Abstract
Zinc-finger proteins, a superfamily of proteins with a typical structural domain that coordinates a zinc ion and binds nucleic acids, participate in the regulation of growth, development, and stress adaptation in plants. Most zinc fingers are C2H2-type or CCCC-type, named after the configuration of cysteine (C) and histidine (H); the less-common CCCH zinc-finger proteins are important in the regulation of plant stress responses. In this review, we introduce the domain structures, classification, and subcellular localization of CCCH zinc-finger proteins in plants and discuss their functions in transcriptional and post-transcriptional regulation via interactions with DNA, RNA, and other proteins. We describe the functions of CCCH zinc-finger proteins in plant development and tolerance to abiotic stresses such as salt, drought, flooding, cold temperatures and oxidative stress. Finally, we summarize the signal transduction pathways and regulatory networks of CCCH zinc-finger proteins in their responses to abiotic stress. CCCH zinc-finger proteins regulate the adaptation of plants to abiotic stress in various ways, but the specific molecular mechanisms need to be further explored, along with other mechanisms such as cytoplasm-to-nucleus shuttling and post-transcriptional regulation. Unraveling the molecular mechanisms by which CCCH zinc-finger proteins improve stress tolerance will facilitate the breeding and genetic engineering of crops with improved traits.
Keywords: abiotic stresses, CCCH zinc-finger proteins, plants, regulation pathways, transcription factor
1. Introduction
Throughout their lives, plants are exposed to various complex environmental conditions [1,2,3,4,5]. Environmental conditions that are not conducive to plant development and growth are collectively referred to as stress [6,7,8]. Generally speaking, stresses include biotic stresses (bacteria, viruses, and insect pests) and abiotic stresses (drought, high and low temperatures, high salt levels, waterlogging and heavy metals) [8,9,10,11]. Abiotic stresses can seriously decrease the growth and yield of crops and are important factors limiting crop yields worldwide [6,12,13,14]. Salt and drought stresses affect 10% of the world’s arable land, reducing global crop yields by more than 50%, whereas biotic stresses such as pests and diseases also result in substantial crop losses [15,16,17].
To adapt to variable environmental conditions, plants have evolved diverse regulatory pathways to receive and respond to different stress signals [18,19]. When a plant is subjected to stress, it activates the corresponding regulatory pathway; the resulting responses help it to survive under the stress [7,20,21,22]. In harsh conditions such as high salt levels, extreme drought and high concentrations of heavy metals or arsenic, halophytes [23,24], xerophytes [25,26] and arsenic hyperaccumulators [27,28] have formed their own unique morphology that resists abiotic stress during long-term evolution. Plant responses to abiotic stresses involve a large number of transcription factors [29,30,31,32]. Results from genome-wide sequencing, differential transcriptome analysis, and functional analysis of numerous genes have shown that the transcription factor families involved in abiotic stress tolerance in plants include bHLH (basic helix-loop-helix) [33,34,35], WRKY [10,36,37,38], bZIP (basic leucine zipper) [39,40,41], homeodomain [42,43], HSF [3,44,45,46], NAC [47,48,49], MYB [50,51,52], MADS-box [53,54,55], AP2/ERF [56,57,58], and zinc-finger proteins [59,60,61]. The large and diverse zinc-finger protein family plays important roles in all aspects of plant growth and development. The first zinc-finger protein (TF IIIA) involved in transcriptional regulation was found in Xenopus laevis oocytes in 1985; since then, zinc fingers with a variety of functions have been found in animals, plants, yeasts, and viruses [62].
Plant genomes encode large numbers of CCCH zinc-finger proteins. For example, genomic surveys identified 68 CCCH zinc-finger protein genes in Arabidopsis thaliana (L.) Heynh [63], 67 in Oryza sativa L. [63], 68 in Zea mays L. [64], 91 in Populus trichocarpa Torr. & Gray [65], 34 in Medicago truncatula Gaertn. [66], 36 in Aegilops tauschii Coss. [67], 80 in Solanum lycopersicum L. [68], 69 in Vitis vinifera L. [69], 58 in Cicer arietinum L. [70], and 103 in Brassica rapa L. ssp. Pekinensis [71]. CCCH zinc-finger protein genes participating in many biological functions such as development and growth have been cloned and studied in leaf senescence in rice (OsDOS) [72], seed germination in Arabidopsis (AtTZF4, AtTZF5, and AtTZF6) [73], flowering time in Medicago sativa L. (MsZFN) [74], cell elongation in Arabidopsis (AtC3H14) [75], secondary xylem formation in poplar (PdC3H17 and PdC3H18, a homolog of AtC3H14) [76], seed storage in rice (OsGZF1) [77], anther development and secondary wall thickening in poplar (C3H14 and C3H15) [78], natural rubber biosynthesis in Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg. (HbCZF1) [79], and oleic acid homeostasis in Brassica napus L. (BnZFP1) [80]. In addition to these processes, CCCH zinc-finger proteins play key roles in regulating the tolerance of plants to abiotic and biotic stresses [81]. Here, we focus on their role in plant responses to abiotic stress.
2. Domain Structure of CCCH Zinc-Finger Proteins
A characteristic feature of zinc-finger proteins is the zinc-finger domain, which forms a finger-shaped tetrahedral structure by binding zinc ions, and is an important domain in eukaryotic transcription factors [82,83]. Based on their structural features, zinc-finger domains can be divided into several different categories [84]. The most common zinc fingers in transcription factors are C2H2-type or CCCC-type, named after the configuration of cysteine (C) and histidine (H) in the zinc finger domain [85]. Compared with other types of zinc finger proteins, CCCH zinc fingers are less common, accounting for about 0.8% of all zinc fingers [86,87], and these have their own specific biological functions.
The CCCH zinc-finger proteins of plants have one to six copies of the conserved CCCH motif [88]. CCCH motifs vary in the number of amino acid residues separating the conserved Cys and His residues. The consensus sequence was originally defined as C-X6–14-C-X4–5-C-X3–4-H [89], but a detailed analysis of CCCH zinc-finger motifs in Arabidopsis and rice identified five CCCH motifs not mentioned in the original classification: C-X4-C-X5-C-X3-H, C-X5-C-X4-C-X3-H, C-X7-C-X6-C-X3-H, C-X8-C-X6-C-X3-H, and C-X15-C-X5-C-X3-H. Consequently, the motif has been redefined as C-X4–15-C-X4–6-C-X3–4-H [63]. The same analysis revealed that CCCH zinc-finger proteins containing the C-X7–8-C-X5-C-X3-H motif in the middle of the protein are the most prevalent, suggesting that this motif may be the original from which the others were derived [65]. With continuous research on CCCH zinc-finger proteins in different plants, it was found that the same pattern is present in other model plants [71]. Furthermore, the same study also found that the CCCH domain of CCCH zinc-finger proteins plays a key role in plant resistance [90].
A protein containing a tandem CCCH zinc-finger (TZF) motif is called a TZF protein [91]. Generally speaking, the TZF motif in animals is composed of two CCCH motifs (C-X7-8-C-X5-C-X3-H) separated by 18 amino acids [92]. However, it is variable in plants compared with animals. For example, TZF protein AtTZF1 in Arabidospsis contains two different motifs, C-X7-C-X5-C-X3-H and C-X5-C-X4-C-X3-H, separated by 16 amino acids [75,93], while in cotton, GhZFP1 also contains two different motifs, C-X8-C-X5-C-X3-H and C-X5-C-X4-C-X3-H zinc, separated by 16 amino acids [84]. Many TZF proteins have a plant unique arginine-rich (RR) region in front of the TZF motif, called RR-TZF, and the function of the RR-TZF family has been more deeply identified and studied in many model plants, including Arabidopsis and rice [94]. Additionally, zinc-finger proteins that do not have tandem motifs are called non-TZF proteins [95]. To date, TZF CCCH zinc-finger genes have been more extensively studied than non-TZF protein genes in plants [81,91].
In addition to the above structures, CCCH zinc-finger proteins have some other domains that are compatible with their biological functions. Many CCCH proteins contain a N- or C-terminal nuclear export signal sequence (NES) and/or nuclear localization sequence (NLS), which set their subcellular localization [84,96], as described below. Moreover, many CCCH zinc-finger proteins can activate transcription in Arabidopsis protoplasts or yeast systems, and domains involved in transcriptional activation have been identified at different positions within their protein sequences, such as the N terminus of the protein [90]. Furthermore, the N terminus of CCCH zinc-finger proteins is highly conserved among plants and can bind to DNA and RNA [97]. Some CCCH proteins participate in RNA metabolism and many of these carry RNA recognition motifs (RRMs) [98]. The different domain structures of CCCH proteins will be described in more detail in the below section, where we discuss protein localization and function.
3. Subcellular Localization of CCCH Zinc-Finger Proteins
To function, proteins must be localized to the appropriate part of the cell; in turn, a protein’s location in a cell can offer clues about its function [99,100]. So far, CCCH zinc-finger proteins show different patterns of localization: some are located in the nucleus, such as GhZFP1 [72], AtTZF11 [101], OsDOS [84], AtZFP1 [21], KHZ1 and KHZ2 [102], SAW1 [103] and OsC3H10 [104]. Some are located in the cytoplasm plasma membrane, such as Oxidation-related Zinc Finger 1 (AtOZF1) [105] and AtOZF2 [106]. Some are located in the cytoplasm, such as ZFP36L3 [107], ZC3H12a [108], and AtTZF2/3 [109], while some can shuttle between the cytoplasm and the nucleus, such as AtTZF1, AtTZF4, AtTZF5, AtTZF6, AtTZF7, and OsLIC [73,93,110,111]. A previous study found that the CCCH zinc-finger proteins ZAP, TTP, CMG1, and TIS11D in animals shuttle between the cytoplasm and the nucleus mainly through their N- or C-terminal NESs and NLSs. The leucine-rich NES also interacts with the nuclear export receptor CRM1 in the nuclear pore complex [96,112,113]. NESs are present in 54 CCCH proteins in Arabidopsis, including the whole 11 proteins of the IX subfamily, and the proteins such as AtTZF1, AtTZF2, AtTZF3, AtTZF4, AtTZF5, AtTZF6, AtTZF7 and AtTZF11 mentioned above were the representative members [63]. The widespread presence of shuttle signals in plant CCCH proteins suggests that they may be intra- and extranuclear shuttle proteins with important roles in signal transduction and stress responses [65]. In addition, AtTZF1, AtTZF4, AtTZF5, AtTZF6 and OsC3H10 co-localize with processing bodies (PBs) and stress granules (SGs) in the cytoplasm [104]. As aggregates of cytoplasmic messenger ribonucleoprotein complexes, PBs and SGs play important roles in the post-transcriptional regulation of genes and have been highly conserved during evolution. PBs and SGs play an important role in plant tolerance to abiotic stress [97]. AtTZF1, AtTZF4, AtTZF5, and AtTZF6 may function through a mechanism associated with PBs and SGs, and this function may be related to the cytoplasmic shuttle characteristics of these CCCH proteins [104,114].
4. Transcriptional and Post-Transcriptional Regulation by CCCH Zinc-Finger Proteins
4.1. Transcriptional Regulation
Many CCCH zinc-finger proteins are important transcriptional regulators, and their nuclear localization is consistent with this function [21,115]. Experiments have found that CCCH-type zinc-finger proteins such as AtTZF1 [93] and PEI1 (AtTZF6) [73,116] in Arabidopsis, and SAW1 [103] and Leaf and Tiller Angle Increased Controller (OsLIC) [110] in rice can bind to DNA in vitro. Conserved domains in CCCH zinc-finger proteins can bind to DNA and enable the zinc-finger protein to regulate downstream genes [77,110].
CCCH zinc-finger proteins can function as activators of transcription [102]. For example, the OsLIC protein of Oryza sativa has transcriptional activation activity in a yeast system, and a typical conserved EELR domain in the C terminus of the protein plays a key role in transcriptional activation [110]. Rice Early heading date 4 (Ehd4) also has detectable transcriptional activation activity in yeast. Deletion of the C-terminal region significantly decreases this activity, and it is speculated that the transcriptional activation domain of Ehd4 is near the C terminus [115]. By contrast, the transcriptional activation domains of some CCCH zinc-finger proteins seem to be N-terminal. For example, the region of AtC3H17 that shows the strongest transcription activation activity is a segment from 1 to 288 amino acids at the N terminus, and conserved glutamate residues in an EELR-like motif at the N terminus of AtC3H17 play a key role in transcriptional activation [117]. Under abiotic stress, these CCCH zinc-finger proteins regulate plant tolerance by directly activating the expression of related target genes. For example, AtZFP1 improves the salt tolerance of plants by activating downstream genes related to salt tolerance, such as SOS1, AtGSTU5, and AtP5CS1 [21]. AtC3H17 improves the salt and oxidative tolerance of plants by regulating ABA-dependent responsive genes RAB18, COR15A, and RD22 [95].
