Abstract
Oxidative phosphorylation (OxPhos) is the basic function of mitochondria, although the landscape of mitochondrial functions is continuously growing to include more aspects of cellular homeostasis. Thanks to the application of -omics technologies to the study of the OxPhos system, novel features emerge from the cataloging of novel proteins as mitochondrial thus adding details to the mitochondrial proteome and defining novel metabolic cellular interrelations, especially in the human brain. We focussed on the diversity of bioenergetics demand and different aspects of mitochondrial structure, functions, and dysfunction in the brain. Definition such as ‘mitoexome’, ‘mitoproteome’ and ‘mitointeractome’ have entered the field of ‘mitochondrial medicine’. In this context, we reviewed several genetic defects that hamper the last step of aerobic metabolism, mostly involving the nervous tissue as one of the most prominent energy-dependent tissues and, as consequence, as a primary target of mitochondrial dysfunction. The dual genetic origin of the OxPhos complexes is one of the reasons for the complexity of the genotype-phenotype correlation when facing human diseases associated with mitochondrial defects. Such complexity clinically manifests with extremely heterogeneous symptoms, ranging from organ-specific to multisystemic dysfunction with different clinical courses. Finally, we briefly discuss the future directions of the multi-omics study of human brain disorders.
Keywords: mitochondria, mitochondrial DNA, nervous tissue, OxPhos complexes, bioenergetics, genomics, proteomics, mitochondrial diseases
1. Introduction
The panoply of mitochondrial functions reflects on highly heterogeneous clinical presentations when an error in a mitochondrial protein or function occurs. Mitochondria are dynamic and mobile organelles representing a hub where exchange of information among the nucleus and other cellular compartments takes place to modulate energy production and metabolites provision to the cell’s specific needs and nutrient availability [1]. The basic function of mitochondria is the generation of more than 90% of cellular energy via the oxidative phosphorylation (OxPhos) system [2] but, in addition, they play many roles in the different types of cells: compartmentalize metabolites for the maintenance of redox homeostasis; function as centers for metabolic waste management [3]; surveil calcium homeostasis [4]; initiate caspase-dependent apoptosis and other intermediate cellular stress response [5]; provide sulfur metabolism and iron-sulfur cluster biogenesis [6,7]; house the synthesis of cardiolipin, steroids, quinone, and heme [8,9]; breakdown fatty acids through β-oxidation; and serve as a metabolic platform for the tricarboxylic acid (TCA), and urea cycles [10]. All these functions include homeostatic regulation of organelle morphology and dynamics [11], quality control [12], and participation in the immune response [13,14]. Alteration of each of the above functions and activities can have different effects according to the specificity of the organ and cell type, but alteration of mitochondrial energy production can impact tissues with the highest energy requirements such as the nervous system, both central (CNS) and peripheral (PNS) [15,16].
The term ‘mitochondrial medicine’ categorizes the ample array of clinical presentations associated with all types of mitochondrial defects having directly or secondarily defect of one or several mitochondrial functions although ‘mitochondrial diseases’ traditionally indicate dysfunction of the OxPhos system [6,17]. The direct link between human disease and the genetic alteration of a mitochondrial function has found a breakthrough with the application of -omics technologies (i.e., genomics, transcriptomics, proteomics, metabolomics, and epigenomics, etc.). Rapidly, high-throughput omics techniques—that is detection of biologically significant differences, even if not high magnitude changes, in a multitude of molecular constituents in organisms supported by sophisticated bioinformatics tools—have allowed progress in cataloging the predicted human mitochondrial proteins thus revealing new details and providing clues to elucidating still unknown basic aspects of mitochondrial structure and function. These novel high-throughput techniques have enhanced the final diagnosis of several mitochondrial disorders. This is a very relevant aspect, especially considering that mitochondrial diseases individually are rare but are probably the most frequent genetic disorder in adults (incidence of 1 in 5000 live births) [18]. More recently, genome editing technology applied to neural cultures and cerebral organoids generated from patients-derived iPSCs is revolutionizing the landscape and offering new opportunities for understanding the pathogenetic effects of mutations in nervous tissue.
This review aims to focus on the dysfunction of OxPhos defects mostly in the nervous system to highlighting the contributions of powerful omics technologies to mitochondrial medicine to land from the laboratory to the clinic.
2. Mitoexome, Mitochondrial Proteome, and Mitointeractome
Before Next-Generation Sequencing (NGS) improved our understanding of how mutations cause diseases, first attempts to identify the mitochondrial proteome were based on ‘cyberscreening’ of available genome databases. This allowed the discovery of few human mitochondrial genes presenting orthologs in lower eukaryotes. An example of the cyberscreening strategy used Saccharomyces cerevisiae proteins as ‘probes’ to identify BCS1, PET112, SCO1, COX15, and COX11, five yeast genes that present orthologs (respectively, BCS1L, GATB, SCO1, COX15, and COX11) in humans [19]. Except for COX11, a COX-assembly, all genes have been implicated in mitochondrial diseases [OMIM 603647.0001-603647.0013; OMIM 603645.0001-603645.0002; OMIM 603644.0001-603644.0002; OMIM 603646.0001-603646.0004], see paragraphs 4.3 and 4.4. To date, whole-exome (WES) and whole-genome (WGS) resequencing have dramatically enhanced the ability to identify the underlying gene mutations in patients with isolated or multiple mitochondrial respiratory chain complex defects [20,21]. The collection of mt genes and coding exons of the 1034 nuclear genes encoding the human mitochondrial proteome is defined as ‘MitoExome’ [22,23]. This multigene panel is useful in performing targeted resequencing of the OxPhos nuclear genes because it includes not only the 77 nuclear structural OxPhos subunits and the 37 mitochondrial (mt) DNA genes including the 13 structural genes for OxPhos subunits [24] but also genes for mitochondrial proteins either already known or not to be associated with a specific mitochondrial disease, including assembly factors and electron carriers’ genes which represent a large fraction of the overall mitochondrial genes that can cause mitochondrial dysfunction [21]. Application of MitoExome resequencing provides novel mutation candidates, enables the discovery of unusual clinical variants [25,26] and new clinical phenotypes [26] (Figure 1). Furthermore, the integration of MitoExome sequencing with the study of mitochondrial proteome potentiates the detection of variants causing protein destabilization and/or aberrantly low expression [27].
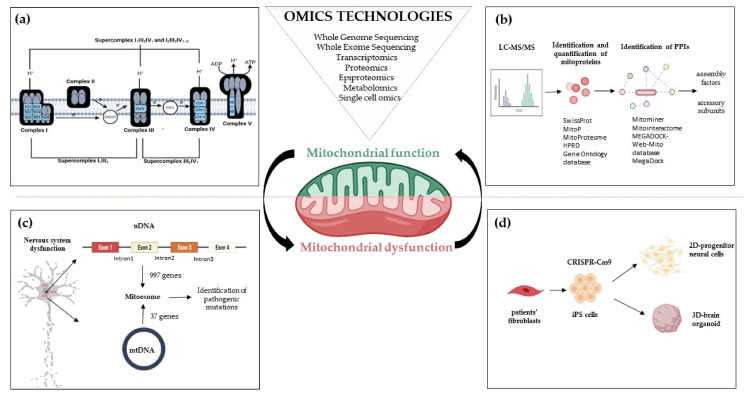
Figure 1.
Omics strategies advance in understanding mitochondrial function and dysfunction in brain disorders related to OxPhos gene mutations. Mitochondrial bioenergetics involves activities whose function and structure have been deeply elucidated by omics technologies; (a) The introduction of high-resolution technologies has been resolutive to deepen the structure of the respiratory chain complexes and supercomplexes; (b) Quantitative proteomics, e.g., LC-MS/MS enable the identification and quantification of mitoproteins and provide large amounts of data. Through Network-based approaches analyzing protein-protein interactions, the huge amount of information allows the discovery of novel accessory subunits and assembly factors of the five multi-subunit enzyme complexes; (c) The re-sequencing carried out with MitoExome increases the possibility of identifying new or previously reported mutations in both mitochondrial and nuclear genes in patients; (d) Novel multi-omics analysis, based on single-cell omics, is applied to two-dimensional (2D) neural cultures and three-dimensional (3D) cerebral organoids generated from patients-derived iPSCs that can be engineered by CRISPR/Cas9. Abbreviations: LC-MS/MS: Liquid Chromatography with tandem mass spectrometry; PPIs: Protein-protein Interactions; nDNA: nuclear DNA; mtDNA: mitochondrial DNA; iPS cells: Induced Pluripotent Stem cells.
Biochemical and ultrastructural characterizations have uncovered the heterogeneity of mitochondria in their function, trafficking patterns, lifespan, and morphology across cell types and different cellular compartments. Different tissues, cell types, and cellular states have unique signatures of protein localization to mitochondria. In the proteomic comparison of the mitochondrial proteins, almost half are found as core components in virtually all tissues, whereas the remaining are tissue-specific [28,29]. The study of mitochondrial proteome starts with the isolation of mt compartment from cells and tissues and stands behind the availability of methodologies to isolate pure mitochondria from different sources to define exactly the function of each protein in each cell type of the human body [30]. The performance of proteomics analysis is driven by the reduction of sample complexity, enhancement of mass spectrometry (MS) power of resolution, and the possibility to reduce the contamination of the sample with non-mitochondrial proteins owed to chemical and physical similarities between mitochondria and other cellular components (e.g., lysosomes). Since the initial rough estimates, it has been suggested that the mammalian mitochondrial proteome encompassed about 1000–1500 distinct proteins—including the 13 mtDNA-encoded proteins [24]—that represent an important subset of the ~20,000 distinct mammalian proteins [31,32] (Figure 2).
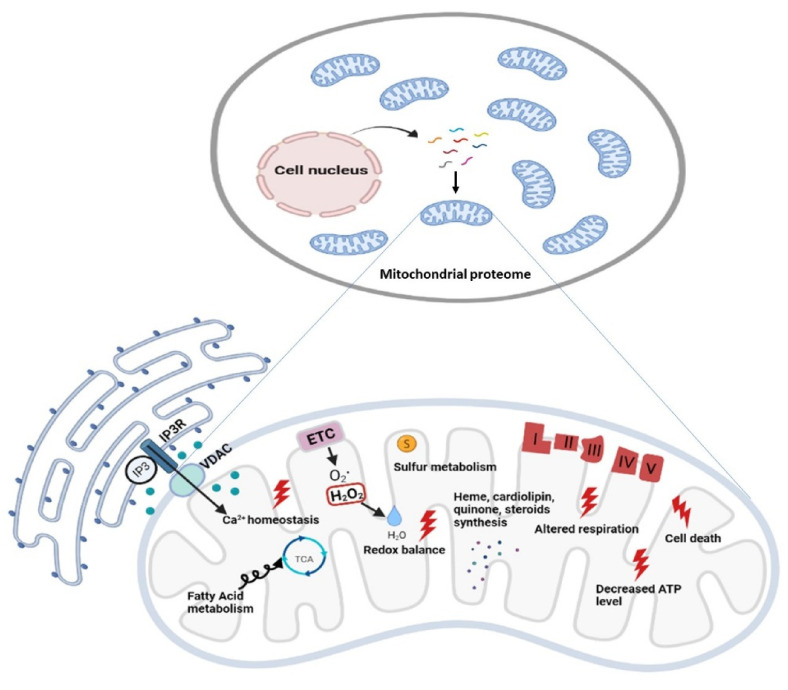
Figure 2.
Functional diversity of mitochondrial proteins and bioenergetics consequences of OxPhos system dysfunction. The mammalian mitochondrial proteome includes both mitochondrial and nuclear DNA- encoded proteins. Most of the proteins required for the various activities in which mitochondria are involved are encoded by the nuclear genome, whereas the mitochondrial energy-producing system, i.e., the OxPhos complexes, has either mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) encoded components. The enlarged mitochondrion shows most of the bioenergetics consequences (indicated by the red bolt lightning) of genetic defects involving the OxPhos complexes. Abbreviations: IP3: Inositol Trisphosphate; IP3R: Inositol Trisphosphate Receptor; VDAC: Voltage-dependent anion channel; ETC: Electron Transport Chain; TCA: Tricarboxylic Acid Cycle; ATP: Adenosine triphosphate.
Quantitative two-dimensional (2D) gels of highly purified mitochondria estimated ~1500 distinct spots [33], a number higher than the ~1000 distinct protein products encoded by the genomes of alpha-proteobacteria, which are the closest living relatives of modern-day mitochondria [34]. Several databases have been used to integrate the experimental data with bioinformatic predictions based on mitochondrial localization or interaction. For example, the MitoProteome is an object-related database developed at the UCSD Supercomputer Center, which contains information on mitochondria-localized proteins [35,36]. Each entry in the MitoProteome corresponds to a gene encoding a protein that is localized within mitochondria and its basic information, along with annotations of isoforms, splice variants, and functions of the corresponding protein. To date, the most comprehensive study elucidating the mitochondrial proteome of different mammalian tissues is represented by the MitoCarta inventory [29,37]. This catalog combines multiple experimental and computational approaches, i.e., mass spectrometry (MS) analysis of mitochondria isolated from 14 mouse tissues, large-scale GFP-fusion microscopy analysis, and bioinformatics using data mining, prediction, evolutionary conservation, and a Bayesian integration of seven additional data sources. The first release was represented by MitoCarta1.0 (http://www.broadinstitute.org/pubs/MitoCarta/; accessed 25 July 2021) which contained about 1000 distinct gene loci [29]. Updated in 2016, MitoCarta 2.0 listed about 1200 genes [37]. Another dedicated database that collected, curated, and annotated information on mitochondrial proteins is the MitoMiner database (http://mitominer.mrc-mbu.cam.ac.uk/; accessed 25 July 2021) [38] (version 4.0, 2018). It is based on the literature and proteomics data based on both LC-MS and 2D gel studies, antibody staining, and other subcellular localization data, and provides a collective score for each protein’s probability to have the mitochondrial association. MitoMiner records mitochondrial proteins from 12 organisms [38]. Using the data contained within MitoMiner, the Integrated Mitochondrial Protein Index (IMPI) was also developed (http://www.mrc-mbu.cam.ac.uk/impi; accessed 25 July 2021). IMPI version Q2 (2018) contains 1626 human genes that encode mitochondrially localized proteins, 1184 known to be mitochondrial and 442 predicted to be mitochondrial. The large amount of information provided by mito-databases as MitoMiner 4.0 v2018 JUN (http://mitominer.mrc-mbu.cam.ac.uk; accessed 25 July 2021), makes it possible to define different score systems for mitochondrial confidence combining data from various mitochondrial and functional annotation databases. These strategies allow increasing the stringency of protein accepted as inherently mitochondrial [39]. An exhaustive list of the major data sources loaded with the latest version and links to the relevant resources is reported in the Data Sources section of the Mitominer (https://mitominer.mrc-mbu.cam.ac.uk/release-4.0/dataCategories.do; accessed 25 July 2021).
More recent advances in the experimental proteomic approaches, specifically in labeling and MS methods, have further expanded and defined the known mitochondrial proteome and have simultaneously revealed the sub-mitochondrial localization of many of them [40,41]. A novel spatial proteomics pipeline demonstrated that many proteins cannot be classified to a single localization as they either transit between compartments or carry out their functional role(s) in multiple locations [41]. The redundant functions, or functions affecting multiple cellular processes, rendered difficult the study and it was estimated that about ~20% of mitochondrial proteins remained uncharacterized [42].
Along with technological progress that has enabled the discovery of approximately 78,120 human proteins [based on The UniProt Knowledgebase (UniProtKB), as of 23 February 2021], derives the challenge of identifying a large amount of potential protein-protein interactions (PPIs). An example of the network-based approaches analyzing protein-protein interaction is represented by MitoInteractome, a web-based portal containing 6549 protein sequences extracted from SwissProt (http://www.expasy.ch/sprot/; accessed 25 July 2021), MitoP (http://www.mitop.de:8080/mitop2/; accessed 25 July 2021), MitoProteome (http://www.mitoproteome.org/; accessed 25 July 2021), HPRD (http://www.hprd.org; accessed 25 July 2021) and Gene Ontology database (http://www.geneontology.org; accessed 25 July 2021). This enables the elucidation of integrative mitochondrial functions and can expedite the discovery of novel interactions which otherwise may have been missed using traditional experimental techniques. MEGADOCK [43,44], a structure-based PPI prediction method, was first developed and then followed the MEGADOCK-Web-Mito database which is a PPI prediction data archive, that includes prediction results for protein pairs of 654 mitochondria-related human proteins [45]. All these approaches have been key in the study of PPI as a means to infer functions for uncharacterized proteins and to enable the discovery of novel proteins, e.g., several complex I assembly factors [46,47] (Figure 1).
For expert reviews on the details about the technical approaches, the required bioinformatics pipelines, and how (multi)omics technologies can help in studying the dysfunction of mitochondrial bioenergetics, see [48,49].
3. Diversity of Bioenergetics Demand in the Brain
The brain relies on glucose metabolism for ATP generation and many other activities and an inappropriate supply of either glucose or oxygen degrades brain function. The principal energy request of the brain is due to activities of the neuronal signaling that include resting and action potentials, glutamate cycling, post-synaptic Ca2, postsynaptic receptors, while the activities of the non-signaling, e.g., turnover of proteins, phospholipids, and nucleic acids, remodeling of the actin in the cytoskeleton, axonal transport, mitochondrial proton leak, etc., are less demanding. Specifically, gray matter and white matter have different energetic requests for non-signaling (30% versus 80%, respectively) and signaling (70% versus 20%, respectively) activities [50,51]. These findings would suggest that the energy demands of signaling activities in gray matter are mainly due to synaptic activity while the energetic demands in white matter satisfy the request of billions of unmyelinated axons and glial cells [50]. Beyond ATP generation, glucose is important for the synthesis of several molecules within the brain, including neurotransmitters and neuromodulators. For these reasons, mitochondria are quite heterogeneous as anatomical localization, activity, and metabolism at regional, cellular, subcellular levels and during differentiation, when the upregulation of mitochondrial metabolism is the basis of cell proliferation in neuronal stem cells and progenitor cells. Although different regions of the brain contain about half as many mitochondria as the heart, the mitochondria of the brain are qualitatively different to support the high metabolic demand that requires, for example, close cooperation between neurons and astrocytes [52]. Astrocytes are metabolically and structurally supportive [52,53,54] and are crucial in neurotransmission [55,56] and behavior [57,58]. The ATP utilized by neurons is produced by the OxPhos process, while most of the energy needs of astrocytes are met by glycolysis [59,60]. The mitochondrial ATP production per molecule of glucose oxidized is ~16 times more than glycolysis. The survival of neurons requires OxPhos [52] and in mature neurons, the local ATP supply provided by mitochondria is used to regulate axonal and dendritic development, axonal regeneration, as well as contributing to synaptic transmission and plasticity. The different energy metabolisms of the two cell types are closely coupled, with astrocytes releasing the glycolytic end-product, lactate, which is used by neighboring neurons to drive OxPhos [61,62].
An example of heterogeneity of mitochondria in metabolic enzyme diversity has been provided by a study comparing the mitochondrial proteome of the three major cerebellar cell types: Granule cells (GC), the most abundant excitatory neuron; Purkinje cells (PC), the major inhibitory neuron of the cerebellum and astrocytes [63]. In the adult cerebellum, ~15% of the annotated mitochondrial proteome was shown to be differentially regulated among the three cell types. Fatty acids were more efficiently metabolized by astrocytic than neuronal mitochondria due to the enrichment of two beta-oxidation enzymes, i.e., short-chain-specific acyl-coenzyme A dehydrogenase and carnitine palmitoyl-transferase 1a, an enzyme that limits the rate of oxidative reactions of long-chain fatty acids [63]. In particular, the mitochondrial proteome of astrocytes showed a remarkable enrichment of peroxisomal proteins, some of which are known to have a double localization (i.e., catalase) [64] or binding to mitochondria (i.e., Eci2 and Pex11b).
In the same work, the mitochondrial calcium uniporter (MCU) [4,63,65] and its regulators were detected mostly in GC [63]. Recent studies suggest that the markedly different modes of ATP production in the neurons and astrocytes reside also in the supra-organization of the mitochondrial respiratory chain in supercomplexes (see Section 4.6 paragraph) able to regulate different rates of respiration and mitochondrial ROS production [66].
The brain mitochondrial proteome is not a unicum also when considering synaptic and non-synaptic mitochondria (sMito and nsMito). Proteomic profiling of sMito vs. nsMito revealed mitochondrial complex I as an upstream regulator of degenerative processes associated with a high range of age-related neuropathologies characterized by synaptic dysfunction [67]. In a separate study, an accurate analysis of quantitative proteomics was performed to differentiate sMito and nsMito using Stable Isotope Labeling with Aminoacids in Cell culture (SILAC) labeled mitochondria from cultured cells as an internal standard. In SILAC, cells are differentially labeled by growing them in a ‘light’ medium, containing normal amino acids, or a ‘heavy’ medium, containing a stable isotope [68]. Significant differential expression was shown for 522 proteins involved in several pathways including the OxPhos system, mitochondrial fission/fusion, calcium transport, and mtDNA replication and maintenance. Lower levels of Pyruvate dehydrogenase (PDH) subunits in the synapse to other parts of the cell and reduced expression of complex I, II, and IV (expect for COX4I2) suggested decreased bioenergetic function of sMito compared to nsMito [68]. Consistent with this finding, sMito exhibited increased age-associated mtDNA deletions and reduced levels of TFAM and mtSod2, suggesting a reduced ability of sMito to withstand ROS, thus providing insights into synaptic mitochondrial susceptibility to damage [68] (Figure 3).
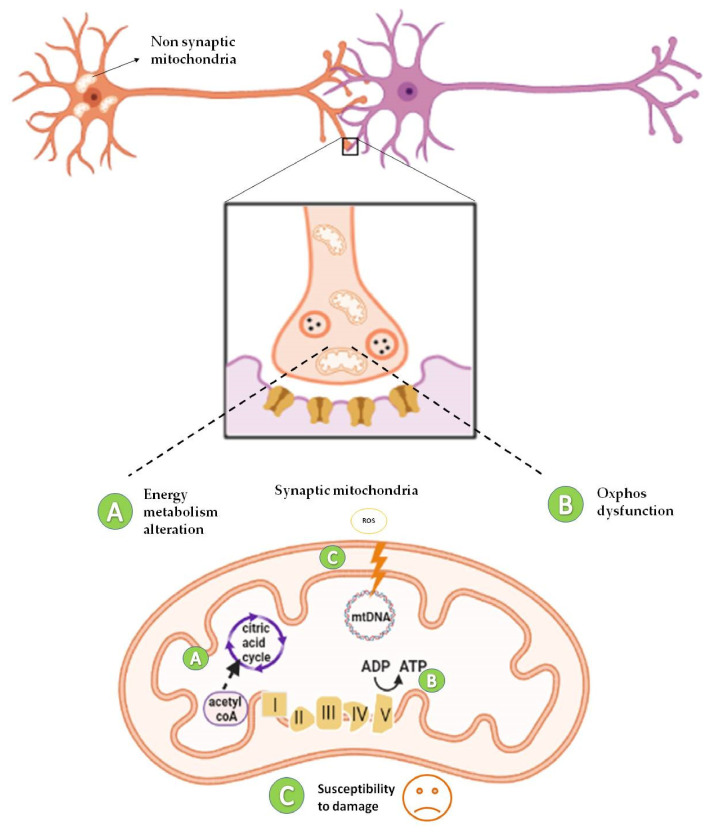
Figure 3.
Diversity of mitochondrial proteome in synaptic and non-synaptic mitochondria. Synaptic mitochondria show defects in energy metabolism due to low levels of Pyruvate dehydrogenase (PDH) subunits [68] (A), reduced expression of complex I [67,68], II, and IV [68] (B), and increased susceptibility to damage (increased mtDNA deletions) [68] (C), compared to non-synaptic mitochondria. ADP: Adenosine diphosphate; ATP: Adenosine triphosphate.
The extreme heterogeneity of mitochondria activities and functioning has been recently shown by a novel and fine imaging approach that specifically allows to label and monitor mitochondrial translation products for microscopic fluorescent imaging. In neuronal cultures, mitochondrial translation was monitored in axonal and dendritic mitochondria as well as in pre-and post-synaptic regions of neurites by specifically labeling the peptides newly synthesized by mitochondrial ribosomes, revealing that not all mitochondria translate to the same extent in different cell types [69]. Finally, the fundamental role of mitochondria during neurogenesis has been recapitulated in the cellular and organoid model of Leigh syndrome (LS), a severe manifestation of mitochondrial disease in children [70]. Mutations in SURF1, a complex IV assembly gene, cause neuronal impairment because of defective metabolic programming of neural progenitor cells (NPCs) that prevents the establishment of neuronal morphogenesis. Using CRISPR/Cas9 engineered SURF1 patient-derived iPSCs, a human model of LS was developed. Single-cell RNA-sequencing and multi-omics analysis revealed compromised neuronal morphogenesis in mutant 2D neural cultures and 3D brain organoids (Figure 1d). The defects already emerged at the level of NPCs, which were unable to shift toward OxPhos and retained a proliferative glycolytic state that fails to instruct neuronal morphogenesis. Interestingly, gene augmentation and PGC1A induction via Bezafibrate treatment inducing mitochondria biogenesis supported the metabolic programming of LS NPCs, leading to restored neuronal morphogenesis [70]. It is interesting to point out that the current understanding of LS is that the disease is caused by neuronal degeneration. This interpretation had led to experimental treatment schemes focused on antioxidants to prevent the build-up of damaging free radicals. The multi-omics analysis in 2D and 3D models adopted by Prigione [70] provided a novel perspective to LS pathology by showing that the disease mechanisms may not necessarily involve a redox imbalance but rather an impairment of neuronal morphogenesis following the loss of NPC commitment. Evidence that Surf1 impairment may affect the neurogenesis was described also in the SURF1-knock out swine model that shows a disorganized cortical structure with several immature neurons and developing of a severe early-onset neurological phenotype [71]. These findings overall suggest that mutations associated with mitochondrial diseases could impair neurogenesis and shift the view of therapeutic approaches that might lead to novel interventions aiming at promoting the reestablishment of physiological neurogenesis [72] rather than merely preventing the degeneration of mature neurons.
