Abstract
Oxygen deficiency (hypoxia) induces adverse effects during biotherapeutic protein production leading to reduced productivity and cell growth. Hypoxic conditions occur during classical batch fermentations using high cell densities or perfusion processes. Here we present an effort to create novel engineered Chinese hamster ovary (CHO) cell lines by exploiting encountered hypoxic bioprocess conditions to reinforce cellular production capacities. After verifying the conservation of the hypoxia-responsive pathway in CHO cell lines by analyzing oxygen sensing proteins HIF1a, HIF1β and VDL, hypoxia-response-elements (HREs) were functionally analyzed and used to create hypoxia-responsive expression vectors. Subsequently engineered hypoxia sensitive CHO cell lines significantly induced protein expression (SEAP) during adverse oxygen limitation encountered during batch fermentations as well as high cell density perfusion processes (2.7 fold). We also exploited this novel cell system to establish a highly effective oxygen shift as innovative bioprocessing strategy using hypoxia induction to improve production titers. Thus, substantial improvements can be made to optimize CHO cell productivity for novel bioprocessing challenges as oxygen limitation, providing an avenue to establish better cell systems by exploiting adverse process conditions for optimized biotherapeutic production.
Keywords: Biotechnology, Cell line engineering, Chinese hamster ovary, Hypoxia, Perfusion, Recombinant protein expression
Highlights
-
•
Demonstration of the capability of CHO cells for oxygen sensing
-
•
Characterization of the most potent hypoxia response elements in CHO cells
-
•
Enhanced productivity of CHO cells exploiting oxygen deprivation conditions
-
•
Introduction of a novel bioprocessing strategy by shifting pO2
1. Introduction
Sufficient oxygen supply is essential for eukaryotic cells to fuel cellular respiration and ensure vital cellular functions (Chance, 1965). The limitation of oxygen under hypoxic conditions impacts diverse physiological process including energy metabolism, autophagy, and cell proliferation (Hubbi and Semenza, 2015a, 2015b). During cellular production of biotherapeutic recombinant proteins, production cell lines encounter adverse hypoxic conditions both during classical batch fermentations and perfusion processes, where high cell densities or volumes of >5000 L are used to ensure high product titers (Kwon et al., 2017; Konstantinov and Cooney, 2015; Janoschek et al., 2019; Li et al., 2010; Huang et al., 2010). These hypoxic conditions are leading to reduced productivity and cell proliferation (Kwon et al., 2017; Janoschek et al., 2019), since industrially used Chinese hamster ovary (CHO) production cell lines (Scarcelli et al., 2017; Wang et al., 2018; Zhong et al., 2019) are currently not optimized for production under hypoxia. This puts a demand on cell engineering to further improve this dominating production cell system. Although many studies have focused on the molecular improvement of CHO cells using engineering approaches to delete detrimental or overexpressing favorable genes (Xu et al., 2011; Kimura and Omasa, 2018; Chung et al., 2017; Laux et al., 2018; Škulj et al., 2014; Lin et al., 2015; Han et al., 2011; Fu et al., 2016), hypoxia adaptation or exploitation has not been addressed so far.
In the human body, oxygen homeostasis is controlled by a sophisticated regulatory system. Hypoxia inducing factor 1α (HIF-1α) is a key transcription factor (Schödel and Ratcliffe, 2019; Lee et al., 2020) degraded during normoxia by hydroxylation, binding of von-Hippel-Lindau (VHL) and subsequent ubiquitination (Tanimoto, 2000) (Fig. 1A). Under hypoxia, a state of insufficient oxygen supply, however, these processes are suppressed and enable HIF-1α to escape destruction. Transcriptional complexes consisting of HIF-1α and HIF-1β heterodimers are formed that bind to specific hypoxia response elements (HREs) at thousands of loci across the genome (Lee et al., 2020; Gonzalez et al., 2019; Javan and Shahbazi, 2017). These induce target gene expression of e.g. phosphoglycerate kinase (PGK1), erythropoietin (EPO) or vascular endothelial growth factor A (VEGF-A) and thereby counteract hypoxia (Li et al., 1996; Stockmann and Fandrey, 2006; Sandner et al., 1997; Kimura et al., 2001; Bergeron et al., 2000; Lee et al., 2019). Previous studies have used HREs for targeted gene therapy against human hypoxic tumors (Javan and Shahbazi, 2017; Binley et al., 2003; Brown and Wilson, 2004; Greco et al., 2002; Shibata et al., 2000)–(Javan and Shahbazi, 2017; Binley et al., 2003; Brown and Wilson, 2004; Greco et al., 2002; Shibata et al., 2000). Varying quantity, orientation and origin of HREs were tested and up to 10 repeats of the VEGF-A, EPO or PGK1 HREs displayed best induction upon hypoxia in human cells (Greco et al., 2002; Shibata et al., 2000). However, HRE elements have not been studied in CHO cell systems.
Fig. 1.
Molecular biological identification of crucial factors for the detection of hypoxia. (A) Hypoxia signaling pathway modified after Mattias Karlén. (B) PCR on cDNA detecting HIF-1α (Lane 1 + 5, expected size 670 bp), HIF-1β (Lane 2 + 6, expected size 490 bp) and VHL (Lane 9 + 11, expected size 470 bp) in unmodified CHO-K1 (Lane 1–4; 9 + 10) and CHO-DG44 (Lane 5–8; 11 + 12). Lane 3–4; 7–8 and 10 + 12 represent negative controls lacking reverse transcriptase. (C) Western blot on HIF-1α (top panel), HIF-1β (middle panel) and VHL (bottom panel) in CHO-K1 and CHO-DG44 using 30 μg of protein per lane.
In the current study, we created a novel hypoxia responding cell system in CHO production cells based on HRE elements and cellular oxygen sensing proteins. We were able to demonstrate substantial improvement of recombinant protein production under hypoxic process conditions using a vector based system which can be used without time- and cost-intensive host cell modifications in industrial settings (Li et al., 2018). Specifically, we first verified the conservation of the hypoxia-responsive pathway in CHO cell lines, functionally analyzed HREs from VEGF and EPO genes and then created hypoxia-responsive expression vectors. We subsequently transfected CHO cells stably with the generated expression vectors and were able to show significantly improved protein expression during oxygen limitation both in batch fermentations and high cell density perfusion processes. Finally, we successfully applied this hypoxia sensing cell system to a novel oxygen shift bioprocessing strategy by using hypoxia induction to improve production titers. Thus, hypoxic process conditions can successfully be exploited to counteract bioprocessing challenges as oxygen limitation.