In addition to transcriptional activators, some other proteins are transcriptional repressors. In plants, GLUB-1-BINDING ZINC FINGER 1 (OsGZF1) from rice regulates the accumulation of gluten via transcriptional inhibition of GluB-1 by binding to GCN4 (TGAGTCA) and PROL (TGCAAAG) motifs in the promoter region of the GluB-1 gene [77]. Additionally, ILA1-interacting protein 4 (IIP4) from rice negatively regulates the expression of MYB61 and CESAs in secondary wall synthesis [118].
4.2. Post-Transcriptional Regulation
In addition to transcriptional regulation, CCCH zinc-finger proteins also participate in post-transcriptional regulation by binding to mRNA [71]. Many plant CCCH zinc-finger proteins have the ability to bind to RNA in vitro, such as AtTZF1 [93], KHZ1 and KHZ2 [102] in Arabidopsis. The human CCCH zinc-finger proteins TTP and BRF1 recognize the AU-rich elements (AREs) of the 3′ untranslated region of target mRNA [119]. An in vitro RNA binding experiment has shown that the CCCH zinc-finger protein OsTZF1 in rice binds to the 3’ untranslated region of mRNA, and OsTZF1 only binds to U homopolymers with the RNA gel electrophoresis mobility shift assay (REMSA) [120]. There are roughly three types of AREs [121]. Type I contains several scattered AUUUA motifs in U-rich regions [122]. Class II contains at least two repeated UUAUUUA (U/A) (U/A) sequences [123]. Class III belongs to the U-rich region, without typical features and the AUUUA motif [124]. Further in vivo experiments in plants have found that AtTZF1 can cause the degradation of ARE-containing mRNA [125]. Research shows that ribonucleoprotein domains or RNA binding domains (RRMs) are present in many CCCH zinc-finger proteins, indicating that these proteins can recognize and bind to RNA [126], and the RRM consensus sequence is Lys⁄Arg-Gly-Phe⁄Tyr-Gly⁄Ala-Phe⁄Tyr-Val⁄Ile⁄Leu-X-Phe⁄Tyr or Ile⁄Val⁄Leu-Phe⁄Tyr-Ile⁄Val⁄Leu-X-Asn-Leu [126]. By contrast, no plant CCCH zinc-finger proteins have the specific amino acids required to specifically recognize the ARE sequence, and the sequences are expected to be rich in U or G [127]. Wang et al. [63] used the three-dimensional structure of the TIS11D CCCH zinc finger as a template [128] to predict the conformation of the Arabidopsis zinc-finger protein AtC3H14 bound to RNA. Each zinc finger has a KTEL(V) residue at the N terminus, which forms a key interface for RNA binding. Each of the two zinc fingers forms a pocket for RNA, which accommodates the two nucleotide residues U6 and U2 of RNA [63]. In further research, the plant-specific TZF motif (RR-TZF) and RR sequence in AtTZF1 are necessary for the protein’s RNA-binding function, and transient expression analysis of plant protoplasts has further confirmed that AtTZF1 can bind to AREs and lead to mRNA degradation [125]. However, the researchers in that study did not use a natural mRNA target, but rather a generic ARE for research, so we lack direct evidence that plant CCCH zinc-finger proteins function in post-transcriptional regulation of gene expression in vivo. Recent research has found that two putative mRNA binding domains, LOTUS/OST-HTH and RRM, were detected in the AtC3H18L protein sequence; this CCCH zinc finger protein has been implicated in stop codon read-through in Arabidopsis [98].
CCCH proteins can regulate gene expression by affecting RNA metabolic processes, including degradation, cleavage, export, and polyadenylation [103]. The RNA metabolic functions of CCCH zinc-finger proteins affect plant development, growth, and abiotic stress responses [118]. In Arabidopsis, the RNA-binding protein HUA1 participates in flower development by regulating the pre-mRNA processing of AGAMOUS [129,130,131]. FRIGIDA-ESSENTIAL 1 (FES1) may promote the winter annual growth habits of Arabidopsis by affecting the mRNA levels of FLOWERING LOCUS C (FLC) in a FRIGIDA-dependent manner [132]. The RNA-binding CCCH zinc-finger protein AtCPSF30 also interacts with calmodulin, but in the presence of calmodulin, its RNA binding activity is reduced; in addition, AtCPSF30 interacts with itself, which may promote RNA processing [133]. Recombinant AtTZF3 and AtTZF2 have RNase activity in vitro, indicating that they may participate in mRNA processing [109]. AtTZF1 may be involved in regulating downstream genes at the post-transcriptional level to integrate plant growth and abiotic stress signals [114]. KHZ1 and KHZ2 may regulate flowering and senescence at the post-transcriptional level [102]. Further studies have found that KHZ1 and KHZ2 can inhibit the splicing efficiency of FLC pre-mRNA and promote flowering through autonomous pathways in Arabidopsis [134].
5. Interactions of CCCH Zinc-Finger Proteins with Other Proteins
CCCH zinc-finger proteins can also exert their functions by interacting with other proteins [135]. The mammalian CCCH zinc-finger protein TTP interacts with many different types of proteins through its N terminus, C terminus, or zinc-finger structure [136]. The mammalian CCCH zinc finger-protein ZAP interacts with the virus Nsp9 protein, thereby inhibiting the replication of the Porcine Reproductive and Respiratory Syndrome virus, and its interaction position is positioned to the zinc-finger domain of ZAP [135]. Because mammalian CCCH zinc finger-proteins and plant CCCH zinc-finger proteins have few homologous sequences except for the zinc-finger domain, protein interactions mediated through the N terminus or C terminus of the protein are not conserved in plant CCCH zinc-finger proteins. Plant CCCH-type zinc-finger proteins mainly interact with other proteins through their zinc-finger domains [84]. In cotton, a yeast two-hybrid screen showed that GhZFP1 mediates plant tolerance through interaction with GZIRD21A and GZIPR5 [84]. Further, these proteins that bind to the CCCH zinc-finger protein in the cytoplasm can shuttle to the nucleus and may activate the plant’s signaling pathway under stress conditions [84]. In Arabidopsis, AtTZF4, AtTZF5, and AtTZF6 interact with MARD1 and RD21A proteins through their zinc-finger motifs [97]. On the other hand, CCCH zinc-finger protein interaction may mediate post-transcriptional regulation in plant development responses [97].
6. CCCH Zinc-Finger Proteins and Plant Hormones
Plant CCCH zinc-finger proteins are effective regulators of hormone-mediated stress responses [91,137]. Many CCCH-type zinc-finger proteins are induced by ABA, gibberellin (GA), and jasmonic acid (JA), and these CCCH-type zinc-finger proteins play important roles in various hormone-mediated signaling pathways [120,138].
In Arabidopsis, the CCCH zinc-finger protein SOMNUS is involved in a signaling pathway mediated by ABA and GA. A loss-of-function som Arabidopsis mutant line has a lower content of endogenous ABA and a higher content of endogenous GA. In vivo experiments found that SOMNUS is directly activated by PIL5, indicating that the SOMNUS regulates ABA and GA metabolism downstream of PIL5. SOMNUS is also involved in plant pigment-mediated signaling pathways, regulating the expression of genes related to hormone metabolism downstream of PIL5 during seed germination [139].
Overexpression lines for AtTZF1, AtTZF4, AtTZF5, and AtTZF6 exhibit a phenotype characteristic of enhanced ABA function and reduced GA function. Loss-of-function mutants for these genes are more sensitive to high salt, cold temperature, and drought stresses [73,140]. AtTZF2 and AtTZF3 also participate in the ABA signaling pathway. Expression of AtTZF2 and AtTZF3 is significantly induced under ABA treatment. AtTZF2 and AtTZF3 Arabidopsis overexpression lines have enhanced tolerance to high salt, osmotic, and ROS stresses. In contrast, the silencing lines for these genes have reduced salt and drought tolerance [105,106,109]. In rice, the ABA-induced CCCH zinc-finger protein OsC3H47 reduces sensitivity to ABA and enhances drought tolerance [141].
JA-mediated signaling pathways may also involve CCCH zinc-finger proteins [72,120,142]. JA can control leaf senescence in plants through the production of ROS [143]. GhTZF1 from cotton, OsTZF1 and OsTZF2 from rice, and AtTZF2 and AtTZF3 from Arabidopsis are all involved in JA-mediated leaf senescence [72,106,120,142]. AtTZF2 and AtTZF3 both participate in plant drought tolerance and growth through ABA and JA signaling pathways. Gene chip analysis and RT-qPCR assays have shown that the differentially expressed genes in Arabidopsis Attzf2 and Attzf3 overexpression lines are involved in JA, ABA, and biotic and abiotic stress processes [109]. Several JA biosynthesis genes and JA response genes are significantly upregulated in OsTZF2 gene silencing lines, while the same genes are downregulated in OsTZF2 overexpression lines, indicating that OsTZF2 negatively regulates the JA signaling pathway [72].
7. The Roles of CCCH Zinc-Finger Proteins in Abiotic Stress
CCCH-type zinc-finger proteins widely participate in plant responses to abiotic stresses such as oxidative, salinity, drought, flooding and cold temperature stresses.
7.1. Oxidative Stress
Many abiotic stresses induce the production of reactive oxygen species (ROS) in plants [144]. Excessive accumulation of ROS will produce oxidative stress, leading to cell death and even plant death [145]. Mitochondria, chloroplasts, and peroxisomes in plant cells are the main production sites of ROS. In the face of abiotic stress, plants have a replication mechanism to regulate the balance of reactive oxygen species, which mainly include enzymatic clearance mechanisms and non-enzymatic clearance mechanisms [146]. The CCCH zinc-finger protein is also involved in this process.
Hydrogen peroxide, abscisic acid and salt stress can significantly induce the expression of AtOZF1. Compared with the wild type, the Arabidopsis atozf1 mutant lines have reduced resistance to oxidative stress, while the overexpression lines improves the antioxidant capacity of the plant. Compared with the wild type, the atozf1 mutant has accumulated more MDA. In addition, the activity of CAT and guaiacol POD in atozf1 mutant is lower, and the expression of antioxidant genes such as APX1 and AtGSTU5 is down-regulated under oxidative stress. Experimental results show that AtOZF1 plays an important role in the resistance of Arabidopsis to oxidative stress [105].
7.2. Salt Stress
More than 8 × 108 hm2 of land worldwide is affected by soil salinization, and this problem continues to worsen [147,148,149,150]. Soil salinization severely affects seed germination, crop growth, and productivity, and is a major factor limiting global agricultural production [151,152,153,154]. NaCl is the main component causing salt stress [155,156,157,158]. High concentrations of salt in the soil reduce the water potential at the root surface, which affects water absorption by the roots and reduces the water use efficiency of plants, leading to osmotic stress and ionic stress, which can generate oxidative stress [159,160,161,162].
Some CCCH-type zinc-finger proteins are induced by salt stress and are closely associated with salt stress tolerance in plants [21]. For example, in rice subjected to various salt treatments, OsC3H33, OsC3H37, and OsC3H50 are all induced [163], and NaCl induces the expression of GhZFP1 in cotton, indicating that these CCCH zinc-finger proteins may function in regulating plant salt stress tolerance [84]. AtSZF1 and AtSZF2 in Arabidopsis [101]; OSC3H33, OSC3H37, OSC3H47 and OSC3H50 in rice [141,163]; GhTZF1 in cotton [142] all regulate the adaptability of plants to salt stress.
CCCH zinc-finger proteins participate in salt tolerance by various mechanisms. One way these proteins improve salt tolerance in plants is by maintaining ion homeostasis. Overexpression of GhZFP1 in transgenic tobacco plants significantly increases their salt tolerance by affecting Na+ homeostasis and K+ acquisition [84]. AtZFP1 improves salt tolerance in Arabidopsis by increasing K+ content and decreasing the Na+/K+ ratio [21].
CCCH zinc-finger proteins also improve the salt tolerance of plants by controlling genes related to salt stress. AtSZF1 and AtSZF2 participate in the response of Arabidopsis to salt stress by negatively regulating the expression of salt stress response genes. The single Arabidopsis mutants atszf1-1 and atszf2-1 show a significant increase in the expression of salt stress-related genes, including KIN1, RD29A, COR15A, and COR47. The Arabidopsis double mutants have significantly reduced salt tolerance, while the overexpression lines for these two genes have significantly improved salt tolerance [101]. Later research has transformed AtSZF2 into soybeans. Under high salt stress, it has been found that the ectopic expression of AtSZF2 gene could significantly reduce the ion leakage and increase the chlorophyll content of transgenic lines. The detection showed that AtSZF2 gene could regulate the expression of ABA/stress response genes in transgenic soybeans, making them salt tolerant. AtZFP1 improves the salt tolerance of plants by activating the downstream genes SOS1, AtGSTU5, and AtP5CS1 [21].
