up.
2.3.7. Outcomes
The primary outcome
-
•
Change in HbA1C (%) from baseline at 6-months
Secondary outcomes
-
•
Degree of changes in clinical parameters (lipid profile, blood pressure, albumin-to- creatinine ratio and Scr) and health services utilization parameters (number and reasons for hospital admission or Emergency Department (ED) visits)
-
•
Process measures (number of visits/FU per patient, pharmacist consultation time per patient, number and type of referrals, number and type of drug related problems)
-
•
Patient medication adherence, diabetes distress and patient satisfaction with pharmacist services
Table 1 shows the scales used to measure these outcomes.
Table 1.
Outcome measures and scales.
| Outcome measures | Scales |
|---|---|
| Clinical parameters |
|
| Process Measures |
|
| Health services utilization | Number and reasons for hospitalization or ED during the study period |
| Patient medication adherence (Arabic version) | The Medication Adherence Report Scale (MARS-5) (Horne et al., 1999) |
| Diabetes distress (Arabic version) | Diabetes Distress Scale (DDS) (Polonsky et al., 2005) |
| Patient satisfaction with pharmacist service (Arabic version) | Patient Satisfaction with Pharmacist Services Questionnaire 2.0 (PSPSQ2.0) (Sakharkar et al., 2015) |
2.3.8. Data collection
Data will be collected using pre-designed and piloted data collection sheets. Demographic characteristics, medical history, past and current medications, vitals, lab tests and outcomes of interest for all patients will be collected by the pharmacist and the principal researcher at baseline, 3, and 6 months.
2.3.9. Questionnaire tools
Three self-administered questionnaires will be used. Medication adherence will be assessed with Medication Adherence Rating Scale (MARS) (Horne et al., 1999). The questionnaire consists of five items and has good validity and reliability across different long-term conditions including diabetes (McAdam-Marx et al., 2014, Wei et al., 2017, Owiredua et al., 2018). The Arabic translations of MARS have proved to be valid and reliable (Alsous et al., 2017). Permission was taken and an agreement with the originator was signed to ensure lawful use of the questionnaire.
Diabetes Distress Survey (DDS) (Polonsky et al., 2005) which is a 17-item questionnaire, will be used to assess the level of emotional distress experienced by the patient. The Arabic version of DDS was found to be a psychometrically sound measure to evaluate diabetes distress among Arab patients with diabetes mellitus (Darawad et al., 2017).
Patient Satisfaction with Pharmacist Services Questionnaire (PSPSQ 2.0) developed by Sakharkar et al. (2015) will be used to evaluate patient satisfaction. It is a valid and reliable questionnaire and consists of 20-items related to three domains. It will be used to measure patients’ satisfaction with the pharmacist-led service conducted among patients with diabetes. (Hassali et al., 2018). Since no Arabic version is available, a translation and cross-cultural adaptation will be carried out during the course of this study. A non-commercial license agreement was signed with the original author to enable the use of PSPSQ 2.0 in this research project.
2.3.10. Data management
There will be two data management stages to ensure the privacy and accuracy of the study data. First, the principal researcher will enter all data into an electronic datasheet in a password-protected server. Second, another member of the research team will confirm and double-check data entry for any potential errors during input. Patients will be coded as numbers, the code key will be saved as a password protected document.
2.3.11. Sample size
Sample size calculation was based on previous research and Cohen’s power tables. The following parameters were considered to calculate the sample size: an effect size of 0.5 in HbA1c at 6 months between the two groups (Wubben and Vivian, 2008) with 80% power at the 5% two-sided significance level. A sample size of 128 patients (64 in each arm) is estimated. Considering a 20% attrition rate, a total sample of 160 (80 patients in each arm) is needed.
2.3.11.1. Statistical analysis
All statistical analyses will be performed using IBM Statistical Package for Social Sciences SPSS to analyze data collected at baseline, 3, and 6 months for both groups. The data will be checked and coded numerically and entered directly in the SPSS. Blinded assessors will analyze all outcomes. A strict intention to treat (ITT) approach will be followed in the analysis to avoid withdrawal bias. For missing data, variable imputation will be used. Continuous normally distributed variables will be compared using appropriate test and reported as means (standard deviations), whereas non-normally distributed variables will be reported as medians (interquartile range). Categorical variables will be compared using appropriate test and reported as numbers (percentages).
