Abstract
Background
Arterial catheterization is frequently performed in neonatal intensive care units with an inherent risk of peripheral ischemic injury, especially in preterm infants. The treatment options following vascular damage involve invasive and non-invasive modalities. The primary objective of this systematic review was to evaluate the evidence of the use of topical nitroglycerine (TNG) either alone or as adjunctive therapy. The secondary aim was to develop an approach to the treatment of catheter induced ischemia in infants based on the available evidence.
Methods
A comprehensive search was conducted of available databases for relevant articles that involved the treatment of peripheral tissue ischemia in neonates with the use of TNG. Citations were restricted to human subjects.
Results
Six hundred and eighty-nine articles were identified, and twenty-seven case reports and case series were compatible with the inclusion and exclusion criteria. Sixty-eight infants out of the 76 published cases (89%) experienced a favorable outcome and 79% (n = 60) demonstrated complete recovery with the topical application of TNG to the ischemic site.
Conclusion
The available evidence demonstrates that TNG is effective for the treatment of peripheral ischemia in neonates after standard conservative measures have failed. However, due to the absence of robust evidence for this therapeutic modality, there are no uniform guidelines regarding the frequency, duration, and safety of TNG use. Planning the management of peripheral ischemia in neonates with TNG should be a multidisciplinary decision that includes close surveillance of blood pressure, methemoglobin levels, and follow up cranial ultrasound.
Keywords: Topical nitroglycerine, Treatment, Neonate, Peripheral ischemia, Outcomes
Abbreviations: TNG, Topical nitroglycerine; NICU, Neonatal intensive care unit; tPA, Tissue plasminogen activator; IVH, Intraventricular hemorrhage; WGA, Weeks gestational age
1. Introduction
Arterial cannulation in neonates is usually performed for frequent blood pressure monitoring and blood sampling. The procedure, while easily executed by skilled neonatal staff, can be associated with serious complications such as vasospasm, thrombosis, embolism, hematoma, infection, peripheral nerve damage, ischemia, and tissue necrosis. (Mosalli et al., 2013, Vasquez et al., 2003, Wong et al., 1992).
Standard, noninvasive measures to reverse peripheral ischemic injury include immediate catheter removal, limb elevation, and application of warm compresses to the non-affected limb to induce reflex vasodilation. These measures may provide temporary resolution but secondary treatment interventions may be necessary, to prevent further complications and irreversible tissue damage (Mosalli et al., 2013). Vasodilators, such as topical nitrogylcerine (TNG) have been utilized as a promising therapeutic option either alone or as adjunctive therapy in the management of neonatal peripheral ischemia due to arterial or venous cannulation, with variable success (Samiee-Zafarghandy et al., 2014, Mosalli et al., 2013). Evidence for using TNG in such scenarios is quite limited (Vasquez et al., 2003) and there is no consensus on the effectiveness, safe dosage, and duration of TNG use in neonates with ischemic injury. A recent systematic review of the topic by (Sushko et al., 2021), addressed the outcomes of peripheral tissue ischemia based on the use of TNG in a small cohort of newborns, without examining its effect relative to a cut-off point for TNG application nor offering algorithmic guidance regarding an approach to treatment. The main objective of this review was to evaluate the evidence on the use of topical nitroglycerine (TNG) either alone or as adjunctive therapy and develop an approach to the treatment of catheter induced ischemia in infants.
2. Methods
A comprehensive literature search was performed of existing databases, including PubMed, Medline, Embase, Web of Science, CINAHL and the Cochrane Library. We included all relevant articles without language restriction from 1980 until March 2021, that comprised neonates up to two months chronological age with peripheral tissue ischemia, that were managed either solely with TNG or as part of adjunctive therapy, with documented follow-up. We excluded articles that did not involve treatment with TNG and animal-based studies. To validate the literature review we used the following keywords and Medical Subject Headings (MeSH) terms: Topical nitroglycerine OR glyceryl trinitrate, AND peripheral skin ischemia OR peripheral tissue ischemia OR iatrogenic skin injury, AND vascular catheterization OR arterial catheterization OR arterial cannulation OR venous catheterization OR venous cannulation OR umbilical arterial catheterization OR umbilical venous catheterization OR central line, AND neonate OR newborn OR infant. The Cochrane central register of controlled trials and the Cochrane Database of Systematic Reviews (Cochrane Library issue 3, March 2021) and the database of abstracts of reviews of effectiveness (DARE) was searched for the topic of interest. All reports were checked for references to additional studies and articles identified from each database were compared, to verify matching and exclude duplication. The first author (RM) reviewed the abstracts of articles from the initial search. If descriptive information from the abstract was limited, the complete article was reviewed for appropriate inclusion criteria. Any identified discrepancies were clarified by discussion with a second co-author (BP) and consensus reached.
The following variables were extracted from the retrieved articles: gestational age, body weight, location, and cause of ischemia, TNG dose and duration of treatment, side effects, adjunctive therapy and outcomes following TNG use.
2.1. Definitions
Preterm infants were defined as those ≤ 37 completed weeks gestational age (wGA) and were sub-classified as late preterm (32–35 wGA), very preterm (28–31 wGA) and extremely preterm (<28 wGA). Term infants were defined as those born at > 37wGA.
2.2. Pharmacokinetics of TNG
Several formulations of TNG are available either as ointment or for transdermal application. RECTIV (nitroglycerine) ointment 0.4%, contains 4 mg nitroglycerine/1g ointment, and is constituted with propylene glycol, lanolin, sorbitan sesquioleate, paraffin wax, and white petrolatum. Nitro-Bid® for topical use contains lactose and 2% nitroglycerine in a base of lanolin, white petrolatum and purified water. Each 2.5 cm squeezed from the tube, contains approximately 15 mg of nitroglycerine. The nitroglycerine patch on the other hand provides continuous, controlled release of nitroglycerine that is linearly dependent upon the area of skin application. Each cm2 of application delivers approximately 0.026 mg of nitroglycerine per hour. Therefore, the 4 cm2, 8 cm2, 16 cm2 and 24 cm2 applications deliver approximately 0.1 mg, 0.2 mg, 0.4 mg, and 0.6 mg of nitroglycerine per hour, respectively and about 11% of nitroglycerine is delivered over 12 h. (Daily Med; US National Library of Medicine). It is likely that the rate and extent of nitroglycerine absorption from ointment will vary with the site and area of the skin application, but these relationships have not been well-studied.
The onset of action of both the patch and the ointment form of nitroglycerine is within 30 min (Imoto et al., 1986). To avoid nitroglycerine tolerance in adults, drug-free intervals of 10–12 h are known to be sufficient, but shorter intervals have not been evaluated nor have they been investigated in the neonatal and pediatric population. Reliable assay techniques for plasma nitroglycerine levels have become recently available, but studies using these techniques to define the pharmacokinetics of nitroglycerine ointment have not been reported. However, the data are consistent and show that nitroglycerine levels rise to steady state within 1–2 h of application of the ointment or patch, and that after removal of nitroglycerine ointment, levels wane with a half-life of about 30 min. Exposure to air quickly reduces the potency of both the nitroglycerine patch or ointment and tubes of ointment should be immediately resealed following use (Drugs.com).