2. Methods
2.1. Cell culture
CHO-DG44 (A1100001, Thermo Fisher, Darmstadt, Germany) and –K1 (CCL-61TM, ATCC) (Chinese hamster ovary) cells were cultured in animal component free SFM4CHO medium (GE Healthcare, Chicago, IL, USA), supplemented with 4 mM L-Glutamine (Lonza, Basel, Switzerland), 10 g/L glucose (Roth, Karlsruhe, Germany) and for CHO-DG44 cells with 2x HT supplement (Thermo Fisher, Darmstadt, Germany). Cultivation was performed at 140 rpm (25 mm orbit) with 5% CO2 and 85% humidity at 37 °C. Cells were passaged every 3–4 days to a viable cell density (VCD) of 0.5 × 106 cells/mL. VCD and viability have been determined via trypan blue exclusion using CEDEX XS (Roche Diagnostics, Mannheim, Germany). Transfection of CHO cells was performed using 15 μg vector using the NEON transfection kit (Thermo Fisher, Waltham, MA, USA). CHO-DG44 and –K1 cell lines stably expressing variants of the pEF-myc-cyto-mCMV-d2GFP (Addgene, Watertown, MA, USA) vector were selected using 500 μg/mL G418 (Genaxxon, Ulm, Germany). In addition, a CHO-DG44 cell line stably expressing the recombinant protein secreted alkaline phosphatase (SEAP) was generated. Therefore, the gene synthesis 5HRE-CMV-SEAP (Genewiz, Leipzig, Germany) was cloned into a pOptivecTM-TOPOTM expression vector (Thermo Fisher, Darmstadt, Germany). Selection was performed by omitting the HT-supplement as dihydrofolate reductase is encoded on the pOptivecTM-TOPOTM expression vector.
2.2. Cultivation under oxygen deprivation conditions
To evaluate the response of CHO cells on hypoxic cultivation conditions, CHO cells were not cultured under shaken conditions but cultivated statically with a seeding density of 0.5 × 106 cells/mL, with 5% CO2 and 85% humidity at 37 °C to induce the expression of d2GFP as an indication for hypoxia. For cultivation at defined O2 concentrations, CHO cells were inoculated in 1 L at a VCD of 0.3 × 106 cells/mL using a 2 L stirred tank bioreactor for batch fermentation (Sartorius, Göttingen, Germany). The bioreactors were controlling pH at 7.15, temperature at 37 °C, stirring speed at 100 rpm and pO2 at 60%. The pO2 set point was stepwise reduced and the d2GFP expression was measured to characterize the inducibility of the transfected HRE constructs using oxygen deprivation conditions. Detailed adjustments of the pO2 are shown in Fig. 3A.
Fig. 3.
Characterization of the response of CHO-K1 and CHO-DG44 cells to defined O2-concentrations. (A) Progression of the controlled oxygen concentration during fermentation in a normoxic batch (dotted line) and a stepwise hypoxic cultivation (solid line). Viable cell densities and viabilities of the cultured CHO-DG44 and –K1 cells are shown in S2. (B) Mean fluorescence of CHO-DG44-Mock (black) and CHO-DG44-5HRE-VEGF (dark green) cells cultured in a batch fermentation over 156 h under normoxic and stepwise hypoxic conditions. Arrow indicates the time point for Fig. 3C. (C) Mean fluorescence of normoxic and hypoxic cultured CHO-DG44-Mock (black bars) and CHO-DG44-5HRE-VEGF (dark green bars) cells after 140 h. (D) Mean fluorescence of CHO-K1-Mock (grey) and CHO-K1-5HRE-VEGF (light green) cells cultured in a batch fermentation over 156 h under normoxic and stepwise hypoxic conditions. Arrow indicates the time point for Fig. 3E. (E) Mean fluorescence of normoxic and hypoxic cultured CHO-K1-Mock (grey bars) and CHO-K1-5HRE-VEGF (light green bars) cells after 140 h. (F) d2GFP expression assessed via qPCR during mild (5%) and strong (1%) hypoxic culture conditions. Fold-changes of d2GFP expression were calculated according to the respective normoxic cultured control cell lines. Cells for RNA isolation were taken 108 h and 156 h after inoculation. (G) Western blot on HIF-1α (top panel) and Actin (bottom panel) in CHO-DG44 (Lane 1–4) and CHO-K1 (Lane 5–8) using 30 μg of protein per lane. Lysates obtained from batch fermentation of normoxic (60% O2) and hypoxic (5% or 1% O2) cultivated CHO-DG44-5HRE-VEGF and CHO-K1-5HRE-VEGF cells after 108 h and 156 h. Statistical analysis was conducted using students t-test [n = 3 replicates; Mean ± SD; * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Fed-batch fermentation
Fed-batch fermentation was performed in a 2 L Biostat ® benchtop bioreactor (Sartorius Stedim Biotech GmbH, Goettingen, Germany). Therefore, 3 × 105 cells/mL were inoculated in 1 L SFM4CHO (GE Healthcare, Chicago, IL, USA), supplemented with 4 mM L-Glutamine (Lonza, Basel, Switzerland) and 10 g/L glucose (Roth, Karlsruhe, Germany) as starting concentrations. Fermentation was performed at 37 °C, pH 7.15, stirring speed at 100 rpm and an initial O2 concentration of 40%. In contrast to the batch fermentation (60% pO2), fed-batch fermentation was conducted at 40% pO2 due to better growth at this condition. During the process, the glucose concentration was maintained at 5 g/L and L-Glutamine at 2 mM after the starting concentrations of the supplements were depleted. Therefore, glucose and L-glutamine concentrations were measured daily from the cell culture supernatant using the Konelab arena 20XT (Thermo Fisher, Darmstadt, Germany). With the measured concentrations, consumption rates and feeding rates could be calculated to maintain the nutrient concentrations within the desired range. The main feed was added by a daily adjusted feeding rate, while the L-glutamine feed was added daily as bolus. The main feed (1 L) consisted of 11.6 g HyCloneTM Cell BoostTM 6 dissolved in 330 mL Millipore water (Cytiva, Marlborough, MA, USA), 20 g/L glucose and 660 mL SFM4CHO. The glutamine feed consisted of 100 mL 200 mM L-glutamine and 100 mL SFM4CHO. Viable cell density and viability was determined daily via trypan blue exclusion and after reaching the static cultivation phase, temperature was reduced to 34 °C. Additionally, in another fermenter temperature was reduced to 34 °C and O2 concentration to 1% to assess the effect of a temperature and oxygen shift on productivity in comparison to the temperature shift alone. Every day cell culture supernatant was taken to determine the expressed SEAP concentration.
2.4. Molecular biology
The starting plasmid 5HRE/d2GFP was a gift from Martin Brown & Thomas Foster (Addgene plasmid # 46926) (Vordermark et al., 2001). Constructs were cloned using HRE sequences of VEGF, EPO and PGK1 published by Javan et al., (2017) (Javan and Shahbazi, 2017). The oligos used are listed in Table 2. For cloning, oligonucleotides were annealed by slowly reducing temperature from 95 °C to 25 °C, phosphorylated using T4 PNK (New England Biolabs, Ipswich, MA, USA) and ligated by T4 ligase (New England Biolabs, Ipswich, MA, USA). Randomly ligated double stranded oligonucleotides have been cloned into the pEF-myc-cyto-mCMV-d2GFP after removing the 5HREs by digesting with XhoI and BglII (both New England Biolabs, Ipswich, MA, USA). Finally, the generated vectors were sequenced to confirm the desired sequence (Eurofins Genomics Europe Sequencing, Constance, Germany).
Table 2.
