Abstract
Monocytes (Mos) and macrophages (Mφs) are key players in the innate immune system and are critical in coordinating the initiation, expansion, and regression of many autoimmune diseases. In addition, they display immunoregulatory effects that impact inflammation and are essential in tissue repair and regeneration. Juvenile idiopathic arthritis (JIA) is an umbrella term describing inflammatory joint diseases in children. Accumulated evidence suggests a link between Mo and Mφ activation and JIA pathogenesis. Accordingly, topics regarding the signals and mechanisms regulating Mo and Mφ activation leading to pathologies in patients with JIA are of great interest. In this review, we critically summarize recent advances in the understanding of how Mo and Mφ activation is involved in JIA pathogenesis and focus on the signaling pathways and mechanisms participating in the related cell activation processes.
Keywords: juvenile idiopathic arthritis, macrophage, monocyte
1. Introduction: Overview of the Pathogenesis of Juvenile Idiopathic Arthritis
Juvenile idiopathic arthritis (JIA) is the leading chronic rheumatic disease of childhood and occurs in children before their sixteenth birthday with symptoms of articular inflammation for at least 6 weeks [1]. Based on the number of joints involved within the first 6 months of disease onset and extra-articular manifestations, JIA is categorized into seven subtypes according to the International League of Associations for Rheumatology (ILAR) classification [2]. Although different subtypes are heterogeneous in presentation, patients with JIA share a common phenotype of inflamed synovial membranes, which can result in growth arrest, articular deformity, and disability [3].
The etiology and pathogenesis of JIA are still elusive. Joint infiltrating inflammatory cells, residential synovial fibroblasts and osteoclasts, antibodies targeting autoantigens, and various inflammatory mediators are some of the critical players that promote the chronic inflammatory process [3,4,5,6]. In recent years, evidence suggests a distinct pathogenesis of systemic onset juvenile idiopathic arthritis (sJIA) compared to that of the oligoarticular and polyarticular forms of JIA [4]. sJIA is an autoinflammatory disease marked by universal inflammation due to a dysregulated innate immune system [2,7,8]. The abnormal activation of various phagocytes, including monocytes (Mos), macrophages (Mφs), and neutrophils, leads to a massive release of the proinflammatory mediators interleukin (IL)-1, IL-6, IL-18, and S100 proteins in sJIA [4]. On the other hand, genetic variants in human leukocyte antigen genes, the identification of cartilage-derived autoantigens, and the imbalance between regulatory T cells and autoreactive type 1 helper T (Th1)/type 17 helper T (Th17) cells in patients with oligoarticular and polyarticular JIA suggest that the disorders in this group are antigen-driven autoimmune diseases mediated by adaptive immune system disorganization [4]. However, despite the presence of autoantibodies and autoreactive lymphocytes, without the participation of innate immune mediators, the adaptive immune system cannot fully account for the development of many autoimmune diseases [7,8,9]. Indeed, an activated Mφ gene expression signature was detected in cells in synovial fluid (SF) from early-onset oligoarticular JIA patients at risk of extending arthritis joint counts [10]. Moreover, a Mo signature was found in the peripheral blood of patients with older-onset oligoarticular JIA [11] and rheumatoid factor (RF)-positive polyarticular JIA [12].
Mos/Mφs are important players in the innate immune system and are critical in coordinating the initiation, expansion, and resolution of many autoimmune diseases [8]. They possess a wide range of inflammatory, immunomodulatory, and tissue-repairing capacities via their secretion of numerous proinflammatory cytokines, growth factors, and proteolytic enzymes to stimulate and recruit effector cells to inflamed tissues [7,8,9]. A growing number of studies have highlighted the role of Mo/Mφ activation and polarization in JIA pathogenesis. In this review, we critically summarize recent advances in the understanding of how Mo and Mφ activation is involved in JIA pathogenesis and focus on the signaling pathways and mechanisms participating in the related cell activation processes.
2. The Origin of Synovial Resident Macrophages
The origin and ontogeny of synovial Mφs are incompletely characterized. Studies suggest that tissue-resident Mφs may be derived from the yolk sac, fetal liver Mos, or bone marrow [13,14]. While evidence has documented that tissue-resident Mφs derived from embryos can sustain themselves for a long period via local proliferation independent of hematopoietic stem cells [13,14,15], the classical mononuclear phagocytic system suggests that tissue Mφs are the final cells of the mononuclear phagocyte lineage derived from circulating Mos originating from the bone marrow [16]. Under inflammatory conditions or physiological stress, circulating Mos are believed to migrate from the bloodstream to tissues in need and to differentiate into dendritic cells or tissue-resident Mφs [16,17,18]. Potent chemokines, such as monocyte chemoattractant protein-1 (MCP-1)/C-C motif chemokine ligand (CCL)2, regulated upon activation, normal T-cell-expressed and, presumably, secreted (RANTES)/CCL5, and C-X-C motif chemokine ligand (CXCL)9 and CXCL10, were found to be significantly elevated in the SF of patients with JIA, driving the chemotactic activity of mononuclear leukocytes [19,20]. Colony-stimulating factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) is also important in this differentiation process [21,22].
3. Characteristics of Monocytes and Macrophages in Juvenile Idiopathic Arthritis
3.1. Monocyte Subsets
Monocytes are a heterogeneous population of leukocytes circulating in the blood until they are recruited to tissues upon signaling. The expression levels of the surface molecules CD14 and CD16 determine the distinct subpopulations of human Mos [23,24]. Classical Mos are characterized by CD14++CD16− expression and represent 80–90% of the Mos in the bloodstream of healthy individuals [25]. Nonclassical CD14+CD16++ Mos and intermediate CD14++CD16+ Mos account for a much smaller proportion of circulating Mos and can expand considerably under inflammatory conditions [25]. For example, while no significant increase in classical and intermediate Mos has been observed in patients with polyarticular JIA, the frequency of intermediate CD14++CD16+ Mos among both the circulating and synovial Mos is expanded in patients diagnosed with enthesitis-related arthritis (ERA) [26,27]. Recently, Schmidt, Cren and Gaur investigated the distribution of Mo subsets in paired SF and blood samples from patients with oligoarticular JIA and ERA. These authors discovered that while classical CD14++CD16− Mos dominate in the circulation, intermediate CD14++CD16+ Mos were highly enriched in oligoarticular JIA and ERA patient SF [28,29,30]. As CD14++CD16+ synovial Mos can be induced by cytokine-rich SF and are found with similar patterns across Mo subsets, reports have suggested that the increased CD16 expression in these cells may likely result from the cytokine milieu of the synovial space and not the recruitment of intermediate CD14++CD16+ Mos from circulation due to their unique features [28,31]. Specifically, cytokines, such as IL-10 and transforming growth factor beta (TGFβ), in SF are potent inducers of CD16, an activating Fcγ receptor (FcγR), expression in Mos [28,29].
As demonstrated by Macaubas et al., the Mo lineage is expanded in patients with active sJIA [30,32]. While increased levels of CD14 and CD16 were found on sJIA Mos in both the flare and quiescence status, the distribution of the CD14+CD16+ Mo subsets was not altered compared to that in healthy controls [30,32]. Both classical CD14++CD16- Mos and CD14+CD16+ Mos are activated in sJIA [30]. The increased expression of CD14, a pattern recognition receptor that binds lipopolysaccharide (LPS) and other microbial molecules, on sJIA Mos was proposed by Srivastava et al. to contribute to the apoptosis resistance of Mos [33].
Seemingly important players in JIA, intermediate Mos are noted for their high surface levels of class II molecules, CD40, CD54, and CD74, which are capable of inducing T cell stimulation and proliferation [34,35]. Upon encountering damage-associated molecular patterns or pathogen-associated molecular patterns, such as LPS, these Mos preferentially produce IL-1β, IL-6, and tumor necrosis factor (TNF)α [36,37]. Furthermore, in response to vascular endothelial growth factor (VEGF), CD14+CD16+ Mos form cell clusters and exhibit proangiogenic behavior [38]. In a rheumatoid arthritis (RA) study, a coculture of intermediate CD14++CD16+ Mos from arthritis patients with naïve T cells skewed the T cells toward pathogenic Th17 cells via the production of IL-23. The increased frequency of IL-17-producing natural killer (NK) cells, CD4, and gamma-delta T cells in patients with ERA was also recently proposed to result from the expansion of intermediate CD14++CD16+ Mos due to their role as the major producer of IL-23, a key cytokine in the pathogenesis of ERA [26].
3.2. Monocyte/Macrophage Polarization
Plasticity and heterogenicity are the hallmarks of Mos/Mφs. Polarization is believed to influence disease progression by altering effector function [37] and has been linked to osteoclastogenesis in RA, to disease severity in osteoarthritis, and to distinct JIA subtypes [29,34,39,40,41]. Demonstrated by the acquisition of distinct functional characteristics directed by the immunological microenvironment and tissue milieu, polarized Mos/Mφs are referred to as classically activated (M1) or alternatively activated (M2) Mφs, mirroring the Th1/Th2 nomenclature [42]. Based on the induction of cytokines and clinical features involving tissue repair, angiogenesis, and immune regulation, alternatively activated Mφs have been further subcategorized [39,43] (Table 1).
Table 1.
Characteristics of monocyte and macrophage polarization in humans.
| Macrophages | Classically Activated (M1) |
Alternatively Activated (M2) | ||
|---|---|---|---|---|
| M2a | M2b | M2c | ||
| Mediators of polarization |
LPS, IFNγ, TNFα, GM-CSF | IL-4, IL-13 | ICs + TLR/IL-1β | IL-10, TGFβ, steroid |
| Surface markers | CD68, CD80, CD86, IL-1R, TLR2, TLR4, iNOs, IFNγR, MHC-IIhigh | CD200R, CD206/MMR, IL-1RII, Dectin-1, MHC-IIlow | CD86, MHC-IIlow | CD163, TLR1, TLR8 |
| Transcription factors and cellular markers |
NFκB, STAT1, STAT5, IRF3, IRF5 | IRF4, PPARγ, STAT6 | IRF4, SOCS3 | IRF4, SOCS3 |
| Produced cytokines |
IL-1α, IL-1β, IL-6, IL-12, IL-18, TNFα, M-CSF | IL-10, TGFβ, IL-1Ra |
IL-1β, IL-6, IL-10, TNFα | IL-10, TGFβ |
| Produced chemokines |
CXCL9, CXCL10, CXCL11 | CCL17, CCL18, CCL22, CCL24 | CCL1, CCL20, CXCL1, CXCL2, CXCL3 | CCL16, CCL18 |
| Features | Proinflammatory, microbicidal, and tumoricidal | endocytic activity, tissue remodeling, and repair | immunoregulation | immunoregulation |
The polarization pattern of Mos/Mφs and its consequences in patients with oligoarticular JIA remain largely unclear. In a recently published report, Schmidt et al. suggested that the inflammatory features of those who suffered from oligoarticular JIA may not fit into the traditional dichotomous polarization categories and should be considered according to their unique pattern [31]. Specifically, compared to circulating Mos, the synovial Mos were polarized with a mixed classically and alternatively activated pattern. This was evidenced by the increased expression of the surface molecules CD40, CD86, and CD206 and by mRNA profiling showing upregulated CD80, signal transducer and activator of transcription (STAT)1, CXCL10, CD206, CCL18, and peroxisome proliferator-activated receptor gamma (PPARγ) expression, but not CD163 or STAT6 expression [31]. Correspondingly, a similar heterogeneous mixture of polarized Mφs is also seen in RA synovium and SF [40,41,44]. Interestingly, even though IL-1β, IL-6, IL-8, and IL-10 were found in JIA SF, SF alone did not induce the in vivo polarization pattern observed [31]. It is therefore hypothesized that Mos might obtain their polarization pattern, at least partially, from an extra-articular environment before migrating to the synovial space [31]. Similarly, the polarization of Mos in sJIA is highly dependent on environmental stimuli [45,46]. Although the molecular mechanism remains to be elucidated, a mixed polarization phenotype was noted in Mos isolated from patients with sJIA, possibly through interferon (IFN)/STAT signaling and the activity of small noncoding RNAs, or microRNAs [30,45,47,48].