Nitroglycerine is 1,2,3-propanetriol trinitrate, an organic nitrate that acts as a vasodilator on both arteries and veins. The first metabolic products of nitroglycerine are inorganic nitrate and the 1,2- and 1,3-dinitroglycerols. The dinitrates are weak vasodilators compared to nitroglycerine, and are further metabolized to mononitrates, glycerol, and carbon dioxide. They have a longer plasma half-life but their contribution to the overall effect of chronic nitroglycerine remains undetermined (Thomas, 2009). The volume of distribution of nitroglycerine is about 3 L/kg, and it is rapidly cleared by the liver, red blood cells and vascular walls at a rate of 1L/kg/min, with a resulting serum half-life of about three minutes (Kleydman et al., 2012).
2.3. Statistical analysis
Data were analyzed using descriptive statistics of frequencies for categorical data and mean as measures of central tendency and dispersion, respectively, for continuous data. The data was not subjected to a more detailed analysis since the treatment strategies and outcomes were described by time periods relative to the use of TNG.
3. Results
Six hundred and eighty-nine journal articles met inclusion criteria for the systematic review. After reviewing the abstracts, and excluding the duplicated articles, a total of 27 eligible case reports and case series comprising 76 subjects were analyzed. One systematic review was identified, and the data were assessed but not included (Sushko et al., 2021; Fig. 1, Table1).
Fig. 1.
Flow diagram of articles selected for ischemia in neonates treated with topical nitroglycerin.
Table 1.
Neonatal peripheral ischemia: cause, location and course of treatment with topical nitroglycerine (n = 76).
| Author/Year | GA (weeks) Weight (g) |
Cause of ischemia | Location of ischemia | Time† (hours) |
2% TNG form, dose | Treatment course |
|---|---|---|---|---|---|---|
| O’Reilly et al., 1988 | ND | Extravasation | Dorsum of the foot | 2.5 | Patches, 25 mg | One dose |
| Denkler and Cohen, 1989 | 34 (1814) | Extravasation | Left hand to low forearm, left foot to knee | Hand: 9.5 Foot: 4 |
Ointment, 13.8 mm/kg | Two doses |
| Wong et al., 1992 | 24 (7 8 0) | PAC | Right hand | 3 | Ointment, 4 mm/kg | 12 h |
| 25 (7 0 0) | PAC | Right hand | 2 | Ointment, 4 mm/kg | 3 h | |
| 26 (6 7 0) | Extravasation | Anterior forearm, and the medial aspect of the arm | 0.5 | Ointment, 4 mm/kg | 24 h | |
| 31 (1565) | Extravasation | Arm, left upper-lateral area of the chest around the anterior axillary | 1.5 | Ointment, 4 mm/kg | 2 h | |
| Varughese, 2001 | 33 (1870) | UAC | Right hip gluteal region | 4 | Patches, 0.4 mg | 30 h |
| Baserga et al., 2002 | 23 (6 6 0) | PAC | Left hand fingers | 1 | Ointment, 4 mm/kg | 30 min |
| 25 (ND) | UAC | Right leg | 1.5 | Ointment, 4 mm/kg | 45 min | |
| 30 (1620) | UAC | Left leg | 1 | Ointment, 4 mm/kg | 30–45 min | |
| Vasquez et al., 2003 | 26 (8 9 6) | PAC | Left hand mid-palm to fingertips | 7 | Ointment, 4 mm/kg | 27 days |
| Maffei et al., 2006 | 29 (8 9 5) | CVC | Left arm | 0.5 | Patches 11 mm/kg |
7 days |
| 32 (2250) | PAC | Right hand | 1.1 | Patches 4 mm/kg |
4 days | |
| Ancora et al., 2006 | 28 (8 8 0) | UVC | Fingers of the right hand and of the feet | 7 | Patches/ Ointment | 5/ 11 days |
| Mousavi et al., 2010 | 32 (1650) | UVC | Right leg and foot | ND | Ointment, 4 mm/kg |
ND |
| Akingbola et al., 2012 | 31 (3600) | PAC | Left lower limb, foot | ND | Ointment, 4 mm/kg | 21 days |
| Kamar et al., 2013 | 25 (5 0 0) | 2 PAC | Left hand, foot | 24 | Spray | 7 days |
| Mosalli et al., 2013 | 25 (5 2 0) | PAC | Middle, ring and index fingers of right hand | 8 | Ointment, >4 mm/kg | 21 days |
| Samiee-Zafarghandy et al., 2014 | 26 (ND) | PVC | Toes, sole, dorsum and ankle of the left foot | 6 days | Ointment, 4 mm/kg | 14 days |
| Teo and Shah, 2015 | 32 (9 7 0) | PAC | Dorsum of the hand | 24 | Patches | 7 days |
| Dilli et al., 2015 | 28 (1000) | UAC | Bilateral lower limb | ND | Ointment, 4 mm/kg | 15 days |
| Parra et al., 2016 | 32 (ND) | Thrombin injection PSA | Right arm | ND | ND | ND |
| ‡Yong et al., 2016 | 28–31 (ND) | UAC | ND | ND | Patch | ND |
| Vivar del Hoyo et al., 2016 | 24 (ND) | UVC, UAC | Left three toes | ND | Ointment, 4 mm/kg | 5–18 days |
| 35 (ND) | PICC, PVC | Fingers | ND | Ointment, 4 mm/kg | 5–18 days | |
| ND§ | PICC, PVC | Fingers | ND | Ointment, 4 mm/kg | 5–18 days | |
| ND§ | PICC, PVC | Fingers | ND | Ointment, 4 mm/kg | 5–18 days | |
| Han et al., 2017 | 24 (7 6 0) | PAC | Five fingers and palm of left hand | 72 | 0.2% glyceryl trinitrate ointment | 29 days |
| Isık et al., 2017 | 34 (2190) | PVC + phenytoin | Right hand | ND | ND | ND |
| Kuttysankaran et al., (2018) | ND§ (3150) | Femoral artery | Left leg | ND | Cream ND | ND |
| Mintoft et al., 2018 | 24 (6 5 0) | PAC | Lower limbs, the thumb and forefinger of the hand | ND | Patches, 18.7 | 2 days |
| 24 (6 1 7) | PAC | Toes on the right foot | Immediate | Patches, 18.7 | 5 days | |
| Weerasekera et al., 2019 | 26 (8 8 0) | PAC | Fingertips of small, middle, ring and index fingers | ND | Ointment, 4 mm/kg | 14 days |
| Zarkesh, 2020 | 37 (2775) | PVC | 4 Fingers | Minutes | Ointment | One hour |
| *Kim et al., 2020 | ≤ 29 - ≥33 (<1000 - >1800) | UAC, UVC, PICC, CVC | ND | 0.03–10.5 days | Patch | 0.03–10.5 days |
| Lavie-Nevo et al., 2020 | 26 (5 6 0) | PAC | Left arm | Several hours | 2% ointment | 5 days |
| Mosalli, 2021 | 25 (7 7 5) | PVC | Right hand | ND | Spray 0.4 mg | 28 days |
†Time of TNG application after ischemic changes appeared.
Term infant but gestation in weeks not reported.
‡Five cases that were all treated with a patch with resolution in only one infant.
*Thirty-six cases were reported in this series. The mean ± standard deviation of TNG application in those with (n = 2) and without necrosis (n = 34) was 10.50 ± 14.85 and 0.03 ± 0.17 days respectively.
CVC; Central venous catheter, GA; gestational age, ND; Not Documented, PA; pseudoaneurysm, PAC; Peripheral arterial catheter, PICC; Peripherally inserted venous catheter, PVC; Peripheral venous catheter, UAC; Umbilical arterial catheter, UVC; Umbilical venous catheter.
