Abstract
Background
There has been a rise in the incidence of injection drug use and associated infective endocarditis.
Methods
The clinical outcomes of 39 patients admitted with injection drug use–associated infective endocarditis were collected with a mean follow-up of 14 months. The outcomes were compared for patients treated medically with those undergoing surgical intervention. Results: The mean age was 39 ± 11 years; 54% were female. Thirty-two patients (82%) had native and 7 (18%) prosthetic infective endocarditis. The tricuspid valve was affected in 17 patients (43%), the mitral in 10 (26%), the aortic in 4 (10%), and multiple valves in 8 (20%). Sixteen (41%) patients underwent surgery, and 23 (59%) were treated with medical therapy. The indications for surgery included heart failure, systemic emboli, recurrent infection, and vegetation size ≥10 mm. Patients undergoing surgery had a higher rate of paravalvular abscess (25% vs 0%, P = 0.02), valve perforation (37% vs 11%, P = 0.04), and mitral valve involvement (44% vs 13%, P = 0.06), whereas medically treated patients had higher tricuspid valve involvement (61% vs 19%, P = 0.02). During follow-up, 26% of medical and 31% of surgical cohort patients died (P = 0.7). Mortality was highest (54%) among those who continued medical management despite an indication for surgery. Univariate predictors of mortality were age (odds ratio [OR] 1.09, 95% confidence interval [CI]: 1.01-1.17; P = 0.02), heart failure (OR 6.9; 95% CI: 1.24-37.49; P = 0.02), septicemia (OR 4.40; 95% CI:0.99-19.54; P = 0.05), and shock (OR 10.8; 95% CI: 1.68-69.92; P = 0.01).
Conclusions
Despite contemporary therapy, patients with injection drug use–associated infective endocarditis remain at high risk of complications and poor clinical outcomes. These findings highlight the need for developing new care pathways and a team approach for effective management.
Résumé
Introduction
Il y a eu une augmentation de l’incidence de l’endocardite infectieuse associée à l’usage de drogues par injection.
Méthodes
Nous avons recueilli au cours d’un suivi moyen de 14 mois les résultats cliniques de 39 patients admis en raison d’une endocardite infectieuse associée à l’usage de drogues par injection. Les résultats ont été comparés pour les patients traités médicalement avec ceux subissant une intervention chirurgicale.
Résultats
L’âge moyen était de 39 ± 11 ans; 54 % étaient des femmes. Trente-deux patients (82 %) avaient une endocardite infectieuse sur valve native et 7 (18 %), une endocardite infectieuse sur prothèse valvulaire. La valve tricuspide était touchée chez 17 patients (43 %), la valve mitrale, chez 10 patients (26 %), la valve aortique, chez 4 patients (10 %), et plusieurs valves, chez 8 patients (20 %). Seize (41 %) patients ont subi une intervention chirurgicale, et 23 (59 %) ont reçu un traitement médical. Les indications d’intervention chirurgicale étaient les suivantes : l’insuffisance cardiaque, les embolies systémiques, l’infection récurrente et la taille de la végétation ≥ 10 mm. Les patients qui avaient subi une intervention chirurgicale ont plus fréquemment eu des abcès paravalvulaires (25 % vs 0 %, P = 0,02), des perforations de valves (37 % vs 11 %, P = 0,04) et des atteintes de la valve mitrale (44 % vs 13 %, P = 0,06), tandis que les patients qui avaient reçu un traitement médical ont plus fréquemment eu des atteintes de la valve tricuspide (61 % vs 19 %, P = 0,02). Durant le suivi, 26 % des patients de la cohorte du traitement médical et 31 % des patients de la cohorte de l’intervention chirurgicale sont morts (P = 0,7). La mortalité était plus élevée (54 %) chez les patients qui poursuivaient la prise en charge médicale malgré l’indication chirurgicale. Les prédicteurs univariés de la mortalité étaient l’âge (rapport de cotes [RC] 1,09, intervalle de confiance [IC] à 95 % : 1,01-1,17; P = 0,02), l’insuffisance cardiaque (RC 6,9; IC à 95 % : 1,24-37,49; P = 0,02), la septicémie (RC 4,40; IC à 95 % : 0,99-19,54; P = 0,05) et le choc (RC 10,8; IC à 95 % : 1,68-69,92; P = 0,01).
Conclusions
En dépit de l’approche thérapeutique contemporaine, les patients atteints d’une endocardite infectieuse associée à l’usage de drogues par injection restent exposés à un risque élevé de complications et de mauvais résultats cliniques. Ces résultats illustrent la nécessité d’élaborer de nouveaux cheminements cliniques et une approche du travail en équipe pour assurer une prise en charge efficace.