Immunoregulatory Mφ activity was found in ERA patients, evidenced by the expression of CD163 [26,47]. CD163 is a scavenger receptor that is enhanced in response to IL-10 [48] and has been linked to the pathogenesis of autoimmune disorders in adults, especially spondyloarthropathies [49]. An increased production of the proinflammatory cytokines IL-1β, IL-6, and TNFα and the immunoregulatory mediators IL-10 and nitrogen oxide was elevated upon the cross-linking of CD163 [37]. In addition to the proinflammation tendency, an immunoregulatory effect of CD163+ Mφs has been suggested as soluble CD163 can suppress the activation and proliferation of T lymphocytes upon stimulation [50]. The anti-inflammatory heme metabolite production results from CD163 hemoglobulin transportation and the production of IL-10 together, also attributed to the immunosuppressive Mφ phenotype [51,52]. Collectively, Mφs with high CD163 expression have been hypothesized to downregulate the inflammatory response in the late inflammation phases [47].
4. Mediators Directing Monocyte/Macrophage Activation and Polarization
4.1. Cytokines
Cytokines play a critical role in the activation and polarization of Mos/Mφs. As summarized in Table 1, while GM-CSF, TNFα, and IFNγ drive these cells to a proinflammatory state, IL-10 is especially important for the immunoregulatory Mo/Mφ polarization in JIA.
4.1.1. GM-CSF
GM-CSF, a hemopoietic growth factor, is secreted by myeloid cells, T and B lymphocytes, and tissue residential cells, such as fibroblasts, chondrocytes, osteoblasts, and epithelial and endothelial cells. GM-CSF interacts with the GM-CSF receptor and activates the Janus kinase (JAK)2-STAT5- suppressor of cytokine signaling (SOCS) as well as mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3 kinase, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), resulting in the activation of tissue-resident cells and the recruitment of inflammatory cells [53]. The production of IL-6 and IL-23 upon GM-CSF receptor signaling activates T cells and promotes the differentiation of Th17 cells to induce additional GM-CSF and IL-17 secretion, forming a feedback loop [53]. While the role of GM-CSF in JIA is not as well studied as that in RA [53], the frequency of GM-CSF-producing T helper cells was significantly enriched among SF mononuclear cells, and the culturing of Th17 cells in the presence of IL-12 resulted in upregulation of GM-CSF and IFNγ, recapitulating the phenotype of GM-CSF-expressing cells within the JIA joints [54].
4.1.2. TNFα
TNFα is a pleiotropic cytokine that promotes the expression of adhesion molecules, inflammatory cytokines, prostaglandin E2, collagenase, and collagen by synovial cells [55]. As Mos/Mφs are the main producers of TNFα, its production serves as an autocrine stimulator and a potent paracrine inducer of inflammatory cytokines, including IL-1, IL-6, IL-8, and GM-CSF [56,57,58]. TNFα triggers NFκB and MAPK activation and/or cell death via apoptosis or necroptosis via either death domain adaptor or TNF receptor associated factor (TRAF) depending on the distinct receptor type (TNFαR1 and TNFαR2) to which it binds [59]. Significantly higher TNFα expression was found in JIA patient plasma without stratifying for disease activity or JIA subtype, and increased levels of serum TNFα and soluble TNFαR1 and TNFαR2 were noted in patients with active sJIA, including those complicated with macrophage activation syndrome (MAS) [20,60]. Upon treatment with TNF blocking agents, the inflammatory Mφs were shifted toward an immunoregulatory phenotype with reduced production of inflammatory cytokines and increased phagocytosis and apoptosis [61,62,63,64].
4.1.3. IFNγ
IFNγ, the sole member of type II IFNs, is produced by multiple cell types, including T cells (Th1 cells in particular), B cells, Mos/Mφs, and NK cells [65]. It regulates more than 9000 genes to orchestrate the production of cytokines and reactive oxygen species, enhance antigen presentation, cellular differentiation, and Mφ activation as well as cell growth and survival [65]. Upon binding to its receptor, JAK1, JAK2, and STAT1 are recruited and activated via phosphorylation to modulate gene transcription [65]. An enhanced responsiveness to IFNγ stimulation was reported with increased STAT1 and/or STAT3 phosphorylation in classical Mos in patients with polyarticular JIA [27]. Although no obvious differences in IFNγ levels were noted in oligoarticular, polyarticular, and sJIA patients under distinct activity statuses when compared to controls [20], altered RNA profiling with upregulation of the IFNγ pathway and increased expression of tripartite motif containing eight was identified in a distinct subpopulation of bone marrow Mφs in sJIA patients complicated with MAS [66]. Interestingly, Macaubas et al. recently discovered impaired IFN/STAT1 signaling in sJIA Mos during active disease, skewing them away from a classically activated phenotype [67]. However, this hyporesponsiveness to IFN was restored and inverted in treated, quiescent subjects [67].
4.1.4. IL-10
IL-10 is a critical cytokine in the negative feedback loop that limits inflammation and increases phagocytosis [68]. IL-10 induction often occurs along with proinflammatory cytokines, although pathways that induce IL-10 often negatively regulate inflammatory effects [68]. The rapid transcription of IL-10 mRNA was reported during proinflammatory Mo/Mφ activation [61]. IL-10 inhibition resulted in a dramatic deprivation in immunoregulatory surface markers with a concomitant increase in inflammatory surface markers [61]. Upon the binding of IL-10 and its receptors (IL10RA and IL10RB), STAT3 signaling is induced through phosphorylation by JAK1 and tyrosine kinase 2 [61,68].
4.2. Toll-Like Receptor Signaling
Toll-like receptors (TLRs) belong to the group of protein recognition receptors and are type I transmembrane proteins serving as the first-line defense against microbes. They recognize both invading pathogens and endogenous danger molecules and are crucial in bridging innate and adaptive immunity [69]. Upon ligand interaction, the dimerization of most TLRs triggers the recruitment of myeloid differentiation primary response protein 88 (MyD88), which interacts with IL-1R-activating kinase (IRAK)4 and IRAK1/IRAK2, activates TRAF6, and enhances the transcriptional activity and production of a number of proinflammatory cytokines and chemokines through the NFκB protein complex [69]. In addition, TLR3 and TLR4 interact with TIR-domain-containing adapter-inducing interferon- (TRIF)β, activate TRAF3, and promote type 1 IFN production [69].
Studies in experimental models have documented the ability of microbial TLR ligands to trigger arthritis in animals [70]. Increased TLR2 and TLR4 expression was found on ERA peripheral blood mononuclear cells (PBMCs) and SF Mos [71], and an increased TLR/IL-1R signature and TLR2 expression were revealed by analyzing gene expression in PBMCs from patients with sJIA [72,73]. Endogenous TLR ligands, such as S100A8/A9 (calprotectin), S100A12, high mobility group box 1, and serum amyloid A, are significantly elevated in JIA cases, particularly in sJIA [74,75,76], and are likely to lead to disease progression [77]. Specifically, the binding of TLR4 with S100A8/A9 on Mos/Mφs was found to induce the transcription of chemokine IFNγ inducible protein 10 (IP-10)/CXCL10 via TRIF signaling [78]. Local injection of exogenic S100A8 into the knee joints of mice resulted in enhanced expression of the FcγR on synovial Mφs via TLR4 [79]. Moreover, in experimental models, S100A8/A9 has been shown to play an essential role in the induction of autoreactive CD8+ T cells and the development of systemic autoimmunity [80]. Interestingly, TLR signaling can be altered according to different disease statuses. During disease remission and off treatment, dysregulated responses to TLR4, TLR8, and TLR7 stimulation were observed in sJIA Mos [81].
4.3. Autoantibodies and Immunocomplexes
By definition, the presence of autoantibodies, RF and anti-citrullinated protein antibodies (ACPA) is the hallmark of RF-positive JIA [82]. RFs are autoantibodies targeting the fragment crystallizable portion of immunoglobulins, mostly in the IgM isotype [83]. ACPAs interact with a variety of citrullinated proteins and are associated with greater articular damage and a poorer response to therapy [83]. They interact with self-antigens, form immunocomplexes (ICs) and induce the production of TNFα or other cytokines by PBMCs via FcγR engagement [5]. Cellular responses were synergized and augmented when RF and ACPA were both presented [5]. In addition to FcγR engagement, ACPA ICs also stimulate Mφs via the dual engagement of TLR4/MyD88 and FcγR for the production of TNFα [84]. Moreover, ACPAs can directly interact with surface-expressed citrullinated proteins on RA Mos to facilitate inflammatory responses through the c-Jun N-terminal kinase and NFκB pathways [84].
4.4. Hypoxia
The hypoxic nature of JIA synovium and the induction of chemokine CCL20 in JIA synovial Mos within the hypoxic milieu of inflamed joints suggests that hypoxia likely increases inflammatory cell infiltration and contributes to the development of local inflammation [85,86]. As the expression of hypoxia-inducible factor (HIF)-1α and HIF-2α was constitutively detected in Mos recruited to inflamed joints in JIA patients, the ability of these key regulators to alter metabolic reactions and protein transcription potentially impacts the activation and polarization of Mos/Mφs in JIA [85]. Specifically, HIF-1α increases the transcription of glycolytic enzymes and promotes the production of the proinflammatory cytokine IL-1β [87]. In addition, decreased myeloid cell joint infiltration and delayed disease progressions were observed in an inflammatory arthritis model utilizing HIF-1α-deficient Mφs [88]. Interestingly, Raggi et al. discovered that synovial Mφs in the hypoxic inflamed joints of oligoarticular JIA patients express high surface levels of triggering receptors expressed on myeloid cells (TREM)-1 [89]. As a hypoxia-inducible gene, TREM-1 reverses the immunoregulatory-polarizing effect of hypoxia and drives proinflammatory reprogramming in a hypoxic microenvironment [89]. Hypoxic synovial Mos in JIA also release VEGF and osteopontin. These proangiogenic mediators within inflamed joints drive neoformation of blood vessels through stimulation of epithelial cell survival, proliferation, and chemotaxis, as well as monocytic cell recruitment and activation [85].