4. Discussion
Neonates, especially those of extremely low gestational age, have an immature hemostatic system, vulnerable and narrow blood vessel diameter and overall are at higher-risk for adverse outcomes including ischæmic complications such as tissue necrosis and gangrene. (Elboraee et al., 2018, Dilli et al., 2015, Beall et al., 2013, Vasquez et al., 2003).
Compared with term infants, preterm infants have higher rates of iatrogenic skin injury (57% versus 3%) respectively (Sardesai et al., 2011). Damage to the blood vessels after arterial or venous catheterization triggers platelet attachment to subendothelial collagen, and induces vasospasm via the production of adenosine diphosphate and thromboxane A2 (Wong et al., 1992). This may manifest clinically as blanched, mottled skin, peripheral cyanosis, weak pulses, and consequently, permanent tissue necrosis if urgent intervention is not instituted (Sadat et al., 2015, Arshad and McCarthy, 2009). The conventional management of peripheral ischemia caused by arterial lines consists of urgent catheter removal, elevation of the affected limb, and induction of reflex vasodilation by applying heat to the contralateral limb (Dilli et al., 2015, Arshad and McCarthy, 2009). In most cases, these combined strategies fall short of improvement and further measures need to be implemented. In several reports, thrombolysis, anticoagulation, and topical hyaluronidase ointment were utilized with limited success and serious attendant complications, such as increased risk of micro embolism and intraventricular hemorrhage (IVH), particularly in preterm neonates (Gault, 1992).
Although limited to case reports and case series, there is growing evidence that TNG may be a promising therapeutic option for peripheral ischemia in infants (Kim et al., 2020, Elboraee et al., 2018, Arshad and McCarthy, 2009, Denkler and Cohen, 1989). To date, there are 27 published case reports and case series involving 76 infants about the use of TNG in the management of tissue ischemia in the neonatal population (Table1). Majority of the cases were preterm neonates (16 of them were extremely preterm with a gestational age less than 28 weeks and 30 were ≤ 29 wGA
Table 2.
Demographic characteristics of infants.
| Descriptor | Neonates, n | Median (range) | |
|---|---|---|---|
| Gender | |||
|
29 | ||
|
24 | ||
| Gestational age, weeks | 26 (23–40) | ||
| Birth weight, g | 1281 (500–3600) | ||
| 5-minute Apgar score | 6 (4–9) | ||
| Delivery | |||
|
48 | ||
|
11 | ||
| Doppler examination | 14 | ||
| *Age at admission, days | 2 (1–60) | ||
| Age at diagnosis, days |
|
*One child was admitted for treatment at 7 weeks and was not included in the range.
Note: Includes information only from studies where data were available.
Table 3.
Outcomes and side effects of topical nitroglycerine in extremely preterm, very preterm, late preterm and term infants.
| First author (year) | Weight (g) | GA (weeks) | Resolution | Side effects |
|---|---|---|---|---|
| Extremely preterm infants | ||||
| (Wong et al., 1992) | 780 | 24 | Partial | ↓BP ↓HR |
| 700 | 25 | Complete | None | |
| 670 | 26 | Complete | ↓BP ↑HR | |
| (Baserga et al., 2002) | 660 | 23 | Complete | ↓BP |
| ND | 25 | Complete | None | |
| (Vasquez et al., 2003) | 896 | 26 | Complete | None |
| (Kamar et al., 2013) | 500 | 25 | Partial | None |
| (Mosalli et al., 2013) | 520 | 25 | Complete | None |
| (Samiee-Zafarghandy et al., 2014) | ND | 26 | Partial | None |
| (Vivar del Hoyo et al., 2016) | ND | 24 | Complete | None |
| (Yong et al., 2016) | ND | <28 (n = 4) | ¶ND | ND |
| (Han et al., 2017) | 760 | 24 | Partial | None |
| (Mintoft et al., 2018) | 650 | 24 | Complete | ↑ MetHb |
| 617 | 24 | Partial | ↑ MetHb | |
| (Weerasekera et al., 2019) | 880 | 26 | Complete | None |
| (Mosalli, 2021) | 775 | 25 | Complete | None |
| (Lavie-Nevo et al., 2020) 560 26 Dry gangrene ND (Kim et al., 2020) < 1000 (n = 22) ≤ 29 (n = 30) ‡Complete (n = 34) ↓BP (n = 4) Very preterm infants | ||||
| (Wong et al., 1992) | 1565 | 31 | Complete | None |
| (Baserga et al., 2002) | 1620 | 30 | Complete | None |
| (Maffei et al., 2006) | 895 | 29 | Complete | None |
| (Ancora et al., 2006) | 880 | 28 | Partial | None |
| (Akingbola et al., 2012) | 3600 | 31 | Partial | None |
| (Dilli et al., 2015) | 1000 | 28 | Complete | Grade 3 ICH |
| (Yong et al., 2016) | ND | 28–31 | ¶ND | ND |
| (Kim et al., 2020) 1000–1800 (n = 13) 30–32 (n = 6) ND ND Late preterm and term infants | ||||
| (O’Reilly et al., 1988) | ND | ND | Complete | None |
| (Denkler and Cohen, 1989) | 1814 | 34 | Complete | None |
| (Varughese, 2001) | 1870 | 33 | Complete | ↓BP |
| (Maffei et al., 2006) | 2250 | 32 | Complete | None |
| (Mousavi et al., 2010) | 1650 | 32 | Death | None |
| (Teo and Shah, 2015) | 970 | 32 | Complete | None |
| (Parra et al., 2016) | ND | 32 | Complete | None |
| (Vivar del Hoyo et al., 2016) | ND | 35 | Complete | None |
| ND | ND§ | Complete | None | |
| ND | ND§ | Complete | None | |
| (Isık et al., 2017) | 2190 | 34 | Reduced arm movement + muscle atrophy | None |
| (Zarkesh, 2020) | 2775 | 37 | Complete | |
§: Term infant but exact gestation unknown; ‡ Outcomes and side effects pertain to the total of 36 extremely preterm and very preterm infants combined in the study; ¶Among the 5 infants reported in this series, 1 had complete recovery and 4 had no response but the reports relative to gestational age were not described.
B.P; Blood pressure, ICH; Intracranial hemorrhage, HR; Heart rate, MetHb: Methemoglobinemia, ND; Not documented.
4.1. Etiology of peripheral vascular ischemia in neonates
Umbilical arterial and venous catheterization is routinely performed in neonates to gain easy vascular access. Arterial catheters are utilized to continuously assess blood pressure, determine respiratory status through blood gas measurements, collect blood samples for laboratory tests, and rarely to administer medications (Sardesai et al., 2011). The radial, ulnar, dorsalis pedis and posterior tibial arteries are the most frequent sites for peripheral arterial catheterization (Mosalli et al., 2013) when umbilical artery catheterization fails. In our review among reports where a direct cause and effect relationship was evident, peripheral arterial catheterization was identified as the source of regional tissue ischemia in 14 infants, followed by peripheral venous catheterization (n = 4) and umbilical arterial catheterization in 4 cases (Table 4). (Dilli et al., 2015) reported a rare incidence of a broken umbilical arterial catheter followed by bilateral lower ischemic extremities. There was one report of ischemic changes after insertion of a brachial artery catheter (Zarkesh, 2020). (Ancora et al., 2006) described an infant who developed necrotic manifestations on the fingers of the right hand and the toes of both feet, after umbilical venous catheterization.