Post-transcriptional regulation is another mechanism by which CCCH zinc-finger proteins improve the salt tolerance of plants. OsTZF1 confers stress tolerance in rice: OsTZF1 overexpression lines show significantly higher tolerance to salt stress than the control and the OsTZF1 silenced lines. OsTZF1 has no transcriptional activation activity, so it does not regulate plant salt tolerance at the transcriptional level [120]. RNA electrophoretic mobility shift assays have shown that OsTZF1 can bind U-rich and ARE motifs of mRNAs, and microarray analysis has shown that OsTZF1 may post-transcriptionally regulate the transcript levels of salt stress-related genes, including Tic32, RNS4, and WRKY45 [120].
CCCH zinc-finger proteins can also increase the ability of plants to scavenge reactive oxygen species (ROS). Under high salt stress, OsTZF1 can reduce ROS damage by regulating genes related to redox homeostasis, such as those coding for ferritin and metallothionin, and genes encoding antioxidant enzymes, such as peroxidase (POD) and glutathione S-transferase (GST) [120]. The overexpression of BoC3H improves the salt tolerance of transgenic Brassica oleracea [164]. BoC3H may improve salt tolerance in broccoli by reducing relative conductivity, hydrogen peroxide (H2O2), and malondialdehyde (MDA), and by increasing the levels of catalase (CAT), POD, and superoxide dismutase (SOD).
CCCH zinc-finger proteins also help plants adjust to high salt environments through the ABA signaling pathway. AtOZF2 overexpression lines significantly improve plant salt resistance, while the antisense atozf2 Arabidopsis line significantly reduces plant salt resistance. Further studies have found that AtOZF2 participates in plant salt resistance through a signaling pathway mediated by ABA insensitive 2 (ABI2) [106]. Under salt stress, the stress response genes RAB18, COR15A, and RD22 in the ABA signaling pathway are significantly upregulated in an AtC3H17 overexpression Arabidopsis line compared with the control. These results indicate that AtC3H17 may regulate plant salt stress responses through an ABA-dependent pathway [95].
7.3. Drought Stress
Under drought conditions, plant root activity decreases, respiration decreases, and root absorption and the transport of water and mineral elements is inhibited [6,165,166,167]. At the same time, leaf growth, leaf area, stomatal index, the opening and closing of stomata, and chlorophyll content decreased, seriously disrupting photosynthesis and respiration [18,168,169,170]. Under drought stress, the ROS levels in plants increase, followed by membrane lipid delipidization or oxidation to form MDA [171,172,173,174]. Drought stress induces the expression of many CCCH zinc-finger protein genes. Through bioinformatics and phylogenetic analysis, 68 CCCH genes in 7 subfamilies have been identified in maize. Among them, 12 CCCH zinc-finger protein genes were found to have putative stress-responsive cis-elements in their promoter regions [64]. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis has found that these genes have five different expression patterns among different tissues and are significantly induced by ABA and drought treatment [64]. In Aegilops tauschii Coss., 36 CCCH zinc-finger family genes have been identified by comprehensive computational analysis, among which AetTZF1 shows the strongest expression under drought stress [67]. Drought and ABA could significantly induce the expression of OsC3H10 in rice [104].
CCCH zinc-finger proteins increase the drought tolerance of plants in a variety of ways. One way is by regulating the function of stomata. AtTZF1 overexpression lines of Arabidopsis are significantly more drought-resistant than the wild type. These drought-tolerant transgenic lines have abnormal stomatal closure and lower stomatal conductance [140]. PeC3H74 is a CCCH zinc-finger protein located on the plasma membrane of Phyllostachys edulis (Carriere) J. Houzeau with self-activating activity. Under drought treatment, the stomata closure rate of the PeC3H74 transgenic Arabidopsis line has been found to be significantly higher than that of the wild type [175].
CCCH zinc-finger proteins directly regulate downstream genes related to drought stress to enhance plant drought tolerance at the transcription level. AetTZF1 transgenic lines of Arabidopsis have a higher germination rate and a stronger root system than the wild type under drought stress. AetTZF1 improves the drought tolerance of the transgenic plants by increasing the expression level of drought stress-related genes, such as CBF1, CBF2, DREB2A, and COR47 [67]. It has been found that the root specific overexpression of OsC3H10 is not enough to induce drought resistance in rice, and the overexpression of OsC3H10 in the whole plant enhances the drought resistance of rice. Transcriptome analysis has shown that OsC3H10 overexpression lines could increase the expression level of stress response related genes, including LATE EMBRYOGENESIS ABUNDANT PROTEINs (LEAs), PATHOGENESIS RELATED GENEs (PRs) and GERMIN-LIKE PROTEINs (GLPs) [104].
CCCH zinc-finger proteins directly regulate downstream genes related to drought stress to enhance plant drought tolerance at the post-transcription level. The expression of Oryza sativa CCCH-tandem zinc finger protein 5 (OsTZF5) can be induced by drought stress [176]. Under drought, OsTZF5 overexpressing lines have shown an improved survival rate and growth retardation. When under the control of the stress-inducible OsNAC6 promoter, OsTZF5 rice overexpressing lines have increased the survival rate of rice under drought stress without growth retardation. The RNA electrophoretic mobility shift assay (REMSA) has shown that OsTZF5 can bind to the 3′ untranslated region of RNA in vitro, which indicates that OsTZF5 may confer plant drought tolerance by regulating RNA. Microarray analysis has shown that compared with the wild type, the OsTZF5 overexpression line has 609 up-regulated genes and 196 down-regulated genes, respectively. Representative genes up-regulated are PR1, salT and GolS2, and representative genes down-regulated are OsSAM3 and flavin monooxygenase [176].
CCCH zinc-finger proteins also enhance drought tolerance through a signaling pathway mediated by ABA. AtTZF2 and AtTZF3 overexpression lines are highly sensitive to ABA and have reduced transpiration, increased drought tolerance, and altered expression of genes related to ABA and abiotic stress processes [109]. Drought stress mimicked by the osmotic stress induced by a 10-day polyethylene glycol (PEG) treatment has been found to significantly inhibit the growth of OsC3H47 overexpressing lines and wild-type rice, and both genotypes showed accelerated leaf senescence and leaf curling. After recovery, the survival rates and PEG-induced drought tolerance of OsC3H47 overexpression lines were significantly higher than those of the control. The CCCH zinc-finger protein OsC3H47 reduces the sensitivity of rice to ABA and improves the drought tolerance of rice. OsC3H47 is located downstream of the ABA signaling pathway and plays an important role in post-transcriptional regulation [141]. PeC3H74 transgenic Arabidopsis seedlings have better root growth than that of the wild type under 10 μM ABA treatment. This indicate that PeC3H74 may enhance the drought tolerance of plants through ABA-dependent signaling pathways [175].
CCCH zinc-finger proteins also enhance drought tolerance through improving ROS scavenging capacity. For example, transformation of Arabidopsis plants with GhTZF1 reduces drought damage and delays leaf senescence by increasing the content of SOD and POD [142]. Compared with the wild type, PeC3H74 transgenic Arabidopsis has significantly improved drought tolerance, including the accumulation of less H2O2 content, less electrolyte leakage, and MDA content [175].
7.4. Flooding Stress
Over the past few decades, droughts and floods have become more frequent, and their impact on the growth and development of crops has become more serious [177,178,179]. Flooding stress significantly inhibits the growth and development of crops, significantly reduces yield and quality, and can kill plants in severe cases [180,181].
CCCH zinc-finger proteins participate in the response to flooding stress in different ways. In rice, hypoxia stress induces the expression of the CCCH zinc-finger genes OsCCCH-Zn-1, OsCCCH-Zn-2, and OsCCCH-Zn-3. The expression levels of OsCCCH-Zn-1 also increase significantly during flooding and in response to ABA. However, this gene is not induced by H2O2, cold stress, or salt stress, indicating that OsCCCH Zn-1 may exclusively regulate responses to flooding stress through the ABA signaling pathway, and rarely participates in responses to other abiotic stresses [182]. The detailed mechanisms of how CCCH zinc-finger proteins function in plant adaptation to flooding need further investigation.
7.5. Cold Stress
Low temperature is one of the abiotic stresses most likely to be encountered during the plant life cycle, and it greatly limits the geographical distribution, growth and development, yield quality, and post-harvest viability of crops [183,184,185,186]. Understanding the physiological and biochemical effects of low temperatures on plants and the molecular mechanisms of plant responses to low temperature stress is essential for breeding crop varieties with improved tolerance to low temperatures [187,188,189,190].
CCCH zinc-finger proteins improve the cold tolerance of plants by directly regulating the expression of downstream cold-related genes as transcription factors. For example, Arabidopsis plants overexpressing AtTZF1 are superior to the wild type in cold tolerance. In one study, under low temperature conditions, the survival rate of AtTZF1 overexpression lines was significantly higher than that of the control. The cold-tolerant phenotype of the Arabidopsis lines were consistent with the upregulated expression of the ABA/low temperature-related genes KIN1, RD29A, and COR15A [140]. DgC3H1 is a nuclear-localized CCCH zinc-finger protein isolated and cloned from Chrysanthemum morifolium Ramat. In another study, the overexpression line improved the low temperature tolerance of Chrysanthemum. The DgC3H1 antisense expression lines reduced the low temperature tolerance of Chrysanthemum. Under low temperature stress, the content of proline and soluble sugar in the DgC3H1 overexpression lines increased, and the activity of POD and SOD increased. In addition, compared with the wild type, low temperature stress-related genes such as DgCOR413, DgDREBa, DgCSD1 and DgCSD2 were up-regulated in the overexpression line and down-regulated in the antisense line. The results showed that DgC3H1 can act as a transcription factor to improve the resistance of Chrysanthemum to cold stress [191].
CCCH zinc-finger proteins also enhance cold tolerance via ABA signaling pathways. In switchgrass (Panicum virgatum L.), low temperature treatment can significantly induce the expression of the CCCH zinc-finger protein PvC3H72. PvC3H72 overexpression transgenic switchgrass has significantly improved cold tolerance compared to wild-type plants at 4 °C. In a study, at −5 °C, the transgenic line had a higher survival rate than the control, as well as a higher relative water content and more stable cell membranes. PvC3H72 improves the cold tolerance of switchgrass transgenic plants by regulating the expression of the ICE1-CBF-COR complex and ABA signaling pathway genes [192].
7.6. Multiple Stresses
Some CCCH zinc-finger proteins also participate in the responses to multiple abiotic stresses. In rice, OsTZF1 can significantly improve the ability of plants to resist salt, drought, and oxidative stress. Significant up-regulation of many biotic and abiotic stress-related genes in OsTZF1 overexpression lines, including disease-related proteins, transcription factors, peroxidase, dehydrin, metal detoxification protein, and ROS scavenging genes has been detected. Further studies have found that as the level of ROS in cells decreases, the expression of genes related to stress also decreases; therefore, OsTZF1 may confer tolerance to abiotic stresses in rice by enhancing tolerance to oxidative stress. In addition, as OsTZF1 can bind to mRNA in vitro, it is speculated that OsTZF1 may regulate abiotic stress through RNA metabolism [120]. In Ipomoea batatas (Linn.) Lamarck, IbC3H18 was cloned and analyzed, and the IbC3H18 overexpression transgenic line was found to have significantly enhanced drought tolerance, salt tolerance, and antioxidant capacity, while an IbC3H18 RNA interference silencing line had significantly reduced tolerance. IbC3H18 can directly bind to the promoter regions of the salt tolerance-related gene SOS5, the active oxygen scavenging gene CCS, and the ABA receptor gene PYL8 to regulate their expression. Based on the results of RNA sequencing and on physiological and biochemical indicators, overexpression of IbC3H18 increases photosynthetic activity by activating the ROS scavenging system and ABA signaling pathway, and maintains the ionic–osmotic balance to enhance the stress tolerance of sweet potatoes [81].
8. Mechanisms That Regulate CCCH Zinc-Finger Proteins under Abiotic Stresses
Plants rapidly respond to a specific abiotic stress based on which type of sensor is stimulated, as this determines which series of responses is activated [193,194]. For instance, the sphingolipid glycosyl inositol phosphorylceramide (GIPC) is involved in sensing salt stress [195], REDUCED HYPEROSMOLALITY INDUCED CALCIUM INCREASE 1 (OSCA1) is an osmotic stress sensor [196], and CHILLING TOLERANCE DIVERGENCE 1 (COLD1) is a cold stress receptor [197,198]. Upon sensing the stress signal, plants produce second messengers (such as a Ca2+ pulse) in the cytoplasm, which act on a series of downstream genes, including CCCH zinc-finger protein genes. However, it is unclear how the upstream stress signals are accurately transmitted to the CCCH zinc finger proteins. The CCCH zinc-finger proteins then regulate plant development and abiotic stress by modulating gene expression, MAPK-mediated protein phosphorylation, and ABA signaling at the transcriptional and post-transcriptional levels via different signaling pathways.