4.5. MicroRNAs
The polarization of Mos/Mφs in sJIA was also found to be driven by epigenetic factors, such as negative transcriptional regulation by microRNAs [50,52,90]. Through a microRNA array analysis comparing the expression of microRNAs in Mos from patients with inactive sJIA, active sJIA, new-onset sJIA, and active polyarticular JIA, Schulert et al. discovered that miR-125a-5p was highly upregulated in active and new-onset sJIA patients and correlated with their systemic features but not the degree of joint involvement [45]. Through microRNA overexpression and inhibition assays, miR-125a-5p was shown to drive Mos toward alternatively activated polarization with an enhanced M2b phenotype, in line with the Mos observed in sJIA in clinical settings [45]. Further investigation suggested that miR-125a-5p and miR-181c overexpression in patients with active sJIA significantly reduced the expression of CD163 on Mφs [91]. Specifically, miR-181 targets CD163 mRNA for degradation and miR-125a-5p decreases IL10RA, the receptor required for IL-10-mediated CD163 expression [91]. Moreover, miR-155 promotes Mφ proinflammatory polarization and suppresses alternatively activated features [92]. miR-155 targets SOCS1 transcription, altering cytokine and surface molecule expression [92]. Compared to controls or patients with clinically inactive sJIA, miR-155 is increased in Mos from children with active sJIA [45]. Together, these data explain how microRNAs aid sJIA Mos toward polarization to an immunoregulatory phenotype [91]. In addition, significantly higher levels of plasma miR-233, a microRNA regulating inflammation, cell differentiation, and oncogenesis, were found in JIA patients. miR-233 promotes the polarization of Mφs toward an immunoregulatory phenotype via direct targeting of PBX/Knotted 1 Homeobox 1 and controls Mφ inflammatory responses by inhibiting NOD-, LRR-, and pyrin domain-containing protein 3 (NLPR3) inflammasome activity [93,94].
5. Effect of Monocyte/Macrophage-Produced Cytokines and Chemokines
Activated Mos and Mφs in JIA synovial space secrete a variety of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-12, IL-18, and IL-23 [12,22,95]. While the levels of these mediators may vary between different JIA subtypes [96], the interactions of these cytokines and chemokines with leukocytes and residential mesenchymal cells, such as synovial fibroblasts, chondrocytes, and osteoclasts contribute JIA pathogenesis [90].
5.1. TNFα
The importance of TNFα in inflammatory arthritis has been highlighted in numerous clinical observations and experimental settings. In addition to its autocrine feedback role in propagating an inflammatory response [56,57,58], TNFα is capable of stimulating fibroblasts to express adhesion molecules, such as intracellular adhesion molecule 1 (ICAM-1), to enhance leukocyte adhesion [56]. Considering that bone destruction is also a hallmark of JIA, TNFα stimulates osteoclast differentiation via NFкB signaling and upregulates several proinflammatory cytokines, including receptor activator of NFκB (RANK), leading to increased RANK/RANK ligand (RANKL) signaling and osteoclast activity [97,98]. Moreover, aside from its soluble form, as a transmembrane protein, TNFα on both Mφs and fibroblasts has been shown to induce arthritis in transgenic mice [95]. Currently, the success of treating JIA patients with various biologics interfering with TNFα signaling has confirmed the importance of TNFα in the pathogenesis of JIA [99,100,101].
5.2. IL-1β
Innate proinflammatory cytokines, such as IL-1, IL-6, IL-18, and TNF, account for many of the features observed in sJIA [46]. Specifically, IL-1β, a pleiotropic proinflammatory cytokine, can upregulate its own transcription as well as that of IL-6 [102]. Moreover, a possible positive feedback loop involving IL-1β and S100 proteins has been proposed to contribute to the perpetuation of chronic inflammation in sJIA [103]. Interestingly, Cepika et al. discovered that the stimulation of Mos in patients with sJIA resulted in an increase in activin receptor signaling, which in turn inhibited IL-1β secretion without altering the accumulation of intracellular IL-1β within sJIA Mos [81]. Moreover, IL-1β induces the expression of ICAM-1 on synovial fibroblasts and activates osteoclasts to display a high degree of resorbing activity [104,105]. The critical role of IL-1β in JIA has been demonstrated by the use of the IL-1β blocking agents anakinra and canakinumab in sJIA treatment with success [106,107,108,109].
5.3. IL-6
IL-6 is a multifunctional cytokine that drives JIA development via immune response regulation, hematopoiesis, and bone metabolism [98]. IL-6 elevation in active sJIA modulates the levels of proteases and their regulators, such as matrix metalloproteinase (MMP)-9 and its tissue inhibitors of metalloproteinases-1, in synoviocytes and chondrocytes [110,111]. IL-6 also alters the cytokine profile in JIA synoviocytes in both a proinflammatory and anti-inflammatory manner [20]. Moreover, IL-6 plays a role in T cell survival and proliferation and promotes the differentiation of Th17 cells [112]. Interestingly, two distinct groups of sJIA patients with specific clinical features were identified based on their IL-6 and IL-18 levels [113]. Patients with dominant IL-6 cytokines have more severe joint disease, and those with dominant IL-18 cytokines are more likely to develop MAS [114]. As the severity of experimental inflammatory arthritis is greatly suppressed in IL-6-/- transgenic mice [115], a promising result was also noted by applying tocilizumab, an IL-6 receptor targeting agent, for the treatment of polyarticular JIA and sJIA [116,117].
5.4. IL-18
In patients with active sJIA, the level of IL-18 is significantly elevated [118]. As documented in the RA joint, IL-18 may induce an inflammatory process because it promotes leukocyte extravasation by upregulating endothelial cell adhesion molecules, releasing chemokines from RA synovial fibroblasts, and serving as a chemoattractant for various leukocytes [119]. Moreover, IL-18 helps develop and maintain the inflammatory pannus by binding and activating endothelial cells and inducing synovial fibroblasts to produce angiogenic chemokines and VEGF, contributing to the vascularity of inflamed pannus [119,120].
5.5. IL-12/IL-23
IL-12 and IL-23 are mainly produced by inflammatory myeloid cells and are critical mediators influencing the differentiation of Th1 and Th17 cells [121]. While IL-23 is specifically important for the development of Th17 and plays a pathogenic role in seronegative spondyloarthropathies, including ankylosing spondylitis (AS), psoriatic arthritis, and ERA [122,123], IL-12 initiates the differentiation of naïve CD4 T cells to Th1 cells and promotes a shift of Th17 cells toward Th17/Th1 and non-classic Th1 cells [124]. These cells are all involved in the pathogenesis of JIA [4,125,126].
Specifically, increased numbers of Th1 and Th17 were reported in the synovial space of patients with oligoarticular JIA and ERA [124,127]. IL-17A and TNFα produced by Th17 and Th1 cells promotes the release of IL-6 and IL-8 by synoviocytes and endothelial cells in the joint space [128]. These cytokines induce expression of adhesion molecules, enhance leukocytes recruitment, and maintain joint inflammation [129]. Moreover, IL-17 produced by Th17 cells stimulates the release of MMPs by synovial fibroblasts and increases osteoclast differentiation, leading to cartilage breakdown and bony erosion [128]. Notably, IL-17 is capable of maintaining articular inflammation independent of TNFα once the arthritis is initiated [125].
5.6. IL-10
IL-10 is a potent cytokine that represses proinflammatory responses and limits unnecessary tissue damage caused by inflammation [126]. IL-10 hinders Mo trafficking into synovial tissue through the downregulation of ICAM-1 expression on synovial cells; suppresses the release and function of the proinflammatory cytokines IL-1, TNFα, and IL-6; and reverses cartilage degradation by mononuclear cells in patients with RA [126,130]. As IL-10 polymorphism confers the susceptibility to JIA [131], animal studies have suggested that insufficient IL-10 production is a mechanism underlying the pathogenesis of sJIA [132].
5.7. Chemokines
Increased levels of chemokines were found in JIA SF, favoring the migration of Mos to the inflamed tissue while promoting their activation and differentiation. While synovial fibroblasts are important sources of proinflammatory cytokines and chemokines [133], synovial Mos/Mφs also secrete a variety of chemokines. CCL2/MCP-1 principally recruits Mos, dendritic cells, and memory T cells to sites of inflammation [19,134]. The expression of CCL18 is upregulated in JIA synovial Mos and is capable of recruiting cells of the adaptive immune system to maintain homeostasis [31,135]. Secreted CCL20, so-called macrophage inflammatory protein-3, is also important in driving Th17 recruitment to the inflamed joints in patients with JIA [92,136,137]. Other chemokines, such as CXCL8 and CXCL10, mediate the recruitment of neutrophils, T lymphocytes, NK cells, dendritic cells, and Mos/Mφs into the joint space, coordinating an inflammatory response [31,96].
5.8. Vascular Endothelial Growth Factor
VEGF is the most critical regulator of angiogenesis and mediates inflammatory and bone-destructive activities [138,139]. Synovial Mos/Mφs in the hypoxic microenvironment are a source of VEGF and the concentration of VEGF was significantly increased in the SF of JIA patients compared with that in the serum [85,140]. While a number of studies have clearly documented a reduction in disease severity and synovial angiogenesis when treating RA patients with VEGF-blocking agents [141], VEGF also serves as a useful marker for assessing the disease activity of oligo/polyarticular JIA during the remission phase [136]. The tapering of medication in oligo/polyarticular JIA is recommended if the level of VEGF remains low [136].
6. Available Treatments and Emerging Therapeutic Opportunities
Based on the clinical relativity and the current knowledge on the pathogenesis of Mos/Mφs in JIA, different strategies have been explored or are currently under investigation for JIA patients and/or have been tested in arthritic animal models. The induction of anti-inflammatory human Mos is a unique property of glucocorticoids [137]. In arthritic rodent models, the local injection of triamcinolone acetonide strongly enhanced Mφ activation toward an immunoregulatory phenotype [142]. This was supported by the enhanced surface expression of CD163 and enhanced IL-10 expression at the mRNA level in ex vivo Mφs [142]. Methotrexate (MTX), a commonly prescribed disease-modifying antirheumatic drug (DMARD) for JIA, exclusively modulates gene expression in proinflammatory Mφs polarized by GM-CSF [143]. Further study revealed that MTX increases the expression of A20, an NFκB suppressor, which inhibits TLR signaling in GM-CSF-polarized Mφs [144]. Moreover, MTX has been reported to dampen the production of TNFα and IL-12 in classically activated Mφs [145]. On the other hand, MTX enhances IL-10 synthesis and inhibits NFκB signaling on immunoregulatory Mφs [145]. Chronic exposure to sulfasalazine, another widely used DMARD, markedly sensitized human Mos/Mφs to steroid treatment via the NFκB signaling pathway, upregulated glucocorticoid receptor α and glucocorticoid expression levels and induced apoptosis [146].
Biological therapies targeting the Mo/Mφ-producing cytokines TNFα, IL-1β, and IL-6 are now recommended as the standard of care for JIA patients with an advanced disease course [147]. While these biologics significantly dampen the proinflammatory response mediated by Mos/Mφs, anti-TNF agents not only inhibit the inflammatory functions of Mφs but also favor the resolution of inflammation by inducing cellular polarization toward alternative features involving the IL-10/STAT3 axis [61]. A decline in Mφs was clearly noted in the inflamed joints of mice shortly after the introduction of infliximab, a TNFα blocking agent [148,149]. Moreover, tocilizumab, an anti-IL6 receptor antibody, shifts Mos/Mφs toward an anti-inflammatory phenotype and induces the apoptosis of Mos [150,151]. Tofacitinib, a small molecule JAK inhibitor, was recently shown to reduce disease flares and improve disease activity and physical function in patients with polyarticular JIA [152]. It abrogates TNF induced STAT1 activation and inhibits proinflammatory mediator production [153].