Infective endocarditis (IE) is a serious medical diagnosis associated with both high short-term mortality and poor survival during long-term follow-up.1, 2, 3 Among the factors contributing to this high complication rate include patient age, comorbidities including presence of injection drug use (IDU), affected cardiac valves, and microbiological profile of the causative organisms. Injection drug users are particularly prone to IE due to the high risk of blood contamination with bacteria and other disease-causing organisms from the skin surface, nonsterile devices, and chemicals from repeated use of injections. Although the tricuspid valve is the most commonly affected, multi-valvular involvement is often observed in IDU patients with Staphylococcus aureus and Pseudomonas as common causative microbes.4
Injection drug use (IDU) with heroin, cocaine, and amphetamines is an ongoing and growing challenge in many industrialized nations. Patients who inject drugs are typically young but have a unique set of comorbidities including hepatitis, liver disease secondary to alcohol abuse, and psychiatric illness5 The average age of IDU-affected individuals is 18 years,6,7 and the median survival time is less than 4 years.8,9 Systemic infection is a common and serious complication of IDU, affecting 60%-80% of IDU practitioners over their lifetime.10 In addition, the risk of infective endocarditis (IE) secondary to IDU (IDU-IE) is estimated at 1%-6% per year, accounting for approximately 5%-10% of overall mortality in this population.11 However, these numbers are likely underestimated due to a lack of long-term follow-up and inadequate outcome data for this population.5 Recent data also suggest an increased incidence of IE that is temporally related to increased opioid use.12 IDU-IE represents a life-threatening diagnosis, and associated morbidity and mortality represent a major societal and economic burden among an otherwise young patient population. There are few publications that have analyzed data for patients with IDU,13,14 and it remains paramount to better understand the role of contemporary management on short- and long-term clinical outcomes in this patient population. Here, we present our institutional experience of medical and surgical management of patients with IDU-IE over an 8-year period.
Methods
Study population
This study was conducted from January 2008 to June 2016 at St Michael’s Hospital, Toronto, Canada, a tertiary care inner-city hospital. The initial data collection for IE case selection was done from an existing administrative database of the hospital. International Classification of Disease (ICD-10) diagnostic codes were used to identify IE. The ICD codes used to identify patients with IE for initial chart review included 133 (acute and subacute IE), 138 (IE valve unspecified), and 139 (endocarditis and heart valve disorder in disease classified elsewhere). Clinical, demographic, operative, and outcome data were retrospectively collected for all patients diagnosed with IDU-IE based on Modified Duke’s criteria.15,16 Medical and surgical management data were extracted for the duration and type of treatment, indication and timing of surgery, and in-hospital and postoperative complications. Follow-up data including vital status, re-hospitalization, and surgical intervention information were collected by reviewing hospital and primary care provider medical records for all patients. In-hospital mortality was defined by death during index hospitalization, and midterm mortality was recorded after discharge from hospital until the end date of study follow-up. IDU-IE was defined as a clinical diagnosis of endocarditis among patients with a history of active injection drug use at the time of presentation. Leukocytosis was defined as an elevated white blood cell count greater than 11,000 per mm3 (11.0 × 109 per L).17 Polymicrobial infection was defined by IE caused by 2 or more causative microorganisms confirmed on at least 2 separate blood cultures. Septicemia was defined as life-threatening organ dysfunction caused by IE.18 This study was approved by the institutional research ethics board, and individual consents were waived.
Statistical analysis
Statistical analyses were performed using STATA version 14.1 (Stata Corp LP, College Station, TX). Univariate analyses were performed with the χ2and Fisher exact test for categorical variables, Student t test for normally distributed continuous variables, and the Mann-Whitney test for continuous variables with nonparametric distribution. Data are presented as mean ± standard deviation (SD). Univariate analysis was performed for each variable to analyze independent influence on survival using the Mantel-Cox regression model expressed with odds ratio (OR), and 95% confidence intervals (CIs). Survival rates were calculated using the Kaplan-Meier method with a log-rank test for comparison. We conducted landmark analyses19 of IDU-IE patients who survived for 3 months postdischarge to account for immortal time bias with regard to medical and surgical treatment cohorts. All statistical tests were 2-tailed, and statistical significance was defined as a P value ≤0.05.