Table 4.
Outcomes relative to the etiology of ischemia and formulations of topical nitroglycerine§.
| Etiology of Ischemia | Topical nitroglycerine effectiveness |
|
|---|---|---|
| Complete recovery n (%) |
Partial no recovery n (%) |
|
| Extravasation, n = 4 | 4(1 0 0) | – |
| Peripheral catheterization, n = 18 | 10 (56) | 6(33) 2(11) |
| Peripheral arterial catheter, n = 14 | 8 (57) | 5(36) 1(7) |
| Peripheral venous catheter, n = 4 | 2(50) | 1(25) 1(25) |
| Umbilical catheterization n = 6 | 4(67) | 1(17) 1(17) |
| Umbilical arterial catheter, n = 4 | 4(1 0 0) | – |
| Umbilical venous catheter, n = 2 | – | 1(50) 1(50) |
| Central venous catheterization n = 1 | 1(1 0 0) | – |
| Peripherally inserted venous catheter and peripheral venous catheter, n = 3 | 3(1 0 0) | – |
| Umbilical arterial catheter and umbilical venous catheter, n = 1 | 1(1 0 0) | – |
| *Formulation (n = 76) | ||
| Topical nitroglycerine ointment/cream, n = 23 | 17 (74) | 4 (17) 2 (9) |
| Topical nitroglycerine patch, n = 48 | 41(85) | 2 (4) 5 (11) |
| Topical nitroglycerine spray, n = 2 | 1(50) | 1(50) - |
| Topical nitroglycerine ointment and patch, n = 1 | – | 1(1 0 0) - |
*In 2 cases the type of nitroglycerine employed was not stated; 1 had complete recovery and 1 had reduced right arm movement with residual muscle atrophy.
Note:§Data only include unique cases where the cause-effect relationship was clearly described
Central and peripheral venous catheter insertion are equally important procedures in neonates to facilitate diagnostic tests and provide supportive treatment. In two case reports, ischemic changes over the upper extremities occurred in four infants related to a central venous catheter. (Maffei et al., 2006) described an axillary artery injury leading to arm ischemia after central venous catheter insertion. (Vivar del Hoyo et al., 2016) reported diminished blood flow to the fingers, following a peripherally inserted central catheter and peripheral venous catheter in three infants. Lavie-Nevo et al., 2020 documented an absent radial pulse in the left arm after infusing 10% dextrose into a peripheral artery and a demarcation line of ischemia occurred after 5 days.
An additional complication of the catheterization procedure in the NICU is the extravasation of intravenous fluids or dopamine. Nearly 3.8% of infants in NICUS in the United Kingdom developed extravasation, that led to tissue necrosis, most notably in extremely preterm neonates (Sardesai et al., 2011, Wilkins and Emmerson, 2004). While some effects of dopamine result from stimulation of dopamine receptors, the prominent cardiovascular effects result from dopamine acting at α1, β1, and β2 adrenergic receptors following vasoconstriction. Accordingly, dopamine extravasation leads to local vasospasm and tissue ischemia (Denkler and Cohen, 1989), that takes 48–72 h after the extravasation to manifest (Beall et al., 2013). Three cases of dopamine extravasation in preterm neonates were reported.
4.2. TNG regimen
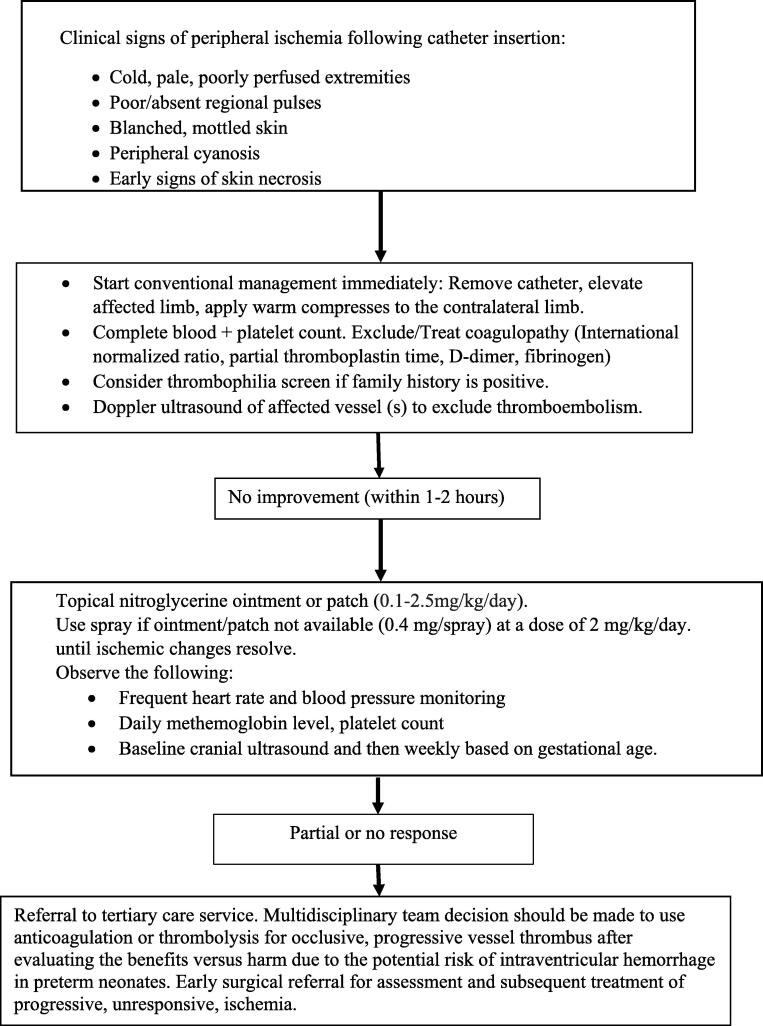
An approach to the use of TNG is outlined in Fig. 2. The enhancement of blood flow after applying TNG to the affected area is speculated to be through the direct effect of nitroglycerine on smooth muscles and the generation of arterial collaterals around the affected area (Dilli et al., 2015, Sardesai et al., 2011, Varughese, 2001). In premature neonates, skin maturation progresses up to four weeks postnatally (Dyer, 2013). The effectiveness of TNG in premature neonates is likely due to the exclusive permeability and fragility of the epidermal skin layer in the first two weeks of life (Weerasekera et al., 2019).
Fig. 2.
An approach to the treatment of peripheral ischemia in infants with topical nitroglycerine.
4.3. TNG dosage
The systemic nitroglycerine level depends on the dose of TNG, and the extent of the skin surface area covered by the treatment modality. The initial dose of 2% TNG was determined to be 4 mm/kg, which is equivalent to the intravenous dose (0.2 to 0.5 ug/kg per minute) to prevent systemic vasodilatation (Baserga et al., 2002, Dilli et al., 2015). An ointment is also more applicable in non-contiguous areas such as joints (Generali and Cada, 2001). The most common dose of ointment employed in 16 subjects was 4 mm/kg (Elboraee et al., 2018, Han et al., 2017, Samiee-Zafarghandy et al., 2014, Akingbola et al., 2012, Sardesai et al., 2011, Mousavi et al., 2010, Vasquez et al., 2003, Baserga et al., 2002, O’Reilly et al., 1988). The physiological effect commenced within one hour and lasted for approximately six hours (Dilli et al., 2015, Baserga et al., 2002). The mean time of the initiation of TNG in all the cases where reported was 26.2 h (range: within minutes – 252 h).