The effectiveness of existing anti-rheumatic regimens and novel therapeutic agents targeting Mos/Mφs in patients with inflammatory arthritis has been investigated. A brief summary of Mo/Mφ-related therapies is shown in Table 2.
Table 2.
Therapeutic agents targeting monocytes/macrophages and their functional activities.
| Agent | Targets | Actions | Developmental Stage | Ref. |
|---|---|---|---|---|
| Etanercept | TNF receptor | Blocks TNFα signaling; shifts Mos/Mφs toward an anti-inflammatory phenotype and induces apoptosis of Mos/Mφs |
JIA–marketing | [61,62] |
| Adalimumab | TNF | Blocks TNFα signaling; shifts Mos/Mφs toward an anti-inflammatory phenotype; induces apoptosis of Mos/Mφs and reduces Mo migration into the joint | polyarticular JIA–marketing | [63,149,154] |
| Infliximab | TNF | Blocks TNFα signaling; shifts Mos/Mφs toward an anti-inflammatory phenotype and induces apoptosis of Mos/Mφs; increases circulating nonclassical Mos and decreases circulating classical Mos; reduces CCR2 and CXCR4 expression on the nonclassical Mo subpopulation |
JIA–marketing | [62,63,64] |
| Certolizumab | TNF | Blocks TNFα signaling; induces HO-1 mRNA and protein production in Mos; inhibits IL-1β production at the mRNA and protein level upon LPS stimulation |
polyarticular JIA–phase III | [154] |
| Anakinra | IL-1β receptor | Blocks IL-1β signaling | sJIA–marketing | [109] |
| Canakinumab | IL-1β | Blocks IL-1β signaling | sJIA–marketing | [106,107] |
| Rilonacept | IL-1β/IL-1α | Blocks IL-1 signaling; skews Mos toward an alternatively activated phenotype |
sJIA–phase II | [155] |
| Tocilizumab | IL-6 receptor | Blocks IL-6 signaling; shifts Mos/Mφs toward an anti-inflammatory phenotype and induces apoptosis of Mos |
sJIA/polyarticular JIA–marketing | [150,151] |
| Sarilumab | IL-6 receptor | Blocks IL-6 signaling | polyarticular JIA–phase II | [156] |
| Ustekinumab | IL-12/IL-23 | Blocks IL-12/IL-23 signaling | psoriatic arthritis–marketing | [157] |
| Secukinumab | IL-17A | Blocks IL-17 signaling; decreases serum IL-6, S100A8, S100A9, VEGF, TNFα, osteopontin, and MMP | ERA/juvenile psoriatic arthritis –phase III |
[158] |
| Ixekizumab | IL-17A | Blocks IL-17 signaling | ERA/juvenile psoriatic arthritis –phase III |
[159] |
| Emapalumab | IFNγ | Blocks IFNγ signaling | sJIA–phase II | [160] |
| Abatacept | CTLA-4 | Blocks ACPA and RF mediated cytokine production in human Mφs; modulates proinflammatory Mφ responses upon cytokine-activated T cell and TLR stimulation |
Polyarticular JIA–marketing | [161,162] |
| Tofacitinib | JAK1/JAK3 | Small molecule that abrogates TNF- induced STAT1 activation; inhibits proinflammatory mediator production |
polyarticular JIA–marketing | [153] |
| Baricitinib | JAK1/JAK2 | Decreases expression of the inflammatory IP-10 and increases IL-10 production | JIA–phase III | [163] |
| Upadacitinib | JAK1 | Selectively targets JAK1 dependent disease drivers such as IL-6 and IFNγ | Polyarticular JIA–phase I | [164] |
| Mavrilimumab (CAM-3001) | GM-CSF receptor α | Blocks GM-CSF signaling and classically activated polarization | RA–phase IIb | [165] |
| Otilimab (MOR103) | GM-CSF | Blocks GM-CSF signaling and classically activated polarization | RA–phase III | [166] |
| Givinostat (ITF2357) | histone deacetylase inhibitor | Prevents LPS-induced TNFα gene transcription and secretion of IL-1β | JIA–phase II | [167] |
| Gamma-linolenic acid | n-6 polyunsaturated fatty acids | Inhibits inflammatory responses through inactivation of NFκB and AP-1 by suppressing oxidative stress and the ERK and JNK signal transduction pathways in LPS-induced Mφs |
JIA-phase I | [168] |
| Sinomenine | plant alkaloid | Attenuates CD11b+F4/80+CD64+ resident Mφs in the synovial tissue and reduces number of CD14+CD16+ circulating Mos | Herbal medicine | [169] |
| Thapsigargin | inhibitor of SERCA | Decreases the number of TNF-induced classically activated Mφs and increases the number of alternatively activated Mφs | preclinical | [170] |
| Withaferin-A | steroidal lactone | Promotes classically activated Mφ to alternatively activated Mφ repolarization | preclinical | [171] |
| Berberine | antimicrobial agent | Increases the proportion of alternatively activated Mφs and decreases the proportion of classically activated Mφs, downregulates HIF-1α expression in synovial Mφs |
Preclinical | [172] |
| Ramucirumab | VEGF | Blocks VEGF signaling | preclinical | [173] |
| Ranibizumab | VEGF | Blocks VEGF signaling | preclinical | [174] |
| 2-benzoyl-phenoxy acetamide | benzophenone analog | Targets VEGF and HIF-1α | preclinical | [175] |
| Paclitaxel (PTX) | tubulin, chemotherapy | Targets VEGF and HIF-1α | preclinical | [176] |
| pLVX-shRNA-HIF-1α | shRNA targeting HIF-1α | Inhibits HIF-1α and VEGF expression, leading to decreased proinflammatory cytokine expression | Preclinical | [177] |
| Clodronate liposomes |
release chlorophosphate | Mφ depletion | Preclinical | [178] |
| Human umbilical cord blood-derived mesenchymal stem cells | stem cells | Polarizes naive Mφs toward an alternatively activated phenotype | preclinical | [179] |
7. Conclusions and Future Perspectives
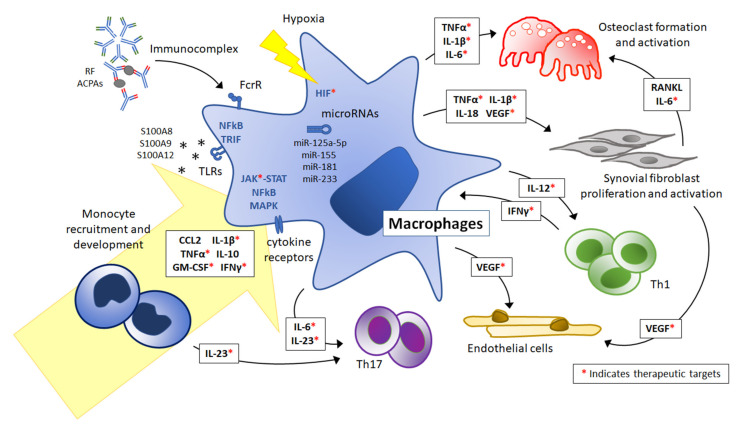
The present review summarizes recent understanding of the phenotype of Mos/Mφs and the mechanisms regulating their activation to elicit inflammation in patients with JIA. As discussed, the activity and phenotype of Mos/Mφs play an important role in pathogenesis across different JIA subtypes. Recruited to the joints by chemokine MCP-1/CCL2 and RANTES, Mos infiltrate the inflamed synovium and add to the tissue-resident Mφ pool orchestrating a local and global inflammatory reaction resulting in tissue damage. Upon the stimulations of cytokines, immunocomplexes, TLR ligands, the hypoxic microenvironment, and microRNAs, a mixed polarization of Mφs toward an inflammatory phenotype was mediated via the JAK-STAT, MAPK, NFκB, TRIF, and HIF signaling pathways. Proinflammatory cytokines, including IL-1β, IL-6, IL-12, IL-18, IL-23, and TNFα were secreted to stimulate activation of osteoclasts and synovial fibroblasts and to promote T lymphocyte polarization. VEGF, in addition, drives synovial angiogenesis, contributing to JIA pathogenesis (Figure 1).
Figure 1.
Therapeutic targets related to monocyte and macrophage activation in juvenile idiopathic arthritis. Monocytes (Mos) were recruited to the joints by CCL2 and differentiated into tissue macrophages (Mφs) under inflammatory conditions. IL-1β, TNFα, IFNγ, GM-CSF, and IL-10 are especially important for the differentiation process. Upon stimulation with cytokines, immunocomplexes, TLR ligands, the hypoxic microenvironment, and microRNAs, the mixed polarization of Mφs toward an inflammatory phenotype was mediated via the JAK-STAT, MAPK, NFκB, TRIF, and HIF signaling pathways. Proinflammatory cytokines, including IL-1β, IL-6, IL-12, IL-18, IL-23, and TNFα were secreted to stimulate activation of osteoclasts and synovial fibroblasts and to promote T lymphocyte polarization. VEGF drives synovial angiogenesis, contributing to JIA pathogenesis. Available therapeutic targets are marked with red asterisks *. Abbreviations: RF, rheumatoid factor; ACPAs, anti-citrullinated protein antibodies; CCL2, monocyte chemoattractant protein 1; TLRs, Toll-like receptors; FcγR, Fc gamma receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription; MAPKs, mitogen-activated protein kinases; TRIF, TIR-domain-containing adapter-inducing interferon; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; HIF, hypoxia-inducible factor; Th1, type 1 T helper cells; Th17, type 17 T helper cells; RANKL, receptor activator of nuclear factor kappa beta ligand.
While investigations into strategies targeting Mo recruitment, Mo/Mφ polarization, cell depletion, and cytokine blockade have been recently performed (Table 2), uncovering the heterogenicity and regulatory mechanisms of Mo/Mφ in JIA pathogenesis is crucially needed for the development of novel approaches aiming at Mo/Mφ for the control of the disease.