Oligonucleotide sequences for molecular biology.
| Oligonucleotide | Sequence [5’ -> 3’] | Gene |
|---|---|---|
| Mock Fw | TCGAGACTAGTCCAGTGA | – |
| Mock Rev | GATCTCACTGGACTAGTC | – |
| VEGF-HRE-pair 1 Fw | TCGAGCCACAGTGCATACGTGGGCTCCAACAGGTCCTCTT | VEGF |
| VEGF-HRE-pair 1 Rev | CTCGACAAGAGGACCTGTTGGAGCCCACGTATGCACTGTGGC | VEGF |
| VEGF-HRE-pair 2 Fw | GTCGAGCCACAGTGCATACGTGGGCTCCAACAGGTCCTCTT | VEGF |
| VEGF-HRE-pair 2 Rev | CTCGACAAGAGGACCTGTTGGAGCCCACGTATGCACTGTGG | VEGF |
| VEGF-HRE-pair 3 Fw | GTCGAGCCACAGTGCATACGTGGGCTCCAACAGGTCCTCTTGTCGA | VEGF |
| VEGF-HRE-pair 3 Rev | GATCTCGACAAGAGGACCTGTTGGAGCCCACGTATGCACTGTGG | VEGF |
| EPO-HRE-pair 1 Fw | TCGAGGCCCTACGTGCTGTCTCACACAGCCTGTCTGAC | EPO |
| EPO-HRE-pair 1 Rev | CTCGACGTCAGACAGGCTGTGTGAGACAGCACGTAGGGCC | EPO |
| EPO-HRE-pair 2 Fw | GTCGAGGCCCTACGTGCTGTCTCACACAGCCTGTCTGAC | EPO |
| EPO-HRE-pair 2 Rev | CTCGACGTCAGACAGGCTGTGTGAGACAGCACGTAGGGC | EPO |
| EPO-HRE-pair 3 Fw | GTCGAGGCCCTACGTGCTGTCTCACACAGCCTGTCTGACGTCGA | EPO |
| EPO-HRE-pair 3 Rev | GATCTCGACGTCAGACAGGCTGTGTGAGACAGCACGTAGGGC | EPO |
2.5. RNA isolation
RNA isolation was performed using the miRNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol with 5 × 106 cells. Purity and concentration of the isolated RNA was assessed using a NanodropTM 1000 Spectrophotometer by absorbance at 260 nm (Thermo Fisher Scientific, Darmstadt, Germany).
2.6. PCR and RT-PCR
After RNA isolation, cDNA was produced using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany) according to the manufactures protocol. The transcription of HIF-1α, HIF-1β and VHL was demonstrated performing PCR on cDNA level with the following primers: HIF-1α Fw (5′-TCCAGTTGCGCTCCTTTGAT-3′); HIF-1α Rev (5′-ATCCATTGATTGCCCCAGCA-3‘); HIF-1β Fw (5’-TTGTCTCGTGTGAGACTGGC-3′); HIF-1β Rev (5-CCAATGGCCACTAGGCAGAA-3′); VHL Fw (5′-GGTCATCTTCTGCAACCGCA-3′) and VHL Rev (5′-AAGCTGGAATTCAGGACCACT-3′). Amplified DNA was separated via gel electrophoresis and detected using Fusion FX imager (Vilber Lourmat, Eberhardszell, Germany). Quantitative real-time PCR on d2GFP was performed using the following primers: d2GFP Fw (5′-CTACCCCGACCACATGAAGC-3′) and d2GFP-Rev (5′-AAGTCGATGCCCTTCAGCTC-3′) with GAPDH as loading control: GAPDH Fw (5′- GACTCTACCCATGGCAAGTTCA-3′); GAPDH Rev (5′-TCGCTCCTGGAAGATGGTGATG-3′). The measurement with SybrGreen Mastermix (Genaxxon, Ulm, Germany) was recorded using a Lightcycler480 (Roche Diagnostics, Mannheim, Germany).
2.7. Flow cytometry analysis
GFP fluorescence of hypoxic and normoxic cultivated CHO cells was measured using MACSQuant Analyzer 10 without further staining (Miltenyi Biotec, Bergisch Gladbach, Germany). Resulting data was analyzed via the MACSQuantifyTM Software.
2.8. Immunoblotting
3 × 106 cells were lysed in 200 μL RIPA buffer (1% v/v NonidetTM P40, 0.5% w/v sodium deoxycholate, ethylenediaminetetraacetic acid 1 mM, all reagents Carl Roth, Karlsruhe, Germany). Protein concentration was assed via BCA-assay according to Walker (1994) (Walker, 1994). The sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using TruPAGETM Precast 4–20% gradient gels (Sigma-Aldich, St. Louis, MO, USA) with 30 μg protein per lane. Blotting of proteins was done on polyvinylidene fluoride membrane for 75 min with 60 mA and immunoblotting with the following antibodies was performed over night at 4 °C: Anti-HIF1-alpha antibody (Bio-Techne, Minneapolis, MN, USA, #NB100-479SS, 1:1000); anti-VHL antibody (Santa Cruz Biotechnology, Dallas, TX, USA, #sc-135657 HRP, 1:200); Anti-ARNT antibody (HIF-1β) (Santa Cruz Biotechnology, Dallas, TX, USA, #sc-17811, 1:200) and anti-β-actin antibody (Sigma-Aldrich, St. Lousis, MO, USA, #A5441-2 ML, 1:1000). The secondary antibodies (anti-mouse IgG- HRP (Sigma Aldrich, St. Louis, MO, USA, #A4416; 1:5000); anti-rabbit IgG-HRP F (ab’)2 (Jackson ImmunoResearch, Cambridge, United Kingdom, #711-036-152, 1:50000)) were incubated for 1 h at room temperature. Detection was performed using Immobilon® Western chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA, USA) and Fusion FX imager (Vilber Lourmat, Eberhartszell, Germany).
2.9. SEAP-assay
To determine SEAP concentration the SEAP reporter gene assay, chemiluminescent (Roche, Mannheim, Germany) was used according to the manufacturer's advice. Therefore, samples were heat inactivated at 65 °C for 30 min and have been diluted 1:50 to 1:300 depending on the SEAP concentration to fit to the linear area of the standard curve. Afterwards, 50 μL heat inactivated sample have been pipetted into a black non-binding flat bottom 96-well plate (Greiner Bio One, Frickenhausen, Germany) and incubated for 5 min with inactivation buffer and freshly prepared substrate was added. After 10 min incubation while gently shaking, luminescence was measured with 1 s integration time via SpectraMacs (Molecular Devices, San Jose, CA, USA).
2.10. Mock perfusion cultivation
For mock perfusion cultivation, CHO-DG44-SEAP and CHO-DG44-5HRE-SEAP cells were inoculated at normal VCD (0.5 × 106 cells/mL) and ultra-high cell density (50 × 106 cells/mL) in a working volume of 10 mL in a 50 mL TubeSpin bioreactor (TPP, Trasadingen, Switzerland). The cells were cultured as described in section 2.1 and cell culture media was exchanged every 24 h by centrifugation at 130×g for 5 min. Finally, the SEAP concentration in the cell culture supernatant of all cell lines was determined after 72 h and the specific productivity determined.