Results
Patient characteristics
Between January 2008 and June 2016, a total of 39 cases were hospitalized with a diagnosis of IDU-IE (Fig. 1). The demographics and baseline characteristics of the study patients are outlined in Table 1. The mean age was 38.6 ± 11.4 years (range: 21-65 years); 21 patients (54%) were female. Prior history of IE was present in 12 patients (31%), and 7 patients (18%) had a diagnosis of prosthetic valve IE. Clinical characteristics associated with IE included congenital cardiac malformation in 1 patient (2.5%), and presence of permanent pacemaker in 1 patient (2.5%). Serological testing was positive for hepatitis B in 4 patients (10%), for hepatitis C in 26 patients (67%), and for human immunodeficiency virus in 4 patients (10%).
Figure 1.
The yearly number of injection drug use–associated infective endocarditis cases admitted from January 2008 to June 2016.
Table 1.
Patient characteristics
| Characteristic | Total (n = 39) | Medical cohort (n = 23) | Surgical cohort (n = 16) | P |
|---|---|---|---|---|
| Age, y | 38.6 ± 11 | 38.4 ± 12 | 39.2 ± 12 | 0.8 |
| Female | 21 (52) | 12 (52) | 9 (56) | 0.8 |
| Diabetes | 2 (5) | 1 (4) | 1 (6) | 1.0 |
| Hypertension | 4 (10) | 2 (9) | 2 (12) | 1.0 |
| Dyslipidemia | 2 (5) | 1 (4) | 1 (6) | 1.0 |
| Smoking | 10 (26) | 3 (13) | 7 (44) | 0.06 |
| Alcohol abuse | 3 (8) | 1 (4) | 2 (12) | 0.5 |
| Previous IE | 12 (31) | 6 (26) | 6 (37) | 1.0 |
| Heart failure | 5 (13) | 2 (9) | 3 (19) | 0.6 |
| Stroke/TIA | 5 (13) | 1 (4) | 4 (25) | 0.1 |
| Pacemaker | 1 (2) | 0 | 1 (6) | 1.0 |
| Renal insufficiency | 4 (10) | 2 (9) | 2 (12) | 1.0 |
| COPD | 3 (8) | 3 (13) | 0 | 0.2 |
| Chronic liver disease | 2 (5) | 1 (4) | 1 (6) | 1.0 |
| Fungal infection | 3 (8) | 2 (9) | 1 (6) | 1.0 |
| Infectious history | ||||
| Hepatitis B | 4 (10) | 4 (17) | 0 | 0.1 |
| Hepatitis C | 26 (67) | 16 (70) | 10 (62) | 0.6 |
| HIV | 4 (10) | 2 (9) | 2 (12) | 1.0 |
Values are n (%) or mean ± standard deviation, unless otherwise indicated.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular accident; DM, diabetes mellitus; DVT, deep vein thrombosis; HIV, human immunodeficiency virus; HT, hypertension; IE, infective endocarditis; TIA, transit ischemic attack.
Clinical presentation
Clinical presentation of the IDU-IE (Supplemental Table S1) included fever (>38 oC) in 33 patients (85%) and cardiac murmur in 8 patients (20.5%). Examination revealed vascular phenomena, including petechia, splinter hemorrhages, and Janeway lesions in, respectively, 2 (5%), 2 (5%), and 1 (2.5%) patient. Splenomegaly was present in 3 (8%) patients. Leukocytosis was present in 6 patients (15%). Systemic complications of IDU-IE included systemic embolization in 14 (36%), a cerebrovascular event in 5 (13%), mycotic aneurysm in 5 (13%), and organ abscess in 8 (20.5%) patients. Eight patients developed congestive heart failure (20.5%), 12 (31%) suffered from septicemia with evolution to shock in 4 patients (10%) and to cardiogenic shock in 1 patient (2.6%).
Cardiac valve involvement
Cardiac valve involvement included mitral valve in 10 (26%), aortic in 4 (10%), tricuspid in 17 (43.5%), and multivalve infection in 8 patients (20.5%) (Supplemental Table S2). Echocardiography demonstrated moderate-to-severe mitral regurgitation in 9 patients (23%), aortic insufficiency in 6 (15%), and tricuspid regurgitation in 20 (51%). Other relevant findings included paravalvular leak in 1 patient (2.5%) and paravalvular abscess in 4 patients (10%; Supplemental Table S3).