Abbreviations
| ACPA | anti-citrullinated protein antibody |
| AS | ankylosing spondylitis |
| CCL | C-C motif chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
| DMARD | disease-modifying antirheumatic drug |
| ERA | enthesitis-related arthritis |
| FcγR | Fcγ receptor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| HIF | hypoxia-inducible factor |
| IC | immunocomplex |
| ICAM-1 | intracellular adhesion molecule 1 |
| IL | interleukin |
| ILAR | International League of Associations for Rheumatology |
| IFN | Interferon |
| IP-10 | IFNγ inducible protein 10 |
| IRAK | IL-1R-activating kinase |
| IRF | interferon regulatory factor |
| JAK | Janus kinase |
| JIA | juvenile idiopathic arthritis |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MAS | macrophage activation syndrome |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | matrix metalloproteinases |
| Mφ | macrophage |
| Mo | monocyte |
| MTX | methotrexate |
| MyD88 | myeloid differentiation primary response protein 88 |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| PBMCs | peripheral blood mononuclear cells |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| RA | rheumatoid arthritis |
| RANTES | regulated upon activation, normal T cell expressed and presumably secreted |
| RF | rheumatoid factor |
| RANK | receptor activator of NFκB |
| RANKL | RANK ligand |
| SF | synovial fluid |
| sJIA | systemic onset JIA |
| SOCS | suppressor of cytokine signaling |
| STAT | signal transducer and activator of transcription |
| TGFβ | transforming growth factor beta |
| Th1 | type 1 helper T cells |
| Th17 | type 17 helper T cells |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| TREM | triggering receptor expressed on myeloid cells |
| TRAF | TNF receptor associated factor |
| VEGF | vascular endothelial growth factor |
Author Contributions
Writing—original draft preparation, C.-Y.W.; writing—review and editing, H.-Y.Y., J.-L.H., and J.-H.L.; supervision, J.-L.H. and J.-H.L.; funding acquisition, C.-Y.W. and J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST 107-2314-B-182A-132-MY3 to Dr. Lai) and Chang Gung Memorial Hospital (CMRPG3J1901-2 and CMRPG3G1191 to Dr. Wu and CMRPG1H0101 to Dr. Lai), Taiwan, R.O.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest related to this study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prakken B., Albani S., Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- 2.Petty R.E., Southwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J., He X., Maldonado-Cocco J., Orozco-Alcala J., Prieur A.M., et al. International League of Associations for, R., International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 3.Petty R.E., Laxer R.M., Lindsley C.B., Wedderburn L., Fuhlbriggezzz R.C., Mellins E.D. Textbook of Pediatric Rheumatology. 8th ed. Elsevier; Philadelphia, PA, USA: 2021. Juvenile Idiopathic Arthritis: Classification and Basic Concepts; pp. 209–215. [Google Scholar]
- 4.Lin Y.T., Wang C.T., Gershwin M.E., Chiang B.L. The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun. Rev. 2011;10:482–489. doi: 10.1016/j.autrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud S.A., Binstadt B.A. Autoantibodies in the Pathogenesis, Diagnosis, and Prognosis of Juvenile Idiopathic Arthritis. Front. Immunol. 2018;9:3168. doi: 10.3389/fimmu.2018.03168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong J.Y., Guan Y.J., Albani S., Arkachaisri T. Recent advances in our understanding of the pathogenesis of juvenile idiopathic arthritis and their potential clinical implications. Expert. Rev. Clin. Immunol. 2018;14:933–944. doi: 10.1080/1744666X.2018.1529757. [DOI] [PubMed] [Google Scholar]
- 7.Laria A., Lurati A., Marrazza M., Mazzocchi D., Re K.A., Scarpellini M. The macrophages in rheumatic diseases. J. Inflamm. Res. 2016;9:1–11. doi: 10.2147/JIR.S82320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma W.T., Gao F., Gu K., Chen D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019;10:1140. doi: 10.3389/fimmu.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma W.T., Chang C., Gershwin M.E., Lian Z.X. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J. Autoimmun. 2017;83:95–112. doi: 10.1016/j.jaut.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Hunter P.J., Nistala K., Jina N., Eddaoudi A., Thomson W., Hubank M., Wedderburn L.R. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum. 2010;62:896–907. doi: 10.1002/art.27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes M.G., Grom A.A., Thompson S.D., Griffin T.A., Luyrink L.K., Colbert R.A., Glass D.N. Biologic similarities based on age at onset in oligoarticular and polyarticular subtypes of juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:3249–3258. doi: 10.1002/art.27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin T.A., Barnes M.G., Ilowite N.T., Olson J.C., Sherry D.D., Gottlieb B.S., Aronow B.J., Pavlidis P., Hinze C.H., Thornton S., et al. Gene expression signatures in polyarticular juvenile idiopathic arthritis demonstrate disease heterogeneity and offer a molecular classification of disease subsets. Arthritis Rheum. 2009;60:2113–2123. doi: 10.1002/art.24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdiguero E.G., Geissmann F. The development and maintenance of resident macrophages. Nat. Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F., Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Prinz M., Erny D., Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017;18:385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S., Pluddemann A. The Mononuclear Phagocytic System. Generation of Diversity. Front. Immunol. 2019;10:1893. doi: 10.3389/fimmu.2019.01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Chang Y., Wei W. Emerging role of targeting macrophages in rheumatoid arthritis: Focus on polarization, metabolism and apoptosis. Cell Prolif. 2020;53:e12854. doi: 10.1111/cpr.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T.C., Kuo M.L., See L.C., Ou L.S., Lee W.I., Chan C.K., Huang J.L. RANTES and monocyte chemoattractant protein 1 as sensitive markers of disease activity in patients with juvenile rheumatoid arthritis: A six-year longitudinal study. Arthritis Rheum. 2006;54:2585–2593. doi: 10.1002/art.21962. [DOI] [PubMed] [Google Scholar]
- 20.De Jager W., Hoppenreijs E.P., Wulffraat N.M., Wedderburn L.R., Kuis W., Prakken B.J. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: A cross-sectional study. Ann. Rheum. Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto D., Miller J., Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J., Liu Y.J., MacPherson G., Randolph G.J., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler-Heitbrock L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passlick B., Flieger D., Ziegler-Heitbrock H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. doi: 10.1182/blood.V74.7.2527.2527. [DOI] [PubMed] [Google Scholar]
- 26.Gaur P., Myles A., Misra R., Aggarwal A. Intermediate monocytes are increased in enthesitis-related arthritis, a category of juvenile idiopathic arthritis. Clin. Exp. Immunol. 2017;187:234–241. doi: 10.1111/cei.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Throm A.A., Moncrieffe H., Orandi A.B., Pingel J.T., Geurs T.L., Miller H.L., Daugherty A.L., Malkova O.N., Lovell D.J., Thompson S.D., et al. Identification of enhanced IFN-gamma signaling in polyarticular juvenile idiopathic arthritis with mass cytometry. JCI Insight. 2018;3:e121544. doi: 10.1172/jci.insight.121544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon B.R., Yoo S.J., Choi Y., Chung Y.H., Kim J., Yoo I.S., Kang S.W., Lee W.W. Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA) PLoS ONE. 2014;9:e109775. doi: 10.1371/journal.pone.0109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawanaka N., Yamamura M., Aita T., Morita Y., Okamoto A., Kawashima M., Iwahashi M., Ueno A., Ohmoto Y., Makino H. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–2586. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 30.Macaubas C., Nguyen K.D., Peck A., Buckingham J., Deshpande C., Wong E., Alexander H.C., Chang S.Y., Begovich A., Sun Y., et al. Alternative activation in systemic juvenile idiopathic arthritis monocytes. Clin. Immunol. 2012;142:362–372. doi: 10.1016/j.clim.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T., Berthold E., Arve-Butler S., Gullstrand B., Mossberg A., Kahn F., Bengtsson A.A., Mansson B., Kahn R. Children with oligoarticular juvenile idiopathic arthritis have skewed synovial monocyte polarization pattern with functional impairment-a distinct inflammatory pattern for oligoarticular juvenile arthritis. Arthritis Res. Ther. 2020;22:186. doi: 10.1186/s13075-020-02279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macaubas C., Nguyen K., Deshpande C., Phillips C., Peck A., Lee T., Park J.L., Sandborg C., Mellins E.D. Distribution of circulating cells in systemic juvenile idiopathic arthritis across disease activity states. Clin. Immunol. 2010;134:206–216. doi: 10.1016/j.clim.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava S., Macaubas C., Deshpande C., Alexander H.C., Chang S.Y., Sun Y., Park J.L., Lee T., Begovich A., Mellins E.D. Monocytes are resistant to apoptosis in systemic juvenile idiopathic arthritis. Clin. Immunol. 2010;136:257–268. doi: 10.1016/j.clim.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler-Heitbrock L., Hofer T.P. Toward a refined definition of monocyte subsets. Front. Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong K.L., Yeap W.H., Tai J.J., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 36.Rossol M., Kraus S., Pierer M., Baerwald C., Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–677. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 37.Fabriek B.O., van Bruggen R., Deng D.M., Ligtenberg A.J., Nazmi K., Schornagel K., Vloet R.P., Dijkstra C.D., van den Berg T.K. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 38.Zawada A.M., Rogacev K.S., Rotter B., Winter P., Marell R.R., Fliser D., Heine G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 39.Murray P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 40.Ambarus C.A., Noordenbos T., de Hair M.J., Tak P.P., Baeten D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012;14:R74. doi: 10.1186/ar3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soler Palacios B., Estrada-Capetillo L., Izquierdo E., Criado G., Nieto C., Municio C., Gonzalez-Alvaro I., Sanchez-Mateos P., Pablos J.L., Corbi A.L., et al. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J. Pathol. 2015;235:515–526. doi: 10.1002/path.4466. [DOI] [PubMed] [Google Scholar]
- 42.Yao Y., Xu X.H., Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandooren B., Noordenbos T., Ambarus C., Krausz S., Cantaert T., Yeremenko N., Boumans M., Lutter R., Tak P.P., Baeten D. Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum. 2009;60:966–975. doi: 10.1002/art.24406. [DOI] [PubMed] [Google Scholar]