Some CCCH zinc-finger proteins function as transcription factors that control various genes at the transcriptional level under abiotic stress [71,192]. CCCH zinc-finger proteins such as PEI1, HUA1, OsDOS, SOMNUS, AtSZF1, AtZFP1, SAW1 and OsC3H10 are located in the nucleus, where they act as transcription factors [13,103,104]. Transcription factors bind to the promoters of stress-related genes as part of a signaling pathway that transmits relevant stress stimuli, initiates the expression of these genes, and helps the plant adapt to stress by producing particular proteins or metabolites [199]. For example, AtSZF1 [101] and AtZFP1 [21] improve the salt tolerance of plants as transcription factors.
CCCH zinc-finger proteins also function at the post-transcriptional level by modulating RNA metabolism in plants subjected to stress. CCCH zinc-finger proteins localize to PBs and SGs, which can combine with specific RNA elements to initiate RNA degradation in PBs and regulate plant development, growth, and stress responses in SGs [140]. Furthermore, AtTZF1, AtTZF4, AtTZF5, AtTZF6, OsTZF1 and OsC3H10 proteins, which shuttle between the cytoplasm and the nucleus, co-localize with PBs and SGs [104,120,140]. For example, AtTZF1 [140] and OsTZF1 [120] improve plant resistance to abiotic stresses by regulating stress-related genes, possibly via RNA metabolism. Although AtTZF1 and OsTZF1 can bind to specific RNA elements, there is currently no evidence that this combination leads to mRNA degradation [114].
Moreover, CCCH zinc-finger proteins improve plant stress tolerance through the ABA signaling pathway. Many CCCH zinc-finger proteins, such as AtTZF1, AtTZF2, AtTZF3, AtTZF4, AtTZF5, AtTZF6, OsC3H47 and OsTZF5, are upregulated in response to ABA [140,141,176]. For example, AtTZF10/11 [101] and AtC3H17 [95] may regulate the adaptability of plants to high-salt conditions through an ABA-dependent signaling pathway. These experimental data indicate that CCCH zinc-finger proteins extensively improve plant stress resistance through ABA signaling.
In addition, CCCH zinc-finger proteins such as GhZFP1 [84] may improve plant stress tolerance through protein interaction. However, GhZFP1 may play different roles in abiotic stress, potentially acting as an RNA binding protein to regulate the RNA metabolism of target genes induced by salt stress [84].
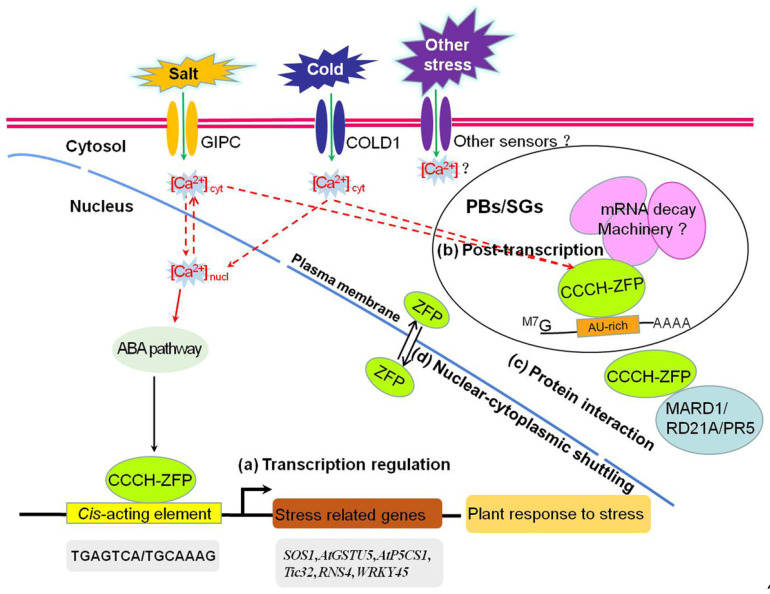
In summary, when plant receptors sense a stress signal, the signal is amplified by secondary messengers and transmitted to CCCH zinc-finger proteins in some unclear way, which improves the stress tolerance of the plant in different ways at the transcriptional and post-transcriptional levels and through protein–protein interactions. A putative regulatory mechanism by which plant CCCH zinc-finger proteins mediate plant abiotic stress responses is shown in Figure 1. In the nucleus, CCCH zinc-finger proteins function as transcription factors that bind to specific cis-elements in the promoters and activate or repress the expression of stress-related genes, improving plant stress resistance through ABA signaling pathways (a). In the cytoplasm, CCCH zinc-finger proteins bind to target mRNAs at U-rich sequences and regulate RNA metabolism in PBs and SGs (b). In the cytoplasm, CCCH zinc-finger proteins can also interact with stress-related proteins, such as MARD1, RD21A, and PR5, to improve the plant’s resistance (c). In addition, CCCH zinc-finger proteins such as ZFPs interact with PBs and SGs shuttling between the cytoplasm and nucleus to improve the plant’s abiotic stress resistance (d). Finally, the gene IDs of CCCH zinc-finger proteins, CCCH motifs, functions related to stress, mode of action, and related references are summarized in Supplementary Table S1.
Figure 1.
Putative regulatory mechanism by which CCCH zinc-finger proteins mediate abiotic stress tolerance in plants. (a) Transcription regulation of CCCH zinc-finger protein in the nucleus under abiotic stress. (b) Post-transcriptional regulation of CCCH zinc-finger protein in processing bodies (PBs) and stress granules (SGs) of the cytoplasm under abiotic stress. (c) Protein interaction of CCCH zinc-finger protein in the cytoplasm under abiotic stress. (d) Shuttling of CCCH zinc-finger protein between the nucleus and cytoplasm under abiotic stress. Solid arrows indicate processes that have been verified, and dotted arrows indicate processes that need to be clarified.
9. Conclusions and Perspectives
Despite our progress in understanding how CCCH zinc-finger proteins contribute to plant responses to abiotic stress, more specific receptors for sensing abiotic stresses, such as salt, osmotic, and low temperature stresses, remain to be identified. Emerging technologies, such as genome-wide association analysis, CRISPR–Cas9 genome editing, and single-cell sequencing will be very helpful for examining the mechanisms by which different CCCH zinc-finger proteins interact with various sensors to improve the abiotic stress tolerance of plants.
More detailed studies are needed to unravel how secondary messengers such as calcium pulses induced by abiotic stress initiate CCCH zinc-finger protein–mediated regulation of post-transcription, protein interaction, and shuttling between the cytoplasm and nucleus, although Ca2+-mediated ABA or MAPKs transcription regulation is clear.
Plant CCCH zinc-finger proteins can be located on PBs and SGs, and bind to specific RNA elements to initiate RNA degradation. However, no natural RNA binding target site has been found on plant CCCH zinc-finger proteins. Therefore, it is necessary to seek the natural RNA binding site of plant CCCH zinc-finger proteins through in vivo experiments. RNA immunoprecipitation (RIP) and cross-linking and immunoprecipitation (CLIP) may be efficient methods to determine the function of plant CCCH zinc-finger proteins in post-transcriptional regulation [75,200].
Most research on plant CCCH zinc-finger proteins has focused on model plants such as Arabidopsis and rice, which are highly sensitive to abiotic stress. Analyzing the sensors, signal transduction, and molecular regulatory networks of CCCH zinc-finger proteins in plants that are extremely tolerant to abiotic stress, such as heavy metals or arsenic hyperaccumulator plants, halophytes and xerophytes, would provide valuable insight into the mechanism by which CCCH zinc-finger proteins contribute to abiotic stress tolerance. Although individual studies have found that the CCCH zinc-finger protein regulates proteins related to heavy metal detoxification [120], specific studies on CCCH zinc-finger proteins’ regulation of plant heavy metal (Cd, Cr, Al, Cu, Zn, etc.) or arsenic stress have not been reported, which shows that there is still a large research space between CCCH zinc-finger proteins and plant heavy metal stress, which is our future research direction.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22158327/s1.
Author Contributions
G.H. wrote the manuscript. Z.Q., Y.L., C.W. and B.W. participated in the writing and modification of this manuscript. G.H. and B.W. conceptualized the idea and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Research Foundation of China (project nos. 32000209 and 31770288), Natural Science Research Foundation of Shandong Province (project no. ZR2020QC031 and ZR2019MC065), and China Postdoctoral Science Foundation (project no. 2020M672114).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is presented in manuscript and in supplementary materials.
Conflicts of Interest
The authors state no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen M., Yang Z., Liu J., Zhu T., Wei X., Fan H., Wang B. Adaptation mechanism of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018;19:3668. doi: 10.3390/ijms19113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding T., Yang Z., Wei X., Yuan F., Yin S., Wang B. Evaluation of salt-tolerant germplasm and screening of the salt-tolerance traits of sweet sorghum in the germination stage. Funct. Plant Biol. 2018;45:1073–1081. doi: 10.1071/FP18009. [DOI] [PubMed] [Google Scholar]
- 3.Li B., Gao K., Ren H., Tang W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018;60:757–779. doi: 10.1111/jipb.12701. [DOI] [PubMed] [Google Scholar]
- 4.Luo X., Wang B., Gao S., Zhang F., Terzaghi W., Dai M. Genome-wide association study dissects the genetic bases of salt tolerance in maize seedlings. J. Integr. Plant Biol. 2019;61:658–674. doi: 10.1111/jipb.12797. [DOI] [PubMed] [Google Scholar]
- 5.Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011;180:169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Yuan F., Guo J., Shabala S., Wang B. Reproductive Physiology of Halophytes: Current Standing. Front. Plant Sci. 2019;9:1954. doi: 10.3389/fpls.2018.01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu C., Feng Z., Yuan F., Han G., Wang B. The SNARE protein LbSYP61 participates in salt secretion in Limonium bicolor. Environ. Exp. Bot. 2020;176:104076. doi: 10.1016/j.envexpbot.2020.104076. [DOI] [Google Scholar]
- 8.Guo J., Dong X., Li Y., Wang B. NaCl treatment markedly enhanced pollen viability and pollen preservation time of euhalophyte Suaeda salsa via up regulation of pollen development-related genes. J. Plant Res. 2020;133:57–71. doi: 10.1007/s10265-019-01148-0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S., Yang Z., Wang M., Song J., Sui N., Fan H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol. Plant. 2014;36:1823–1830. doi: 10.1007/s11738-014-1555-3. [DOI] [Google Scholar]
- 10.Jiang J., Ma S., Ye N., Jiang M., Cao J., Zhang J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017;59:86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- 11.Zubair M., Khan Q.U., Mirza N., Sarwar R., Khan A.A., Baloch M.S., Fahad S., Shah A.N. Physiological response of spinach to toxic heavy metal stress. Environ. Sci. Pollut. Res. Int. 2019;26:31667–31674. doi: 10.1007/s11356-019-06292-7. [DOI] [PubMed] [Google Scholar]
- 12.Song Y., Li J., Liu M., Meng Z., Liu K., Sui N. Nitrogen increases drought tolerance in maize seedlings. Funct. Plant Biol. 2019;46:350–359. doi: 10.1071/FP18186. [DOI] [PubMed] [Google Scholar]
- 13.Wang W., Zheng H., Wang Y., Han G., Sui N. Overexpression of CCCH zinc finger protein gene delays flowering time and enhances salt tolerance in Arabidopsis by increasing fatty acid unsaturation. Acta Physiol. Plant. 2018;40:196. doi: 10.1007/s11738-018-2775-8. [DOI] [Google Scholar]
- 14.Li X., Zeng R., Liao H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016;58:193–202. doi: 10.1111/jipb.12434. [DOI] [PubMed] [Google Scholar]
- 15.Guo J., Lu C., Zhao F., Gao S., Wang B. Improved reproductive growth of euhalophyte Suaeda salsa under salinity is correlated with altered phytohormone biosynthesis and signal transduction. Funct. Plant Biol. 2020;47:170–183. doi: 10.1071/FP19215. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q., Liu R., Ma Y., Song J. Physiological and molecular evidence for Na+ and Cl− exclusion in the roots of two Suaeda salsa populations. Aquat. Bot. 2018;146:1–7. doi: 10.1016/j.aquabot.2018.01.001. [DOI] [Google Scholar]