4. Structure, Assembly, and Disorders of Bioenergetics Complexes
The development of mito-omics-based approaches has been crucial in understanding the functional and bioenergetic consequences of mutations responsible for the onset of primary mitochondrial diseases. The OxPhos is the enzymatic machinery by which mitochondria produce the ATP needed by the cells. The reactions are performed by five multimeric enzyme complexes (EC): Complex I (EC 1.6.5.3) or NADH-Ubiquinone Reductase, CI, 45 subunits; Complex II (EC 1.3.5.1) or Succinate-Ubiquinone Oxidoreductase, CII, 4 subunits; Complex III (EC 1.10.2.2) or Ubiquinol: cytochrome c (cyt c) oxidoreductase, CIII, 10 subunits; Complex IV (EC 1.9.31) or Cyt c oxidase (COX), CIV, 13 subunits; Complex V (EC 3.6.14) or ATP synthase, CV, 16 subunits; and two-electron transport carriers, namely, ubiquinone (coenzyme Q, CoQ) and cyt c [73]. Reactions catalyzed by CI, CIII, and CIV result in the release of protons in the inner membrane space, thereby creating the proton gradient needed for ATP synthase activity. The correct function of the OxPhos system depends on the concerted action of several chaperones and other assembly factors that play essential roles in the formation, regulation, and stability of the five complexes and the mobile electron carriers, and nucleotide transporters [74]. Assembly factors of CI, CII, CIII, and CV have been classified as early-stage factors, acting in the structural assembly of individual subunits and sub-complexes, and late-stage accessory factors, called LYRM (leucine-tyrosine-arginine motif) proteins, controlling the incorporation and/or activation of last subunits and/or cofactors (i.e., Fe-S clusters). The human mitoproteome contains at least 12 LYRM proteins [75].
The OxPhos system is under a dual genetic control: 13 subunits are of mtDNA origin [24] and the remaining are encoded by the nuclear DNA (nDNA) [76]. MtDNA is a small circular genome [24] that encodes only 13 mitochondrial proteins, 22 mt-tRNAs, and 2 mt-rRNAs. Hence, the nuclear-encoded mitochondrial proteome requires sophisticated machinery for the transport into mitochondria [77,78,79]. Over the last years, a growing number of human proteins involved in mtDNA replication, and expression have been identified owing to the study of primary mitochondrial diseases. The coordination between the two genomes is crucial for mtDNA integrity, copy number regulation, and mitochondrial protein synthesis because mutations in nuclear genes encoding proteins for mtDNA replication and maintenance may affect its integrity and properties [80]. Dedicated reviews on these topics, including also the specific mechanisms regulating mtDNA replication [81], transcription [82], and translation [83,84] are available elsewhere.
Genetically, the mitochondrial diseases associated with the OxPhos system are split into two broad genetic categories: disorders due to mutations in the mtDNA, observing the rules of mitochondrial genetics; disorders due to mutations in the nDNA, transmitted as a Mendelian trait [6,85]. To date, mutations in both mitochondrial and nuclear genomes have been reported to cause mitochondrial disease manifesting with characteristic leukoencephalopathy and other clinical phenotypes either multisystemic or with single tissue involvement [86,87,88].
Since the first descriptions of mtDNA mutations [89,90,91], the number of mutations has been growing more and more until it counts over 1000 heteroplasmic rearrangements (large deletions/duplications) (http://mitobreak.portugene.com; accessed 25 July 2021), and over 500-point mutations possibly pathogenic among the 700 variants reported, which affect all mtDNA genes (https://www.mitomap.org; accessed 25 July 2021). A few major clinical phenotypes in adults have been recently reviewed [92]: LHON [91,93]; Neuropathy, ataxia, retinitis pigmentosa (NARP)/maternally inherited Leigh syndrome (MIILS) [94,95]; Maternally inherited nonsyndromic deafness, associated or not with aminoglycosides use [96]; Myoclonus, epilepsy, ragged-red-fibers syndrome (MERRF) [97,98]; Mitochondrial encephalopathy, lactic acidosis stroke-like syndrome (MELAS) [99,100]; Chronic progressive external ophthalmoplegia (CPEO) spectrum [89]; Kearns–Sayre syndrome (KSS) [101,102] and Pearson’s syndrome [103,104]. LHON and NARP/MILS are disorders that affect single OxPhos complex, complex I in LHON [105], and complex V in NARP/MILS [106], respectively. All these phenotypes are maternally inherited, displaying the hallmarks of mitochondrial diseases including variability of the phenotype, incomplete penetrance, and overlapping clinical features. The exception is represented by CPEO/KSS/Pearson associated with single mtDNA deletions, which are mostly sporadic [107,108].
Herein, we will provide some rapid information on structure, assembly, and disorders related to each of the OxPhos complexes. All the details of complexes assembly, including the factors, the interacting module/function, the associated clinical phenotypes, and the references have been adapted from [47,74,109,110].
4.1. NADH–Ubiquinone Oxidoreductase–Complex I
NADH–Ubiquinone Oxidoreductase (Complex I, CI) couples the electron transfer of the two electrons derived from NADH oxidation to the ubiquinone with the translocation of four protons into the intermembrane space (IMS) [111,112,113]. Most of the molecular studies of mitochondrial diseases have focused on Complex I, which is the largest and most complicated among the respiratory complexes. Of 45 subunits, seven are encoded by the mtDNA (MT-ND1-6 and MT-ND4L), and the remaining, including the dual copy of the acyl-carrier protein NDUFAB1 [114], are encoded by nDNA [114,115]. Structurally, CI is an L-shaped complex that is composed of two domains: the hydrophilic head protruding into the matrix and the hydrophobic part within the inner mitochondrial membrane (IMM) [116]. Fourteen core subunits, conserved from bacteria to humans, perform catalytic activities [114,117,118]. Seven core subunits in the hydrophilic arm contain the redox-active centers: a non-covalently bound FMN and seven Fe–S clusters [119]. All the seven mtDNA-encoded CI subunits are in the hydrophobic arm and form the proton channels [115]. The remaining 30 subunits are ‘supernumerary’ but important for assembly and stability [120]. Most accessory subunits are only found in eukaryotic complex I. A notable exception is represented by subunits NDUFS4, NDUFS6, and NDUFA12 that are already present in complex I from α proteobacteria [121].
The complete mammalian CI structure has been elucidated [111,122] and determined by X-ray crystallography [117,123] and cryo-EM [118,124,125,126,127,128,129]. It is organized in six independent modules, N, Q, ND1/PP-a, ND2/PP-b, ND4/PD, and ND5/PD-b, that, assisted by specific assembly factors, are incorporated in a specific order [130]. The overall L-shaped CI structure derives from the assembly of the N- and Q modules in the peripheral arm, and ND1, ND2, ND4, and ND5 modules in the P part of the membrane arm forming, at the hinge between the two arms, the channel of the CoQ binding site (Q-module) [119,120]. The N module, situated at the head of the hydrophilic part, contains the NADH-binding site and a flavomononucleotide (FMN) cofactor which oxidizes NADH to release two electrons [130]; the Q module for Q reduction, situated in the hydrophilic arm, contains eight Fe–S clusters where electrons flow to reach ubiquinone [130]. The N and Q modules form the peripheral arm containing the seven “core” subunits (NDUFV1, NDUFV2, NDUFS1, NDUFS2, NDUFS3, NDUFS7, and NDUFS8) whereas the 30 accessory subunits are necessary to stabilize the enzyme [131]. The P-module constitutes the membrane arm and is composed of the seven mtDNA-encoded proteins: ND1- ND4, ND4L, ND5, and ND6, involved in proton translocation [132]. Specific factors assisting the preassembly of the modules and the role of protein import machinery are summarized in Table 1.
Table 1.
Complex I assembly factors with interacting module/function, associated clinical phenotypes, and references. Adapted from [47,74,110,133].
| Assembly Factors | CI Interacting Module/Function |
Associated Clinical Phenotypes | References |
|---|---|---|---|
| ACAD9 | ND2/PP-b module Component of MCIA complex, necessary for insertion of ND2 |
Cardiorespiratory depression, hypertrophic cardiomyopathy, encephalopathy, and severe lactic acidosis | [134,135] |
| ECSIT | ND2/PP-b module Component of MCIA complex, necessary for insertion of ND2 |
- | [136] |
| FOXRED1 | ND4/PD module | Leigh syndrome, congenital lactic acidosis, athetoid movements of the limbs in early childhood, hypotonia and cerebellar atrophy, mitochondrial respiratory CI deficiency associated with Leigh syndrome, encephalocardiomyopathy, or ataxia |
[137,138,139] |
| ATP5SL/DMAC2 | ND4/PD module | - | [140] |
| TMEM70 | ND4/PD module | Neonatal mitochondrial encephalocardiomyopathy, mitochondrial CV deficiency, nuclear type 2, occasionally facial dysmorphisms and CI deficiency | [141,142,143,144,145,146] |
| NDUFAF1 | N module, ND1 Component of MCIA complex, necessary for insertion of ND2 |
Hypertrophic cardiomyopathy, developmental delay, lactic acidosis, hypotonia, and Wolff–Parkinson–White syndrome | [147,148] |
| NDUFAF2 | N module. Stabilization of pre-CI or 830 kDa subcomplex |
Ataxia, lethargy, nystagmus, hypotonia, optic atrophy, and episodic respiratory, insufficiency, generic encephalopathic syndromes, or Leigh syndrome | [149] |
| NDUFAF3/C3ORF60 | Q module | Macrocephaly, weak cry, no eye contact, wide anterior fontanel and axial hypotonia |
[150] |
| NDUFAF4/C6ORF66 | Q module | Severe encephalopathy and antenatal Cardiomyopathy |
[151] |
| NDUFAF5/C20ORF7 | Not known. Catalyze hydroxylation of NDUFS7 and dimethylation of NDUFS2 of the Q module |
Facial dysmorphism, progressive lactic acidosis and neurological defects, severe early-onset encephalopathy | [152,153] |
| NDUFAF6 | Not known. Maintain a normal level of mt-ND1 subunit |
Focal seizures, decreased movement and strength, ataxia, lactic acidosis, and Leigh syndrome | [29,154,155,156,157,158] |
| NDUFAF7 | Not known. Catalyze dimethylation of NDUFS2 of the Q module |
- | [159,160] |
| NDUFAF8/C17ORF89 | Not known. Stabilization of NDUFAF5 |
Leigh syndrome | [161] |
| NUBPL | Supposed to interact with the developing N module and possibly Q module. Insertion of iron-sulfur clusters in N and Q module subunits |
Infantile onset hepatopathy, renal tubular acidosis, developmental delay, short stature, leukoencephalopathy, myopathy, nystagmus, and ataxia | [162,163,164] |
| TIMMDC1/C3ORF1 | ND1/PP-a Insertion of ND1 |
Infantile onset hypotonia, failure to thrive, delayed or minimal psychomotor development, sensorineural deafness, dysmetria, dyskinetic movements, peripheral neuropathy, nystagmus, and Leigh syndrome |
[140,165,166] |
| TMEM126A | ND4 module Component of MCIA complex, necessary for building the intermediate ND2 module |
Autosomal recessive optic atrophy | [167,168,169,170,171] |
| TMEM126B | ND2/PP-b module Component of MCIA complex, necessary for building the intermediate ND2 module |
Exercise intolerance, muscle weakness, myalgia, early-onset renal tubular acidosis, and hypertrophic cardiomyopathy | [172,173,174] |
| TMEM186 | ND2/PP-b module- Interact strongly with newly synthesized ND3 |
- | [175] |
| DMAC1/TMEM261 | ND5/PD-b | - | [120] |
| COA1/MITRAC15 | ND2/PP-b module | - | [175] |
| COA7 | - | Autosomal recessive spinocerebellar ataxia with axonal neuropathy type 3 | [176] |
| LYRM-2 | NADH-Dehydrogenase module Maturation of N-module |
- | [177] |
A wide range of pathological phenotypes of the nervous system has been found to affect CI stability/activity both involving mitochondrial- and nuclear-encoded subunits [6]. Many pathological variants in the seven mtDNA encoded subunits, MT-ND1-6 and ND4L have been associated with a wide spectrum of syndromes with the age of onset occurring mostly during late childhood or early adulthood [178,179,180,181]. Mutations in three MT-ND genes are the main cause of Leber’s hereditary optic neuropathy (LHON) [OMIM 535 000], the most common mtDNA inherited disease [182]. LHON is one cause of bilateral acute or subacute, painless loss of central vision in young men (more than 80% of LHON patients are male, because of degeneration of retinal ganglion cell layers [183,184]. Important clues to understanding the pathogenesis of LHON, which is characterized by yet poorly understood genetic and environmental factors affecting the incomplete penetrance, have been obtained by analysis of mtDNA copy number and by proteomics approaches [185,186,187,188]. Mitochondrial DNA copy number is a key factor in differentiating LHON affected individuals from the unaffected mutation carriers [185,186,187,188]. A mitochondrial proteomic profile of 11778G>A mutant fibroblasts using 2-Dimensional Polyacrylamide Gel Electrophoresis (2-DE) and MS [189] disclosed that most of the mitochondrial proteins–including those involved in intermediary metabolic processes, nucleoid-related proteins, chaperones, cristae remodeling ones, and an antioxidant enzyme–were down-regulated, and some OxPhos subunits were altered [189]. The major bioenergetics consequences, particularly of MT-ND4 and MT-ND1 mutations, resulted in CI-dependent reduction of ATP synthesis and redox balance leading to increased ROS levels and decreased antioxidant enzyme activities [190,191,192].
The main pathological mutations found in structural CI subunits are summarized in Table 2.
Table 2.
Complex II subunits with location, associated clinical phenotypes, and references. Adapted from [47,74,110,133].
| Subunits | Location | Associated Clinical Phenotypes | References |
|---|---|---|---|
| MTND1 | ND1-module | Leber optic atrophy, MELAS syndrome, dystonia, spasticity, and myopathy | [193,194,195] |
| MTND2 | ND2-module | Leber optic atrophy | [196] |
| MTND3 | ND2-module | Infantile encephalopathy and Leigh syndrome | [197] |
| MTND4 | ND4-module | Leber optic atrophy and MELAS syndrome | [198,199] |
| MTND4L | ND2-module | Leber optic atrophy | [200] |
| MTND5 | ND5-module | Leber optic atrophy and MELAS syndrome | [201,202] |
| MTND6 | ND2-module | Leber optic atrophy and MELAS syndrome | [201,203] |
| NDUFV1 | N-module | Severe encephalopathy and neurologic abnormalities | [204,205] |
| NDUFV2 | N-module | Hypertrophic cardiomyopathy, truncal hypotonia, and encephalopathy | [206] |
| NDUFV3 | N-module | Complex I deficiency | - |
| NDUFS1 | N-module | Growth retardation, axial hypotonia, hepatomegaly, dystonia, and persistent hyperlactatemia | [205] |
| NDUFS2 | Q-module | Neonatal lactic acidosis and hypertrophic cardiomyopathy | [207] |
| NDUFS3 | Q-module | Leigh syndrome, severe axial dystonia with oral and pharyngeal motor dysfunction, dysphagia and a tetraparetic syndrome |
[208] |
| NDUFS4 | Q-module | Muscular hypotonia, absence of visual and auditive attention, and cardiac defects | [209] |
| NDUFS6 | Q-module | Fatal infantile lactic acidosis, neonatal myopathy, encephalopathy, and lactic acidosis | [210,211] |
| NDUFS7 | Q-module | Leigh syndrome, feeding problems, dysarthria, and ataxia | [212] |
| NDUFS8 | Q-module | Leigh syndrome, poor feeding, and episodes of apnea and cyanosis | [213] |
| NDUFA11 | ND2-module | Fatal infantile metabolic acidosis, brain atrophy, no motor development and hypertrophic cardiomyopathy |
[214] |
| NDUFA1 | ND1-module | Leigh syndrome, hypotonia, nystagmus, generalized choreoathetosis, and decreased reflexes |
[215] |
| NDUFA2 | N-module | Leigh syndrome, hypertrophic cardiomyopathy, and developmental delay | [216] |
| NDUFA3 | ND1-module | - | - |
| NDUFA5 | Q-module | - | - |
| NDUFA6/LYRM-6 | LYR protein | Auditory and optic neuropathy, mitochondrial-related infantile death, brain disorder, leukoencephalopathy | [217] |
| NDUFA7 | N-module | - | - |
| NDUFA8 | IMS protein (ND1-module) |
Intrauterine growth retardation, respiratory insufficiency, lactic acidosis and hypoglycemia |
[178] |
| NDUFA9 | Q-module | Severe neonatal hypotonia, dysmorphic features, epilepsy, and signs of brainstem involvement |
[218] |
| NDUFA10 | ND2-module | Leigh syndrome | - |
| NDUFA11 | ND2-module | Encephalocardiomyopathy and fatal infantile lactic acidemia, neuromuscular disorder | - |
| NDUFA12 | N-module | Respiratory and metabolic acidosis, hearing loss, apneas, and retinitis pigmentosa | [219] |
| NDUFA13 | ND1-module | Leigh syndrome, progressive loss of motor abilities, scoliosis, and dystonia | [220] |
| NDUFB1 | ND4-module | - | - |
| NDUFB2 | ND5-module | - | - |
| NDUFB3 | ND5-module | Delayed development, hypotonia, poor eye contact, abnormal eye movements, poor feeding, encephalopathy, and hearing loss |
[221] |
| NDUFB4 | ND4-module | - | - |
| NDUFB5 | ND4-module | - | - |
| NDUFB6 | ND5-module | - | - |
| NDUFB7 | ND5-module | - | - |
| NDUFB8 | ND5-module | Encephalopathy, myopathy, hypotonia, developmental delay, and lactic acidosis, mitochondrial Complex I Deficiency in Individuals with Leigh-like Encephalomyopathy | [222] |
| NDUFB9/LYRM-3 | LYR protein | Leigh syndrome, respiratory failure, seizures, hypotonia, cardiac hypertrophy, failureto thrive and severely delayed psychomotor development |
[221] |
| NDUFB10 | IMS protein(ND4 module) | Progressive hypotonia associated with increased serum lactate | [223] |
| NDUFB11 | ND4-module | Lethal complex I deficiency, X-linked microphthalmia with linear skin defects (MLS) syndrome | [224,225,226] |
| NDUFC1 | ND2-module | - | - |
| NDUFC2 | ND2-module | X-linked microphthalmia with linear skin defects (MLS) syndrome, cardiomyopathy and other congenital anomalies |
[227] |
| NDUFS5 | IMS protein (ND2 module) |
- | - |
Quantitative proteomics has revealed the importance of the 30 non-catalytically active supernumerary subunits of CI. Pathological variants causing CI deficiency have been described in NDUFAF1 [CIA30], ACAD9, and TMEM126B that together with ECSIT, COA1 and TMEM186, form the Mitochondrial Complex I Intermediate Assembly (MCIA) [172] important for the biogenesis of the ND2-module. NDUFAF3 (C3ORF60) and NDUFAF4 (C6ORF66) working together in the assembly of the Q-module, have been found mutated in different cases of infantile mitochondrial disease [150,151,228,229,230,231].
The gene NDUFS4 (NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, NM_002495.2), is a hotspot for pathogenic mutations. Inactivation of the NDUFS4 gene is known to cause mostly, Leigh or Leigh-like syndrome [232,233,234,235,236,237,238,239,240], a rare disease with a prevalence of roughly 1:40.000 live births [241,242]. Unfortunately, the prognosis of NDUFS4-linked LS is poor. Loss of NDUFS4 affects complex I assembly and causes detrimental structural changes in assembled complex I [232,243]. Several pieces of evidence have suggested that NDUFS4 plays a role in the late stage of complex I assembly [233,235,244]. NDUFS4 knock out mouse models [245,246], human and murine cell lines, and more recently induced pluripotent stem cells (iPSCs) from LS patients carrying mtDNA mutations in the NDUFS4 [70] have been set up to explore strategies to counteract pathophysiological consequences of complex I deficiency. LS patient-derived neural cells have shown defective bioenergetics [247,248], decreased protein synthesis [249], impaired mitochondrial calcium homeostasis [248,250], and abnormal corticogenesis [251]. The presence of defective neurite outgrowth has been confirmed also in neural progenitor cells (NPCs) carrying mutations in the NDUFS4 as well as in the SURF1 (Surfeit locus protein 1, NM_003172.2) genes, another well-known cause of LS [252,253,254].
Structural subunits and specific factors assisting the assembly associated with human diseases are summarized in Table 1 and Table 2.
4.2. Succinate–Ubiquinone Oxidoreductase–Complex II
Succinate dehydrogenase (SDH, complex II, CII), a ~120 kDa integral membrane complex, participates in both the TCA cycle and the respiratory chain. CII transfers the electrons to CoQ and does not contribute to proton pumping across the mitochondrial membrane. All four subunits are encoded by the nuclear genome. The largest hydrophilic domain is a heterodimer composed of SDHA and SDHB that protrude toward the matrix and contain the redox-active groups’ flavin adenine dinucleotide (FAD(H2)) and three Fe–S clusters, respectively. The smaller hydrophobic domain is composed of SDHC and SDHD and contains two CoQ binding sites [255] providing reduction of ubiquinone to ubiquinol, the mobile electron carrier that links to CIII. Four specific chaperones [SDH assembly factor 1–4 (SDHAF1–4)] participate in the stabilization and incorporation of the prosthetic groups into each of the structural subunits SDHA, SDHB, and SDHC + SDHD [130,256]. In the late stage of assembly of CII, ACN9, similarly to LYRM-8 (also known as SDHAF1), is important for the formation and stabilization of CII throughout the insertion or retention of the Fe-S centers within the protein backbones and FMC1 (Formation of mitochondrial complex V assembly factor 1) [257].
CII defects are quite rare and represent less than 10% of OxPhos deficiency cases [258]. Different forms of encephalopathy and rare neuroendocrine tumors are the two main pathological manifestations that can originate from mutations in CII subunits or assembly factors. Mutations in SDHA, encoding the 70 kDa Flavoprotein subunit, have also been found in rare cases of Leigh syndrome [259,260,261,262,263,264]. Ultrarare association of bi-genomic variants in the SDHB and mitochondrial MT-CYB genes has been described in a patient with clinical and metabolic features of a ME-LAS-like syndrome [265].
The main pathological mutations found in CII subunits or assembly factors are summarized in Table 3.
Table 3.
Complex II subunits and assembly factors with function, associated clinical phenotypes, and references. Adapted from [74,133].
| Subunits | Function | Associated Clinical Phenotypes | References |
|---|---|---|---|
| SDHA | CII subunit | Leigh syndrome, neonatal dilated cardiomyopathy, catecholamine-secreting extra-adrenal paraganglioma |
[259,260,261,262,263,264,265,266,267] |
| SDHB | CII subunit | Paraganglioma, pheochromocytoma, gastrointestinal stromal tumors | [268,269] |
| SDHC | CII subunit | Paraganglioma, gastric stromal sarcoma | [270,271] |
| SDHD | CII subunit | Paraganglioma, pheochromocytoma, gastric stromal sarcoma | [271,272] |
| Assembly Factors | |||
| SDHAF1/LYRM-8 | Insert Fe/S clusters into mature SDHB | Leukoencephalopathy, spastic quadriplegia, psychomotor regression | [257] |
| SDHAF2 | Insert FAD cofactor into apo-protein SDHA | Paraganglioma and pheochromocytomas | [270,272,273,274,275,276] |
| SDHAF3/NDUFV1/LYRM-10 | Maintain SHDB stability | Familial and sporadic pheochromocytomas and paraganglioma |
[277] |
| SDHAF4 | Protect the subunit from auto-oxidation and facilitates the assembly with SDHB | Vagal paragangliomas | [278] |
4.3. Ubiquinol: Cytochrome C Oxidoreductase–Complex III
The ubiquinol: cytochrome c oxidoreductase (cytochrome bc1, complex III, CIII) constitutes the central part of the respiratory chain. CIII receives two electrons through reduced CoQ (CoQH2) and transfers them, one at a time, to cytochrome c, by cytochrome b (MT-CYB-human nomenclature), which contains two binding sites with CoQ and two heme b groups; UQCRFS1, the Rieske Fe-S protein; and CYC1, containing heme c. Each of the two ‘monomers’ is composed of 10 different subunits and associate as a symmetric dimer [279]. The complex assembly starts with the synthesis, membrane insertion, and hemylation of cytochrome b, mediated by UQCC1–3 in humans [280,281,282], followed by the sequential incorporation of the remaining subunits into a dimeric pre-CIII2 [282]. MZM1L (LYRM7), BCS1L, and tetratricopeptide repeat domain-containing protein 19 (TTC19) are the three assembly factors, known to be involved in the stabilization, incorporation, and metabolism of UQCRFS1 [283,284,285,286,287,288,289,290]. LYRM7 chaperone binds the Rieske protein before its incorporation as the last step of the biogenesis of the nascent CIII dimer (CIII2), acted by BCS1L [284,286,291].
The first mutations found in CIII were identified in MT-CYB, the only subunit encoded by mtDNA [292,293,294,295]. Most of these pathological variants were found in heteroplasmy and mainly associated with late-onset sporadic myopathy and exercise intolerance [292,293,294,295,296,297,298]. Other MT-CYB mutations were associated with histiocytoid cardiomyopathy [299], parkinsonism and MELAS overlap syndrome [293], or multisystem disorders [300,301,302,303].
Among the cases of CIII deficiency of nuclear origin are mutations in assembly factors [304] and the most common are nonsense and missense mutations in TTC19 [305], LYRM7 [306], and BCS1L [307], which cause defective CIII assembly/stability and decreased ubiquinol:cyt c oxidoreductase activity. Interestingly, a shuttle of electrons from NADH and/or ubiquinol to CIII, pyocyanin, has been used to efficiently recover mitochondrial function thus ameliorating bioenergetic efficiency in fibroblasts derived from patients’ dysfunction due to TTC19, BCS1L, and LYRM7 [291].
The main pathological mutations found in CIII subunits or assembly factors are summarized in Table 4.
Table 4.