2.4. Phase two: The qualitative study
2.4.1. Study design
An individual face-to-face semi-structured interview will be conducted with a sample from patients who were randomized to the intervention arm (MTM group) to explore their experience with the programme. It is noteworthy that the qualitative study will not contribute towards answering the effectiveness question (the primary aim of the study); rather, it will help to understand and explain how the intervention work.
2.4.2. Patients recruitment and eligibility criteria
As mentioned earlier, an embedded design will be used for this study, and therefore, the process of patient recruitment for the qualitative phase is nested within the recruitment process for the quantitative phase. The sampling frame consists of patients who satisfy the inclusion/exclusion criteria and indicate their willingness to continue with the qualitative part. The principal researcher and the patient will decide on the date and time of the interview.
2.4.3. Inclusion criteria
Patients who completed the 6-months MTM programme
2.4.4. Exclusion criteria
Patients not willing to participate in interview.
2.4.5. Description and delivery of the interview
All interviews will be conducted by the first author face to face in the clinic. Each interview will last between 20 and 45 min. Patients will be interviewed within two weeks after completing the MTM programme.
At the beginning of the interview, the purpose of the interview, measures taken to ensure confidentiality, the format and the expected length of the interview will be clarified and explained to the patient. Permission will be also obtained to audio record the interview. If the patient agrees, a written consent form will be obtained. The interviews will be audio taped using two digital audio recorders.
A semi-structured topic guide will be used to ensure uniformity, based on the literature review and study objectives. Topic guide was designed to cover the following areas: expectations from the service; efficacy of the service (did it help? How?); quality of the service; interaction with pharmacist (time given for consultation, engaging patient in discussion and designing of therapeutic plan, listening to and understanding the problem); their opinion in the role of pharmacist in MTM programme; and overall satisfaction (experience compared to other services in past, aspects of the service which need improvement etc.). Patient will also be given the chance to express any additional views at the end of the interview.
2.4.6. Data management
After each interview, data from both audio recorders will be transferred to a password protected server. Once transferred, the audio recordings will be deleted from both recorders. All interviews will be transcribed verbatim by a professional transcribing office and the audio files will be uploaded to a secure online server, which will be only accessible to the assigned transcriber. All interviews will be transcribed by a single transcriber. Once transcriptions are received, the principal researcher will listen to the audio recordings to check the transcriptions for accuracy.
2.4.7. Sample size
In the qualitative part of the study, data saturation or informational redundancy will guide sample size (Lincoln and Guba, 1985). The principal researcher will continue to interview patients until no new information is emerging during the interviews.
2.4.8. Qualitative data analysis
For this study, thematic analysis will be used to obtain various perspectives on the research question related to the explore patients' views around their experiences with the community pharmacist-led MTM programme. Thematic analysis is a method used for ‘identifying, analysing, and reporting patterns (themes) within the data’. The strategies will be used for the analysis largely followed the approach proposed by Braun and Clarke (2006). Braun and Clarke approach is systematically and explicitly apply the principles of undertaking qualitative analysis to a series of interconnected stages that guide the process. Braun and Clarke (2006) point out that patterns are identified through a rigorous process of data familiarisation, data coding and theme searching, revision, defining and naming and finally report development (Braun and Clarke, 2006)
2.5. Consent form
Verbal and written consent will be obtained from all patients by the research coordinator before randomization. Since there are two phases of the study, two patient information sheets with consents forms, one general and one specifically for the qualitative phase, were developed to provide patients with the necessary information to make an informed decision whether to participate in the research. Patients will be reminded that they are free to withdraw at any time, and that their data will be stored securely and anonymously. For physicians who will participate in the management should have verbal consent.
2.6. Patient confidentiality and data security
All necessary measures will be taken to ensure the confidentiality of study patients and data protection. Access to data will be under strict privacy and security throughout the study duration, publication and at any public presentation. Only individuals authorized by the principal researcher will allow access to the study database.
3. Discussion
The study protocol is developed as a mixed methods approach to illustrate the feasibility and challenges associated with an implement new programme in Saudi community pharmacy setting. The study aims to assess the effectiveness of providing MTM by community pharmacists and explore patients' views around their experiences with the programme.
We anticipate that the evidence generated from this study will help to affirm the role of the pharmacist as an integral member of primary care services to maximize the quality of provided care and to develop a framework for MTM service that matches the specific needs of the people in Saudi Arabia. The study will also provide evidence on the impact of providing extended patient-centred care activities; particularly to control chronic diseases, improve community health, and build the capacity to fill the gap in primary health care services.