- 45.Schulert G.S., Fall N., Harley J.B., Shen N., Lovell D.J., Thornton S., Grom A.A. Monocyte MicroRNA Expression in Active Systemic Juvenile Idiopathic Arthritis Implicates MicroRNA-125a-5p in Polarized Monocyte Phenotypes. Arthritis Rheumatol. 2016;68:2300–2313. doi: 10.1002/art.39694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellins E.D., Macaubas C., Grom A.A. Pathogenesis of systemic juvenile idiopathic arthritis: Some answers, more questions. Nat. Rev. Rheumatol. 2011;7:416–426. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharya S., Yadav A., Aggarwal A. Evidence for M2 macrophage activation in patients with enthesitis-related arthritis category of juvenile idiopathic arthritis. Clin. Rheumatol. 2019;38:1715–1719. doi: 10.1007/s10067-018-04408-x. [DOI] [PubMed] [Google Scholar]
- 48.Sulahian T.H., Hogger P., Wahner A.E., Wardwell K., Goulding N.J., Sorg C., Droste A., Stehling M., Wallace P.K., Morganelli P.M., et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 49.Baeten D., Demetter P., Cuvelier C.A., Kruithof E., Van Damme N., De Vos M., Veys E.M., De Keyser F. Macrophages expressing the scavenger receptor CD163: A link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J. Pathol. 2002;196:343–350. doi: 10.1002/path.1044. [DOI] [PubMed] [Google Scholar]
- 50.Frings W., Dreier J., Sorg C. Only the soluble form of the scavenger receptor CD163 acts inhibitory on phorbol ester-activated T-lymphocytes, whereas membrane-bound protein has no effect. FEBS Lett. 2002;526:93–96. doi: 10.1016/S0014-5793(02)03142-3. [DOI] [PubMed] [Google Scholar]
- 51.Kristiansen M., Graversen J.H., Jacobsen C., Sonne O., Hoffman H.J., Law S.K., Moestrup S.K. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 52.Moestrup S.K., Moller H.J. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 53.Avci A.B., Feist E., Burmester G.R. Targeting GM-CSF in rheumatoid arthritis. Clin. Exp. Rheumatol. 2016;34:39–44. [PubMed] [Google Scholar]
- 54.Piper C., Pesenacker A.M., Bending D., Thirugnanabalan B., Varsani H., Wedderburn L.R., Nistala K. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014;66:1955–1960. doi: 10.1002/art.38647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swidrowska-Jaros J., Orczyk K., Smolewska E. Macrophages—Silent enemies in juvenile idiopathic arthritis. Postepy. Hig. Med. Dosw. 2016;70:743–750. doi: 10.5604/17322693.1208887. [DOI] [PubMed] [Google Scholar]
- 56.Chin J.E., Winterrowd G.E., Krzesicki R.F., Sanders M.E. Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990;33:1776–1786. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- 57.Haworth C., Brennan F.M., Chantry D., Turner M., Maini R.N., Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: Regulation by tumor necrosis factor-alpha. Eur. J. Immunol. 1991;21:2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 58.Butler D.M., Maini R.N., Feldmann M., Brennan F.M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur. Cytokine Netw. 1995;6:225–230. [PubMed] [Google Scholar]
- 59.Webster J.D., Vucic D. The Balance of TNF Mediated Pathways Regulates Inflammatory Cell Death Signaling in Healthy and Diseased Tissues. Front. Cell. Dev. Biol. 2020;8:365. doi: 10.3389/fcell.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Benedetti F., Pignatti P., Massa M., Sartirana P., Ravelli A., Cassani G., Corti A., Martini A. Soluble tumour necrosis factor receptor levels reflect coagulation abnormalities in systemic juvenile chronic arthritis. Br. J. Rheumatol. 1997;36:581–588. doi: 10.1093/rheumatology/36.5.581. [DOI] [PubMed] [Google Scholar]
- 61.Degboe Y., Rauwel B., Baron M., Boyer J.F., Ruyssen-Witrand A., Constantin A., Davignon J.L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019;10:3. doi: 10.3389/fimmu.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catrina A.I., Trollmo C., af Klint E., Engstrom M., Lampa J., Hermansson Y., Klareskog L., Ulfgren A.K. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: Extended report. Arthritis Rheum. 2005;52:61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 63.Vos A.C., Wildenberg M.E., Arijs I., Duijvestein M., Verhaar A.P., de Hertogh G., Vermeire S., Rutgeerts P., van den Brink G.R., Hommes D.W. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm. Bowel Dis. 2012;18:401–408. doi: 10.1002/ibd.21818. [DOI] [PubMed] [Google Scholar]
- 64.Aeberli D., Kamgang R., Balani D., Hofstetter W., Villiger P.M., Seitz M. Regulation of peripheral classical and non-classical monocytes on infliximab treatment in patients with rheumatoid arthritis and ankylosing spondylitis. RMD Open. 2016;2:e000079. doi: 10.1136/rmdopen-2015-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato M. New insights into IFN-gamma in rheumatoid arthritis: Role in the era of JAK inhibitors. Immunol. Med. 2020;43:72–78. doi: 10.1080/25785826.2020.1751908. [DOI] [PubMed] [Google Scholar]
- 66.Schulert G.S., Pickering A.V., Do T., Dhakal S., Fall N., Schnell D., Medvedovic M., Salomonis N., Thornton S., Grom A.A. Monocyte and bone marrow macrophage transcriptional phenotypes in systemic juvenile idiopathic arthritis reveal TRIM8 as a mediator of IFN-gamma hyper-responsiveness and risk for macrophage activation syndrome. Ann. Rheum. Dis. 2020;80:617–625. doi: 10.1136/annrheumdis-2020-217470. [DOI] [PubMed] [Google Scholar]
- 67.Macaubas C., Wong E., Zhang Y., Nguyen K.D., Lee J., Milojevic D., Shenoi S., Stevens A.M., Ilowite N., Saper V., et al. Altered signaling in systemic juvenile idiopathic arthritis monocytes. Clin. Immunol. 2016;163:66–74. doi: 10.1016/j.clim.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 69.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joosten L.A., Koenders M.I., Smeets R.L., Heuvelmans-Jacobs M., Helsen M.M., Takeda K., Akira S., Lubberts E., van de Loo F.A., van den Berg W.B. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: Critical role of myeloid differentiation factor 88. J. Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 71.Myles A., Aggarwal A. Expression of Toll-like receptors 2 and 4 is increased in peripheral blood and synovial fluid monocytes of patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis. Rheumatology. 2011;50:481–488. doi: 10.1093/rheumatology/keq362. [DOI] [PubMed] [Google Scholar]
- 72.Fall N., Barnes M., Thornton S., Luyrink L., Olson J., Ilowite N.T., Gottlieb B.S., Griffin T., Sherry D.D., Thompson S., et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 73.Pascual V., Allantaz F., Arce E., Punaro M., Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganeva M., Fuehner S., Kessel C., Klotsche J., Niewerth M., Minden K., Foell D., Hinze C.H., Wittkowski H. Trajectories of disease courses in the inception cohort of newly diagnosed patients with JIA (ICON-JIA): The potential of serum biomarkers at baseline. Pediatr. Rheumatol. Online J. 2021;19:64. doi: 10.1186/s12969-021-00553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schierbeck H., Pullerits R., Pruunsild C., Fischer M., Holzinger D., Laestadius A., Sundberg E., Harris H.E. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J. Rheumatol. 2013;40:1604–1613. doi: 10.3899/jrheum.120987. [DOI] [PubMed] [Google Scholar]
- 76.Dev S., Singh A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J. Fam. Med. Prim. Care. 2019;8:2129–2133. doi: 10.4103/jfmpc.jfmpc_339_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung J.Y., Kim J.W., Suh C.H., Kim H.A. Roles of Interactions Between Toll-Like Receptors and Their Endogenous Ligands in the Pathogenesis of Systemic Juvenile Idiopathic Arthritis and Adult-Onset Still’s Disease. Front. Immunol. 2020;11:583513. doi: 10.3389/fimmu.2020.583513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J., Vodovotz Y., Fan L., Li Y., Liu Z., Namas R., Barclay D., Zamora R., Billiar T.R., Wilson M.A., et al. Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. FASEB J. 2015;29:250–262. doi: 10.1096/fj.14-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Lent P.L., Grevers L.C., Schelbergen R., Blom A., Geurts J., Sloetjes A., Vogl T., Roth J., van den Berg W.B. S100A8 causes a shift toward expression of activatory Fcgamma receptors on macrophages via toll-like receptor 4 and regulates Fcgamma receptor expression in synovium during chronic experimental arthritis. Arthritis Rheum. 2010;62:3353–3364. doi: 10.1002/art.27654. [DOI] [PubMed] [Google Scholar]
- 80.Loser K., Vogl T., Voskort M., Lueken A., Kupas V., Nacken W., Klenner L., Kuhn A., Foell D., Sorokin L., et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 81.Cepika A.M., Banchereau R., Segura E., Ohouo M., Cantarel B., Goller K., Cantrell V., Ruchaud E., Gatewood E., Nguyen P., et al. A multidimensional blood stimulation assay reveals immune alterations underlying systemic juvenile idiopathic arthritis. J. Exp. Med. 2017;214:3449–3466. doi: 10.1084/jem.20170412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martini A., Ravelli A., Avcin T., Beresford M.W., Burgos-Vargas R., Cuttica R., Ilowite N.T., Khubchandani R., Laxer R.M., Lovell D.J., et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019;46:190–197. doi: 10.3899/jrheum.180168. [DOI] [PubMed] [Google Scholar]
- 83.Wu C.Y., Yang H.Y., Luo S.F., Lai J.H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021;22:686. doi: 10.3390/ijms22020686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C.Y., Yang H.Y., Lai J.H. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int. J. Mol. Sci. 2020;21:4015. doi: 10.3390/ijms21114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bosco M.C., Delfino S., Ferlito F., Puppo M., Gregorio A., Gambini C., Gattorno M., Martini A., Varesio L. The hypoxic synovial environment regulates expression of vascular endothelial growth factor and osteopontin in juvenile idiopathic arthritis. J. Rheumatol. 2009;36:1318–1329. doi: 10.3899/jrheum.080782. [DOI] [PubMed] [Google Scholar]
- 86.Bosco M.C., Delfino S., Ferlito F., Battaglia F., Puppo M., Gregorio A., Gambini C., Gattorno M., Martini A., Varesio L. Hypoxic synovial environment and expression of macrophage inflammatory protein 3gamma/CCL20 in juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1833–1838. doi: 10.1002/art.23516. [DOI] [PubMed] [Google Scholar]
- 87.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cramer T., Yamanishi Y., Clausen B.E., Forster I., Pawlinski R., Mackman N., Haase V.H., Jaenisch R., Corr M., Nizet V., et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raggi F., Pelassa S., Pierobon D., Penco F., Gattorno M., Novelli F., Eva A., Varesio L., Giovarelli M., Bosco M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017;8:1097. doi: 10.3389/fimmu.2017.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shingu M., Nagai Y., Isayama T., Naono T., Nobunaga M., Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin. Exp. Immunol. 1993;94:145–149. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Do T., Tan R., Bennett M., Medvedovic M., Grom A.A., Shen N., Thornton S., Schulert G.S. MicroRNA networks associated with active systemic juvenile idiopathic arthritis regulate CD163 expression and anti-inflammatory functions in macrophages through two distinct mechanisms. J. Leukoc. Biol. 2018;103:71–85. doi: 10.1002/JLB.2A0317-107R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niu X., Schulert G.S. Functional Regulation of Macrophage Phenotypes by MicroRNAs in Inflammatory Arthritis. Front. Immunol. 2019;10:2217. doi: 10.3389/fimmu.2019.02217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bauernfeind F., Rieger A., Schildberg F.A., Knolle P.A., Schmid-Burgk J.L., Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 94.Chen Q., Wang H., Liu Y., Song Y., Lai L., Han Q., Cao X., Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1beta production in macrophages by targeting STAT3. PLoS ONE. 2012;7:e42971. doi: 10.1371/journal.pone.0042971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Georgopoulos S., Plows D., Kollias G. Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J. Inflamm. 1996;46:86–97. [PubMed] [Google Scholar]