Complex III subunits and assembly factors with function, associated clinical phenotypes, and references. Adapted from [74,133].
| Subunits | Function | Associated Clinical Phenotypes | References |
|---|---|---|---|
| UQCRC1 | CIII subunit | Parkinsonism with polyneuropathy | [308] |
| UQCRC2 | CIII subunit | Hypoglycemia, lactic acidosis, ketosis, and hyperammonemia | [309] |
| MT-CYB | CIII subunit | Leber optic atrophy, exercise intolerance, encephalomyopathy, cardiomyopathy, and multisystemic disorder, histiocytosis cardiomyopathy, parkinsonism, and MELAS overlap syndrome | [293,294,299,300,310,311] |
| CYC1 | CIII subunit | Neurologic deterioration, insulin-responsive hyperglycemia, ketoacidosis with increased serum lactate, liver failure, and hyperammonemia |
[312] |
| UQCRFS1 | CIII subunit | Cardiomyopathy and alopecia totalis | [313] |
| UQCRH | CIII subunit | - | - |
| UQCRB | CIII subunit | Gastroenteritis, liver enlargement, hypoglycemia, and metabolic acidosis but normal psychomotor development at age 4, hepatopathy |
[314] |
| UQCRQ | CIII subunit | Severe neurologic phenotype, early-onset severe encephalopathy | [315] |
| UQCR10 | CIII subunit | - | - |
| UQCR11 | CIII subunit | - | - |
| Assembly Factors | |||
| UQCC1 | Cytochrome b assembly factor | - | - |
| UQCC2 | Cytochrome b assembly factor | Intrauterine growth retardation, neonatal lactic acidosis and renal tubular dysfunction | [281,316] |
| UQCC3 | Cytochrome b assembly factor | Lactic acidosis, hypoglycemia, hypotonia, and delayed development | [282] |
| VPS53 | Heme lyase (Cytochrome c1) | Complicated hereditary spastic paraparesis | [317] |
| BCS1L | AAA-ATPase involved in Rieske protein incorporation. Stabilization, incorporation, and metabolism of UQCRFS1 |
GRACILE Syndrome, Bjornstad Syndrome, myopathy, encephalopathy, proximal tubulopathy, and liver failure | [26,288,304,318,319,320,321,322,323] |
| MZM1L/LYRM-7 | Matrix protein involved in Rieske protein incorporation. Stabilization, incorporation, and metabolism of UQCRFS1 | Neurological decompensation and regression, leukoencephalopathy and liver failure, infantile CIII deficiency associated with cavitating leukoencephalopathy metabolic decompensation | [306,324,325,326] |
| TTC19 | Rieske protein metabolism Stabilization, incorporation, and metabolism of UQCRFS1 | Progressive encephalopathy, ataxia, spastic paraparesis, and psychiatric phenotype | [305,327,328,329,330] |
4.4. Cytochrome C Oxidase–Complex IV
Cytochrome c oxidase (COX, complex IV, CIV) is the terminal complex of the ETC. The enzyme transfers electrons from cytochrome c to molecular oxygen. In humans, it is composed of 14 subunits, with the NDUFA4, the most recently discovered subunit initially attributed to CI [331,332], found to be incorporated in the structure of monomeric human CIV [333]. Only two, MT-CO1 and MT-CO2, are catalytical subunits. MTCO1 contains three prosthetic groups: cytochrome a3 and CuB, which form the bi-nuclear center that binds oxygen, and cytochrome a. MT-CO2 incorporates the CuA center [334]. MT-CO3 is necessary to provide additional stability to the enzyme while it undergoes turnover [335]. Subunits such as COX4, 5A, 5B, 6A, 6B, 6C, 7A, 7B, 7C, 8A are believed to play a role in stabilizing the structure of the complex. The cytochrome c oxidase complex is unique among the ETC complexes to have tissue, developmental and species-specific isoforms for COX subunits 4, 6A, 6B, 7A, 7B, and 8A [336,337].
CIV assembly grows with a modular process through the incorporation of modules formed by different subunits and defined by each of the mtDNA-encoded core subunits [130,338,339]. Any subunit of complex IV could carry mutations and rise a mitochondriopathy [337,340,341,342]. Mutations in the MT-CO1, MT-CO2, and MT-CO3 are causative of COX deficiency and mitochondrial disease with an extreme clinical heterogeneity (Table 5).
Table 5.
| Subunits | Associated Clinical Phenotypes | References |
|---|---|---|
| MTCO1 | MELAS syndrome, myopathy, myoglobinuria, motor neuron disease, exercise intolerance, epilepsy, multisystem disorders, deafness, LHON, or mitochondrial sensorineural hearing loss |
[343,344,345,346,347] |
| MTCO2 | Encephalomyopathy, LHON, myopathy, hypertrophic cardiomyopathy | [348,349,350,351] |
| MTCO3 | MIDD, LHON, myopathy, Leigh disease, myoglobinuria, sporadic bilateral optic neuropathy, rhabdomyolysis, encephalopathy |
[352,353,354,355,356,357] |
| COX4I1 | Short stature, poor weight gain, mild dysmorphic features, Fanconi anemia, Leigh-like syndrome | [358,359] |
| COX4I2 | Exocrine pancreatic insufficiency, dyserythropoietic anemia, calvarial hyperostosis | [360] |
| COX5A | Early-onset pulmonary arterial hypertension, lactic acidemia, failure to thrive | [361] |
| COX6A1 | Charcot–Marie–Tooth disease | [362] |
| COX6A2 | Muscle weakness and hypotonia, cardiomyopathy | [363] |
| COX6B1 | Severe infantile encephalomyopathy | [341,342] |
| COX7A1 | Failure to thrive, encephalopathy, hypotonia | [364] |
| COX7B | Microphthalmia with linear skin lesions | [365] |
| COX8A | Leigh-like syndrome presenting with leukodystrophy and severe epilepsy | [366] |
| NDUFA4 | Leigh syndrome | [331] |
Pathological variants in ‘supernumerary’ COX subunits have been reported in tissue and development-specific isoforms [336]. Among the assembly factors, the most representative is SURF1, the functional absence of which causes LS [252,253,276] or even Charcot–Marie–Tooth disease [367]. The elucidation of the pathogenetic mechanism has received an impulse recently [70]. Mutations in COX10, which catalyzes the farnesylation of a vinyl group of heme b, cause LS and other forms of the fatal early-onset neurological syndrome [368,369,370]. Mutations in COX15, which catalyzes the subsequent step of heme synthesis, cause variable clinical presentations [371,372,373]. Copper delivery to the active sites of MT-CO1 and MT-CO2 involves factors essential for COX activity [130,374]. SCO1, SCO2, and COA6 have been found mutated in patients showing CIV deficiency and fatal outcomes [338,368,375,376,377,378,379,380,381,382,383,384,385,386]. Among complex IV proteins, COX6B1 assists CIV assembly, working as a linking subunit at the dimeric interface of CIV [387].
The specific functions of the remaining proteins (all associated with human diseases, see Table 6) are known only in part and require additional studies.
Table 6.
Complex IV assembly factors with function, associated clinical phenotypes, and references. Adapted from [74,133].
| Assembly Factors | Function | Associated Clinical Phenotypes | References |
|---|---|---|---|
| RNA Stability and Translation | |||
| TACO1 | Translational activator of mitochondria encoded MTCO1 | Leigh syndrome | [388,389] |
| LRPPRC | Mitochondrial mRNA stability | French Canadian type of Leigh syndrome | [390] |
| FASTKD2 | Involved in post-transcriptional RNA maturation, ribosome biogenesis and translation |
Brain atrophy, epilepsy, delayed psychomotor development, bilateral optic atrophy, spastic hemiparesis, cardiomyopathy |
[391,392,393] |
| Heme a Biosynthesis and Insertion | |||
| COX10 | Heme a synthesis (conversion of heme b into heme o) | Leigh syndrome, encephalopathy, cardiomyopathy, sensorineural deafness, and metabolic acidosis | [369,370,394,395] |
| COX15 | Heme a synthesis (conversion of heme o into heme a) | Leigh syndrome, encephalopathy, cardiomyopathy, sensorineural deafness, and metabolic acidosis | [369,371,373,396,397] |
| SURF1 | Involved in the insertion or stabilization of heme a3 | Leigh syndrome, Charcot–Marie–Tooth disease | [252,253,276,367,398] |
| Copper Metabolism and Insertion | |||
| COA5/C2ORF64 | Involved in the unknown step of CIV biogenesis |
Fatal infantile cardioencephalomyopathy | [399] |
| COA6/C1ORF31 | Copper homeostasis and transport to CIV | Fatal infantile cardioencephalopathy | [385,386,400] |
| SCO1 | Incorporation of copper atoms (biogenesis of CuA center) | Cardioencephalomyopathy, Leigh syndrome-like symptoms, spinal muscular atrophy-like presentations, Charcot–Marie–Tooth disease type 4, CIV deficiency, neonatal hepatopathy, encephalopathy with hepatopathy and cardiomyopathy, pure encephalopathy, metabolic syndrome with exclusively fatal lactic acidosis | [375,381,383,395,401,402] |
| SCO2 | Incorporation of copper atoms (biogenesis of CuA center) | Cardioencephalomyopathy, Leigh syndrome-like symptoms, spinal muscular atrophy-like presentations, Charcot–Marie–Tooth disease type 4, CIV deficiency, cardiac hypertrophy | [377,378,379,380,381] |
| COX11 | Copper chaperone | Coloboma, Ocular, With or Without Hearing Impairment, Cleft Lip/Palate, And/Or Mental Retardation and Spinal Muscular Atrophy, Distal, X-Linked 3 | [403] |
| COX16 | MTCO2 maturation | - | [404,405] |
| COX17 | Copper transfer | - | [406] |
| COX19 | Stabilization of COX11 | - | [407,408] |
| COX20 | Stabilization of MT-CO2 | Cerebellar ataxia | [409,410,411] |
| Assembly | |||
| COA3/MITRAC12 | Required for MTCO1 stability and assembly, involved in translational regulation of MTCO1 and prevention of MTCO1 aggregation before assembly | Mild phenotype, exercise intolerance, peripheral neuropathy, obesity, and short stature | [412,413,414,415] |
| COA7 | Unknown | Ataxia and peripheral neuropathy, cognitive impairments, leukodystrophy | [176,416] |
| COX14/C12ORF62 | MTCO1 stability and assembly; avoids MTCO1 aggregation | Severe lactic acidosis and dysmorphic features | [417] |
| CMC1 | Stabilizes the interaction between MTCO1, COX14, and COA3 | [418] | |
| COX20/FAM36A | MTCO2 chaperone for copper metalation | Growth delay, hypotonia, cerebellar ataxia | [410,411,419] |
| PET100 | Stabilizes MT-CO2 module | Early-onset psychomotor delay, seizures, hypotonia, Leigh syndrome, CIV deficiency, and fatal infantile lactic acidosis | [420,421,422] |
| PET117 | Assembly factor: possible role in Cox15 oligomerization and function, stabilizes MT-CO2 module | Neurodevelopmental regression and bulbar lesions | [423,424,425] |
| MR-1S | Interacts with PET117 and PET100, | - | [339] |
| APOPT1/COA8 | intermediates assembly steps Putative role in CIV protection from ROS damage, enhances CIV biogenesis | Leukodystrophy, neurological signs | [426,427,428] |
| COX18 | Promotes the translocation of MTCO2 globular domain through the IMM | Isolated COX deficiency in infancy | [429,430,431] |
| COX19 | Stabilization of COX11 | Isolated COX deficiency in infancy | [407,408,431] |
| COA-X | Putative assembly factor | - | [432] |
| HIGD2A | Promotes incorporation of MT-CO3 module | - | - |
4.5. ATP Synthase–Complex V
ATP synthase (Complex V, CV) is the enzyme that catalyzes the synthesis of ATP required as an energy source for various cellular processes from ADP and phosphate utilizing the proton-motive force generated through electron transfer. ATP synthase F1FO consists of two functional domains: the hydrophilic domain F1 facing the matrix which serves for the production of ATP and the FO domain facing the membrane which serves to translocate protons [433,434]. The proton translocation leads to the rotational movement of the c ring in the FO domain which is connected to the catalytic subunit F1 by the peripheral stalk (PS). The human CV is composed of 29 proteins of 18 kinds, including the Inhibitory factor 1, IF1, in which only FO-ATP6 and ATP8 are mtDNA encoded [435]. The complete structure of the dimeric and monomeric mammalian mitochondrial F1Fo-ATP synthase has been just recently resolved by Cryo-EM [436,437].
The assembly pathway of human CV is also modular [433,435,438,439] since three subcomplexes, F1 module, c-ring, and PS are formed individually and then associate together. The assembly starts from the three alpha and three beta subunits that make up the F1 domain to which the other subunits subsequently bind. The eight units of the c-ring assemble inside the IMM. When these two sub-complexes join, the PS subunits also bind, followed by the membrane domain’s remaining subunits, which include MT-ATP6 and MT-ATP8 [130]. To date, only three assembly factors are known, including ATPAF1 and ATPAF2, that binds and stabilizes subunit beta [440] and subunit alpha [441], respectively.
Pathogenic mutations have been reported both in mtDNA and nDNA encoded ATP synthase subunits. The coding sequences of two Fo subunits are overlapping in the human mtDNA and pathological variants in both are the cause of sporadic and maternally inherited mitochondrial disease (Table 7).
Table 7.
Complex V subunits and assembly factors with function, associated clinical phenotypes, and references. Adapted from [74,133].
| Subunits | Location | Associated Clincial Phenotypes |
References |
|---|---|---|---|
| MT-ATP6 | Fo domain | Mitochondrial CV deficiency Neuropathy, Ataxia and Retinitis Pigmentosa (NARP) syndrome Leigh syndrome Adult-onset ataxia and polyneuropathy Bilateral striatal necrosis Motor neuron syndrome Mitochondrial myopathy, lactic acidosis, and sideroblastic anemia |
[94,95,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457] |
| MT-ATP8 | Fo domain | Mitochondrial CV deficiency Valproate-induced reversible brain atrophy Hypertrophic cardiomyopathy |
[458,459] |
| MT-ATP6/8 overlap region |
Fo domain | Mitochondrial CV deficiency Infantile hypertrophic cardiomyopathy |
[457] |
| ATP5F1A | F1 domain | Mitochondrial CV deficiency Combined OXPHOS deficiency Fatal infantile encephalopathy |
[460,461] |
| ATP5F1D | F1 domain | Mitochondrial CV deficiency Metabolic decompensation with lactic acidosis, hypoglycemia, hyperammonemia, and 3-methylglutaconic aciduria, encephalopathy |
[462] |
| ATP5F1E | F1 domain | Mitochondrial CV deficiency Neonatal-onset lactic acidosis, 3-methylglutaconic aciduria, mild mental retardation, hypertrophic cardiomyopathy, and peripheral neuropathy |
[463] |
| Assembly Factors | |||
| ATPAF1 | Binds and stabilIzes subunit beta of F1 Domain |
Asthma in children | [464] |
| ATPAF2 | Binds and stabilizes subunit alpha of F1 domain | Degenerative encephalopathy, elevated lactate levels, developmental delay | [465] |
| TMEM70 | Unknown | Neonatal mitochondrial encephalocardiomyopathy Mitochondrial CV deficiency, nuclear type 2 Occasionally facial dysmorphisms CI deficiency |
[141,142,143,144,145,146] |
Mutations in MT-ATP6 have been identified in neuropathy, ataxia, and retinitis pigmentosa syndrome (NARP) [94] and maternally inherited Leigh syndrome (MILS) [442,466]. NARP is a slowly progressive form that manifests in adulthood, while MILS is an early onset, highly disabling, often fatal disease. In many cases, NARP and MILS are associated with the 8993 T > C or T > G mutation [443,467,468]. The T > G transversion usually presents with a more severe form that correlates with the degree of heteroplasmy of the mutation in post-mitotic tissues [95,468]. Until now, only three of the sixteen nucleus-encoded CV subunits and three assembly factors (e.g., ATPAF2; ATP12 and TMEM70) have been associated with mitochondrial disease (see Table 7).
4.6. Respiratory Supercomplexes
OxPhos complexes associate with each other resulting in the formation of higher-order structures which have been called supercomplexes (SC). Complexes IV and V can form dimers and oligomers [469,470,471] and based on the size and composition of the subunits, the main SCs that have been recognized have the following stoichiometries: III2IV1, I1III2, I1III2IV1, and I2III2IV1-2. In particular, the association of complexes I, III2 and IV, SC I1III2IV1, considered as a functional unit capable of transferring electrons from NADH to O2, is defined as the ‘respirasome’ [472] whereas the supercomplex I2III2IV2 has been named as ‘respiratory megacomplex’ [129]. High-resolution Cryo-EM structures of the respirasome of several mammalian species, including humans, have been recently resolved [129,473,474,475,476]. The respirasome organization was supposed to be functionally advantageous making electron transfer from CI to CIV through CIII2 more efficient and decreasing the formation of deleterious ROS [477,478,479,480]. It has been suggested that the functional unit of OxPhos is composed of the dimer of ATP synthase flanked by the adenine nucleotide and the phosphate transporters, located at the apices of cristae and the CI-CIII2-CIV supercomplexes organized along the cristae membrane to perform the electron transfer and proton translocation [481].
The fact that the biogenesis of CIII2 and CIV occurs independently but the CI assembly does not can be an explanation for the reason why defects in CIII2 and CIV may result in secondary effects on CI assembly. When the defect is originated from mutations in CI components, the manifestation is almost always an isolated CI deficiency [209,482]. High-throughput proteomics techniques have recently been applied in human cybrids holo-CIII2-deficient, demonstrating the loss of SCs containing CIII2 and CI when the CIII2 is not fully assembled. In this model, the combination of null CIII and markedly reduced CI enzymatic activity, confirmed the well-established connection between CIII2 deficiency and hampered assembly process in CI [483].
In astrocytes, most of CI is free, resulting in poor mitochondrial respiration but high ROS production; while, in neurons, CI is mostly embedded into supercomplexes, thus resulting in high mitochondrial respiration and low ROS production [66]. Notably, crest-shaping proteins, as well as the proteins of mitochondrial contact sites and the cristae organization system complex (MICOS) are essential for the assembly and functionality of the OxPhos system [484]. Understanding the structure and assembly of SCs is very crucial to explain those cases of combined respiratory chain deficiency.
For expert reviews on the issue of the relationship between crest dynamics and bioenergetics, refer to [485,486]. For a detailed review of the formation and function of SCs, see [487].
5. Conclusions
An integrative approach that combines multi-omics data could represent a strategic way to solve, at least in part, the complexity of mitochondrial diseases and mitochondrial medicine highlighting the interrelationships of the involved OxPhos complexes and their functions, and the knowledge about genotype-phenotype correlation. However, the science behind combined omic approaches, will need the integration of data from genomics, transcriptomics, proteomics, and metabolomics, to include also the novel approaches looking to the epiproteome, the set of all post-translational modifications made to proteins comprising an organelle, a cell, or an organism, that provide the link between metabolism, mitochondrial proteome, and the two cellular genomes. The recent application of CRISPR/Cas9 technology to patient-specific iPSCs, to generate neural cultures and cerebral organoids is providing patient-specific cellular and tissue models that allow the investigation of the defects of neuronal morphogenesis caused by specific mutations (Figure 1). Thus, in the multi-omic era, the opportunity to understand the cause of each mitochondrial disease becomes ever more tangible.
Acknowledgments
We thank the patients’ association MITOCON and UILDM (Unione Italiana Lotta alla Distrofia Muscolare) and CollaGe-associazione-Genitori-Manzoni-Poli-Molfetta.
Author Contributions
Conceptualization, P.Z. and V.P.; writing—original draft preparation, P.Z.; S.D. and V.P.; writing, review, supervision and editing, P.Z. and V.P.; supervision, V.P. and F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from REGIONE PUGLIA-MALATTIE RARE-Petruzzella Del. N.246 10.09.2019_UPB-SMBNOS and by donations of Parents’ Associations (to VP). This study received partial financial support from the Italian Ministry of Health-Ricerca Corrente, MITO-NEXT, Mit-OMICS (to FMS), Fondazione Telethon (Grant numbers GUP09004). The authors alone are responsible for the content and writing.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandel N.S. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green D.R., Galluzzi L., Kroemer G. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorman G.S., Chinnery P.F., DiMauro S., Hirano M., Koga Y., McFarland R., Suomalainen A., Thorburn D.R., Zeviani M., Turnbull D.M. Mitochondrial diseases. Nat. Rev. Dis. Prim. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 7.Muraresku C.C., McCormick E.M., Falk M.J. Mitochondrial disease: Advances in Clinical diagnosis, management, therapeutic development, and preventative strategies. Curr. Genet. Med. Rep. 2018;6:62–72. doi: 10.1007/s40142-018-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piel R.B., Dailey H.A., Medlock A.E. The mitochondrial heme metabolon: Insights into the complex(Ity) of heme synthesis and distribution. Mol. Genet. Metab. 2019;128:198–203. doi: 10.1016/j.ymgme.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Anderson A.J., Jackson T.D., Stroud D.A., Stojanovski D. Mitochondria—Hubs for regulating cellular biochemistry: Emerging concepts and networks. Open Biol. 2019;9:190126. doi: 10.1098/rsob.190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 12.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills E.L., Kelly B., O’Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 14.Tiku V., Tan M.-W., Dikic I. Mitochondrial functions in infection and immunity. Trends Cell Biol. 2020;30:263–275. doi: 10.1016/j.tcb.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schapira A.H. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 16.DiMauro S., Schon E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 17.Luft R. The development of mitochondrial medicine. Proc. Natl. Acad. Sci. USA. 1994;91:8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Morgia C., Maresca A., Caporali L., Valentino M.L., Carelli V. Mitochondrial diseases in adults. J. Intern. Med. 2020;287:592–608. doi: 10.1111/joim.13064. [DOI] [PubMed] [Google Scholar]
- 19.Petruzzella V., Tiranti V., Fernandez P., Ianna P., Carrozzo R., Zeviani M. Identification and characterization of human CDNAs specific to BCS1, PET112, SCO1, COX15, and COX11—Five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics. 1998;54:494–504. doi: 10.1006/geno.1998.5580. [DOI] [PubMed] [Google Scholar]
- 20.Stenton S.L., Prokisch H. Advancing genomic approaches to the molecular diagnosis of mitochondrial disease. Essays Biochem. 2018;62:399–408. doi: 10.1042/EBC20170110. [DOI] [PubMed] [Google Scholar]
- 21.Stenton S.L., Prokisch H. Genetics of mitochondrial diseases: Identifying mutations to help diagnosis. EBioMedicine. 2020;56:102784. doi: 10.1016/j.ebiom.2020.102784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo S.E., Compton A.G., Hershman S.G., Lim S.C., Lieber D.S., Tucker E.J., Laskowski A., Garone C., Liu S., Jaffe D.B., et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 2012;4:118ra10. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plutino M., Chaussenot A., Rouzier C., Ait-El-Mkadem S., Fragaki K., Paquis-Flucklinger V., Bannwarth S. Targeted next generation sequencing with an extended gene panel does not impact variant detection in mitochondrial diseases. BMC Med. Genet. 2018;19:57. doi: 10.1186/s12881-018-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H.L., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 25.Garone C., Donati M.A., Sacchini M., Garcia-Diaz B., Bruno C., Calvo S., Mootha V.K., DiMauro S. Mitochondrial encephalomyopathy due to a novel mutation in ACAD9. JAMA Neurol. 2013;70:1177–1179. doi: 10.1001/jamaneurol.2013.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oláhová M., Berti C.C., Collier J.J., Alston C.L., Jameson E., Jones S.A., Edwards N., He L., Chinnery P.F., Horvath R., et al. Molecular genetic investigations identify new clinical phenotypes associated with BCS1L-related mitochondrial disease. Hum. Mol. Genet. 2019;28:3766–3776. doi: 10.1093/hmg/ddz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenton S.L., Kremer L.S., Kopajtich R., Ludwig C., Prokisch H. The diagnosis of inborn errors of metabolism by an integrative “multi-omics’’ approach: A perspective encompassing genomics, transcriptomics, and proteomics. J. Inherit. Metab. Dis. 2020;43:25–35. doi: 10.1002/jimd.12130. [DOI] [PubMed] [Google Scholar]
- 28.Mootha V.K., Bunkenborg J., Olsen J.V., Hjerrild M., Wisniewski J.R., Stahl E., Bolouri M.S., Ray H.N., Sihag S., Kamal M., et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/S0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 29.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.-E., Walford G.A., Sugiana C., Boneh A., Chen W.K., et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonczarowska-Jorge H., Zahedi R.P., Sickmann A. The proteome of baker’s yeast mitochondria. Mitochondrion. 2017;33:15–21. doi: 10.1016/j.mito.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Clamp M., Fry B., Kamal M., Xie X., Cuff J., Lin M.F., Kellis M., Lindblad-Toh K., Lander E.S. Distinguishing protein-coding and noncoding genes in the human genome. Proc. Natl. Acad. Sci. USA. 2007;104:19428–19433. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponomarenko E.A., Poverennaya E.V., Ilgisonis E.V., Pyatnitskiy M.A., Kopylov A.T., Zgoda V.G., Lisitsa A.V., Archakov A.I. The size of the human proteome: The width and depth. Int. J. Anal. Chem. 2016;2016:7436849. doi: 10.1155/2016/7436849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez M.F., Kristal B.S., Chernokalskaya E., Lazarev A., Shestopalov A.I., Bogdanova A., Robinson M. High-throughput profiling of the mitochondrial proteome using affinity fractionation and automation. Electrophoresis. 2000;21:3427–3440. doi: 10.1002/1522-2683(20001001)21:16<3427::AID-ELPS3427>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Karlberg O., Canbäck B., Kurland C.G., Andersson S.G. The dual origin of the yeast mitochondrial proteome. Yeast. 2000;17:170–187. doi: 10.1155/2000/597406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotter D. MitoProteome: Mitochondrial protein sequence database and annotation system. Nucleic Acids Res. 2004;32:D463–D467. doi: 10.1093/nar/gkh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guda P., Subramaniam S., Guda C. Mitoproteome: Human heart mitochondrial protein sequence database. Methods Mol Biol. 2007;357:375–383. doi: 10.1385/1-59745-214-9:375. [DOI] [PubMed] [Google Scholar]