Development and implementation of a community pharmacist-led MTM programme will not help improve patient outcomes for patients with diabetes but also will provide robust evidence to further develop various community pharmacist-led innovative services in Saudi Arabia. Strengthening of community pharmacy services is an integral part of the Saudi Vision 2030 for the transformation of healthcare with an aim of shifting pharmaceutical care services from inpatient to community settings. Developing robust research evidence is critical in ensuring suitability of such services that is why both quantitative and qualitive will be used in this study.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al Nahdi, 2018. Health Educational Clinics. https://nahdi.sa/en/social-sustainability/health-educational-clinics (accessed 25 December 2020).
- Alanazi A.S., Alfadl A.A., Hussain A.S. Pharmaceutical care in the community pharmacies of Saudi Arabia: present status and possibilities for improvement. SJMMS. 2016;4(1):9–14. doi: 10.4103/1658-631X.170881. https://www.sjmms.net/article.asp?issn=1658-631X;year=2016;volume=4;issue=1;spage=9;epage=14;aulast=Alanazi [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Arifi M.N. Patients’ perception, views and satisfaction with pharmacists’ role as health care provider in community pharmacy setting at Riyadh, Saudi Arabia. SPJ. 2012;20(4):323–330. doi: 10.1016/j.jsps.2012.05.007. https://www.sciencedirect.com/science/article/pii/S1319016412000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadl A.A., Alrasheedy A.A., Alhassun M.S. Evaluation of medication counseling practice at community pharmacies in Qassim region, Saudi Arabia. SPJ. 2018;26(2):258–262. doi: 10.1016/j.jsps.2017.12.002. https://pubmed.ncbi.nlm.nih.gov/30166925/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad M. Youth experience with community pharmacy services and their perceptions toward implementation of medication therapy management services by community pharmacists in the Western region of Saudi Arabia. TIRS. 2019;53(1):95–99. doi: 10.1177/2168479018769299. https://pubmed.ncbi.nlm.nih.gov/29714597/ [DOI] [PubMed] [Google Scholar]
- Al-Hassan M. Community pharmacy practice in Saudi Arabia: an overview. Internet J. Pharmacol. 2009;9(1) http://ispub.com/IJPHARM/9/1/5301# [Google Scholar]
- Al-Hassan M. A look at community pharmacy practice in Saudi Arabia. J. Med. Sci. 2009;3(3):111–114. http://docsdrive.com/pdfs/medwelljournals/rjmsci/2009/111-114.pdf [Google Scholar]
- Alomi, Y.A., 2017. New pharmacy model for Vision 2030 in Saudi Arabia. JPPCM. 3 (3), 194–196. https://www.researchgate.net/publication/317698117_New_Pharmacy_Model_for_Vision_2030_in_Saudi_Arabia.
- Al-Saleh H., Al-Houtan T., Al-Odaill K., Al-Mutairi B., Al-Muaybid M., Al-Falah T., Ashraf Nazir M. Role of community pharmacists in providing oral health advice in the eastern province of Saudi Arabia. Saudi Dent J. 2017;29:123–128. doi: 10.1016/j.sdentj.2017.03.004. https://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC5502909&blobtype=pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsous, M., Alhalaiqa, F., Abu Farha, R., Abdel Jalil, M., McElnay, J., Horne, R., 2017. Reliability and validity of Arabic translation of Medication Adherence Report Scale (MARS) and Beliefs about Medication Questionnaire (BMQ)–specific for use in children and their parents. PLOS One. 12(2), e0171863. http://dx.doi.10.1371/journal.pone.0171863. https://pubmed.ncbi.nlm.nih.gov/28192467/. [DOI] [PMC free article] [PubMed]
- Al-Tannir M., Alharbi A.I., Alfawaz A.S., Zahran R.I., AlTannir M. Saudi adults satisfaction with community pharmacy services. SpringerPlus. 2016;5(1):774. doi: 10.1186/s40064-016-2442-8. https://europepmc.org/article/pmc/4912500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Pharmacists Association and the National Association of Chain Drug Stores Foundation, 2008. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). J. Am. Pharm. Assoc. 48, 341–353. https://www.pharmacist.com/sites/default/files/files/mtm_in_pharm_practice_v2_0.pdf. [DOI] [PubMed]