Microbiological profile
Infective agents were isolated in 38 (97%) patients. S. aureus was identified as the causative agent in 29 patients (74%); 5 (17%) were methicillin-resistant, and 24 (83%) were methicillin-sensitive strains. Non–S. aureus agents were isolated in 13 (34%) patients (coagulase-negative Staphylococcus, n = 2; Streptococcus, n = 5; Pseudomonas, n = 3; HACEK organisms, n = 2; Enterococcus, n = 1) and fungal infection in 3 patients. Polymicrobial infections were documented in 7 (18%) patients.
Clinical management
All patients received antibiotic therapy during the index admission extended for 4 weeks in 6 (15%), 6 weeks in 24 (61.5%), and >6 weeks in 5 (13%) patients. Fourteen patients (38.5%) were managed with a single antimicrobial agent, whereas dual antibiotics were used in 7 patients. Eight patients had 3 antibiotics, and a combination of 4 or more antibiotics were given in 10 patients (25.6%). Medical therapy in patients and sensitivity to the antibiotics are shown in Supplemental Table S4.
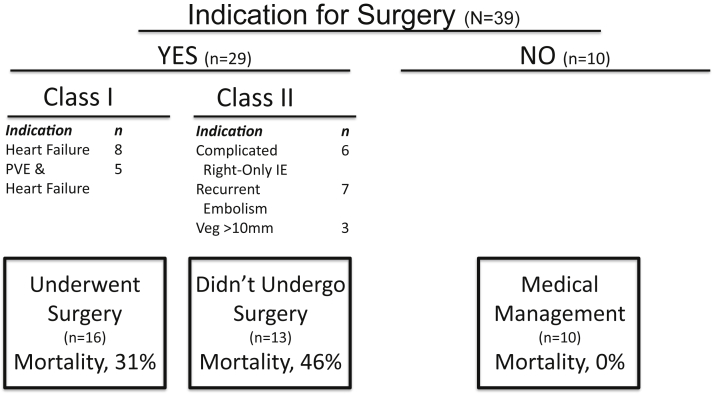
Seventy-four percent of patients (29 of 39) had a surgical indication, of which 55% (16 of 29) underwent surgery, and 45% (13/29) were treated medically (Fig. 2). Patients undergoing surgery were more likely to have paravalvular abscess (25% vs 0%, P = 0.02), valve perforation (36.5% vs 11%, P = 0.04), and a trend toward mitral valve involvement (44% vs 17%, P = 0.06). The decision to treat patients medically despite presence of a surgical indication was related to prohibitive surgical risk (heart team consensus) in 7 patients, history of ongoing IDU in 2 patients, and treatment refusal in 4 patients. All patients were seen by the inpatient psychiatric service and post-discharge follow-up was recommended. The average length of hospital stay for the overall cohort was 24 days (range: 5-114 days). Nineteen patients were transferred to another hospital or rehabilitation facility after index hospitalization, whereas 14 were discharged home.
Figure 2.
Indications of surgery and mortality for injection drug use–associated infective endocarditis patients. IE, infective endocarditis; PVE, prosthetic valve IE; Veg, vegetation.
Cardiac surgery in IDU-IE
With respect to timing of surgery, 13 patients (81%) had surgery during the index hospitalization (9 patients within 2 weeks and 4 patients after 2 weeks), and 3 patients had late surgery following failure of medical management. The mean time for early surgery during index hospitalization was 19 days (range: 2-114 days). Surgical interventions included mitral valve in 5 patients, tricuspid valve in 3, and multivalve surgery in 4. reoperative surgery was performed in 4 patients (25%; Supplemental Table S5). All patients undergoing surgical intervention underwent pre and/or intraoperative transesophageal echocardiography examination.
Clinical outcomes
A total of 6 (15%) patients died during the index hospitalization. These deaths were related to sepsis/multi-organ failure (n = 3), cardiac decompensation (n = 2), and cerebral hemorrhage (n = 1). Of 29 patients with clinical indications for surgery, mortality was 54% for patients who continued medical management despite an indication for surgery and 25% for those who underwent surgical intervention. No patients died among the cohort treated with medical management and without an indication for surgery.