- 37.Calvo S.E., Clauser K.R., Mootha V.K. MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith A.C., Robinson A.J. MitoMiner v3.1, an update on the mitochondrial proteomics database. Nucleic Acids Res. 2016;44:D1258–D1261. doi: 10.1093/nar/gkv1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doccini S., Morani F., Nesti C., Pezzini F., Calza G., Soliymani R., Signore G., Rocchiccioli S., Kanninen K.M., Huuskonen M.T., et al. Proteomic and functional analyses in disease models reveal CLN5 protein involvement in mitochondrial dysfunction. Cell Death Discov. 2020;6:18. doi: 10.1038/s41420-020-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung V., Lam S.S., Udeshi N.D., Svinkina T., Guzman G., Mootha V.K., Carr S.A., Ting A.Y. Correction: Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. eLife. 2019;8:e50707. doi: 10.7554/eLife.50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geladaki A., Kočevar Britovšek N., Breckels L.M., Smith T.S., Vennard O.L., Mulvey C.M., Crook O.M., Gatto L., Lilley K.S. Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics. Nat. Commun. 2019;10:331. doi: 10.1038/s41467-018-08191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung A.Y., Floyd B.J., Pagliarini D.J. Systems biochemistry approaches to defining mitochondrial protein function. Cell Metab. 2020;31:669–678. doi: 10.1016/j.cmet.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohue M., Matsuzaki Y., Uchikoga N., Ishida T., Akiyama Y. MEGADOCK: An all-to-all protein-protein interaction prediction system using tertiary structure data. Protein Pept. Lett. 2013;21:766–778. doi: 10.2174/09298665113209990050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohue M., Shimoda T., Suzuki S., Matsuzaki Y., Ishida T., Akiyama Y. MEGADOCK 4.0: An ultra–high-performance protein–protein docking software for heterogeneous supercomputers. Bioinformatics. 2014;30:3281–3283. doi: 10.1093/bioinformatics/btu532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi T., Matsuzaki Y., Yanagisawa K., Ohue M., Akiyama Y. MEGADOCK-Web: An integrated database of high-throughput structure-based protein-protein interaction predictions. BMC Bioinform. 2018;19:62. doi: 10.1186/s12859-018-2073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Floyd B.J., Wilkerson E.M., Veling M.T., Minogue C.E., Xia C., Beebe E.T., Wrobel R.L., Cho H., Kremer L.S., Alston C.L., et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol. Cell. 2016;63:621–632. doi: 10.1016/j.molcel.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formosa L.E., Dibley M.G., Stroud D.A., Ryan M.T. Building a complex complex: Assembly of mitochondrial respiratory chain complex I. Semin. Cell Dev. Biol. 2018;76:154–162. doi: 10.1016/j.semcdb.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Labory J., Fierville M., Ait-El-Mkadem S., Bannwarth S., Paquis-Flucklinger V., Bottini S. Multi-omics approaches to improve mitochondrial disease diagnosis: Challenges, advances, and perspectives. Front. Mol. Biosci. 2020;7:590842. doi: 10.3389/fmolb.2020.590842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S., Ince-Dunn G., Suomalainen A., Elo L.L. Integrative omics approaches provide biological and clinical insights: Examples from mitochondrial diseases. J. Clin. Investig. 2020;130:20–28. doi: 10.1172/JCI129202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y., Herman P., Rothman D.L., Agarwal D., Hyder F. Evaluating the gray and white matter energy budgets of human brain function. J. Cereb. Blood Flow Metab. 2018;38:1339–1353. doi: 10.1177/0271678X17708691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokoloff L., Reivich M., Kennedy C., Rosiers M.H.D., Patlak C.S., Pettigrew K.D., Sakurada O., Shinohara M. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 52.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Barros L.F., Courjaret R., Jakoby P., Loaiza A., Lohr C., Deitmer J.W. Preferential transport and metabolism of glucose in bergmann glia over purkinje cells: A multiphoton study of cerebellar slices. Glia. 2009;57:962–970. doi: 10.1002/glia.20820. [DOI] [PubMed] [Google Scholar]
- 54.Jha M.K., Morrison B.M. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp. Neurol. 2018;309:23–31. doi: 10.1016/j.expneurol.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parpura V., Basarsky T.A., Liu F., Jeftinija K., Jeftinija S., Haydon P.G. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 56.Xin W., Bonci A. Functional astrocyte heterogeneity and implications for their role in shaping neurotransmission. Front. Cell. Neurosci. 2018;12:141. doi: 10.3389/fncel.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliveira J.F., Sardinha V.M., Guerra-Gomes S., Araque A., Sousa N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015;38:535–549. doi: 10.1016/j.tins.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Allen N.J. Star power: Astrocytes regulate behavior. Cell. 2019;177:1091–1093. doi: 10.1016/j.cell.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 59.Herrero-Mendez A., Almeida A., Fernández E., Maestre C., Moncada S., Bolaños J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat. Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 60.Bittner C.X., Valdebenito R., Ruminot I., Loaiza A., Larenas V., Sotelo-Hitschfeld T., Moldenhauer H., San Martin A., Gutierrez R., Zambrano M., et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouzier-Sore A.-K., Voisin P., Canioni P., Magistretti P.J., Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J. Cereb. Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 62.Allaman I., Bélanger M., Magistretti P.J. Astrocyte–neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Fecher C., Trovò L., Müller S.A., Snaidero N., Wettmarshausen J., Heink S., Ortiz O., Wagner I., Kühn R., Hartmann J., et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 2019;22:1731–1742. doi: 10.1038/s41593-019-0479-z. [DOI] [PubMed] [Google Scholar]
- 64.Petrova V.Y., Drescher D., Kujumdzieva A.V., Schmitt M.J. Dual Targeting of yeast catalase A to peroxisomes and mitochondria. Biochem. J. 2004;380:393–400. doi: 10.1042/bj20040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Fabuel I., Le Douce J., Logan A., James A.M., Bonvento G., Murphy M.P., Almeida A., Bolaños J.P. Complex I Assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. USA. 2016;113:13063–13068. doi: 10.1073/pnas.1613701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graham L.C., Eaton S.L., Brunton P.J., Atrih A., Smith C., Lamont D.J., Gillingwater T.H., Pennetta G., Skehel P., Wishart T.M. Proteomic profiling of neuronal mitochondria reveals modulators of synaptic architecture. Mol. Neurodegener. 2017;12:77. doi: 10.1186/s13024-017-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stauch K.L., Purnell P.R., Fox H.S. Quantitative proteomics of synaptic and nonsynaptic mitochondria: Insights for synaptic mitochondrial vulnerability. J. Proteome Res. 2014;13:2620–2636. doi: 10.1021/pr500295n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yousefi R., Fornasiero E.F., Cyganek L., Montoya J., Jakobs S., Rizzoli S.O., Rehling P., Pacheu-Grau D. Monitoring mitochondrial translation in living cells. EMBO Rep. 2021;22:e51635. doi: 10.15252/embr.202051635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inak G., Rybak-Wolf A., Lisowski P., Pentimalli T.M., Jüttner R., Glažar P., Uppal K., Bottani E., Brunetti D., Secker C., et al. Defective metabolic programming impairs early neuronal morphogenesis in neural cultures and an organoid model of leigh syndrome. Nat. Commun. 2021;12:1929. doi: 10.1038/s41467-021-22117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quadalti C., Brunetti D., Lagutina I., Duchi R., Perota A., Lazzari G., Cerutti R., Di Meo I., Johnson M., Bottani E., et al. SURF1 knockout cloned pigs: Early onset of a severe lethal phenotype. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018;1864:2131–2142. doi: 10.1016/j.bbadis.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bottani E., Lamperti C., Prigione A., Tiranti V., Persico N., Brunetti D. Therapeutic approaches to treat mitochondrial diseases: ‘’One-size-fits-all’’ and ‘’precision medicine’’ strategies. Pharmaceutics. 2020;12:1083. doi: 10.3390/pharmaceutics12111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papa S., Martino P.L., Capitanio G., Gaballo A., De Rasmo D., Signorile A., Petruzzella V. The oxidative phosphorylation system in mammalian mitochondria. In: Scatena R., Bottoni P., Giardina B., editors. Advances in Mitochondrial Medicine. Volume 942. Springer; Dordrecht, The Netherlands: 2012. pp. 3–37. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez-Vizarra E., Zeviani M. Mitochondrial disorders of the OXPHOS system. FEBS Lett. 2021;595:1062–1106. doi: 10.1002/1873-3468.13995. [DOI] [PubMed] [Google Scholar]
- 75.Tang J.X., Thompson K., Taylor R.W., Oláhová M. Mitochondrial OXPHOS biogenesis: Co-regulation of protein synthesis, Import, and assembly pathways. Int. J. Mol. Sci. 2020;21:3820. doi: 10.3390/ijms21113820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergman O., Ben-Shachar D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) deficits in schizophrenia: Possible interactions with cellular processes. Can. J. Psychiatry. 2016;61:457–469. doi: 10.1177/0706743716648290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chacinska A., Koehler C.M., Milenkovic D., Lithgow T., Pfanner N. Importing mitochondrial proteins: Machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carapito C., Kuhn L., Karim L., Rompais M., Rabilloud T., Schwenzer H., Sissler M. Two proteomic methodologies for defining N-termini of mature human mitochondrial aminoacyl-TRNA synthetases. Methods. 2017;113:111–119. doi: 10.1016/j.ymeth.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Russell O.M., Gorman G.S., Lightowlers R.N., Turnbull D.M. Mitochondrial diseases: Hope for the future. Cell. 2020;181:168–188. doi: 10.1016/j.cell.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 80.Rusecka J., Kaliszewska M., Bartnik E., Tońska K. Nuclear genes involved in mitochondrial diseases caused by instability of mitochondrial DNA. J. Appl. Genet. 2018;59:43–57. doi: 10.1007/s13353-017-0424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yasukawa T., Kang D. An overview of mammalian mitochondrial DNA replication mechanisms. J. Biochem. 2018;164:183–193. doi: 10.1093/jb/mvy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barshad G., Marom S., Cohen T., Mishmar D. Mitochondrial DNA transcription and its regulation: An evolutionary perspective. Trends Genet. 2018;34:682–692. doi: 10.1016/j.tig.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Boczonadi V., Ricci G., Horvath R. Mitochondrial DNA transcription and translation: Clinical syndromes. Essays Biochem. 2018;62:321–340. doi: 10.1042/EBC20170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kummer E., Ban N. Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 2021;22:307–325. doi: 10.1038/s41580-021-00332-2. [DOI] [PubMed] [Google Scholar]
- 85.Wallace D.C. Bioenergetics in human evolution and disease: Implications for the origins of biological complexity and the missing genetic variation of common diseases. Philos. Trans. R. Soc. B Biol. Sci. 2013;368:20120267. doi: 10.1098/rstb.2012.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DiMauro S., Schon E.A., Carelli V., Hirano M. The clinical maze of mitochondrial neurology. Nat. Rev. Neurol. 2013;9:429–444. doi: 10.1038/nrneurol.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallace D.C. Mitochondrial Genetic medicine. Nat. Genet. 2018;50:1642–1649. doi: 10.1038/s41588-018-0264-z. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira C.R., Rahman S., Keller M., Zschocke J., ICIMD Advisory Group. Abdenur J., Ali H., Artuch R., Ballabio A., Barshop B., et al. An international classification of inherited metabolic disorders (ICIMD) J. Inherit. Metab. Dis. 2021;44:164–177. doi: 10.1002/jimd.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holt I.J., Harding A.E., Morgan-Hughes J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 90.Wallace D., Singh G., Lott M., Hodge J., Schurr T., Lezza A., Elsas L., Nikoskelainen E. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 91.Wallace D.C., Zheng X., Lott M.T., Shoffner J.M., Hodge J.A., Kelley R.I., Epstein C.M., Hopkins L.C. Familial mitochondrial encephalomyopathy (MERRF): Genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988;55:601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- 92.Carelli V., La Morgia C. Clinical syndromes associated with MtDNA mutations: Where we stand after 30 years. Essays Biochem. 2018;62:235–254. doi: 10.1042/EBC20170097. [DOI] [PubMed] [Google Scholar]
- 93.Leber T. Ueber hereditäre und congenital-angelegte sehnervenleiden. Graefe’s Arch. Clin. Exp. Ophthalmol. 1871;17:249–291. doi: 10.1007/BF01694557. [DOI] [Google Scholar]
- 94.Holt I.J., Harding A.E., Petty R.K., Morgan-Hughes J.A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 1990;46:428–433. [PMC free article] [PubMed] [Google Scholar]
- 95.Tatuch Y., Christodoulou J., Feigenbaum A., Clarke J.T., Wherret J., Smith C., Rudd N., Petrova-Benedict R., Robinson B.H. Heteroplasmic MtDNA mutation (T----G) at 8993 can cause leigh disease when the percentage of abnormal MtDNA is high. Am. J. Hum. Genet. 1992;50:852–858. [PMC free article] [PubMed] [Google Scholar]
- 96.Prezant T.R., Agapian J.V., Bohlman M.C., Bu X., Öztas S., Qiu W.-Q., Arnos K.S., Cortopassi G.A., Jaber L., Rotter J.I., et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic–induced and non–syndromic deafness. Nat. Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 97.Fukuhara N., Tokiguchi S., Shirakawa K., Tsubaki T. Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities): Disease entity or a syndrome? J. Neurol. Sci. 1980;47:117–133. doi: 10.1016/0022-510X(80)90031-3. [DOI] [PubMed] [Google Scholar]
- 98.Shoffner J.M., Lott M.T., Lezza A.M.S., Seibel P., Ballinger S.W., Wallace D.C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA TRNALys mutation. Cell. 1990;61:931–937. doi: 10.1016/0092-8674(90)90059-N. [DOI] [PubMed] [Google Scholar]
- 99.Pavlakis S.G., Phillips P.C., DiMauro S., De Vivo D.C., Rowland L.P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: A distinctive clinical syndrome. Ann. Neurol. 1984;16:481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- 100.Goto Y., Nonaka I., Horai S. A Mutation in the TRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 101.Kearns T.P., Sayre G.P. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: Unusual syndrome with histologic study in one of two cases. AMA. Arch. Ophthalmol. 1958;60:280–289. doi: 10.1001/archopht.1958.00940080296016. [DOI] [PubMed] [Google Scholar]
- 102.Zeviani M., Moraes C.T., DiMauro S., Nakase H., Bonilla E., Schon E.A., Rowland L.P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988;38:1339. doi: 10.1212/WNL.38.9.1339. [DOI] [PubMed] [Google Scholar]
- 103.Pearson H.A., Lobel J.S., Kocoshis S.A., Naiman J.L., Windmiller J., Lammi A.T., Hoffman R., Marsh J.C. A new syndrome of refractory sideroblastic anemia with vacuolization of marrow precursors and exocrine pancreatic dysfunction. J. Pediatr. 1979;95:976–984. doi: 10.1016/S0022-3476(79)80286-3. [DOI] [PubMed] [Google Scholar]
- 104.Rötig A., Cormier V., Blanche S., Bonnefont J.P., Ledeist F., Romero N., Schmitz J., Rustin P., Fischer A., Saudubray J.M. Pearson’s marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J. Clin. Investig. 1990;86:1601–1608. doi: 10.1172/JCI114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carelli V., Ghelli A., Ratta M., Bacchilega E., Sangiorgi S., Mancini R., Leuzzi V., Cortelli P., Montagna P., Lugaresi E., et al. Leber’s hereditary optic neuropathy: Biochemical effect of 11778/ND4 and 3460/ND1 mutations and correlation with the mitochondrial genotype. Neurology. 1997;48:1623–1632. doi: 10.1212/WNL.48.6.1623. [DOI] [PubMed] [Google Scholar]
- 106.Tatuch Y., Robinson B.H. The mitochondrial DNA mutation at 8993 associated with NARP slows the rate of ATP synthesis in isolated lymphoblast mitochondria. Biochem. Biophys. Res. Commun. 1993;192:124–128. doi: 10.1006/bbrc.1993.1390. [DOI] [PubMed] [Google Scholar]
- 107.Bernes S.M., Bacino C., Prezant T.R., Pearson M.A., Wood T.S., Fournier P., Fischel-Ghodsian N. Identical mitochondrial DNA deletion in mother with progressive external ophthalmoplegia and son with Pearson marrow-pancreas syndrome. J. Pediatr. 1993;123:598–602. doi: 10.1016/S0022-3476(05)80962-X. [DOI] [PubMed] [Google Scholar]
- 108.Shanske S., Tang Y., Hirano M., Nishigaki Y., Tanji K., Bonilla E., Sue C., Krishna S., Carlo J.R., Willner J., et al. Identical mitochondrial DNA deletion in a woman with ocular myopathy and in her son with Pearson syndrome. Am. J. Hum. Genet. 2002;71:679–683. doi: 10.1086/342482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mukherjee S., Ghosh A. Molecular mechanism of mitochondrial respiratory chain assembly and its relation to mitochondrial diseases. Mitochondrion. 2020;53:1–20. doi: 10.1016/j.mito.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Dang Q.-C.L., Phan D.H., Johnson A.N., Pasapuleti M., Alkhaldi H.A., Zhang F., Vik S.B. Analysis of human mutations in the supernumerary subunits of complex I. Life. 2020;10:296. doi: 10.3390/life10110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirst J. Mitochondrial complex I. Ann. Rev. Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 112.Ripple M.O., Kim N., Springett R. Mammalian complex I pumps 4 protons per 2 electrons at high and physiological proton motive force in living cells*. J. Biol. Chem. 2013;288:5374–5380. doi: 10.1074/jbc.M112.438945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Efremov R.G., Baradaran R., Sazanov L.A. The architecture of respiratory complex I. Nature. 2010;465:441–445. doi: 10.1038/nature09066. [DOI] [PubMed] [Google Scholar]
- 114.Zhu J., Vinothkumar K.R., Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–358. doi: 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vinothkumar K.R., Zhu J., Hirst J. Architecture of mammalian respiratory complex I. Nature. 2014;515:80–84. doi: 10.1038/nature13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clason T., Ruiz T., Schägger H., Peng G., Zickermann V., Brandt U., Michel H., Radermacher M. The structure of eukaryotic and prokaryotic complex I. J. Struct. Biol. 2010;169:81–88. doi: 10.1016/j.jsb.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baradaran R., Berrisford J.M., Minhas G.S., Sazanov L.A. Crystal structure of the entire respiratory complex I. Nature. 2013;494:443–448. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agip A.-N.A., Blaza J.N., Bridges H.R., Viscomi C., Rawson S., Muench S.P., Hirst J. Cryo-EM structures of complex I from mouse heart mitochondria in two biochemically defined states. Nat. Struct. Mol. Biol. 2018;25:548–556. doi: 10.1038/s41594-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirst J., Roessler M.M. Energy conversion, redox catalysis and generation of reactive oxygen species by respiratory complex I. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1857:872–883. doi: 10.1016/j.bbabio.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stroud D.A., Surgenor E.E., Formosa L.E., Reljic B., Frazier A.E., Dibley M.G., Osellame L.D., Stait T., Beilharz T.H., Thorburn D.R., et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature. 2016;538:123–126. doi: 10.1038/nature19754. [DOI] [PubMed] [Google Scholar]
- 121.Yip C., Harbour M.E., Jayawardena K., Fearnley I.M., Sazanov L.A. Evolution of respiratory complex I. J. Biol. Chem. 2011;286:5023–5033. doi: 10.1074/jbc.M110.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sazanov L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015;16:375–388. doi: 10.1038/nrm3997. [DOI] [PubMed] [Google Scholar]
- 123.Zickermann V., Wirth C., Nasiri H., Siegmund K., Schwalbe H., Hunte C., Brandt U. Mechanistic Insight from the crystal structure of mitochondrial complex I. Science. 2015;347:44–49. doi: 10.1126/science.1259859. [DOI] [PubMed] [Google Scholar]
- 124.Parey K., Wirth C., Vonck J., Zickermann V. Respiratory complex I—Structure, mechanism and evolution. Curr. Opin. Struct. Biol. 2020;63:1–9. doi: 10.1016/j.sbi.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 125.Kampjut D., Sazanov L.A. The coupling mechanism of mammalian respiratory complex I. Science. 2020;370:eabc4209. doi: 10.1126/science.abc4209. [DOI] [PubMed] [Google Scholar]
- 126.Grba D.N., Hirst J. Mitochondrial complex I structure reveals ordered water molecules for catalysis and proton translocation. Nat. Struct. Mol. Biol. 2020;27:892–900. doi: 10.1038/s41594-020-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klusch N., Senkler J., Yildiz Ö., Kühlbrandt W., Braun H.-P. A ferredoxin bridge connects the two arms of plant mitochondrial complex I. Plant Cell. 2021;33:2072–2091. doi: 10.1093/plcell/koab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soufari H., Parrot C., Kuhn L., Waltz F., Hashem Y. Specific features and assembly of the plant mitochondrial complex I revealed by Cryo-EM. Nat. Commun. 2020;11:5195. doi: 10.1038/s41467-020-18814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo R., Zong S., Wu M., Gu J., Yang M. Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell. 2017;170:1247–1257. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 130.Signes A., Fernandez-Vizarra E. Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem. 2018;62:255–270. doi: 10.1042/EBC20170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vartak R.S., Semwal M.K., Bai Y. An update on complex I assembly: The assembly of players. J. Bioenerg. Biomembr. 2014;46:323–328. doi: 10.1007/s10863-014-9564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carroll J., Ding S., Fearnley I.M., Walker J.E. Post-translational modifications near the quinone binding site of mammalian complex I*. J. Biol. Chem. 2013;288:24799–24808. doi: 10.1074/jbc.M113.488106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Protasoni M., Zeviani M. Mitochondrial structure and bioenergetics in normal and disease conditions. Int. J. Mol. Sci. 2021;22:586. doi: 10.3390/ijms22020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nouws J., Nijtmans L., Houten S.M., van den Brand M., Huynen M., Venselaar H., Hoefs S., Gloerich J., Kronick J., Hutchin T., et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab. 2010;12:283–294. doi: 10.1016/j.cmet.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Haack T.B., Danhauser K., Haberberger B., Hoser J., Strecker V., Boehm D., Uziel G., Lamantea E., Invernizzi F., Poulton J., et al. Exome sequencing identifies ACAD9 mutations as a cause of complex I deficiency. Nat. Genet. 2010;42:1131–1134. doi: 10.1038/ng.706. [DOI] [PubMed] [Google Scholar]
- 136.Vogel R.O., Janssen R.J., van den Brand M.A.M., Dieteren C.E.J., Verkaart S., Koopman W.J.H., Willems P.H.G.M., Pluk W., van den Heuvel L.P.W.J., Smeitink J.A.M., et al. Cytosolic signaling protein ecsit also localizes to mitochondria where it interacts with chaperone NDUFAF1 and functions in complex I assembly. Genes Dev. 2007;21:615–624. doi: 10.1101/gad.408407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rendón O.Z., Antonicka H., Horvath R., Shoubridge E.A. A mutation in the flavin adenine dinucleotide-dependent oxidoreductase FOXRED1 results in cell-type-specific assembly defects in oxidative phosphorylation complexes I and II. Mol. Cell. Biol. 2016;36:2132–2140. doi: 10.1128/MCB.00066-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Formosa L.E., Mimaki M., Frazier A.E., McKenzie M., Stait T.L., Thorburn D.R., Stroud D.A., Ryan M.T. Characterization of mitochondrial FOXRED1 in the assembly of respiratory chain complex I. Hum. Mol. Genet. 2015;24:2952–2965. doi: 10.1093/hmg/ddv058. [DOI] [PubMed] [Google Scholar]
- 139.Calvo S.E., Tucker E.J., Compton A.G., Kirby D.M., Crawford G., Burtt N.P., Rivas M., Guiducci C., Bruno D.L., Goldberger O.A., et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 2010;42:851–858. doi: 10.1038/ng.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andrews B., Carroll J., Ding S., Fearnley I.M., Walker J.E. Assembly factors for the membrane arm of human complex I. Proc. Natl. Acad. Sci. USA. 2013;110:18934–18939. doi: 10.1073/pnas.1319247110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Čížková A., Stránecký V., Mayr J.A., Tesařová M., Havlíčková V., Paul J., Ivánek R., Kuss A.W., Hansíková H., Kaplanová V., et al. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat. Genet. 2008;40:1288–1290. doi: 10.1038/ng.246. [DOI] [PubMed] [Google Scholar]