- 17.Qi Y., Li J., Chen C., Li L., Zheng X., Liu J., Zhu T., Pang C., Wang B., Chen M. Adaptive growth response of exotic Elaeagnus angustifolia L. to indigenous saline soil and its beneficial effects on the soil system in the Yellow River Delta, China. Trees-Struct. Funct. 2018;32:1723–1735. doi: 10.1007/s00468-018-1746-4. [DOI] [Google Scholar]
- 18.Wu Q., Wang M., Shen J., Chen D., Zheng Y., Zhang W. ZmOST1 mediates abscisic acid regulation of guard cell ion channels and drought stress responses. J. Integr. Plant Biol. 2019;61:478–491. doi: 10.1111/jipb.12714. [DOI] [PubMed] [Google Scholar]
- 19.Qin Y.J., Wu W.H., Wang Y. ZmHAK5 and ZmHAK1 function in K+ uptake and distribution in maize under low K+ conditions. J. Integr. Plant Biol. 2019;61:691–705. doi: 10.1111/jipb.12756. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y., Song Y., Zheng H., Zhang Y., Guo J., Sui N. NADP-Malate Dehydrogenase of Sweet Sorghum Improves Salt Tolerance of Arabidopsis thaliana. J. Agric. Food Chem. 2018;66:5992–6002. doi: 10.1021/acs.jafc.8b02159. [DOI] [PubMed] [Google Scholar]
- 21.Han G., Wang M., Yuan F., Sui N., Song J., Wang B. The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol. Biol. 2014;86:237–253. doi: 10.1007/s11103-014-0226-5. [DOI] [PubMed] [Google Scholar]
- 22.Huang J., Yang M., Zhang X. The function of small RNAs in plant biotic stress response. J. Integr. Plant Biol. 2016;58:312–327. doi: 10.1111/jipb.12463. [DOI] [PubMed] [Google Scholar]
- 23.Rozentsvet O.A., Nesterov V.N., Bogdanova E.S., Rozenberg G.S. Physiological and Biochemical Strategies of Adaptation in Halophytes. Dokl. Biol. Sci. 2020;492:83–85. doi: 10.1134/S0012496620030072. [DOI] [PubMed] [Google Scholar]
- 24.Matinzadeh Z., Akhani H., Abedi M., Palacio S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019;141:259–278. doi: 10.1016/j.plaphy.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Xi J.J., Chen H.Y., Bai W.P., Yang R.C., Yang P.Z., Chen R.J., Hu T.M., Wang S.M. Sodium-Related Adaptations to Drought: New Insights From the Xerophyte Plant Zygophyllum xanthoxylum. Front. Plant Sci. 2018;9:1678. doi: 10.3389/fpls.2018.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boukhris A., Laffont-Schwob I., Folzer H., Rabier J., Mezghani I., Salducci M.D., Tatoni T., Chaieb M. Tolerance strategies of two Mediterranean native xerophytes under fluoride pollution in Tunisia. Environ. Sci. Pollut. Res. Int. 2018;25:34753–34764. doi: 10.1007/s11356-018-3431-y. [DOI] [PubMed] [Google Scholar]
- 27.Souri Z., Karimi N., Sandalio L.M. Arsenic Hyperaccumulation Strategies: An Overview. Front. Cell Dev. Biol. 2017;5:67. doi: 10.3389/fcell.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T., Yan H., He Z. Advances in molecular mechanisms of arsenic hyperaccumulation of Pteris vittata L. Chin. J. Biotechnol. 2020;36:397–406. doi: 10.13345/j.cjb.190374. [DOI] [PubMed] [Google Scholar]
- 29.Sun X., Zheng H., Sui N. Regulation mechanism of long non-coding RNA in plant response to stress. Biochem. Biophys. Res. Commun. 2018;503:402–407. doi: 10.1016/j.bbrc.2018.07.072. [DOI] [PubMed] [Google Scholar]
- 30.Meng C., Sui N. Overexpression of maize MYB-IF35 increases chilling tolerance in Arabidopsis. Plant Physiol. Biochem. 2019;135:167–173. doi: 10.1016/j.plaphy.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y., Li J.Q., Yan J.Y., Yuan J.J., Li G.X., Wu Y.R., Xu J.M., Huang R.F., Harberd N.P., Ding Z.J. Ethylene promotes seed iron storage during Arabidopsis seed maturation via ERF95 transcription factor. J. Integr. Plant Biol. 2020;62:1193–1212. doi: 10.1111/jipb.12986. [DOI] [PubMed] [Google Scholar]
- 32.Tan Z., Wen X., Wang Y. Betula platyphylla BpHOX2 transcription factor binds to different cis-acting elements and confers osmotic tolerance. J. Integr. Plant Biol. 2020;62:1762–1779. doi: 10.1111/jipb.12994. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Klatte M., Jakoby M., Baumlein H., Weisshaar B., Bauer P. Iron deficiency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana. Planta. 2007;226:897–908. doi: 10.1007/s00425-007-0535-x. [DOI] [PubMed] [Google Scholar]
- 34.Li L., Gao W., Peng Q., Zhou B., Kong Q., Ying Y., Shou H. Two soybean bHLH factors regulate response to iron deficiency. J. Integr. Plant Biol. 2018;60:608–622. doi: 10.1111/jipb.12651. [DOI] [PubMed] [Google Scholar]
- 35.Meng F., Yang C., Cao J., Chen H., Pang J., Zhao Q., Wang Z., Qing Fu Z., Liu J. A bHLH transcription activator regulates defense signaling by nucleo-cytosolic trafficking in rice. J. Integr. Plant Biol. 2020;62:1552–1573. doi: 10.1111/jipb.12922. [DOI] [PubMed] [Google Scholar]
- 36.Song H., Wang P., Hou L., Zhao S., Zhao C., Xia H., Li P., Zhang Y., Bian X., Wang X. Global Analysis of WRKY Genes and Their Response to Dehydration and Salt Stress in Soybean. Front. Plant Sci. 2016;7:9. doi: 10.3389/fpls.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Y., Li J., Sui Y., Han G., Zhang Y., Guo S., Sui N. The sweet sorghum SbWRKY50 is negatively involved in salt response by regulating ion homeostasis. Plant Mol. Biol. 2020;102:603–614. doi: 10.1007/s11103-020-00966-4. [DOI] [PubMed] [Google Scholar]
- 38.Li C.X., Yan J.Y., Ren J.Y., Sun L., Xu C., Li G.X., Ding Z.J., Zheng S.J. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Biol. 2020;62:1176–1192. doi: 10.1111/jipb.12888. [DOI] [PubMed] [Google Scholar]
- 39.Zou M., Guan Y., Ren H., Zhang F., Chen F. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol. Biol. 2008;66:675–683. doi: 10.1007/s11103-008-9298-4. [DOI] [PubMed] [Google Scholar]
- 40.Zong W., Yang J., Fu J., Xiong L. Synergistic regulation of drought-responsive genes by transcription factor OsbZIP23 and histone modification in rice. J. Integr. Plant Biol. 2020;62:723–729. doi: 10.1111/jipb.12850. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Lu X., Malik W.A., Chen X., Wang J., Wang D., Wang S., Chen C., Guo L., Ye W. Differentially expressed bZIP transcription factors confer multi-tolerances in Gossypium hirsutum L. Int. J. Biol. Macromol. 2020;146:569–578. doi: 10.1016/j.ijbiomac.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Wei W., Zhang Y., Tao J., Chen H., Li Q., Zhang W., Ma B., Lin Q., Zhang J., Chen S. The Alfin-like homeodomain finger protein AL5 suppresses multiple negative factors to confer abiotic stress tolerance in Arabidopsis. Plant J. 2015;81:871–883. doi: 10.1111/tpj.12773. [DOI] [PubMed] [Google Scholar]
- 43.Brandt R., Cabedo M., Xie Y., Wenkel S. Homeodomain leucine-zipper proteins and their role in synchronizing growth and development with the environment. J. Integr. Plant Biol. 2014;56:518–526. doi: 10.1111/jipb.12185. [DOI] [PubMed] [Google Scholar]
- 44.Chen S.-S., Jiang J., Han X.-J., Zhang Y.-X., Zhuo R.-Y. Identification, expression analysis of the Hsf family, and characterization of class A4 in Sedum Alfredii hance under cadmium stress. Int. J. Mol. Sci. 2018;19:1216. doi: 10.3390/ijms19041216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton E.A., Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 2013;12:112–120. doi: 10.1111/acel.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M., Zhu J., Dong Z. Immediate transcriptional responses of Arabidopsis leaves to heat shock. J. Integr. Plant Biol. 2020 doi: 10.1111/jipb.12990. [DOI] [PubMed] [Google Scholar]
- 47.Hong Y., Zhang H., Huang L., Li D., Song F. Overexpression of a Stress-Responsive NAC Transcription Factor Gene ONAC022 Improves Drought and Salt Tolerance in Rice. Front. Plant Sci. 2016;7:4. doi: 10.3389/fpls.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang B., Wei J., Song N., Wang N., Zhao J., Kang Z. A novel wheat NAC transcription factor, TaNAC30, negatively regulates resistance of wheat to stripe rust. J. Integr. Plant Biol. 2018;60:432–443. doi: 10.1111/jipb.12627. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J., Liu J.S., Meng F.N., Zhang Z.Z., Long H., Lin W.H., Luo X.M., Wang Z.Y., Zhu S.W. ANAC005 is a membrane-associated transcription factor and regulates vascular development in Arabidopsis. J. Integr. Plant Biol. 2016;58:442–451. doi: 10.1111/jipb.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Q., Luo Q., Wang R., Zhang F., He Y., Zhang Y., Qiu D., Li K., Chang J., Yang G. A Wheat R2R3-type MYB Transcription Factor TaODORANT1 Positively Regulates Drought and Salt Stress Responses in Transgenic Tobacco Plants. Front. Plant Sci. 2017;8:1374. doi: 10.3389/fpls.2017.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., Liu G., Jia J., Zhao G., Xia C., Zhang L., Li F., Zhang Q., Dong C., Gao S. The wheat MYB-related transcription factor TaMYB72 promotes flowering in rice. J. Integr. Plant Biol. 2016;58:701–704. doi: 10.1111/jipb.12461. [DOI] [PubMed] [Google Scholar]
- 52.Guo H., Wang L., Yang C., Zhang Y., Zhang C., Wang C. Identification of novel cis-elements bound by BplMYB46 involved in abiotic stress responses and secondary wall deposition. J. Integr. Plant Biol. 2018;60:1000–1014. doi: 10.1111/jipb.12671. [DOI] [PubMed] [Google Scholar]
- 53.Chen R., Ma J., Luo D., Hou X., Ma F., Zhang Y., Meng Y., Zhang H., Guo W. CaMADS, a MADS-box transcription factor from pepper, plays an important role in the response to cold, salt, and osmotic stress. Plant Sci. 2019;280:164–174. doi: 10.1016/j.plantsci.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Meng Q., Li X., Zhu W., Yang L., Liang W., Dreni L., Zhang D. Regulatory network and genetic interactions established by OsMADS34 in rice inflorescence and spikelet morphogenesis. J. Integr. Plant Biol. 2017;59:693–707. doi: 10.1111/jipb.12594. [DOI] [PubMed] [Google Scholar]
- 55.Haas M., Schreiber M., Mascher M. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. J. Integr. Plant Biol. 2019;61:204–225. doi: 10.1111/jipb.12737. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y.-L., Zhang C.-L., Wang G.-L., Wang Y.-X., Qi C.-H., You C.-X., Li Y.-Y., Hao Y.-J. Apple AP2/EREBP transcription factor MdSHINE2 confers drought resistance by regulating wax biosynthesis. Planta. 2019;249:1627–1643. doi: 10.1007/s00425-019-03115-4. [DOI] [PubMed] [Google Scholar]
- 57.Zeng L., Yin Y., You C., Pan Q., Xu D., Jin T., Zhang B., Ma H. Evolution and protein interactions of AP2 proteins in Brassicaceae: Evidence linking development and environmental responses. J. Integr. Plant Biol. 2016;58:549–563. doi: 10.1111/jipb.12439. [DOI] [PubMed] [Google Scholar]
- 58.Liu C., Zhang T.Z. Functional diversifications of GhERF1 duplicate genes after the formation of allotetraploid cotton. J. Integr. Plant Biol. 2019;61:60–74. doi: 10.1111/jipb.12764. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H., Ni L., Liu Y., Wang Y., Zhang A., Tan M., Jiang M. The C2H2-type Zinc Finger Protein ZFP182 is Involved in Abscisic Acid-Induced Antioxidant Defense in Rice F. J. Integr. Plant Biol. 2012;54:500–510. doi: 10.1111/j.1744-7909.2012.01135.x. [DOI] [PubMed] [Google Scholar]
- 60.Yang L., Liu Q., Liu Z., Yang H., Wang J., Li X., Yang Y. Arabidopsis C3HC4-RING finger E3 ubiquitin ligase AtAIRP4 positively regulates stress-responsive abscisic acid signaling. J. Integr. Plant Biol. 2016;58:67–80. doi: 10.1111/jipb.12364. [DOI] [PubMed] [Google Scholar]