4.4. TNG formulations and duration of use
Twenty-three patients received TNG ointment or cream (Table 4). A total of 48 patients had TNG in the form of patches (Kim et al., 2020, Mintoft et al., 2018, Yong et al., 2016, Teo and Shah, 2015, Mousavi et al., 2010); Maffei et al., 2006, Varughese, 2001, O’Reilly et al., 1988; Table 4). Two patients received TNG spray (Mosalli, 2021, Kamar et al., 2013). (Mosalli, 2021) used nitroglycerine spray at 0.4 mg /dose (total of 2 mg/kg /day) for 28 days while (Kamar et al., 2013) employed a 2% TNG spray every 8 h for 7 days (cumulative dose not reported; Table 1, Table 4). TNG patches and ointment were employed in a single case and they were deemed to be similar in efficacy (Ancora et al., 2006).
There was no consensus on the appropriate duration of TNG use and corresponding outcomes as it ranged from a single dose (O’Reilly et al., 1988) to 29 days (Han et al., 2017) based on a multiple dose regimen of TNG ointment only. (Baserga et al., 2002), used TNG for less than 50 min with rapid recovery and resolution of the ischemia. In the report by (Wong et al., 1992) patients recovered within hours. Resolution was observed in approximately one week in three case reports (Teo and Shah, 2015, Kamar et al., 2013, Maffei et al., 2006). Fifteen patients were treated for longer than one week (Mosalli, 2021, Kim et al., 2020, Weerasekera et al., 2019, Han et al., 2017, Vivar del Hoyo et al., 2016, Dilli et al., 2015, Samiee-Zafarghandy et al., 2014, Mosalli et al., 2013, Akingbola et al., 2012, Ancora et al., 2006, Vasquez et al., 2003). (Kamar et al., 2013) recommended that treatment should be individualized based on the severity of ischemic involvement and the efficacy of nitroglycerine, and therapy continued until ischemic changes are clinically resolved.
4.5. Outcomes with the use of TNG
Of the reported cases among extremely preterm, very preterm, and late preterm/term infants, 62.5% (10/16), 66.7% (4/6) and 83% (10/12) respectively, had full resolution of the ischemic tissue injuries after applying TNG (Table 3). The use of TNG patches versus ointment and spray was associated with 85%, 74% and 50% complete skin resolution, respectively. (Table 4).
There were eleven cases with incomplete resolution of ischemia following the use of TNG in various scenarios (Lavie-Nevo et al., 2020, Kim et al., 2020, Mintoft et al., 2018, Han et al., 2017, Isık et al., 2017, Samiee-Zafarghandy et al., 2014, Kamar et al., 2013, Akingbola et al., 2012, Ancora et al., 2006, Wong et al., 1992), 4 cases with no response (Yong et al., 2016) and two deaths (Kim et al., 2020, Mousavi et al., 2010). The residual lesions involved arm necrosis (Lavie-Nevo et al., 2020), peripheral necrosis of the hand (Wong et al., 1992) and digits (Kim et al., 2020, Mintoft et al., 2018, Han et al., 2017, Samiee-Zafarghandy et al., 2014, Kamar et al., 2013, Ancora et al., 2006) and muscle atrophy (Isık et al., 2017). Despite improvement in generalized blood flow to the respective areas with the use of TNG ointment, spray, patches, or a combination thereof, persistent and/or permanent necrosis of finger tips or toes (Kim et al., 2020, Han et al., 2017, Kamar et al., 2013, Akingbola et al., 2012), loss of digital tips (Mintoft et al., 2018), and complete digits or distal phalanges (Samiee-Zafarghandy et al., 2014, Ancora et al., 2006) and an autoamputaion (Lavie-Nevo et al., 2020) occurred. The latter serious sequelae may be variably ascribed to delayed TNG treatment (Lavie-Nevo et al., 2020, Kim et al., 2020, Han et al., 2017, Samiee-Zafarghandy et al., 2014, Kamar et al., 2013, Ancora et al., 2006).
4.6. Outcomes with early TNG treatment (within 4 h of injury)
Fifteen patients who received nitroglycerine early had better outcomes as demonstrated in the reports (Zarkesh, 2020, Weerasekera et al., 2019, Mintoft et al., 2018, Han et al., 2017, Maffei et al., 2006, Baserga et al., 2002, Varughese, 2001, Wong et al., 1992, Denkler and Cohen, 1989, O’Reilly et al., 1988). The mean time of the early initiation of TNG application where documented was 1.8 h (range: within minutes – 4 h).
(Zarkesh, 2020) reported that after brachial artery catheterization, full recovery of 4 ischemic fingers occurred within one hour of applying TNG ointment to the affected area and warm compresses to the contralateral upper extremity. (Weerasekera et al., 2019) documented recovery of right-hand digital ischemia and associated motor function following TNG application within 3 h of an arterial-related injury. Two premature infants of 24 wGA were treated immediately with TNG patches by (Mintoft et al., 2018) for radial and posterior tibial arterial ischemia. One infant completely recovered while the other experienced sloughing of the tips of the toes of the right foot which did not require amputation. (Han et al., 2017) employed 0.2% TNG and humidification to treat ischemic injuries of the left fingers and thumb, 12 h post radial artery catheterization. Following treatment, residual dry gangrene of a fingertip and a compromised fingertip tendon was evident. (Maffei et al., 2006) treated two infants with TNG patches (Triniplas 5 mg/7cm2) within 30–70 min after left axillary and radial artery ischemia occurred. In both cases arterial pulses were restored with complete recovery of the peripheral digital circulation. (Baserga et al., 2002) reported on three cases that were treated with TNG ointment within 90 min of the ischemic insult after conventional management had failed. Recovery in all patients occurred over a period of 30–45 min. The preterm neonate in the article by (Varughese, 2001) had ischemia in the right hip and gluteal region after an umbilical artery placement. TNG patches were applied within 4 h of the insult and the ischemic changes resolved in 30 h. At discharge, the neonate had completely recovered. (Wong et al., 1992) documented the use of (4 mm/kg) ointment in four neonates. Two of them had ischemia caused by radial artery catheterization, and the other two patients had ischemia resulting from dopamine extravasation in the right hand, forearm, chest, and axilla. Perfusion returned within hours. (Denkler and Cohen, 1989) employed two doses of 2% TNG ointment (13.8 mm/kg) to manage extravasation with remarkable improvement in the ischemic sites after a few minutes. (O’Reilly et al., 1988) commenced nitroglycerine within 2.5 h of the ischemic injury. The glyceryl trinitrate 25 mg patch (5 mg TNG release per 24 h) was applied for one hour to treat extravasation and resulted in complete improvement with resolution of perfusion.
4.7. Outcomes following delayed TNG treatment (after 4 h of injury)
There was a delay of more than 4 h in the initiation of TNG in nine case reports and one case series (Lavie-Nevo et al., 2020, Kim et al., 2020, Teo and Shah, 2015, Samiee-Zafarghandy et al., 2014, Mosalli et al., 2013, Kamar et al., 2013, Ancora et al., 2006, Vasquez et al., 2003, Denkler and Cohen, 1989). The mean time of the delay in the initiation of TNG application where reported was 36.9 h (range: 7–252 h).