- 96.Saxena N., Aggarwal A., Misra R. Elevated concentrations of monocyte derived cytokines in synovial fluid of children with enthesitis related arthritis and polyarticular types of juvenile idiopathic arthritis. J. Rheumatol. 2005;32:1349–1353. [PubMed] [Google Scholar]
- 97.Luo G., Li F., Li X., Wang Z.G., Zhang B. TNFalpha and RANKL promote osteoclastogenesis by upregulating RANK via the NFkappaB pathway. Mol. Med. Rep. 2018;17:6605–6611. doi: 10.3892/mmr.2018.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akioka S. Interleukin-6 in juvenile idiopathic arthritis. Mod. Rheumatol. 2019;29:275–286. doi: 10.1080/14397595.2019.1574697. [DOI] [PubMed] [Google Scholar]
- 99.Armaroli G., Klein A., Ganser G., Ruehlmann M.J., Dressler F., Hospach A., Minden K., Trauzeddel R., Foeldvari I., Kuemmerle-Deschner J., et al. Long-term safety and effectiveness of etanercept in JIA: An 18-year experience from the BiKeR registry. Arthritis Res.Ther. 2020;22:258. doi: 10.1186/s13075-020-02326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lovell D.J., Brunner H.I., Reiff A.O., Jung L., Jarosova K., Nemcova D., Mouy R., Sandborg C., Bohnsack J.F., Elewaut D., et al. Long-term outcomes in patients with polyarticular juvenile idiopathic arthritis receiving adalimumab with or without methotrexate. RMD Open. 2020;6:e001208. doi: 10.1136/rmdopen-2020-001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruperto N., Brunner H.I., Pacheco-Tena C., Louw I., Vega-Cornejo G., Spindler A.J., Kingsbury D.J., Schmeling H., Borzutzky A., Cuttica R., et al. Open-Label Phase 3 Study of Intravenous Golimumab in Patients With Polyarticular Juvenile Idiopathic Arthritis. Rheumatology. 2021;67:2759–2770. doi: 10.1093/rheumatology/keab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson D.C., Marinov A.D., Blair H.C., Bushnell D.S., Thompson S.D., Chaly Y., Hirsch R. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frosch M., Ahlmann M., Vogl T., Wittkowski H., Wulffraat N., Foell D., Roth J. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:883–891. doi: 10.1002/art.24349. [DOI] [PubMed] [Google Scholar]
- 104.Shiratori T., Kyumoto-Nakamura Y., Kukita A., Uehara N., Zhang J., Koda K., Kamiya M., Badawy T., Tomoda E., Xu X., et al. IL-1beta Induces Pathologically Activated Osteoclasts Bearing Extremely High Levels of Resorbing Activity: A Possible Pathological Subpopulation of Osteoclasts, Accompanied by Suppressed Expression of Kindlin-3 and Talin-1. J. Immunol. 2018;200:218–228. doi: 10.4049/jimmunol.1602035. [DOI] [PubMed] [Google Scholar]
- 105.Yang C.M., Luo S.F., Hsieh H.L., Chi P.L., Lin C.C., Wu C.C., Hsiao L.D. Interleukin-1beta induces ICAM-1 expression enhancing leukocyte adhesion in human rheumatoid arthritis synovial fibroblasts: Involvement of ERK, JNK, AP-1, and NF-kappaB. J. Cell Physiol. 2010;224:516–526. doi: 10.1002/jcp.22153. [DOI] [PubMed] [Google Scholar]
- 106.Nishimura K., Hara R., Umebayashi H., Takei S., Iwata N., Imagawa T., Shimizu M., Tomiita M., Seko N., Kitawaki T., et al. Efficacy and safety of canakinumab in systemic juvenile idiopathic arthritis: 48-week results from an open-label phase III study in Japanese patients. Mod. Rheumatol. 2021;31:226–234. doi: 10.1080/14397595.2020.1783163. [DOI] [PubMed] [Google Scholar]
- 107.Brunner H.I., Quartier P., Alexeeva E., Constantin T., Kone-Paut I., Marzan K., Schneider R., Wulffraat N.M., Chasnyk V., Tirosh I., et al. Efficacy and Safety of Canakinumab in Patients With Systemic Juvenile Idiopathic Arthritis With and Without Fever at Baseline: Results From an Open-Label, Active-Treatment Extension Study. Arthritis Rheumatol. 2020;72:2147–2158. doi: 10.1002/art.41436. [DOI] [PubMed] [Google Scholar]
- 108.Monteagudo L.A., Boothby A., Gertner E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020;2:276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atemnkeng Ntam V., Klein A., Horneff G. Safety and efficacy of anakinra as first-line or second-line therapy for systemic onset juvenile idiopathic arthritis—data from the German BIKER registry. Expert Opin. Drug Saf. 2021;20:93–100. doi: 10.1080/14740338.2021.1843631. [DOI] [PubMed] [Google Scholar]
- 110.Silacci P., Dayer J.M., Desgeorges A., Peter R., Manueddu C., Guerne P.A. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J. Biol. Chem. 1998;273:13625–13629. doi: 10.1074/jbc.273.22.13625. [DOI] [PubMed] [Google Scholar]
- 111.Sarma P.K., Misra R., Aggarwal A. Elevated serum receptor activator of NFkappaB ligand (RANKL), osteoprotegerin (OPG), matrix metalloproteinase (MMP)3, and ProMMP1 in patients with juvenile idiopathic arthritis. Clin. Rheumatol. 2008;27:289–294. doi: 10.1007/s10067-007-0701-3. [DOI] [PubMed] [Google Scholar]
- 112.Dienz O., Rincon M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimizu M., Nakagishi Y., Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine. 2013;61:345–348. doi: 10.1016/j.cyto.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu M., Nakagishi Y., Inoue N., Mizuta M., Ko G., Saikawa Y., Kubota T., Yamasaki Y., Takei S., Yachie A. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin. Immunol. 2015;160:277–281. doi: 10.1016/j.clim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Boe A., Baiocchi M., Carbonatto M., Papoian R., Serlupi-Crescenzi O. Interleukin 6 knock-out mice are resistant to antigen-induced experimental arthritis. Cytokine. 1999;11:1057–1064. doi: 10.1006/cyto.1999.0502. [DOI] [PubMed] [Google Scholar]
- 116.De Benedetti F., Brunner H.I., Ruperto N., Kenwright A., Wright S., Calvo I., Cuttica R., Ravelli A., Schneider R., Woo P., et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 117.Brunner H.I., Ruperto N., Zuber Z., Cuttica R., Keltsev V., Xavier R.M., Burgos-Vargas R., Penades I.C., Silverman E.D., Espada G., et al. Efficacy and Safety of Tocilizumab for Polyarticular-Course Juvenile Idiopathic Arthritis in the Open-Label Two-Year Extension of a Phase III Trial. Arthritis Rheumatol. 2021;73:530–541. doi: 10.1002/art.41528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mizuta M., Shimizu M., Inoue N., Ikawa Y., Nakagishi Y., Yasuoka R., Iwata N., Yachie A. Clinical significance of interleukin-18 for the diagnosis and prediction of disease course in systemic juvenile idiopathic arthritis. Rheumatology. 2021;60:2421–2426. doi: 10.1093/rheumatology/keaa634. [DOI] [PubMed] [Google Scholar]
- 119.Volin M.V., Koch A.E. Interleukin-18: A mediator of inflammation and angiogenesis in rheumatoid arthritis. J. Interferon Cytokine Res. 2011;31:745–751. doi: 10.1089/jir.2011.0050. [DOI] [PubMed] [Google Scholar]
- 120.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teng M.W., Bowman E.P., McElwee J.J., Smyth M.J., Casanova J.L., Cooper A.M., Cua D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 122.Bridgewood C., Sharif K., Sherlock J., Watad A., McGonagle D. Interleukin-23 pathway at the enthesis: The emerging story of enthesitis in spondyloarthropathy. Immunol. Rev. 2020;294:27–47. doi: 10.1111/imr.12840. [DOI] [PubMed] [Google Scholar]
- 123.Tsukazaki H., Kaito T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int. J. Mol. Sci. 2020;21:6401. doi: 10.3390/ijms21176401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maggi L., Mazzoni A., Cimaz R., Liotta F., Annunziato F., Cosmi L. Th17 and Th1 Lymphocytes in Oligoarticular Juvenile Idiopathic Arthritis. Front. Immunol. 2019;10:450. doi: 10.3389/fimmu.2019.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koenders M.I., Lubberts E., van de Loo F.A., Oppers-Walgreen B., van den Bersselaar L., Helsen M.M., Kolls J.K., Di Padova F.E., Joosten L.A., van den Berg W.B. Interleukin-17 acts independently of TNF-alpha under arthritic conditions. J. Immunol. 2006;176:6262–6269. doi: 10.4049/jimmunol.176.10.6262. [DOI] [PubMed] [Google Scholar]
- 126.Ouyang W., Rutz S., Crellin N.K., Valdez P.A., Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 127.Mahendra A., Misra R., Aggarwal A. Th1 and Th17 Predominance in the Enthesitis-related Arthritis Form of Juvenile Idiopathic Arthritis. J. Rheumatol. 2009;36:1730–1736. doi: 10.3899/jrheum.081179. [DOI] [PubMed] [Google Scholar]
- 128.Beringer A., Miossec P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019;15:491–501. doi: 10.1038/s41584-019-0243-5. [DOI] [PubMed] [Google Scholar]
- 129.Griffin G.K., Newton G., Tarrio M.L., Bu D.X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F.W., Croce K.J., et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Waal Malefyt R., Abrams J., Bennett B., Figdor C.G., de Vries J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harsini S., Saghazadeh A., Nedjat S., Rezaei N. Associations between interleukin-10 polymorphisms and susceptibility to juvenile idiopathic arthritis: A systematic review and meta-analysis. Eur. Cytokine Netw. 2018;29:16–26. doi: 10.1684/ecn.2018.0404. [DOI] [PubMed] [Google Scholar]
- 132.Imbrechts M., Avau A., Vandenhaute J., Malengier-Devlies B., Put K., Mitera T., Berghmans N., Burton O., Junius S., Liston A., et al. Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis. J. Immunol. 2018;201:2654–2663. doi: 10.4049/jimmunol.1800468. [DOI] [PubMed] [Google Scholar]
- 133.Brescia A.C., Simonds M.M., Sullivan K.E., Rose C.D. Secretion of pro-inflammatory cytokines and chemokines and loss of regulatory signals by fibroblast-like synoviocytes in juvenile idiopathic arthritis. Proteom. Clin. Appl. 2017;11:1600088. doi: 10.1002/prca.201600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pavkova Goldbergova M., Lipkova J., Pavek N., Gatterova J., Vasku A., Soucek M., Nemec P. RANTES, MCP-1 chemokines and factors describing rheumatoid arthritis. Mol. Immunol. 2012;52:273–278. doi: 10.1016/j.molimm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Schraufstatter I.U., Zhao M., Khaldoyanidi S.K., Discipio R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology. 2012;135:287–298. doi: 10.1111/j.1365-2567.2011.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamasaki Y., Takei S., Imanaka H., Nerome Y., Kubota T., Nonaka Y., Akaike H., Takezaki T., Kawano Y. Prediction of long-term remission of oligo/polyarticular juvenile idiopathic arthritis with S100A12 and vascular endothelial growth factor. Mod. Rheumatol. 2016;26:551–556. doi: 10.3109/14397595.2015.1109784. [DOI] [PubMed] [Google Scholar]
- 137.Tsianakas A., Varga G., Barczyk K., Bode G., Nippe N., Kran N., Roth J., Luger T.A., Ehrchen J., Sunderkoetter C. Induction of an anti-inflammatory human monocyte subtype is a unique property of glucocorticoids, but can be modified by IL-6 and IL-10. Immunobiology. 2012;217:329–335. doi: 10.1016/j.imbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 138.Swidrowska-Jaros J., Smolewska E. A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent. Eur. J. Immunol. 2018;43:325–330. doi: 10.5114/ceji.2018.80052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim H.R., Kim K.W., Kim B.M., Cho M.L., Lee S.H. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PLoS ONE. 2015;10:e0124909. doi: 10.1371/journal.pone.0124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vignola S., Picco P., Falcini F., Sabatini F., Buoncompagni A., Gattorno M. Serum and synovial fluid concentration of vascular endothelial growth factor in juvenile idiopathic arthritides. Rheumatology. 2002;41:691–696. doi: 10.1093/rheumatology/41.6.691. [DOI] [PubMed] [Google Scholar]