- 61.Li S., Chen H., Hou Z., Li Y., Yang C., Wang D., Song C.P. Screening of abiotic stress-responsive cotton genes using a cotton full-length cDNA overexpressing Arabidopsis library. J. Integr. Plant Biol. 2020;62:998–1016. doi: 10.1111/jipb.12861. [DOI] [PubMed] [Google Scholar]
- 62.Han G., Lu C., Guo J., Qiao Z., Sui N., Qiu N., Wang B. C2H2 Zinc Finger Proteins: Master Regulators of Abiotic Stress Responses in Plants. Front. Plant Sci. 2020;11:115. doi: 10.3389/fpls.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang D., Guo Y., Wu C., Yang G., Li Y., Zheng C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008;9:44. doi: 10.1186/1471-2164-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng X., Zhao Y., Cao J., Zhang W., Jiang H., Li X., Ma Q., Zhu S., Cheng B. CCCH-type zinc finger family in maize: Genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS ONE. 2012;7:e40120. doi: 10.1371/journal.pone.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chai G., Hu R., Zhang D., Qi G., Zuo R., Cao Y., Chen P., Kong Y., Zhou G. Comprehensive analysis of CCCH zinc finger family in poplar (Populus trichocarpa) BMC Genom. 2012;13:253. doi: 10.1186/1471-2164-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang C., Zhang H., Zhao Y., Jiang H., Zhu S., Cheng B., Xiang Y. Genome-wide analysis of the CCCH zinc finger gene family in Medicago truncatula. Plant Cell Rep. 2013;32:1543–1555. doi: 10.1007/s00299-013-1466-6. [DOI] [PubMed] [Google Scholar]
- 67.Jiang A.-L., Xu Z.-S., Zhao G.-Y., Cui X.-Y., Chen M., Li L.-C., Ma Y.-Z. Genome-wide analysis of the C3H zinc finger transcription factor family and drought responses of members in Aegilops tauschii. Plant Mol. Biol. Report. 2014;32:1241–1256. doi: 10.1007/s11105-014-0719-z. [DOI] [Google Scholar]
- 68.Xu R. Genome-wide analysis and identification of stress-responsive genes of the CCCH zinc finger family in Solanum lycopersicum. Mol. Genet. Genom. 2014;289:965–979. doi: 10.1007/s00438-014-0861-1. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Zhong Y., Cheng Z.M. Evolution and Expression Analysis of the CCCH Zinc Finger Gene Family in Vitis vinifera. Plant Genome. 2014;7 doi: 10.3835/plantgenome2014.05.0019. [DOI] [Google Scholar]
- 70.Pradhan S., Kant C., Verma S., Bhatia S. Genome-wide analysis of the CCCH zinc finger family identifies tissue specific and stress responsive candidates in chickpea (Cicer arietinum L.) PLoS ONE. 2017;12:e0180469. doi: 10.1371/journal.pone.0180469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pi B., He X., Ruan Y., Jang J.-C., Huang Y. Genome-wide analysis and stress-responsive expression of CCCH zinc finger family genes in Brassica rapa. BMC Plant Biol. 2018;18:373. doi: 10.1186/s12870-018-1608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong Z., Li M., Yang W., Xu W., Xue Y. A Novel Nuclear-Localized CCCH-Type Zinc Finger Protein, OsDOS, Is Involved in Delaying Leaf Senescence in Rice. Plant Physiol. 2006;141:1376–1388. doi: 10.1104/pp.106.082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bogamuwa S., Jang J. The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ. 2013;36:1507–1519. doi: 10.1111/pce.12084. [DOI] [PubMed] [Google Scholar]
- 74.Chao Y., Zhang T., Yang Q., Kang J., Sun Y., Gruber M.Y., Qin Z. Expression of the alfalfa CCCH-type zinc finger protein gene MsZFN delays flowering time in transgenic Arabidopsis thaliana. Plant Sci. 2014;215:92–99. doi: 10.1016/j.plantsci.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Kim W.C., Kim J.Y., Ko J.H., Kang H., Kim J., Han K.H. AtC3H14, a plant-specific tandem CCCH zinc-finger protein, binds to its target mRNAs in a sequence-specific manner and affects cell elongation in Arabidopsis thaliana. Plant J. 2014;80:772–784. doi: 10.1111/tpj.12667. [DOI] [PubMed] [Google Scholar]
- 76.Chai G., Qi G., Cao Y., Wang Z., Yu L., Tang X., Yu Y., Wang D., Kong Y., Zhou G. Poplar PdC3H17 and PdC3H18 are direct targets of PdMYB3 and PdMYB21, and positively regulate secondary wall formation in Arabidopsis and poplar. New Phytol. 2014;203:520–534. doi: 10.1111/nph.12825. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y., Sun A., Wang M., Zhu Z., Ouwerkerk P.B.F. Functions of the CCCH type zinc finger protein OsGZF1 in regulation of the seed storage protein GluB-1 from rice. Plant Mol. Biol. 2014;84:621–634. doi: 10.1007/s11103-013-0158-5. [DOI] [PubMed] [Google Scholar]
- 78.Chai G., Kong Y., Zhu M., Yu L., Qi G., Tang X., Wang Z., Cao Y., Yu C., Zhou G. Arabidopsis C3H14 and C3H15 have overlapping roles in the regulation of secondary wall thickening and anther development. J. Exp. Bot. 2015;66:2595–2609. doi: 10.1093/jxb/erv060. [DOI] [PubMed] [Google Scholar]
- 79.Guo D., Yi H.-Y., Li H.-L., Liu C., Yang Z.-P., Peng S.-Q. Molecular characterization of HbCZF1, a Hevea brasiliensis CCCH-type zinc finger protein that regulates hmg1. Plant Cell Rep. 2015;34:1569–1578. doi: 10.1007/s00299-015-1809-6. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Zhang Z., Xiong T., Xiong X., Wu X., Guan C., Xiao G. The CCCH-type transcription factor BnZFP1 is a positive regulator to control oleic acid levels through the expression of diacylglycerol O-acyltransferase 1 gene in Brassica napus. Plant Physiol. Biochem. 2018;132:633–640. doi: 10.1016/j.plaphy.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H., Gao X., Zhi Y., Li X., Zhang Q., Niu J., Wang J., Zhai H., Zhao N., Li J. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019;223:1918–1936. doi: 10.1111/nph.15925. [DOI] [PubMed] [Google Scholar]
- 82.Han G., Wei X., Dong X., Wang C., Sui N., Guo J., Yuan F., Gong Z., Li X., Zhang Y. Arabidopsis ZINC FINGER PROTEIN1 Acts Downstream of GL2 to Repress Root Hair Initiation and Elongation by Directly Suppressing bHLH Genes. Plant Cell. 2020;32:206–225. doi: 10.1105/tpc.19.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan A., Wu M., Zhao Y., Zhang A., Liu B., Schiefelbein J., Gan Y. Involvement of C2H2 zinc finger proteins in the regulation of epidermal cell fate determination in Arabidopsis. J. Integr. Plant Biol. 2014;56:1112–1117. doi: 10.1111/jipb.12221. [DOI] [PubMed] [Google Scholar]
- 84.Guo Y.H., Yu Y.P., Wang D., Wu C.A., Yang G.D., Huang J.G., Zheng C.C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009;183:62–75. doi: 10.1111/j.1469-8137.2009.02838.x. [DOI] [PubMed] [Google Scholar]
- 85.Matthews J.M., Sunde M. Zinc fingers--folds for many occasions. IUBMB Life. 2002;54:351–355. doi: 10.1080/15216540216035. [DOI] [PubMed] [Google Scholar]
- 86.Brown R.S. Zinc finger proteins: Getting a grip on RNA. Curr. Opin. Struct. Biol. 2005;15:94–98. doi: 10.1016/j.sbi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Liang J., Song W., Tromp G., Kolattukudy P.E., Fu M. Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS ONE. 2008;3:e2880. doi: 10.1371/journal.pone.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blackshear P.J. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2001;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 89.Berg J.M., Shi Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 90.Zhuang Y., Wang C., Zhang Y., Chen S., Wang D., Liu Q., Zhou G., Chai G. Overexpression of PdC3H17 Confers Tolerance to Drought Stress Depending on Its CCCH Domain in Populus. Front. Plant Sci. 2020;10:1748. doi: 10.3389/fpls.2019.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bogamuwa S., Jang J. Tandem CCCH Zinc Finger Proteins in Plant Growth, Development and Stress Response. Plant Cell Physiol. 2014;55:1367–1375. doi: 10.1093/pcp/pcu074. [DOI] [PubMed] [Google Scholar]
- 92.Blackshear P.J., Phillips R.S., Lai W.S. Zinc Finger Proteins. Springer; Berlin/Heidelberg, Germany: 2005. Tandem CCCH zinc finger proteins in mRNA binding; pp. 80–90. [Google Scholar]
- 93.Pomeranz M., Hah C., Lin P., Kang S.G., Finer J.J., Blackshear P.J., Jang J. The Arabidopsis Tandem Zinc Finger Protein AtTZF1 Traffics between the Nucleus and Cytoplasmic Foci and Binds Both DNA and RNA. Plant Physiol. 2010;152:151–165. doi: 10.1104/pp.109.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Orso F., De Leonardis A.M., Salvi S., Gadaleta A., Ruberti I., Cattivelli L., Morelli G., Mastrangelo A.M. Conservation of AtTZF1, AtTZF2, and AtTZF3 homolog gene regulation by salt stress in evolutionarily distant plant species. Front. Plant Sci. 2015;6:394. doi: 10.3389/fpls.2015.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seok H., Nguyen L.V., Park H., Tarte V.N., Ha J., Lee S., Moon Y. Arabidopsis non-TZF gene AtC3H17 functions as a positive regulator in salt stress response. Biochem. Biophys. Res. Commun. 2018;498:954–959. doi: 10.1016/j.bbrc.2018.03.088. [DOI] [PubMed] [Google Scholar]
- 96.Murata T., Yoshino Y., Morita N., Kaneda N. Identification of nuclear import and export signals within the structure of the zinc finger protein TIS11. Biochem. Biophys. Res. Commun. 2002;293:1242–1247. doi: 10.1016/S0006-291X(02)00363-7. [DOI] [PubMed] [Google Scholar]
- 97.Bogamuwa S., Jang J. Plant Tandem CCCH Zinc Finger Proteins Interact with ABA, Drought, and Stress Response Regulators in Processing-Bodies and Stress Granules. PLoS ONE. 2016;11:e0151574. doi: 10.1371/journal.pone.0151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu L., Liu T., Xiong X., Liu W., Yu Y., Cao J. AtC3H18L is a stop-codon read-through gene and encodes a novel non-tandem CCCH zinc-finger protein that can form cytoplasmic foci similar to mRNP granules. Biochem. Biophys. Res. Commun. 2020;528:140–145. doi: 10.1016/j.bbrc.2020.05.081. [DOI] [PubMed] [Google Scholar]
- 99.Yeats T.H., Bacic A., Johnson K.L. Plant glycosylphosphatidylinositol anchored proteins at the plasma membrane-cell wall nexus. J. Integr. Plant Biol. 2018;60:649–669. doi: 10.1111/jipb.12659. [DOI] [PubMed] [Google Scholar]
- 100.Wang W., Wei X., Jiao G., Chen W., Wu Y., Sheng Z., Hu S., Xie L., Wang J., Tang S., et al. GBSS-BINDING PROTEIN, encoding a CBM48 domain-containing protein, affects rice quality and yield. J. Integr. Plant Biol. 2020;62:948–966. doi: 10.1111/jipb.12866. [DOI] [PubMed] [Google Scholar]
- 101.Sun J., Jiang H., Xu Y., Li H., Wu X., Xie Q., Li C. The CCCH-Type Zinc Finger Proteins AtSZF1 and AtSZF2 Regulate Salt Stress Responses in Arabidopsis. Plant Cell Physiol. 2007;48:1148–1158. doi: 10.1093/pcp/pcm088. [DOI] [PubMed] [Google Scholar]
- 102.Yan Z., Jia J., Yan X., Shi H., Han Y. Arabidopsis KHZ1 and KHZ2, two novel non-tandem CCCH zinc-finger and K-homolog domain proteins, have redundant roles in the regulation of flowering and senescence. Plant Mol. Biol. 2017;95:549–565. doi: 10.1007/s11103-017-0667-8. [DOI] [PubMed] [Google Scholar]
- 103.Wang B., Fang R., Chen F., Han J., Liu Y., Chen L., Zhu Q. A novel CCCH-type zinc-finger protein SAW1 activates OsGA20ox3 to regulate gibberellin homeostasis and anther development in rice. J. Integr. Plant Biol. 2020;62:1594–1606. doi: 10.1111/jipb.12924. [DOI] [PubMed] [Google Scholar]
- 104.Seong S.Y., Shim J.S., Bang S.W., Kim J.K. Overexpression of OsC3H10, a CCCH-Zinc Finger, Improves Drought Tolerance in Rice by Regulating Stress-Related Genes. Plants. 2020;9:1298. doi: 10.3390/plants9101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang P., Chung M., Ju H., Na H., Lee D.J., Cheong H., Kim C.S. Physiological characterization of the Arabidopsis thaliana Oxidation-related Zinc Finger 1, a plasma membrane protein involved in oxidative stress. J. Plant Res. 2011;124:699–705. doi: 10.1007/s10265-010-0397-3. [DOI] [PubMed] [Google Scholar]