- Amien F., Myburgh N.G., Butler N. Location of community pharmacies and prevalence of oral conditions in the Western Cape Province. Health SA Gesondheid. 2013;18(1):9–15. https://www.researchgate.net/publication/262743257_Location_of_community_pharmacies_and_prevalence_of_oral_conditions_in_the_Western_Cape_Province [Google Scholar]
- Bernsten, C., Bjorkman, I., Caramona, M., Crealey, G., Frokjaer, B., Grundberger, E., Gustafsson, T., Henman, M., Herborg, H., Hughes, C., Mcelnay, J., Magner, M., Van mil, F., Schaeffer, M., Silva, S., Sondergaard, B., Sturgess, I., Tromp, D., Vivero, l., Winterstein, A., 2001. Pharmaceutical care of the elderly in europe research group. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs Aging. 18(1). 63–77. https://pubmed.ncbi.nlm.nih.gov/11232739/. [DOI] [PubMed]
- Braun V., Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. https://www.researchgate.net/publication/235356393_Using_thematic_analysis_in_psychology [Google Scholar]
- Cheema E., Sutcliffe P., Weickert M.O., Singer D.R.J. A randomised controlled trial of the impact of structured written and verbal advice by community pharmacists on improving hypertension education and control in patients with high blood pressure. Eur J Clin Pharmacol. 2018;74(11):1391–1395. doi: 10.1007/s00228-018-2519-0. https://pubmed.ncbi.nlm.nih.gov/30022334/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darawad M.W., Hammad S., Samarkandi O.A., Hamdan-Mansour A.M., Khalil A.A. Evaluating the Psychometric Properties of the Arabic Version of the Diabetes Distress Scale. J. Psychosoc. Nurs. Ment. Health Serv. 2017;55(9):43–51. doi: 10.3928/02793695-20170818-12. https://www.researchgate.net/publication/319429313_Evaluating_the_Psychometric_Properties_of_the_Arabic_Version_of_the_Diabetes_Distress_Scale [DOI] [PubMed] [Google Scholar]
- De Oliveira D.R., Brummel A.R., Miller D.B. Medication Therapy Management: 10 Years of Experience in a Large Integrated Health Care System. J Manag Care Pharm. 2010;16(3):185–195. doi: 10.18553/jmcp.2010.16.3.185. https://www.researchgate.net/publication/42439800_Medication_Therapy_Management_10_Years_of_Experience_in_a_Large_Integrated_Health_Care_System [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J.A., Shaw J.P. Community pharmacists’ perspectives on pharmaceutical care implementation in New Zealand. Pharm. World Sci. 2002;24(6):224–230. doi: 10.1023/a:1021526425458. https://pubmed.ncbi.nlm.nih.gov/12512154/ [DOI] [PubMed] [Google Scholar]
- Giberson, S., Yoder, S., Lee, M.P., 2011. Improving patients and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General. http://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf (accessed: 22 February 2020).
- Gillani, S.W., ur Rahman, S.A., Mohammad Abdul, M.I., Syed Sulaiman, S.A., 2017. Assessment of community pharmacists’ perceptions of healthcare services in Saudi Arabia. JPHSR. 8, 269–74. https://onlinelibrary.wiley.com/doi/full/10.1111/jphs.12183.
- Hadi M.A., Alldred D.P., Briggs M., Closs S.J. Mixed methods research in pharmacy practice: Basics and beyond (Part 1) IJPP. 2013;21:341–345. doi: 10.1111/ijpp.12010. https://pubmed.ncbi.nlm.nih.gov/23418918/ [DOI] [PubMed] [Google Scholar]
- Hadi M.A., Alldred D.P., Briggs M., Closs S.J. Mixed-methods research in Pharmacy Practice: Recommendations for quality reporting (Part 2) IJPP. 2014;22:96–100. doi: 10.1111/ijpp.12015. https://pubmed.ncbi.nlm.nih.gov/23419033/ [DOI] [PubMed] [Google Scholar]
- Hadi M.A., Closs S.J. Applications of mixed-methods methodology in Clinical Pharmacy research. Int. J. Clin. Pharm. 2016;38(3):635–640. doi: 10.1007/s11096-015-0231-z. https://pubmed.ncbi.nlm.nih.gov/26659085/ [DOI] [PubMed] [Google Scholar]
- Hadi M.A., Phillip A.D., Closs S.J., Marczewski K., Briggs M. A mixed-methods evaluation of a nurse-pharmacist–managed pain clinic: Design, rationale and limitations. CPJ. 2013;146(4):197–201. doi: 10.1177/1715163513490400. https://journals.sagepub.com/doi/pdf/10.1177/1715163513490400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassali, M.A., Saleem, F., Verma, A.K., Choy, W.Y., Nouri, A.I., Asmani, M.F.M., 2018. Translation and Validation of Patient Satisfaction with Pharmacist Services Questionnaire (PSPSQ 2.0). J. Young Pharm. 10(4), 427–432. https://www.researchgate.net/publication/328017467_Translation_and_Validation_of_Patient_Satisfaction_with_Pharmacist_Services_Questionnaire_PSPSQ_20.