- 141.Koch A.E. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 2003;62:60–67. doi: 10.1136/ard.62.suppl_2.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siebelt M., Korthagen N., Wei W., Groen H., Bastiaansen-Jenniskens Y., Muller C., Waarsing J.H., de Jong M., Weinans H. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res. Ther. 2015;17:352. doi: 10.1186/s13075-015-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Municio C., Soler Palacios B., Estrada-Capetillo L., Benguria A., Dopazo A., Garcia-Lorenzo E., Fernandez-Arroyo S., Joven J., Miranda-Carus M.E., Gonzalez-Alvaro I., et al. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann. Rheum. Dis. 2016;75:2157–2165. doi: 10.1136/annrheumdis-2015-208736. [DOI] [PubMed] [Google Scholar]
- 144.Municio C., Dominguez-Soto A., Fuentelsaz-Romero S., Lamana A., Montes N., Cuevas V.D., Campos R.G., Pablos J.L., Gonzalez-Alvaro I., Puig-Kroger A. Methotrexate limits inflammation through an A20-dependent cross-tolerance mechanism. Ann. Rheum. Dis. 2018;77:752–759. doi: 10.1136/annrheumdis-2017-212537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gremese E., Alivernini S., Tolusso B., Zeidler M.P., Ferraccioli G. JAK inhibition by methotrexate (and csDMARDs) may explain clinical efficacy as monotherapy and combination therapy. J. Leukoc. Biol. 2019;106:1063–1068. doi: 10.1002/JLB.5RU0519-145R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oerlemans R., Vink J., Dijkmans B.A., Assaraf Y.G., van Miltenburg M., van der Heijden J., Ifergan I., Lems W.F., Scheper R.J., Kaspers G.J., et al. Sulfasalazine sensitises human monocytic/macrophage cells for glucocorticoids by upregulation of glucocorticoid receptor alpha and glucocorticoid induced apoptosis. Ann. Rheum. Dis. 2007;66:1289–1295. doi: 10.1136/ard.2006.060509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Giancane G., Alongi A., Ravelli A. Update on the pathogenesis and treatment of juvenile idiopathic arthritis. Curr. Opin. Rheumatol. 2017;29:523–529. doi: 10.1097/BOR.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 148.Onuora S. Experimental arthritis: Anti-TNF kills the macrophage response. Nat. Rev. Rheumatol. 2018;14:64. doi: 10.1038/nrrheum.2017.207. [DOI] [PubMed] [Google Scholar]
- 149.Huang Q.Q., Birkett R., Doyle R., Shi B., Roberts E.L., Mao Q., Pope R.M. The Role of Macrophages in the Response to TNF Inhibition in Experimental Arthritis. J. Immunol. 2018;200:130–138. doi: 10.4049/jimmunol.1700229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tono T., Aihara S., Hoshiyama T., Arinuma Y., Nagai T., Hirohata S. Effects of anti-IL-6 receptor antibody on human monocytes. Mod. Rheumatol. 2015;25:79–84. doi: 10.3109/14397595.2014.914016. [DOI] [PubMed] [Google Scholar]
- 151.Obeng J.A., Amoruso A., Camaschella G.L., Sola D., Brunelleschi S., Fresu L.G. Modulation of human monocyte/macrophage activity by tocilizumab, abatacept and etanercept: An in vitro study. Eur. J. Pharmacol. 2016;780:33–37. doi: 10.1016/j.ejphar.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 152.Ruperto N., Synoverska O., Ting T., Abud-Mendoza C., Spindler A., Vyzhga Y., Marzan K., Keltsev V., Tirosh I., Imundo L., et al. OP0291TOFACITINIB for the treatment of polyarticular course juvenile idiopathic arthritis: Results of a phase 3, randomised, double-blind, placebo-controlled withdrawal study. Ann. Rheum. Dis. 2020;79:180–181. doi: 10.1136/annrheumdis-2020-eular.396. [DOI] [Google Scholar]
- 153.Boyle D.L., Soma K., Hodge J., Kavanaugh A., Mandel D., Mease P., Shurmur R., Singhal A.K., Wei N., Rosengren S., et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1311–1316. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boyer J.F., Baron M., Constantin A., Degboe Y., Cantagrel A., Davignon J.L. Anti-TNF certolizumab pegol induces antioxidant response in human monocytes via reverse signaling. Arthritis Res. Ther. 2016;18:56. doi: 10.1186/s13075-016-0955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Gupta S., Ilstad-Minnihan A., Ayyangar S., Hay A.D., Pascual V., Ilowite N.T., Macaubas C., Mellins E.D. Interleukin-1 in monocyte activation phenotypes in systemic juvenile idiopathic arthritis: Observations from a clinical trial of rilonacept, an interleukin-1 inhibitor. Clin. Immunol. 2018;194:9–18. doi: 10.1016/j.clim.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Benedetti F.D., Penadés I.C., Pérez N.E.R., Maschan A., Quartier P., Żuber Z., Stanislav M., Barria R., Garulo D.C., Cornejo G.V., et al. FRI0549SARILUMAB, a human monoclonal antibody to the interleukin-6 (il-6) receptor, in polyarticular-course juvenile idiopathic arthritis (pcjia): A 12-week multinational open-label dose-finding study. Ann. Rheum. Dis. 2019;78:969–970. [Google Scholar]
- 157.Reich K., Yasothan U., Kirkpatrick P. Ustekinumab. Nat. Rev. Drug Discov. 2009;8:355–356. doi: 10.1038/nrd2878. [DOI] [PubMed] [Google Scholar]
- 158.Kaaij M.H., Helder B., van Mens L.J.J., van de Sande M.G.H., Baeten D.L.P., Tas S.W. Anti-IL-17A treatment reduces serum inflammatory, angiogenic and tissue remodeling biomarkers accompanied by less synovial high endothelial venules in peripheral spondyloarthritis. Sci. Rep. 2020;10:21094. doi: 10.1038/s41598-020-78204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mease P.J., van der Heijde D., Ritchlin C.T., Okada M., Cuchacovich R.S., Shuler C.L., Lin C.Y., Braun D.K., Lee C.H., Gladman D.D., et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Al-Salama Z.T. Emapalumab: First Global Approval. Drugs. 2019;79:99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- 161.Bozec A., Luo Y., Engdahl C., Figueiredo C., Bang H., Schett G. Abatacept blocks anti-citrullinated protein antibody and rheumatoid factor mediated cytokine production in human macrophages in IDO-dependent manner. Arthritis Res. Ther. 2018;20:24. doi: 10.1186/s13075-018-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wenink M.H., Santegoets K.C., Platt A.M., van den Berg W.B., van Riel P.L., Garside P., Radstake T.R., McInnes I.B. Abatacept modulates proinflammatory macrophage responses upon cytokine-activated T cell and Toll-like receptor ligand stimulation. Ann. Rheum. Dis. 2012;71:80–83. doi: 10.1136/annrheumdis-2011-200348. [DOI] [PubMed] [Google Scholar]
- 163.Magnol M., Rauwel B., Sayegh S., Diallo K., Baron M., Lacassagne E., Boyer J.-F., Ruyssen-Witrand A., Constantin A., Davignon J.-L., et al. AB0040JAK inhibitors—Baricitinib and tofacitinib—Modulate the in vitro inflammatory and alternative polarizations of macrophages. Ann. Rheum. Dis. 2019;78:1486–1487. [Google Scholar]
- 164.Parmentier J.M., Voss J., Graff C., Schwartz A., Argiriadi M., Friedman M., Camp H.S., Padley R.J., George J.S., Hyland D., et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takeuchi T., Tanaka Y., Close D., Godwood A., Wu C.Y., Saurigny D. Efficacy and safety of mavrilimumab in Japanese subjects with rheumatoid arthritis: Findings from a Phase IIa study. Mod. Rheumatol. 2015;25:21–30. doi: 10.3109/14397595.2014.896448. [DOI] [PubMed] [Google Scholar]
- 166.Greven D.E., Cohen E.S., Gerlag D.M., Campbell J., Woods J., Davis N., van Nieuwenhuijze A., Lewis A., Heasmen S., McCourt M., et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1924–1930. doi: 10.1136/annrheumdis-2014-205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leoni F., Fossati G., Lewis E.C., Lee J.K., Porro G., Pagani P., Modena D., Moras M.L., Pozzi P., Reznikov L.L., et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol. Med. 2005;11:1–15. doi: 10.2119/2006-00005.Dinarello. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang C.S., Sun H.L., Lii C.K., Chen H.W., Chen P.Y., Liu K.L. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-kappaB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation. 2010;33:46–57. doi: 10.1007/s10753-009-9157-8. [DOI] [PubMed] [Google Scholar]
- 169.Liu W., Zhang Y., Zhu W., Ma C., Ruan J., Long H., Wang Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front. Immunol. 2018;9:2228. doi: 10.3389/fimmu.2018.02228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun W., Zhang H., Wang H., Chiu Y.G., Wang M., Ritchlin C.T., Kiernan A., Boyce B.F., Xing L. Targeting Notch-Activated M1 Macrophages Attenuates Joint Tissue Damage in a Mouse Model of Inflammatory Arthritis. J. Bone Miner. Res. 2017;32:1469–1480. doi: 10.1002/jbmr.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sultana F., Neog M.K., Rasool M. Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surf. B. Biointerfaces. 2017;155:349–365. doi: 10.1016/j.colsurfb.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 172.Yu Y., Cai W., Zhou J., Lu H., Wang Y., Song Y., He R., Pei F., Wang X., Zhang R., et al. Anti-arthritis effect of berberine associated with regulating energy metabolism of macrophages through AMPK/ HIF-1alpha pathway. Int. Immunopharmacol. 2020;87:106830. doi: 10.1016/j.intimp.2020.106830. [DOI] [PubMed] [Google Scholar]
- 173.Abdel-Maged A.E., Gad A.M., Wahdan S.A., Azab S.S. Efficacy and safety of Ramucirumab and methotrexate co-therapy in rheumatoid arthritis experimental model: Involvement of angiogenic and immunomodulatory signaling. Toxicol. Appl. Pharmacol. 2019;380:114702. doi: 10.1016/j.taap.2019.114702. [DOI] [PubMed] [Google Scholar]
- 174.Abdel-Maged A.E., Gad A.M., Abdel-Aziz A.K., Aboulwafa M.M., Azab S.S. Comparative study of anti-VEGF Ranibizumab and Interleukin-6 receptor antagonist Tocilizumab in Adjuvant-induced Arthritis. Toxicol. Appl. Pharmacol. 2018;356:65–75. doi: 10.1016/j.taap.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 175.Shankar J., Thippegowda P.B., Kanum S.A. Inhibition of HIF-1alpha activity by BP-1 ameliorates adjuvant induced arthritis in rats. Biochem. Biophys. Res. Commun. 2009;387:223–228. doi: 10.1016/j.bbrc.2009.01.086. [DOI] [PubMed] [Google Scholar]
- 176.Xu J., Feng Z., Chen S., Zhu J., Wu X., Chen X., Li J. Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels. Clin. Rheumatol. 2019;38:19–27. doi: 10.1007/s10067-017-3646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu Y., Zhang T., Chen J., Cheng W., Chen J., Zheng Z., Lin J., Zhu G., Zhang Y., Bai X., et al. Downregulation of Hypoxia-Inducible Factor-1alpha by RNA Interference Alleviates the Development of Collagen-Induced Arthritis in Rats. Mol. Ther. Nucleic. Acids. 2020;19:1330–1342. doi: 10.1016/j.omtn.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barrera P., Blom A., van Lent P.L., van Bloois L., Beijnen J.H., van Rooijen N., de Waal Malefijt M.C., van de Putte L.B., Storm G., van den Berg W.B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43:1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 179.Shin T.H., Kim H.S., Kang T.W., Lee B.C., Lee H.Y., Kim Y.J., Shin J.H., Seo Y., Won Choi S., Lee S., et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. doi: 10.1038/cddis.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]