- 106.Huang P., Ju H., Min J., Zhang X., Chung J., Cheong H., Kim C.S. Molecular and Physiological Characterization of the Arabidopsis thaliana Oxidation-Related Zinc Finger 2, a Plasma Membrane Protein Involved in ABA and Salt Stress Response Through the ABI2-Mediated Signaling Pathway. Plant Cell Physiol. 2012;53:193–203. doi: 10.1093/pcp/pcr162. [DOI] [PubMed] [Google Scholar]
- 107.Frederick E.D., Ramos S.B.V., Blackshear P.J. A Unique C-terminal Repeat Domain Maintains the Cytosolic Localization of the Placenta-specific Tristetraprolin Family Member ZFP36L3. J. Biol. Chem. 2008;283:14792–14800. doi: 10.1074/jbc.M801234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsushita K., Takeuchi O., Standley D.M., Kumagai Y., Kawagoe T., Miyake T., Satoh T., Kato H., Tsujimura T., Nakamura H. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 109.Lee S.-j., Jung H.J., Kang H., Kim S.Y. Arabidopsis Zinc Finger Proteins AtC3H49/AtTZF3 and AtC3H20/AtTZF2 are Involved in ABA and JA Responses. Plant Cell Physiol. 2012;53:673–686. doi: 10.1093/pcp/pcs023. [DOI] [PubMed] [Google Scholar]
- 110.Wang L., Xu Y., Zhang C., Ma Q., Joo S., Kim S., Xu Z., Chong K. OsLIC, a Novel CCCH-Type Zinc Finger Protein with Transcription Activation, Mediates Rice Architecture via Brassinosteroids Signaling. PLoS ONE. 2008;3:e3521. doi: 10.1371/journal.pone.0003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blanvillain R., Wei S., Wei P., Kim J.H., Ow D.W. Stress tolerance to stress escape in plants: Role of the OXS2 zinc-finger transcription factor family. EMBO J. 2011;30:3812–3822. doi: 10.1038/emboj.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phillips R.S., Ramos S.B., Blackshear P.J. Members of the tristetraprolin family of tandem CCCH zinc finger proteins exhibit CRM1-dependent nucleocytoplasmic shuttling. J. Biol. Chem. 2002;277:11606–11613. doi: 10.1074/jbc.M111457200. [DOI] [PubMed] [Google Scholar]
- 113.Liu L., Chen G., Ji X., Gao G. ZAP is a CRM1-dependent nucleocytoplasmic shuttling protein. Biochem. Biophys. Res. Commun. 2004;321:517–523. doi: 10.1016/j.bbrc.2004.06.174. [DOI] [PubMed] [Google Scholar]
- 114.Jang J. Arginine-rich motif-tandem CCCH zinc finger proteins in plant stress responses and post-transcriptional regulation of gene expression. Plant Sci. 2016;252:118–124. doi: 10.1016/j.plantsci.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 115.Gao H., Zheng X., Fei G., Chen J., Jin M., Ren Y., Wu W., Zhou K., Sheng P., Zhou F. Ehd4 Encodes a Novel and Oryza-Genus-Specific Regulator of Photoperiodic Flowering in Rice. PLoS Genet. 2013;9:e1003281. doi: 10.1371/journal.pgen.1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Z., Thomas T.L. PEI1, an Embryo-Specific Zinc Finger Protein Gene Required for Heart-Stage Embryo Formation in Arabidopsis. Plant Cell. 1998;10:383–398. doi: 10.1105/tpc.10.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seok H.-Y., Woo D.-H., Park H.-Y., Lee S.-Y., Tran H.T., Lee E.-H., Vu Nguyen L., Moon Y.-H. AtC3H17, a Non-Tandem CCCH Zinc Finger Protein, Functions as a Nuclear Transcriptional Activator and Has Pleiotropic Effects on Vegetative Development, Flowering and Seed Development in Arabidopsis. Plant Cell Physiol. 2016;57:603–615. doi: 10.1093/pcp/pcw013. [DOI] [PubMed] [Google Scholar]
- 118.Zhang D., Xu Z., Cao S., Chen K., Li S., Liu X., Gao C., Zhang B., Zhou Y. An Uncanonical CCCH-Tandem Zinc-Finger Protein Represses Secondary Wall Synthesis and Controls Mechanical Strength in Rice. Mol. Plant. 2018;11:163–174. doi: 10.1016/j.molp.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Barreau C., Paillard L., Osborne H.B. AU-rich elements and associated factors: Are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jan A., Maruyama K., Todaka D., Kidokoro S., Abo M., Yoshimura E., Shinozaki K., Nakashima K., Yamaguchi-Shinozaki K. OsTZF1, a CCCH-tandem zinc finger protein, confers delayed senescence and stress tolerance in rice by regulating stress-related genes. Plant Physiol. 2013;161:1202–1216. doi: 10.1104/pp.112.205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen C.Y., Shyu A.B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/S0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 122.Bakheet T., Frevel M., Williams B.R., Greer W., Khabar K.S. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bakheet T., Williams B.R., Khabar K.S. ARED 2.0: An update of AU-rich element mRNA database. Nucleic Acids Res. 2003;31:421–423. doi: 10.1093/nar/gkg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng S.S., Chen C.Y., Shyu A.B. Functional characterization of a non-AUUUA AU-rich element from the c-jun proto-oncogene mRNA: Evidence for a novel class of AU-rich elements. Mol. Cell. Biol. 1996;16:1490–1499. doi: 10.1128/MCB.16.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qu J., Kang S.G., Wang W., Musier-Forsyth K., Jang J.C. The Arabidopsis thaliana tandem zinc finger 1 (AtTZF1) protein in RNA binding and decay. Plant J. 2014;78:452–467. doi: 10.1111/tpj.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maris C., Dominguez C., Allain F.H.T. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272:2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 127.Maldonadobonilla L.D., Eschenlippold L., Gagozachert S., Tabassum N., Bauer N., Scheel D., Lee J. The Arabidopsis Tandem Zinc Finger 9 Protein Binds RNA and Mediates Pathogen-Associated Molecular Pattern-Triggered Immune Responses. Plant Cell Physiol. 2014;55:412–425. doi: 10.1093/pcp/pct175. [DOI] [PubMed] [Google Scholar]
- 128.Hudson B.P., Martinez-Yamout M.A., Dyson H.J., Wright P.E. Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d. Nat. Struct. Mol. Biol. 2004;11:257. doi: 10.1038/nsmb738. [DOI] [PubMed] [Google Scholar]
- 129.Cheng Y., Kato N., Wang W., Li J., Chen X. Two RNA Binding Proteins, HEN4 and HUA1, Act in the Processing of AGAMOUS Pre-mRNA in Arabidopsis thaliana. Dev. Cell. 2003;4:53–66. doi: 10.1016/S1534-5807(02)00399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguezcazorla E., Ortunomiquel S., Candela H., Baileysteinitz L.J., Yanofsky M.F., Martinezlaborda A., Ripoll J.J., Vera A. Ovule identity mediated by pre-mRNA processing in Arabidopsis. PLoS Genet. 2018;14:e1007182. doi: 10.1371/journal.pgen.1007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li J., Jia D., Chen X. HUA1, a Regulator of Stamen and Carpel Identities in Arabidopsis, Codes for a Nuclear RNA Binding Protein. Plant Cell. 2001;13:2269–2281. doi: 10.1105/tpc.010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmitz R.J., Hong L., Michaels S., Amasino R.M. FRIGIDA-ESSENTIAL 1 interacts genetically with FRIGIDA and FRIGIDA-LIKE 1 to promote the winter-annual habit of Arabidopsis thaliana. Development. 2005;132:5471–5478. doi: 10.1242/dev.02170. [DOI] [PubMed] [Google Scholar]
- 133.Delaney K.J., Xu R., Zhang J., Li Q.Q., Yun K.-Y., Falcone D.L., Hunt A.G. Calmodulin interacts with and regulates the RNA-binding activity of an Arabidopsis polyadenylation factor subunit. Plant Physiol. 2006;140:1507–1521. doi: 10.1104/pp.105.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan Z., Shi H., Liu Y., Jing M., Han Y. KHZ1 and KHZ2, novel members of the autonomous pathway, repress the splicing efficiency of FLC pre-mRNA in Arabidopsis. J. Exp. Bot. 2020;71:1375–1386. doi: 10.1093/jxb/erz499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao Y., Song Z., Bai J., Liu X., Nauwynck H., Jiang P. ZAP, a CCCH-Type Zinc Finger Protein, Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication and Interacts with Viral Nsp9. J. Virol. 2019;93:e00001-19. doi: 10.1128/JVI.00001-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brooks S.A., Blackshear P.J. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta. 2013;1829:666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raja V., Majeed U., Kang H., Andrabi K.I., John R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017;137:142–157. doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- 138.Verma V., Ravindran P., Kumar P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim D.H., Yamaguchi S., Lim S., Oh E., Park J., Hanada A., Kamiya Y., Choi G. SOMNUS, a CCCH-Type Zinc Finger Protein in Arabidopsis, Negatively Regulates Light-Dependent Seed Germination Downstream of PIL5. Plant Cell. 2008;20:1260–1277. doi: 10.1105/tpc.108.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin P., Pomeranz M., Jikumaru Y., Kang S.G., Hah C., Fujioka S., Kamiya Y., Jang J. The Arabidopsis tandem zinc finger protein AtTZF1 affects ABA- and GA-mediated growth, stress and gene expression responses. Plant J. 2011;65:253–268. doi: 10.1111/j.1365-313X.2010.04419.x. [DOI] [PubMed] [Google Scholar]
- 141.Wang W., Liu B., Xu M., Jamil M., Wang G. ABA-induced CCCH tandem zinc finger protein OsC3H47 decreases ABA sensitivity and promotes drought tolerance in Oryza sativa. Biochem. Biophys. Res. Commun. 2015;464:33–37. doi: 10.1016/j.bbrc.2015.05.087. [DOI] [PubMed] [Google Scholar]
- 142.Zhou T., Yang X., Wang L., Xu J., Zhang X. GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol. Biol. 2014;85:163–177. doi: 10.1007/s11103-014-0175-z. [DOI] [PubMed] [Google Scholar]
- 143.Hung K.T., Hsu Y.T., Kao C.H. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Physiol. Plant. 2006;127:293–303. doi: 10.1111/j.1399-3054.2006.00662.x. [DOI] [Google Scholar]
- 144.Nadarajah K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int J. Mol. Sci. 2020;21:5208. doi: 10.3390/ijms21155208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Choudhury F.K., Rivero R.M., Blumwald E., Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 146.Gong Z., Xiong L., Shi H., Yang S., Herrera-Estrella L.R., Xu G., Chao D.Y., Li J., Wang P.Y., Qin F., et al. Plant abiotic stress response and nutrient use efficiency. Sci. China. Life Sci. 2020;63:635–674. doi: 10.1007/s11427-020-1683-x. [DOI] [PubMed] [Google Scholar]
- 147.Guo J., Li Y., Han G., Song J., Wang B. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 2017;45:350–361. doi: 10.1071/FP17181. [DOI] [PubMed] [Google Scholar]
- 148.Song J., Wang B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015;115:541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sui N., Tian S., Wang W., Wang M., Fan H. Overexpression of Glycerol-3-Phosphate Acyltransferase from Suaeda salsa Improves Salt Tolerance in Arabidopsis. Front. Plant Sci. 2017;8:1337. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deng Y., Bao J., Yuan F., Liang X., Feng Z., Wang B. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016;79:391–399. doi: 10.1007/s10725-015-0143-x. [DOI] [Google Scholar]
- 151.Han G., Yuan F., Guo J., Zhang Y., Sui N., Wang B. AtSIZ1 improves salt tolerance by maintaining ionic homeostasis and osmotic balance in Arabidopsis. Plant Sci. 2019;285:55–67. doi: 10.1016/j.plantsci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 152.Liu S., Wang W., Li M., Wan S., Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol. Plant. 2017;39:207. doi: 10.1007/s11738-017-2501-y. [DOI] [Google Scholar]