After inadvertent brachial artery cannulation, Lavie-Nevo et al., 2020 applied 2% TNG for 5 days to the affected left arm several hours following the ischemic injury, that resulted in necrosis, dry gangrene and subsequent autoamputation. Thirty-six infants were treated with nitroglycerine patches by Kim et al., 2020, seven or more hours post peripheral arterial-related ischemia. Two preterm infants (6%) developed necrosis-one involving tips of the fingers, while the other died. The overall mortality in this study of peripheral arterial ischemia was remarkably high (n = 14; 41%). Risk factors that were positively associated with ischemia in the univariate logistic regression analysis were patent ductus arteriosus and prolonged use of inotropes, ventilation, and umbilical arterial catheters that increased the risk 1.8–7.5-fold each extra day of the respective intervention. Lavie-Nevo et al., 2020 applied TNG ointment several hours after the ischemic event which resulted in dry gangrene and autoamputation of the of the left forearm. (Teo and Shah, 2015) utilized anticoagulation for 12 h for digital ischemia that occurred after the insertion of a right radial arterial catheter. A glyceryl trinitrate patch was then applied to the cubital fossa with gradual improvement, but with persistent index finger-tip necrosis, 13 days later. Doppler ultrasonography confirmed return of the distal arterial flow in the hand without any side effects. (Samiee-Zafarghandy et al., 2014) reported on an extremely premature infant with peripheral ischemia secondary to severe vascular spasm. The patient had serious comorbidities including intraventricular hemorrhage and an intracutaneous hematoma, that resulted in a delay in treatment for six days. TNG (4 mm/kg) ointment, was applied every six hours for two weeks. Partial improvement occurred and nitroglycerine was discontinued but restarted within two hours due to the recurrence of ischemia. This resulted in permanent loss of the second and third toes of the left foot. (Mosalli et al., 2013) reported an extremely preterm infant born at 25 wGA with peripheral ischemic changes over the fingers of the right hand following brachial artery catheterization. TNG was applied four hours after the appearance of the ischemic changes for seven days. By the end of the treatment course, there was full resolution of the ischemic manifestations. Of note, the infant had comorbidities of metabolic acidosis and renal failure prior to the ischemic event. (Kamar et al., 2013) used a 2% nitroglycerine spray to ensure equal spread across two ischemic injuries in the left hand and foot, in an extremely premature infant (25 wGA; birth weight, 500 g). The spray was utilized every eight hours for one week. Follow up assessment showed suboptimal effect and partial improvement with permanent tissue necrosis of the fourth finger of the hand and the third toe of the foot. (Ancora et al., 2006) treated a preterm neonate born at 28 wGA, who had an ischemic insult due to an umbilical venous catheter placement. Treatment was delayed because TNG ointment was unavailable. Patches were applied every six hours for five days followed by ointment application until the eleventh day of life. Seven days after TNG was discontinued, necrosis appeared at the distal phalanges of one finger and one toe that was managed by amputation. Necrosis also occurred in the nail beds of the right foot. The patient had other co-morbidities such as necrotizing enterocolitis. Six months follow up demonstrated normal motor function. (Vasquez et al., 2003) initiated TNG treatment at 7 h of age for peripheral digital ischemia in a premature infant of 26 wGA. Therapy continued for 27 days and completely resolution of necrotic fingertips was documented at 8 months of age. Similarly, (Denkler and Cohen, 1989) effectively treated a 34 wGA preterm infant at 4 and 9.5 h of age with TNG ointment for dopamine induced ischemia in the left leg and hand, respectively. Complete recovery in both limbs was reported.
4.8. Adjunct therapy with TNG
In most cases, further adjunctive management was required but no standard guideline exists on when to commence additional pharmacotherapy in the management of vascular induced ischemic injury. The use of anticoagulation and thrombolysis as adjunctive therapy was reported by six authors. (Akingbola et al., 2012) described the use of high dose tissue plasminogen activator (tPA), fresh frozen plasma and 2% TNG, to manage life-threatening ischemic complications after femoral artery catheterization in a two-month-old infant. Complete recovery resulted except for a small area of dry gangrene. (Dilli et al., 2015) managed an infant with fresh frozen plasma, heparin infusion, and tPA, without marked improvement. Therefore, intravenous nitroglycerine infusion and 2% TNG were started. Three days later, a cranial ultrasound revealed grade 3 IVH. Accordingly, intravenous nitroglycerine and tPA were withdrawn, heparin was replaced by low molecular weight heparin, and TNG discontinued on day 15 after full recovery of the ischemia. Neurological and motor function was normal at three months of age. There was uncertainty if the IVH was a side effect of the nitroglycerine or the other medications. Heparin alone or with thrombin (Parra et al., 2016) or tPA (Yong et al., 2016) was used with TNG resulting in complete recovery in two cases, residual phalangeal necrosis (n = 2; Yong et al., 2016) and muscle atrophy (n = 1; Isık et al., 2017). Ischemia associated with peripheral artery catheterization, resulted in full recovery without side effects, after using nitroglycerine patches for one week combined with unfractionated heparin (Teo and Shah, 2015). The TNG dose and frequency of patch applications were not specified. A multidisciplinary decision to use anticoagulation or thrombolysis should be made only after evaluating the benefits versus harm, due to the potential risk of IVH in preterm neonates.
(Han et al., 2017) were the only investigators who utilized 100% local humidification (through an LG moistened, H-716CP humidifier) along with 0.2% glyceryl trinitrate ointment to treat a premature newborn with five ischemic fingers following peripheral arterial catherization. The affected area was checked every eight hours throughout the 29 days of treatment to detect infections and high temperature burns. Humidification played two roles. First, the high temperature (37.5 °C) induced vasodilation of the affected area. Second, the area remained consistently moisturized which may have facilitated the proliferation of keratin cells and the growth of fibroblasts that accelerate epithelial formation, resulting in a significantly smaller wound scar. No side effects were observed, and the infant fully recovered except for dry gangrene of the fourth fingertip, which was removed.
4.8.1. Adverse events with TNG
Approximately one-quarter of the cases (12/76; 16%) developed side effects consistent with the description by (Bogaert, 1987) such as a decrease in the mean arterial blood pressure (7/76; 9%, [Sardesai et al., 2011, Baserga et al., 2002, Varughese, 2001, Wong et al., 1992]), and methemoglobinemia (2/76; 3%, [Mintoft et al., 2018]).
One of the cases that developed a mild transient decrease in blood pressure, had spontaneous resolution within thirty minutes (Sardesai et al., 2011). In the case report by (Varughese, 2001), the decrease in blood pressure was managed with dopamine infusion. The infant reported by (Wong et al., 1992) had co-morbidities and was hemodynamically unstable prior to the use of TNG. His hypotension was managed with an adjustment of dopamine infusion. Blood pressure reduction and changes in heart rate are well-known side effects of nitroglycerine caused by the vasodilatory action of nitric oxide (van Reempts and Van Overmeire, 1990, Chu et al., 1984). Other side effects documented were tachycardia and bradycardia which did not require any medical intervention (Wong et al., 1992).
Methemoglobinemia is a rare side effect first reported after the application of TNG patches in two preterm neonates for 2–5 days (Mintoft et al., 2018). This resulted in severe hypoxia as methemoglobin has a high affinity for oxygen, which was treated by oxygen supplementation and removal of the patches. The exact threshold that results in methemoglobinemia remains unclear, since one extremely premature neonate utilized ten patches during TNG treatment.