- Health sector transformation strategy, 2017. Saudi Ministry of Health. https://www.moh.gov.sa/en/Ministry/vro/Documents/Healthcare-Transformation-Strategy.pdf (accessed: 1 January 2021).
- Horne, R., Weinman, J., Hankins, M., 1999. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health. 14(1), 1–24. https://www.tandfonline.com/doi/abs/10.1080/08870449908407311.
- Jodar-Sanchez, F., Malet-Larrea, A., Martin, J.J., Garcia-Mochon, L., Lopez del Amo, M.P., Martinez-Martinez, F., Gastelurrutia-Garralda, M.A., García-Cárdenas, V., Sabater-Hernández, D., Sáez-Benito, L., Benrimoj, S.I., 2015 Cost-Utility Analysis of a Medication Review with Follow-Up Service for Older Adults with Polypharmacy in Community Pharmacies in Spain: The conSIGUE Program. Pharmacoeconomics, 33(6). 599–610. https://pubmed.ncbi.nlm.nih.gov/25774017/. [DOI] [PubMed]
- Lincoln Y.S., Guba E.G. SAGE; Newbury Park, CA: 1985. Naturalistic Inquiry. [Google Scholar]
- McAdam-Marx C., Bellows B.K., Unni S., Mukherjee J., Wygant G., Iloeje U., Liberman J.N., Ye X., Bloom F.J., Brixner D.I. Determinants of glycaemic control in a practice setting: the role of weight loss and treatment adherence (The DELTA Study) Int. J. Clin. Pract. 2014;68(11):1309–1317. doi: 10.1111/ijcp.12502. https://pubmed.ncbi.nlm.nih.gov/25113816/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of health, 2019. Healthcare Licensing Services. https://www.moh.gov.sa/en/eServices/Licences/Pages/Licences.aspx (accessed: 2 March 2020).
- Mossialos E., Courtin E., Naci H., Benrimoj S., Bouvy M., Farris K., Noyce P., Sketris I. From “retailers” to health care providers: Transforming the role of community pharmacists in chronic disease management. Health Policy. 2015;119(5):628–639. doi: 10.1016/j.healthpol.2015.02.007. https://pubmed.ncbi.nlm.nih.gov/25747809/ [DOI] [PubMed] [Google Scholar]
- Mott D.A., Martin B., Breslow R., Michaels B., Kirchner J., Mahoney J., Margolis A. Impact of a medication therapy management intervention targeting medications associated with falling Results of a pilot study. JAPhA. 2003;56(1):22–28. doi: 10.1016/j.japh.2015.11.001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4743551/pdf/nihms748963.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid A. Knowledge attitude and practice towards pharmaceutical care in community pharmacy in Saudi Arabia. Br J Med Med Res. 2016;15(9):1–9. https://www.researchgate.net/publication/303324703_Knowledge_Attitude_and_Practice_towards_Pharmaceutical_Care_in_Community_Pharmacy_in_Saudi_Arabia [Google Scholar]
- Owiredua, C., Quarshie, EN-B., Atorkey. P., 2018. Living with diabetes: an exploratory study of illness representation and medication adherence in Ghana. Cogent Med. 5(1),1463599. file:///C:/Users/basma/Downloads/Livingwithdiabetes-AnexploratorystudyofillnessrepresentationandmedicationadherenceinGhana%20(2).pdf.