- 142.Sánchez-Caballero L., Elurbe D.M., Baertling F., Guerrero-Castillo S., van den Brand M., van Strien J., van Dam T.J.P., Rodenburg R., Brandt U., Huynen M.A., et al. TMEM70 functions in the assembly of complexes I and V. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020;1861:148202. doi: 10.1016/j.bbabio.2020.148202. [DOI] [PubMed] [Google Scholar]
- 143.Catteruccia M., Verrigni D., Martinelli D., Torraco A., Agovino T., Bonafé L., D’Amico A., Donati M.A., Adorisio R., Santorelli F.M., et al. Persistent pulmonary arterial hypertension in the newborn (PPHN): A frequent manifestation of TMEM70 defective patients. Mol. Genet. Metab. 2014;111:353–359. doi: 10.1016/j.ymgme.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 144.Staretz-Chacham O., Wormser O., Manor E., Birk O.S., Ferreira C.R. TMEM70 deficiency: Novel mutation and hypercitrullinemia during metabolic decompensation. Am. J. Med. Genet. 2019;179:1293–1298. doi: 10.1002/ajmg.a.61138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hirono K., Ichida F., Nishio N., Ogawa-Tominaga M., Fushimi T., Feichtinger R.G., Mayr J.A., Kohda M., Kishita Y., Okazaki Y., et al. Mitochondrial complex deficiency by novel compound heterozygous TMEM70 variants and correlation with developmental delay, undescended testicle, and left ventricular noncompaction in a Japanese patient: A case report. Clin. Case Rep. 2019;7:553–557. doi: 10.1002/ccr3.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spiegel R., Khayat M., Shalev S.A., Horovitz Y., Mandel H., Hershkovitz E., Barghuti F., Shaag A., Saada A., Korman S.H., et al. TMEM70 mutations are a common cause of nuclear encoded ATP synthase assembly defect: Further delineation of a new syndrome. J. Med. Genet. 2011;48:177–182. doi: 10.1136/jmg.2010.084608. [DOI] [PubMed] [Google Scholar]
- 147.Vogel R.O., Janssen R.J.R.J., Ugalde C., Grovenstein M., Huijbens R.J., Visch H.-J., van den Heuvel L.P., Willems P.H., Zeviani M., Smeitink J.A.M., et al. Human mitochondrial complex I assembly is mediated by NDUFAF1. FEBS J. 2005;272:5317–5326. doi: 10.1111/j.1742-4658.2005.04928.x. [DOI] [PubMed] [Google Scholar]
- 148.Dunning C.J.R., McKenzie M., Sugiana C., Lazarou M., Silke J., Connelly A., Fletcher J.M., Kirby D.M., Thorburn D.R., Ryan M.T. Human CIA30 is involved in the early assembly of mitochondrial complex I and mutations in its gene cause disease. EMBO J. 2007;26:3227–3237. doi: 10.1038/sj.emboj.7601748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ogilvie I., Ogilvie I., Kennaway N.G., Shoubridge E.A. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. J. Clin. Investig. 2005;115:2784–2792. doi: 10.1172/JCI26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saada A., Edvardson S., Rapoport M., Shaag A., Amry K., Miller C., Lorberboum-Galski H., Elpeleg O. C6ORF66 is an assembly factor of mitochondrial complex I. Am. J. Hum. Genet. 2008;82:32–38. doi: 10.1016/j.ajhg.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saada A., Vogel R.O., Hoefs S.J., van den Brand M.A., Wessels H.J., Willems P.H., Venselaar H., Shaag A., Barghuti F., Reish O., et al. Mutations in NDUFAF3 (C3ORF60), encoding an NDUFAF4 (C6ORF66)-interacting complex I assembly protein, cause fatal neonatal mitochondrial disease. Am. J. Hum. Genet. 2009;84:718–727. doi: 10.1016/j.ajhg.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sugiana C., Pagliarini D.J., McKenzie M., Kirby D.M., Salemi R., Abu-Amero K.K., Dahl H.-H.M., Hutchison W.M., Vascotto K.A., Smith S.M., et al. Mutation of C20orf7 disrupts complex I assembly and causes lethal neonatal mitochondrial disease. Am. J. Hum. Genet. 2008;83:468–478. doi: 10.1016/j.ajhg.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rhein V.F., Carroll J., Ding S., Fearnley I.M., Walker J.E. NDUFAF5 Hydroxylates NDUFS7 at an early stage in the assembly of human complex I. J. Biol. Chem. 2016;291:14851–14860. doi: 10.1074/jbc.M116.734970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McKenzie M., Tucker E.J., Compton A.G., Lazarou M., George C., Thorburn D.R., Ryan M.T. Mutations in the gene encoding C8orf38 block complex I assembly by inhibiting production of the mitochondria-encoded subunit ND1. J. Mol. Biol. 2011;414:413–426. doi: 10.1016/j.jmb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 155.Bianciardi L., Imperatore V., Fernandez-Vizarra E., Lopomo A., Falabella M., Furini S., Galluzzi P., Grosso S., Zeviani M., Renieri A., et al. Exome sequencing coupled with MRNA analysis identifies NDUFAF6 as a leigh gene. Mol. Genet. Metab. 2016;119:214–222. doi: 10.1016/j.ymgme.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 156.Catania A., Ardissone A., Verrigni D., Legati A., Reyes A., Lamantea E., Diodato D., Tonduti D., Imperatore V., Pinto A.M., et al. Compound heterozygous missense and deep intronic variants in NDUFAF6 unraveled by exome sequencing and MRNA analysis. J. Hum. Genet. 2018;63:563–568. doi: 10.1038/s10038-018-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baide-Mairena H., Gaudó P., Marti-Sánchez L., Emperador S., Sánchez-Montanez A., Alonso-Luengo O., Correa M., Grau A.M., Ortigoza-Escobar J.D., Artuch R., et al. Mutations in the mitochondrial complex I assembly factor NDUFAF6 cause isolated bilateral striatal necrosis and progressive dystonia in childhood. Mol. Genet. Metab. 2019;126:250–258. doi: 10.1016/j.ymgme.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Hartmannová H., Piherová L., Tauchmannová K., Kidd K., Acott P.D., Crocker J.F.S., Oussedik Y., Mallet M., Hodaňová K., Stránecký V., et al. Acadian variant of fanconi syndrome is caused by mitochondrial respiratory chain complex I deficiency due to a non-coding mutation in complex I assembly factor NDUFAF6. Hum. Mol. Genet. 2016;25:4062–4079. doi: 10.1093/hmg/ddw245. [DOI] [PubMed] [Google Scholar]
- 159.Carilla-Latorre S., Gallardo M.E., Annesley S.J., Calvo-Garrido J., Graña O., Accari S.L., Smith P.K., Valencia A., Garesse R., Fisher P.R., et al. MidA is a putative methyltransferase that is required for mitochondrial complex I function. J. Cell Sci. 2010;123:1674–1683. doi: 10.1242/jcs.066076. [DOI] [PubMed] [Google Scholar]
- 160.Rhein V.F., Carroll J., Ding S., Fearnley I.M., Walker J.E. NDUFAF7 methylates arginine 85 in the NDUFS2 subunit of human complex I. J. Biol. Chem. 2013;288:33016–33026. doi: 10.1074/jbc.M113.518803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alston C.L., Veling M.T., Heidler J., Taylor L.S., Alaimo J.T., Sung A.Y., He L., Hopton S., Broomfield A., Pavaine J., et al. Pathogenic bi-allelic mutations in NDUFAF8 cause leigh syndrome with an isolated complex I deficiency. Am. J. Hum. Genet. 2020;106:92–101. doi: 10.1016/j.ajhg.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sheftel A.D., Stehling O., Pierik A.J., Netz D.J.A., Kerscher S., Elsässer H.-P., Wittig I., Balk J., Brandt U., Lill R. Human Ind1, an iron-sulfur cluster assembly factor for respiratory complex I. Mol. Cell. Biol. 2009;29:6059–6073. doi: 10.1128/MCB.00817-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bych K., Kerscher S., Netz D.J.A., Pierik A.J., Zwicker K., Huynen M.A., Lill R., Brandt U., Balk J. The iron–sulphur protein Ind1 is required for effective complex I assembly. EMBO J. 2008;27:1736–1746. doi: 10.1038/emboj.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Protasoni M., Bruno C., Donati M.A., Mohamoud K., Severino M., Allegri A., Robinson A.J., Reyes A., Zeviani M., Garone C. Novel compound heterozygous pathogenic variants in nucleotide-binding protein like protein (NUBPL) cause leukoencephalopathy with multi-systemic involvement. Mol. Genet. Metab. 2020;129:26–34. doi: 10.1016/j.ymgme.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Guarani V., Paulo J., Zhai B., Huttlin E.L., Gygi S.P., Harper J.W. TIMMDC1/C3orf1 functions as a membrane-embedded mitochondrial complex I assembly factor through association with the MCIA complex. Mol. Cell. Biol. 2014;34:847–861. doi: 10.1128/MCB.01551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kremer L.S., Bader D.M., Mertes C., Kopajtich R., Pichler G., Iuso A., Haack T.B., Graf E., Schwarzmayr T., Terrile C., et al. Genetic diagnosis of mendelian disorders via RNA sequencing. Nat. Commun. 2017;8:15824. doi: 10.1038/ncomms15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Désir J., Coppieters F., Van Regemorter N., De Baere E., Abramowicz M., Cordonnier M. TMEM126A mutation in a Moroccan family with autosomal recessive optic atrophy. Mol. Vis. 2012;18:1849–1857. [PMC free article] [PubMed] [Google Scholar]
- 168.Hanein S., Perrault I., Roche O., Gerber S., Khadom N., Rio M., Boddaert N., Jean-Pierre M., Brahimi N., Serre V., et al. TMEM126A, encoding a mitochondrial protein, is mutated in autosomal-recessive nonsyndromic optic atrophy. Am. J. Hum. Genet. 2009;84:493–498. doi: 10.1016/j.ajhg.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kloth K., Synofzik M., Kernstock C., Schimpf-Linzenbold S., Schuettauf F., Neu A., Wissinger B., Weisschuh N. Novel Likely Pathogenic Variants in TMEM126A identified in non-syndromic autosomal recessive optic atrophy: Two case reports. BMC Med. Genet. 2019;20:62. doi: 10.1186/s12881-019-0795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.La Morgia C., Caporali L., Tagliavini F., Palombo F., Carbonelli M., Liguori R., Barboni P., Carelli V. First TMEM126A missense mutation in an italian proband with optic atrophy and deafness. Neurol. Genet. 2019;5:e329. doi: 10.1212/NXG.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meyer E., Michaelides M., Tee L.J., Robson A.G., Rahman F., Pasha S., Luxon L.M., Moore A.T., Maher E.R. Nonsense mutation in TMEM126A causing autosomal recessive optic atrophy and auditory neuropathy. Mol. Vis. 2010;16:650–664. [PMC free article] [PubMed] [Google Scholar]
- 172.Heide H., Bleier L., Steger M., Ackermann J., Dröse S., Schwamb B., Zörnig M., Reichert A.S., Koch I., Wittig I., et al. Complexome profiling identifies TMEM126B as a component of the mitochondrial complex I assembly complex. Cell Metab. 2012;16:538–549. doi: 10.1016/j.cmet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 173.Sánchez-Caballero L., Ruzzenente B., Bianchi L., Assouline Z., Barcia G., Metodiev M.D., Rio M., Funalot B., van den Brand M.A.M., Guerrero-Castillo S., et al. Mutations in complex I assembly factor TMEM126B result in muscle weakness and isolated complex I deficiency. Am. J. Hum. Genet. 2016;99:208–216. doi: 10.1016/j.ajhg.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alston C.L., Compton A.G., Formosa L.E., Strecker V., Oláhová M., Haack T.B., Smet J., Stouffs K., Diakumis P., Ciara E., et al. Biallelic mutations in TMEM126B cause severe complex I deficiency with a variable clinical phenotype. Am. J. Hum. Genet. 2016;99:217–227. doi: 10.1016/j.ajhg.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guerrero-Castillo S., Baertling F., Kownatzki D., Wessels H.J., Arnold S., Brandt U., Nijtmans L. The assembly pathway of mitochondrial respiratory chain complex I. Cell Metab. 2017;25:128–139. doi: 10.1016/j.cmet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 176.Martinez Lyons A., Ardissone A., Reyes A., Robinson A.J., Moroni I., Ghezzi D., Fernandez-Vizarra E., Zeviani M. COA7 (C1orf163/RESA1) mutations associated with mitochondrial leukoencephalopathy and cytochrome C oxidase deficiency. J. Med. Genet. 2016;53:846–849. doi: 10.1136/jmedgenet-2016-104194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dibley M.G., Formosa L.E., Lyu B., Reljic B., McGann D., Muellner-Wong L., Kraus F., Sharpe A.J., Stroud D.A., Ryan M.T. The mitochondrial acyl-carrier protein interaction network highlights important roles for LYRM family members in complex I and mitoribosome assembly. Mol. Cell. Proteom. 2020;19:65–77. doi: 10.1074/mcp.RA119.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bugiani M., Invernizzi F., Alberio S., Briem E., Lamantea E., Carrara F., Moroni I., Farina L., Spada M., Donati M.A., et al. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 179.Malfatti E., Bugiani M., Invernizzi F., de Souza C.F.-M., Farina L., Carrara F., Lamantea E., Antozzi C., Confalonieri P., Sanseverino M.T., et al. Novel mutations of ND genes in complex I deficiency associated with mitochondrial encephalopathy. Brain. 2007;130:1894–1904. doi: 10.1093/brain/awm114. [DOI] [PubMed] [Google Scholar]
- 180.Fassone E., Rahman S. Complex I deficiency: Clinical features, biochemistry and molecular genetics. J. Med. Genet. 2012;49:578–590. doi: 10.1136/jmedgenet-2012-101159. [DOI] [PubMed] [Google Scholar]
- 181.Rodenburg R.J. Mitochondrial complex I-linked disease. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1857:938–945. doi: 10.1016/j.bbabio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 182.Man P.Y.-W., Griffiths P.G., Brown D.T., Howell N., Turnbull D.M., Chinnery P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003;72:333–339. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carelli V., Rugolo M., Sgarbi G., Ghelli A., Zanna C., Baracca A., Lenaz G., Napoli E., Martinuzzi A., Solaini G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: A model of mitochondrial neurodegeneration. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004;1658:172–179. doi: 10.1016/j.bbabio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 184.Yu-Wai-Man P., Griffiths P.G., Chinnery P.F. Mitochondrial optic neuropathies—Disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Giordano C., Iommarini L., Giordano L., Maresca A., Pisano A., Valentino M.L., Caporali L., Liguori R., Deceglie S., Roberti M., et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2014;137:335–353. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bianco A., Martínez-Romero I., Bisceglia L., D’Agruma L., Favia P., Ruiz-Pesini E., Guerriero S., Montoya J., Petruzzella V. Mitochondrial DNA copy number differentiates the Leber’s hereditary optic neuropathy affected individuals from the unaffected mutation carriers. Brain. 2016;139:e1. doi: 10.1093/brain/awv216. [DOI] [PubMed] [Google Scholar]
- 187.Bianco A., Bisceglia L., Russo L., Palese L.L., D’Agruma L., Emperador S., Montoya J., Guerriero S., Petruzzella V. High mitochondrial DNA copy number is a protective factor from vision loss in heteroplasmic Leber’s hereditary optic neuropathy (LHON) Investig. Opthalmol. Vis. Sci. 2017;58:2193. doi: 10.1167/iovs.16-20389. [DOI] [PubMed] [Google Scholar]
- 188.Bianco A., Valletti A., Longo G., Bisceglia L., Montoya J., Emperador S., Guerriero S., Petruzzella V. Mitochondrial DNA copy number in affected and unaffected LHON mutation carriers. BMC Res. Notes. 2018;11:911. doi: 10.1186/s13104-018-4025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tun A.W., Chaiyarit S., Kaewsutthi S., Katanyoo W., Chuenkongkaew W., Kuwano M., Tomonaga T., Peerapittayamongkol C., Thongboonkerd V., Lertrit P. Profiling the mitochondrial proteome of Leber’s hereditary optic neuropathy (LHON) in Thailand: Down-regulation of bioenergetics and mitochondrial protein quality control pathways in fibroblasts with the 11778G>A mutation. PLoS ONE. 2014;9:e106779. doi: 10.1371/journal.pone.0106779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lenaz G., Baracca A., Carelli V., D’Aurelio M., Sgarbi G., Solaini G. Bioenergetics of mitochondrial diseases associated with MtDNA mutations. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004;1658:89–94. doi: 10.1016/j.bbabio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 191.Brown M.D., Trounce I.A., Jun A.S., Allen J.C., Wallace D.C. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. J. Biol. Chem. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- 192.Floreani M., Napoli E., Martinuzzi A., Pantano G., De Riva V., Trevisan R., Bisetto E., Valente L., Carelli V., Dabbeni-Sala F. Antioxidant defences in cybrids harboring MtDNA mutations associated with Leber’s hereditary optic neuropathy: Antioxidant defences in LHON cybrids. FEBS J. 2005;272:1124–1135. doi: 10.1111/j.1742-4658.2004.04542.x. [DOI] [PubMed] [Google Scholar]
- 193.Simon D.K., Friedman J., Breakefield X.O., Jankovic J., Brin M.F., Provias J., Bressman S.B., Charness M.E., Tarsy D., Johns D.R., et al. A heteroplasmic mitochondrial complex I gene mutation in adult-onset dystonia. Neurogenetics. 2003;4:199–205. doi: 10.1007/s10048-003-0150-3. [DOI] [PubMed] [Google Scholar]
- 194.Kirby D.M. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J. Med. Genet. 2004;41:784–789. doi: 10.1136/jmg.2004.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Howell N., Bindoff L.A., McCullough D.A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D.M. Leber hereditary optic neuropathy: Identification of the same mitochondrial ND1 mutation in six pedigrees. Am. J. Hum. Genet. 1991;49:939–950. [PMC free article] [PubMed] [Google Scholar]
- 196.Johns D.R., Berman J. Alternative, simultaneous complex I mitochondrial DNA mutations in Leber’s hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 1991;174:1324–1330. doi: 10.1016/0006-291X(91)91567-V. [DOI] [PubMed] [Google Scholar]
- 197.McFarland R., Kirby D.M., Fowler K.J., Ohtake A., Ryan M.T., Amor D.J., Fletcher J.M., Dixon J.W., Collins F.A., Turnbull D.M., et al. De novo mutations in the mitochondrial ND3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann. Neurol. 2004;55:58–64. doi: 10.1002/ana.10787. [DOI] [PubMed] [Google Scholar]
- 198.Torroni A., Petrozzi M., D’Urbano L., Sellitto D., Zeviani M., Carrara F., Carducci C., Leuzzi V., Carelli V., Barboni P., et al. Haplotype and phylogenetic analyses suggest that one european-specific MtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am. J. Hum. Genet. 1997;60:1107–1121. [PMC free article] [PubMed] [Google Scholar]
- 199.Lertrit P., Noer A.S., Jean-Francois M.J., Kapsa R., Dennett X., Thyagarajan D., Lethlean K., Byrne E., Marzuki S. A new disease-related mutation for mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) syndrome affects the ND4 subunit of the respiratory complex I. Am. J. Hum. Genet. 1992;51:457–468. [PMC free article] [PubMed] [Google Scholar]
- 200.Brown M.D., Starikovskaya E., Derbeneva O., Hosseini S., Allen J.C., Mikhailovskaya I.E., Sukernik R.I., Wallace D.C. The role of MtDNA background in disease expression: A new primary LHON mutation associated with western eurasian haplogroup. J. Hum. Genet. 2002;110:130–138. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- 201.Brown M.D., Voljavec A.S., Lott M.T., Macdonald I., Wallace D.C. Leber’s hereditary optic neuropathy: A model for mitochondrial neurodegenerative diseases. FASEB J. 1992;6:2791–2799. doi: 10.1096/fasebj.6.10.1634041. [DOI] [PubMed] [Google Scholar]
- 202.Liolitsa D., Rahman S., Benton S., Carr L.J., Hanna M.G. Is the mitochondrial complex I ND5 gene a hot-spot for MELAS causing mutations? Ann. Neurol. 2003;53:128–132. doi: 10.1002/ana.10435. [DOI] [PubMed] [Google Scholar]
- 203.Ravn K., Wibrand F., Hansen F.J., Horn N., Rosenberg T., Schwartz M. An MtDNA mutation, 14453G→A, in the NADH dehydrogenase subunit 6 associated with severe MELAS syndrome. Eur. J. Hum. Genet. 2001;9:805–809. doi: 10.1038/sj.ejhg.5200712. [DOI] [PubMed] [Google Scholar]
- 204.Schuelke M., Smeitink J., Mariman E., Loeffen J., Plecko B., Trijbels F., Stöckler-Ipsiroglu S., van den Heuvel L. Mutant NDUFV1 subunit of mitochondrial complex I causes leukodystrophy and myoclonic epilepsy. Nat. Genet. 1999;21:260–261. doi: 10.1038/6772. [DOI] [PubMed] [Google Scholar]
- 205.Bénit P., Chretien D., Kadhom N., de Lonlay-Debeney P., Cormier-Daire V., Cabral A., Peudenier S., Rustin P., Munnich A., Rötig A. Large-scale deletion and point mutations of the nuclear NDUFV1 and NDUFS1 genes in mitochondrial complex I deficiency. Am. J. Hum. Genet. 2001;68:1344–1352. doi: 10.1086/320603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bénit P., Beugnot R., Chretien D., Giurgea I., De Lonlay-Debeney P., Issartel J.-P., Corral-Debrinski M., Kerscher S., Rustin P., Rötig A., et al. Mutant NDUFV2 subunit of mitochondrial complex I causes early onset hypertrophic cardiomyopathy and encephalopathy: NDUFV2 and cardiomyopathy/encephalopathy. Hum. Mutat. 2003;21:582–586. doi: 10.1002/humu.10225. [DOI] [PubMed] [Google Scholar]
- 207.Loeffen J., Elpeleg O., Smeitink J., Smeets R., Stöckler-Ipsiroglu S., Mandel H., Sengers R., Trijbels F., van den Heuvel L. Mutations in the complex I NDUFS2 gene of patients with cardiomyopathy and encephalomyopathy. Ann. Neurol. 2001;49:195–201. doi: 10.1002/1531-8249(20010201)49:2<195::AID-ANA39>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 208.Benit P., Slama A., Cartault F., Giurgea I., Chretien D., Lebon S., Marsac C., Munnich A., Rotig A., Rustin P. Mutant NDUFS3 Subunit of mitochondrial complex I causes leigh syndrome. J. Med. Genet. 2004;41:14–17. doi: 10.1136/jmg.2003.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Budde S.M.S., van den Heuvel L.P.W.J., Janssen A.J., Smeets R.J.P., Buskens C.A.F., DeMeirleir L., Van Coster R., Baethmann M., Voit T., Trijbels J.M.F., et al. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem. Biophys. Res. Commun. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- 210.Spiegel R., Shaag A., Mandel H., Reich D., Penyakov M., Hujeirat Y., Saada A., Elpeleg O., Shalev S.A. Mutated NDUFS6 is the cause of fatal neonatal lactic acidemia in caucasus jews. Eur. J. Hum. Genet. 2009;17:1200–1203. doi: 10.1038/ejhg.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Taylor R.W., Pyle A., Griffin H., Blakely E.L., Duff J., He L., Smertenko T., Alston C.L., Neeve V.C., Best A., et al. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312:68. doi: 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Smeitink J., van den Heuvel L. Human mitochondrial complex I in health and disease. Am. J. Hum. Genet. 1999;64:1505–1510. doi: 10.1086/302432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Loeffen J., Smeitink J., Triepels R., Smeets R., Schuelke M., Sengers R., Trijbels F., Hamel B., Mullaart R., van den Heuvel L. The first nuclear-encoded complex I mutation in a patient with Leigh syndrome. Am. J. Hum. Genet. 1998;63:1598–1608. doi: 10.1086/302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Berger I., Hershkovitz E., Shaag A., Edvardson S., Saada A., Elpeleg O. Mitochondrial complex I deficiency caused by a deleterious NDUFA11 mutation. Ann. Neurol. 2008;63:405–408. doi: 10.1002/ana.21332. [DOI] [PubMed] [Google Scholar]
- 215.Fernandez-Moreira D., Ugalde C., Smeets R., Rodenburg R.J.T., Lopez-Laso E., Ruiz-Falco M.L., Briones P., Martin M.A., Smeitink J.A.M., Arenas J. X-Linked NDUFA1 gene mutations associated with mitochondrial encephalomyopathy. Ann. Neurol. 2007;61:73–83. doi: 10.1002/ana.21036. [DOI] [PubMed] [Google Scholar]
- 216.Hoefs S.J.G., Dieteren C.E.J., Distelmaier F., Janssen R.J.R.J., Epplen A., Swarts H.G.P., Forkink M., Rodenburg R.J., Nijtmans L.G., Willems P.H., et al. NDUFA2 complex I mutation leads to Leigh disease. Am. J. Hum. Genet. 2008;82:1306–1315. doi: 10.1016/j.ajhg.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alston C.L., Heidler J., Dibley M.G., Kremer L.S., Taylor L.S., Fratter C., French C.E., Glasgow R.I.C., Feichtinger R.G., Delon I., et al. Bi-Allelic mutations in NDUFA6 establish its role in early-onset isolated mitochondrial complex I deficiency. Am. J. Hum. Genet. 2018;103:592–601. doi: 10.1016/j.ajhg.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van den Bosch B.J.C., Gerards M., Sluiter W., Stegmann A.P.A., Jongen E.L.C., Hellebrekers D.M.E.I., Oegema R., Lambrichs E.H., Prokisch H., Danhauser K., et al. Defective NDUFA9 as a novel cause of neonatally fatal complex I disease. J. Med. Genet. 2012;49:10–15. doi: 10.1136/jmedgenet-2011-100466. [DOI] [PubMed] [Google Scholar]
- 219.Ostergaard E., Rodenburg R.J., van den Brand M., Thomsen L.L., Duno M., Batbayli M., Wibrand F., Nijtmans L. Respiratory chain complex I deficiency due to NDUFA12 mutations as a new cause of Leigh syndrome. J. Med. Genet. 2011;48:737–740. doi: 10.1136/jmg.2011.088856. [DOI] [PubMed] [Google Scholar]
- 220.Angebault C., Charif M., Guegen N., Piro-Megy C., de Camaret B.M., Procaccio V., Guichet P.-O., Hebrard M., Manes G., Leboucq N., et al. Mutation in NDUFA13/GRIM19 leads to early onset hypotonia, dyskinesia and sensorial deficiencies, and mitochondrial complex I instability. Hum. Mol. Genet. 2015;24:3948–3955. doi: 10.1093/hmg/ddv133. [DOI] [PubMed] [Google Scholar]
- 221.Haack T.B., Madignier F., Herzer M., Lamantea E., Danhauser K., Invernizzi F., Koch J., Freitag M., Drost R., Hillier I., et al. Mutation screening of 75 candidate genes in 152 complex I deficiency cases identifies pathogenic variants in 16 genes including NDUFB9. J. Med. Genet. 2012;49:83–89. doi: 10.1136/jmedgenet-2011-100577. [DOI] [PubMed] [Google Scholar]