- 153.Yang Z., Li J., Liu L., Xie Q., Sui N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020;10:1722. doi: 10.3389/fpls.2019.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feng Z., Deng Y., Fan H., Sun Q., Sui N., Wang B. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica. 2014;52:313–320. doi: 10.1007/s11099-014-0032-y. [DOI] [Google Scholar]
- 155.Sui N., Yang Z., Liu M., Wang B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genom. 2015;16:534. doi: 10.1186/s12864-015-1760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yuan F., Leng B., Wang B. Progress in Studying Salt Secretion from the Salt Glands in Recretohalophytes: How Do Plants Secrete Salt? Front. Plant Sci. 2016;7:977. doi: 10.3389/fpls.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun X., Lin L., Sui N. Regulation mechanism of microRNA in plant response to abiotic stress and breeding. Mol. Biol. Rep. 2019;46:1447–1457. doi: 10.1007/s11033-018-4511-2. [DOI] [PubMed] [Google Scholar]
- 158.Wu H., Guo J., Wang C., Li K., Zhang X., Yang Z., Li M., Wang B. An Effective Screening Method and a Reliable Screening Trait for Salt Tolerance of Brassica napus at the Germination Stage. Front. Plant Sci. 2019;10:530. doi: 10.3389/fpls.2019.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Z., Zheng H., Wei X., Song J., Wang B., Sui N. Transcriptome analysis of sweet Sorghum inbred lines differing in salt tolerance provides novel insights into salt exclusion by roots. Plant Soil. 2018;430:423–439. doi: 10.1007/s11104-018-3736-0. [DOI] [Google Scholar]
- 160.Zhao Y., Yang Y., Song Y., Li Q., Song J. Analysis of storage compounds and inorganic ions in dimorphic seeds of euhalophyte Suaeda salsa. Plan. Physiol. Biochem. 2018;130:511–516. doi: 10.1016/j.plaphy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 161.Yuan F., Leng B., Zhang H., Wang X., Han G., Wang B. A WD40-Repeat Protein From the Recretohalophyte Limonium bicolor Enhances Trichome Formation and Salt Tolerance in Arabidopsis. Front. Plant Sci. 2019;10:1456. doi: 10.3389/fpls.2019.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Y., Guo Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018;60:796–804. doi: 10.1111/jipb.12689. [DOI] [PubMed] [Google Scholar]
- 163.Jamil M., Iqbal W., Bangash A., Rehman S.U., Imran Q.M., Rha E.S. Constitutive expression of OSC3H33, OSC3H50 and OSC3H37 genes in rice under salt stress. Pak. J. Bot. 2010;42:4003–4009. [Google Scholar]
- 164.Jiang M., Jiang J., Miao L., He C. Over-expression of a C3H-type zinc finger gene contributes to salt stress tolerance in transgenic broccoli plants. Plant Cell Tissue Organ. Cult. 2017;130:239–254. doi: 10.1007/s11240-017-1218-3. [DOI] [Google Scholar]
- 165.Ma Y., Yang Y., Liu R., Li Q., Song J. Adaptation of euhalophyte Suaeda salsa to nitrogen starvation under salinity. Plant Physiol. Biochem. 2020;146:287–293. doi: 10.1016/j.plaphy.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 166.Tang K., Zhao L., Ren Y., Yang S., Zhu J.K., Zhao C. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 2020;62:258–263. doi: 10.1111/jipb.12918. [DOI] [PubMed] [Google Scholar]
- 167.Qi J., Song C.P., Wang B., Zhou J., Kangasjärvi J., Zhu J.K., Gong Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018;60:805–826. doi: 10.1111/jipb.12654. [DOI] [PubMed] [Google Scholar]
- 168.Sun X., Wang Y., Sui N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018;503:397–401. doi: 10.1016/j.bbrc.2018.07.123. [DOI] [PubMed] [Google Scholar]
- 169.Wei X., Yang Z., Han G., Zhao X., Yin S., Yuan F., Wang B. The developmental dynamics of the sweet sorghum root transcriptome elucidate the differentiation of apoplastic barriers. Plant Signal. Behav. 2020;15:1724465. doi: 10.1080/15592324.2020.1724465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abid M., Ali S., Qi L.K., Zahoor R., Tian Z., Jiang D., Snider J.L., Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Sci. Rep. 2018;8:4615. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S.W., Saud S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng H., Yang Z., Wang W., Guo S., Li Z., Liu K., Sui N. Transcriptome analysis of maize inbred lines differing in drought tolerance provides novel insights into the molecular mechanisms of drought responses in roots. Plant Physiol. Biochem. 2020;149:11–26. doi: 10.1016/j.plaphy.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 173.Chang Y.N., Zhu C., Jiang J., Zhang H., Zhu J.K., Duan C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020;62:563–580. doi: 10.1111/jipb.12901. [DOI] [PubMed] [Google Scholar]
- 174.Sun X., Han G., Meng Z., Lin L., Sui N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019;14:e1644596. doi: 10.1080/15592324.2019.1644596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen F., Liu H.-L., Wang K., Gao Y.-M., Wu M., Xiang Y. Identification of CCCH Zinc Finger Proteins Family in Moso Bamboo (Phyllostachys edulis), and PeC3H74 Confers Drought Tolerance to Transgenic Plants. Front. Plant Sci. 2020;11:1697. doi: 10.3389/fpls.2020.579255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Selvaraj M.G., Jan A., Ishizaki T., Valencia M., Dedicova B., Maruyama K., Ogata T., Todaka D., Yamaguchi-Shinozaki K., Nakashima K., et al. Expression of the CCCH-tandem zinc finger protein gene OsTZF5 under a stress-inducible promoter mitigates the effect of drought stress on rice grain yield under field conditions. Plant Biotechnol. J. 2020;18:1711–1721. doi: 10.1111/pbi.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baileyserres J., Voesenek L.A.C.J. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 178.Song J., Shi G., Gao B., Fan H., Wang B. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol. Plant. 2011;141:343–351. doi: 10.1111/j.1399-3054.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 179.Wang L., Gao J., Zhang Z., Liu W., Cheng P., Mu W., Su T., Chen S., Chen F., Jiang J. Overexpression of CmSOS1 confers waterlogging tolerance in Chrysanthemum. J. Integr. Plant Biol. 2020;62:1059–1064. doi: 10.1111/jipb.12889. [DOI] [PubMed] [Google Scholar]
- 180.Yang J.C., Li M., Xie X.Z., Han G.L., Sui N., Wang B.S. Deficiency of phytochrome B alleviates chilling-induced photoinhibition in rice. Am. J. Bot. 2013;100:1860–1870. doi: 10.3732/ajb.1200574. [DOI] [PubMed] [Google Scholar]
- 181.Zhou Y., Tan W.J., Xie L.J., Qi H., Yang Y.C., Huang L.P., Lai Y.X., Tan Y.F., Zhou D.M., Yu L.J., et al. Polyunsaturated linolenoyl-CoA modulates ERF-VII-mediated hypoxia signaling in Arabidopsis. J. Integr. Plant Biol. 2020;62:330–348. doi: 10.1111/jipb.12875. [DOI] [PubMed] [Google Scholar]
- 182.Pandey D.M., Kim S. Identification and expression analysis of hypoxia stress inducible CCCH-type zinc finger protein genes in rice. J. Plant Biol. 2012;55:489–497. doi: 10.1007/s12374-012-0384-4. [DOI] [Google Scholar]
- 183.Sui N. Photoinhibition of Suaeda salsa to chilling stress is related to energy dissipation and water-water cycle. Photosynthetica. 2015;53:207–212. doi: 10.1007/s11099-015-0080-y. [DOI] [Google Scholar]
- 184.Ding Y., Lv J., Shi Y., Gao J., Hua J., Song C., Gong Z., Yang S. EGR2 phosphatase regulates OST1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 2019;38:e99819. doi: 10.15252/embj.201899819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lu X., Zhou Y., Fan F., Peng J., Zhang J. Coordination of light, circadian clock with temperature: The potential mechanisms regulating chilling tolerance in rice. J. Integr. Plant Biol. 2020;62:737–760. doi: 10.1111/jipb.12852. [DOI] [PubMed] [Google Scholar]
- 186.Guo X., Liu D., Chong K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018;60:745–756. doi: 10.1111/jipb.12706. [DOI] [PubMed] [Google Scholar]
- 187.Sui N., Wang Y., Liu S., Yang Z., Wang F., Wan S. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front. Plant Sci. 2018;9:7. doi: 10.3389/fpls.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang D., Guo X., Xu Y., Li H., Ma L., Yao X., Weng Y., Guo Y., Liu C.M., Chong K. OsCIPK7 point-mutation leads to conformation and kinase-activity change for sensing cold response. J. Integr. Plant Biol. 2019;61:1194–1200. doi: 10.1111/jipb.12800. [DOI] [PubMed] [Google Scholar]
- 189.Zhao M., Wang L., Wang J., Jin J., Zhang N., Lei L., Gao T., Jing T., Zhang S., Wu Y. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J. Integr. Plant Biol. 2020;62:1461–1468. doi: 10.1111/jipb.12937. [DOI] [PubMed] [Google Scholar]
- 190.Liu Y., Xu C., Zhu Y., Zhang L., Chen T., Zhou F., Chen H., Lin Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018;60:173–188. doi: 10.1111/jipb.12614. [DOI] [PubMed] [Google Scholar]
- 191.Bai H., Lin P., Li X., Liao X., Wan L., Yang X., Luo Y., Zhang L., Zhang F., Liu S. DgC3H1, a CCCH zinc finger protein gene, confers cold tolerance in transgenic chrysanthemum. Sci. Hortic. 2021;281:109901. doi: 10.1016/j.scienta.2021.109901. [DOI] [Google Scholar]
- 192.Xie Z., Lin W., Yu G., Cheng Q., Xu B., Huang B. Improved cold tolerance in switchgrass by a novel CCCH-type zinc finger transcription factor gene, PvC3H72, associated with ICE1–CBF–COR regulon and ABA-responsive genes. Biotechnol. Biofuels. 2019;12:224. doi: 10.1186/s13068-019-1564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meng X., Zhou J., Sui N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018;13:149–154. doi: 10.1515/biol-2018-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J., Tergel T., Chen J., Yang J., Kang Y., Qi Z. Arabidopsis transcriptional response to extracellular Ca2+ depletion involves a transient rise in cytosolic Ca2+ J. Integr. Plant Biol. 2015;57:138–150. doi: 10.1111/jipb.12218. [DOI] [PubMed] [Google Scholar]
- 195.Jiang Z., Zhou X., Tao M., Yuan F., Liu L., Wu F., Wu X., Xiang Y., Niu Y., Liu F. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature. 2019;572:341–346. doi: 10.1038/s41586-019-1449-z. [DOI] [PubMed] [Google Scholar]
- 196.Yuan F., Yang H., Xue Y., Kong D., Ye R., Li C., Zhang J., Theprungsirikul L., Shrift T., Krichilsky B. Corrigendum: OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2015;519:378. doi: 10.1038/nature14305. [DOI] [PubMed] [Google Scholar]
- 197.Ma Y., Dai X., Xu Y., Luo W., Zheng X., Zeng D., Pan Y., Lin X., Liu H., Zhang D. COLD1 Confers Chilling Tolerance in Rice. Cell. 2015;160:1209–1221. doi: 10.1016/j.cell.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 198.Liu J., Shi Y., Yang S. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018;60:780–795. doi: 10.1111/jipb.12657. [DOI] [PubMed] [Google Scholar]
- 199.Zhang C., Lei Y., Lu C., Wang L., Wu J. MYC2, MYC3, and MYC4 function additively in wounding-induced jasmonic acid biosynthesis and catabolism. J. Integr. Plant Biol. 2020;62:1159–1175. doi: 10.1111/jipb.12902. [DOI] [PubMed] [Google Scholar]
- 200.Marchese D., de Groot N.S., Lorenzo Gotor N., Livi C.M., Tartaglia G.G. Advances in the characterization of RNA-binding proteins. Wiley Interdiscip. Rev. RNA. 2016;7:793–810. doi: 10.1002/wrna.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is presented in manuscript and in supplementary materials.