2.11. Statistical analysis
Statistical differences between data was determined using GraphPad Prism 6 software. ONE-WAY-ANOVA test with Bonferroni correction was conducted to calculate statistical differences of the mean between groups. All values of groups are shown as mean ± SD.
3. Results
3.1. Conservation of the hypoxia-responsive pathway in CHO cells
In order to take advantage of hypoxia conditions encountered during high density cultivation to enhance productivity, we aimed at the creation of a hypoxia inducible expression system, similar to the regulation of hypoxia sensitive genes during oxygen deprivation conditions (Fig. 1A) (Kaelin et al., 2019). Hypoxia sensing and responsive pathways and especially HREs are currently unexplored in CHO cells. Since mainly CHO-K1 and CHO-DG44 cells are used for the industrial production of biopharmaceuticals (Xu et al., 2011; Kimura and Omasa, 2018), these cell lines were examined for the expression of the essential hypoxia related factors HIF-1α, HIF-1β and VHL. For both cell lines HIF-1α, HIF-1β and VHL RNA expression was detected using RT-PCR analysis (Fig. 1B). In addition, protein expression of HIF-1β and VHL was detected for both CHO-K1 and CHO-DG44 cells cultured under normoxic conditions, however, the unstable HIF-1α could only be detected in CHO-DG44 cells cultivated under hypoxic culture conditions (Fig. 1C). These data indicate the presence of key factors of the hypoxia sensing pathway in CHO production cells.
Since the transcription factors HIF-1α and HIF-1β directly bind to the HRE promotor elements to induce transcriptional response, protein sequences were analyzed for conservation of crucial domains (Supplementary 1). Critical areas like the DNA-binding domain, the HIF-1β heterodimerization domain and the nuclear localization signal were identical across all isoforms and species, while homology of the entire proteins reached at least 77% across all five variants. Especially the identical DNA-binding domains may allow for hamster HIF-1α to bind to vector based human HREs introduced into CHO cells.
3.2. Construction of hypoxia sensing cell systems
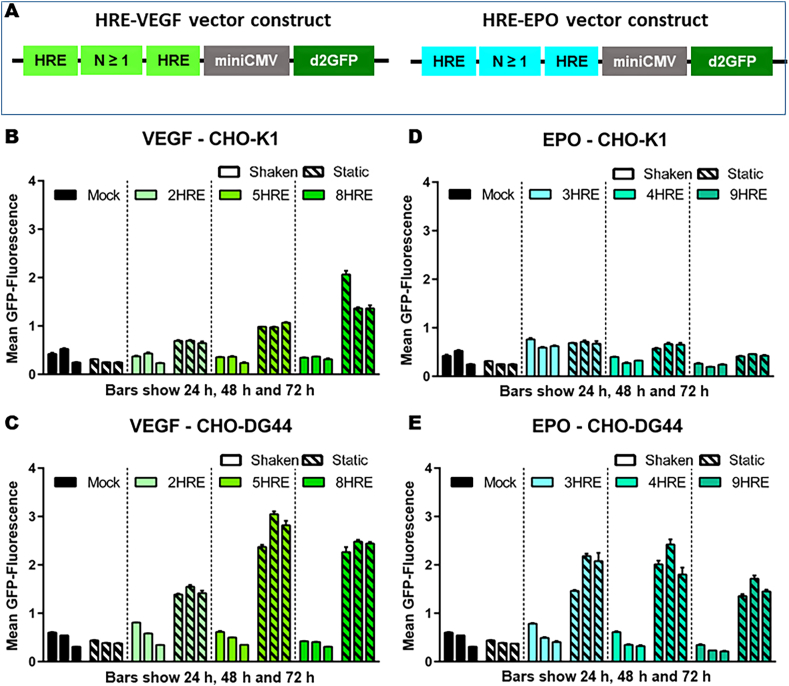
For the construction of hypoxia responsive vectors to selectively drive protein expression under hypoxic culture conditions, HRE building blocks were selected from human origin including the hypoxia sensitive vascular endothelial growth factor A (VEGF-A) and erythropoietin (EPO) genes (Javan and Shahbazi, 2017). Subsequently, several expression vectors were designed carrying a minimal CMV promotor flanked by varying repetitions of VEGF- and EPO-HRE sequences controlling the expression of the unstable d2GFP (half-life 2 h) as a quantifiable marker for protein expression (Fig. 2A). For all resulting vectors (Table 1), stable cell pools for CHO-K1 and DG44 cell lines were generated by transfection and antibiotic selection for the validation of a hypoxia sensitive expression system. To verify the functionality of the generated cell lines expressing d2GFP under the control of hypoxia inducible promotors, cells were initially cultivated in small scale batch fermentations under normal shaken and oxygen deprivation static conditions. d2GFP expression was measured daily using flow cytometry.
Fig. 2.
Identification of the most promising vector constructs under undefined hypoxic conditions. (A) Schematic vector construct. (B + C) Flow cytometry analysis of static or shaken cultivated CHO-K1 (B) and CHO-DG44 (C) pools, stably expressing destabilized GFP under the control HREs from VEGF origin. Blank bars stand for shaken, while striped bars represent static cultured pools. The three bars represent 24 h, 48 h and 72 h after inoculation. (D + E) Flow cytometry analysis of static or shaken cultivated CHO-K1 (D) and CHO-DG44 (E) pools, stably expressing destabilized GFP under the control of HREs from or EPO origin. Blank bars stand for shaken, while striped bars represent static cultured pools. The three bars represent 24 h, 48 h and 72 h after inoculation. [n = 3 replicates; Mean ± SD].
Table 1.
Generated vector constructs for the analysis of HRE functionality.
| Name | Vector backbone | Gene origin | HRE repetitions |
|---|---|---|---|
| Mock | pEF-myc-cyto-mCMV-d2GFP | – | – |
| 2HRE-VEGF | pEF-myc-cyto-mCMV-d2GFP | VEGF | 2 |
| 5HRE-VEGF | pEF-myc-cyto-mCMV-d2GFP | VEGF | 5 |
| 8HRE-VEGF | pEF-myc-cyto-mCMV-d2GFP | VEGF | 8 |
| 3HRE-EPO | pEF-myc-cyto-mCMV-d2GFP | EPO | 3 |
| 4HRE-EPO | pEF-myc-cyto-mCMV-d2GFP | EPO | 4 |
| 9HRE-EPO | pEF-myc-cyto-mCMV-d2GFP | EPO | 9 |
When culturing CHO-K1 cells expressing d2GFP under the control of VEGF-HREs under normal shaken conditions, these showed no induction of d2GFP expression irrespective of expression construct, with a minimal mean-fluorescence of around 0.3–0.5 between 24 h and 72 h (Fig. 2B). In contrast, the statically cultured CHO-K1 cells showed a strongly increased d2GFP expression with mean fluorescence of 0.7 (2HRE-VEGF), 1.0 (5HRE-VEGF) and up to 2.0 (8HRE-VEGF) over time, except for the mock control (Fig. 2B). Comparable or even slightly pronounced induced expression results were obtained with CHO-DG44 cells, where mock expressing or shaken cultured cells showed no induced d2GFP expression with values between 0.3 and 0.6, whereas a strong increase in fluorescence intensity was detected with statically cultivated 2HRE-VEGF (1.4), 5HRE-VEGF (3.0) and 8HRE-VEGF (2.4) CHO-DG44 cells (Fig. 2C). When using CHO-K1 cells expressing hypoxia inducible constructs with 3, 4 or 9 HREs derived from the EPO gene, only a slight increase in d2GFP expression was observed for static (0.4–0.7) compared to mock or shaken (0.2–0.5) cultured cells (Fig. 2D). However, CHO-DG44 cells expressing EPO-HRE constructs showed again a remarkably strong induction of d2GFP expression under/with static, hypoxia imitating conditions (1.5–2.2) in comparison to the shaken cultured control cells (0.3–0.6) (Fig. 2E).