- 222.Piekutowska-Abramczuk D., Assouline Z., Mataković L., Feichtinger R.G., Koňařiková E., Jurkiewicz E., Stawiński P., Gusic M., Koller A., Pollak A., et al. NDUFB8 mutations cause mitochondrial complex I deficiency in individuals with Leigh-like encephalomyopathy. Am. J. Hum. Genet. 2018;102:460–467. doi: 10.1016/j.ajhg.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Friederich M.W., Erdogan A.J., Coughlin C.R., Elos M.T., Jiang H., O’Rourke C.P., Lovell M.A., Wartchow E., Gowan K., Chatfield K.C., et al. Mutations in the accessory subunit NDUFB10 result in isolated complex I deficiency and illustrate the critical role of intermembrane space import for complex I holoenzyme assembly. Hum. Mol. Genet. 2016;26:702–716. doi: 10.1093/hmg/ddw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van Rahden V.A., Fernandez-Vizarra E., Alawi M., Brand K., Fellmann F., Horn D., Zeviani M., Kutsche K. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am. J. Hum. Genet. 2015;96:640–650. doi: 10.1016/j.ajhg.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reinson K., Kovacs-Nagy R., Õiglane-Shlik E., Pajusalu S., Nõukas M., Wintjes L.T., van den Brandt F.C.A., Brink M., Acker T., Ahting U., et al. Diverse phenotype in patients with complex I deficiency due to mutations in NDUFB11. Eur. J. Med. Genet. 2019;62:103572. doi: 10.1016/j.ejmg.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 226.Kohda M., Tokuzawa Y., Kishita Y., Nyuzuki H., Moriyama Y., Mizuno Y., Hirata T., Yatsuka Y., Yamashita-Sugahara Y., Nakachi Y., et al. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12:e1005679. doi: 10.1371/journal.pgen.1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Alahmad A., Nasca A., Heidler J., Thompson K., Oláhová M., Legati A., Lamantea E., Meisterknecht J., Spagnolo M., He L., et al. Bi-allelic pathogenic variants in NDUFC2 cause early-onset Leigh syndrome and stalled biogenesis of complex I. EMBO Mol. Med. 2020;12:e12619. doi: 10.15252/emmm.202012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Baertling F., Sánchez-Caballero L., Timal S., van den Brand M.A., Ngu L.H., Distelmaier F., Rodenburg R.J., Nijtmans L.G. Mutations in mitochondrial complex I assembly factor NDUFAF3 cause Leigh syndrome. Mol. Genet. Metab. 2017;120:243–246. doi: 10.1016/j.ymgme.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 229.Baertling F., Sánchez-Caballero L., van den Brand M.A.M., Wintjes L.T., Brink M., van den Brandt F.A., Wilson C., Rodenburg R.J.T., Nijtmans L.G.J. NDUFAF4 variants are associated with Leigh syndrome and cause a specific mitochondrial complex I assembly defect. Eur. J. Hum. Genet. 2017;25:1273–1277. doi: 10.1038/ejhg.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ishiyama A., Muramatsu K., Uchino S., Sakai C., Matsushima Y., Makioka N., Ogata T., Suzuki E., Komaki H., Sasaki M., et al. NDUFAF3 variants that disrupt mitochondrial complex I assembly may associate with cavitating leukoencephalopathy. Clin. Genet. 2018;93:1103–1106. doi: 10.1111/cge.13215. [DOI] [PubMed] [Google Scholar]
- 231.Ugarteburu O., Teresa Garcia-Silva M., Aldamiz-Echevarria L., Gort L., Garcia-Villoria J., Tort F., Ribes A. Complex I deficiency, due to NDUFAF4 mutations, causes severe mitochondrial dysfunction and is associated to early death and dysmorphia. Mitochondrion. 2020;55:78–84. doi: 10.1016/j.mito.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 232.Petruzzella V., Vergari R., Puzziferri I., Boffoli D., Lamantea E., Zeviani M., Papa S. A nonsense mutation in the NDUFS4 gene encoding the 18 KDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum. Mol. Genet. 2001;10:529–535. doi: 10.1093/hmg/10.5.529. [DOI] [PubMed] [Google Scholar]
- 233.Petruzzella V., Papa S. Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: The NDUFS4 gene. Gene. 2002;286:149–154. doi: 10.1016/S0378-1119(01)00810-1. [DOI] [PubMed] [Google Scholar]
- 234.Petruzzella V., Panelli D., Torraco A., Stella A., Papa S. Mutations in the NDUFS4 gene of mitochondrial complex I alter stability of the splice variants. FEBS Lett. 2005;579:3770–3776. doi: 10.1016/j.febslet.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 235.Scacco S., Petruzzella V., Budde S., Vergari R., Tamborra R., Panelli D., van den Heuvel L.P., Smeitink J.A., Papa S. Pathological mutations of the human NDUFS4 gene of the 18-KDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J. Biol. Chem. 2003;278:44161–44167. doi: 10.1074/jbc.M307615200. [DOI] [PubMed] [Google Scholar]
- 236.Lamont R.E., Beaulieu C.L., Bernier F.P., Sparkes R., Innes A.M., Jackel-Cram C., Ober C., Parboosingh J.S., Lemire E.G. A novel NDUFS4 frameshift mutation causes Leigh disease in the Hutterite population. Am. J. Med. Genet. 2017;173:596–600. doi: 10.1002/ajmg.a.37983. [DOI] [PubMed] [Google Scholar]
- 237.Budde S.M.S., van den Heuvel L.P.W.J., Smeets R.J.P., Skladal D., Mayr J.A., Boelen C., Petruzzella V., Papa S., Smeitink J.A.M. Clinical heterogeneity in patients with mutations in the NDUFS4 gene of mitochondrial complex I. J. Inherit. Metab. Dis. 2003;26:813–815. doi: 10.1023/B:BOLI.0000010003.14113.af. [DOI] [PubMed] [Google Scholar]
- 238.Leshinsky-Silver E., Lebre A.-S., Minai L., Saada A., Steffann J., Cohen S., Rötig A., Munnich A., Lev D., Lerman-Sagie T. NDUFS4 mutations cause Leigh syndrome with predominant brainstem involvement. Mol. Genet. Metab. 2009;97:185–189. doi: 10.1016/j.ymgme.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 239.Ortigoza-Escobar J.D., Oyarzabal A., Montero R., Artuch R., Jou C., Jiménez C., Gort L., Briones P., Muchart J., López-Gallardo E., et al. Ndufs4 related Leigh syndrome: A case report and review of the literature. Mitochondrion. 2016;28:73–78. doi: 10.1016/j.mito.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 240.Finsterer J., Zarrouk-Mahjoub S. NDUFS4-related Leigh syndrome in Hutterites. Am. J. Med. Genet. 2017;173:1450–1451. doi: 10.1002/ajmg.a.38225. [DOI] [PubMed] [Google Scholar]
- 241.Rahman S., Blok R.B., Dahl H.-H.M., Danks D.M., Kirby D.M., Chow C.W., Christodoulou J., Thorburn D.R. Leigh Syndrome: Clinical features and biochemical and DNA abnormalities. Ann. Neurol. 1996;39:343–351. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- 242.Leigh D. Subacute necrotizing encephalomyelopathy in an infant. J. Neurol. Neurosurg. Psychiatry. 1951;14:216–221. doi: 10.1136/jnnp.14.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Assouline Z., Jambou M., Rio M., Bole-Feysot C., de Lonlay P., Barnerias C., Desguerre I., Bonnemains C., Guillermet C., Steffann J., et al. A constant and similar assembly defect of mitochondrial respiratory chain complex I allows rapid identification of NDUFS4 mutations in patients with Leigh syndrome. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012;1822:1062–1069. doi: 10.1016/j.bbadis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 244.Lazarou M., McKenzie M., Ohtake A., Thorburn D.R., Ryan M.T. Analysis of the assembly profiles for mitochondrial—and nuclear-DNA-encoded subunits into complex I. Mol. Cell. Biol. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Breuer M.E., Willems P.H.G.M., Smeitink J.A.M., Koopman W.J.H., Nooteboom M. Cellular and animal models for mitochondrial complex I deficiency: A focus on the NDUFS4 subunit. IUBMB Life. 2013;65:202–208. doi: 10.1002/iub.1127. [DOI] [PubMed] [Google Scholar]
- 246.Ingraham C.A., Burwell L.S., Skalska J., Brookes P.S., Howell R.L., Sheu S.-S., Pinkert C.A. NDUFS4: Creation of a mouse model mimicking a complex I disorder. Mitochondrion. 2009;9:204–210. doi: 10.1016/j.mito.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ma H., Folmes C.D.L., Wu J., Morey R., Mora-Castilla S., Ocampo A., Ma L., Poulton J., Wang X., Ahmed R., et al. Metabolic rescue in pluripotent cells from patients with MtDNA disease. Nature. 2015;524:234–238. doi: 10.1038/nature14546. [DOI] [PubMed] [Google Scholar]
- 248.Galera-Monge T., Zurita-Díaz F., Canals I., Grønning Hansen M., Rufián-Vázquez L., Ehinger J.K., Elmér E., Martin M.A., Garesse R., Ahlenius H., et al. Mitochondrial dysfunction and calcium dysregulation in Leigh syndrome induced pluripotent stem cell derived neurons. Int. J. Mol. Sci. 2020;21:3191. doi: 10.3390/ijms21093191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zheng X., Boyer L., Jin M., Kim Y., Fan W., Bardy C., Berggren T., Evans R.M., Gage F.H., Hunter T. Alleviation of neuronal energy deficiency by MTOR inhibition as a treatment for mitochondria-related neurodegeneration. eLife. 2016;5:e13378. doi: 10.7554/eLife.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lorenz C., Lesimple P., Bukowiecki R., Zink A., Inak G., Mlody B., Singh M., Semtner M., Mah N., Auré K., et al. Human IPSC-derived neural progenitors are an effective drug discovery model for neurological MtDNA disorders. Cell Stem Cell. 2017;20:659–674.e9. doi: 10.1016/j.stem.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 251.Romero-Morales A., Rastogi A., Temuri H., Rasmussen M., McElroy G.S., Hsu L., Almonacid P.M., Milis B.A., Chandel N., Cartailler J.-P., et al. Human iPSC-derived cerebral organoids model features of leigh syndrome and reveal abnormal corticogenesis. Cell Biol. 2020 doi: 10.2139/ssrn.3611282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu Z., Yao J., Johns T., Fu K., Bie I.D., Macmillan C., Cuthbert A.P., Newbold R.F., Wang J., Chevrette M., et al. SURF1, Encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998;20:337–343. doi: 10.1038/3804. [DOI] [PubMed] [Google Scholar]
- 253.Tiranti V., Hoertnagel K., Carrozzo R., Galimberti C., Munaro M., Granatiero M., Zelante L., Gasparini P., Marzella R., Rocchi M., et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 1998;63:1609–1621. doi: 10.1086/302150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saneto R., Ruhoy I. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2014;7:221. doi: 10.2147/TACG.S46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sun F., Huo X., Zhai Y., Wang A., Xu J., Su D., Bartlam M., Rao Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 256.Van Vranken J.G., Na U., Winge D.R., Rutter J. Protein-mediated assembly of succinate dehydrogenase and its cofactors. Crit. Rev. Biochem. Mol. Biol. 2015;50:168–180. doi: 10.3109/10409238.2014.990556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ghezzi D., Goffrini P., Uziel G., Horvath R., Klopstock T., Lochmüller H., D’Adamo P., Gasparini P., Strom T.M., Prokisch H., et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009;41:654–656. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- 258.Munnich A., Rustin P. Clinical spectrum and diagnosis of mitochondrial disorders. Am. J. Med. Genet. 2001;106:4–17. doi: 10.1002/ajmg.1391. [DOI] [PubMed] [Google Scholar]
- 259.Bourgeron T., Rustin P., Chretien D., Birch-Machin M., Bourgeois M., Viegas-Péquignot E., Munnich A., Rötig A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995;11:144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- 260.Parfait B., Chretien D., Rötig A., Marsac C., Munnich A., Rustin P. Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum. Genet. 2000;106:236–243. doi: 10.1007/s004399900218. [DOI] [PubMed] [Google Scholar]
- 261.Van Coster R., Seneca S., Smet J., Van Hecke R., Gerlo E., Devreese B., Van Beeumen J., Leroy J.G., De Meirleir L., Lissens W. Homozygous Gly555Glu mutation in the nuclear-encoded 70 KDa flavoprotein gene causes instability of the respiratory chain complex II. Am. J. Med. Genet. 2003;120A:13–18. doi: 10.1002/ajmg.a.10202. [DOI] [PubMed] [Google Scholar]
- 262.Pagnamenta A.T., Hargreaves I.P., Duncan A.J., Taanman J.-W., Heales S.J., Land J.M., Bitner-Glindzicz M., Leonard J.V., Rahman S. Phenotypic variability of mitochondrial disease caused by a nuclear mutation in complex II. Mol. Genet. Metab. 2006;89:214–221. doi: 10.1016/j.ymgme.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 263.Horvath R., Abicht A., Holinski-Feder E., Laner A., Gempel K., Prokisch H., Lochmüller H., Klopstock T., Jaksch M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) J. Neurol. Neurosurg. Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jain-Ghai S., Cameron J.M., Al Maawali A., Blaser S., MacKay N., Robinson B., Raiman J. Complex II deficiency—A case report and review of the literature. Am. J. Med. Genet. 2013;161:285–294. doi: 10.1002/ajmg.a.35714. [DOI] [PubMed] [Google Scholar]
- 265.Nesti C., Meschini M.C., Meunier B., Sacchini M., Doccini S., Romano A., Petrillo S., Pezzini I., Seddiki N., Rubegni A., et al. Additive effect of nuclear and mitochondrial mutations in a patient with mitochondrial encephalomyopathy. Hum. Mol. Genet. 2015;24:3248–3256. doi: 10.1093/hmg/ddv078. [DOI] [PubMed] [Google Scholar]
- 266.Levitas A., Muhammad E., Harel G., Saada A., Caspi V.C., Manor E., Beck J.C., Sheffield V., Parvari R. Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase. Eur. J. Hum. Genet. 2010;18:1160–1165. doi: 10.1038/ejhg.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Burnichon N., Brière J.-J., Libé R., Vescovo L., Rivière J., Tissier F., Jouanno E., Jeunemaitre X., Bénit P., Tzagoloff A., et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Astuti D., Latif F., Dallol A., Dahia P.L.M., Douglas F., George E., Sköldberg F., Husebye E.S., Eng C., Maher E.R. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Janeway K.A., Kim S.Y., Lodish M., Nosé V., Rustin P., Gaal J., Dahia P.L.M., Liegl B., Ball E.R., Raygada M., et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Baysal B.E., Willett-Brozick J., Filho P., Lawrence E.C., Myers E.N., Ferrell R. An alu-mediated partial SDHC deletion causes familial and sporadic paraganglioma. J. Med. Genet. 2004;41:703–709. doi: 10.1136/jmg.2004.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.McWhinney S.R., Pasini B., Stratakis C.A. Familial gastrointestinal stromal tumors and germ-line mutations. N. Engl. J. Med. 2007;357:1054–1056. doi: 10.1056/NEJMc071191. [DOI] [PubMed] [Google Scholar]
- 272.Baysal B.E., Ferrell R.E., Willett-Brozick J.E., Lawrence E.C., Myssiorek D., Bosch A., Van Der Mey A., Taschner P., Rubinstein W.S., Myers E.N., et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 273.Hao H.-X., Khalimonchuk O., Schraders M., Dephoure N., Bayley J.-P., Kunst H., Devilee P., Cremers C.W.R.J., Schiffman J.D., Bentz B.G., et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sköldberg F., Grimelius L., Woodward E.R., Rorsman F., Van Schothorst E.W., Winqvist O., Karlsson F.A., Åkerström G., Kämpe O., Husebye E.S. A family with hereditary extra-adrenal paragangliomas without evidence for mutations in the von Hippel-Lindau disease or Ret genes: Hereditary extra-adrenal paraganglioma. Clin. Endocrinol. 1998;48:11–16. doi: 10.1046/j.1365-2265.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- 275.Lussey-Lepoutre C., Buffet A., Gimenez-Roqueplo A.-P., Favier J. Mitochondrial deficiencies in the predisposition to paraganglioma. Metabolites. 2017;7:17. doi: 10.3390/metabo7020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ghezzi D., Zeviani M. Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem. 2018;62:271–286. doi: 10.1042/EBC20170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dwight T., Na U., Kim E., Zhu Y., Richardson A.L., Robinson B.G., Tucker K.M., Gill A.J., Benn D.E., Clifton-Bligh R.J., et al. Analysis of SDHAF3 in familial and sporadic pheochromocytoma and paraganglioma. BMC Cancer. 2017;17:497. doi: 10.1186/s12885-017-3486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kudryavtseva A.V., Kalinin D.V., Pavlov V.S., Savvateeva M.V., Fedorova M.S., Pudova E.A., Kobelyatskaya A.A., Golovyuk A.L., Guvatova Z.G., Razmakhaev G.S., et al. Mutation profiling in eight cases of vagal paragangliomas. BMC Med. Genom. 2020;13:115. doi: 10.1186/s12920-020-00763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Iwata S., Lee J.W., Okada K., Lee J.K., Iwata M., Rasmussen B., Link T.A., Ramaswamy S., Jap B.K. Complete structure of the 11-subunit bovine mitochondrial cytochrome Bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 280.Hildenbeutel M., Hegg E.L., Stephan K., Gruschke S., Meunier B., Ott M. Assembly factors monitor sequential hemylation of cytochrome b to regulate mitochondrial translation. J. Cell Biol. 2014;205:511–524. doi: 10.1083/jcb.201401009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tucker E.J., Wanschers B.F.J., Szklarczyk R., Mountford H.S., Wijeyeratne X.W., van den Brand M.A.M., Leenders A.M., Rodenburg R.J., Reljić B., Compton A.G., et al. Mutations in the UQCC1-interacting protein, UQCC2, cause human complex III deficiency associated with perturbed cytochrome b protein expression. PLoS Genet. 2013;9:e1004034. doi: 10.1371/journal.pgen.1004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wanschers B.F.J., Szklarczyk R., van den Brand M.A.M., Jonckheere A., Suijskens J., Smeets R., Rodenburg R.J., Stephan K., Helland I.B., Elkamil A., et al. A mutation in the human CBP4 ortholog UQCC3 impairs complex III assembly, activity and cytochrome b stability. Hum. Mol. Genet. 2014;23:6356–6365. doi: 10.1093/hmg/ddu357. [DOI] [PubMed] [Google Scholar]
- 283.Bottani E., Cerutti R., Harbour M.E., Ravaglia S., Dogan S.A., Giordano C., Fearnley I.M., D’Amati G., Viscomi C., Fernandez-Vizarra E., et al. TTC19 plays a husbandry role on UQCRFS1 turnover in the biogenesis of mitochondrial respiratory complex III. Mol. Cell. 2017;67:96–105. doi: 10.1016/j.molcel.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 284.Atkinson A., Smith P., Fox J.L., Cui T.-Z., Khalimonchuk O., Winge D.R. The LYR protein Mzm1 functions in the insertion of the rieske Fe/S protein in yeast mitochondria. Mol. Cell. Biol. 2011;31:3988–3996. doi: 10.1128/MCB.05673-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cui T.-Z., Smith P.M., Fox J.L., Khalimonchuk O., Winge D.R. Late-stage maturation of the Rieske Fe/S protein: Mzm1 stabilizes Rip1 but does not facilitate its translocation by the AAA ATPase Bcs1. Mol. Cell. Biol. 2012;32:4400–4409. doi: 10.1128/MCB.00441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sánchez E., Lobo T., Fox J.L., Zeviani M., Winge D.R., Fernández-Vizarra E. LYRM7/MZM1L is a UQCRFS1 chaperone involved in the last steps of mitochondrial complex III assembly in human cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013;1827:285–293. doi: 10.1016/j.bbabio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cruciat C.-M., Hell K., Fölsch H., Neupert W., Stuart R.A. Bcs1p, an AAA-family member, is a chaperone for the assembly of the cytochrome Bc1 complex. EMBO J. 1999;18:5226–5233. doi: 10.1093/emboj/18.19.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fernandez-Vizarra E., Bugiani M., Goffrini P., Carrara F., Farina L., Procopio E., Donati A., Uziel G., Ferrero I., Zeviani M. Impaired complex III assembly associated with BCS1L gene mutations in isolated mitochondrial encephalopathy. Hum. Mol. Genet. 2007;16:1241–1252. doi: 10.1093/hmg/ddm072. [DOI] [PubMed] [Google Scholar]
- 289.Tang W.K., Borgnia M.J., Hsu A.L., Esser L., Fox T., de Val N., Xia D. Structures of AAA protein translocase Bcs1 suggest translocation mechanism of a folded protein. Nat. Struct. Mol. Biol. 2020;27:202–209. doi: 10.1038/s41594-020-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wagener N., Ackermann M., Funes S., Neupert W. A pathway of protein translocation in mitochondria mediated by the AAA-ATPase Bcs1. Mol. Cell. 2011;44:191–202. doi: 10.1016/j.molcel.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 291.Peruzzo R., Corrà S., Costa R., Brischigliaro M., Varanita T., Biasutto L., Rampazzo C., Ghezzi D., Leanza L., Zoratti M., et al. Exploiting pyocyanin to treat mitochondrial disease due to respiratory complex III dysfunction. Nat. Commun. 2021;12:2103. doi: 10.1038/s41467-021-22062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Andreu A.L., Bruno C., Dunne T.C., Tanji K., Shanske S., Sue C.M., Krishna S., Hadjigeorgiou G.M., Shtilbans A., Bonilla E., et al. A nonsense mutation (G15059A) in the cytochrome b gene in a patient with exercise intolerance and myoglobinuria. Ann. Neurol. 1999;45:127–130. doi: 10.1002/1531-8249(199901)45:1<127::AID-ART20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 293.Andreu A.L., Hanna M.G., Reichmann H., Bruno C., Penn A.S., Tanji K., Pallotti F., Iwata S., Bonilla E., Lach B., et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 1999;341:1037–1044. doi: 10.1056/NEJM199909303411404. [DOI] [PubMed] [Google Scholar]
- 294.De Coo I.F., Renier W.O., Ruitenbeek W., Ter Laak H.J., Bakker M., Schägger H., Van Oost B.A., Smeets H.J. A 4-base pair deletion in the mitochondrial cytochrome b gene associated with parkinsonism/MELAS overlap syndrome. Ann. Neurol. 1999;45:130–133. doi: 10.1002/1531-8249(199901)45:1<130::AID-ART21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 295.Keightley J.A., Anitori R., Burton M.D., Quan F., Buist N.R.M., Kennaway N.G. Mitochondrial encephalomyopathy and complex III deficiency associated with a stop-codon mutation in the cytochrome b gene. Am. J. Hum. Genet. 2000;67:1400–1410. doi: 10.1086/316900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Andreu A.L., Bruno C., Shanske S., Shtilbans A., Hirano M., Krishna S., Hayward L., Systrom D.S., Brown R.H., DiMauro S. Missense mutation in the MtDNA cytochrome b gene in a patient with myopathy. Neurology. 1998;51:1444–1447. doi: 10.1212/WNL.51.5.1444. [DOI] [PubMed] [Google Scholar]
- 297.Lamantea E., Carrara F., Mariotti C., Morandi L., Tiranti V., Zeviani M. A novel nonsense mutation (Q352X) in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul. Disord. 2002;12:49–52. doi: 10.1016/S0960-8966(01)00244-9. [DOI] [PubMed] [Google Scholar]
- 298.Mancuso M., Filosto M., Stevens J.C., Patterson M., Shanske S., Krishna S., DiMauro S. Mitochondrial myopathy and complex III deficiency in a patient with a new stop-codon mutation (G339X) in the cytochrome b gene. J. Neurol. Sci. 2003;209:61–63. doi: 10.1016/S0022-510X(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 299.Andreu A.L., Checcarelli N., Iwata S., Shanske S., Dimauro S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr. Res. 2000;48:311–314. doi: 10.1203/00006450-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 300.Wibrand F., Ravn K., Schwartz M., Rosenberg T., Horn N., Vissing J. Multisystem disorder associated with a missense mutation in the mitochondrial cytochromeb gene. Ann. Neurol. 2001;50:540–543. doi: 10.1002/ana.1224. [DOI] [PubMed] [Google Scholar]
- 301.Schuelke M., Krude H., Finckh B., Mayatepek E., Janssen A., Schmelz M., Trefz F., Trijbels F., Smeitink J. Septo-optic dysplasia associated with a new mitochondrialcytochrome b mutation. Ann. Neurol. 2002;51:388–392. doi: 10.1002/ana.10151. [DOI] [PubMed] [Google Scholar]
- 302.Ghelli A., Tropeano C.V., Calvaruso M.A., Marchesini A., Iommarini L., Porcelli A.M., Zanna C., De Nardo V., Martinuzzi A., Wibrand F., et al. The cytochrome b p.278Y>C mutation causative of a multisystem disorder enhances superoxide production and alters supramolecular interactions of respiratory chain complexes. Hum. Mol. Genet. 2013;22:2141–2151. doi: 10.1093/hmg/ddt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Carossa V., Ghelli A., Tropeano C.V., Valentino M.L., Iommarini L., Maresca A., Caporali L., La Morgia C., Liguori R., Barboni P., et al. A novel in-frame 18-Bp microdeletion in MT-CYB causes a multisystem disorder with prominent exercise intolerance. Hum. Mutat. 2014;35:954–958. doi: 10.1002/humu.22596. [DOI] [PubMed] [Google Scholar]
- 304.Fernández-Vizarra E., Zeviani M. Nuclear gene mutations as the cause of mitochondrial complex III deficiency. Front. Genet. 2015;6:134. doi: 10.3389/fgene.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ghezzi D., Arzuffi P., Zordan M., Da Re C., Lamperti C., Benna C., D’Adamo P., Diodato D., Costa R., Mariotti C., et al. Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nat. Genet. 2011;43:259–263. doi: 10.1038/ng.761. [DOI] [PubMed] [Google Scholar]
- 306.Invernizzi F., Tigano M., Dallabona C., Donnini C., Ferrero I., Cremonte M., Ghezzi D., Lamperti C., Zeviani M. A homozygous mutation in LYRM 7/ MZM 1 L associated with early onset encephalopathy, lactic acidosis, and severe reduction of mitochondrial complex III activity. Hum. Mutat. 2013;34:1619–1622. doi: 10.1002/humu.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.de Lonlay P., Valnot I., Barrientos A., Gorbatyuk M., Tzagoloff A., Taanman J.-W., Benayoun E., Chrétien D., Kadhom N., Lombès A., et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat. Genet. 2001;29:57–60. doi: 10.1038/ng706. [DOI] [PubMed] [Google Scholar]
- 308.Lin C.-H., Tsai P.-I., Lin H.-Y., Hattori N., Funayama M., Jeon B., Sato K., Abe K., Mukai Y., Takahashi Y., et al. Mitochondrial UQCRC1 mutations cause autosomal dominant parkinsonism with polyneuropathy. Brain. 2020;143:3352–3373. doi: 10.1093/brain/awaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Miyake N., Yano S., Sakai C., Hatakeyama H., Matsushima Y., Shiina M., Watanabe Y., Bartley J., Abdenur J.E., Wang R.Y., et al. Mitochondrial complex III deficiency caused by a homozygous UQCRC2 mutation presenting with neonatal-onset recurrent metabolic decompensation. Hum. Mutat. 2013;34:446–452. doi: 10.1002/humu.22257. [DOI] [PubMed] [Google Scholar]
- 310.Brown M.D., Voljavec A.S., Lott M.T., Torroni A., Yang C.C., Wallace D.C. Mitochondrial DNA complex I and III mutations associated with Leber’s hereditary optic neuropathy. Genetics. 1992;130:163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bouzidi M.F., Schägger H., Collombet J.-M., Carrier H., Flocard F., Quard S., Mousson B., Godinot C. Decreased expression o ubiquinol-cytochrome c reductase subunits in patients exhibiting mitochondrial myopathy with progressive exercise intolerance. Neuromuscul. Disord. 1993;3:599–604. doi: 10.1016/0960-8966(93)90123-2. [DOI] [PubMed] [Google Scholar]