Despite an in-depth review, our study has several limitations that merit consideration. First, the data were assembled from case reports and case series with inherent heterogeneity that cannot be accounted for statistically. Second, based on the case design, the published reports may be fraught with publication bias that favor positive outcomes. However, our review also encompassed subjects that incurred a broad spectrum of adverse events and serious sequelae. Third, although all the cases were correctly ascertained, alternative causation eliminated, and adequate description and follow-up provided, a therapeutic TNG challenge-rechallenge was not performed except by (Akingbola et al., 2012), nor a dose-response effect determined in any of the studies. We attempted to partly address this issue in our review by exploring a potential cut-off time (greater or less than 4 h) where TNG may have a more beneficial effect. Fourth, the quality of evidence derived from TNG cases is low because reporting is subject to imprecision, lack of standardization of the inception cohort, indirectness due to plausible confounding from the use of adjunct therapy and the inability to infer a direct cause and effect relationship between TNG and resolution of ischemia (Balshem et al., 2011).
5. Conclusion
In comparison to systemic heparinization and other invasive interventions, TNG may be a promising alternative therapeutic modality that appears to be relatively safe in the management of peripheral ischemia in neonates secondary to vascular cannulation. The advantage lies in the fact that TNG can be applied locally with limited systemic effects, which makes it a favorable choice. Overall, the early application of TNG resulted in significant and rapid improvement, particularly in premature neonates where surgical intervention may prove difficult. However, current evidence for the generalized use of TNG as a recommendation for all cases of peripheral ischemia in neonates is weak, since the data are based solely on case reports. The optimal dose, time, place of application relative to the affected site and treatment duration to elicit complete, safe recovery without adverse effects, varies and remains unclear in the absence of robust, prospective, studies compared to other available treatment modalities. In general, side effects appear minor but close surveillance of blood pressure, methemoglobin levels, together with follow-up cranial ultrasounds are important in the care of extremely premature infants. Planning the management of peripheral ischemia in neonates should be multidisciplinary, weighing the benefit and adverse events associated with individual therapies. TNG is a valid treatment option for peripheral ischemia in very preterm neonates as an alternative strategy when surgical intervention is not possible, but further concrete evidence of its benefit in this regard is awaited.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Rafat Mosalli: Data curation, Writing - original draft. Wed Khayyat: Data curation, Writing - original draft. Sarah Al Qarni: Data curation, Writing - original draft. Amirah Al Matrafi: Data curation, Writing - original draft. Mohamed El Baz: Data curation, Writing - original draft. Bosco Paes: Data curation, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akingbola O., Singh D., Steiner R., Frieberg E., Petrescu M. High-dose tissue plasminogen activator, topical nitroglycerin, and heparin for severe ischemic injury in a neonate. Clin. Pediatr. (Phila) 2012;51:1095–1098. doi: 10.1177/0009922811423312. [DOI] [PubMed] [Google Scholar]
- Ancora G., Soffritti S., Faldella G. Diffuse and severe ischemic injury of the extremities: a complication of umbilical vein catheterization. Am. J. Perinatol. 2006;23:341–344. doi: 10.1055/S-2006-946721. [DOI] [PubMed] [Google Scholar]
- Arshad, A., McCarthy, M.J., 2009. Management of Limb Ischaemia in the Neonate and Infant. Eur. J. Vasc. Endovasc. Surg. https://doi.org/10.1016/j.ejvs.2009.03.010. [DOI] [PubMed]
- Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., Guyatt G.H. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Baserga M.C., Puri A., Sola A. The use of topical nitroglycerin ointment to treat peripheral tissue ischemia secondary to arterial line complications in neonates. J. Perinatol. 2002;22:416–419. doi: 10.1038/sj.jp.7210713. [DOI] [PubMed] [Google Scholar]
- Beall V., Hall B., Mulholland J.T., Gephart S.M. Neonatal extravasation: An overview and algorithm for evidence-based treatment. Newborn Infant Nurs. Rev. 2013;13:189–195. doi: 10.1053/j.nainr.2013.09.001. [DOI] [Google Scholar]
- Bogaert M.G. Clinical pharmacokinetics of glyceryl trinitrate following the use of systemic and topical preparations. Clin. Pharmacokinet. 1987;12:1–11. doi: 10.2165/00003088-198712010-00001. [DOI] [PubMed] [Google Scholar]
- Chu L.C., Gale R.M., Schmitt L.G., Shaw J.E. Nitroglycerin concentration in plasma: Comparison between transdermal therapeutic system and ointment. Angiology. 1984;35:545–552. doi: 10.1177/000331978403500901. [DOI] [PubMed] [Google Scholar]
- Daily Med; US National Library of Medicine. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e98a2692-1cb9-4994-98d8-1d8d9ef1a256#. Accessed April 14th, 2021.
- Denkler K.A., Cohen B.E. Reversal of dopamine extravasation injury with topical nitroglycerin ointment. Plast. Reconstr. Surg. 1989;84:811–813. doi: 10.1097/00006534-198911000-00017. [DOI] [PubMed] [Google Scholar]
- Dilli D., Özyazici E., Fettah N., Kaya O., Pala Akdoan M., Zenciroğlu A., Okumuş N., Güzoğlu N. Rupture and displacement of umbilical catheter: bilateral arterial occlusion in in a very low birth weight preterm. Arch. Argent. Pediatr. 2015;113:e283–e285. doi: 10.5546/aap.2015.eng.e283. [DOI] [PubMed] [Google Scholar]
- Drugs.com. Available at: https://www.drugs.com/pro/nitro-bid.html#s-34090-1. Accessed April 15th, 2021.
- Dyer, J.A., 2013. Newborn skin care. Semin. Perinatol. https://doi.org/10.1053/j.semperi.2012.11.008. [DOI] [PubMed]
- Elboraee M.S., Toye J., Ye X.Y., Shah P.S., Aziz K. Association between umbilical catheters and neonatal outcomes in extremely preterm infants. Am. J. Perinatol. 2018;35:233–241. doi: 10.1055/s-0037-1606607. [DOI] [PubMed] [Google Scholar]
- Gault D.T. Vascular compromise in newborn infants. Arch Dis Child. 1992;67(4 Spec No):463–467. doi: 10.1136/adc.67.4_spec_no.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generali J., Cada D.J. Nitroglycerin (Topical): Extravasation treatment. Hospital pharmacy. 2001;36:1091–1095. [Google Scholar]
- Han Y.S., Song S., Sung T.J., Chun J. Successful management of severe peripheral tissue ischemia after arterial catheterization in micro preemies using humidification & topical nitroglycerin. Neonatal Med. 2017;24:197–201. doi: 10.5385/nm.2017.24.4.197. [DOI] [Google Scholar]
- Imoto S., Kuramoto M., Iwabuch K., Nagal H., Shimpo K. Percutaneous chronic toxicity study of 10% nitroglycerin (NT-1 ointment) in rabbits. J Toxicol Sci. 1986;11(Suppl 2):59–70. doi: 10.2131/jts.11.supplementii_31. [DOI] [PubMed] [Google Scholar]
- Isık, D.U., Demirel, N., Erol, S., Ünal, S., Ahmet Yağmur Başet, A.Y., 2017. A rare complication of phenytoin infusion in newborn: purple glove syndrome. Balkan Med J. 34, 584-585. doi: 10.4274/balkanmedj.2017.0480. [DOI] [PMC free article] [PubMed]
- Kamar R., van Vonderen J.J., Lopriore E., Te Pas A.B. Nitroglycerin for severe ischaemic injury after peripheral arterial line in a preterm infant. Acta Paediatr. 2013;102:e144–e145. doi: 10.1111/apa.12141. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee J.W., Kim D.Y. Analysis of characteristics of peripheral arterial ischemia in premature babies and effects of nitroglycerin patch application. Child Health Nurs Res. 2020;26:434–444. doi: 10.4094/chnr.2020.26.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleydman K., Cohen J.L., Marmur E. Nitroglycerin: a review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg. 2012;38:1889–1897. doi: 10.1111/dsu.12001. [DOI] [PubMed] [Google Scholar]
- Kuttysankaran, R., Ramaiah, S., Popescu, O., 2018. Spontaneous femoral artery thrombosis in a newborn infant: case report. Perinatologia. 1, 22-24. doi: 10.26416/Peri.2.1.2018.1649.