These data indicated that the VEGF-HRE constructs induced protein expression in both cell lines more reliable than the EPO-HRE variants and that CHO-DG44 cells responded stronger to static and hypoxic cultivation conditions with both VEGF- and EPO-HRE constructs than CHO-K1 cells. In addition, a correlation between HRE number and expression response was observed, which was saturated between 5 and 8 HRE repetitions. Therefore, the 5HRE-VEGF construct was chosen for further developments due to its reliable and strong induction of protein expression under oxygen limitation.
3.3. Hypoxia induction during scale-up batch fermentation with defined oxygen concentrations
After identifying the 5HRE-VEGF vector construct as the most responsive and reliable HRE vector system under oxygen deprivation conditions, its performance was further characterized in a scale up batch fermentation under defined oxygen concentrations. Both stable mock and 5HRE-VEGF expressing CHO-K1 and CHO-DG44 cell pools were cultivated as batch in a 2 L-bioreactor under normoxic conditions (60% pO2) for 156 h (Fig. 3A). In addition, all cell lines were cultured in a batch fermentation where oxygen concentrations were switched to hypoxia starting after 12 h to 40% pO2 and continuing in a stepwise manner to 10%, at 60 h, 5% at 84 h and 1% at 108 h. For all fermentations, hypoxia induced d2GFP fluorescence was recorded at regular intervals by flow cytometry. CHO-DG44-mock and CHO-DG44-5HRE-VEGF cells displayed identical mean fluorescence over the first 72 h of cultivation (Fig. 3B). However, after a reduction to 5% pO2 the CHO-DG44-5HRE-VEGF cells showed a clear induction of d2GFP expression to about 0.6, which again increased significantly to 1.5 mean fluorescence at an oxygen concentration of 1%. In contrast, all control cells showed continuously decreasing values throughout the fermentation (Fig. 3B). When comparing the mean fluorescence of DG44-5HRE-VEGF cells cultured under normoxic and hypoxic conditions, a highly significant and nearly 10-fold induction of the d2GFP signal could be detected after 140 h, while no significant induction was observed for control cell lines (Fig. 3C).
When culturing CHO-K1 cell lines under the same as above described normoxic and hypoxic fermentation conditions, all cell lines showed for the first 84 h h a comparable pattern of low fluorescence signal of about 0.5 decreasing to a value of about 0.25 (Fig. 3D). After switching to hypoxia with a decrease to 5% pO2, CHO-K1-5HRE-VEGF cells displayed a slight increase in mean fluorescence to 0.3, which was further increased to 0.5 after a reduction to 1% pO2. For all control cell lines the fluorescence signal continued to decrease to about 0.15 at 5% pO2 and 0.1 at 1% pO2 (Fig. 3E). When comparing the mean fluorescence of CHO-K1-5HRE-VEGF cell lines after 140 h a significant stronger 3-fold d2GFP expression could be observed under hypoxic condition, while no induction was observed for control cell lines (Fig. 3E). Interestingly, the hypoxic cultivation using pO2 concentrations of 5% or 1% had no significant negative effect on growth or viability of CHO-DG44 or CHO-K1 cells (Supplementary Fig. 2).
Furthermore, when monitoring d2GFP at transcript level by qPCR, a significant increase in expression was observed for CHO-DG44 5HRE-VEGF cells at pO2 concentrations of 5% after 108 h or 1% after 156 h when compared to cells cultured at 60% pO2 after 108 h and 156 h, respectively (Fig. 3F). However, no significant increase in d2GFP RNA expression was observed for CHO-K1-5HRE-VEGF cells when cultured during oxygen deprivation conditions, which mirrors the lower induction at protein level (Fig. 3F and D). In addition, to validate the molecular basis for the observe induction of protein expression under hypoxic conditions, the protein expression of unstable HIF-1α as the oxygen sensing protein binding to the HREs was analyzed in normoxic (60% pO2) and hypoxic (5% and 1% pO2) cultivated cells. While under normoxia no expression was observed, HIF-1α was detected in CHO-DG44-5HRE-VEGF cells cultured under hypoxic conditions with a slight signal at 5% pO2 and a stronger stabilization at 1% pO2 (Fig. 3G). On the other hand, no expression of the unstable protein HIF-1α was determined via Western blot for CHO-K1-5HRE-VEGF cells under normoxia or hypoxia, which is in agreement with the lower response to hypoxia of CHO-K1 cells. These data generated for fermentations under controlled O2 conditions indicate a successful establishment of a hypoxia inducible vector system by using a 5HRE-VEGF vector construct to drive protein expression under oxygen limitation.
3.4. Exploiting hypoxia responsive induction for increased recombinant protein production during perfusion processing
The above established vector system responsive to hypoxic culture conditions may represent an excellent tool to exploit O2 deprivation encountered e.g. under high cell density conditions during perfusion cultures. To validate the potential of the hypoxia responsive vector system to improve recombinant protein expression under hypoxia, novel vector constructs were constructed now using a full CMV promotor as used industrially to drive protein expression. Here, the 5HRE-VEGF element was inserted upstream of a full CMV promotor to drive the expression of the recombinant model protein SEAP (Fig. 4A). After transfection and selection, resulting stable cell pools expressing SEAP under the control of a full CMV with 5HRE-VEGF elements (CHO-DG44-5HRE-VEGF-SEAP) and mock cell pools (CHO-DG44-SEAP) were cultured under O2 deprivation with very high cell density. Both cell lines were inoculated with a normal (0.5 × 106 cells/mL) and ultra-high (50 × 106 cells/mL) cell density and a batch cultivation was performed including a daily replacement of cell culture medium mimicking a perfusion process. The VCDs during the experiment are shown in Supplemental Fig. 3. After 3 media replacements the specific productivities were calculated revealing a slight decrease in specific productivity of ultra-high inoculated mock CHO-DG44-SEAP cells to around 80% when compared to the normally inoculated control (Fig. 4B). In contrast, CHO-DG44-5HRE-VEGF-SEAP cells did not decrease but significantly increased their specific productivity during mock-perfusion conditions of about 2.7-fold when compared to normally cultured cells leading to a significantly better performance during the mock-perfusion of cells carrying 5HREs of the gene VEGF (Fig. 4B).