- 312.Gaignard P., Menezes M., Schiff M., Bayot A., Rak M., de Baulny H.O., Su C.-H., Gilleron M., Lombes A., Abida H., et al. Mutations in CYC1, encoding cytochrome C1 subunit of respiratory chain complex III, cause insulin-responsive hyperglycemia. Am. J. Hum. Genet. 2013;93:384–389. doi: 10.1016/j.ajhg.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gusic M., Schottmann G., Feichtinger R.G., Du C., Scholz C., Wagner M., Mayr J.A., Lee C.-Y., Yépez V.A., Lorenz N., et al. Bi-allelic UQCRFS1 variants are associated with mitochondrial complex iii deficiency, cardiomyopathy, and alopecia totalis. Am. J. Hum. Genet. 2020;106:102–111. doi: 10.1016/j.ajhg.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Haut S., Brivet M., Touati G., Rustin P., Lebon S., Garcia-Cazorla A., Saudubray J.M., Boutron A., Legrand A., Slama A. A deletion in the human QP-C gene causes a complex III deficiency resulting in hypoglycaemia and lactic acidosis. Hum. Genet. 2003;113:118–122. doi: 10.1007/s00439-003-0946-0. [DOI] [PubMed] [Google Scholar]
- 315.Barel O., Shorer Z., Flusser H., Ofir R., Narkis G., Finer G., Shalev H., Nasasra A., Saada A., Birk O.S. Mitochondrial complex III deficiency associated with a homozygous mutation in UQCRQ. Am. J. Hum. Genet. 2008;82:1211–1216. doi: 10.1016/j.ajhg.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Feichtinger R.G., Brunner-Krainz M., Alhaddad B., Wortmann S.B., Kovacs-Nagy R., Stojakovic T., Erwa W., Resch B., Windischhofer W., Verheyen S., et al. Combined respiratory chain deficiency and UQCC2 mutations in neonatal encephalomyopathy: Defective supercomplex assembly in complex III deficiencies. Oxidative Med. Cell. Longev. 2017;2017:7202589. doi: 10.1155/2017/7202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hausman-Kedem M., Ben-Shachar S., Menascu S., Geva K., Sagie L., Fattal-Valevski A. VPS53 gene is associated with a new phenotype of complicated hereditary spastic paraparesis. Neurogenetics. 2019;20:187–195. doi: 10.1007/s10048-019-00586-1. [DOI] [PubMed] [Google Scholar]
- 318.Baker R.A., Priestley J.R.C., Wilstermann A.M., Reese K.J., Mark P.R. Clinical spectrum of BCS1L mitopathies and their underlying structural relationships. Am. J. Med. Genet. 2019;179:373–380. doi: 10.1002/ajmg.a.61019. [DOI] [PubMed] [Google Scholar]
- 319.Visapää I., Fellman V., Varilo T., Palotie A., Raivio K.O., Peltonen L. Assignment of the locus for a new lethal neonatal metabolic syndrome to 2q33-37. Am. J. Hum. Genet. 1998;63:1396–1403. doi: 10.1086/302123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Siddiqi S., Siddiq S., Mansoor A., Oostrik J., Ahmad N., Kazmi S.A.R., Kremer H., Qamar R., Schraders M. Novel mutation in AAA domain of BCS1L causing Bjornstad syndrome. J. Hum. Genet. 2013;58:819–821. doi: 10.1038/jhg.2013.101. [DOI] [PubMed] [Google Scholar]
- 321.Hinson J.T., Fantin V.R., Schönberger J., Breivik N., Siem G., McDonough B., Sharma P., Keogh I., Godinho R., Santos F., et al. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N. Engl. J. Med. 2007;356:809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- 322.Gil-Borlado M.C., González-Hoyuela M., Blázquez A., García-Silva M.T., Gabaldón T., Manzanares J., Vara J., Martín M.A., Seneca S., Arenas J., et al. Pathogenic Mutations in the 5′ untranslated region of BCS1L MRNA in mitochondrial complex III deficiency. Mitochondrion. 2009;9:299–305. doi: 10.1016/j.mito.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 323.Blázquez A., Gil-Borlado M.C., Morán M., Verdú A., Cazorla-Calleja M.R., Martín M.A., Arenas J., Ugalde C. Infantile mitochondrial encephalomyopathy with unusual phenotype caused by a novel BCS1L mutation in an isolated complex III-deficient patient. Neuromuscul. Disord. 2009;19:143–146. doi: 10.1016/j.nmd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 324.Dallabona C., Abbink T.E.M., Carrozzo R., Torraco A., Legati A., van Berkel C.G.M., Niceta M., Langella T., Verrigni D., Rizza T., et al. LYRM7 mutations cause a multifocal cavitating leukoencephalopathy with distinct MRI appearance. Brain. 2016;139:782–794. doi: 10.1093/brain/awv392. [DOI] [PubMed] [Google Scholar]
- 325.Hempel M., Kremer L.S., Tsiakas K., Alhaddad B., Haack T.B., Löbel U., Feichtinger R.G., Sperl W., Prokisch H., Mayr J.A., et al. LYRM7—Associated complex III deficiency: A clinical, molecular genetic, MR tomographic, and biochemical study. Mitochondrion. 2017;37:55–61. doi: 10.1016/j.mito.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 326.Kremer L.S., L’hermitte-Stead C., Lesimple P., Gilleron M., Filaut S., Jardel C., Haack T.B., Strom T.M., Meitinger T., Azzouz H., et al. Severe respiratory complex III defect prevents liver adaptation to prolonged Fasting. J. Hepatol. 2016;65:377–385. doi: 10.1016/j.jhep.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Morino H., Miyamoto R., Ohnishi S., Maruyama H., Kawakami H. Exome sequencing reveals a novel TTC19 mutation in an autosomal recessive spinocerebellar ataxia patient. BMC Neurol. 2014;14:5. doi: 10.1186/1471-2377-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nogueira C., Barros J., Sá M.J., Azevedo L., Taipa R., Torraco A., Meschini M.C., Verrigni D., Nesti C., Rizza T., et al. Novel TTC19 mutation in a family with severe psychiatric manifestations and complex III deficiency. Neurogenetics. 2013;14:153–160. doi: 10.1007/s10048-013-0361-1. [DOI] [PubMed] [Google Scholar]
- 329.Habibzadeh P., Inaloo S., Silawi M., Dastsooz H., Farazi Fard M.A., Sadeghipour F., Faghihi Z., Rezaeian M., Yavarian M., Böhm J., et al. A novel TTC19 mutation in a patient with neurological, psychological, and gastrointestinal impairment. Front. Neurol. 2019;10:944. doi: 10.3389/fneur.2019.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mordaunt D.A., Jolley A., Balasubramaniam S., Thorburn D.R., Mountford H.S., Compton A.G., Nicholl J., Manton N., Clark D., Bratkovic D., et al. Phenotypic variation of TTC19—deficient mitochondrial complex III deficiency: A case report and literature review. Am. J. Med. Genet. 2015;167:1330–1336. doi: 10.1002/ajmg.a.36968. [DOI] [PubMed] [Google Scholar]
- 331.Balsa E., Marco R., Perales-Clemente E., Szklarczyk R., Calvo E., Landázuri M.O., Enríquez J.A. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012;16:378–386. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 332.Pitceathly R.D.S., Rahman S., Wedatilake Y., Polke J.M., Cirak S., Foley A.R., Sailer A., Hurles M.E., Stalker J., Hargreaves I., et al. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 2013;3:1795–1805. doi: 10.1016/j.celrep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zong S., Wu M., Gu J., Liu T., Guo R., Yang M. Structure of the intact 14-subunit human cytochrome c oxidase. Cell Res. 2018;28:1026–1034. doi: 10.1038/s41422-018-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hill B.C. The sequence of electron carriers in the reaction of cytochromec oxidase with oxygen. J. Bioenerg. Biomembr. 1993;25:115–120. doi: 10.1007/BF00762853. [DOI] [PubMed] [Google Scholar]
- 335.Sharma V., Ala-Vannesluoma P., Vattulainen I., Wikström M., Róg T. Role of subunit III and its lipids in the molecular mechanism of cytochrome c oxidase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015;1847:690–697. doi: 10.1016/j.bbabio.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 336.Sinkler C.A., Kalpage H., Shay J., Lee I., Malek M.H., Grossman L.I., Hüttemann M. Tissue- and condition-specific isoforms of mammalian cytochrome c oxidase subunits: From function to human disease. Oxidative Med. Cell. Longev. 2017;2017:1534056. doi: 10.1155/2017/1534056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kadenbach B., Hüttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion. 2015;24:64–76. doi: 10.1016/j.mito.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 338.Nijtmans L.G.J., Taanman J.-W., Muijsers A.O., Speijer D., Van den Bogert C. Assembly of cytochrome-c oxidase in cultured human cells. Eur. J. Biochem. 1998;254:389–394. doi: 10.1046/j.1432-1327.1998.2540389.x. [DOI] [PubMed] [Google Scholar]
- 339.Vidoni S., Harbour M.E., Guerrero-Castillo S., Signes A., Ding S., Fearnley I.M., Taylor R.W., Tiranti V., Arnold S., Fernandez-Vizarra E., et al. MR-1S interacts with pet100 and pet117 in module-based assembly of human cytochrome c oxidase. Cell Rep. 2017;18:1727–1738. doi: 10.1016/j.celrep.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 340.Rak M., Bénit P., Chrétien D., Bouchereau J., Schiff M., El-Khoury R., Tzagoloff A., Rustin P. Mitochondrial cytochrome c oxidase deficiency. Clin. Sci. 2016;130:393–407. doi: 10.1042/CS20150707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Massa V., Fernandez-Vizarra E., Alshahwan S., Bakhsh E., Goffrini P., Ferrero I., Mereghetti P., D’Adamo P., Gasparini P., Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 2008;82:1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Abdulhag U.N., Soiferman D., Schueler-Furman O., Miller C., Shaag A., Elpeleg O., Edvardson S., Saada A. Mitochondrial complex IV deficiency, caused by mutated COX6B1, is associated with encephalomyopathy, hydrocephalus and cardiomyopathy. Eur. J. Hum. Genet. 2015;23:159–164. doi: 10.1038/ejhg.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lamperti C., Diodato D., Lamantea E., Carrara F., Ghezzi D., Mereghetti P., Rizzi R., Zeviani M. MELAS-like encephalomyopathy caused by a new pathogenic mutation in the mitochondrial DNA encoded cytochrome c oxidase subunit I. Neuromuscul. Disord. 2012;22:990–994. doi: 10.1016/j.nmd.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 344.Valente L., Piga D., Lamantea E., Carrara F., Uziel G., Cudia P., Zani A., Farina L., Morandi L., Mora M., et al. Identification of novel mutations in five patients with mitochondrial encephalomyopathy. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009;1787:491–501. doi: 10.1016/j.bbabio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 345.Comi G.P., Bordoni A., Salani S., Franceschina L., Sciacco M., Prelle A., Fortunato F., Zeviani M., Napoli L., Bresolin N., et al. Cytochromec oxidase subunit I microdeletion in a patient with motor neuron disease. Ann. Neurol. 1998;43:110–116. doi: 10.1002/ana.410430119. [DOI] [PubMed] [Google Scholar]
- 346.D’Aurelio M., Pallotti F., Barrientos A., Gajewski C.D., Kwong J.Q., Bruno C., Beal M.F., Manfredi G. In vivo regulation of oxidative phosphorylation in cells harboring a stop-codon mutation in mitochondrial DNA-encoded cytochrome c oxidase subunit I. J. Biol. Chem. 2001;276:46925–46932. doi: 10.1074/jbc.M106429200. [DOI] [PubMed] [Google Scholar]
- 347.Nishigaki Y., Ueno H., Coku J., Koga Y., Fujii T., Sahashi K., Nakano K., Yoneda M., Nonaka M., Tang L., et al. Extensive screening system using suspension array technology to detect mitochondrial DNA point mutations. Mitochondrion. 2010;10:300–308. doi: 10.1016/j.mito.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Clark K.M., Taylor R.W., Johnson M.A., Chinnery P.F., Chrzanowska-Lightowlers Z.M.A., Andrews R.M., Nelson I.P., Wood N.W., Lamont P.J., Hanna M.G., et al. An MtDNA mutation in the initiation codon of the cytochrome c oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am. J. Hum. Genet. 1999;64:1330–1339. doi: 10.1086/302361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Abu-Amero K.K., Bosley T.M. Mitochondrial abnormalities in patients with LHON-like optic neuropathies. Investig. Opthalmol. Vis. Sci. 2006;47:4211–4220. doi: 10.1167/iovs.06-0295. [DOI] [PubMed] [Google Scholar]
- 350.Rahman S., Taanman J.-W., Cooper J.M., Nelson I., Hargreaves I., Meunier B., Hanna M.G., García J.J., Capaldi R.A., Lake B.D., et al. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am. J. Hum. Genet. 1999;65:1030–1039. doi: 10.1086/302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wei Y.-L., Yu C.-A., Yang P., Li A.-L., Wen J.-Y., Zhao S.-M., Liu H.-X., Ke Y.-N., Campbell W., Zhang Y.-G., et al. Novel mitochondrial DNA mutations associated with chinese familial hypertrophic cardiomyopathy. Clin. Exp. Pharmacol. Physiol. 2009;36:933–939. doi: 10.1111/j.1440-1681.2009.05183.x. [DOI] [PubMed] [Google Scholar]
- 352.Tabebi M., Mkaouar-Rebai E., Mnif M., Kallabi F., Ben Mahmoud A., Ben Saad W., Charfi N., Keskes-Ammar L., Kamoun H., Abid M., et al. A novel mutation MT-COIII m.9267G>C and MT-COI m.5913G>A mutation in mitochondrial genes in a Tunisian family with maternally inherited diabetes and deafness (MIDD) associated with sever nephropathy. Biochem. Biophys. Res. Commun. 2015;459:353–360. doi: 10.1016/j.bbrc.2015.01.151. [DOI] [PubMed] [Google Scholar]
- 353.Horvath R., Scharfe C., Hoeltzenbein M., Do B.H., Schröder C., Warzok R., Vogelgesang S., Lochmüller H., Müller-Höcker J., Gerbitz K.D., et al. Childhood onset mitochondrial myopathy and lactic acidosis caused by a stop mutation in the mitochondrial cytochrome c oxidase III gene. J. Med. Genet. 2002;39:812–816. doi: 10.1136/jmg.39.11.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mkaouar-Rebai E., Ellouze E., Chamkha I., Kammoun F., Triki C., Fakhfakh F. Molecular-clinical correlation in a family with a novel heteroplasmic Leigh syndrome missense mutation in the mitochondrial cytochrome c oxidase III gene. J. Child Neurol. 2011;26:12–20. doi: 10.1177/0883073810371227. [DOI] [PubMed] [Google Scholar]
- 355.Bosley T.M., Brodsky M.C., Glasier C.M., Abu-Amero K.K. Sporadic bilateral optic neuropathy in children: The role of mitochondrial abnormalities. Investig. Opthalmol. Vis. Sci. 2008;49:5250. doi: 10.1167/iovs.08-2193. [DOI] [PubMed] [Google Scholar]
- 356.Marotta R., Chin J., Kirby D.M., Chiotis M., Cook M., Collins S.J. Novel single base pair COX III subunit deletion of mitochondrial DNA associated with rhabdomyolysis. J. Clin. Neurosci. 2011;18:290–292. doi: 10.1016/j.jocn.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 357.Hanna M.G., Nelson I.P., Rahman S., Lane R.J.M., Land J., Heales S., Cooper M.J., Schapira A.H.V., Morgan-Hughes J.A., Wood N.W. Cytochrome c oxidase deficiency associated with the first stop-codon point mutation in human MtDNA. Am. J. Hum. Genet. 1998;63:29–36. doi: 10.1086/301910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Abu-Libdeh B., Douiev L., Amro S., Shahrour M., Ta-Shma A., Miller C., Elpeleg O., Saada A. Mutation in the COX4I1 gene is associated with short stature, poor weight gain and increased chromosomal breaks, simulating fanconi anemia. Eur. J. Hum. Genet. 2017;25:1142–1146. doi: 10.1038/ejhg.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pillai N.R., AlDhaheri N.S., Ghosh R., Lim J., Streff H., Nayak A., Graham B.H., Hanchard N.A., Elsea S.H., Scaglia F. Biallelic variants in COX4I1 associated with a novel phenotype resembling Leigh syndrome with developmental regression, intellectual disability, and seizures. Am. J. Med. Genet. 2019;179:2138–2143. doi: 10.1002/ajmg.a.61288. [DOI] [PubMed] [Google Scholar]
- 360.Shteyer E., Saada A., Shaag A., Al-Hijawi F.A., Kidess R., Revel-Vilk S., Elpeleg O. Exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis are caused by a mutation in the COX4I2 gene. Am. J. Hum. Genet. 2009;84:412–417. doi: 10.1016/j.ajhg.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Baertling F., Al-Murshedi F., Sánchez-Caballero L., Al-Senaidi K., Joshi N.P., Venselaar H., van den Brand M.A., Nijtmans L.G., Rodenburg R.J. Mutation in mitochondrial complex IV subunit COX5A causes pulmonary arterial hypertension, lactic acidemia, and failure to thrive. Hum. Mutat. 2017;38:692–703. doi: 10.1002/humu.23210. [DOI] [PubMed] [Google Scholar]
- 362.Tamiya G., Makino S., Hayashi M., Abe A., Numakura C., Ueki M., Tanaka A., Ito C., Toshimori K., Ogawa N., et al. A mutation of COX6A1 causes a recessive axonal or mixed form of charcot-marie-tooth disease. Am. J. Hum. Genet. 2014;95:294–300. doi: 10.1016/j.ajhg.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Inoue M., Uchino S., Iida A., Noguchi S., Hayashi S., Takahashi T., Fujii K., Komaki H., Takeshita E., Nonaka I., et al. COX6A2 variants cause a muscle-specific cytochrome c oxidase deficiency. Ann. Neurol. 2019;86:193–202. doi: 10.1002/ana.25517. [DOI] [PubMed] [Google Scholar]
- 364.Vondrackova A., Vesela K., Hansikova H., Docekalova D.Z., Rozsypalova E., Zeman J., Tesarova M. High-resolution melting analysis of 15 genes in 60 patients with cytochrome-c oxidase deficiency. J. Hum. Genet. 2012;57:442–448. doi: 10.1038/jhg.2012.49. [DOI] [PubMed] [Google Scholar]
- 365.Indrieri A., van Rahden V.A., Tiranti V., Morleo M., Iaconis D., Tammaro R., D’Amato I., Conte I., Maystadt I., Demuth S., et al. Mutations in COX7B cause microphthalmia with linear skin lesions, an unconventional mitochondrial disease. Am. J. Hum. Genet. 2012;91:942–949. doi: 10.1016/j.ajhg.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hallmann K., Kudin A.P., Zsurka G., Kornblum C., Reimann J., Stüve B., Waltz S., Hattingen E., Thiele H., Nürnberg P., et al. Loss of the smallest subunit of cytochrome c oxidase, COX8A, causes Leigh-like syndrome and epilepsy. Brain. 2016;139:338–345. doi: 10.1093/brain/awv357. [DOI] [PubMed] [Google Scholar]
- 367.Echaniz-Laguna A., Ghezzi D., Chassagne M., Mayencon M., Padet S., Melchionda L., Rouvet I., Lannes B., Bozon D., Latour P., et al. SURF1 deficiency causes demyelinating charcot-marie-tooth disease. Neurology. 2013;81:1523–1530. doi: 10.1212/WNL.0b013e3182a4a518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Valnot I., Von Kleist-Retzow J.-C., Barrientos A., Gorbatyuk M., Taanman J.-W., Mehaye B., Rustin P., Tzagoloff A., Munnich A., Rotig A. A mutation in the human heme A: Farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- 369.Antonicka H., Pankratz N., Nichols W.C., Uniacke S.K., Halter C., Murrell J., Rudolph A., Shults C.W., Conneally P.M., Foroud T. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003;12:2693–2702. doi: 10.1093/hmg/ddg284. [DOI] [PubMed] [Google Scholar]
- 370.Coenen M.J.H., van den Heuvel L.P., Ugalde C., ten Brinke M., Nijtmans L.G.J., Trijbels F.J.M., Beblo S., Maier E.M., Muntau A.C., Smeitink J.A.M. Cytochromec oxidase biogenesis in a patient with a mutation in COX10 gene. Ann. Neurol. 2004;56:560–564. doi: 10.1002/ana.20229. [DOI] [PubMed] [Google Scholar]
- 371.Antonicka H., Mattman A., Carlson C.G., Glerum D.M., Hoffbuhr K.C., Leary S.C., Kennaway N.G., Shoubridge E.A. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003;72:101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Alfadhel M., Lillquist Y.P., Waters P.J., Sinclair G., Struys E., McFadden D., Hendson G., Hyams L., Shoffner J., Vallance H.D. Infantile cardioencephalopathy due to a COX15 gene defect: Report and review. Am. J. Med. Genet. 2011;155:840–844. doi: 10.1002/ajmg.a.33881. [DOI] [PubMed] [Google Scholar]
- 373.Bugiani M., Tiranti V., Farina L., Uziel G., Zeviani M. Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency. J. Med. Genet. 2005;42:e28. doi: 10.1136/jmg.2004.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Fernández-Vizarra E., Tiranti V., Zeviani M. Assembly of the oxidative phosphorylation system in humans: What we have learned by studying its defects. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009;1793:200–211. doi: 10.1016/j.bbamcr.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 375.Leary S.C., Antonicka H., Sasarman F., Weraarpachai W., Cobine P.A., Pan M., Brown G.K., Brown R., Majewski J., Ha K.C.H., et al. Novel mutations in SCO1 as a cause of fatal infantile encephalopathy and lactic acidosis. Hum. Mutat. 2013;34:1366–1370. doi: 10.1002/humu.22385. [DOI] [PubMed] [Google Scholar]
- 376.Brix N., Jensen J.M., Pedersen I.S., Ernst A., Frost S., Bogaard P., Petersen M.B., Bender L. Mitochondrial disease caused by a novel homozygous mutation (Gly106del) in the SCO1 gene. Neonatology. 2019;116:290–294. doi: 10.1159/000499488. [DOI] [PubMed] [Google Scholar]
- 377.Papadopoulou L.C., Sue C.M., Davidson M.M., Tanji K., Nishino I., Sadlock J.E., Krishna S., Walker W., Selby J., Glerum D.M., et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 378.Jaksch M. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:795–801. doi: 10.1093/hmg/9.5.795. [DOI] [PubMed] [Google Scholar]
- 379.Sue C.M., Karadimas C., Checcarelli N., Tanji K., Papadopoulou L.C., Pallotti F., Guo F.L., Shanske S., Hirano M., De Vivo D.C., et al. Differential features of patients with mutations in two COX assembly genes, SURF-1 and SCO2. Ann. Neurol. 2000;47:589–595. doi: 10.1002/1531-8249(200005)47:5<589::AID-ANA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 380.Jaksch M., Horvath R., Horn N., Auer D.P., Macmillan C., Peters J., Gerbitz K.-D., Kraegeloh-Mann I., Muntau A., Karcagi V., et al. Homozygosity (E140K) in SCO2 causes delayed infantile onset of cardiomyopathy and neuropathy. Neurology. 2001;57:1440–1446. doi: 10.1212/WNL.57.8.1440. [DOI] [PubMed] [Google Scholar]
- 381.Pronicki M., Kowalski P., Piekutowska-Abramczuk D., Taybert J., Karkucinska-Wieckowska A., Szymanska-Debinska T., Karczmarewicz E., Pajdowska M., Migdal M., Milewska-Bobula B., et al. A homozygous mutation in the SCO2 gene causes a spinal muscular atrophy like presentation with stridor and respiratory insufficiency. Eur. J. Paediatr. Neurol. 2010;14:253–260. doi: 10.1016/j.ejpn.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 382.Pronicka E., Piekutowska-Abramczuk D., Szymańska-Dębińska T., Bielecka L., Kowalski P., Łuczak S., Karkucińska-Więckowska A., Migdał M., Kubalska J., Zimowski J., et al. The natural history of SCO2 deficiency in 36 Polish children confirmed the genotype-phenotype correlation. Mitochondrion. 2013;13:810–816. doi: 10.1016/j.mito.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 383.Rebelo A.P., Saade D., Pereira C.V., Farooq A., Huff T.C., Abreu L., Moraes C.T., Mnatsakanova D., Mathews K., Yang H., et al. SCO2 mutations cause early-onset axonal charcot-marie-tooth disease associated with cellular copper deficiency. Brain. 2018;141:662–672. doi: 10.1093/brain/awx369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Barcia G., Assouline Z., Pennisi A., Gitiaux C., Schiff M., Boddaert N., Munnich A., Bonnefont J.-P., Rötig A. Cytochrome c oxidase deficiency caused by biallelic SCO2 mutations in two sibs with cerebellar ataxia and progressive peripheral axonal neuropathy. Mol. Genet. Metab. Rep. 2019;21:100528. doi: 10.1016/j.ymgmr.2019.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ghosh A., Trivedi P.P., Timbalia S.A., Griffin A.T., Rahn J.J., Chan S.S.L., Gohil V.M. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of coa6 deficiency. Hum. Mol. Genet. 2014;23:3596–3606. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Baertling F., Brand M.A.V.D., Hertecant J.L., Al-Shamsi A., Heuvel L.P.V.D., Distelmaier F., Mayatepek E., Smeitink J.A., Nijtmans L.G., Rodenburg R. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum. Mutat. 2015;36:34–38. doi: 10.1002/humu.22715. [DOI] [PubMed] [Google Scholar]
- 387.Salvador-Severo K., Gómez-Caudillo L., Quezada H., de García-Trejo J.J., Cárdenas-Conejo A., Vázquez-Memije M.E., Minauro-Sanmiguel F. Mitochondrial proteomic profile of complex IV deficiency fibroblasts: Rearrangement of oxidative phosphorylation complex/supercomplex and other metabolic pathways. Bol. Méd. Del Hosp. Infant. México. 2017;74:175–180. doi: 10.1016/j.bmhimx.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 388.Weraarpachai W., Antonicka H., Sasarman F., Seeger J., Schrank B., Kolesar J.E., Lochmüller H., Chevrette M., Kaufman B.A., Horvath R., et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset leigh syndrome. Nat. Genet. 2009;41:833–837. doi: 10.1038/ng.390. [DOI] [PubMed] [Google Scholar]
- 389.Oktay Y., Güngör S., Zeltner L., Wiethoff S., Schöls L., Sonmezler E., Yilmaz E., Munro B., Bender B., Kernstock C., et al. Confirmation of TACO1 as a Leigh syndrome disease gene in two additional families. J. Neuromuscul. Dis. 2020;7:301–308. doi: 10.3233/JND-200510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Xu F., Morin C., Mitchell G., Ackerley C., Robinson B.H. The role of the LRPPRC (Leucine-Rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: Mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III MRNA. Biochem. J. 2004;382:331–336. doi: 10.1042/BJ20040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Antonicka H., Shoubridge E.A. Mitochondrial RNA granules are centers for posttranscriptional RNA processing and ribosome biogenesis. Cell Rep. 2015;10:920–932. doi: 10.1016/j.celrep.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 392.Ghezzi D., Saada A., D’Adamo P., Fernandez-Vizarra E., Gasparini P., Tiranti V., Elpeleg O., Zeviani M. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 2008;83:415–423. doi: 10.1016/j.ajhg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wei X., Du M., Li D., Wen S., Xie J., Li Y., Chen A., Zhang K., Xu P., Jia M., et al. Mutations in FASTKD2 are associated with mitochondrial disease with multi-OXPHOS deficiency. Hum. Mutat. 2020;41:961–972. doi: 10.1002/humu.23985. [DOI] [PubMed] [Google Scholar]
- 394.Nobrega M.P., Nobrega F.G., Tzagoloff A. COX10 codes for a protein homologous to the ORF1 product of paracoccus denitrificans and is required for the synthesis of yeast cytochrome oxidase. J. Biol. Chem. 1990;265:14220–14226. doi: 10.1016/S0021-9258(18)77289-X. [DOI] [PubMed] [Google Scholar]