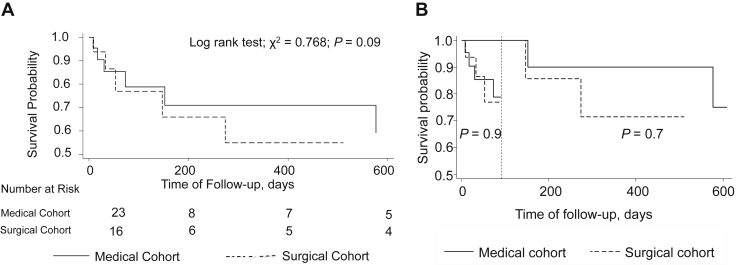
At a mean follow-up of 14 months, 11 of 39 patients (28%) had died. There was no difference in mortality between the surgically treated (31%) and medically treated (26%) patients for the follow-up period (P = 0.09; Fig. 3A). When analysis was limited to patients who survived the 3 months post discharge, again no significant difference in mortality was observed between the surgical and medical management cohorts (P = 0.7; Fig. 3B). A total of 8 patients (2 from the initial surgical cohort, 6 from the medical cohort) experienced recurrent IE during the follow-up period. Five were treated medically, and surgery was performed in 3 patients, including 2 re-do surgical procedures.
Figure 3.
(A) Posthospital discharge survival of medically and surgically managed injection drug use–associated infective endocarditis patients. (B) Short-term and midterm landmark survival analysis between medically and surgically managed injection drug use–associated infective endocarditis patients.
Univariate predictors of mortality are age (OR 1.09, 95% CI: 1.01-1.17; P = 0.02), congestive heart failure (OR 6.9, 95% CI: 1.24-37.49; P = 0.02), septicemia (OR 4.40, 95% CI: 0.99-19.54; P = 0.05), and septic shock (OR 10.8, 95% CI: 1.68-69.92; P = 0.01); these are shown in Table 2. Major complications of IE secondary to IDU did not differ between the medical and surgical cohorts (Supplemental Table S6).
Table 2.
Univariate regression analysis of predictors of in-hospital and midterm mortality in infective endocarditis secondary to injection drug use
| Characteristic | Univariate (logistic regression) |
||
|---|---|---|---|
| Odds ratio | 95% CI | P | |
| Age | 1.09 | 1.01, 1.17 | 0.02 |
| Gender | 0.63 | 0.15, 2.54 | 0.51 |
| Fungal infection | 6.00 | 0.48, 74.29 | 0.16 |
| S. aureus infection | 0.26 | 0.06, 1.21 | 0.08 |
| Persistent infection | 1.12 | 0.23, 5.45 | 0.88 |
| PVE | 4.76 | 0.86, 26.48 | 0.07 |
| Vegetation ≥10 mm (1.0 cm) | 1.04 | 0.26, 4.22 | 0.96 |
| Stroke/TIA | 2.70 | 0.15, 47.39 | 0.49 |
| Mycotic aneurysm | 4.87 | 0.69, 34.50 | 0.11 |
| Heart failure | 6.94 | 1.29, 37.49 | 0.02 |
| Valvular perforation | 3.43 | 0.68, 17.35 | 0.13 |
| Valvular abscess | 2.89 | 0.35, 23.63 | 0.32 |
| Cardiac arrest | 4.87 | 0.69, 34.50 | 0.11 |
| Prolonged ventilation | 6.00 | 0.48, 74.29 | 0.16 |
| Systemic emboli | 1.04 | 0.26, 4.22 | 0.96 |
| Systemic abscess | 1.73 | 0.33, 8.91 | 0.51 |
| Septicemia | 4.40 | 0.99, 19.54 | 0.05 |
| Shock | 10.83 | 1.68, 69.92 | 0.01 |
| Pneumonia | 0.60 | 0.06, 6.06 | 0.66 |
| Respiratory failure | 1.30 | 0.11, 15.98 | 0.83 |
| Acute kidney injury | 1.30 | 0.11, 15.98 | 0.83 |
| Dialysis | 1.00 | 1.00, 1.00 | - |
| Bleeding/transfusion | 2.25 | 0.41, 12.28 | 0.34 |
CI, confidence interval; PVE, prosthetic valve infective endocarditis; S. aureus, Staphylococcus aureus; TIA, transient ischemic attack.
Discussion
In the present study, we describe the clinical profile, management, and outcome of 39 patients with IDU-IE. The findings show the following: (i) patients with ISU-IE were generally young, with few chronic illnesses; (ii) 40% of the IDU-IE cohort underwent surgical management; (iii) the primary indications for surgery were heart failure, systemic emboli, recurrent infection, and vegetation ≥10 mm; (iv) patients with tricuspid valve involvement only were more likely to be treated non-surgically; (v) the highest mortality occurred in patients with a surgical indication who were treated medically; (vi) age, heart failure, sepsis, and septic shock were independent risk factors for mortality.