Fig. 4.
Increased SEAP expression exploiting hypoxia during high cell density cultivations. (A) Schematic vector construct for SEAP expression using 5HREs of VEGF origin and a complete CMV. (B) Specific productivity of CHO-DG44-SEAP and CHO-DG44-5HRE-SEAP cells cultured in ultra-high cell density compared to normally cultured cells.
3.5. Oxygen shift as a novel bioprocessing strategy
During bioprocessing, a temperature shift from 37 °C to 34 °C is an established strategy to increase titers, prolong cultivation time and improve the product quality. After showing the potential of the oxygen responsive vector system to improve productivity under high cell density conditions, we now explored its potential to improve the production capacity of CHO cells during classical fermentation processes when applying an oxygen shift to further induce the vector system. Therefore, a fed-batch fermentation with CHO-DG44-5HRE-VEGF-SEAP cells was performed in a 2 L benchtop bioreactor, where cells were cultured under standard conditions (37 °C, 40% pO2) until reaching the static growth phase. At this point both a temperature shift to 34 °C alone and in combination with an oxygen shift to 1% pO2 was conducted. After reducing temperature or temperature and oxygen concentration, VCD remained stable for both cultures, with one fermentation showing a slightly lower VCD before the condition shift (Fig. 5A). Interestingly, a nearly identical viability for both culture conditions of >90% was observed over 12 days, demonstrating the ability of CHO cells to be cultured under strong oxygen deprivation conditions (Fig. 5B). During the process, CHO cells cultured with an oxygen shift to 1% pO2 consumed slightly more glucose and less L-glutamine (Supplemental Fig. S4). When analyzing recombinant protein titer of both fermentations for the first 6 days before shifting the conditions, a comparable concentration of around 13 μg/mL of produced protein was monitored (Fig. 5C) with similar specific productivities of approximately 1.7 pg/cell/day (Fig. 5D). However, after shifting temperature or temperature and oxygen concentration SEAP expression was induced for both conditions, however, to a significantly stronger extent when adjusting pO2 concentration to 1% (Fig. 5C and D). Under these conditions, the hypoxia inducible system led to a SEAP titer of around 460 μg/mL (Fig. 5C) and a nearly 2-fold higher specific productivity than non-induced cells of approximately 20 pg/cell/day SEAP (Fig. 5D).
Fig. 5.
Establishment of a novel bioprocess strategy conducting an oxygen shift to induce recombinant protein expression. (A + B) Viable cell density (A) and viability (B) of CHO-DG44-5HRE-SEAP cells during fed-batch fermentation. (C) SEAP concentration during fed-batch fermentation secreted by CHO-DG44-5HRE-SEAP cells with and without hypoxic shift to 1% O2. (D) Specific productivity of CHO-DG44-5HRE-SEAP cells is shown in average of all days before the temperature and oxygen shift and after the shift. Statistical analysis was conducted using one-way ANOVA with Bonferroni correction [n = 3 replicates; Mean ± SD; * = p < 0.05; ** = p < 0.01].
4. Discussion
Driven by increasing challenges for CHO producer cell lines confronted with the need for improved product titer or the expression of complex format recombinant proteins, intensified continuous high cell density perfusion processes are becoming highly attractive alternatives to common fed-batch fermentations (Gagnon et al., 2018). This next generation biomanufacturing platform ensures a longer process, higher cell densities and better product quality (Kwon et al., 2017; Konstantinov and Cooney, 2015) as well as higher yield, leading to substantial cost and time savings (Janoschek et al., 2019; Xu et al., 2017). However, these initially beneficial characteristics are leading to new challenges for CHO cells such as cellular aggregation, medium acidification and increased cell densities (Ruffieux et al., 1998; Xiu et al., 1999; Michl et al., 2019; Jing et al., 2016; Kishishita et al., 2015; Shojaosadati et al., 2008). Especially high cell densities were shown to initiate oxygen deprivation conditions affecting CHO cell productivity and counteracting improved recombinant protein titer (Ruffieux et al., 1998; Xiu et al., 1999; Michl et al., 2019; Jing et al., 2016; Kishishita et al., 2015). To counteract this drawback of continuous bioprocessing, popular solutions have been increased agitation or gassing rates entailing their own drawbacks as elevated shear stress for agitation or higher costs and a certain toxicity for CHO cells for pure oxygen gassing (Shojaosadati et al., 2008; Lee, 1996; Baez and Shiloach, 2014). However, cell engineering approaches have not been undertaken to adapt CHO cells to novel continuous bioprocessing conditions.
Here, we aimed to engineer CHO production cell lines by developing a vector system exploiting limiting oxygen conditions during high cell density cultivation to increase CHO cell productivity without the need for complex and cost intensive host cell modifications. We showed that 5 repeats of HRE derivatives of VEGF-A origin are superior enhancers for recombinant protein expression in CHO cells when induced by oxygen deprivation conditions. This was observed not only in combination with a minimalCMV promotor, but also for a full CMV promotor driving the expression of a recombinant model protein. Using these established enhancer elements, perfusion processes may benefit massively by exploiting the accompanying induced hypoxia. Oxygen limitation in high cell density cultivations is a well-known problem resulting in reduced specific productivities and low overall titers (Kwon et al., 2017; Janoschek et al., 2019; Shojaosadati et al., 2008; Ozturk, 1996). While conservative solutions as increased agitation or gassing rates failed in restoring CHO productivity with additional drawbacks on cell viability and protein quality (Shojaosadati et al., 2008; Lee, 1996; Baez and Shiloach, 2014), hypoxia induction by the utilization of HREs even increased specific productivity during perfusion experiments to around 2.7-fold in our study.
A further attractive option enabled by the utilization of HREs is the application of novel bioprocessing strategies for discontinuous fed-batch processes. Standard industrial fed-batch processes apply a shift of temperature from 37 °C to lower temperatures after cells reach the static cultivation phase (McHugh et al., 2020). Thereby, the longevity of the bioprocess can be enhanced combined with an increased final product titer as temperature reduction leads to a favorable redistribution of nutrients for therapeutic product synthesis (McHugh et al., 2020; Avello et al., 2017; Bollati-Fogolín et al., 2008). We hypothesized, that using a HREs containing vector system, hypoxia could be exploited by adjustment of oxygen setpoints after cells reach the static phase to induce and drive hypoxia responsive expression of the recombinant protein. Interestingly, our data demonstrate that when the oxygen shift is performed in parallel with temperature reduction after cells reach the static cultivation phase, a nearly 2-fold increase of specific productivity can be achieved by exploiting hypoxia in contrast to common fed-batch strategies.
While hypoxia was in our study exploited for improved productivity, Lieske et al. recently described the hypoxia signaling pathway to be involved in the phenotypic instability of CHO cells leading to a loss of titer and notably significantly upregulated HIF-1α expression levels over time (Lieske et al., 2019). Although phenotypic instability of industrially used CHO cell lines is one of the major challenges, only little is known about the underlying molecular mechanisms (Bailey et al., 2012). However, by introducing HRE elements in recombinant expression vectors relevant binding proteins may occupy these elements and thereby even improve long-term expression of the gene of interest in CHO cells to counteract phenotypic instability by taking advantage of upregulated HIF-1α expression levels.