- Lavie-Nevo, K., Molad, M., Eidelman, M., 2020. Ischaemic limb injury and autoamputation complicating peripheral cannula insertion. Arch Dis Child Fetal Neonatal Ed.fetalneonatal-2020-318899. doi: 10.1136/archdischild-2020-318899. [DOI] [PubMed]
- Maffei G., Rinaldi M., Rinaldi G. Resolution of peripheral tissue ischemia secondary to arterial vasospasm following treatment with a topical nitroglycerin device in two newborns: case reports. J. Perinat. Med. 2006;34:252. doi: 10.1515/jpm.2006.047. [DOI] [PubMed] [Google Scholar]
- Mintoft A., Williams E., Harris C., Kennea N., Greenough A. Methemoglobinemia during the use of glyceryl trinitrate patches in neonates: Two case reports. AJP Rep. 2018;8:e227–e229. doi: 10.1055/s-0038-1669945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosalli R., Elbaz M., Paes B. Topical nitroglycerine for neonatal arterial associated peripheral ischemia following cannulation: A case report and comprehensive literature review. Case Rep. Pediatr. 2013;1–7 doi: 10.1155/2013/608516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosalli, R., 2021. Successful use of nitroglycerine spray to treat a neonate with ischemic injury. Pediatr. Int. Mar 25. PMID: 33764568. doi: 10.1111/ped. 14530. [DOI] [PubMed]
- O’Reilly C., Mckay F.M.A., Duffty P., Lloyd D.J. Glyceryl trinitrate in skin necrosis caused by extravasation of parenteral nutrition. Lancet. 1988;2:565–566. doi: 10.1016/S0140-6736(88)92682-7. [DOI] [PubMed] [Google Scholar]
- Mousavi S.A., Sahebpour A.A., Shahmohammadi S. Neonatal gangrene of the extremity: a complication of umbilical catheterization. Pak. J Med Sci. 2010;26:488–489. www.pjms.com.pk. [Google Scholar]
- Parra, D., Zuker, R., Connolly, B., 2016. Brachial pseudoaneurysm of the neonate with partial response to thrombin injections and late spontaneous thrombosis and regression during expectant management. BJR. Case Rep. 2:20150383. doi: 10.1259/bjrcr.20150383. [DOI] [PMC free article] [PubMed]
- Sadat U., Hayes P.D., Varty K. Acute limb ischemia in pediatric population secondary to peripheral vascular cannulation: Literature review and recommendations. Vasc. Endovascular Surg. 2015;49:142–147. doi: 10.1177/1538574415604059. [DOI] [PubMed] [Google Scholar]
- Samiee-Zafarghandy S., Van Den Anker J.N., Ben Fadel N. Topical nitroglycerin in neonates with tissue injury: A case report and review of the literature. Paediatr. Child Health. 2014;19:9–12. doi: 10.1093/pch/19.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai S.R., Kornacka M.K., Walas W., Ramanathan R. Iatrogenic skin injury in the neonatal intensive care unit. J. Matern Fetal Neonatal Med. 2011;24:197–203. doi: 10.3109/14767051003728245. [DOI] [PubMed] [Google Scholar]
- Sushko K., Litalien C., Ferruccio L., Gilpin A., Mazer-Amirshahi M., Chan A.K., van den Anker J., Lacaze-Masmonteil T., Samiee-Zafarghandy S. Topical nitroglycerin ointment as salvage therapy for peripheral tissue ischemia in newborns: a systematic review. CMAJ. Open. 2021;9:E252–E260. doi: 10.9778/cmajo.20200129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo, M.C., Shah, V.A., 2015. Digital ischaemia following inadvertent arterial cannulation of a peripherally inserted central catheter in a very low birth weight infant. Singapore Med. J. 56, 482–483. https://doi.org/10.11622/smedj.2015125. [DOI] [PMC free article] [PubMed]
- Thomas, P.D.R., 2009. Physicians’ Desk Reference (63rd ed). Montvale, NJ. 205, 217, 299, 2966.
- van Reempts P., Van Overmeire B. Topical use of nitroglycerin ointment in neonates. J. Pediatr. 1990;116:155. doi: 10.1016/s0022-3476(05)81671-3. [DOI] [PubMed] [Google Scholar]
- Varughese, M., 2001. Successful use of topical nitroglycerine in ischaemia associated with umbilical arterial line in a neonate. J. Perinatol. 556–558. https://doi: 10.1038/sj.jp.7210567. [DOI] [PubMed]
- Vasquez P., Burd A., Mehta R., Hiatt M., Hegyi T. Resolution of peripheral artery catheter-induced ischemic injury following prolonged treatment with topical nitroglycerin ointment in a newborn: A case report. J. Perinatol. 2003;23:348–350. doi: 10.1038/sj.jp.7210870. [DOI] [PubMed] [Google Scholar]
- Vivar del Hoyo, P., Sánchez Ruiz, P., Ludeña del Río, M., López-Menchero Oliva, J.C., García Cabezas, M.Á., 2016. Use of topical nitroglycerin in newborns with ischaemic injuries after vascular cannulation. An. Pediatría (Barc). 85, 155–156. https://doi:10.1016/j.anpedi.2016.01.024. [DOI] [PubMed]
- Weerasekera M., Lakmini C., Imbulana N.S., Hettiarachchi K.R., Sampath G.U. Topical nitroglycerine in management of peripheral ischaemia in a neonate following arterial cannulation. Sri Lanka J. Child Health. 2019;48:263–265. doi: 10.4038/sljch.v48i3.8765. [DOI] [Google Scholar]
- Wilkins C.E., Emmerson A.J.B. Extravasation injuries on regional neonatal units. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89 doi: 10.1136/adc.2003.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.F., McCulloch L.M., Sola A. Treatment of peripheral tissue ischemia with topical nitroglycerin ointment in neonates. J. Pediatr. 1992;121:980–983. doi: 10.1016/S0022-3476(05)80356-7. [DOI] [PubMed] [Google Scholar]
- Yong J., Rajan C., Molnar Z., Adams E., Hall G., Qureshi A., Bhatnagar N. Single UK tertiary neonatal unit experiences of neonatal arterial thrombosis [abstract] Br J Haematol. 2016;173(Suppl 1):5–178. [Google Scholar]
- Zarkesh, M.R., 2020. Successful use of topical nitroglycerine and warm compress for peripheral ischemia following arterial blood sampling in a neonate. World J. Adv. Res. Rev. 7, 206–209. https://doi.org/10.30574/wjarr.2020.7.2.0296.