The IDU-IE patient population differs from the general IE population—they are younger, with fewer chronic illnesses but a greater prevalence of infectious diseases (eg, hepatitis B and C, human immunodeficiency virus), immunocompromised states, and psychiatric illness. Although we did not formally document social history, it is well recognized that such patients have limited social supports and often inadequate primary health care.4,20 The complexity of these patients is evidenced by the prolonged length of hospital stay and the fact that many required transfer to another hospital or rehabilitation facility to facilitate recovery. Consistent with other series, we note that there is a higher prevalence of S. aureus and polymicrobial infections.10,21, 22, 23, 24 The latter explains the need for multiple antibiotic agents in a significant proportion of cases. We showed a nonsignificant trend of higher mortality with S. aureus infection, comparable with other series.9,11,25 In keeping with previously published studies, the most common valvular involvement was tricuspid, followed mitral and aortic.10,14,24,26, 27, 28, 29 Although, the exact reason the tricuspid valve is most commonly affected is not known, it is hypothesized that repeated and prolonged exposure of the right-sided valve, via contaminated needles, drugs, or unsterile skin, facilitates microbial growth and IE progression.30 Medical management with antibiotics was the primary management strategy for IE limited to the tricuspid valve. Current evidence suggests that more than 85% of cases of right-sided infection, and uncomplicated cases, can be successfully treated with oral or parenteral antibiotic therapy.13,31,32
Similar to the general recommendations for IE, surgery in the IDU-IE cohort should be reserved for heart failure, sepsis or recurrent infection despite antibiotic therapy, and presence of abscess or large vegetation.33 However, valve surgery in IDU-IE patients requires careful consideration owing to high risk of recidivism, recurrent infection, and challenges for managing oral anticoagulation in patients with drug addiction.34, 35, 36 Where applicable, valve repair is the recommended treatment of choice to minimize introduction of intra-cardiac foreign materials, particularly in the tricuspid position where some degree of regurgitation is usually well tolerated.4 We favor the use of bioprosthetic valves in patients with IDU-IE, to avoid the need for oral anticoagulation and due to the unfortunately poor midterm survival in this patient population.
A major finding of our study was an overall mortality of 28% during follow-up, with no significant difference between the surgical vs nonsurgical cohorts. This percentage is in line with previously published series with mortality ranges of 5% to 40%.11,23,28,37, 38, 39 We also analyzed short-term and long-term survival between the medical and surgical groups through landmark analysis, and our results illustrate no statistically significant difference between the 2 groups. The low midterm survival in IDU-IE patients is likely a result of endocarditis-related complications, such as embolism, sepsis, cardiac decompensation, and respiratory failure, compounded by the complications of illicit drug use.7,40 An important finding is that medical treatment of patients with a surgical indication was associated with a very high mortality. On the other hand, medical treatment of patients without a surgical indication resulted in no midterm deaths, albeit the majority of these patients had isolated and uncomplicated tricuspid valve involvement. These findings highlight the importance of close monitoring of all patients admitted with IDU-IE and an early consideration of surgery for appropriate clinical indications. Whether to proceed with a major cardiac surgery intervention in high-risk patients with multiple comorbidities and high rates of recidivism is often a difficult decision. However, a multidisciplinary approach and a team consensus, as suggested in Figure 4, is the best approach for optimum surgical outcomes for IDU-IE patients.
Figure 4.
Proposed management for injection drug use–associated infective endocarditis patients using an “endocarditis team”–based approach.
Patients with IDU-IE can be conceptualized as having 2 parallel illnesses: infective endocarditis and drug addiction.4 In most cases, ongoing drug addiction or recidivism are the limiting factors for the long-term survival of these patients.6,8,41 and are the main reasons for reluctance for reoperative surgery for recurrent cases of IDU-IE. Therefore, the importance of an initial psychiatric evaluation, intensive addiction therapy in-hospital, and an ongoing drug rehabilitation program after discharge, including treatment of psychiatric illnesses, cannot be overemphasized.4 Any inner-city hospital or institution serving IDU-IE patients can greatly benefit from the establishment of a multidisciplinary “endocarditis team” with input from surgery, cardiology, infectious disease, psychiatry, social work, and other services as necessary to effectively manage these high-risk patients, especially from a drug abuse and addiction standpoint (Fig. 4). Involvement and “buy-in” from the patient, and utilization of a family support system, are also integral for the success of any IDU-IE treatment paradigm.