Cell line engineering is an attractive tool to adapt production cell lines to novel challenges. Our demonstration of engineered exploitation of hypoxia for improved bioproduction highlights that adverse process conditions can be successfully converted into productive traits and even enable the application of novel bioprocessing strategies for optimized production.
Author statement
Nikolas Zeh: Conceptualization, Methodology, Investigation, Writing - Original Draft; Patrick Schlossbauer: Investigation, Writing - Review & Editing; Nadja Raab: Investigation, Writing - Review & Editing; Florian Klingler: Investigation, Writing - Review & Editing; René Handrick: Supervision, Writing - Review & Editing; Kerstin Otte: Conceptualization, Supervision, Writing - Review & Editing.
Funding/acknowledgement statement
Nikolas Zeh is funded by the German Federal Ministry of Education and Research, funding program Forschung an Fachhochschulen, contract number 13FH162PA6.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mec.2021.e00181.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Avello V., Tapia B., Vergara M., Acevedo C., Berrios J., Reyes J.G. Impact of sodium butyrate and mild hypothermia on metabolic and physiological behaviour of CHO TF 70R cells. Electron. J. Biotechnol. 2017;27:55–62. doi: 10.1016/j.ejbt.2017.03.008. [DOI] [Google Scholar]
- Baez A., Shiloach J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microb. Cell Factories. 2014;13:181. doi: 10.1186/s12934-014-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L.A., Hatton D., Field R., Dickson A.J. Determination of Chinese hamster ovary cell line stability and recombinant antibody expression during long-term culture. Biotechnol. Bioeng. 2012;109:2093–2103. doi: 10.1002/bit.24485. [DOI] [PubMed] [Google Scholar]
- Bergeron M., Gidday J.M., Yu A.Y., Semenza G.L., Ferriero D.M., Sharp F.R. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann. Neurol. 2000;48 285–296. doi:10.1002/1531-8249(200009)48:3<285::AID-ANA2>3.0.CO;2–8. [PubMed] [Google Scholar]
- Binley K., Askham Z., Martin L., Spearman H., Day D., Kingsman S. Hypoxia-mediated tumour targeting. Gene Ther. 2003;10:540–549. doi: 10.1038/sj.gt.3301944. [DOI] [PubMed] [Google Scholar]
- Bollati-Fogolín M., Forno G., Nimtz M., Conradt H.S., Etcheverrigaray M., Kratje R. Temperature reduction in cultures of hGM-CSF-expressing CHO cells: effect on productivity and product quality. Biotechnol. Prog. 2008;21:17–21. doi: 10.1021/bp049825t. [DOI] [PubMed] [Google Scholar]
- Brown J.M., Wilson W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Canc. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J. Gen. Physiol. 1965:49. doi: 10.1085/jgp.49.1.163. Suppl:163-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C., Wang Q., Yang S., Ponce S.A., Kirsch B.J., Zhang H. Combinatorial genome and protein engineering yields monoclonal antibodies with hypergalactosylation from CHO cells. Biotechnol. Bioeng. 2017;114:2848–2856. doi: 10.1002/bit.26375. [DOI] [PubMed] [Google Scholar]
- Fu T., Zhang C., Jing Y., Jiang C., Li Z., Wang S. Regulation of cell growth and apoptosis through lactate dehydrogenase C over-expression in Chinese hamster ovary cells. Appl. Microbiol. Biotechnol. 2016;100:5007–5016. doi: 10.1007/s00253-016-7348-4. [DOI] [PubMed] [Google Scholar]
- Gagnon M., Nagre S., Wang W., Hiller G.W. Shift to high-intensity, low-volume perfusion cell culture enabling a continuous, integrated bioprocess. Biotechnol. Prog. 2018;34:1472–1481. doi: 10.1002/btpr.2723. [DOI] [PubMed] [Google Scholar]
- Gonzalez F.J., Xie C., Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2019;15:21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco O., Marples B., Dachs G.U., Williams K.J., Patterson A.V., Scott S.D. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 2002;9:1403–1411. doi: 10.1038/sj.gt.3301823. [DOI] [PubMed] [Google Scholar]
- Han Y.K., Ha T.K., Kim Y.-G., Lee G.M. Bcl-xL overexpression delays the onset of autophagy and apoptosis in hyperosmotic recombinant Chinese hamster ovary cell cultures. J. Biotechnol. 2011;156:52–55. doi: 10.1016/j.jbiotec.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Huang Y.-M., Hu W., Rustandi E., Chang K., Yusuf-Makagiansar H., Ryll T. Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol. Prog. 2010;26:1400–1410. doi: 10.1002/btpr.436. [DOI] [PubMed] [Google Scholar]
- Hubbi M.E., Semenza G.L. An essential role for chaperone-mediated autophagy in cell cycle progression. Autophagy. 2015;11:850–851. doi: 10.1080/15548627.2015.1037063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbi M.E., Semenza G.L. Regulation of cell proliferation by hypoxia-inducible factors. Am. J. Physiol. Physiol. 2015;309:C775–C782. doi: 10.1152/ajpcell.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoschek S., Schulze M., Zijlstra G., Greller G., Matuszczyk J. A protocol to transfer a fed-batch platform process into semi-perfusion mode: the benefit of automated small-scale bioreactors compared to shake flasks as scale-down model. Biotechnol. Prog. 2019;35 doi: 10.1002/btpr.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javan B., Shahbazi M. Hypoxia-inducible tumour-specific promoters as a dual-targeting transcriptional regulation system for cancer gene therapy. Ecancermedicalscience. 2017;11:751. doi: 10.3332/ecancer.2017.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Zhang C., Fu T., Jiang C., Ma K., Zhang D. Combination of dextran sulfate and recombinant trypsin on aggregation of Chinese hamster ovary cells. Cytotechnology. 2016;68:241–248. doi: 10.1007/s10616-014-9774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W.G., Ratcliffe P.J., Semenza G.L., William, Kaelin G., Jr., Sir Peter J., Ratcliffe, Gregg L. Semenza “for their discoveries of how cells sense and adapt to oxygen availability.”. In: The Nobel Prize. 2019 https://www.nobelprize.org/prizes/medicine/2019/press-release/ [Google Scholar]
- Kimura S., Omasa T. Genome sequence comparison between Chinese hamster ovary (CHO) DG44 cells and mouse using end sequences of CHO BAC clones based on BAC-FISH results. Cytotechnology. 2018;70:1399–1407. doi: 10.1007/s10616-018-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Weisz A., Ogura T., Hitomi Y., Kurashima Y., Hashimoto K. Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J. Biol. Chem. 2001;276:2292–2298. doi: 10.1074/jbc.M008398200. [DOI] [PubMed] [Google Scholar]