- 395.Valnot I., Osmond S., Gigarel N., Mehaye B., Amiel J., Cormier-Daire V., Munnich A., Bonnefont J.-P., Rustin P., Rötig A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am. J. Hum. Genet. 2000;67:1104–1109. doi: 10.1016/S0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Glerum D.M., Muroff I., Jin C., Tzagoloff A. COX15 codes for a mitochondrial protein essential for the assembly of yeast cytochrome oxidase. J. Biol. Chem. 1997;272:19088–19094. doi: 10.1074/jbc.272.30.19088. [DOI] [PubMed] [Google Scholar]
- 397.Oquendo C.E., Antonicka H., Shoubridge E., Reardon W., Brown G.K. Functional and genetic studies demonstrate that mutation in the COX15 gene can cause Leigh syndrome. J. Med. Genet. 2004;41:540–544. doi: 10.1136/jmg.2003.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Smith D., Gray J., Mitchell L., Antholine W.E., Hosler J.P. Assembly of cytochrome-c oxidase in the absence of assembly protein Surf1p leads to loss of the active site heme. J. Biol. Chem. 2005;280:17652–17656. doi: 10.1074/jbc.C500061200. [DOI] [PubMed] [Google Scholar]
- 399.Huigsloot M., Nijtmans L.G., Szklarczyk R., Baars M.J.H., van den Brand M.A.M., HendriksFranssen M.G.M., van den Heuvel L.P., Smeitink J.A.M., Huynen M.A., Rodenburg R.J.T. A mutation in C2orf64 causes impaired cytochrome c oxidase assembly and mitochondrial cardiomyopathy. Am. J. Hum. Genet. 2011;88:488–493. doi: 10.1016/j.ajhg.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Stroud D.A., Maher M.J., Lindau C., Vögtle F.-N., Frazier A.E., Surgenor E., Mountford H., Singh A.P., Bonas M., Oeljeklaus S., et al. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of MtDNA-encoded COX2. Hum. Mol. Genet. 2015;24:5404–5415. doi: 10.1093/hmg/ddv265. [DOI] [PubMed] [Google Scholar]
- 401.Leary S.C., Kaufman B.A., Pellecchia G., Guercin G.-H., Mattman A., Jaksch M., Shoubridge E.A. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 402.Stiburek L., Vesela K., Hansikova H., Hulkova H., Zeman J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am. J. Physiol. Cell Physiol. 2009;296:C1218–C1226. doi: 10.1152/ajpcell.00564.2008. [DOI] [PubMed] [Google Scholar]
- 403.Hiser L., Di Valentin M., Hamer A.G., Hosler J.P. Cox11p is required for stable formation of the CuBand magnesium centers of cytochrome c oxidase. J. Biol. Chem. 2000;275:619–623. doi: 10.1074/jbc.275.1.619. [DOI] [PubMed] [Google Scholar]
- 404.Cerqua C., Morbidoni V., Desbats M.A., Doimo M., Frasson C., Sacconi S., Baldoin M.C., Sartori G., Basso G., Salviati L., et al. COX16 is required for assembly of cytochrome c oxidase in human cells and is involved in copper delivery to COX2. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018;1859:244–252. doi: 10.1016/j.bbabio.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 405.Carlson C.G., Barrientos A., Tzagoloff A., Glerum D.M. Cox16 encodes a novel protein required for the assembly of cytochrome oxidase in saccharomyces cerevisiae. J. Biol. Chem. 2003;278:3770–3775. doi: 10.1074/jbc.M209893200. [DOI] [PubMed] [Google Scholar]
- 406.Glerum D.M., Shtanko A., Tzagoloff A. Characterization of, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 1996;271:14504–14509. doi: 10.1074/jbc.271.24.14504. [DOI] [PubMed] [Google Scholar]
- 407.Bode M., Woellhaf M.W., Bohnert M., van der Laan M., Sommer F., Jung M., Zimmermann R., Schroda M., Herrmann J.M. Redox-regulated dynamic interplay between Cox19 and the copper-binding protein Cox11 in the intermembrane space of mitochondria facilitates biogenesis of cytochrome c oxidase. Mol. Biol Cell. 2015;26:2385–2401. doi: 10.1091/mbc.E14-11-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nobrega M.P., Bandeira S.C.B., Beers J., Tzagoloff A. Characterization of COX19, a widely distributed gene required for expression of mitochondrial cytochrome oxidase. J. Biol. Chem. 2002;277:40206–40211. doi: 10.1074/jbc.M207348200. [DOI] [PubMed] [Google Scholar]
- 409.Naess K., Bruhn H., Stranneheim H., Freyer C., Wibom R., Mourier A., Engvall M., Nennesmo I., Lesko N., Wredenberg A., et al. Clinical presentation, genetic etiology, and coenzyme Q10 levels in 55 children with combined enzyme deficiencies of the mitochondrial respiratory chain. J. Pediatr. 2021;228:240–251.e2. doi: 10.1016/j.jpeds.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 410.Szklarczyk R., Wanschers B.F.J., Nijtmans L.G., Rodenburg R.J., Zschocke J., Dikow N., van den Brand M.A.M., Hendriks-Franssen M.G.M., Gilissen C., Veltman J.A., et al. A mutation in the FAM36A gene, the human ortholog of COX20, impairs cytochrome c oxidase assembly and is associated with ataxia and muscle hypotonia. Hum. Mol. Genet. 2013;22:656–667. doi: 10.1093/hmg/dds473. [DOI] [PubMed] [Google Scholar]
- 411.Doss S., Lohmann K., Seibler P., Arns B., Klopstock T., Zühlke C., Freimann K., Winkler S., Lohnau T., Drungowski M., et al. Recessive dystonia-ataxia syndrome in a Turkish family caused by a COX20 (FAM36A) mutation. J. Neurol. 2014;261:207–212. doi: 10.1007/s00415-013-7177-7. [DOI] [PubMed] [Google Scholar]
- 412.Clemente P., Peralta S., Cruz-Bermudez A., Echevarría L., Fontanesi F., Barrientos A., Fernandez-Moreno M.A., Garesse R. HCOA3 stabilizes cytochrome c oxidase 1 (COX1) and promotes cytochrome c oxidase assembly in human mitochondria*. J. Biol. Chem. 2013;288:8321–8331. doi: 10.1074/jbc.M112.422220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Mick D.U., Vukotic M., Piechura H., Meyer H.E., Warscheid B., Deckers M., Rehling P. Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J. Cell Biol. 2010;191:141–154. doi: 10.1083/jcb.201007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Mick D.U., Dennerlein S., Wiese H., Reinhold R., Pacheu-Grau D., Lorenzi I., Sasarman F., Weraarpachai W., Shoubridge E.A., Warscheid B., et al. MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell. 2012;151:1528–1541. doi: 10.1016/j.cell.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 415.Ostergaard E., Weraarpachai W., Ravn K., Born A.P., Jønson L., Duno M., Wibrand F., Shoubridge E.A., Vissing J. Mutations in COA3 cause isolated complex IV deficiency associated with neuropathy, exercise intolerance, obesity, and short stature. J. Med. Genet. 2015;52:203–207. doi: 10.1136/jmedgenet-2014-102914. [DOI] [PubMed] [Google Scholar]
- 416.Higuchi Y., Okunushi R., Hara T., Hashiguchi A., Yuan J., Yoshimura A., Murayama K., Ohtake A., Ando M., Hiramatsu Y., et al. Mutations in COA7 cause spinocerebellar ataxia with axonal neuropathy. Brain. 2018;141:1622–1636. doi: 10.1093/brain/awy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Weraarpachai W., Sasarman F., Nishimura T., Antonicka H., Auré K., Rötig A., Lombès A., Shoubridge E.A. Mutations in C12orf62, a factor that couples COX I synthesis with cytochrome c oxidase assembly, cause fatal neonatal lactic acidosis. Am. J. Hum. Genet. 2012;90:142–151. doi: 10.1016/j.ajhg.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Bourens M., Barrientos A. A CMC 1 knockout reveals translation-independent control of human mitochondrial complex IV biogenesis. EMBO Rep. 2017;18:477–494. doi: 10.15252/embr.201643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Hell K., Tzagoloff A., Neupert W., Stuart R.A. Identification of Cox20p, a novel protein involved in the maturation and assembly of cytochrome oxidase subunit 2. J. Biol. Chem. 2000;275:4571–4578. doi: 10.1074/jbc.275.7.4571. [DOI] [PubMed] [Google Scholar]
- 420.Church C., Chapon C., Poyton R.O. Cloning and characterization of PET100, a gene required for the assembly of yeast cytochrome c oxidase. J. Biol. Chem. 1996;271:18499–18507. doi: 10.1074/jbc.271.31.18499. [DOI] [PubMed] [Google Scholar]
- 421.Lim S.C., Smith K.R., Stroud D.A., Compton A.G., Tucker E.J., Dasvarma A., Gandolfo L.C., Marum J.E., McKenzie M., Peters H.L., et al. A founder mutation in PET100 causes isolated complex IV deficiency in Lebanese individuals with leigh syndrome. Am. J. Hum. Genet. 2014;94:209–222. doi: 10.1016/j.ajhg.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Oláhová M., Haack T.B., Alston C.L., Houghton J.A., He L., Morris A.A., Brown G.K., McFarland R., Chrzanowska-Lightowlers Z.M., Lightowlers R.N., et al. A truncating PET100 variant causing fatal infantile lactic acidosis and isolated cytochrome c oxidase deficiency. Eur. J. Hum. Genet. 2015;23:935–939. doi: 10.1038/ejhg.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Taylor N.G., Swenson S., Harris N.J., Germany E.M., Fox J.L., Khalimonchuk O. The assembly factor Pet117 couples heme a synthase activity to cytochrome oxidase assembly. J. Biol. Chem. 2017;292:1815–1825. doi: 10.1074/jbc.M116.766980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.McEwen J.E., Hong K.H., Park S., Preciado G.T. Sequence and chromosomal localization of two PET genes required for cytochrome c oxidase assembly in saccharomyces cerevisiae. Curr. Genet. 1993;23:9–14. doi: 10.1007/BF00336742. [DOI] [PubMed] [Google Scholar]
- 425.Renkema G.H., Visser G., Baertling F., Wintjes L.T., Wolters V.M., van Montfrans J., de Kort G.A.P., Nikkels P.G.J., van Hasselt P.M., van der Crabben S.N., et al. Mutated PET117 causes complex IV deficiency and is associated with neurodevelopmental regression and medulla oblongata lesions. Hum. Genet. 2017;136:759–769. doi: 10.1007/s00439-017-1794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Signes A., Cerutti R., Dickson A.S., Benincá C., Hinchy E.C., Ghezzi D., Carrozzo R., Bertini E., Murphy M.P., Nathan J.A., et al. APOPT 1/ COA 8 assists COX assembly and is oppositely regulated by UPS and ROS. EMBO Mol. Med. 2019;11:e9582. doi: 10.15252/emmm.201809582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Melchionda L., Damseh N.S., Abu Libdeh B.Y., Nasca A., Elpeleg O., Zanolini A., Ghezzi D. A novel mutation in TTC19 associated with isolated Complex III deficiency, cerebellar hypoplasia, and bilateral basal ganglia lesions. Front. Genet. 2014;5:397. doi: 10.3389/fgene.2014.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Hedberg-Oldfors C., Darin N., Thomsen C., Lindberg C., Oldfors A. COX deficiency and leukoencephalopathy due to a novel homozygous APOPT1/COA8 mutation. Neurol. Genet. 2020;6:e464. doi: 10.1212/NXG.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Souza R.L., Green-Willms N.S., Fox T.D., Tzagoloff A., Nobrega F.G. Cloning and characterization of COX18, ASaccharomyces cerevisiae PET gene required for the assembly of cytochrome oxidase. J. Biol. Chem. 2000;275:14898–14902. doi: 10.1074/jbc.275.20.14898. [DOI] [PubMed] [Google Scholar]
- 430.Bourens M., Barrientos A. Human mitochondrial cytochrome c oxidase assembly factor COX18 acts transiently as a membrane insertase within the subunit 2 maturation module. J. Biol. Chem. 2017;292:7774–7783. doi: 10.1074/jbc.M117.778514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sacconi S., Trevisson E., Pistollato F., Baldoin M.C., Rezzonico R., Bourget I., Desnuelle C., Tenconi R., Basso G., DiMauro S., et al. HCOX18 and HCOX19: Two human genes involved in cytochrome c oxidase assembly. Biochem. Biophys. Res. Commun. 2005;337:832–839. doi: 10.1016/j.bbrc.2005.09.127. [DOI] [PubMed] [Google Scholar]
- 432.Legati A., Reyes A., Nasca A., Invernizzi F., Lamantea E., Tiranti V., Garavaglia B., Lamperti C., Ardissone A., Moroni I., et al. New genes and pathomechanisms in mitochondrial disorders unraveled by NGS technologies. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1857:1326–1335. doi: 10.1016/j.bbabio.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 433.Jonckheere A.I., Smeitink J.A.M., Rodenburg R.J.T. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012;35:211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Abrahams J.P., Leslie A.G.W., Lutter R., Walker J.E. Structure at 2.8 Â resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 435.He J., Ford H.C., Carroll J., Douglas C., Gonzales E., Ding S., Fearnley I.M., Walker J.E. Assembly of the membrane domain of ATP synthase in human mitochondria. Proc. Natl. Acad. Sci. USA. 2018;115:2988–2993. doi: 10.1073/pnas.1722086115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Spikes T.E., Montgomery M.G., Walker J.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. USA. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Pinke G., Zhou L., Sazanov L.A. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat. Struct. Mol. Biol. 2020;27:1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 438.Nijtmans L.G.J., Klement P., Houštěk J., van den Bogert C. Assembly of mitochondrial ATP synthase in cultured human cells: Implications for mitochondrial diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1995;1272:190–198. doi: 10.1016/0925-4439(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 439.Wittig I., Meyer B., Heide H., Steger M., Bleier L., Wumaier Z., Karas M., Schägger H. Assembly and oligomerization of human ATP synthase lacking mitochondrial subunits a and A6L. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010;1797:1004–1011. doi: 10.1016/j.bbabio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 440.Wang Z.G., Ackerman S.H. The assembly factor Atp11p binds to the beta-subunit of the mitochondrial F(1)-ATPase. J. Biol. Chem. 2000;275:5767–5772. doi: 10.1074/jbc.275.8.5767. [DOI] [PubMed] [Google Scholar]
- 441.Wang Z.-G., Sheluho D., Gatti D.L., Ackerman S.H. The alpha -subunit of the mitochondrial F1 ATPase interacts directly with the assembly factor Atp12p. EMBO J. 2000;19:1486–1493. doi: 10.1093/emboj/19.7.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Shoffner J.M., Fernhoff P.M., Krawiecki N.S., Caplan D.B., Holt P.J., Koontz D.A., Takei Y., Newman N.J., Ortiz R.G., Polak M., et al. Subacute necrotizing encephalopathy: Oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology. 1992;42:2168. doi: 10.1212/WNL.42.11.2168. [DOI] [PubMed] [Google Scholar]
- 443.White S.L., Shanske S., Biros I., Warwick L., Dahl H.M., Thorburn D.R., Di Mauro S. Two cases of prenatal analysis for the pathogenic T to G substitution at nucleotide 8993 in mitochondrial DNA. Prenat. Diagn. 1999;19:1165–1168. doi: 10.1002/(SICI)1097-0223(199912)19:12<1165::AID-PD719>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 444.Burrage L.C., Tang S., Wang J., Donti T.R., Walkiewicz M., Luchak J.M., Chen L.-C., Schmitt E.S., Niu Z., Erana R., et al. Mitochondrial myopathy, lactic acidosis, and sideroblastic anemia (MLASA) plus associated with a novel de novo mutation (m.8969G>A) in the mitochondrial encoded ATP6 gene. Mol. Genet. Metab. 2014;113:207–212. doi: 10.1016/j.ymgme.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Rantamäki M.T., Soini H.K., Finnilä S.M., Majamaa K., Udd B. Adult-onset ataxia and polyneuropathy caused by mitochondrial 8993T-C mutation. Ann. Neurol. 2005;58:337–340. doi: 10.1002/ana.20555. [DOI] [PubMed] [Google Scholar]
- 446.Craig K., Elliott H.R., Keers S.M., Lambert C., Pyle A., Graves T.D., Woodward C., Sweeney M.G., Davis M.B., Hanna M.G., et al. Episodic ataxia and hemiplegia caused by the 8993T->C mitochondrial DNA mutation. J. Med. Genet. 2007;44:797–799. doi: 10.1136/jmg.2007.052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Pfeffer G., Blakely E.L., Alston C.L., Hassani A., Boggild M., Horvath R., Samuels D.C., Taylor R.W., Chinnery P.F. Adult-onset spinocerebellar ataxia syndromes due to MTATP6 mutations. J. Neurol. Neurosurg. Psychiatry. 2012;83:883–886. doi: 10.1136/jnnp-2012-302568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.De Meirleir L., Seneca S., Lissens W., Schoentjes E., Desprechins B. Bilateral striatal necrosis with a novel point mutation in the mitochondrial ATPase 6 gene. Pediatr. Neurol. 1995;13:242–246. doi: 10.1016/0887-8994(95)00184-H. [DOI] [PubMed] [Google Scholar]
- 449.Thyagarajan D., Shanske S., Vazquez -Memije M., Devivo D., Dimauro S. A novel mitochondrial ATPase 6 point mutation in familial bilateral striatal necrosis. Ann. Neurol. 1995;38:468–472. doi: 10.1002/ana.410380321. [DOI] [PubMed] [Google Scholar]
- 450.Brum M., Semedo C., Guerreiro R., Pinto Marques J. Motor neuron syndrome as a new phenotypic manifestation of mutation 9185T>C in gene MTATP6. Case Rep. Neurol. Med. 2014;2014:701761. doi: 10.1155/2014/701761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Takahashi S., Makita Y., Oki J., Miyamoto A., Yanagawa J., Naito E., Goto Y., Okuno A. De novo MtDNA Nt 8993 (T—G) mutation resulting in leigh syndrome. Am. J. Hum. Genet. 1998;62:717–719. doi: 10.1086/301751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Vilarinho L., Barbot C., Carrozzo R., Calado E., Tessa A., Dionisi-Vici C., Guimarães A., Santorelli F.M. Clinical and molecular findings in four new patients harbouring the MtDNA 8993T’C mutation. J. Inherit. Metab. Dis. 2001;24:883–884. doi: 10.1023/A:1013908728445. [DOI] [PubMed] [Google Scholar]
- 453.Carrozzo R., Tessa A., Vazquez-Memije M.E., Piemonte F., Patrono C., Malandrini A., Dionisi-Vici C., Vilarinho L., Villanova M., Schagger H., et al. The T9176G MtDNA mutation severely affects ATP production and results in Leigh syndrome. Neurology. 2001;56:687–690. doi: 10.1212/WNL.56.5.687. [DOI] [PubMed] [Google Scholar]
- 454.Lopez-Gallardo E., Solano A., Herrero-Martin M.D., Martinez-Romero I., Castano-Perez M.D., Andreu A.L., Herrera A., Lopez-Perez M.J., Ruiz-Pesini E., Montoya J. NARP syndrome in a patient harbouring an insertion in the MT-ATP6 gene that results in a truncated protein. J. Med. Genet. 2008;46:64–67. doi: 10.1136/jmg.2008.060616. [DOI] [PubMed] [Google Scholar]
- 455.D’Aurelio M., Vives-Bauza C., Davidson M.M., Manfredi G. Mitochondrial DNA background modifies the bioenergetics of NARP/MILS ATP6 mutant cells. Hum. Mol. Genet. 2010;19:374–386. doi: 10.1093/hmg/ddp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.de Coo I.F., Smeets H.J., Gabreëls F.J., Arts N., van Oost B.A. Isolated case of mental retardation and ataxia due to a de novo mitochondrial T8993G mutation. Am. J. Hum. Genet. 1996;58:636–638. [PMC free article] [PubMed] [Google Scholar]
- 457.Ware S.M., El-Hassan N., Kahler S.G., Zhang Q., Ma Y.-W., Miller E., Wong B., Spicer R.L., Craigen W.J., Kozel B.A., et al. Infantile cardiomyopathy caused by a mutation in the overlapping region of mitochondrial ATPase 6 and 8 genes. J. Med. Genet. 2009;46:308–314. doi: 10.1136/jmg.2008.063149. [DOI] [PubMed] [Google Scholar]
- 458.Galimberti C.A., Diegoli M., Sartori I., Uggetti C., Brega A., Tartara A., Arbustini E. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology. 2006;67:1715–1717. doi: 10.1212/01.wnl.0000242882.58086.9a. [DOI] [PubMed] [Google Scholar]
- 459.Jonckheere A.I., Hogeveen M., Nijtmans L., van den Brand M., Janssen A., Diepstra H., van den Brandt F., van den Heuvel B., Hol F., Hofste T., et al. A novel mitochondrial ATP8 gene mutation in a patient with apical hypertrophic cardiomyopathy and neuropathy. J Med Genet. 2008;45:129–133. doi: 10.1136/jmg.2007.052084. [DOI] [PubMed] [Google Scholar]
- 460.Jonckheere A.I., Renkema G.H., Bras M., van den Heuvel L.P., Hoischen A., Gilissen C., Nabuurs S.B., Huynen M.A., de Vries M.C., Smeitink J.A.M., et al. A complex V ATP5A1 defect causes fatal neonatal mitochondrial encephalopathy. Brain. 2013;136:1544–1554. doi: 10.1093/brain/awt086. [DOI] [PubMed] [Google Scholar]
- 461.Lieber D.S., Calvo S.E., Shanahan K., Slate N.G., Liu S., Hershman S.G., Gold N.B., Chapman B.A., Thorburn D.R., Berry G.T., et al. Targeted exome sequencing of suspected mitochondrial disorders. Neurology. 2013;80:1762–1770. doi: 10.1212/WNL.0b013e3182918c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Oláhová M., Yoon W.H., Thompson K., Jangam S., Fernandez L., Davidson J.M., Kyle J.E., Grove M.E., Fisk D.G., Kohler J.N., et al. Biallelic mutations in ATP5F1D, which encodes a subunit of ATP synthase, cause a metabolic disorder. Am. J. Hum. Genet. 2018;102:494–504. doi: 10.1016/j.ajhg.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Mayr J.A., Havlickova V., Zimmermann F., Magler I., Kaplanova V., Jesina P., Pecinova A., Nuskova H., Koch J., Sperl W., et al. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 subunit. Hum. Mol. Genet. 2010;19:3430–3439. doi: 10.1093/hmg/ddq254. [DOI] [PubMed] [Google Scholar]
- 464.Schauberger E.M., Ewart S.L., Arshad S.H., Huebner M., Karmaus W., Holloway J.W., Friderici K.H., Ziegler J.T., Zhang H., Rose-Zerilli M.J., et al. Identification of ATPAF1 as a novel candidate gene for asthma in children. J. Allergy Clin. Immunol. 2011;128:753–760. doi: 10.1016/j.jaci.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.De Meirleir L. Respiratory Chain Complex V Deficiency due to a mutation in the assembly gene ATP12. J. Med. Genet. 2004;41:120–124. doi: 10.1136/jmg.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.de Vries D.D., van Engelen B.G.M., Gabreëls F.J.M., Ruitenbeek W., van Oost B.A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome: MtDNA mutation in Leigh’s syndrome. Ann. Neurol. 1993;34:410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]
- 467.White S.L., Shanske S., McGill J.J., Mountain H., Geraghty M.T., DiMauro S., Dahl H.-H.M., Thorburn D.R. Mitochondrial DNA mutations at nucleotide 8993 show a lack of tissue- or age-related variation. J. Inherit. Metab. Dis. 1999;22:899–914. doi: 10.1023/A:1005639407166. [DOI] [PubMed] [Google Scholar]
- 468.houštěk j., pícková a., vojtíšková a., mráček t., pecina p., ješina p. mitochondrial diseases and genetic defects of ATP synthase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006;1757:1400–1405. doi: 10.1016/j.bbabio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 469.Schägger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim. Biophys. Acta (BBA)-Bioenerg. 2002;1555:154–159. doi: 10.1016/S0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 470.Wittig I., Schägger H. Structural organization of mitochondrial ATP synthase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008;1777:592–598. doi: 10.1016/j.bbabio.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 471.Mourier A., Matic S., Ruzzenente B., Larsson N.-G., Milenkovic D. The respiratory chain supercomplex organization is independent of COX7a2l isoforms. Cell Metab. 2014;20:1069–1075. doi: 10.1016/j.cmet.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Acín-Pérez R., Fernández-Silva P., Peleato M.L., Pérez-Martos A., Enriquez J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 473.Gu J., Wu M., Guo R., Yan K., Lei J., Gao N., Yang M. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. doi: 10.1038/nature19359. [DOI] [PubMed] [Google Scholar]
- 474.Wu M., Gu J., Guo R., Huang Y., Yang M. Structure of mammalian respiratory supercomplex I 1 III 2 IV 1. Cell. 2016;167:1598–1609. doi: 10.1016/j.cell.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 475.Letts J.A., Fiedorczuk K., Sazanov L.A. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 476.Sousa J.S., Mills D.J., Vonck J., Kühlbrandt W. Functional asymmetry and electron flow in the bovine respirasome. eLife. 2016;5:e21290. doi: 10.7554/eLife.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Lapuente-Brun E., Moreno-Loshuertos R., Acín-Pérez R., Latorre-Pellicer A., Colás C., Balsa E., Perales-Clemente E., Quirós P.M., Calvo E., Rodríguez-Hernández M.A., et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 478.Genova M.L., Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014;1837:427–443. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 479.Lenaz G., Tioli G., Falasca A.I., Genova M.L. Complex I function in mitochondrial supercomplexes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1857:991–1000. doi: 10.1016/j.bbabio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 480.Calvo E., Cogliati S., Hernansanz-Agustín P., Loureiro-López M., Guarás A., Casuso R.A., García-Marqués F., Acín-Pérez R., Martí-Mateos Y., Silla-Castro J., et al. Functional role of respiratory supercomplexes in mice: SCAF1 relevance and segmentation of the Q pool. Sci. Adv. 2020;6:eaba7509. doi: 10.1126/sciadv.aba7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Capitanio G., Papa F., Papa S. The allosteric protein interactions in the proton-motive function of mammalian redox enzymes of the respiratory chain. Biochimie. 2021;189:1–12. doi: 10.1016/j.biochi.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 482.Loeffen J.L.C.M., Smeitink J.A.M., Trijbels J.M.F., Janssen A.J.M., Triepels R.H., Sengers R.C.A., van den Heuvel L.P. Isolated complex I deficiency in children: Clinical, biochemical and genetic aspects. Hum. Mutat. 2000;15:123–134. doi: 10.1002/(SICI)1098-1004(200002)15:2<123::AID-HUMU1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 483.Protasoni M., Pérez-Pérez R., Lobo-Jarne T., Harbour M.E., Ding S., Peñas A., Diaz F., Moraes C.T., Fearnley I.M., Zeviani M., et al. Respiratory supercomplexes act as a platform for complex III -mediated maturation of human mitochondrial complexes I and IV. EMBO J. 2020;39:e102817. doi: 10.15252/embj.2019102817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Pernas L., Scorrano L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 485.Cogliati S., Enriquez J.A., Scorrano L. Mitochondrial cristae: Where beauty meets functionality. Trends Biochem. Sci. 2016;41:261–273. doi: 10.1016/j.tibs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 486.Silva Ramos E., Larsson N.-G., Mourier A. Bioenergetic roles of mitochondrial fusion. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1857:1277–1283. doi: 10.1016/j.bbabio.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 487.Letts J.A., Sazanov L.A. Clarifying the supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017;24:800–808. doi: 10.1038/nsmb.3460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.