Limitations
The present study has important limitations. The data are from a single, tertiary care inner-city hospital and findings may not reflect experiences in other suburban and rural centers. This is an observational study based on retrospectively collected data with a relatively small sample size, limiting our ability to reach definitive conclusions on optimal management strategies. We were unable to collect data on either the types of illicit drug(s) used by the patients in our study or the nature and extent of addiction treatment received, social support mechanisms, or the socioeconomic status of the patient, all of which may affect the effectiveness of behavioral modification efforts. These important variables have important implications for the ability of patients to overcome the underlying addictions and undergo surgery, and thus for their overall prognosis.
Conclusions
Patients with IDU-IE were young, had few chronic illnesses, and remained at high risk of complications, including persistent infection, heart failure, sepsis, and death. The tricuspid valve was the most commonly affected valve in IDU-IE, but multivalve involvement was not uncommon. More than two-thirds of patients with IDU-IE developed an indication for surgery, but surgery could be performed in only half of eligible patients. Midterm clinical outcomes were poor for both medical and surgical cohorts but remained particularly unfavorable among those with an indication for surgical intervention treated medically. Advanced age, heart failure, and shock were independently associated with higher mortality. Despite contemporary management, IDU-IE continues to be a major management challenge and is associated with significant morbidity and mortality.
Funding Sources
The authors have no funding to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was approved by the institutional research ethics board, and individual consents were waived.
See page 901 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.02.015.
Supplementary Material
References
- 1.Hoen B., Alla F., Selton-Suty C., et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 2.Mylonakis E., Calderwood S.B. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 3.Cabell C.H., Jollis J.G., Peterson G.E., et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162:90–94. doi: 10.1001/archinte.162.1.90. [DOI] [PubMed] [Google Scholar]
- 4.Yanagawa B., Bahji A., Lamba W., et al. Endocarditis in the setting of IDU: multidisciplinary management. Curr Opin Cardiol. 2018;33:140–147. doi: 10.1097/HCO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 5.Ratnasingham S., Cairney J., Manson H., et al. The burden of mental illness and addiction in Ontario. Can J Psychiatry. 2013;58:529–537. doi: 10.1177/070674371305800908. [DOI] [PubMed] [Google Scholar]
- 6.Smyth B., Fan J., Hser Y.-I. Life expectancy and productivity loss among narcotics addicts thirty-three years after index treatment. J Addictive Dis. 2006;25:37–47. doi: 10.1300/J069v25n04_04. [DOI] [PubMed] [Google Scholar]
- 7.Joe G.W., Simpson D.D. Mortality rates among opioid addicts in a longitudinal study. Am J Public Health. 1987;77:347–348. doi: 10.2105/ajph.77.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth B., Hoffman V., Fan J., Hser Y.-I. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med. 2007;44:369–374. doi: 10.1016/j.ypmed.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabkin D.G., Mokadam N.A., Miller D.W., et al. Long-term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg. 2012;93:51–57. doi: 10.1016/j.athoracsur.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Miro J.M., del Rio A., Mestres C.A. Infective endocarditis in intravenous drug abusers and HIV-1 infected patients. Infect Dis Clin North Am. 2002;16:273–295. doi: 10.1016/s0891-5520(01)00008-3. vii-viii. [DOI] [PubMed] [Google Scholar]
- 11.Miro J.M., del Rio A., Mestres C.A. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin. 2003;21:167–184. doi: 10.1016/s0733-8651(03)00025-0. v-vi. [DOI] [PubMed] [Google Scholar]
- 12.Weir M.A., Slater J., Jandoc R., et al. The risk of infective endocarditis among people who inject drugs: a retrospective, population-based time series analysis. CMAJ. 2019;191:E93–E99. doi: 10.1503/cmaj.180694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shetty N., Nagpal D., Koivu S., Mrkobrada M. Surgical and medical management of isolated tricuspid valve infective endocarditis in intravenous drug users. J Card Surg. 2016;31:83–88. doi: 10.1111/jocs.12682. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Bautista C., Lopez J., Garcia-Granja P.E., et al. Current profile of infective endocarditis in intravenous drug users: the prognostic relevance of the valves involved. Int J Cardiol. 2015;187:472–474. doi: 10.1016/j.ijcard.2015.03.368. [DOI] [PubMed] [Google Scholar]
- 15.Li J.S., Sexton D.J., Mick N., et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin R.J., Lee B.L., Sande M.A., Chambers H.F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2:1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- 17.Riley L.K., Rupert J. Evaluation of patients with leukocytosis. Am Fam Phys. 2015;92:1004–1011. [PubMed] [Google Scholar]