- Kishishita S., Katayama S., Kodaira K., Takagi Y., Matsuda H., Okamoto H. Optimization of chemically defined feed media for monoclonal antibody production in Chinese hamster ovary cells. J. Biosci. Bioeng. 2015;120:78–84. doi: 10.1016/j.jbiosc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Konstantinov K.B., Cooney C.L., White Paper on Continuous Bioprocessing May 20–21 2014 Continuous manufacturing symposium. J. Pharmacol. Sci. 2015;(104):813–820. doi: 10.1002/jps.24268. [DOI] [PubMed] [Google Scholar]
- Kwon T., Prentice H., De Oliveira J., Madziva N., Warkiani M.E., Hamel J.-F.P. Microfluidic cell retention device for perfusion of mammalian suspension culture. Sci. Rep. 2017;7:6703. doi: 10.1038/s41598-017-06949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux H., Romand S., Nuciforo S., Farady C.J., Tapparel J., Buechmann-Moeller S. Degradation of recombinant proteins by Chinese hamster ovary host cell proteases is prevented by matriptase-1 knockout. Biotechnol. Bioeng. 2018;115:2530–2540. doi: 10.1002/bit.26731. [DOI] [PubMed] [Google Scholar]
- Lee S.Y. High cell-density culture of Escherichia coli. Trends Biotechnol. 1996;14:98–105. doi: 10.1016/0167-7799(96)80930-9. [DOI] [PubMed] [Google Scholar]
- Lee J.W., Ko J., Ju C., Eltzschig H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019;51:1–13. doi: 10.1038/s12276-019-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Ko H.P., Whitlock J.P. Induction of phosphoglycerate kinase 1 gene expression by hypoxia. J. Biol. Chem. 1996;271:21262–21267. doi: 10.1074/jbc.271.35.21262. [DOI] [PubMed] [Google Scholar]
- Li F., Vijayasankaran N., Shen A. (Yijuan), Kiss R, Amanullah A. Cell culture processes for monoclonal antibody production. mAbs. 2010;2:466–479. doi: 10.4161/mabs.2.5.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tian Z., Xu D., Wang X., Wang T. Construction strategies for developing expression vectors for recombinant monoclonal antibody production in CHO cells. Mol. Biol. Rep. 2018;45:2907–2912. doi: 10.1007/s11033-018-4351-0. [DOI] [PubMed] [Google Scholar]
- Lieske P.L., Wei W., Crowe K.B., Figueroa B., Zhang L. HIF-1 signaling pathway implicated in phenotypic instability in a Chinese hamster ovary production cell line. Biotechnol. J. 2019 doi: 10.1002/biot.201900306. e1900306. [DOI] [PubMed] [Google Scholar]
- Lin N., Brooks J., Sealover N., George H.J., Kayser K.J. Overexpression of Serpinb1 in Chinese hamster ovary cells increases recombinant IgG productivity. J. Biotechnol. 2015;193:91–99. doi: 10.1016/j.jbiotec.2014.10.040. [DOI] [PubMed] [Google Scholar]
- McHugh K.P., Xu J., Aron K.L., Borys M.C., Li Z.J. Effective temperature shift strategy development and scale confirmation for simultaneous optimization of protein productivity and quality in Chinese hamster ovary cells. Biotechnol. Prog. 2020;36:1–11. doi: 10.1002/btpr.2959. [DOI] [PubMed] [Google Scholar]
- Michl J., Park K.C., Swietach P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun. Biol. 2019;2:144. doi: 10.1038/s42003-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk S.S. Engineering challenges in high density cell culture systems. Cytotechnology. 1996;22:3–16. doi: 10.1007/BF00353919. [DOI] [PubMed] [Google Scholar]
- Ruffieux P.-A., von Stockar U., Marison I.W. Measurement of volumetric (OUR) and determination of specific (qO2) oxygen uptake rates in animal cell cultures. J. Biotechnol. 1998;63:85–95. doi: 10.1016/S0168-1656(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Sandner P., Wolf K., Bergmaier U., Gess B., Kurtz A. Induction of VEGF and VEGF receptor gene expression by hypoxia: divergent regulation in vivo and in vitro. Kidney Int. 1997;51:448–453. doi: 10.1038/ki.1997.60. [DOI] [PubMed] [Google Scholar]
- Scarcelli J.J., Shang T.Q., Iskra T., Allen M.J., Zhang L. Strategic deployment of CHO expression platforms to deliver Pfizer's Monoclonal Antibody Portfolio. Biotechnol. Prog. 2017;33:1463–1467. doi: 10.1002/btpr.2493. [DOI] [PubMed] [Google Scholar]
- Schödel J., Ratcliffe P.J. Mechanisms of hypoxia signalling: new implications for nephrology. Nat. Rev. Nephrol. 2019;15:641–659. doi: 10.1038/s41581-019-0182-z. [DOI] [PubMed] [Google Scholar]
- Shibata T., Giaccia A.J., Brown J.M. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- Shojaosadati S.A., Varedi Kolaei S.M., Babaeipour V., Farnoud A.M. Recent advances in high cell density cultivation for production of recombinant protein. Iran. J. Biotechnol. 2008;6:63–84. http://www.ijbiotech.com/article_7048.html Available. [Google Scholar]
- Škulj M., Pezdirec D., Gaser D., Kreft M., Zorec R. Reduction in C-terminal amidated species of recombinant monoclonal antibodies by genetic modification of CHO cells. BMC Biotechnol. 2014;14:76. doi: 10.1186/1472-6750-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann C., Fandrey J., HYPOXIA-INDUCED ERYTHROPOIETIN PRODUCTION A paradigm for oxygen-regulated gene expression. Clin. Exp. Pharmacol. Physiol. 2006;33:968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- Tanimoto K. Mechanism of regulation of the hypoxia-inducible factor-1alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermark D., Shibata T., Brown J.M. Green fluorescent protein is a suitable reporter of tumor hypoxia despite an oxygen requirement for chromophore formation. Neoplasia. 2001;3:527–534. doi: 10.1038/sj.neo.7900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- Wang B., Albanetti T., Miro-Quesada G., Flack L., Li L., Klover J. High-throughput screening of antibody-expressing CHO clones using an automated shaken deep-well system. Biotechnol. Prog. 2018;34:1460–1471. doi: 10.1002/btpr.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu Z.L., Deckwer W.D., Zeng A.P. Estimation of rates of oxygen uptake and carbon dioxide evolution of animal cell culture using material and energy balances. Cytotechnology. 1999;29:159–166. doi: 10.1023/A:1008004618163. 10.1023/A:1008004618163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Nagarajan H., Lewis N.E., Pan S., Cai Z., Liu X. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011;29:735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Gavin J., Jiang R., Chen H. Bioreactor productivity and media cost comparison for different intensified cell culture processes. Biotechnol. Prog. 2017;33:867–878. doi: 10.1002/btpr.2415. [DOI] [PubMed] [Google Scholar]
- Zhong X., Ma W., Meade C.L., Tam A.S., Llewellyn E., Cornell R. Transient CHO expression platform for robust antibody production and its enhanced N-glycan sialylation on therapeutic glycoproteins. Biotechnol. Prog. 2019;35 doi: 10.1002/btpr.2724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.