- Planas L.G., Crosby K.M., Farmer K.C., Harrison D.L. Evaluation of a diabetes management program using selected HEDIS measures. J Am Pharm Assoc. 2012;52:e130–e138. doi: 10.1331/JAPhA.2012.11148. https://pubmed.ncbi.nlm.nih.gov/23224336/ [DOI] [PubMed] [Google Scholar]
- Polonsky W.H., Fisher L., Earles J., Dudl R.J., Lees J., Mullan J., Jackson R.A. Assessing psychosocial distress in diabetes: Development of the diabetes distress scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. https://pubmed.ncbi.nlm.nih.gov/15735199/ [DOI] [PubMed] [Google Scholar]
- Rasheed M.K., Hasan S.S., Babar Z.U.D. Community pharmacist's knowledge, attitude, roles and practices towards patient-centred care in Saudi Arabia: a systematic review of the literature: A Systematic Review of the Literature. JPHSR. 2019;10(1):101–115. doi: 10.1111/jphs.12264. [DOI] [Google Scholar]
- Sakharkar, P., Bounthavong, M., Hirsch, J.D., Morello, C.M., Chen, T.C., Law, A.V., 2015. Development and validation of PSPSQ 2.0 measuring patient satisfaction with pharmacist services. Res. Social Adm. Pharm. 11(4), 487–498. https://pubmed.ncbi.nlm.nih.gov/25481330/. [DOI] [PubMed]
- Saudi Vision 2030, 2016. http://www.vision2030.gov.sa (accessed:1 February 2020).
- Schulz M., Griese-Mammen N., Anker S.D., Koehler F., Ihle P., Ruckes C., Schumacher P.M., Trenk D., Böhm M., Laufs U. Pharmacy-based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM-CHF randomized controlled trial. EJHF. 2019;21(8):1012–1021. doi: 10.1002/ejhf.1503. https://pubmed.ncbi.nlm.nih.gov/31129917/ [DOI] [PubMed] [Google Scholar]
- Smith M., Giuliano M.R., Starkowski M.P. In Connecticut: improving patient medication management in primary care. Health Aff. (Millwood). 2011;30(4):646–654. doi: 10.1377/hlthaff.2011.0002. https://pubmed.ncbi.nlm.nih.gov/21471485/ [DOI] [PubMed] [Google Scholar]
- Stewart, K., George, J., Mc namara, K.P., Jackson, S.L., Peterson, G.M., Bereznicki, L.R., Gee, P.R., Hughes, J.D., Bailey, M.J., Hsueh, Y.A., McDowell, J.M., Bortoletto, D.A., Lau, R., 2014. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial). J. Clin. Pharm. Ther. 39, 527–534. https://europepmc.org/article/med/24943987. [DOI] [PubMed]
- Tsuyuki, R.T., AL Hamarneh, Y.N., Jones, C.A., Hemmelgarn, B.R., 2016. The Effectiveness of Pharmacist Interventions on Cardiovascular Risk: The Multicenter Randomized Controlled RxEACH Trial. J. Am. Coll. Cardiol. 67(24). 2846–2854. https://pubmed.ncbi.nlm.nih.gov/27058907/. [DOI] [PubMed]
- Verdoorn S., Kwint H.F., Blom J.W., Gussekloo J., Bouvy M.L. Effects of a clinical medication review focused on personal goals, quality of life, and health problems in older persons with polypharmacy: A randomised controlled trial (DREAMeR-study) PLoS Med. 2019;16(5) doi: 10.1371/journal.pmed.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfaty, 2020. About Wasfaty https://wasfaty.sa/about/ (accessed:1 December 2020).
- Wei, L., Champman, S., Li, X., Li, X., Li, S., Chen, R., Bo, N., Chater, A., Horne1, R., 2017. Beliefs about medicines and nonadherence in patients with stroke, diabetes mellitus and rheumatoid arthritis: a cross-sectional study in China. BMJ Open. 7(10). e017293 . http://dx.doi.10.1136/bmjopen-2017-017293. https://pubmed.ncbi.nlm.nih.gov/28982826/. [DOI] [PMC free article] [PubMed]
- World Health Organization. Regional Office for the Eastern Mediterranean, 2013. Country cooperation strategy for WHO and Saudi Arabia 2012 - 2016. World Health Organization. Regional Office for the Eastern Mediterranean. https://apps.who.int/iris/handle/10665/113227.
- Wubben, D.P., Vivian, E.M., 2008. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 28(4), 421–436. http://europepmc.org/article/med/18363526. [DOI] [PubMed]