- 18.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park H.S., Gross C.P., Makarov D.V., Yu J.B. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–1373. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Reisberg B.E. Infective endocarditis in the narcotic addict. Prog Cardiovasc Dis. 1979;22:193–204. doi: 10.1016/0033-0620(79)90023-9. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha N.K., Jue J., Hussain S.T., et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100:875–882. doi: 10.1016/j.athoracsur.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Murdoch D.R., Corey G.R., Hoen B., et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew J., Addai T., Anand A., et al. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug users. Arch Intern Med. 1995;155:1641–1648. [PubMed] [Google Scholar]
- 24.Chambers H.F., Korzeniowski O.M., Sande M.A. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983;62:170–177. [PubMed] [Google Scholar]
- 25.Weymann A., Borst T., Popov A.F., et al. Surgical treatment of infective endocarditis in active intravenous drug users: a justified procedure? J Cardiothorac Surg. 2014;9:58. doi: 10.1186/1749-8090-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musci M., Siniawski H., Pasic M., et al. Surgical treatment of right-sided active infective endocarditis with or without involvement of the left heart: 20-year single center experience∗. Eur J Cardio-Thorac Surg. 2007;32:118–125. doi: 10.1016/j.ejcts.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Moss R., Munt B. Injection drug use and right sided endocarditis. Heart. 2003;89:577–581. doi: 10.1136/heart.89.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung H.C., Chen S.C., Chan K.C., et al. Retrospective evaluation of infective endocarditis over ten years in Taiwan. J Heart Valve Dis. 2013;22:248–256. [PubMed] [Google Scholar]
- 29.Chambers H.F., Morris D.L., Täuber M.G., Modin G. Cocaine use and the risk for endocarditis in intravenous drug users. Ann Intern Med. 1987;106:833–836. doi: 10.7326/0003-4819-106-6-833. [DOI] [PubMed] [Google Scholar]
- 30.Jain V., Yang M.H., Kovacicova-Lezcano G. Infective endocarditis in an urban medical center: association of individual drugs with valvular involvement. J Infect. 2008;57:132–138. doi: 10.1016/j.jinf.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Mertz D., Viktorin N., Wolbers M., et al. Appropriateness of antibiotic treatment in intravenous drug users, a retrospective analysis. BMC Infect Dis. 2008;8:42. doi: 10.1186/1471-2334-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinosoglou K., Apostolakis E., Marangos M., Pasvol G. Native valve right sided infective endocarditis. Eur J Intern Med. 2013;24:510–519. doi: 10.1016/j.ejim.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 33.DiMaio J.M., Salerno T.A., Bernstein R., et al. Ethical obligation of surgeons to noncompliant patients: can a surgeon refuse to operate on an intravenous drug-abusing patient with recurrent aortic valve prosthesis infection? Ann Thorac Surg. 2009;88:1–8. doi: 10.1016/j.athoracsur.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 34.Meszaros K., Nujic S., Sodeck G.H., et al. Long-term results after operations for active infective endocarditis in native and prosthetic valves. Ann Thorac Surg. 2012;94:1204–1210. doi: 10.1016/j.athoracsur.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 35.Grubitzsch H., Schaefer A., Melzer C., et al. Outcome after surgery for prosthetic valve endocarditis and the impact of preoperative treatment. J Thorac Cardiovasc Surg. 2014;148:2052–2059. doi: 10.1016/j.jtcvs.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Baddour L.M., Wilson W.R., Bayer A.S., et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 37.Taghavi S., Clark R., Jayarajan S.N., et al. Surgical management of tricuspid valve endocarditis in systemically infected patients. J Heart Valve Dis. 2013;22:578–583. [PubMed] [Google Scholar]
- 38.Leitman M., Dreznik Y., Tyomkin V., et al. Vegetation size in patients with infective endocarditis. Eur Heart J Cardiovasc Imaging. 2012;13:330–338. doi: 10.1093/ejechocard/jer253. [DOI] [PubMed] [Google Scholar]
- 39.Hecht S.R., Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med. 1992;117:560–566. doi: 10.7326/0003-4819-117-7-560. [DOI] [PubMed] [Google Scholar]
- 40.Perucci C.A., Davoli M., Rapiti E., Abeni D.D., Forastiere F. Mortality of intravenous drug users in Rome: a cohort study. Am J Public Health. 1991;81:1307–1310. doi: 10.2105/ajph.81.10.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanagawa B., Pettersson G.B., Habib G., et al. Surgical management of infective endocarditis complicated by embolic stroke: practical recommendations for clinicians. Circulation. 2016;134:1280–1292. doi: 10.1161/CIRCULATIONAHA.116.024156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.